Associations between Fossil Beetles and Other Organisms
Abstract
:1. Introduction
2. Materials and Methods
3. Results
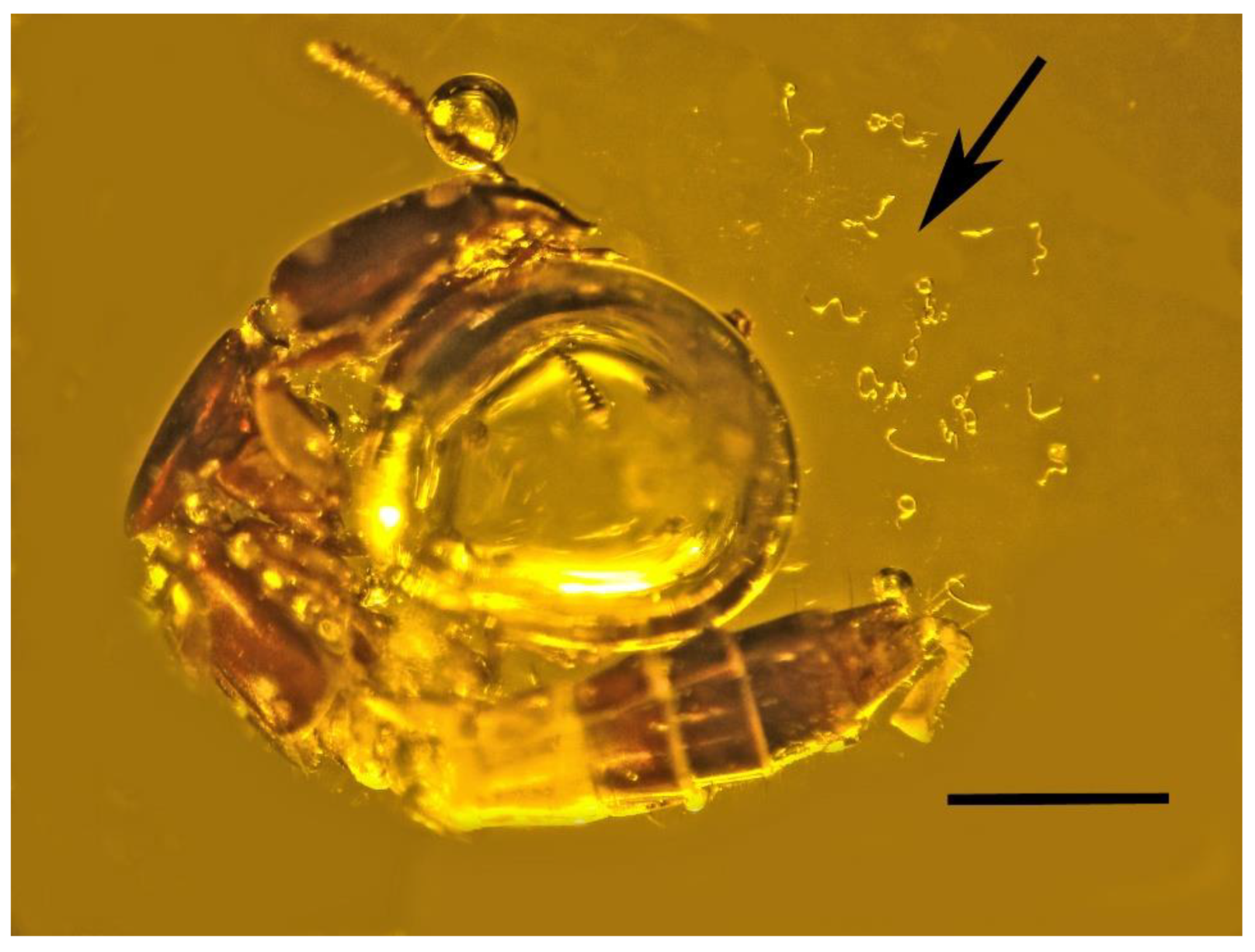
3.1. Associations with Mites (Arachnida: Acari)
3.2. Associations with Pseudoscorpions (Arachnida: Pseudoscorpiones)
3.3. Associations with Spiders (Arachnida: Araneae)
3.4. Associations with Termites (Blattodea: Rhinotermitidae)
3.5. Associations with Insect Parasites and Predators
3.6. Associations with Fungi
3.7. Associations with Flowering Plants
3.8. Associations with Vertebrates
3.9. Associations with Nematodes (Nematoda)
4. Discussion
5. Conclusions
Funding
Conflicts of Interest
References
- Evans, A.V.; Bellamy, C.L. An Inordinate Fondness for Beetles; Henry Holt and Company: New York, NY, USA, 1996; pp. 1–208. [Google Scholar]
- Boucot, A.J. Evolutionary Paleobiology of Behavior and Co-Evolution; Elsevier: Amsterdam, The Netherlands, 1990; pp. 1–725. [Google Scholar]
- Boucot, A.J.; Poinar, G.O., Jr. Fossil Behavior Compendium; CRC Press: Boca Raton, FL, USA, 2010; pp. 1–424. [Google Scholar]
- Poinar, G.O., Jr.; Hess, R. Preservative qualities of recent and fossil resins: Electron micrograph studies on tissue preserved in Baltic amber. J. Baltic Studies 1985, 16, 222–230. [Google Scholar] [CrossRef]
