Abstract
Lower Eocene fluvial strata in the Chuckanut Formation preserve abundant bird and mammal tracks. Reptile trace fossils include footprints from a small turtle (ichnogenus Chelonipus), and several Crocodylian trackways that consist of irregularly spaced footprints associated with linear tail drag marks. The latter trackways represent “punting” locomotion, where a submerged Crocodylian used intermittent substrate contacts to provide forward motion of their neutrally buoyant bodies. Two adjacent sandstone blocks preserve Crocodylian trace fossils that are named herein as a new ichnogenus and ichnospecies Anticusuchipes amnis. Two other Crocodylian trackways lack sufficient detail for ichnotaxonomic assignment.
Keywords:
Chuckanut Formation; Crocodylian; footprints; ichnology; trace fossils; tracks; turtle; Washington 1. Introduction
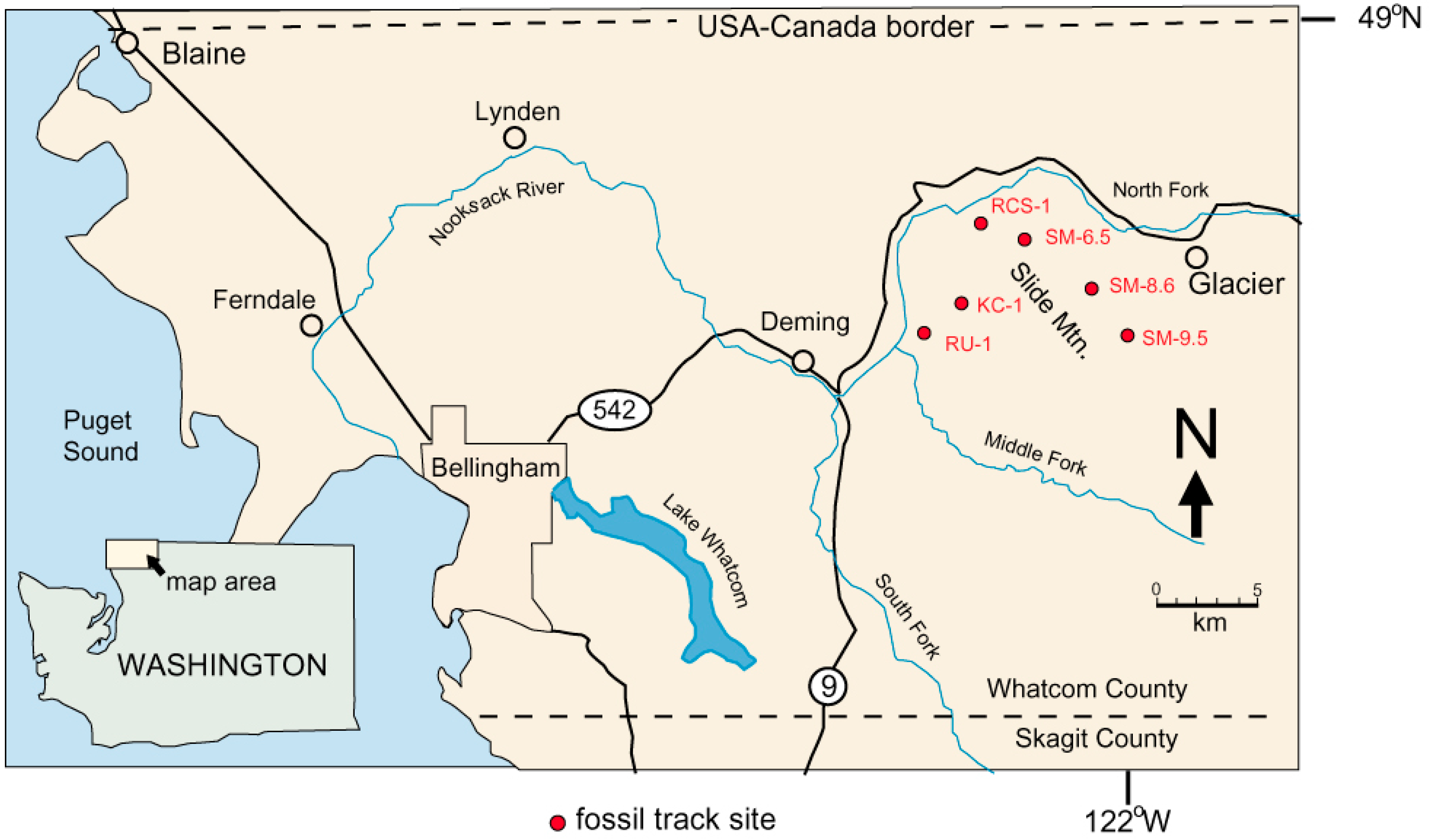
Birds and animals are represented by a diverse variety of footprints preserved on bedding planes in gently-dipping Chuckanut Formation strata in the Mount Baker foothills in Western Whatcom County, Washington, USA (Figure 1, Table 1). These locations are in a forested area that has been subject to extensive commercial logging, where road construction yields new bedrock exposures. Landslides also reveal fossiliferous bedrock. The discovery of these Eocene tracks is noteworthy because evidence of vertebrate life is scarce in Paleogene continental sedimentary rocks in the Pacific Northwest. This paper describes reptile tracks; avian and mammalian trace fossils will be described in subsequent reports.
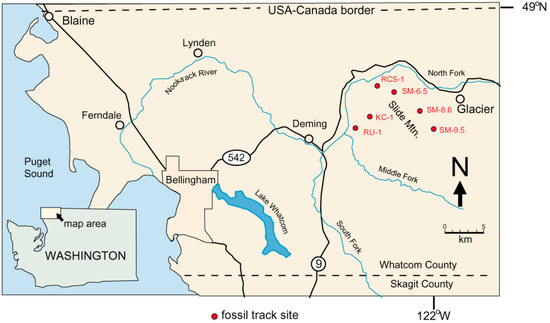
Figure 1.
Chuckanut Formation fossil track locations. Reptile trace fossils are known only from Western Washington University (WWU)-KC-1 turtle trackway) and WWU-RCS-1 (Crocodylian tracks) sites.

Table 1.
Reptile track locations. GPS locations are from WGS 84 datum.
These discoveries are noteworthy given the scarcity of reports of Cenozoic reptile tracks. For perspective, a comprehensive bibliography of 584 references describing Cenozoic vertebrate tracks [1] comprise 1% Amphibia, 2% Reptilia, 32.7% Aves, and 64.3% Mammalia. The only known turtle tracks are two Eocene ichnospecies, Chelonipus chardronicus and C. parvus from Texas USA [2], and two Paleocene Crocodylian ichnotaxa, Albertasuchipes russellia from Alberta, Canada, [3], and Boreaosuchipes hanksi from North Dakota, USA [4]. Our report establishes a new Crocodylian ichnotaxon, Anticusuchipes amnis, and describes a turtle trackway as Chelonipus isp. For ichnotaxonomic purposes, the term crocodyle is used include members of three families: Crocodylidae (true crocodiles), Alligatoridae (alligators and caimen), and Gavailidae (gharail and false gharail).
1.1. Geologic Setting
The Chuckanut Formation consists of beds of conglomerate, arkosic sandstone, siltstone, and coal that unconformably overlie Paleozoic and Mesozoic metamorphic basement rocks. These fluvial sediments were deposited on a broad floodplain that existed prior to the mid-Tertiary uplift of the North Cascade Range [5,6]. Isolated exposures extend along fault zones to connect the main outcrop belt on the west side of the Cascade Range with the Swauk Formation in Central Washington. Correlative strata also extend north into British Columbia where they are called the Huntingdon Formation [7]. Mustoe and Gannaway [8] suggested that this far-flung outcrop distribution is evidence of a large depositional basin that was dissected by strike-slip faulting.
The Chuckanut Formation is one of the thickest nonmarine sedimentary formations in North America, but extensive forest cover restricts measurement of continuous stratigraphic sections. Estimates of the total thickness of the formation in the main outcrop belt in northwest Washington range from 3000 m [9] to 8300 m [10]. Recent mapping suggests a minimum thickness of 4000–5000 m [11].
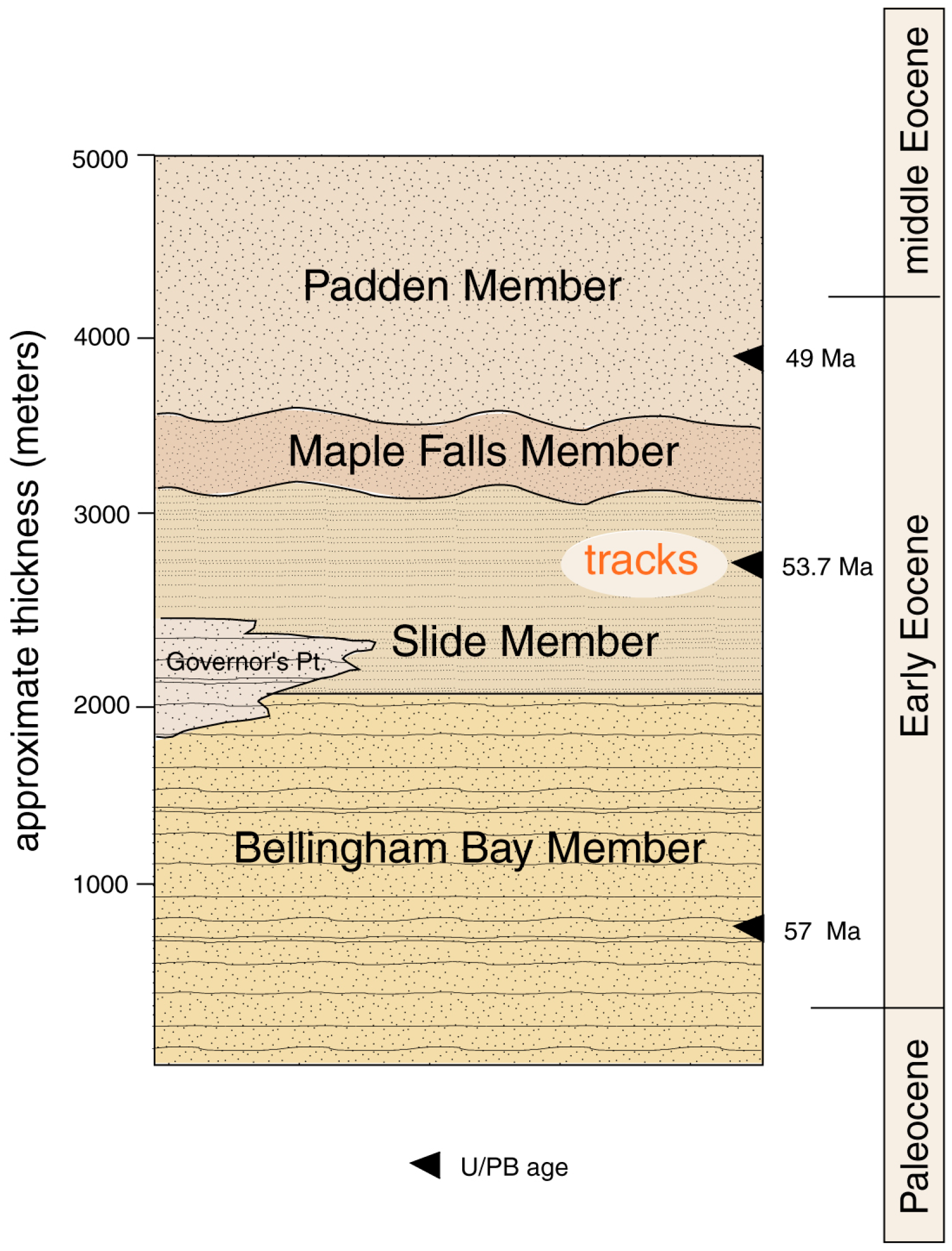
Several schemes have been proposed for dividing the Chuckanut Formation into stratigraphic members [5,8,11]. The basic architecture is a three-component system composed of the basal Late Paleocene-Early Eocene Bellingham Bay Member, overlain in the Mount Baker foothills by the Early Eocene Slide Member (Figure 2). The third component is the middle Eocene-Late Eocene Padden Member, which is perhaps separated from underlying strata by an unconformity. The stratigraphic positions of two minor members remain uncertain [11].
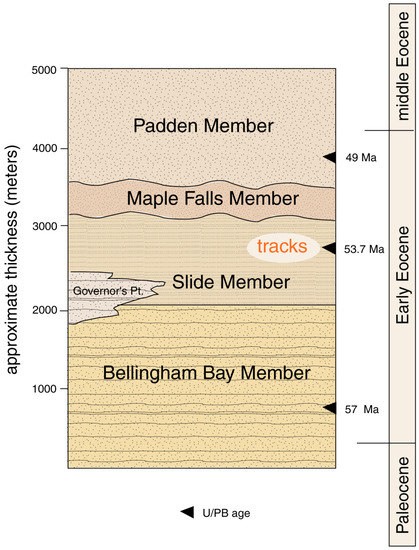
Figure 2.
Stratigraphy of the Chuckanut Formation.
All track fossils that have so far been discovered have come from Slide Member strata in the Mount Baker foothills. These discoveries are related to the gentle dip of Chuckanut strata in this part of the main outcrop belt, where outcrops commonly expose bedding plane surfaces where tracks are likely to be preserved. In contrast, other stratigraphic members largely comprise steeply-dipping strata, where bedrock exposures show bedding edges rather than bedding planes.
Locations are shown in Table 1. Reptile trace fossils have found only at two locations, WWU-KC-1 (turtle trackway) and WWU-RCS-1 (Crocodylian tracks). The WWU-KC-1 site has been described in detail [12]. The WWU-RCS-1 site is at a major landslide that occurred 9 January 2009 in the headwaters of Racehorse Creek. The landslide (Figure 3) extends over 1 km in length, with an estimated 5 × 105 m3 of displaced rock distributed over an elevation range of 800 m [13]. Fossil tracks occur in sandstone blocks scattered over the scattered over a large part of the slide area, but originating in a stratigraphic zone that contains a tephra horizon that yielded a U-Pb age of 53.676 ± 0.023 Ma [11].

