Origin of the Vanadiferous Serpentine–Magnetite Rocks of the Mt. Sorcerer Area, Lac Doré Layered Intrusion, Chibougamau, Québec
Abstract
1. Introduction
2. Geological Context
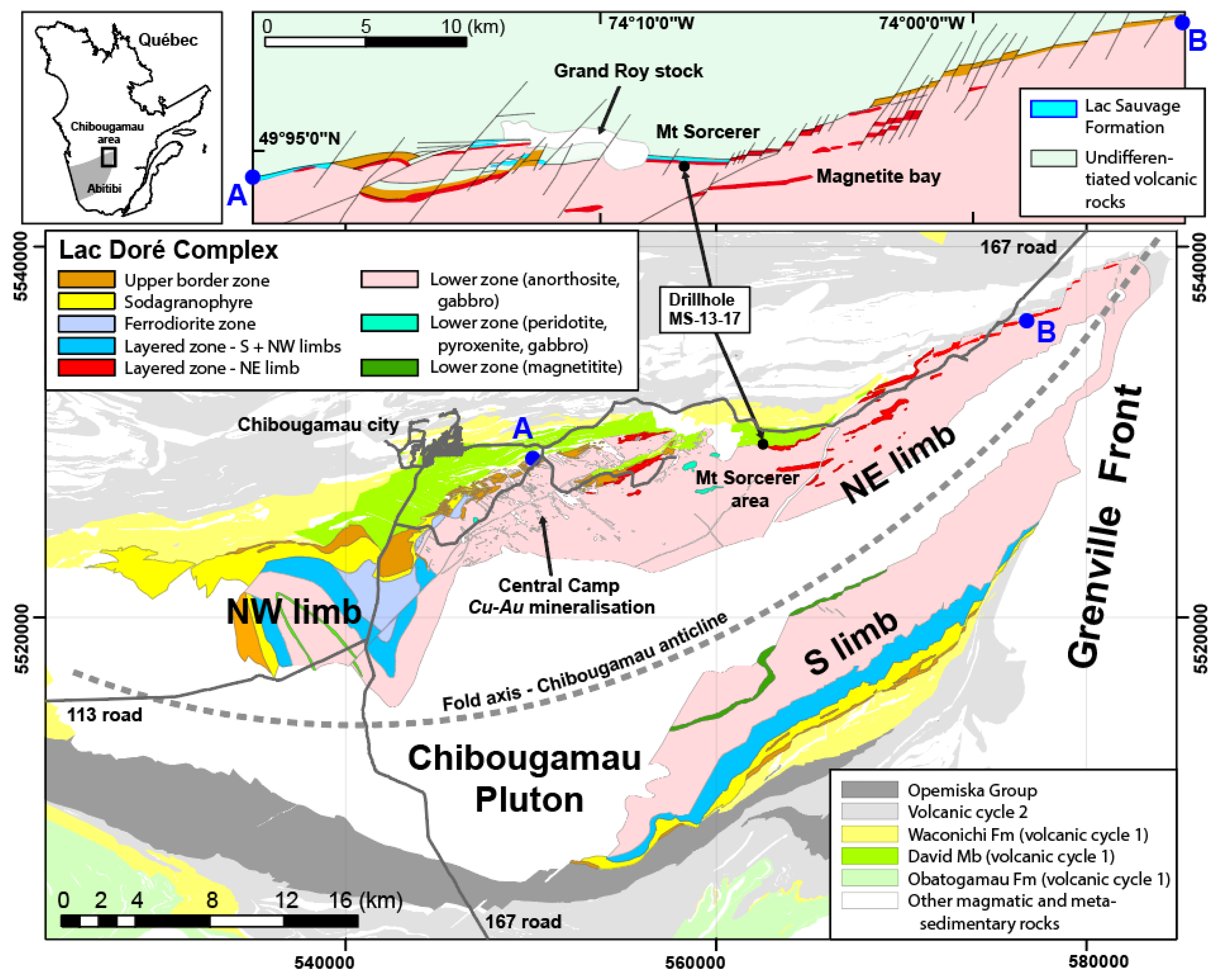