- Iturralde-Vinent, M.S.; MacPhee, R.D.E. Age and paleographic origin of Dominican amber. Science 1996, 273, 1850–1852. [Google Scholar] [CrossRef]
- Schlee, D. Das Bernstein-Kabinett. Stuttgart Beitrage Natur (C) 1990, 28, 1–100. [Google Scholar]
- Berggren, W.A.; Van Couvering, J.A.H. The late Neogene. Palaeogeo. Palaeoclimat. Palaeoecol. 1974, 16, 1–216. [Google Scholar]
- Cruickshank, R.D.; Ko, K. Geology of an amber locality in the Hukawng Valley, northern Myanmar. J. Asian Earth Sci. 2003, 21, 441–455. [Google Scholar] [CrossRef]
- Shi, G.; Grimaldi, D.A.; Harlow, G.E.; Wang, J.; Wang, J.; Yang, M.; Lei, W.; Li, Q.; Li, X. Age constraint on Burmese amber based on U–Pb dating of zircons. Cretac. Res. 2012, 37, 155–163. [Google Scholar] [CrossRef]
- Krantz, G.W.; Poinar, G.O., Jr. Mites, nematodes and the multimillion dollar weevil. J. Natural History 2004, 38, 135–141. [Google Scholar] [CrossRef]
- Poinar, G., Jr.; Legalov, A.A. Bicalcasura maculata n. gen., n. sp. (Curculionoidea: Dryophthoridae) with a list of weevils described from Dominican amber. Hist. Biol. 2013, 26, 449–453. [Google Scholar] [CrossRef]