Figure 3.
Racehorse Creek landslide, locality WWU-RCS-1.
1.2. Paleoenvironment and Paleoclimate
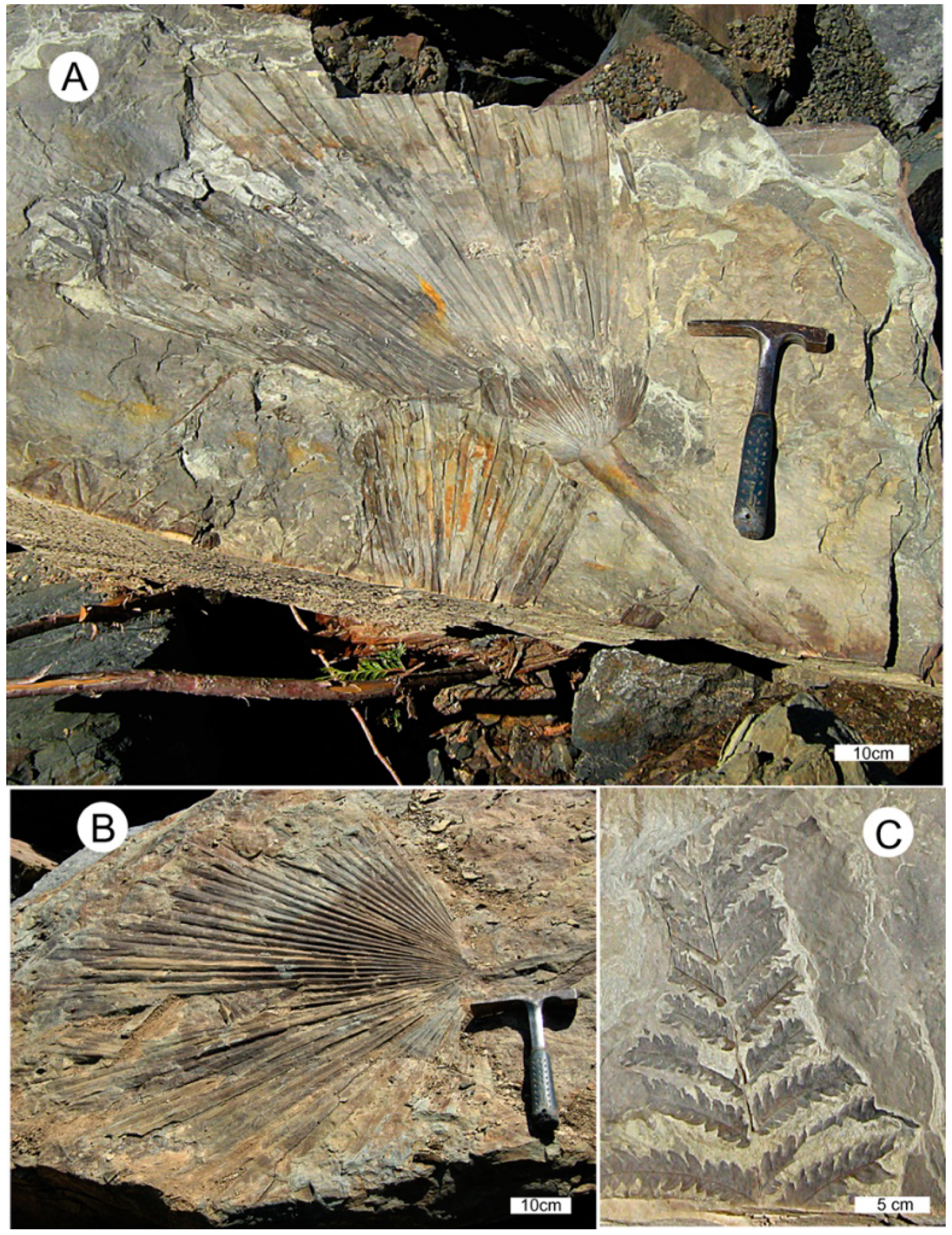
The common occurrence of footprints from a diverse assemblage of birds and animals at a single locality, often on the same bedding plane, shows that these creatures lived contemporaneously. Track fossils are typically preserved in sandstone that originated as point bar deposits bordering the ancient meandering river, and the track-makers likely utilized the riverbank as a source of water and food. The abundance of invertebrate traces in subaqueous strata suggests the availability of a rich nutrient supply for wading birds, which are represented by several avian ichnogenera. Nearby fine-grained facies originated as overbank deposits that contain abundant sub-tropical rain forest plant fossils. Two of the most common plant fossils are Sabalites palm fronds, and fronds of a tree fern, Cyathea pinnata Pabst (Figure 4). More than 30 species of angiosperms are represented by leaf and seed fossils. Mustoe and Gannaway [8] used the CLAMP multivariate analysis method of Wolfe [14] to calculate a mean annual temperature (MAT) of 16°, and an estimated annual precipitation of 150–250 cm for Slide Member strata. Breedlovestrout [11] employed the leaf margin analysis method [15] to calculate a MAT of 19.8° for the same fossil assemblage, and 21.9° for specimens from a nearby site.
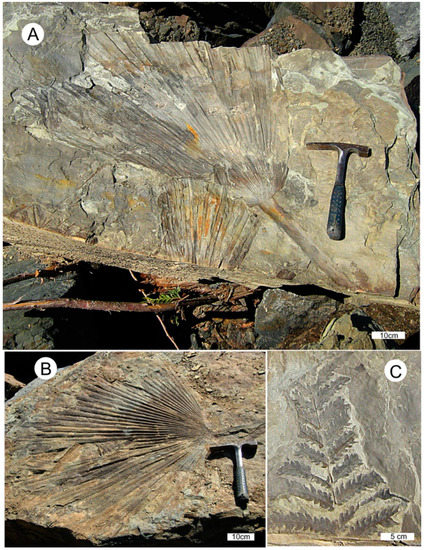
Figure 4.
Semi-tropical plant fossils at Racehorse Creek landslide, WWU locality RCS-1. (A,B) Sabalites palm fronds; (C) Cyathea pinnata tree fern frond.
1.3. Previous Work
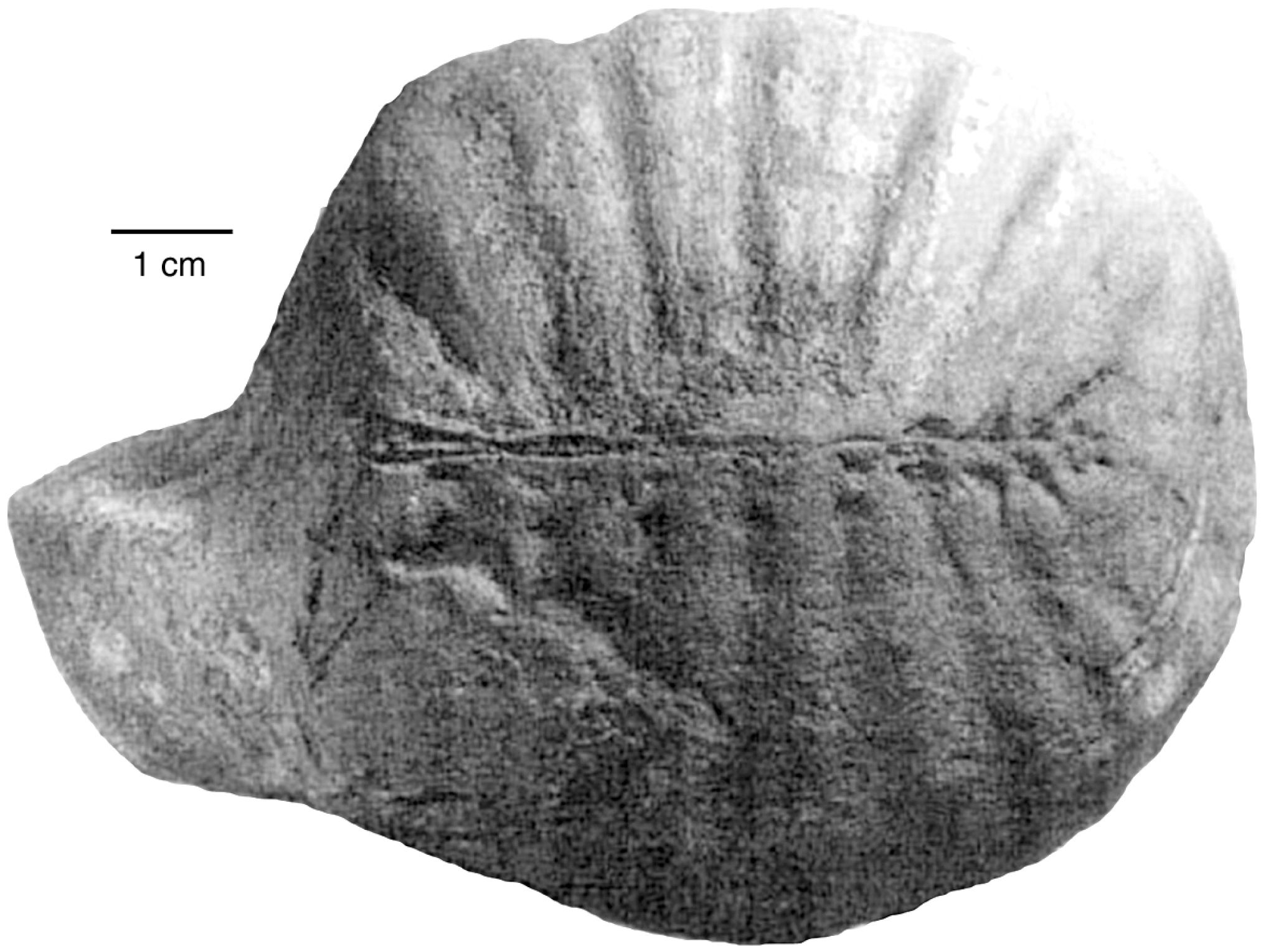
The first discovery of vertebrate footprints in the Paleocene/early Eocene Chuckanut Formation was made in 1990, when logging road construction in the Mt. Baker foothills exposed a trackway of a heron-like bird [12]. In later years, other tracks were found at several nearby localities [4,6,8]. Although bird and mammal tracks are the most common vertebrate trace fossils in the Chuckanut Formation, reptiles are represented. The only known vertebrate skeletal fossil from the Chuckanut Formation is the carapace of a small pond turtle (Figure 5) [16,17]. Reptile trace fossils include a turtle trackway preserved in the slab found in 1990 that contains heron-like tracks, and Crocodylian tracks that were exposed by the 2009 Racehorse Creek landslide. These trace fossils are described in detail in this report.
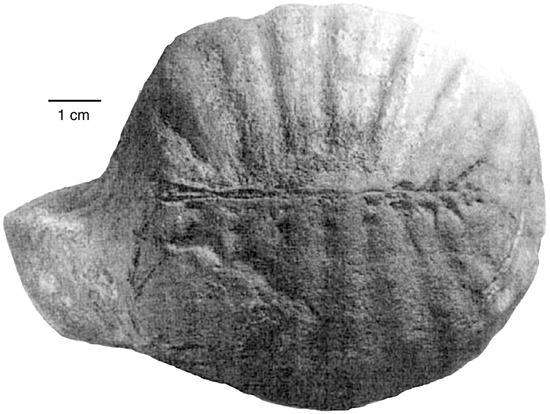
Figure 5.
Cast of a carapace of an early Eocene trionychid turtle from the Chuckanut Formation near Bellingham, Washington USA. Adapted from [17].
2. Methods
Trace fossils were collected as a collaborative effort by geoscientists and avocational paleontology enthusiasts. Fossils from Chuckanut Formation sites described in this report are presently housed at the Western Washington University Geology Department, and at the University of Washington Burke Museum of History and Culture. This joint venture has caused collecting locations to have been assigned duplicate identifications using both WWU and UWBM catalog numbers. At present, all Crocodylian trace fossils are archived at Western Washington University in Bellingham, WA USA. The WWU-KC-1 slab that preserves heron-like tracks and a turtle trackway was quarried by a team from the University of Washington, and the specimen is presently at the Burke Museum in Seattle, WA USA. At the WWU-RCS-1 site, trackway geometries were recorded by photography and by tracing the surface features on a flexible sheet of transparent plastic. Footprint molds were made using latex or silicone. One original reptile track slab was transported for display at the WWU Geology Department. Other reptile tracks are on large blocks that remain at the site; taxonomic descriptions utilize silicone molds and plaster casts as type specimens. Unless otherwise noted, all photographs and illustrations were created by the author.
3. Ichonotaxonomy
Ichnotaxonomic names are traditionally based strictly on morphology. Many articles have been devoted to this topic [18,19,20,21,22,23]. The method of “pure ichnotaxonomy” is virtually a necessity for classifying invertebrate trace fossils, which may include burrows, feeding traces, marks left insect larvae, and a host of other features, some of which are of enigmatic origin. Strategies for the ichnotaxonomy of vertebrate footprints have often been different from the classification methods used for invertebrate trace fossils. The International Code of Zoological Nomenclature [22,23] is based on the philosophy that trace fossils are named based strictly on morphology, without consideration of possible track-makers. However, vertebrate tracks provide an indication of foot anatomy, and the skeletal structures are known for a multitude of ancient and extant creatures. Therefore, it is a common practice to speculate on the identity of the track-maker. One approach has been to identify vertebrate tracks at the Class or Order level, but to create ichnofamily and ichnogenus names to classify the trace fossils. The result is a classification scheme that mirrors conventional zoological classification, but uses ichnotaxonomic categories.
