Viroids-First—A Model for Life on Earth, Mars and Exoplanets
Abstract
1. Potential for Life in the Universe
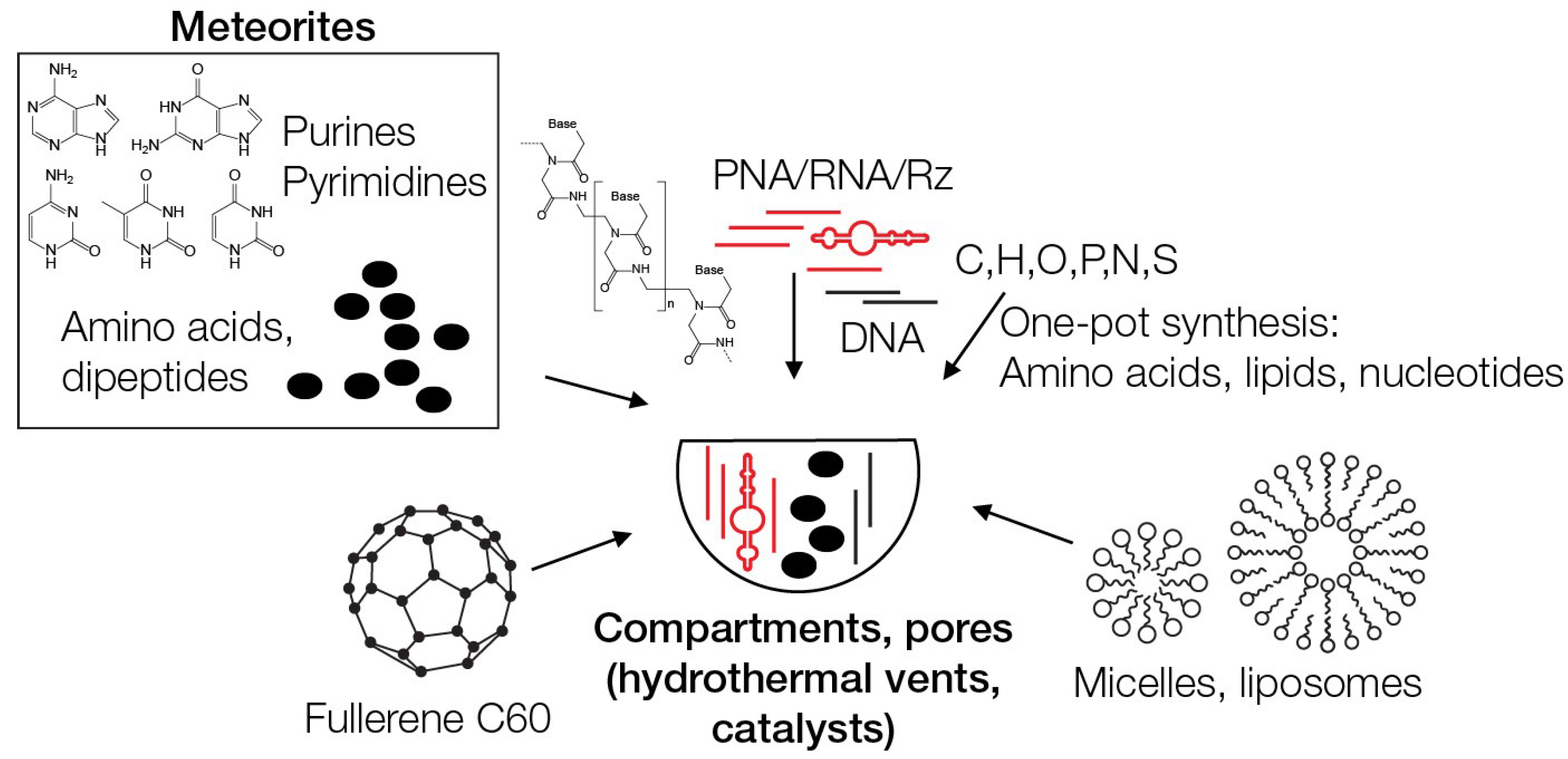
2. Meteorites and Early Earth
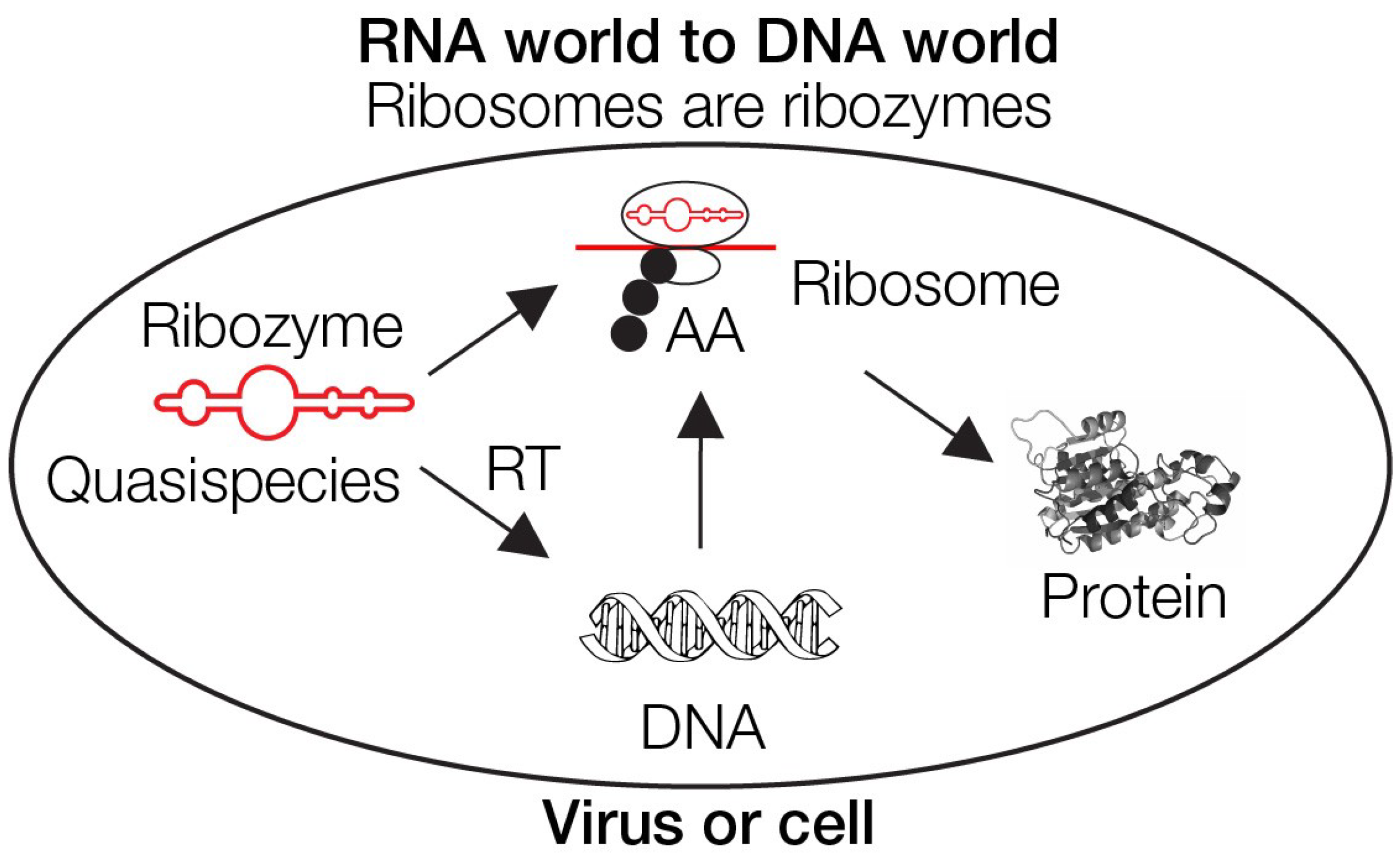
3. The RNA world
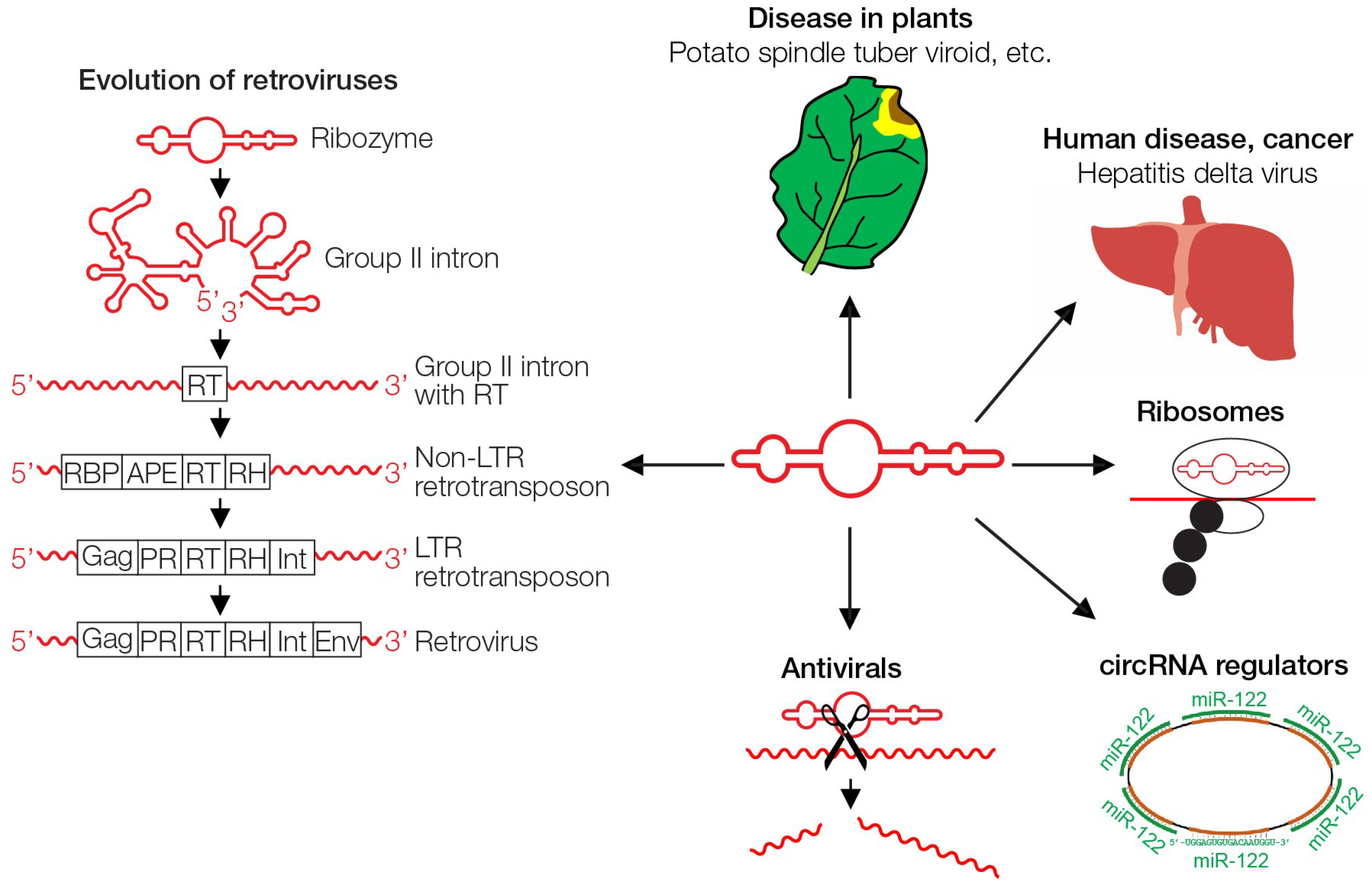
4. Viroids
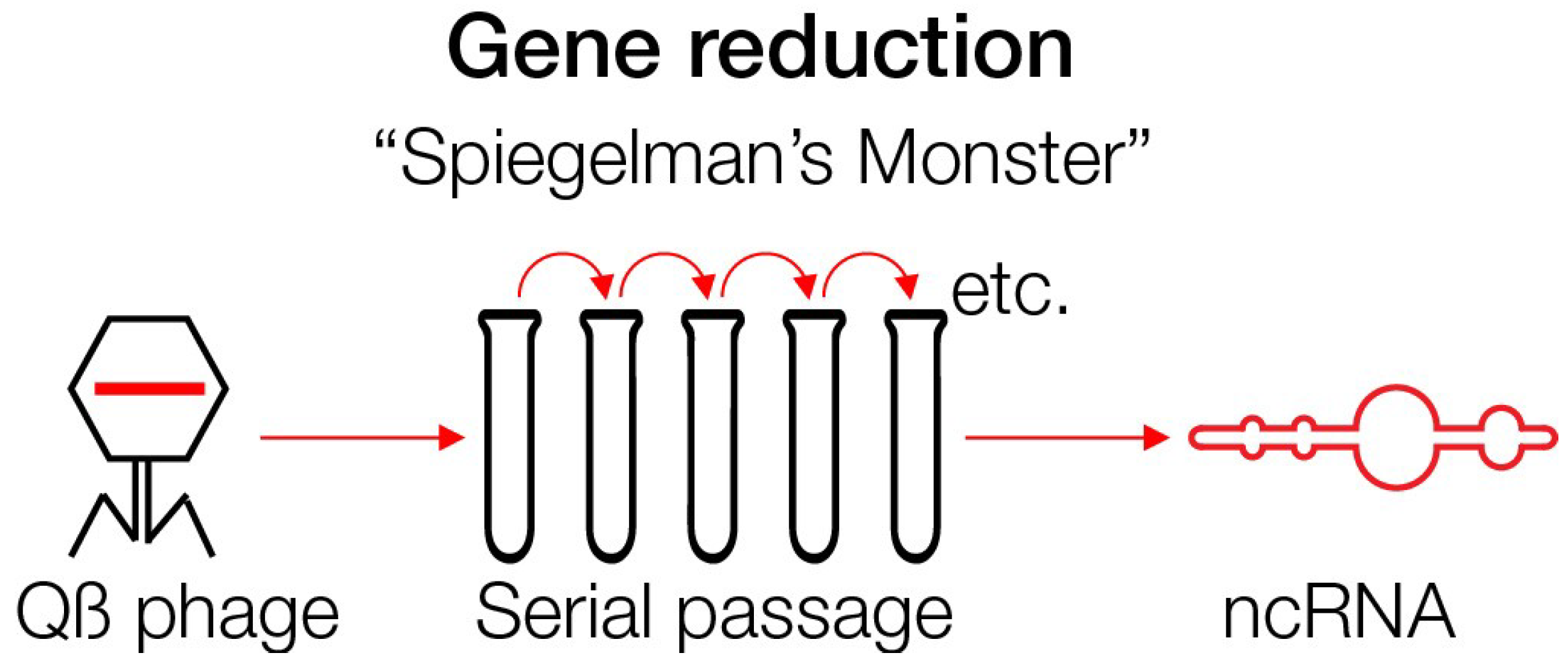
5. Spiegelman’s Monster
6. Endosymbiosis and Giant Viruses