- Poinar, G.O., Jr.; Curcic, B.P.M.; Cokendolpher, J.C. Arthropod phoresy involving pseudoscorpions in the past and present. Acta Arachnol. 1998, 47, 79–96. [Google Scholar] [CrossRef]
- Yamamoto, S.; Maruyama, M.; Parker, J. Evidence for social parasites of early insect societies by Cretaceous rove beetles. Nat. Commun. 2016, 7, 13658. [Google Scholar] [CrossRef]
- Poinar, G., Jr.; Shaw, S.R. Endoparasitism of a Cretaceous adult weevil by a euphorine wasp (Hymenoptera: Braconidae). N. Jb. Geol. Pal. A. 2016, 282, 109–113. [Google Scholar] [CrossRef]
- Poinar, G.O., Jr. Stenaspidiotus microptilus n. gen., n. sp. (Coleoptera: Chrysomelidae: Chrysomelinae) in Dominican amber, with evidence of tachinid (Diptera: Tachinidae) oviposition. Hist. Biol. 2012, 25, 101–105. [Google Scholar] [CrossRef]
- Poinar, G.O., Jr. Meloe dominicanus n. sp. (Coleoptera: Meloidae) phoretic on the bee Proplebia dominicana (Hymenoptera: Apidae) in Dominican amber. Proc. Entomol. Soc. Washington 2009, 111, 145–150. [Google Scholar] [CrossRef]
- Poinar, G.O., Jr.; Marshall, C.; Buckley, R. One hundred million years of chemical warfare by insects. J. Chem. Ecol. 2007, 33, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Franc, V. Mycetophilous beetles (Coleoptera: Mycetophila)-indicators of well preserved ecosystems. Biologia 1997, 52, 181–186. [Google Scholar]