The foundation of this approach is the work of O.S. Vialov (also spelled “Vyalov” in some citations), who assigned fossil tracks as ichnospecies within broadly-defined ichnogenera [24,25]. For example, in the Class Aves, all fossil bird tracks were given the ichnogenus name Avipeda; carnivore footprints were given the name Bestiopeda. Vialov’s groundbreaking work is widely cited but his monographs are difficult to access and are published in Russian. Fortunately, a recent English language translation is readily available [26]. English versions are also available for two other articles [27,28].
Vialov’s taxonomy was first used to describe trace fossils from the Russia and Eastern Europe, but later applied to Miocene bird and mammal tracks from Nevada, USA [29]. Subsequently, a compromise strategy has been introduced, using ichnologically-based families into higher-level “natural” taxonomic categories that are inferred from osteological characteristics [30]. In this report, presumed reptile footprints are classified by Class and Order. Lower level identifications are made using ichnotaxonomic criteria.
4. Chuckanut Formation Turtle Tracks
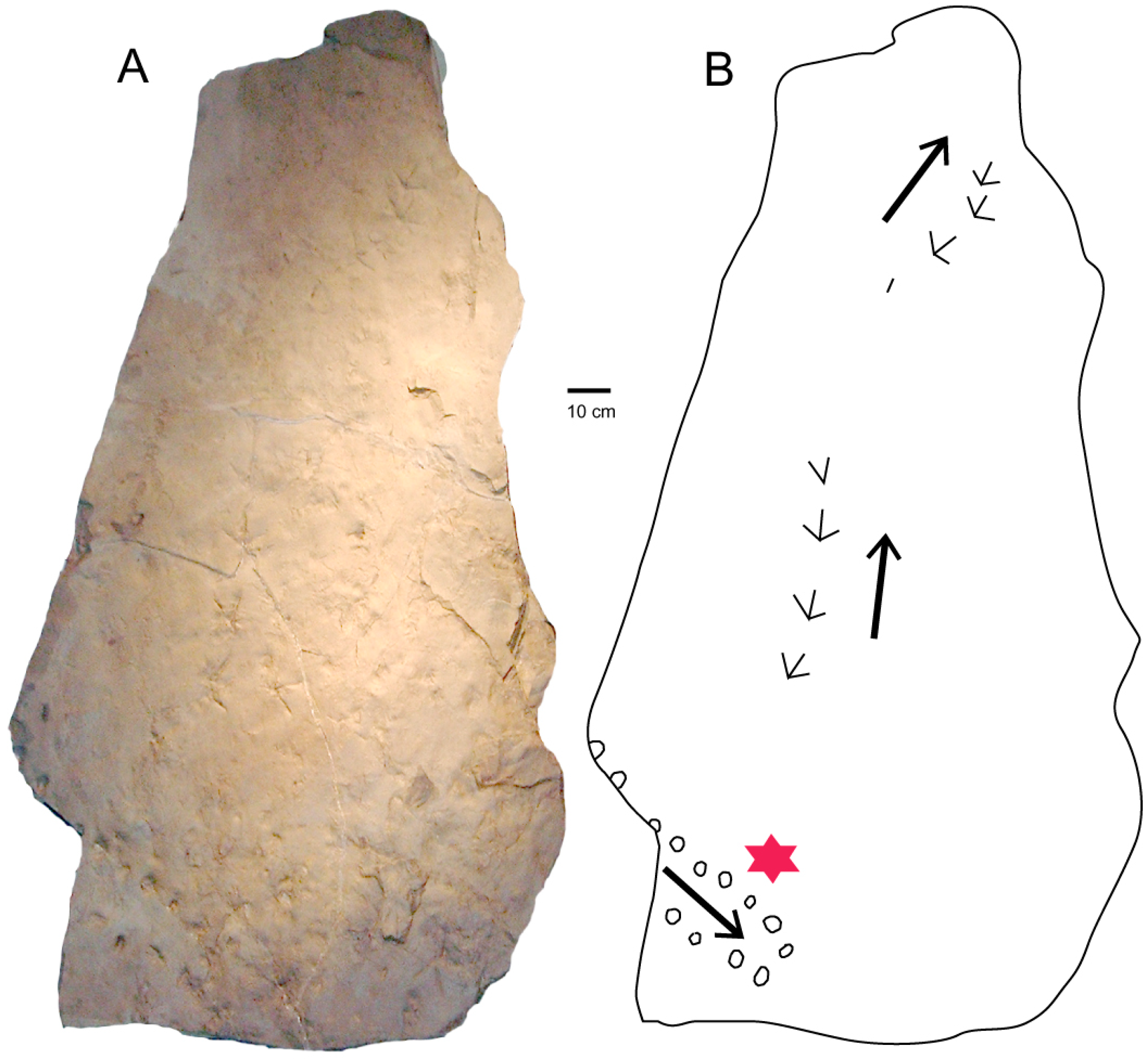
This 62-cm long trackway (Figure 6) comprises 14 footprints preserved on a sandstone bedding plane that also contains well-preserved footprints from a heron-like bird. The sharply-preserved tracks indicate the ancient bird was walking on wet riverbank sediment, suggesting that the nearby turtle tracks were made by an animal crawling was crawling on similar terrain.
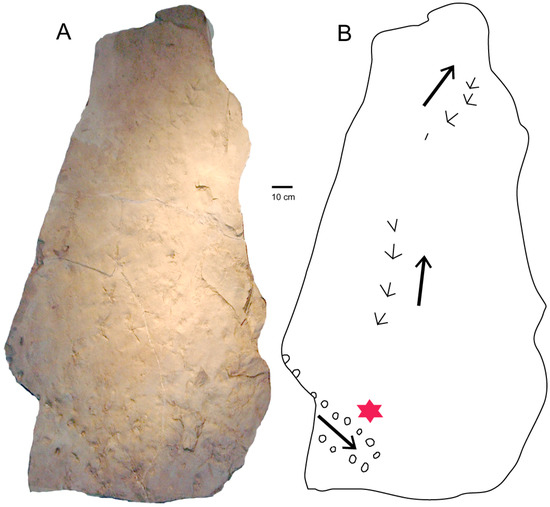
Figure 6.
(A) Slab found in 1990 at WWU-KC-1 site preserves a turtle trackway and footprints of a heron-like bird. (B) Turtle trackway marked with star. Arrows show travel directions. The specimen is now at the University of Washington Burke Museum of History and Culture, Seattle, WA, USA.
Distal margins of the impressions show faint claw marks, indicating the direction of travel. The ~15 mm average width of the trackway (Figure 7) suggests that the animal had a carapace width of ~12 cm, based on the ambulatory patterns of modern pond turtles (Figure 8). Tracks alternate in size. Possibly the smaller impressions are manus imprints, pes impressions being larger. Based on this interpretation, the average stride length would be ~17 cm, yielding a width/stride value of 1.13. For comparison, the stride/width values for two Eocene turtles from Texas are 0.55 for Chelonipus chadronicus, and 1.18 for C. parvus [2].
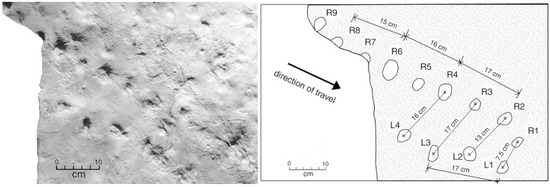
Figure 7.
Chuckanut Formation turtle trackway and drawing showing travel direction and dimensions.

Figure 8.
Pond turtle (Family Emydidae: Terrapenecarolina), showing sprawling gait typical of amphibious turtles.
4.1. Systematic Ichnology
Class Reptilia
Order Testudines Linnaeus 1758.
Morphofamily Chelonipedidae Sarjeant and Langston 1994 [2].
Ichnogenus Chelonipus Rűhle von Vilienstern 1939 [31], emended by Sarjeant and Langston 1994 [2].
Diagnosis: Quadripedal footprints with trackways very broad and stride short. Semiplantigrade to digitigrade; footprints broader than long and digits stiff and very short, bearing short, often blunt claws.
4.2. Comments
Turtles have a long evolutionary history, with a body plan that has remained relatively constant since the Mesozoic. For this reason, their footprints provide little information about the taxonomic identity of the track maker; tracks are described as a single ichnogenus, Chelonipus. The oldest examples date from the late Early Triassic of Wyoming and Utah, USA, and the early Middle Triassic of Germany [32]. The Chuckanut Formation turtle trackway is preserved in fluvial sandstone, an environment that suggests the track maker may have been a member of the Superfamilies Trionychoidea or Testidinoidea. The former group is represented in the Chuckanut Formation by skeletal remains [17]. Trioncychid turtles are common Eocene fossils in Europe and North America; extant members have worldwide range. However, the semitropical Eocene paleoenvironment may have provided a favorable habitat for some members of Testudinoidea, e.g., Family Geoemydidae, whose members presently inhabit aquatic environments in warm areas of Asia, Europe, and North Africa. Alternately, the Chuckanut trackway may represent the Family Emydidae, a group of pond and marsh-dwelling turtles that have a long history in North America, including examples from the Upper Cretaceous, Paleocene, and Eocene [33].
5. Crocodylian Trace Fossils
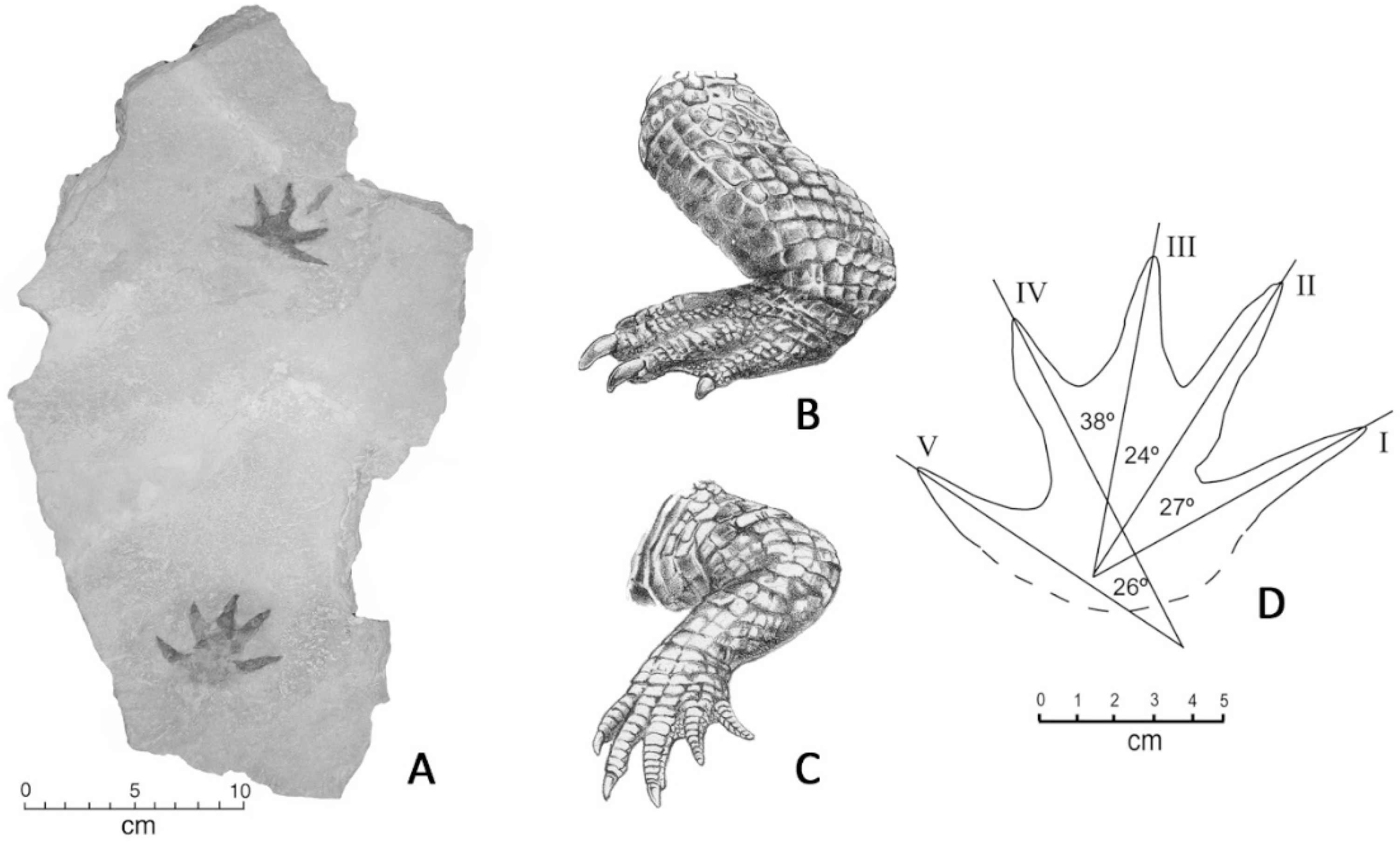
Crocodylian trace fossils from the Chuckanut Formation have been observed on sandstone blocks were exposed by the 2003 Racehorse Creek landslide. The first specimen to be found preserves a pair of plantigrade manus imprints that show acuminate terminations of the five digits (Figure 9).
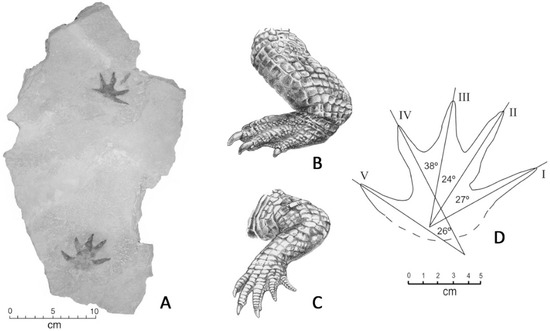
Figure 9.