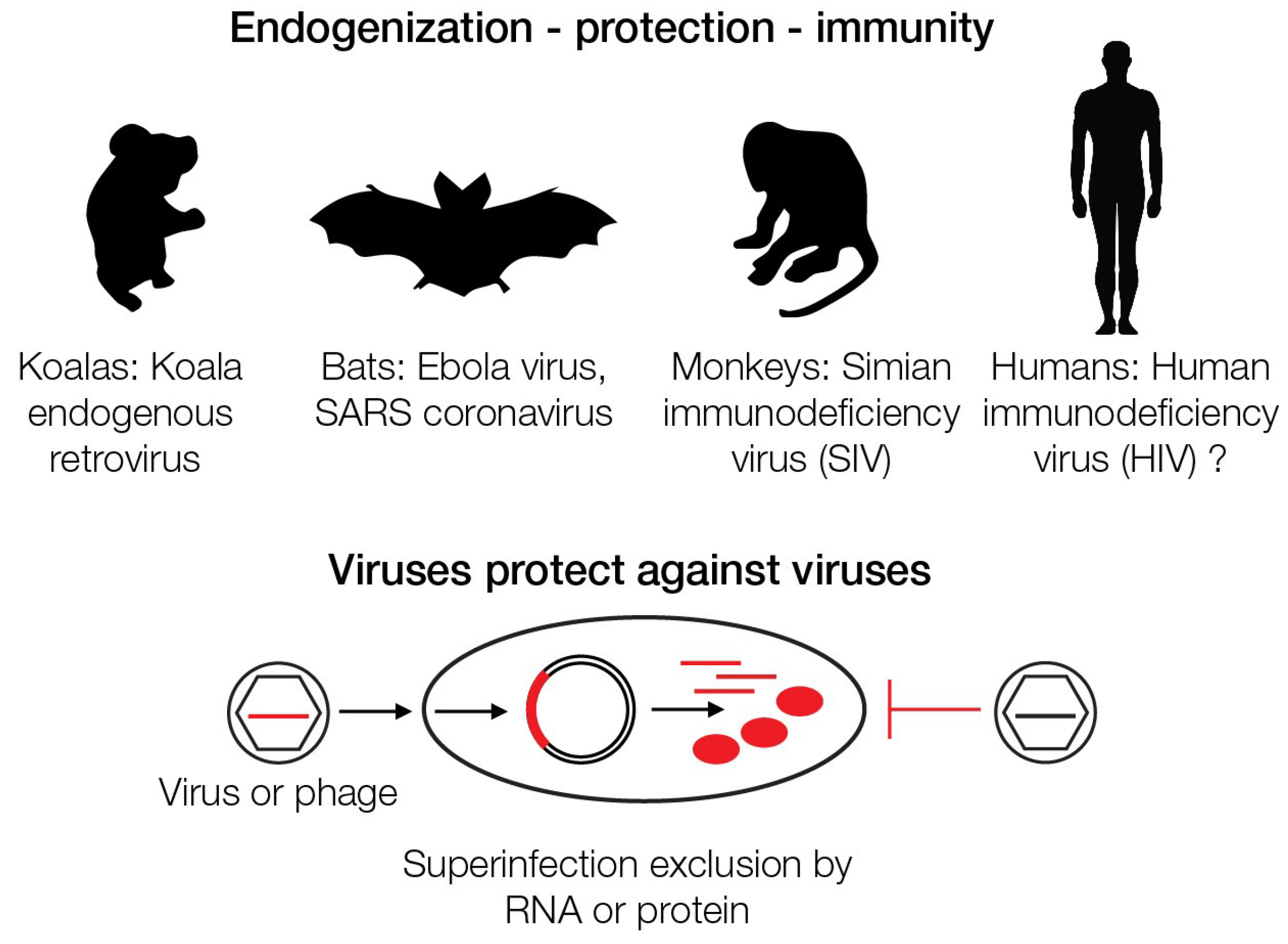
7. Retroviruses
8. Habitable zones
9. Archaea and Archaeal Viruses
10. Spores and Survival under Extreme Conditions
11. Outlook—Including Viral Signatures may Help in the Search for Extraterrestrial Life
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mayor, M.; Queloz, D. A Jupiter-mass companion to a solar-type star. Nature 1995, 378, 355–359. [Google Scholar] [CrossRef]
- Kasting, J.F.; Whitmire, D.P.; Reynolds, R.T. Habitable zones around main sequence stars. Icarus 1993, 101, 108–128. [Google Scholar] [CrossRef]
- Southey, R. The Three Bears. 1837. Available online: https://etc.usf.edu/lit2go/68/fairy-tales-and-other-traditional-stories/5105/the-three-bears/ (accessed on 13 March 2019).