- Harrington, T.C. Ecology and evolution of mycophagous bark beetles and their fungal partners. In Insect-fungal Associations: Ecology and Evolution; Vega, F.E., Blackwell, M., Eds.; Oxford University Press: Oxford, England, 2005; pp. 257–291. [Google Scholar]
- Poinar, G.O., Jr.; Vega, F.E. A mid-Cretaceous ambrosia fungus, Paleoambrosia entomophila gen. nov. et sp. nov. (Ascomycota: Ophiostomatales) in Burmese (Myanmar) amber, and evidence for a femoral mycangium. Fungal. Biol. 2018, 122, 1159–1162. [Google Scholar] [CrossRef]
- Poinar, G.O., Jr.; Vega, F.E.; Legalov, A.A. New subfamily of ambrosia beetles (Coleoptera: Platypodidae) from mid-Cretaceous Burmese amber. Hist. Biol. 2018, 1–6. [Google Scholar] [CrossRef]
- Lichtwardt, R.W. The Trichomycetes: Fungal Associates of Arthropods; Springer Verlag: New York, NY, USA, 1986; pp. 1–343. [Google Scholar]
- Poinar, G.; Vega, F.E. A mid-Cretaceous trichomycete, Priscadvena corymbosa gen. et sp. nov., in Burmese amber. Fungal. Biol. 2019. [Google Scholar] [CrossRef]
- Poinar, G., Jr. Beetles with orchid pollinaria in Dominican and Mexican amber. Am. Èntomol. 2016, 62, 180–185. [Google Scholar] [CrossRef]