Manus imprints. Specimen WWU-TR-063, excavated November, 2010. A. trackways; B. Pes of modern crocodile, showing three clawed digits; C. Manus of modern crocodile, showing four clawed digits; D. Dimensions of Chuckanut manus impression.
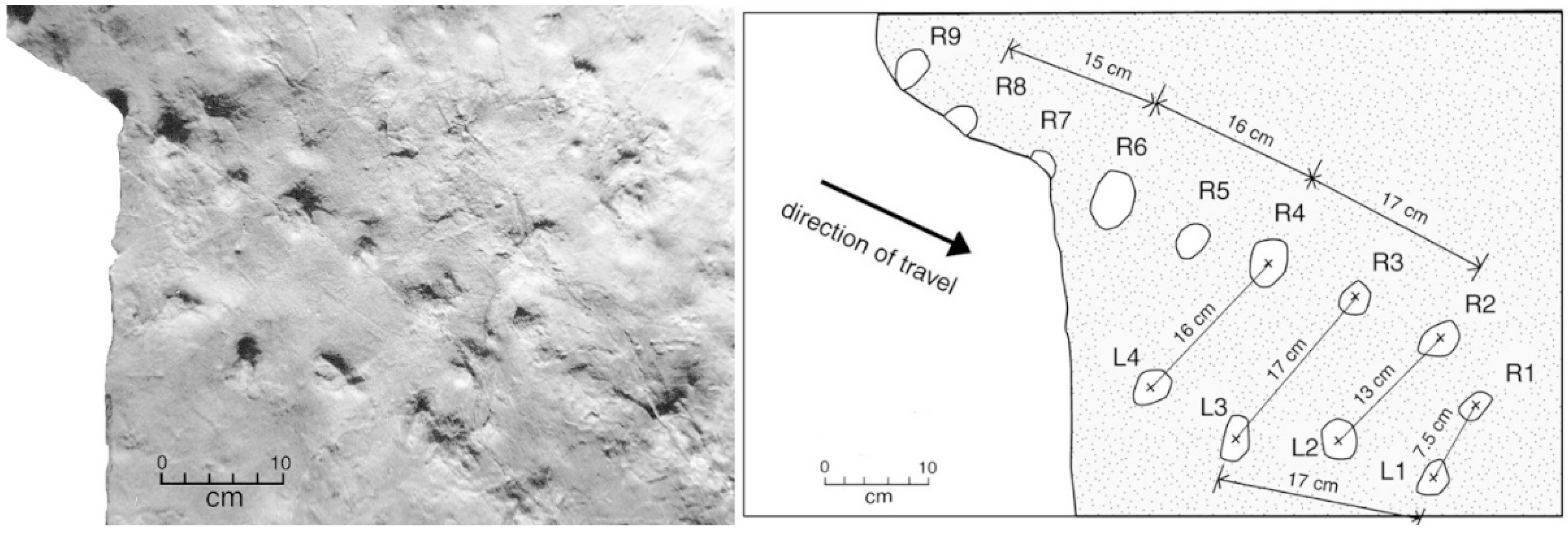
Three other trackway shows smaller footprints associated with tail drag marks. On one landslide block, the upper surface is a bedding plane that has a prominent sinuous drag mark, with shallow impressions of several bilateral footprints bordering the central groove (Figure 10).

Figure 10.
Crocodylian trackway preserving multiple footprints on either side of a prominent drag mark. Tracks are highlighted with chalk.
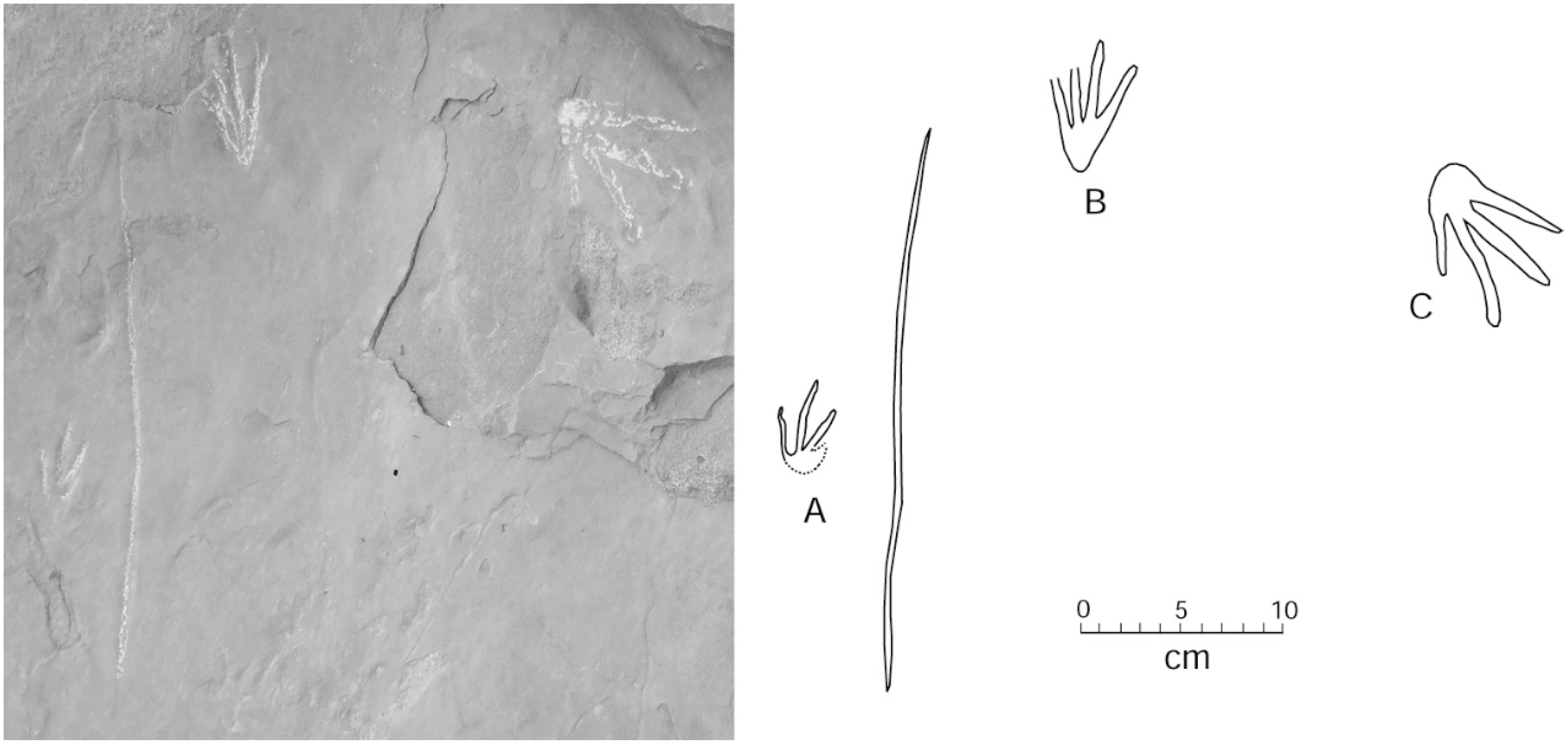
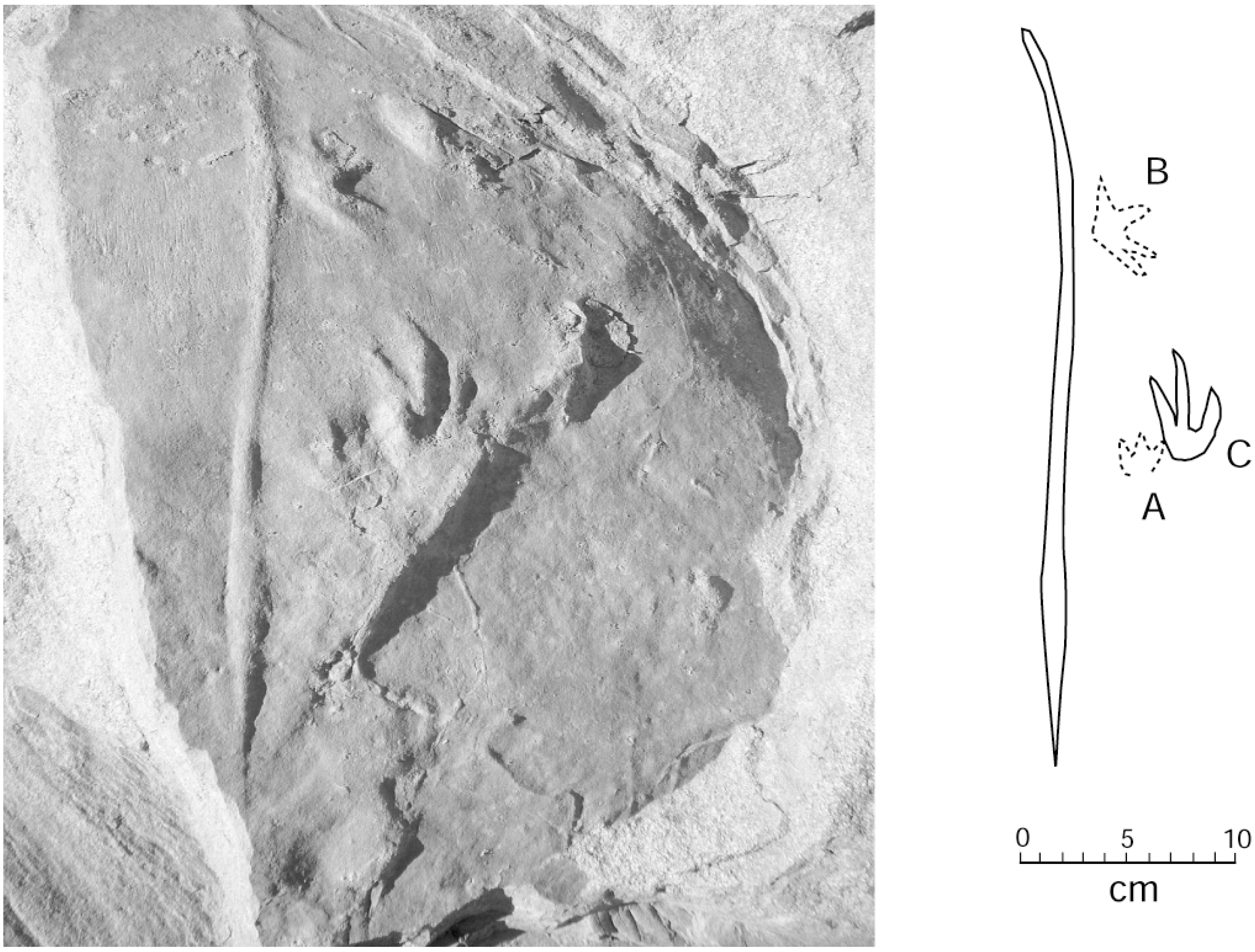
Two adjacent sandstone blocks discovered in 2015 preserve trackways where small footprints are positioned in association with a linear drag mark (Figure 11 and Figure 12). Lithologic similarities in the two blocks suggest the possibility that both trackways may have originally been contiguous.
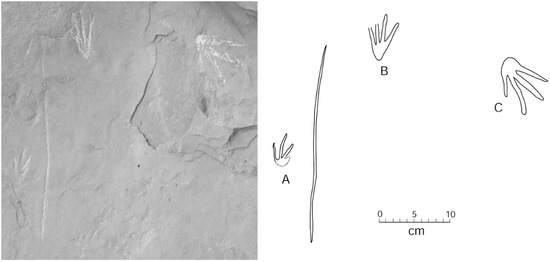
Figure 11.
Trackways showing multiple footprints and tail drag marks. Specimen WWU-TR-073. A. left manus; B. right pes. Both footprints are in proximity to a central tail drag mark. C. right manus from a larger Crocodylian traveling in a different direction. Footprints are highlighted with chalk.
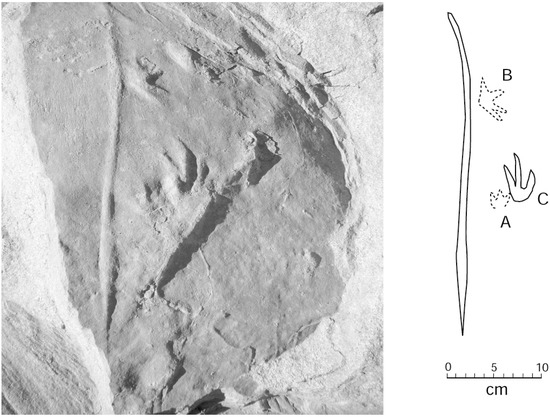
Figure 12.
Specimen WWU-TR-074. Prominent drag mark with digitigrades footprints. A, B. right manus; C. right pes.
Interpretation of these trackways is based on locomotion patterns that have been observed for modern Crocodylians. For terrestrial travel, these reptiles may use “low walking” and “high walking” styles, depending on the position of the body relative to the substrate. In both modes, footprints typically occur in regular patterns, with a prominent central drag mark (Figure 13) [34,35,36]. This type of trackway may also be made by Crocodylians wading in shallow water, where much of the body weight is carried by the feet [37].

Figure 13.
Left by modern American Alligator (Alligator mississippiensis) from Florida, USA, showing manus and pes imprints and central tail drag mark. Photo courtesy of Kim Trebatowski.
Very different trackways are produced when Crocodylians are submerged, when buoyancy causes their feet to bear very little weight [38]. Webbed hind feet may be used to push off the bottom, and to paddle the animal forward. Front feet can be used to probe for surface obstacles, and to facilitate changes in travel direction. The use of front feet for detecting substrate irregularities is valuable as a source of protection to avoid accidentally injury to the tender underbelly. The tail extends distally as the crocodyle glides forward. During forward travel, the feet may only intermittently make contact with the substrate, causing footprint sequences to be irregular compared to terrestrial or shallow water locomotion. The distances between successive tracks may be lengthened when water currents or wave action facilitate forward propulsion. This type of submerged locomotion, described as “punting”, has previously been reported for crustacea [39,40] and skates (Condricthyes) [41]. The transition to swimming occurs when foot contact ceases, and the crocodyle moves forward primarily by oscillations of the powerful tail. A common form of locomotion on wet substrates is by belly sliding. Swim tracks are typically strongly digitigrade manus impressions, sometimes being only scrape marks left when clawed digits gouged soft sediment [42].