2.1. Chibougamau Area
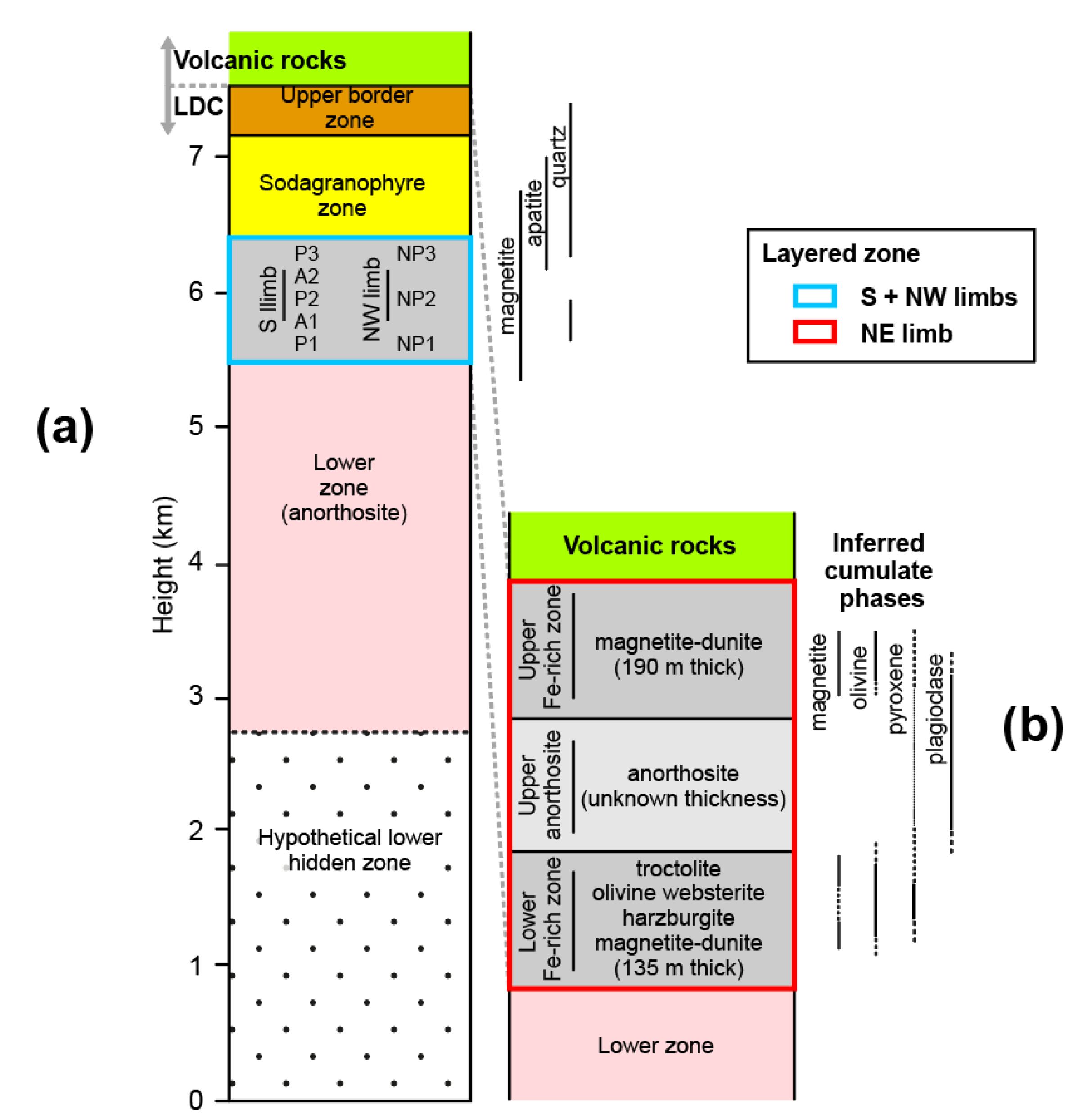
2.2. Geology of the LDC