- Chaboo, C.S.; Brown, C.G.; Funk, D.J. Faecal case architecture in the gibbosus species group of Neochlamisus Karren, 1972 (Coleoptera: Chrysomelidae: Cryptocephalinae: Chlamisini). Zoöl. J. Linn. Soc. 2008, 152, 315–351. [Google Scholar] [CrossRef]
- Poinar, G.O., Jr. The Evolutionary History of Nematodes; Brill: Leiden, The Netherlands, 2011; pp. 1–429. [Google Scholar]
- Poinar, G.O., Jr.; Brodzinsky, J. Fossil evidence of a nematode (Tylenchida) parasitic in Staphylinidae (Coleoptera). Nematologica 1986, 31, 353–355. [Google Scholar]
- Wirth, S.F.; Weis, O.; Pernek, M. Comparison of phoretic mites associated with bark beetles Ips typographys and Ips cembrae from Central Croatia. Sumarski List 2016, 11–12, 549–560. [Google Scholar]
- Kistner, D.H. New species of termitophilous Trichopseniinae (Coleoptera: Staphylinidae) found with Mastotermes darwiniensis in Australia and in Dominican amber. Sociobiology 1998, 31, 51–64. [Google Scholar]
- Harris, W.V. African termites of the genus Schedorhinotermes (Isoptera: Rhinotermitidae) and associated termitophiles (Lepidoptera: Tineidae). System. Entomol. 1968, 37, 103–113. [Google Scholar] [CrossRef]