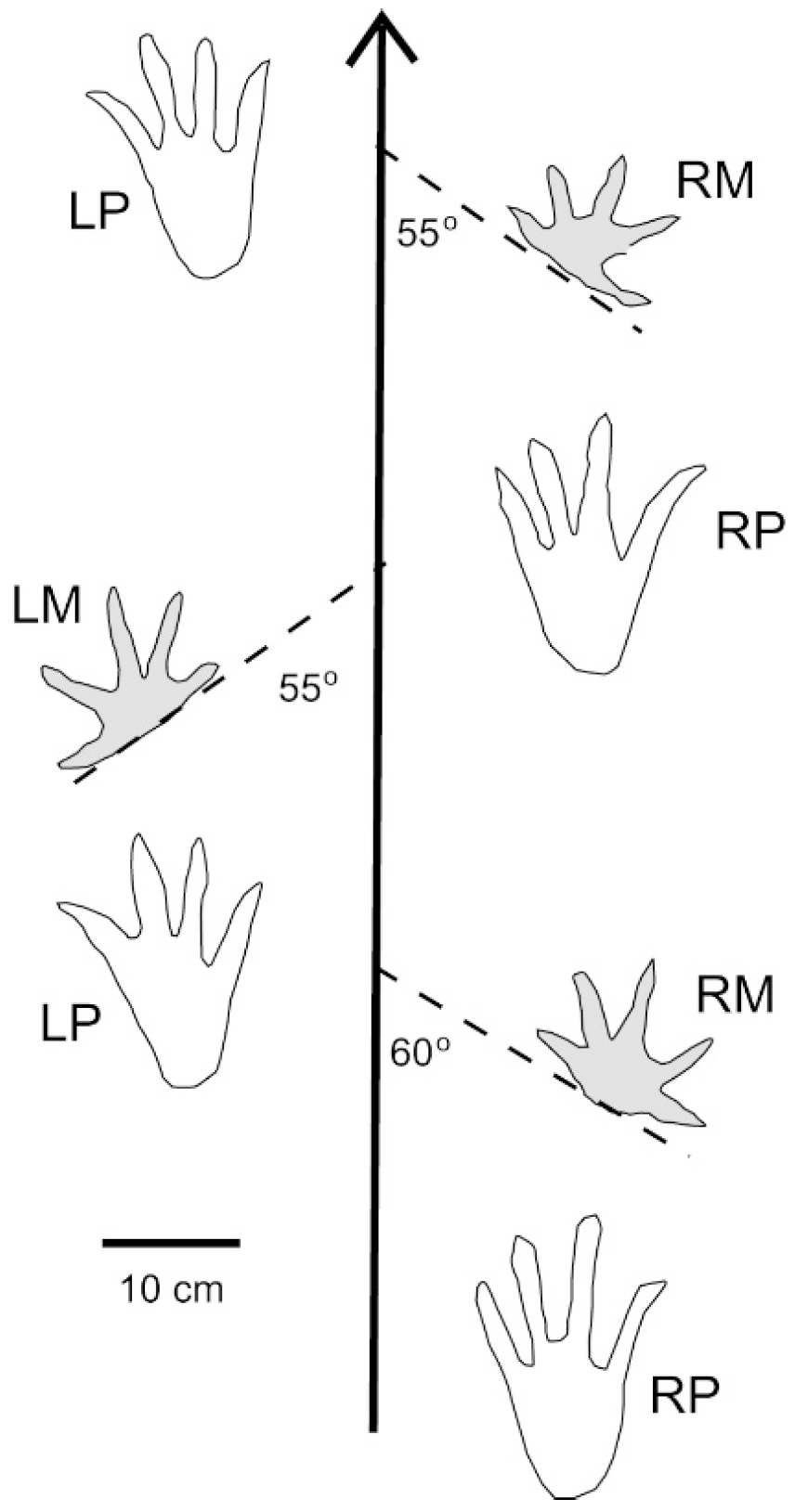
The tracks shown in Figure 9 can be interpreted as manus imprints produced by a “punting” Crocodylian. The discordant angles of the two imprints is consistent with tracks made by modern crocodyles, where the splayed gait causes manus imprints too occur at broad angles relative the direction of travel (Figure 14). The absence of pes imprints and central tail drag suggest that the animal was swimming rather than bottom walking (Figure 15).
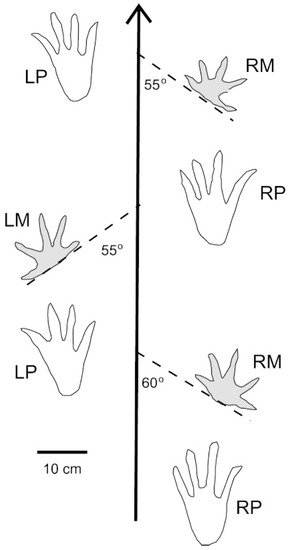
Figure 14.
Trackway of modern American Crocodile (Crocodylus acutus) showing angular relationships of manus imprints (shaded tracks) to direction of travel (arrow). Adapted from [43].
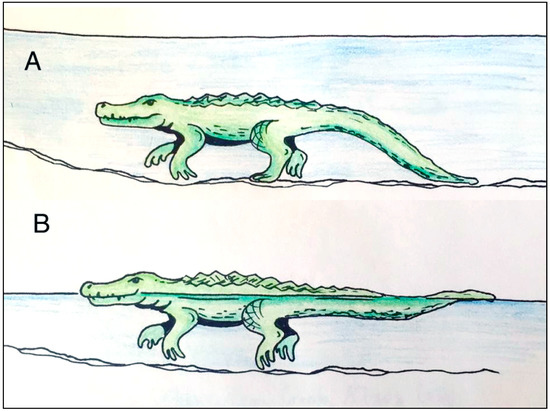
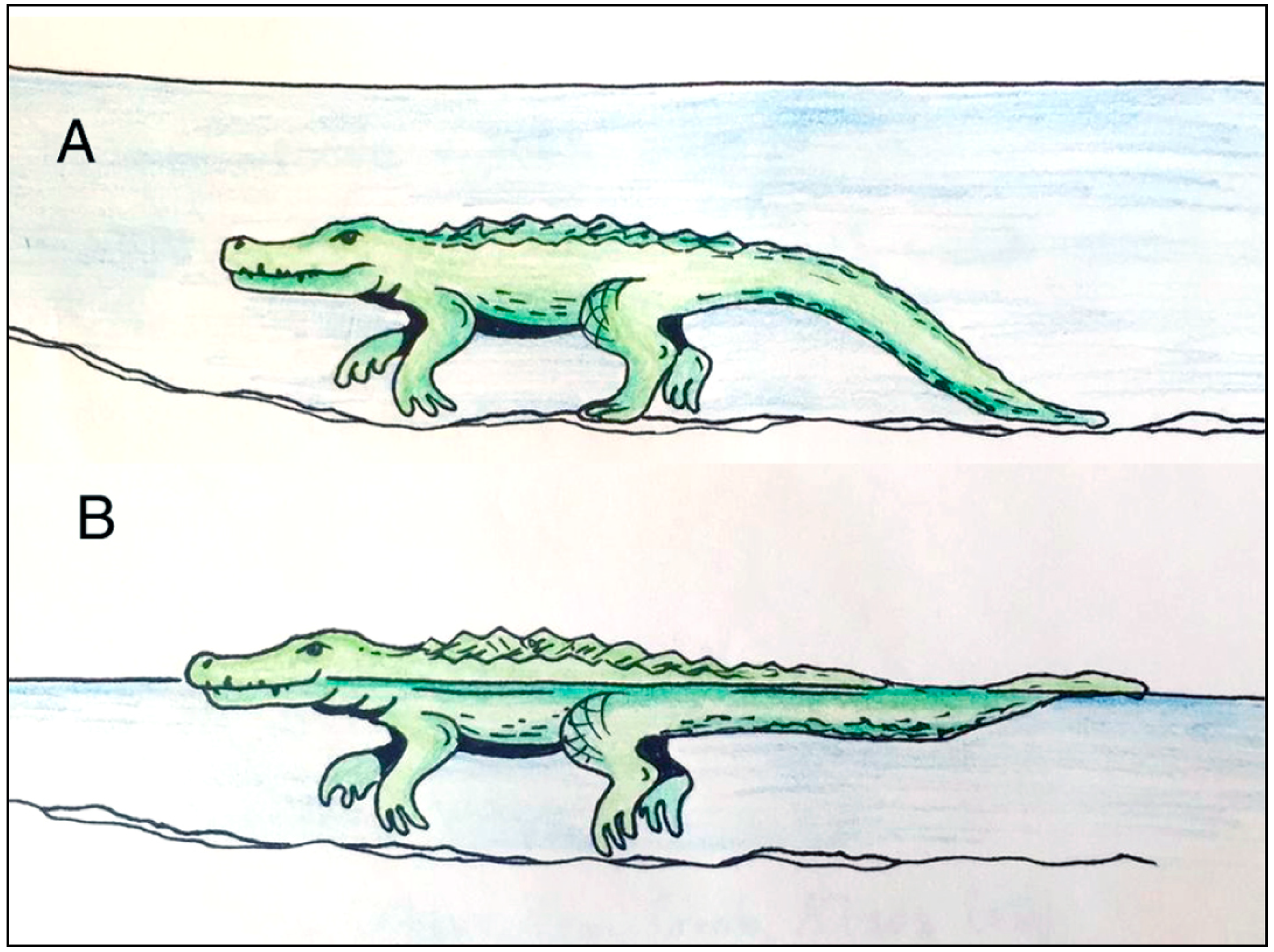
Figure 15.
Locomotion patterns of crocodiles travelling in water. A. Bottom walking, with intermittent foot contact and linear tail drag, B. Swimming in shallow water, with possible intermittent foot contact and tail undulations for propulsion. Drawing by Jessie Thoreson, 2019.
For the other trackways (Figure 10, Figure 11 and Figure 12), the long distances between tracks, the irregular distribution, and the shallowness of the impressions are suggestive of punting rather than bottom walking.
Ichnotaxonomy of Crocodylian Tracks
Crocodylian trace fossils found at the Chuckanut Formation Racehorse Creek landslide location include manus and pes impressions, and associated tail drag marks, but in two specimens these trace fossils lack sufficient information for reliable ichnotaxonomic assignment (Figure 9 and Figure 10). Two trackways preserved on adjacent landslide blocks (Figure 11 and Figure 12) provide better detail. The irregular pattern of the footprints is presumably evidence of punting-style locomotion of a small submerged crocodyle, resulting in trackways that are dissimilar to the more regular footprint patterns that occur during terrestrial or shallow water walking. However, Crocodylian “swim tracks” (e.g., punting tracks) are common in the geologic record, suggesting that these irregular footprint patterns are a typical phenomenon.
Class Reptilia
Order Crocodylia Wermuth 1953 [44] Crocodilia Owen 1842 [45]
Ichnogenus Anticusuchipes amnis ichnogen et ichnosp. nov.
Included ichnospecies: The ichnogenus is presently known to include the type ichnospecies.
Type ichnospecies: Anticusuchipes amnis Mustoe, herein. Early Eocene, Slide Stratigraphic Member, Chuckanut Formation, western Whatcom County, Washington USA.
Etymology: Latin: Anticus, ancient; suchi, referring to Eosuchia, the basal clade for Crocodylians; pes, foot; amnis, river. The name thus roughly translates as “ancient river crocodyle footprint”.