- Milliken, R.E.; Li, S. Remote detection of widespread indigenous water in lunar pyroclastic deposits. Nat. Geosci. 2017, 10, 561–565. [Google Scholar] [CrossRef]
- Gillon, M.; Jehin, E.; Lederer, S.M.; Delrez, L.; De Wit, J.; Burdanov, A.; Van Grootel, V.; Burgasser, A.J.; Triaud, A.H.M.J.; Opitom, C.; et al. Temperate Earth-sized planets transiting a nearby ultracool dwarf star. Nature 2016, 533, 221–224. [Google Scholar] [CrossRef]
- Wu, J.; Desch, S.J.; Schaefer, L.; Elkins-Tanton, L.T.; Pahlevan, K.; Buseck, P.R. Origin of Earth’s Water: Chondritic Inheritance Plus Nebular Ingassing and Storage of Hydrogen in the Core. J. Geophys. Res. 2018, 123, 2691–2712. [Google Scholar] [CrossRef]
- Kan, S.B.; Lewis, R.D.; Chen, K.; Arnold, F.H. Directed evolution of cytochrome c for carbon-silicon bond formation: Bringing silicon to life. Science 2016, 354, 1048–1051. [Google Scholar] [CrossRef] [PubMed]
- Madhusudhan, N.; Lee, K.K.M.; Mousis, O. A possible carbon-rich interior in super-earth 55 Cancri e. Astrophys. J. Lett. 2012, 759, L40. [Google Scholar] [CrossRef]
- Rains Liquid Iron and Glass—Hubble Reveals 1st Images of an Exoplanet Atmosphere. Available online: https://dailygalaxy.com/2016/02/rains-liquid-iron-and-glass-hubble-reveals-1st-images-of-an-exoplanet-atmosphere/ (accessed on 19 February 2016).
- O’Malley-James, J.T.; Raven, J.A.; Cockell, C.S.; Greaves, J.S. Life and light: Exotic photosynthesis in binary and multiple-star systems. Astrobiology 2012, 12, 115–124. [Google Scholar] [CrossRef]
- Eberle, U. Geo Kompakt; Gruner&Jahr GmbH: Hamburg, Germany, 2014; pp. 122–138. [Google Scholar]
- Meierhenrich, U. Amino Acids and the Asymmetry of Life; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Koga, T.; Naraoka, H. A new family of extraterrestrial amino acids in the Murchison meteorite. Sci. Rep. 2017, 7, 636. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.H.; Percivalle, C.; Ritson, D.J.; Duffy, C.D.; Sutherland, J.D. Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism. Nat. Chem. 2015, 7, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Kirby, E.N.; Guhathakurta, P.; Simon, J.D.; Geha, M.C.; Rockosi, C.M.; Sneden, C.; Cohen, J.G.; Sohn, S.T.; Majewski, S.R.; Siegel, M. Multi-element abundance measurements from medium-resolution spectra. II. Catalog of stars in Milky Way dwarf satellite galaxies. Astrophys. J. Suppl. Ser. 2010, 191, 352–375. [Google Scholar] [CrossRef]
- Küppers, B.O. The Computability of the World, How Far Can Science Take Us? The Frontiers Collection; Springer International Publishing AG: Gewerbestrasse, Switzerland, 2018. [Google Scholar]
- Nam, I.; Nam, H.G.; Zare, R.N. Abiotic synthesis of purine and pyrimidine ribonucleosides in aqueous microdroplets. Proc. Natl. Acad. Sci. USA 2018, 115, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Szostak, J.W.; Bartel, D.P.; Luisi, P.L. Synthesizing life. Nature 2001, 409, 387–390. [Google Scholar] [CrossRef]
- Joyce, G.F. The antiquity of RNA-based evolution. Nature 2002, 418, 214–221. [Google Scholar] [CrossRef]
- Benner, S.A. Defining life. Astrobiology 2010, 10, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.S.; Szostak, J.W. In vitro selection of functional nucleic acids. Annu. Rev. Biochem. 1999, 68, 611–647. [Google Scholar] [CrossRef] [PubMed]
- Brackett, D.M.; Dieckmann, T. Aptamer to ribozyme: The intrinsic catalytic potential of a small RNA. Chembiochem 2006, 7, 839–843. [Google Scholar] [CrossRef]
- Dyson, F. Origins of Life, 2nd ed.; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Lincoln, T.A.; Joyce, G.F. Self-sustained replication of an RNA enzyme. Science 2009, 323, 1229–1232. [Google Scholar] [CrossRef]
- Dreher, T.W. Role of tRNA-like structures in controlling plant virus replication. Virus Res. 2009, 139, 217–229. [Google Scholar] [CrossRef]
- Dreher, T.W. Viral tRNAs and tRNA-like structures. Wiley Interdiscip. Rev. RNA 2010, 1, 402–414. [Google Scholar] [CrossRef]
- Crick, F.H. The origin of the genetic code. J. Mol. Biol. 1968, 38, 367–379. [Google Scholar] [CrossRef]
- Cech, T.R. Structural biology. The ribosome is a ribozyme. Science 2000, 289, 878–879. [Google Scholar] [CrossRef] [PubMed]
- Ban, N.; Beckmann, R.; Cate, J.H.; Dinman, J.D.; Dragon, F.; Ellis, S.R.; Lafontaine, D.L.; Lindahl, L.; Liljas, A.; Lipton, J.M.; et al. A new system for naming ribosomal proteins. Curr. Opin. Struct. Biol. 2014, 24, 165–169. [Google Scholar] [CrossRef]
- Harish, A.; Caetano-Anollés, G. Ribosomal history reveals origins of modern protein synthesis. PLoS ONE 2012, 7, e32776. [Google Scholar] [CrossRef]
- McGinness, K.E.; Joyce, G.F. In search of an RNA replicase ribozyme. Chem Biol 2003, 10, 5–14. [Google Scholar] [CrossRef]
- Szostak, J.W. An optimal degree of physical and chemical heterogeneity for the origin of life? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 2894–2901. [Google Scholar] [CrossRef]
- Shen, L.; Ji, H.F. Small cofactors may assist protein emergence from RNA world: Clues from RNA-protein complexes. PLoS ONE 2011, 6, e22494. [Google Scholar] [CrossRef]
- Martin, W.; Baross, J.; Kelley, D.; Russell, M.J. Hydrothermal vents and the origin of life. Nat. Rev. Microbiol. 2008, 6, 805–814. [Google Scholar] [CrossRef]
- Attwater, J.; Wochner, A.; Pinheiro, V.B.; Coulson, A.; Holliger, P. Ice as a protocellular medium for RNA replication. Nat. Commun. 2010, 1, 76. [Google Scholar] [CrossRef]
- Gilbert, W. Origin of life: The RNA world. Nature 1986, 319, 618. [Google Scholar] [CrossRef]
- Campillo-Balderas, J.A.; Lazcano, A.; Becerra, A. Viral Genome Size Distribution Does Not Correlate with the Antiquity of the Host Lineages. Front. Ecol. Evol. 2015, 3, 143. [Google Scholar] [CrossRef]