- Clausen, C.P. Entomophagous Insects; Hafner Publishing Company: New York, NY, USA, 1962; pp. 1–688. [Google Scholar]
- Poinar, G.O., Jr. Entomogenous Nematodes; E. J. Brill: Leiden, The Netherlands, 1975; pp. 1–317. [Google Scholar]

























| Beetle Group | M | P | S | P+P | F | A | V | N |
|---|---|---|---|---|---|---|---|---|
| Brentidae | +D | |||||||
| Cantharidae | +B | |||||||
| Chrysomelidae | +D | +D | ||||||
| Colydinae | +D | |||||||
| Curculionidae | +D | +D | +D | |||||
| Dermestidae | +B | |||||||
| Dryophthoridae | +D | |||||||
| Elateridae | +D | +B | ||||||
| Histeridae | +D | |||||||
| Ithyceridae | +B | |||||||
| Lampyridae | +D | |||||||
| Meloidae | +D | |||||||
| Platypodinae | +D | +D | +D | +D | +B | +D | ||
| Pselaphinae | +D | |||||||
| Ptilodactylidae | +Me | |||||||
| Staphylinidae | +D | +D | +D |
| Figure Number and Subject | Type of Amber | Location of Specimen |
|---|---|---|
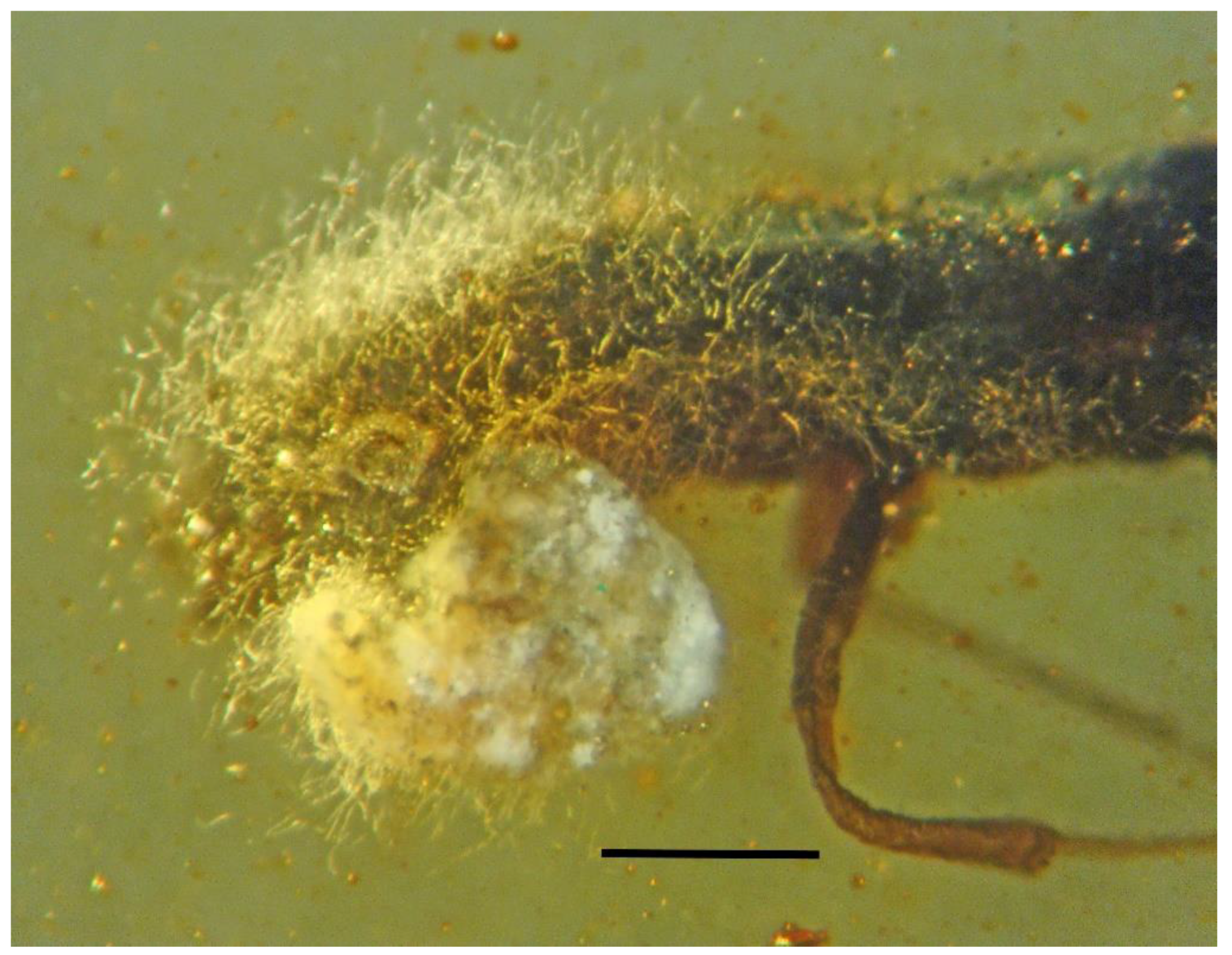
| 1. Mites on beetle | Dominican | PAC-Sy-1-5 |
| 2. Mites on beetle | Dominican | PAC-Sy-1-164 |
| 3. Mites adjacent to beetle | Dominican | PAC-Sy-1-5 |
| 4. Mite holding beetle leg | Dominican | PAC-Sy-1-5 |
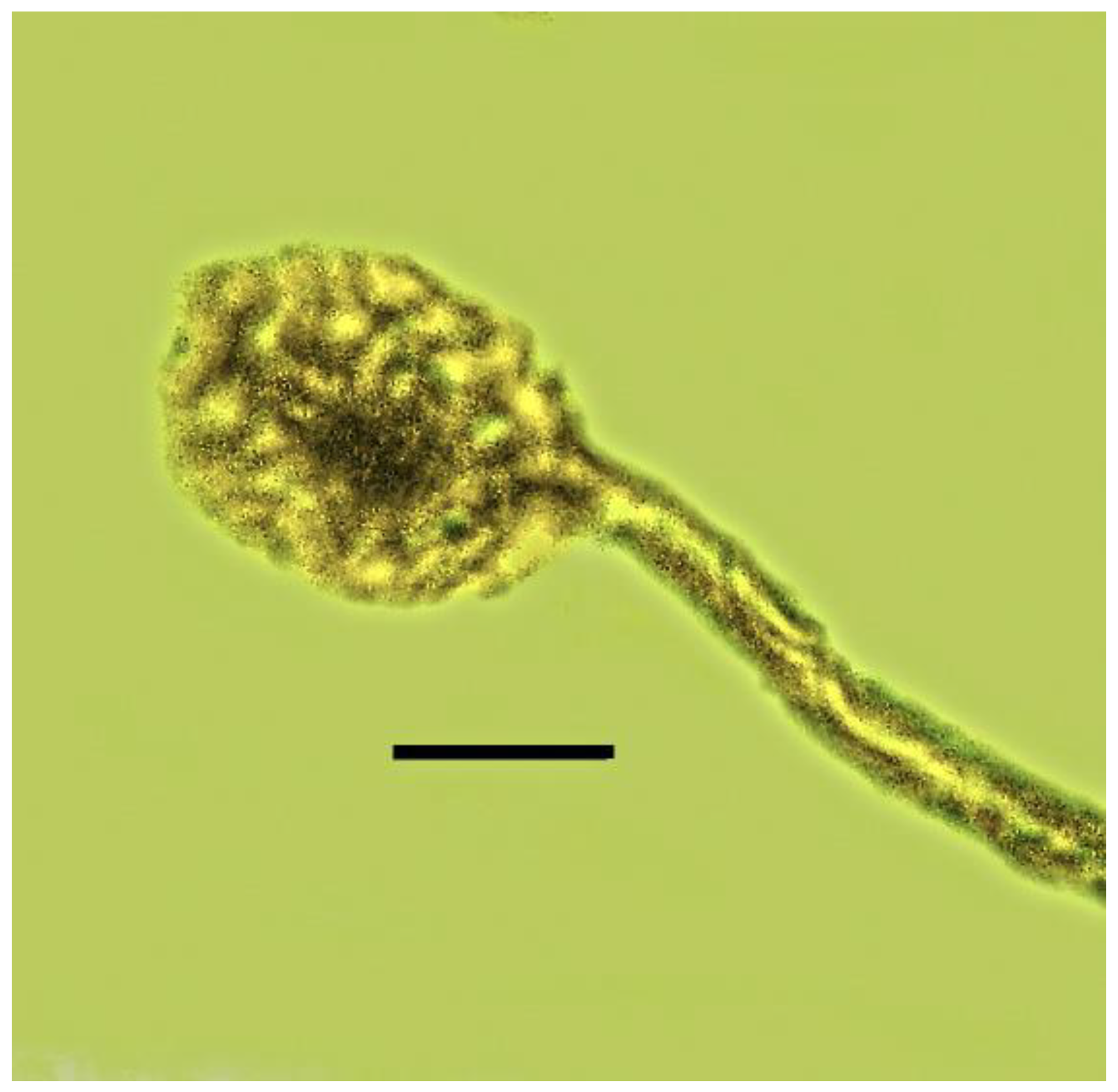
| 5. Mites on weevil | Dominican | PAC-C-7-413 |