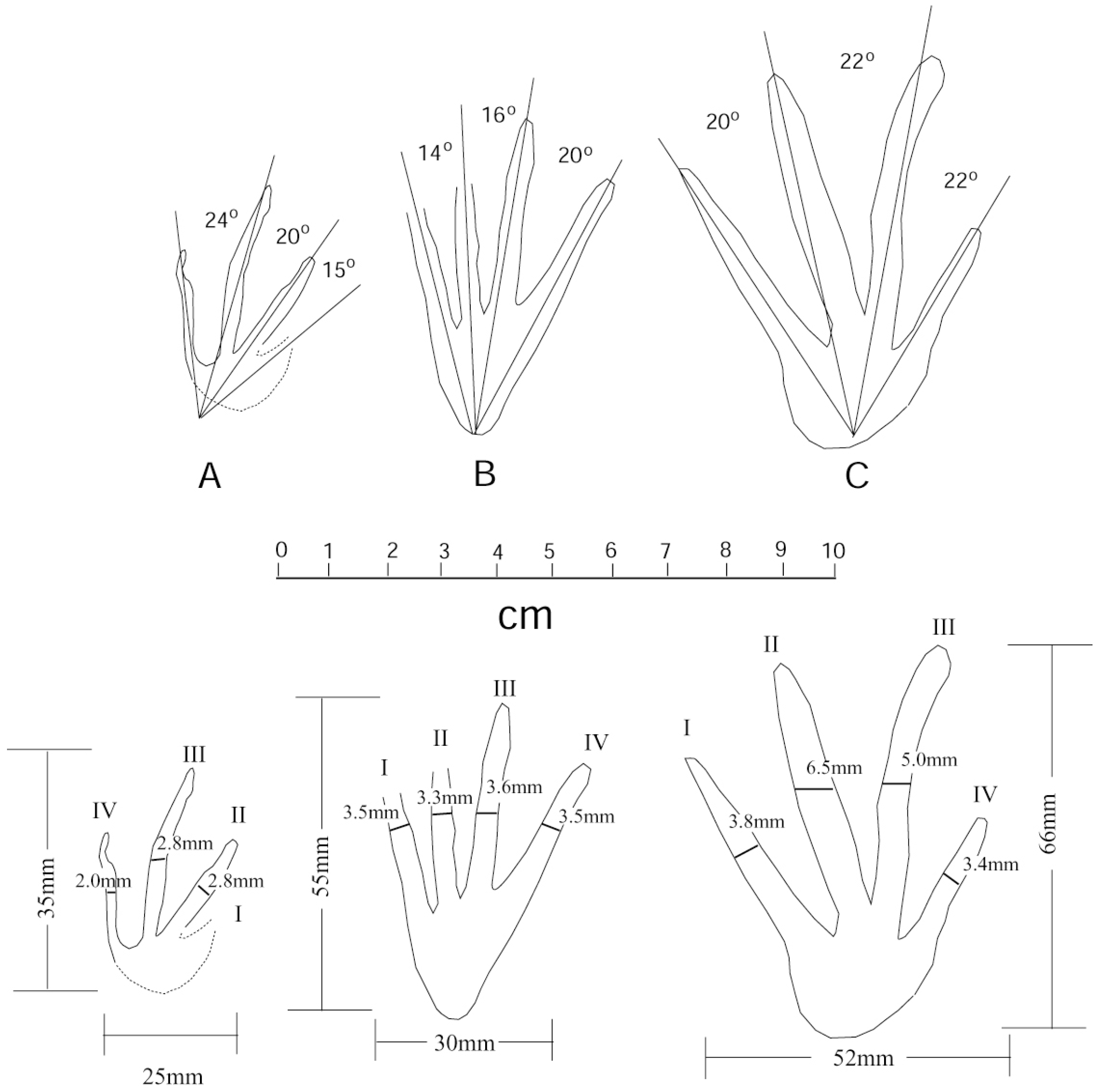
Diagnosis: Tetradactyl tracks with slender elongate digits with apices that range from rounded to sharply pointed. Claws are not distinctly evident at the digital apices. Heel pad are rounded. Tracks are plantigrade to digitigrades. Manus impressions are much smaller than pes impressions. Detailed measurements are shown in Figure 16. The Chuckanut Formation footprints are distinctively different from previously-described Crocodylian tracks (Figure 17). Footprints of Crocodylopodus [46] and Batrachopus [47] are much smaller than Anticusichipes; Mehliella [48], Albertasuchipes [3], and an unnamed track from USA [49] are much larger. The shapes of all of these ichnogenera are unlike that of Anticusichipes. Mesozoic Crocodylian tracks that are comparable in size are Hatcherichnus [50] and Kuanguanpus [51], but these footprints are much broader and have shorter, wider digit impressions than Indosuchipes or Anticusuchipes. The most notable difference between Indosuchipes and Anticusuchipes is the shape of the heel pad, which is broadly rounded and prominent in the former ichnogenus, and smaller and narrow for Anticusichipes. An unnamed track from Portugal [52] is more similar in shape, but digit impressions of the latter fossil are short and triangular, compared to the slender elongated digits characteristic of Indosuchipes and Anticusuchipes.
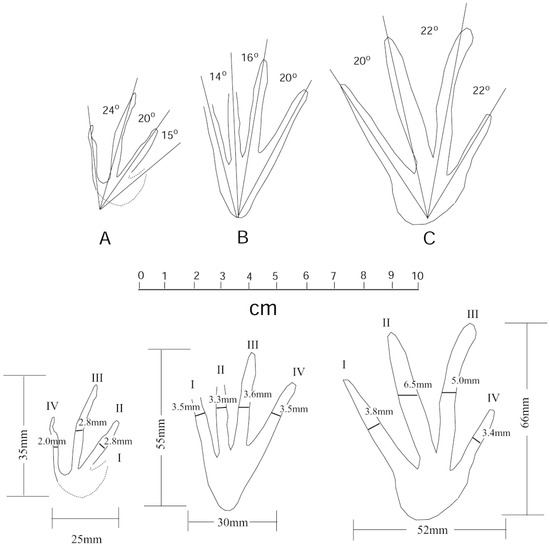
Figure 16.
Sketches of Anticusuchipes amnis footprints A–C, showing interdigital angles and track dimensions, specimen WWU-TR-073. Track images have been rotated to show parallel orientations. The original positions are shown in Figure 8.

Figure 17.
Outlines of crocdylian tracks of Mesozoic and Cenozoic age. A. Early Cretaceous Laiyangpus, China [51]; B. Late Jurassic Crocodylopodus [46]; C. Early Cretaceous, Thailand [53]; D. Early Jurassic Batrachopus [47]; E. Late Jurassic, Portugal [52]; F. Miocene, Characichnos, Czech Republic [42]; G. mid-Cretaceous, Mehiella, USA [48]; H. Paleocene Albertasuchipes, Canada [3]; I. Late Jurassic Hatcherichnus, USA [50]; J. Late Paleocene Borealosuchipes, USA [4]; K. Late Cretaceous, USA [49]; L. Middle Jurassic, Kuanguanopus China [51]; M. Oligocene, Indosuchipes, [54]; N. Anticusuchipes amnis Mustoe, ichnogen. and ichnosp. nov., Eocene, northwest Washington.
Anticusuchipes amnis
Holotype: Western Washington University specimen WWW-TR-073, represented by a plaster cast made from a silicone mold archived at the W.W.U Geology Department archives, Bellingham, WA USA.
Diagnosis: Same as for the ichnogenus
Horizon and locality: Chuckanut Formation, Slide Stratigraphic Member (Early Eocene), Racehorse Creek landslide, WWU-RCS-1, Whatcom County, Washington USA.
Remarks: Tracks A and B are positioned on either side of a central drag mark. Footprint A is a left manus, and the larger footprint B is a right pes. Track C is not part of this trackway, being the right manus print of a larger individual travelling in a different direction. For Crocodylians, footprint size is not a reliable parameter for ichotaxonomy, because even the largest individuals began their life as tiny hatchlings. Also, apparent track sizes and shapes may be affected by the sedimentary characteristics of the substrate. Trace fossils in specimen WWU-TR-074 are not as well preserved as WWU-TR-073. In the former specimen, three footprints are located to the left of the drag mark and appear to represent and two incomplete manus imprints (Figure 12A,B) one pes impression (Figure 12C). Possibly these trace fossils represent the same track-maker as the holotype specimen WWU-TR-073; both trackways appear to occur on a single bedding plane that is now represented by two adjacent landslide blocks. Alternately, the two trackways may have been made by two individuals of the same approximate size. The small sizes of these tracks are suggestive of juveniles, consistent with the common tendency for extant Crocodylians to live in proximity [55].
6. Discussion
The Crocodylian crown group dates to the Cretaceous, but the earliest known crocodylomorph footprints are Triassic. Lockley et al. [56] reviewed the evolutionary history of Crocodylians and described modern members. Crocodylomorph tracks have broad geographic distribution in Triassic and Jurassic strata. The most abundant footprints have been found in Cretaceous formations, most notably the Albian-Cenomania Dakota Group of Colorado, Kansas, and New Mexico, USA, where Crocodylian tracks are known from 70 sites. Unlike non-avian dinosaurs, Crocodylians survived the great K/T extinction. These reptiles clearly existed throughout the Cenozoic, as evidenced by extant members that comprise three groups: Alligatoridae (8 species), Crocodylidae (14 species), and Gavailidae (1 species). However, Cenozoic tracks have rarely been reported. McCrea et al. [3] described Paleocene Crocodylian tracks from the Paleocene of Alberta, Canada, and Erickson [4] illustrated similar footprints from North Dakota, USA. Miocene scrape marks from Czech Republic were interpreted to have been made by a Crocodylian [42].
Modern Crocodylians inhabit tropical regions, where they dwell in rivers, lakes, swamps, and coastal estuaries. They are endemic to southern North America, Central America, South America, Africa, Australia, and Southern Asia; the fossil record is reviewed by Brochu [57]. Scarcity of North American Cenozoic Crocodylian fossils is probably related to the worldwide decline in temperatures that began in the late Eocene. Because Crocodylians require subtropical or tropical climate, this climatic decline would have greatly reduced the geographic extent of suitable habitat. At low latitudes, tropical environments allowed Crocodylians to remain successful throughout the Cenozoic. In Asia, saltwater crocodiles (Crocodylus porosus) are endemic to coastal areas of the Indo-Pacific region, and the freshwater gharial (Gavialis gangeticus) inhabits rivers in Northern India. The presence of Oligocene Crocodylian trace fossils in Manipur, Northeast India [54] is evidence that these reptiles have a long history in a region where the equatorial latitude provided continued warm temperatures. In South America, Crocodylians reached a zenith in the late Cenozoic, with 14 species present in the late Miocene, compared to 10 extant taxa [58]. Africa is today inhabited by one native crocodile, Crocodylus (Nile Crocodile), with known ancestors that date to the late Miocene [59].
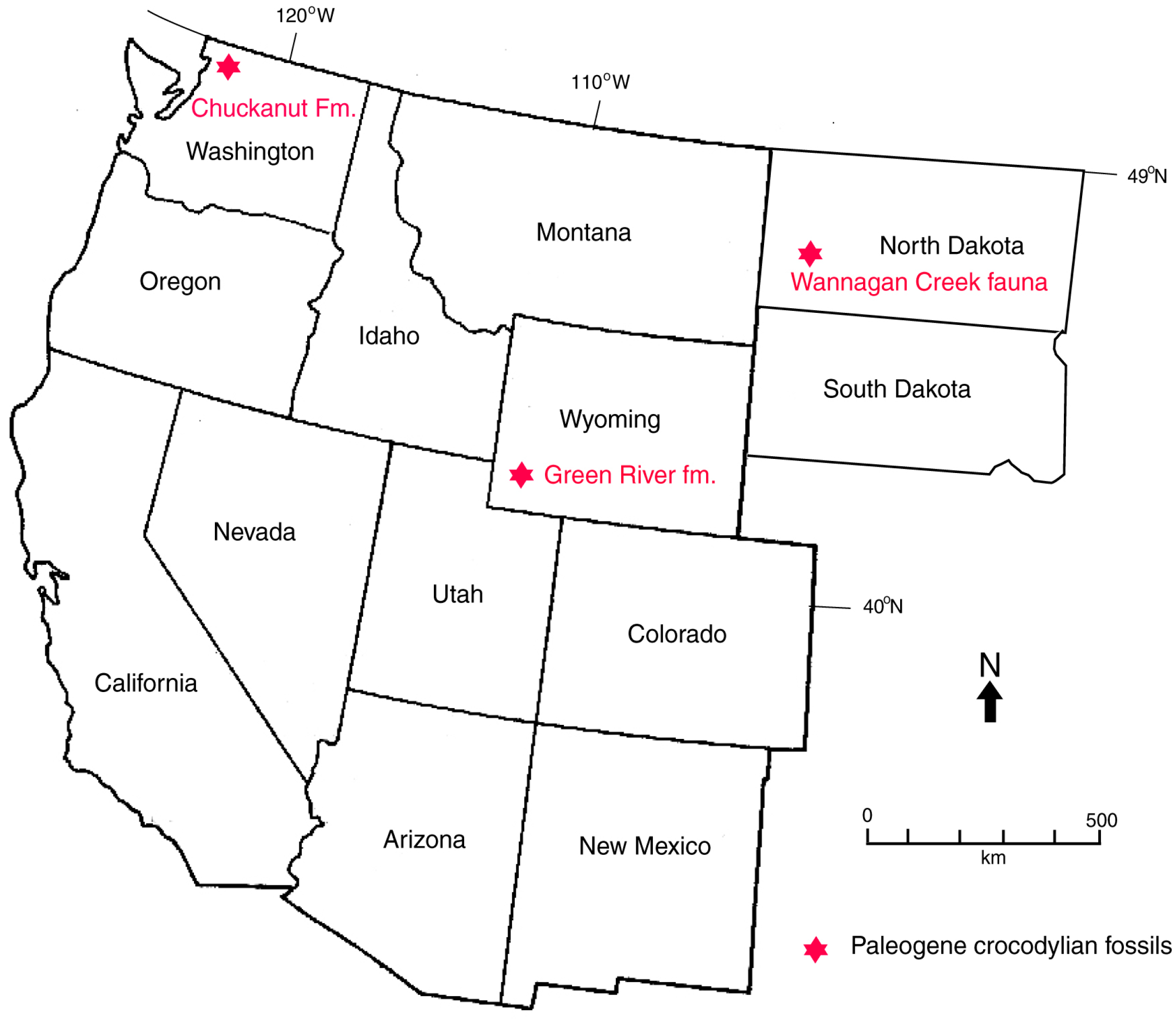
In North America, Cretaceous and Paleogene crocodyllian fossils occur as far north as Wyoming, but the only modern representatives are the American alligator (Alligator mississippiensis), whose range extends from Florida to Texas, and the American crocodile (Crocodylus acutus), which inhabits Florida. In Northwest Washington, USA, the subtropical climate of the coastal lowlands during the Eocene would have provided a favorable environment for Crocodylians. Their presence in fluvial Chuckanut Fm. strata indicates that these Crocodylians were river dwellers. Early Eocene Crocodylians from the Green River in Wyoming, USA provide possible analogs for the Chuckanut Formation track-makers. In both occurrences, Crocodylians inhabited fluvial/lacustrine habitats, and the formations are age correlative (Figure 18). Teeth, schutes, and coprolites are the most common Crocodylian fossils in the Green River basin sediments, but articulated skeletons have been found [60,61]. Plant fossils indicate that the early Eocene paleoclimate of central Wyoming was warm temperate to subtropical, comparable to the Chuckanut Formation. The ancestral history of these Cenozoic Crocodylians is enigmatic because of the scarcity of Paleocene sedimentary formations. However, Crocodylians are represented at the Wannagan Creek fossil quarry in North Dakota, USA by skeletal remains of at least 80 individuals [4,62,63,64].