2.3. Mt. Sorcerer Area
2.4. Evolution of the LDC Magma Chamber
3. Samples
4. Methodology
5. Results
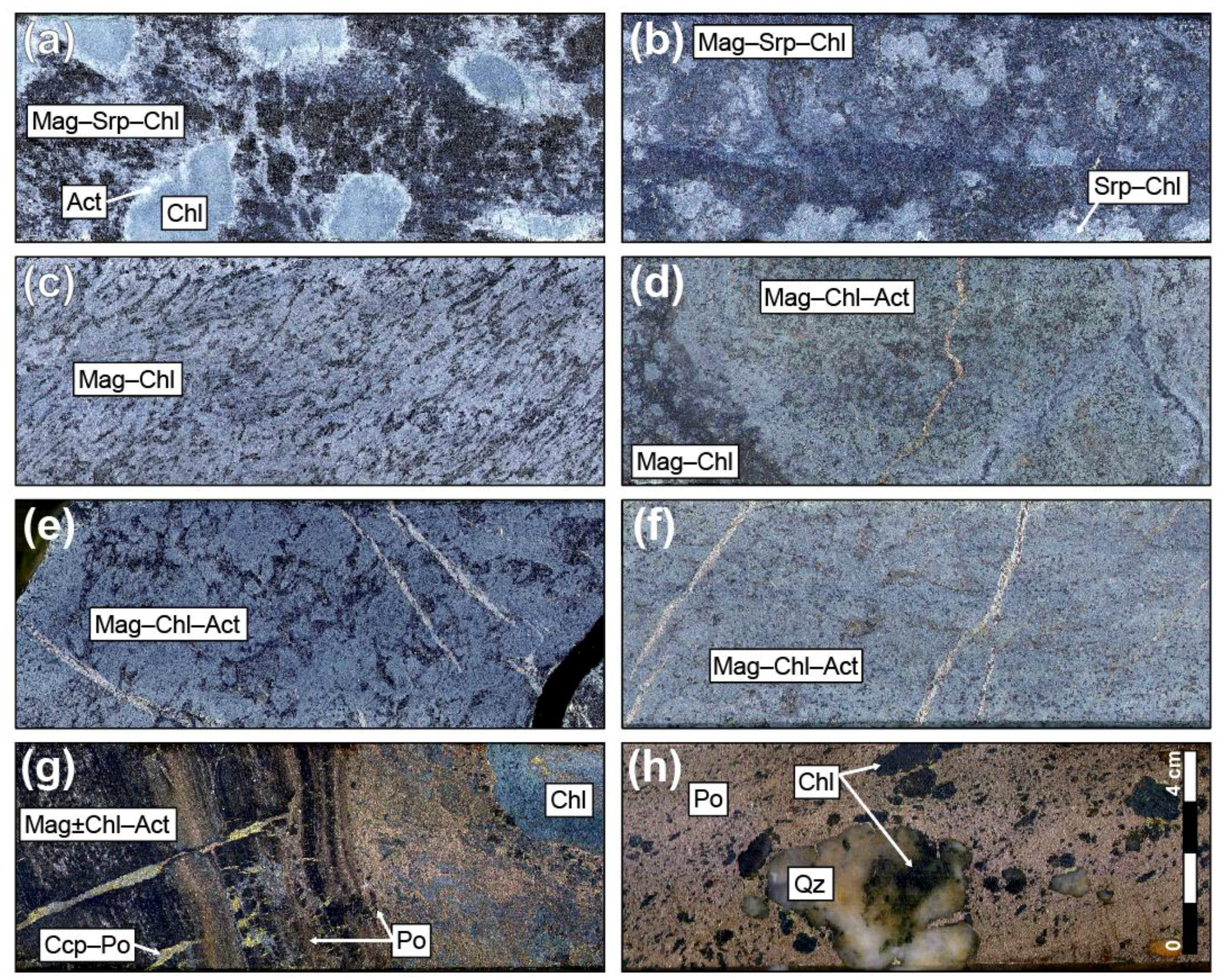
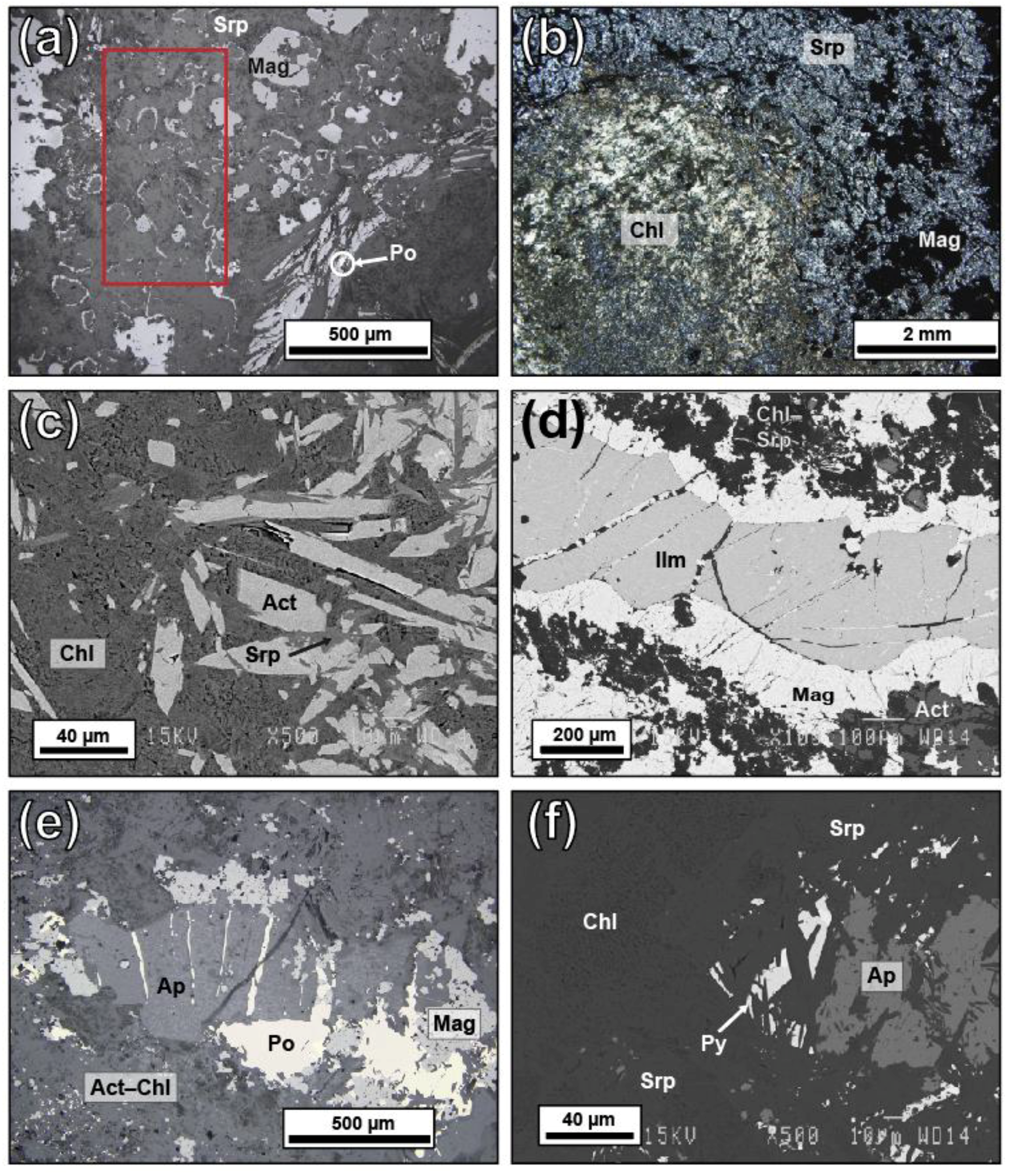