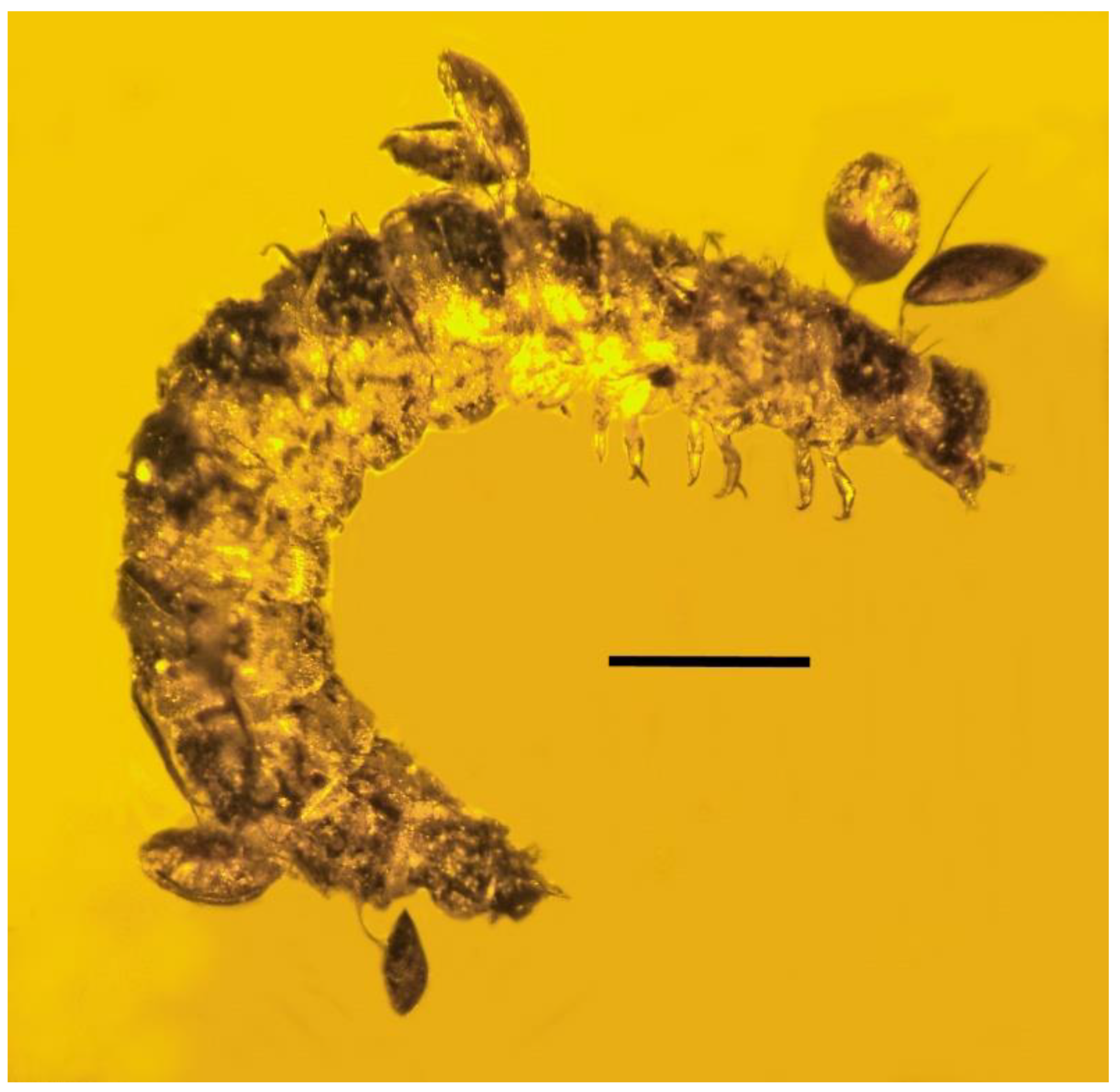
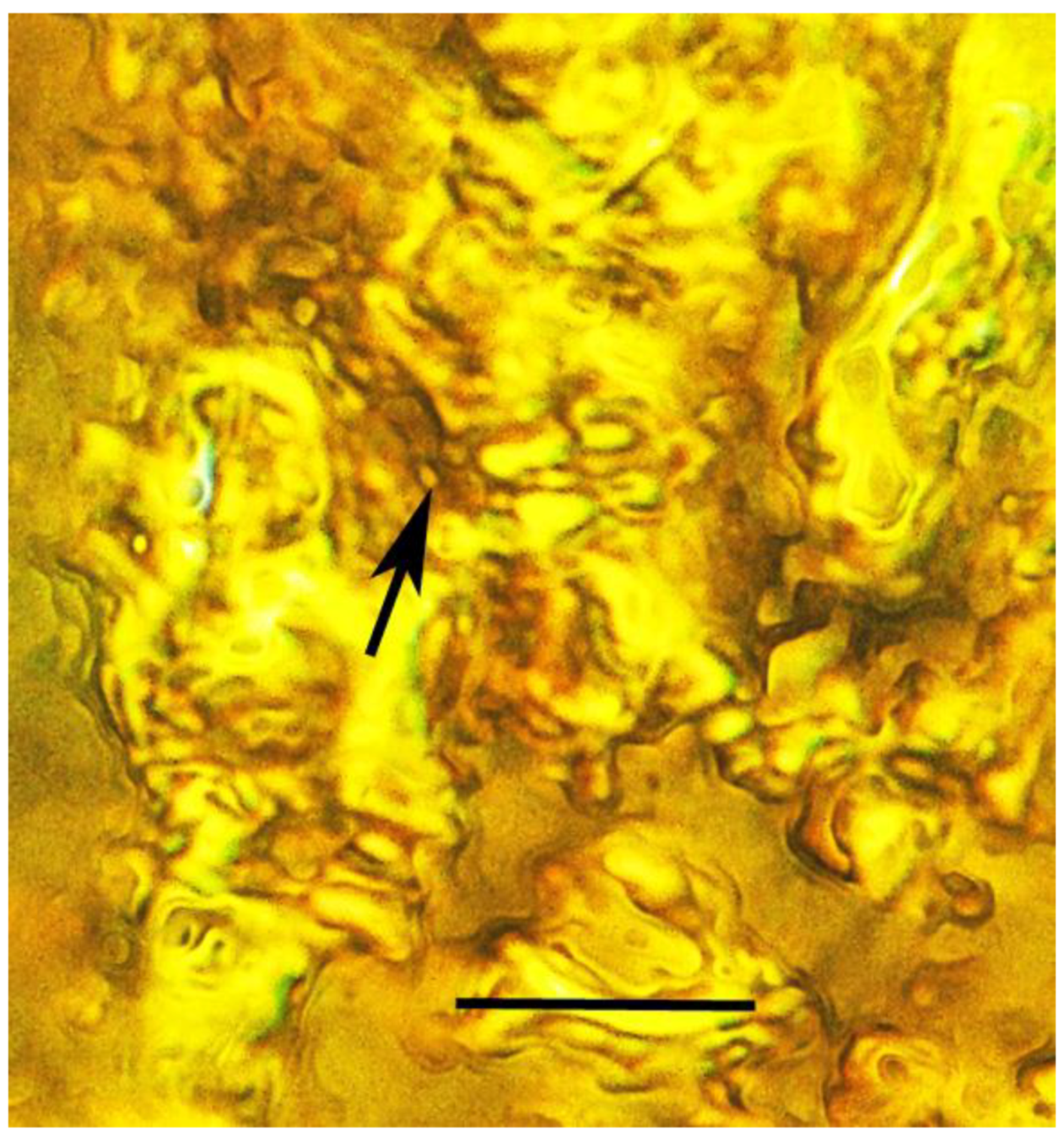
| 6. Mites on beetle larva | Dominican | PAC-Sy-1-27 |
| 7. Mites on beetle larva | Dominican | PAC-Sy-1-27 |
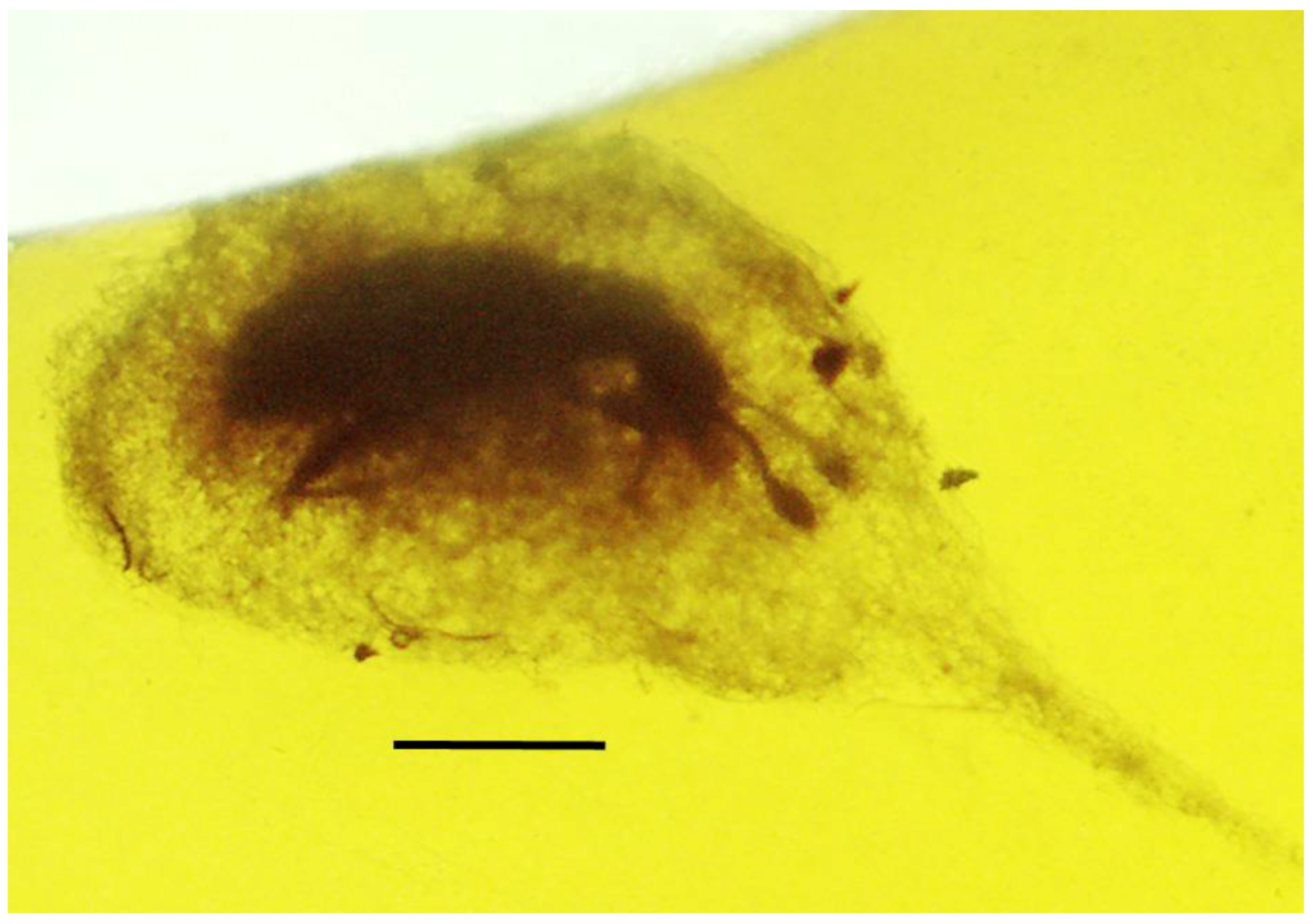
| 8. Pseudoscorpion on beetle | Dominican | PAC-Sy-1-15 |
| 9. Pseudoscorpion on beetle | Dominican | PAC-Sy-1-15 |
| 10. Beetle in spider cocoon | Dominican | PAC-Sy-1-58 |
| 11. Beetle in spider web | Dominican | PAC-Sy-1-58 |
| 12. Beetle in spider web | Dominican | PAC-Sy-1-58 |
| 13. Beetle with termite | Dominican | PAC-Sy-1-24 |
| 14. beetle and moth | Dominican | PAC-Sy-1-186 |
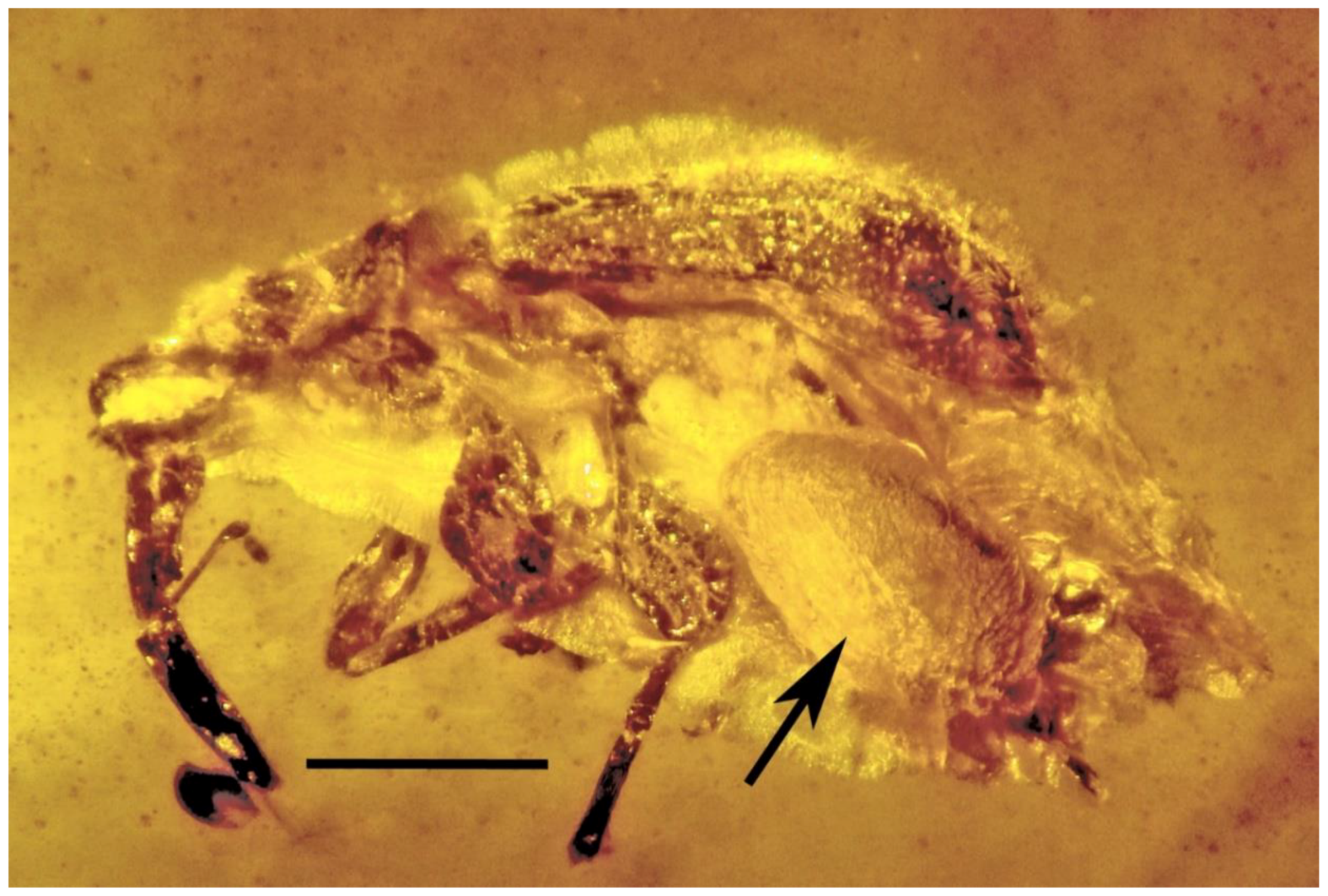
| 15. Parasitized weevil | Burmese | PAC- B-C-48B |
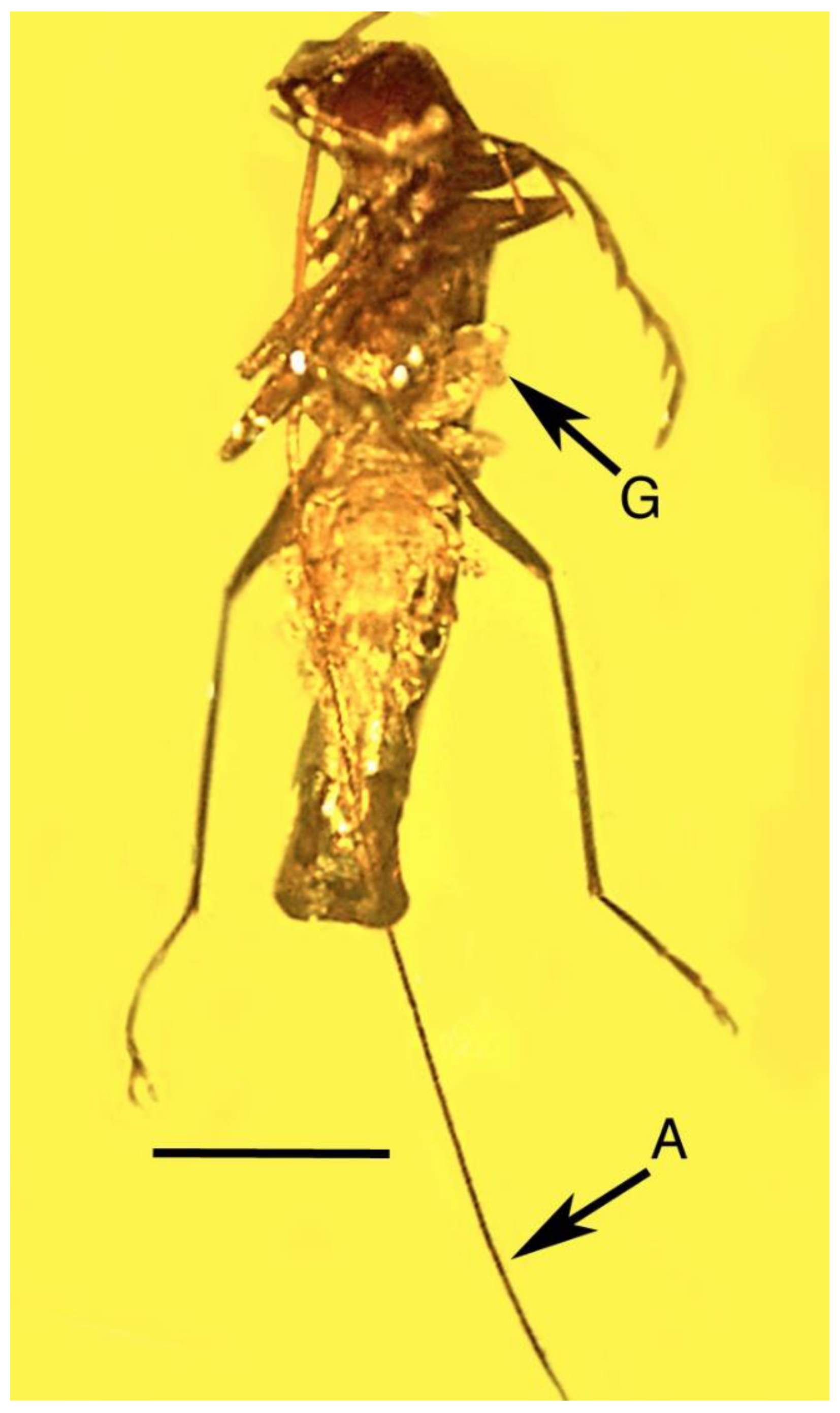
| 16. Parasitized click beetle | Dominican | PAC-Sy-1-131 |
| 17. Wasp cocoons | Dominican | PAC-Sy-1-131 |
| 18. Fly egg on beetle | Dominican | PAC-C-7-305F |
| 19. Bug attacking beetle | Dominican | PAC-Sy-1-135 |
| 20. Beetle on stingless bee | Dominican | PAC-Sy-1-14 |
| 21. Chemical warfare | Burmese | Buckley collection ABS66 |
| 22. Beetle with fungus | Burmese | PAC-B-F-7 |
| 23. Fungus on beetle | Burmese | PAC-B-F-7 |
| 24. Beetle mycangium | Burmese | PAC-B-F-7 |