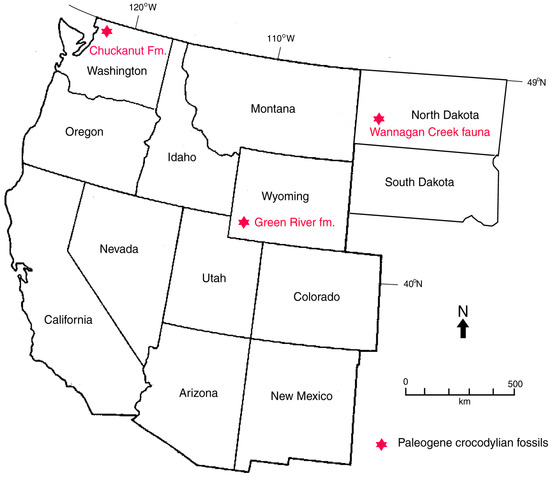
Figure 18.
Location of Paleogene Crocodylian fossil localities in Western USA.
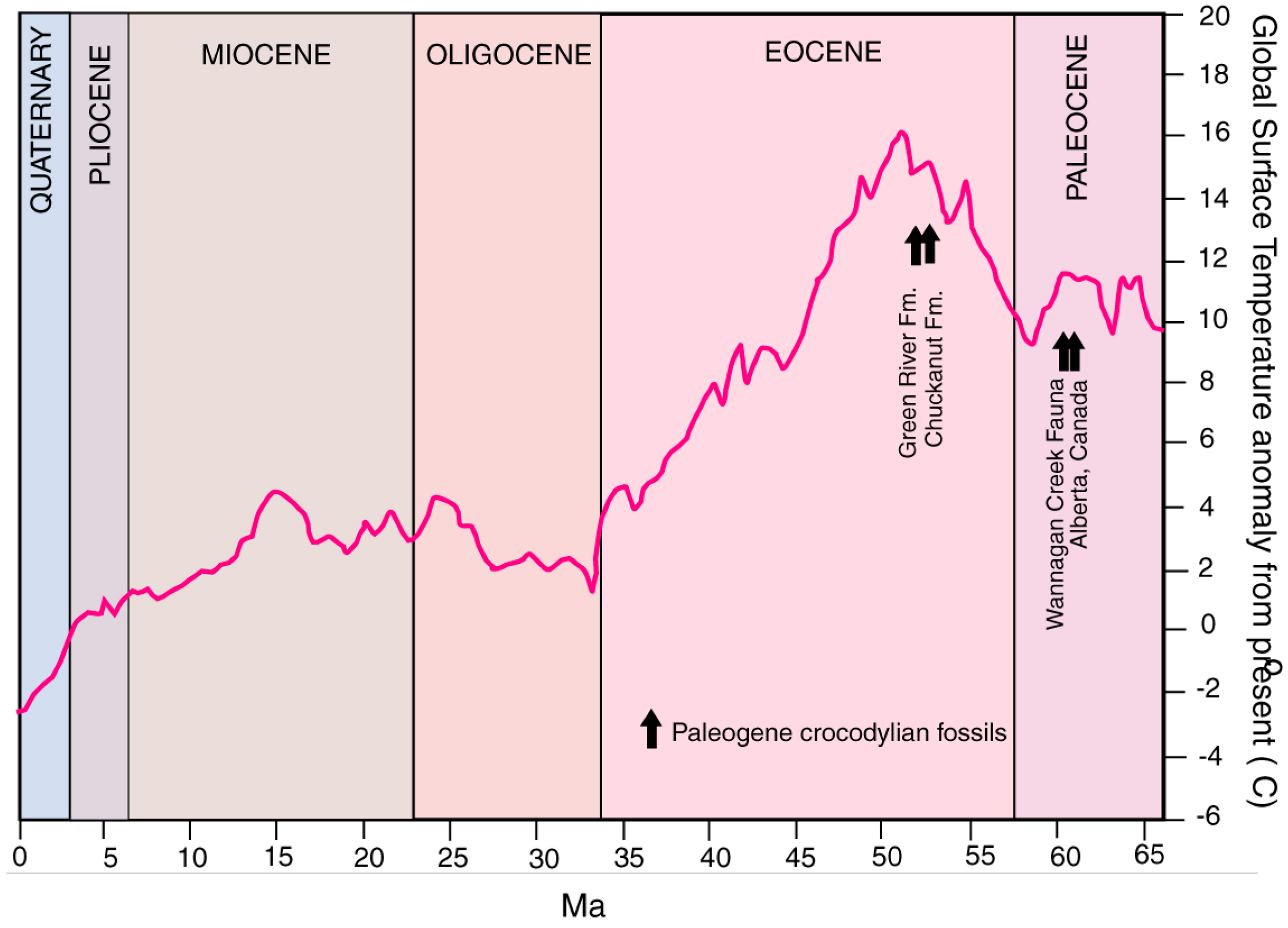
Favorable habitat conditions for crocodiles depends on local environmental factors, e.g., the presence of suitable bodies of water and adequate food. From a geographic standpoint, tectonic forces may be involved, because marine coasts and low elevation inland lakes and rivers are required by Crocodylians. However, the dominant factor is temperature. Beginning in the late Eocene, progressive climatic cooling beginning in the late Eocene explains the demise of Crocodylians at high latitudes in North America (Figure 19). Extant members are limited to the warm climate of the Southeastern US. For perspective, modern Alligator misssissipiensis stops eating at temperatures below 16 °C, but can survive freezing conditions. In contrast, Crocodylus acutus succumbs to hypothermia at 7 °C [65].
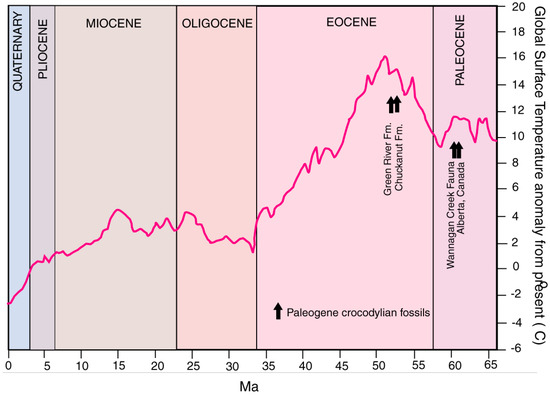
Figure 19.
Mean global surface temperatures during the Cenozoic, and age of Paleogene Crocodylian fossils from North America. Paleoclimate data adapted from [67,68].
Compared to Crocodylians, turtles have broader climatic tolerance; extant species native to Washington and Oregon, USA, are Actinemys marmarota (Western Pond Turtle) and Chrysemys picta (Painted Turtle). A non-native taxon, Trachemys scripta (Pond Slider) has appeared as a result of the release of turtles that had originally been sold as pets. Other non-native forms include Cheleydra serpentina (Snapping Turtle) and Apalone spinifera (Spiny Softshell). Leatherback Turtles (Dermochelys coriacea) are regular off the Washington coast. Other sea turtles, including Green (Chelonia mydas), Loggerhead (Caretta caretta) and Pacific Ridley (Lepidochelys olivacea) are much rarer, but a few have been found washed up on northwest beaches [66]. Warm Paleogene climate likely provided favorable habitat, but turtles have a well-documented record as evolutionary survivors.
Funding
This research received no external funding.
Acknowledgments
Spencer Lucas provided helpful advice during manuscript preparation. Brennan Martens and Emmet Martens assisted with field work at the WWU-RCS-1 locality. Brennan Martens deserves special credit for discovering Crocodylian trackways (Figure 11 and Figure 12) at a site that had previously received numerous visits by geoscientists. At age 14 at the time of the 2015 discovery, Brennan Martens displayed admirable observational skills. Following the original 2009 visit to the WWU-RCS-1 site, dozens of volunteers have assisted in searching the extensive landslide in pursuit of fossils, resulting in recovery of many specimens, and preparation of molds of specimens on blocks that are too big to transport. Particular credit goes to Tadd Dillhoff, Wes Gannaway, Don Hopkins, Keith Kemplin, Dave Tucker, and Jared Watson, who all made many visits to the site.
Conflicts of Interest
The authors declare no conflict of interest.
References
- McDonald, H.G.; White, R.S.; Lockley, M.G.; Mustoe, G.E. An indexed bibliography of Cenezoic vertebrate tracks. In Cenozoic Vertebrate Tracks and Traces; Milàn, J., Lucas, S.G., Spielmann, J.A., Lockkley, M.G., Eds.; New Mexico Museum of Natural History and Science Bulletin: Albuquerque, NM, USA, 2007; Volume 42, pp. 275–302. [Google Scholar]
- Sarjeant, W.A.S.; Langston, W., Jr. Vertebrate footprints and invertebrate traces from the Chardronian (Late Eocene) of Trans-Pecos, Texas. In Texas Memorial Museum Bulletin 36; University of Texas: Austin, TX, USA, 1994; p. 86. [Google Scholar]
- McCrea, R.T.; Pemberton, S.G.; Currie, P.J. New ichnotaxa of mammal and reptile tracks from the upper Paleocene of Alberta. Ichnos 2004, 11, 323–339. [Google Scholar] [CrossRef]
- Erickson, B.R. Crocodile and arthropod tracks from the Late Paleocene Wannagan Creek fauna of North Dakota, USA. Ichnos 2005, 12, 303–308. [Google Scholar] [CrossRef]
- Johnson, S.Y. Stratigraphy, age, and paleogeography of the Eocene Chuckanut Formation, northwest Washington. Can. J. Earth Sci. 1984, 21, 92–106. [Google Scholar] [CrossRef]
- Johnson, S.Y. Cyclic fluvial sedimentation in a rapidly subsiding basin. Sediment. Geol. 1984, 38, 361–391. [Google Scholar] [CrossRef]
- Mustard, P.S.; Rouse, G.E. Stratigraphy and evolution of Tertiary Georgia Basin and subadjacent Upper Cretaceous sedimentary rocks, southwestern British Columbia and northwestern Washington. In Geology and Geological Hazards of the Vancouver Region, Southwestern British Columbia; Monger, J.W.H., Ed.; Geological Survey of Canada Bulletin: Ottawa, ON, Canada, 1994; Volume 481, pp. 97–169. [Google Scholar]
- Mustoe, G.E.; Gannaway, W.L. Paleogeography and paleontology of the early Tertiary Chuckanut Formation, northwest Washington. Wash. Geol. 1997, 25, 1–18. [Google Scholar]
- Haugerud, R. Preliminary report on significant thrusting and extension of the early Tertiary Chuckanut Formation, NW Washington. In Slave-Northern Cordillera Lithospheric Evolution (SNORCLE) and Cordilleran Tectonics Workshop; Cook, F., Erdmer, P., Eds.; Lithoprobe Report; University of British Columbia: Vancouver, BC, Canada, 1998; p. 203. [Google Scholar]
- Mustoe, G.E.; Dillhoff, R.M.; Dillhoff, T.A. Geology and paleontology of the early Tertiary Chuckanut Formation. In Geological Society of America Field Guide 9: Floods, Faults, and Fire. Geological Field Trips in Washington State and Southwest British Columbia; Stelling, P., Tucker, D.S., Eds.; Geological Society of America: Boulder, CO, USA, 2007; pp. 121–135. [Google Scholar]
- Breedlovesrout, R.L. Paleofloristic Studies in the Paleogene Chuckanut Basin, Western Washington, USA. Unpublished Ph.D. dissertation, University of Idaho, Moscow, Russia, 2011; p. 953. [Google Scholar]
- Mustoe, G.E. Eocene bird tracks from the Chuckanut Formation, northwest Washington. Can. J. Earth Sci. 1993, 30, 987–990. [Google Scholar] [CrossRef]
- Crider, J.G.; Tucker, D.S.; Clark, D.H.; Linneman, S.R. The 2009 Racehorse Creek Landslide: Forensic Dynamics of a Large, Complex Catastrophic Mass Movement. Geol. Soc. Am. Abstr. Programs 2009, 41, 498. [Google Scholar]
- Wolfe, J.A. A Method of Obtaining Climatic Parameters from Tertiary Leaf Assemblages; 5 plates; US Geological Survey Bulletin: Reston, VA, USA, 1993; Volume 2040, p. 71.