- Grew, E.S.; Bada, J.L.; Hazem, R.M. Borate minerals and origin of the RNA world. Orig. Life Evol. Biophys. 2011, 41, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Flint, S.J.; Enquist, L.W.; Krug, R.M.; Racaniello, V.R.; Skalka, A.M. Principles of Virology, 4th ed.; ASM Press: Washington, DC, USA, 2015. [Google Scholar]
- Ritson, D.J.; Sutherland, J.D. Conversion of biosynthetic precursors of RNA to those of DNA by photoredox chemistry. J. Mol. Evol. 2014, 78, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Samanta, B.; Joyce, G.F. A reverse transcriptase ribozyme. eLife 2017, 6, e31153. [Google Scholar] [CrossRef]
- Cojocaru, R.; Unrau, P.J. Transitioning to DNA genomes in an RNA world. eLife 2017, 6, e32330. [Google Scholar] [CrossRef] [PubMed]
- Horning, D.P.; Joyce, G.F. Amplification of RNA by an RNA polymerase ribozyme. Proc. Natl. Acad. Sci. USA 2016, 113, 9786–9791. [Google Scholar] [CrossRef] [PubMed]
- Yamagami, T.; Ishino, S.; Kawarabayasi, Y.; Ishino, Y. Mutant Taq DNA polymerases with improved elongation ability as a useful reagent for genetic engineering. Front. Microbiol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Mohr, G.; Perlman, P.S.; Lambowitz, A.M. Evolutionary relationships among group II intron-encoded proteins and identification of a conserved domain that may be related to maturase function. Nucleic Acids Res. 1993, 21, 4991–4997. [Google Scholar] [CrossRef]
- Simon, D.M.; Zimmerly, S. A diversity of uncharacterized reverse transcriptases in bacteria. Nucleic Acids Res. 2008, 36, 7219–7229. [Google Scholar] [CrossRef]
- Moelling, K.; Broecker, F.; Russo, G.; Sunagawa, S. RNase H As Gene Modifier, Driver of Evolution and Antiviral Defense. Front. Microbiol. 2017, 8, 1745. [Google Scholar] [CrossRef]
- Deveson, I.W.; Hardwick, S.A.; Mercer, T.R.; Mattick, J.S. The Dimensions, Dynamics, and Relevance of the Mammalian Noncoding Transcriptome. Trends Genet. 2017, 33, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA circles function as efficient microRNA sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef]
- Diener, T.O. Viroids: “living fossils” of primordial RNAs? Biol. Direct 2016, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Gross, H.J.; Domdey, H.; Lossow, C.; Jank, P.; Raba, M.; Alberty, H.; Sänger, H.L. Nucleotide sequence and secondary structure of potato spindle tuber viroid. Nature 1978, 273, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.; Hernández, C.; Martínez de Alba, A.E.; Daròs, J.A.; Di Serio, F. Viroids and viroid-host interactions. Annu. Rev. Phytopathol. 2005, 43, 117–139. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.; Gago-Zachert, S.; Serra, P.; Sanjuán, R.; Elena, S.F. Viroids: Survivors from the RNA world? Annu. Rev. Microbiol. 2014, 68, 395–414. [Google Scholar] [CrossRef]
- Broecker, F.; Moelling, K. Evolution of immune systems from viruses and transposable elements. Front. Microbiol. 2019, 10, 51. [Google Scholar] [CrossRef]
- Hegedus, K.; Palkovics, L.; Tóth, E.K.; Dallmann, G.; Balázs, E. The DNA form of a retroviroid-like element characterized in cultivated carnation species. J. Gen. Virol. 2001, 82, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.M. Replication of the hepatitis delta virus RNA genome. Adv. Virus Res. 2009, 74, 103–121. [Google Scholar]
- AbouHaidar, M.G.; Venkataraman, S.; Golshani, A.; Liu, B.; Ahmad, T. Novel coding, translation, and gene expression of a replicating covalently closed circular RNA of 220 nt. Proc. Natl. Acad. Sci. USA 2014, 111, 14542–14547. [Google Scholar] [CrossRef]
- Pachuk, C.J.; Yoon, K.; Moelling, K.; Coney, L.R. Selective cleavage of bcr-abl chimeric RNAs by a ribozyme targeted to non-contiguous sequences. Nucleic Acids Res. 1994, 22, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Müller, G.; Strack, B.; Dannull, J.; Sproat, B.S.; Surovoy, A.; Jung, G.; Moelling, K. Amino acid requirements of the nucleocapsid protein of HIV-1 for increasing catalytic activity of a Ki-ras ribozyme in vitro. J. Mol. Biol. 1994, 242, 422–429. [Google Scholar] [CrossRef]
- Ding, B.; Qin, Y.; Chen, M. Nucleocapsid proteins: Roles beyond viral RNA packaging. Wiley Interdiscip. Rev. RNA 2016, 7, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.L. A production of amino acids under possible primitive earth conditions. Science 1953, 117, 528–529. [Google Scholar] [CrossRef]
- Miller, S.L.; Urey, H.C. Organic compound synthesis on the primitive earth. Science 1959, 130, 245–251. [Google Scholar] [CrossRef] [PubMed]
- La Scola, B.; Audic, S.; Robert, C.; Jungang, L.; de Lamballerie, X.; Drancourt, M.; Birtles, R.; Claverie, J.M.; Raoult, D. A giant virus in amoebae. Science 2003, 299, 2033. [Google Scholar] [CrossRef] [PubMed]
- Schulz, F.; Yutin, N.; Ivanova, N.N.; Ortega, D.R.; Kwon, T.; Vierheilig, J.; Daims, H.; Horn, M.; Wagner, M.; Jensen, G.J.; et al. Giant viruses with an expanded complement of translation system components. Science 2017, 356, 82–85. [Google Scholar] [CrossRef]
- La Scola, B.; Desnues, C.; Pagnier, I.; Robert, C.; Barrassi, L.; Fournous, G.; Merchat, M.; Suzan-Monti, M.; Forterre, P.; Koonin, E.; et al. The virophage as a unique parasite of the giant mimivirus. Nature 2008, 455, 100–105. [Google Scholar] [CrossRef]
- Karapetyan, Y.E. Viruses do replicate in cell-free systems. Proc. Natl. Acad. Sci. USA 2012, 109, E461. [Google Scholar] [CrossRef]
- Moelling, K. Viruses: More Friends than Foes; World Scientific Press: Singapore, 2017. [Google Scholar]
- Moelling, K. Are viruses our oldest ancestors? EMBO Rep. 2012, 13, 1033. [Google Scholar] [CrossRef]
- Moelling, K. What contemporary viruses tell us about evolution: A personal view. Arch. Virol. 2013, 158, 1833–1848. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, L.P. Viruses and the Evolution of Life; American Society of Microbiology Press: Washington, DC, USA, 2005. [Google Scholar]