| 25. Fungus on click beetle | Burmese | PAC-B-F-9 |
| 26. Weevil with orchid | Dominican | PAC-C-1-191 |
| 27. Beetle with orchid | Mexican | PAC- C-1-156 |
| 28. Beetle larva with case | Dominican | PAC-C-7-52 |
| 29. Beetle larva by bird | Burmese | PAC-B-V-3 |
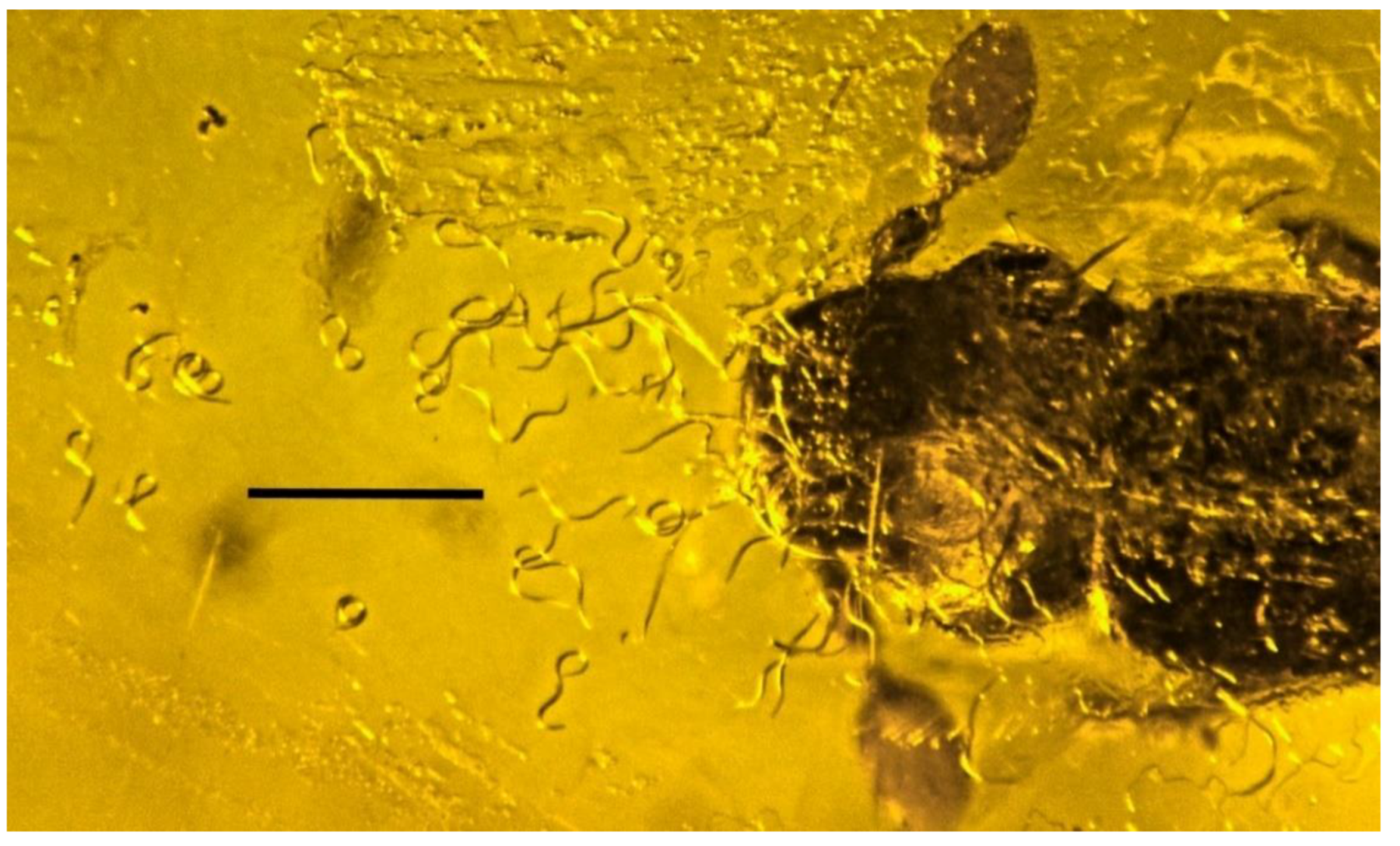
| 30. Nematode on beetle | Dominican | PAC-N-3-19 |
| 31. Nematode parasite | Dominican | PAC-N-3-60 |
| 32. Nematode parasite | Dominican | PAC-N-3-41 |
| 33. Nematode parasite | Dominican | PAC-N-3-9 |
| 34. Nematode parasite | Dominican | PAC-N-3-17 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poinar, G. Associations between Fossil Beetles and Other Organisms. Geosciences 2019, 9, 184. https://doi.org/10.3390/geosciences9040184
Poinar G. Associations between Fossil Beetles and Other Organisms. Geosciences. 2019; 9(4):184. https://doi.org/10.3390/geosciences9040184
Chicago/Turabian StylePoinar, George. 2019. "Associations between Fossil Beetles and Other Organisms" Geosciences 9, no. 4: 184. https://doi.org/10.3390/geosciences9040184
APA StylePoinar, G. (2019). Associations between Fossil Beetles and Other Organisms. Geosciences, 9(4), 184. https://doi.org/10.3390/geosciences9040184