- Wilf, P. When are leaves good thermometers? A new case for leaf margin Analysis. Paleobiology 1997, 23, 213–215. [Google Scholar] [CrossRef]
- Mustoe, G.E.; Pevear, D.R. Vertebrate fossils from the Chuckanut Formation of northwest Washington. Northwest Sci. 1983, 5, 3–18. [Google Scholar]
- Mustoe, G.E.; Girouard, S.P., Jr. A fossil trionychid turtle from the early Tertiary Chuckanut Formation of northwestern Washington. Northwest Sci. 2001, 75, 211–218. [Google Scholar]
- Simpson, S. The morphological classification of trace fossils. In The Study of Trace Fossils; Frey, R.W., Ed.; Springer-Verlag: New York, NY, USA, 1975; pp. 39–54. [Google Scholar]
- Ekdale, A.A.; Bromley, R.G.; Pemberton, S.G. Ichnology: The Use of Trace Fossils in Sedimentology and Stratigraphy; SEPM Society for Sedimentary Geology: Tulsa, OK, USA, 1984. [Google Scholar]
- Bertling, M.; Braddy, S.J.; Bromley, R.G.; Demathieu, G.R.; Genise, J.; Mikuláš, R.; Nielsen, J.K.; Nielsen, K.S.; Rindsberg, A.K.; Schlirf, M.; et al. Names for trace fossils: A uniform approach. Lethaia 2006, 39, 265–286. [Google Scholar] [CrossRef]
- Bertling, M. What’s in a name? Nomenclature, systematic, ichnotaxonomy. In Trace Fossils; Miller, W., III, Ed.; Chapter 5; Elsevier: Amsterdam, The Netherlands, 2007; pp. 81–91. [Google Scholar]
- Rindsberg, A.K. Ichnological consequences of the 1985 International Code of Zoological Nomenclature. Ichnos 1990, 1, 59–63. [Google Scholar] [CrossRef]
- Rindsberg, A.K. Construction of ichnogeneric names. Ann. Sociatis Geol. Pol. 2015, 85, 529–549. [Google Scholar] [CrossRef]
- Vialov, O.S. Stratigrafiya Neogeonovykh molass Predkarpatskovo progroba (Stratigraphy of the Neogne molasse of the PreCarpathian basin). In Akadimya Nauk Urainskoy SSR Institut Geologii I Geokhimii Goryuchikh Iskopaymykh; Naukkova Dumka: Kiev, Ukraine, 1965; p. 165. [Google Scholar]
- Vialov, O.S. Sledy zxhinedeyatelnosti organizmov I ikh paleoentologischeskoe znacheniye (Traces of living organisms and their paleontological significance). In Akadimya Nauk Urainskoy SSR Institut Geologii I Geokhimii Goryuchikh Iskopaymykh; Naukova Dumka: Kiev, Ukraine, 1966; p. 219. [Google Scholar]
- Lucas, S.G. Cenozoic vertebrate footprint ichnotaxa named by O.S. Vyalov in 1965 and 1966. In Cenozoic Vertebrate Tracks and Traces; Lucas, S.G., Spielmann, J.H., Lockley, M.G., Eds.; 34 plates; New Mexico Museum of Natural History and Science Bulletin: Albuquerque, NM, USA, 2007; Volume 42, pp. 113–117. [Google Scholar]
- Vialov, O.S. The classification of fossil traces of life. In Proceedings of the 24th International Congress, Montreal, QC, Canada, 21–26 August 1972; pp. 71–78. [Google Scholar]
- Palii, V.M. The contribution of O.S. Vialov to the development of ichnological classification and nomenclature. Stratigr. Geol. Correl. 2013, 21, 249–251. [Google Scholar] [CrossRef]
- Scrivner, P.J.; Bottjer, D.J. Neogne avian and mammalian tracks from Death Valley National Monument, California: Their context, classification, and preservation. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1986, 57, 285–331. [Google Scholar] [CrossRef]
- Haubold, H. Ichnia Amphibiorum et Reptiliorum Fossilium. Handbuch der Paläoherpatologie; part 18; Gustav Fischer: Stuttgart, Germany, 1971; p. 123. [Google Scholar]
- Rühle von Lilienstern, H. Fährten und Spüren im Chirotherium−Sandstein von Südthüringen. Fortschr. Geol. Paläontol. 1939, 12, 93–387. [Google Scholar]
- Lichtig, A.J.; Lucas, S.G.; Klein, H.; Lovelace, D.M. Triassic turtle tracks and the origin of turtles. Hist. Biol. 2018, 30, 1112–1122. [Google Scholar] [CrossRef]
- Ernst, C.H.; Lovich, J.E. Turtles of the United States and Canada; Johns Hopkins University Press: Baltimore, MD, USA, 2009; ISBN 9780801891212. [Google Scholar]
- Milán, J.; Hedegaard, R. Interspecific variation in tracks and trackways from extant Crocodylians. In Crocodyle Tracks and Traces; Milàn, J., Lucas, S.G., Spielmann, J.A., Eds.; New Mexico Museum of Natural History and Science Bulletin: Albuquerque, NM, USA, 2010; Volume 51, pp. 15–30. [Google Scholar]
- Kumagai, C.J.; Farlow, J.O. Observations on traces of the American crocodile (Crocodylus acutus) from northwestern Costa Rica. In Crocodyle Tracks and Traces; Milàn, J., Lucas, S.G., Spielmann, J.A., Eds.; New Mexico Museum of Natural History and Science Bulletin: Albuquerque, NM, USA, 2010; Volume 51, pp. 41–50. [Google Scholar]
- Farlow, J.O.; Elsey, R.M. Footprints and trackways of the American alligator. Rockefeller Wildlife Refuge, Louisiana. In Crocodyle Tracks and Traces; Milàn, J., Lucas, S.G., Spielmann, J.A., Eds.; New Mexico Museum of Natural History and Science Bulletin: Albuquerque, NM, USA, 2010; Volume 51, pp. 31–40. [Google Scholar]
- Grigg, G.; Kirshner, D. Biology and Evolution of Crocodylians; Cornell University Press: Ithaca, NY, USA, 2015; p. 649. [Google Scholar]
- Farlow, J.O.; Robinson, N.J.; Turner, M.L.; Black, J.; Gatesy, S.M. Footfall pattern of a bottom-walking crocodile (Crocodylus acutus). Palaios 2018, 33, 406–413. [Google Scholar] [CrossRef]
- Martinez, M.M. Issues for aquatic pedestrian locomotion. Am. Zool. 1996, 36, 619–627. [Google Scholar] [CrossRef]
- Martinez, M.M.; Full, R.J.; Koehl, M.A.R. Underwater punting by an intertidal crab: A novel gait revealed by the kinematics of pedestrian locomotion in air versus water. J. Exp. Biol. 1998, 201, 2609–2623. [Google Scholar]
- Koester, D.M.; Spiritu, C.P. Punting: An usual mode of locomotion in the little skate, Leucoraja erinaceae (Chondricthes: Rajidae). Copea 2003, 2003, 553–561. [Google Scholar] [CrossRef]
- Mikuláš, R.; Dvořák, Z. Charachichnos isp., probable Crocodylian swim traces from the Miocene of Ceseké basin, Czech Republic. In Crocodyle Tracks and Traces; Milàn, J., Lucas, S.G., Spielmann, J.A., Eds.; New Mexico Museum of Natural History and Science Bulletin: Albuquerque, NM, USA, 2010; Volume 51, pp. 121–136. [Google Scholar]
- Farlow, J.O.; Robinson, N.J.; Kumagai, C.J.; Paladino, F.V.; Falkingham, P.L.; Esey, R.M.; Martin, A.J. Trackways of the American Crocodile (Crocodylus acutus) in northwestern Costa Rica: Implications for Crocodylian ichnology. Ichnos 2017, 9, 1–36. [Google Scholar] [CrossRef]
- Weymouth, H. Systematik der Rezenten Krokodile. Mitt. Mus. Berl. 1953, 29, 275–514. [Google Scholar]
- Owen, R. Report on British fossil reptiles. Rep. Br. Assoc. Adv. Sci. 1842, 9, 60–204. [Google Scholar]
- Avanzini, M.; Piñuela, L.; Garcia-Ramos, J.C. Preservational morphotypes of Crocodylopus from the Late Jurassic of Asgturia (Spain). In Crocodyle Tracks and Traces; Milàn, J., Lucas, S.G., Spielmann, J.A., Eds.; New Mexico Museum of Natural History and Science Bulletin: Albuquerque, NM, USA, 2010; Volume 51, pp. 239–244. [Google Scholar]
- Olsen, K.; Padian, P.E. Earliest records of Batrachopus from the southwestern United States, and a revision of some early Mesozoic crocodylomorph inchnogenera. In The Beginning of the Age of Dinosaurs; Padian, K., Ed.; Cambridge University Press: Cambridge, UK, 1986; pp. 259–273. [Google Scholar]
- Lockley, M.G. A solution to the Mehiella mystery: Tracking, naming, identifying, and measuring the first Crocodylian trackway reported from the Cretaceous (Dakota Group, Colorado). In Crocodyle Tracks and Traces; Milàn, J., Lucas, S.G., Spielmann, J.A., Eds.; New Mexico Museum of Natural History and Science Bulletin: Albuquerque, NM, USA, 2010; Volume 51, pp. 157–174. [Google Scholar]
- Lockley, M.G.; Foster, J.R. An assemblage of probably Crocodylian traces and associated dinosaur tracks from the lower Morrison Formation (Upper Jurassic) of eastern Utah. In Crocodyle Tracks and Traces; Milàn, J., Lucas, S.G., Spielmann, J.A., Eds.; New Mexico Museum of Natural History and Science Bulletin: Albuquerque, NM, USA, 2010; Volume 51, pp. 93–98. [Google Scholar]
- Avanzini, M.A.; Piñuela, L.A.; Ruiz-Omeñaca, J.I.; Garcia-Ramos, J.C. The crocodile track Hatcherichnus from the Upper Jurassic of Aturia (Spain). In Crocodyle Tracks and Traces; Milàn, J., Lucas, S.G., Spielmann, J.A., Eds.; New Mexico Museum of Natural History and Science Bulletin: Albuquerque, NM, USA, 2009; Volume 51, pp. 89–92. [Google Scholar]
- Lockley, M.G.; Li, R.; Matxkawa, M.; Li, J. Tracking Chinese Crocodylians: Kuangyanpus, Laiyangpus, and implications for naming Crocodylian and Crocodylian-like tracks and associated ichnofacies. In Crocodyle Tracks and Traces; Milàn, J., Lucas, S.G., Spielmann, J.A., Eds.; New Mexico Museum of Natural History and Science Bulletin: Albuquerque, NM, USA, 2010; Volume 51, pp. 99–108. [Google Scholar]
- Mateus, O.; Milán, J. First record of Crocodyle and Pterosaur tracks in the upper Jurassic of Portugal. In Crocodyle Tracks and Traces; Milàn, J., Lucas, S.G., Spielmann, J.A., Eds.; New Mexico Museum of Natural History and Science Bulletin: Albuquerque, NM, USA, 2010; Volume 51, pp. 83–88. [Google Scholar]
- Le Leuf, J.; Lauprasert, K.; Suteethorn, S.; Souillat, V.; Suteethorn, V.; Buffetaut, E. Late Early Cretaceous crocodyliform trackways from northeastern Thailand. In Crocodyle Tracks and Traces; Milàn, J., Lucas, S.G., Spielmann, J.A., Eds.; New Mexico Museum of Natural History and Science Bulletin: Albuquerque, NM, USA, 2010; Volume 51, pp. 175–178. [Google Scholar]
- Rajkumar, H.S.; Mustoe, G.E.; Khaidem, K.S.; Soibam, I. Crocodylian tracks from Lower Eocene flysch deposits of the Barail Group, Manipur, India. Ichnos 2015, 22, 122–131. [Google Scholar] [CrossRef]
- Brandt, M.E. Coral Disease Epizootiology in the Florida Keys (U.S.A.) and Cayman Islands (British West Indies), and the Development of the Simulation of Infected Corals Model. Ph.D. Thesis, University of Miami, Coral Gables, FL, USA, December 2017. [Google Scholar]
- Lockley, M.G.; Lucas, S.G.; Milàn, J.; Harris, J.D.; Avenzini, M.; Foster, J.R.; Spielmann, J. The fossil record of Crocodylian tracks and traces: An overview. In Crocodyle Tracks and Traces; Milàn, J., Lucas, S.G., Spielmann, J.A., Eds.; New Mexico Museum of Natural History and Science Bulletin: Albuquerque, NM, USA, 2010; Volume 51, pp. 1–14. [Google Scholar]
- Brochu, C.A. Phylogenetic approaches toward Crocodylian history. Annu. Rev. Earth Planet. Sci. 2003, 31, 357–397. [Google Scholar] [CrossRef]
- Scheyer, T.M.; Aguilera, O.A.; Delfino, M.; Fortier, D.C.; Carlini, A.A.; Sánchez, R.; Carillo-Briceño, J.D.; Quiroz, L.; Sánchez-Villagra, M.R. Crocodylian diversity peak and extinction in the late Cenozoic of the northern Neotropics. Nat. Commun. 2013, 4, 1–9. [Google Scholar] [CrossRef]
- Brochu, C.A.; Storrs, G.W. A giant crocodile from the Plio-Pleistocene of Kenya, the phylogenetic relationship of Neogene African crocodylines, and the antiquity of Crocodylus in Africa. J. Vertebr. Paleontol. 2012, 32, 587–602. [Google Scholar] [CrossRef]
- Grande, L. Paleontology of the Green River Formation, with a Review of Fish Fauna; Geological Survey of Wyoming Bulletin 63: Laramie, WY, USA, 1984; Volume 63, pp. 198–203.
- Grande, L. The Lost World of Fossil Lake; University of Chicago Press: Chicago, IL, USA, 2013; pp. 210–213. ISBN 13: 978-0-226-92296-6. [Google Scholar]
- Erickson, B.R. Wannagonosuchus, a new alligator from the Paleocene of North America. J. Paleontol. 1982, 56, 492–506. [Google Scholar]
- Erickson, B.R. The Wannagan Creek Quarry and Its Reptilian Fauna (Bullion Creek Formation, Paleocene) in Billins County, North Dakota; Report of Investigations No. 72, 1982; North Dakota Geological Survey: Grand Forks, ND, USA, 1982.
- Erisckson, B.R. Fossil Lake Wannegan (Paleocene:Tiffanian), Billings County, North Dakota; Series No. 87; North Dakota Geological Survey Misc: Grand Forks, ND, USA, 1999.
- Lance, V.A. Alligator physiology and life history: The importance of temperature. Exp. Gerontol. 2003, 38, 801–805. [Google Scholar] [CrossRef]
- Washington Herp Alas, Washington Department of Natural Resources. 2017. Available online: http://www1.dnr.wa.gov/nhp/refdesk/herp/speciesmain.html (accessed on 16 June 2019).
- Zachos, J.; Pagani, M.; Sloan, L.; Thomas, E.; Billups, K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 2001, 282, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Zachos, J.C.; Dickens, G.R.; Zeebe, R.E.; Zeebe, R.E. An early Cenozoic perspective on greenhouse warming and carbon-cycle dynamics. Nature 2008, 451, 279–283. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).