- Villarreal, L.P.; Witzany, G. That is life: Communicating RNA networks from viruses and cells in continuous interaction. Ann. N. Y. Acad. Sci. 2019. [Google Scholar] [CrossRef]
- Vasas, V.; Szathmary, E.; Santosa, M. Lack of evolvability in self-sustaining autocatalytic networks: A constraint on the metabolism-fist path to the origin of life. Proc. Natl. Acad. Sci. USA 2010, 107, 1470–1475. [Google Scholar] [CrossRef] [PubMed]
- Nasir, A.; Caetano-Anollés, G. A phylogenomic data-driven exploration of viral origins and evolution. Sci. Adv. 2015, 1, e1500527. [Google Scholar] [CrossRef]
- Segré, D.; Ben-Eli, D.; Deamer, D.W.; Lancet, D. The lipid world. Orig. Life Evol. Biosph. 2001, 31, 119–145. [Google Scholar] [CrossRef] [PubMed]
- Andras, P.; Andras, C. The origin of life—The ‘protein interaction world’ hypothesis: Protein interactions were the first form of self-reproducing life and nucleic acids evolved later as memory molecules. Med. Hypotheses 2005, 64, 678–688. [Google Scholar] [CrossRef]
- Hayward, A. Origin of the retroviruses: When, where, and how? Curr. Opin. Virol. 2017, 25, 23–27. [Google Scholar] [CrossRef]
- Orgel, L.E. Prebiotic chemistry and the origin of the RNA world. Crit. Rev. Biochem. Mol. Biol. 2004, 39, 99–123. [Google Scholar]
- Lacey, J.C.; Cook, G.W.; Mullins, D.W. Concepts related to the origin of coded protein synthesis. CHEMTRACTS-Biochem. Mol. Biol. 1999, 12, 398–418. [Google Scholar]
- Kauffman, S.A. The Origins of Order; Oxford Univ Press: New York, NY, USA, 1993. [Google Scholar]
- Anet, F.A. The place of metabolism in the origin of life. Curr. Opin. Chem. Biol. 2004, 8, 654–659. [Google Scholar] [CrossRef]
- Segré, D.; Ben-Eli, D.; Lancet, D. Compositional genomes: Prebiotic information transfer in mutually catalytic noncovalent assemblies. Proc. Natl. Acad. Sci. USA 2000, 97, 4112–4117. [Google Scholar] [CrossRef] [PubMed]
- Spiegelman, S.; Haruna, I.; Holland, I.B.; Beaudreau, G.; Mills, D. The synthesis of a self-propagating and infectious nucleic acid with a purified enzyme. Proc. Natl. Acad. Sci. USA 1965, 54, 919–927. [Google Scholar] [CrossRef]
- Sumper, M.; Luce, R. Evidence for de novo production of self-replicating and environmentally adapted RNA structures by bacteriophage Qbeta replicase. Proc. Natl. Acad. Sci. USA 1975, 72, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Eigen, M. From Strange Simplicity to Complex Familiarity; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Margulis, L. Origin of Eukaryotic Cells; Yale University Press: New Haven, CT, USA, 1970. [Google Scholar]
- Margulis, L. Symbiosis in Cell Evolution, 2nd ed.; W.H. Freeman and Co.: New York, NY, USA, 1993. [Google Scholar]
- Segata, N. Gut microbiome: Westernization and the disappearance of intestinal diversity. Curr. Biol. 2015, 25, R611–R613. [Google Scholar] [CrossRef] [PubMed]
- Schnorr, S.L.; Candela, M.; Rampelli, S.; Centanni, M.; Consolandi, C.; Basaglia, G.; Turroni, S.; Biagi, E.; Peano, C.; Severgnini, M.; et al. Gut microbiome of the Hadza hunter-gatherers. Nat. Commun. 2014, 5, 3654. [Google Scholar] [CrossRef]
- Carrapiço, F. How symbiogenic is evolution? Theory Biosci. 2010, 129, 135–139. [Google Scholar] [CrossRef]
- Schatz, G. Mitochondria: Beyond oxidative phosphorylation. Biochim. Biophys. Acta 1995, 1271, 123–126. [Google Scholar]
- Ogata, H.; Audic, S.; Renesto-Audiffren, P.; Fournier, P.E.; Barbe, V.; Samson, D.; Roux, V.; Cossart, P.; Weissenbach, J.; Claverie, J.M.; Raoult, D. Mechanisms of evolution in Rickettsia conorii and R. prowazekii. Science 2001, 293, 2093–2098. [Google Scholar] [CrossRef]
- Koonin, E.V.; Yutin, N. Evolution of the large nucleocytoplasmic DNA viruses of eukaryotes and convergent origins of viral gigantism. Adv. Virus Res. 2019, 103, 167–202. [Google Scholar] [PubMed]
- Brandes, M.; Linial, M. Giant Viruses-Big Surprises. Viruses 2019, 11, 404. [Google Scholar] [CrossRef]
- Mölling, K.; Bolognesi, D.P.; Bauer, H.; Büsen, W.; Plassmann, H.W.; Hausen, P. Association of viral reverse transcriptase with an enzyme degrading the RNA moiety of RNA-DNA hybrids. Nat. New Biol. 1971, 234, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Hingamp, P.; Kojima, K.K.; Villar, E.; Romac, S.; Veluchamy, A.; Boccara, M.; Jaillon, O.; Iudicone, D.; Bowler, C.; et al. Reverse transcriptase genes are highly abundant and transcriptionally active in marine plankton assemblages. ISME J. 2016, 10, 1134–1146. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.G.; Chen, L.; Ji, H.F.; Chen, Z.H.; Yang, F.R.; Wang, L.; Qu, G.; Jiang, Y.Y.; Ji, C.; Zhang, H.Y. Characters of very ancient proteins. Biochem. Biophys. Res. Commun. 2008, 366, 607–611. [Google Scholar] [CrossRef]
- Majorek, K.A.; Dunin-Horkawicz, S.; Steczkiewicz, K.; Muszewska, A.; Nowotny, M.; Ginalski, K.; Bujnicki, J.M. The RNase H-like superfamily: New members, comparative structural analysis and evolutionary classification. Nucleic Acids Res. 2014, 42, 4160–4179. [Google Scholar] [CrossRef]
- Eigen, M. Selforganization of matter and the evolution of biological macromolecules. Naturwissenschaften 1971, 58, 465–523. [Google Scholar] [CrossRef] [PubMed]
- Girard, A.; Sachidanandam, R.; Hannon, G.J.; Carmell, M.A. A germline-specific class of small RNAs bind mammalian Piwi proteins. Nature 2006, 442, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; FitzHugh, W.; et al. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar] [PubMed]
- Dewannieux, M.; Heidmann, T. Endogenous retroviruses: Acquisition, amplification and taming of genome invaders. Curr. Opin. Virol. 2013, 3, 646–656. [Google Scholar] [CrossRef]