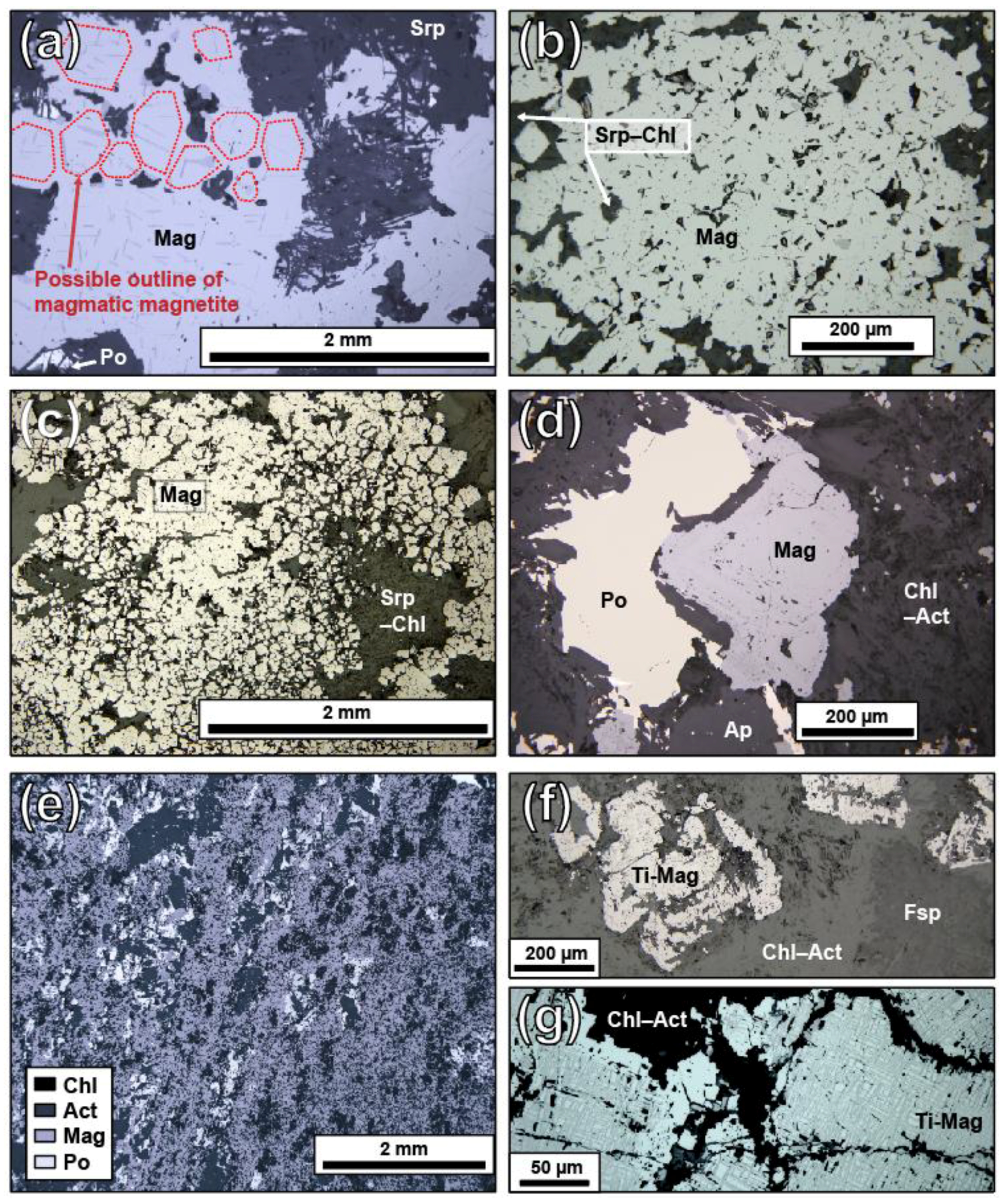
5.1. Petrographic Descriptions
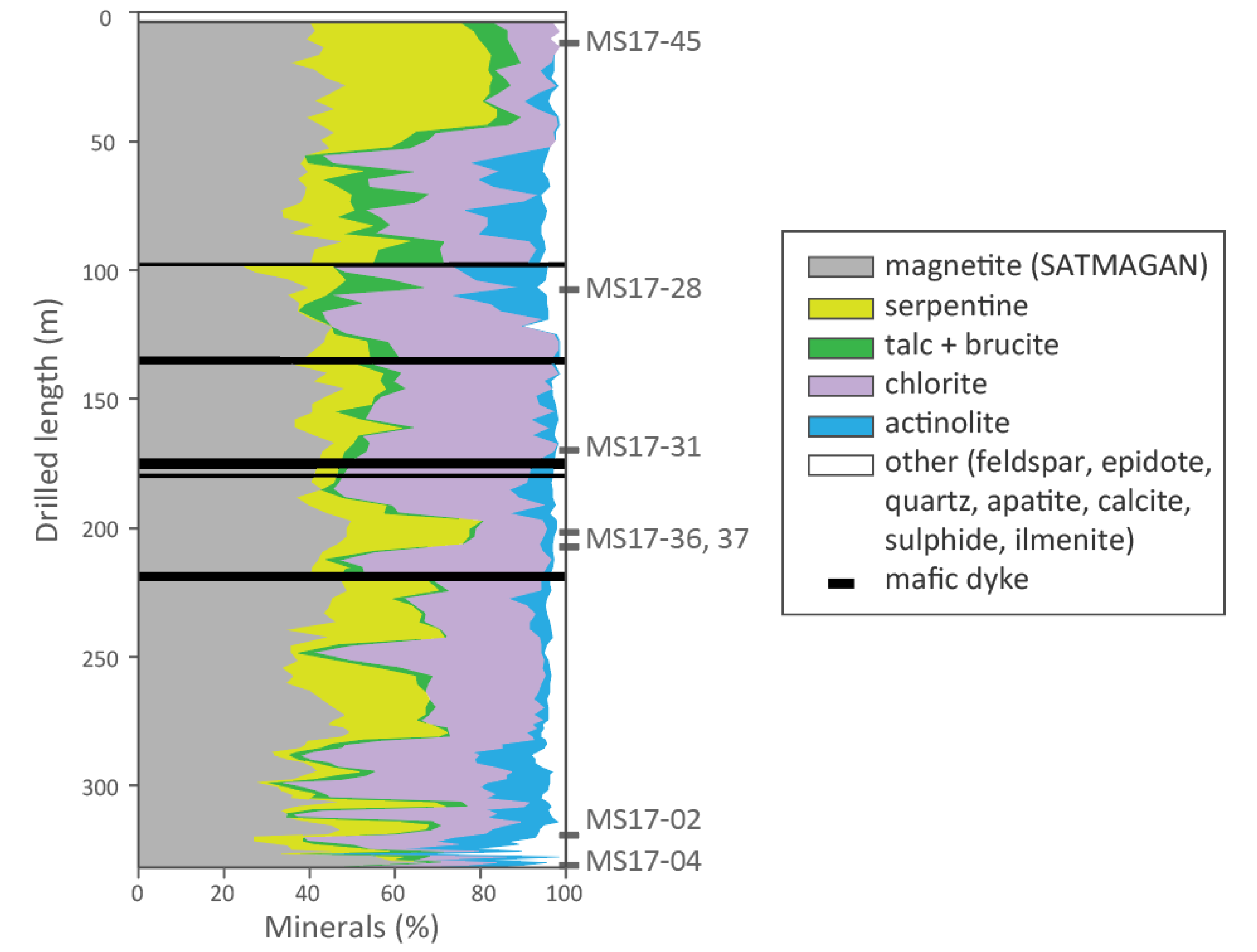