- Dupressoir, A.; Lavialle, C.; Heidmann, T. From ancestral infectious retroviruses to bona fide cellular genes: Role of the captured syncytins in placentation. Placenta 2012, 33, 663–671. [Google Scholar] [CrossRef]
- Tarlinton, R.E. Koala Retrovirus Endogenisation in Action. In Viruses: Essential Agents of Life; Witzany, G., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 283–291. [Google Scholar]
- Li, H.; Nykoluk, M.; Li, L.; Liu, L.R.; Omange, R.W.; Soule, G.; Schroeder, L.T.; Toledo, N.; Kashem, M.A.; Correia-Pinto, J.F.; et al. Natural and cross-inducible anti-SIV antibodies in Mauritian cynomologus macaques. PLoS ONE 2017, 12, e0186079. [Google Scholar]
- Li, H.; Li, L.; Liu, L.R.; Omange, R.W.; Toledo, N.; Kashem, M.A.; Hai, Y.; Liang, B.; Plummer, F.A.; Luo, M. Hypothetical endogenous SIV-like antigens in Mauritian cynomologus macaques. Bioinformation 2018, 14, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Hayman, D.T. Bats as viral reservoirs. Annu. Rev. Virol. 2016, 3, 77–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, L.B.; Hou, Z.W.; Kang, X.J.; Xie, Q.D.; Yu, X.J.; Ma, M.F.; Ma, B.L.; Wang, Z.S.; Lei, Y.; et al. The integrated HIV-1 provirus in patient sperm chromosome and its transfer into the early embryo by fertilization. PLoS ONE 2011, 6, e28586. [Google Scholar] [CrossRef]
- McKay, C.P. The search for life in our Solar System and the implications for science and society. Philos. Trans. A Math. Phys. Eng. Sci. 2011, 369, 594–606. [Google Scholar] [CrossRef]
- Postberg, F.; Khawaja, N.; Abel, B.; Choblet, G.; Glein, C.R.; Gudipati, M.S.; Henderson, B.L.; Hsu, H.-W.; Kempf, S.; Klenner, F.; et al. Macromolecular organic compounds from the depths of Enceladus. Nature 2018, 558, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Gaind, N. Mars probe poised to solve red planet’s methane mystery. Nature 2018, 556, 419. [Google Scholar] [CrossRef] [PubMed]
- McKay, C.P. Titan as the Abode of life. Life 2016, 6, 8. [Google Scholar] [CrossRef]
- Jones, B.M.; Kaiser, R.I.; Strazzulla, G. Carbonic acid as a reserve of carbon dioxide on icy moons: The formation of carbon dioxide (CO2) in a polar environment. Astrophys. J. 2014, 788, 170. [Google Scholar] [CrossRef]
- Craddock, R.A.; Howard, A.D. The case for rainfall on a warm, wet early Mars. JGR Planets 2002, 107, 21-1–21-6. [Google Scholar] [CrossRef]
- Tyler, R.H. Strong ocean tidal flow and heating on moons of the outer planets. Nature 2008, 456, 770–772. [Google Scholar] [CrossRef]
- Raulin, F.; Owen, T. Organic chemistry and exobiology on Titan. Space Sci. Rev. 2002, 104, 377–394. [Google Scholar] [CrossRef]
- Europlanet 2020 Planetary Field Analogue Sites. Available online: http://www.europlanet-2020-ri.eu/research-infrastructure/field-and-lab-visits/ta1-planetary-field-analogue-sites-pfa (accessed on 25 May 2019).
- de Vera, J.P.; Alawi, M.; Backhaus, T.; Baqué, M.; Billi, D.; Böttger, U.; Berger, T.; Bohmeier, M.; Cockell, C.; Demets, R.; et al. Limits of Life and the Habitability of Mars: The ESA Space Experiment BIOMEX on the ISS. Astrobiology 2019, 19, 145–157. [Google Scholar] [CrossRef] [PubMed]
- de la Torre Noetzel, R.; Miller, A.Z.; de la Rosa, J.M.; Pacelli, C.; Onofri, S.; García Sancho, L.; Cubero, B.; Lorek, A.; Wolter, D.; de Vera, J.P. Cellular Responses of the Lichen Circinaria gyrosa in Mars-Like Conditions. Front. Microbiol. 2018, 9, 308. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Serra, I.; Vasyunin, A.I.; Caselli, P.; Marcelino, P.; Billot, N.; Viti, S.; Testi, L.; Vastel, C.; Lefloch, B.; Bachiller, R. The spatial distribution of complex organic molecules in the L1544 pre-stellar core. Astrophys. J. Lett. 2016, 830, L6. [Google Scholar] [CrossRef] [PubMed]
- Billi, D.; Verseux, C.; Fagliarone, C.; Napoli, A.; Baqué, M.; de Vera, J.P. A Desert Cyanobacterium under Simulated Mars-like Conditions in Low Earth Orbit: Implications for the Habitability of Mars. Astrobiology 2019, 19, 158–169. [Google Scholar] [CrossRef]
- Westall, F.; Hickman-Lewis, K.; Hinman, N.; Gautret, P.; Campbell, K.A.; Bréhéret, J.G.; Foucher, F.; Hubert, A.; Sorieul, S.; Dass, A.V.; et al. A Hydrothermal-Sedimentary Context for the Origin of Life. Astrobiology 2018, 18, 259–293. [Google Scholar] [CrossRef] [PubMed]
- Ip, C.L.; Loose, M.; Tyson, J.R.; De Cesare, M.; Brown, B.L.; Jain, M.; Leggett, R.M.; Eccles, D.A.; Zalunin, V.; Urban, J.M.; et al. MinION Analysis and Reference Consortium: Phase 1 data release and analysis. F1000Research 2015, 4, 1075. [Google Scholar] [CrossRef] [PubMed]
- Rampelotto, P.H. Extremophiles and extreme environments. Life 2013, 3, 482–485. [Google Scholar] [CrossRef]
- Ptchelkine, D.; Gillum, A.; Mochizuki, T.; Lucas-Staat, S.; Liu, Y.; Krupovic, M.; Phillips, S.E.V.; Prangishvili, D.; Huiskonen, J.T. Unique architecture of thermophilic archaeal virus APBV1 and its genome packaging. Nat. Commun. 2017, 8, 1436. [Google Scholar] [CrossRef]
- Krupovic, M.; Cvirkaite-Krupovic, V.; Iranzo, J.; Prangishvili, D.; Koonin, E.V. Viruses of archaea: Structural, functional, environmental and evolutionary genomics. Virus Res. 2018, 244, 181–193. [Google Scholar] [CrossRef]
- Cano, R.J.; Borucki, M.K. Revival and Identification of Bacterial Spores in 25- to 40-Million-Year-Old Dominican Amber. Science 1995, 268, 1060–1064. [Google Scholar] [CrossRef] [PubMed]
- Vreeland, R.H.; Rosenzweig, W.D.; Powers, D.W. Isolation of a 250 million-year-old halotolerant bacterium from a primary salt crystal. Nature 2000, 407, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Crick, F.H.; Orgel, L.E. Directed panspermia. Icarus 1973, 19, 341–346. [Google Scholar] [CrossRef]
- Steele, E.J.; Al-Mufti, S.; Augustyn, K.A.; Chandrajith, R.; Coghlan, J.P.; Coulson, S.G.; Ghosh, S.; Gillman, M.; Gorczynski, R.M.; Klyce, B.; et al. Cause of Cambrian Explosion—Terrestrial or Cosmic? Prog. Biophys. Mol. Biol. 2018, 136, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Moelling, K. Commentary to: Cause of Cambrian explosion—Terrestrial or cosmic? Steele, E.J. et al. Prog. Biophys. Mol. Biol. 2018, 136, 24. [Google Scholar] [CrossRef] [PubMed]
- Weronika, E.; Łukasz, K. Tardigrades in Space Research—Past and Future. Orig. Life Evol. Biosph. 2017, 47, 545–553. [Google Scholar] [CrossRef]
- Rothschild, L.J.; Mancinelli, R.L. Life in extreme environments. Nature 2001, 409, 1092–1101. [Google Scholar] [CrossRef]
- Shatilovich, A.V.; Tchesunov, A.V.; Neretina, T.V.; Grabarnik, I.P.; Gubin, S.V.; Vishnivetskaya, T.A.; Onstott, T.C.; Rivkina, E.M. Viable Nematodes from Late Pleistocene Permafrost of the Kolyma River Lowland. Dokl. Biol. Sci. 2018, 480, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Shtarkman, Y.M.; Koçer, Z.A.; Edgar, R.; Veerapaneni, R.S.; D’Elia, T.; Morris, P.F.; Rogers, S.O. Subglacial Lake Vostok (Antarctica) accretion ice contains a diverse set of sequences from aquatic, marine and sediment-inhabiting bacteria and eukarya. PLoS ONE 2013, 8, e67221. [Google Scholar] [CrossRef]
- Makarova, K.S.; Aravind, L.; Wolf, Y.I.; Tatusov, R.L.; Minton, K.W.; Koonin, E.V.; Daly, M.J. Genome of the Extremely Radiation-Resistant Bacterium Deinococcus radiodurans Viewed from the Perspective of Comparative Genomics. Microbiol. Mol. Biol. Rev. 2001, 65, 44–79. [Google Scholar] [CrossRef]
- Dessau, M.; Goldhill, D.; McBride, R.; Turner, P.E.; Modis, Y. Selective pressure causes an RNA virus to trade reproductive fitness for increased structural and thermal stability of a viral enzyme. PLoS Genet. 2012, 8, e1003102. [Google Scholar] [CrossRef]
- Singhal, S.; Guerrero, C.M.L.; Whang, S.G.; McClure, E.M.; Busch, H.G.; Kerr, B. Adaptations of an RNA virus to increasing thermal stress. PLoS ONE 2017, 12, e0189602. [Google Scholar] [CrossRef] [PubMed]
- Bolduc, B.; Shaughnessy, D.P.; Wolf, Y.I.; Koonin, E.V.; Roberto, F.F.; Young, M. Identification of novel positive-strand RNA viruses by metagenomic analysis of archaea-dominated Yellowstone hot springs. J. Virol. 2012, 86, 5562–5573. [Google Scholar] [CrossRef] [PubMed]
- Stamos, J.L.; Lentzsch, A.M.; Lambowitz, A.M. Structure of a thermostable group II intron reverse transcriptase with template-primer and its functional and evolutionary implications. Mol. Cell 2017, 68, 926–939. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.C. The other microbiome. Proc. Natl. Acad. Sci. USA 2013, 110, 2682–2684. [Google Scholar] [CrossRef] [PubMed]
- Hahn, J.; Haug, P. Traces of achaebacteria in ancient sediments. Syst. Appl. Microbiol. 1986, 7, 178–183. [Google Scholar] [CrossRef]
- Bell, E.A.; Boehnke, P.; Harrison, T.M.; Mao, W.L. Potentially biogenic carbon preserved in a 4.1 billion-year-old zircon. Proc. Natl. Acad. Sci. USA 2015, 112, 14518–14521. [Google Scholar] [CrossRef] [PubMed]
- Berliner, A.J.; Mochizuki, T.; Stedman, K.M. Astrovirology: Viruses at Large in the Universe. Astrobiology 2018, 18, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Sogo, J.M.; Koller, T.; Diener, T.O. Potato spindle tuber viroid. X. Visualization and size determination by electron microscopy. Virology 1973, 55, 70–80. [Google Scholar] [CrossRef]
- Vardi, A.; Van Mooy, B.A.S.; Fredricks, H.F.; Popendorf, K.; Ossolinski, J.E.; Haramaty, L.; Bidle, K.D. Viral glycosphingolipids induce lytic infection and cell death in marine phytoplankton. Science 2009, 326, 861–865. [Google Scholar] [CrossRef]
- Suttle, C.A. Marine viruses–major players in the global ecosystem. Nat. Rev. Microbiol. 2007, 5, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Copley, S.D.; Smith, E.; Morowitz, H.J. The origin of the RNA world: Co-evolution of genes and metabolism. Bioorg. Chem. 2007, 35, 430–443. [Google Scholar] [CrossRef] [PubMed]
- Cech, T.R. The RNA world in context. Cold Spring Harb. Perspect. Biol. 2012, 4, a006742. [Google Scholar] [CrossRef] [PubMed]
| Theory | Central Ideas |
|---|---|
| Viruses-first | Virus-like entities have evolved from complex molecules like nucleic acids and proteins [68,69,70,71]. Viruses contributed to the evolution of cellular life. The concept is compatible with the proposed ancient RNA world and the replication first approach [50,77], and with the “viroids-first” view described here. |
| Proteins-first | Life emerged from a self-reproducing system of interacting proteins [75]. Concentrated peptide interactors surrounded by lipid membranes formed protocells [78]. Nucleic acids evolved later and stored the information of protein/peptide interactions. |
| Metabolism-first | Metabolic networks arose before nucleic acids [72,79,80]. Complex homeostatic metabolic reactions occurred in micellar structures that divided by fission and were capable of Darwinian evolution [72,81]. The combined catalytic reactions of such micelles can be regarded as “compositional genomes”. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moelling, K.; Broecker, F. Viroids-First—A Model for Life on Earth, Mars and Exoplanets. Geosciences 2019, 9, 241. https://doi.org/10.3390/geosciences9050241
Moelling K, Broecker F. Viroids-First—A Model for Life on Earth, Mars and Exoplanets. Geosciences. 2019; 9(5):241. https://doi.org/10.3390/geosciences9050241
Chicago/Turabian StyleMoelling, Karin, and Felix Broecker. 2019. "Viroids-First—A Model for Life on Earth, Mars and Exoplanets" Geosciences 9, no. 5: 241. https://doi.org/10.3390/geosciences9050241
APA StyleMoelling, K., & Broecker, F. (2019). Viroids-First—A Model for Life on Earth, Mars and Exoplanets. Geosciences, 9(5), 241. https://doi.org/10.3390/geosciences9050241