5.2. Mineral Proportions
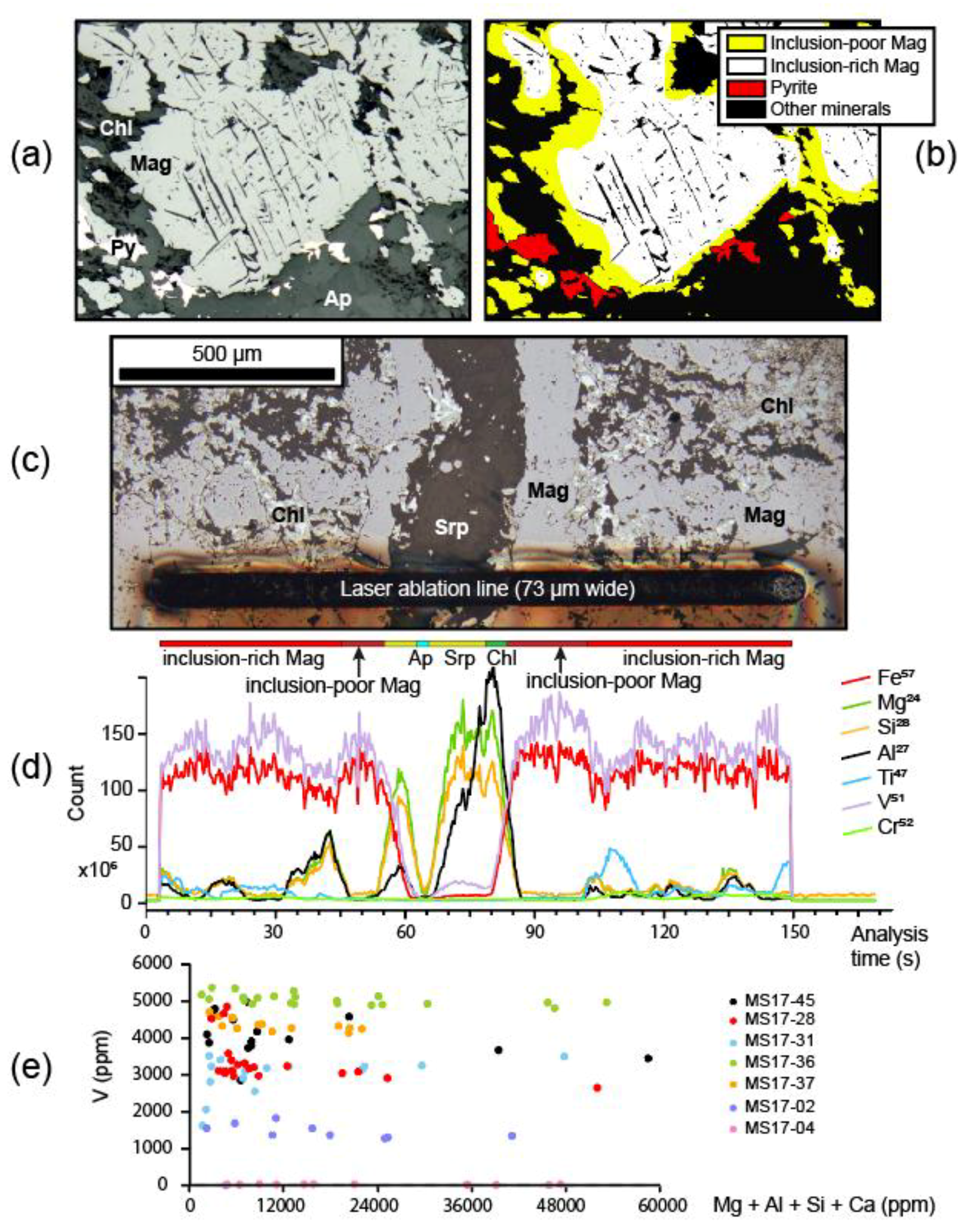
5.3. Magnetite Texture
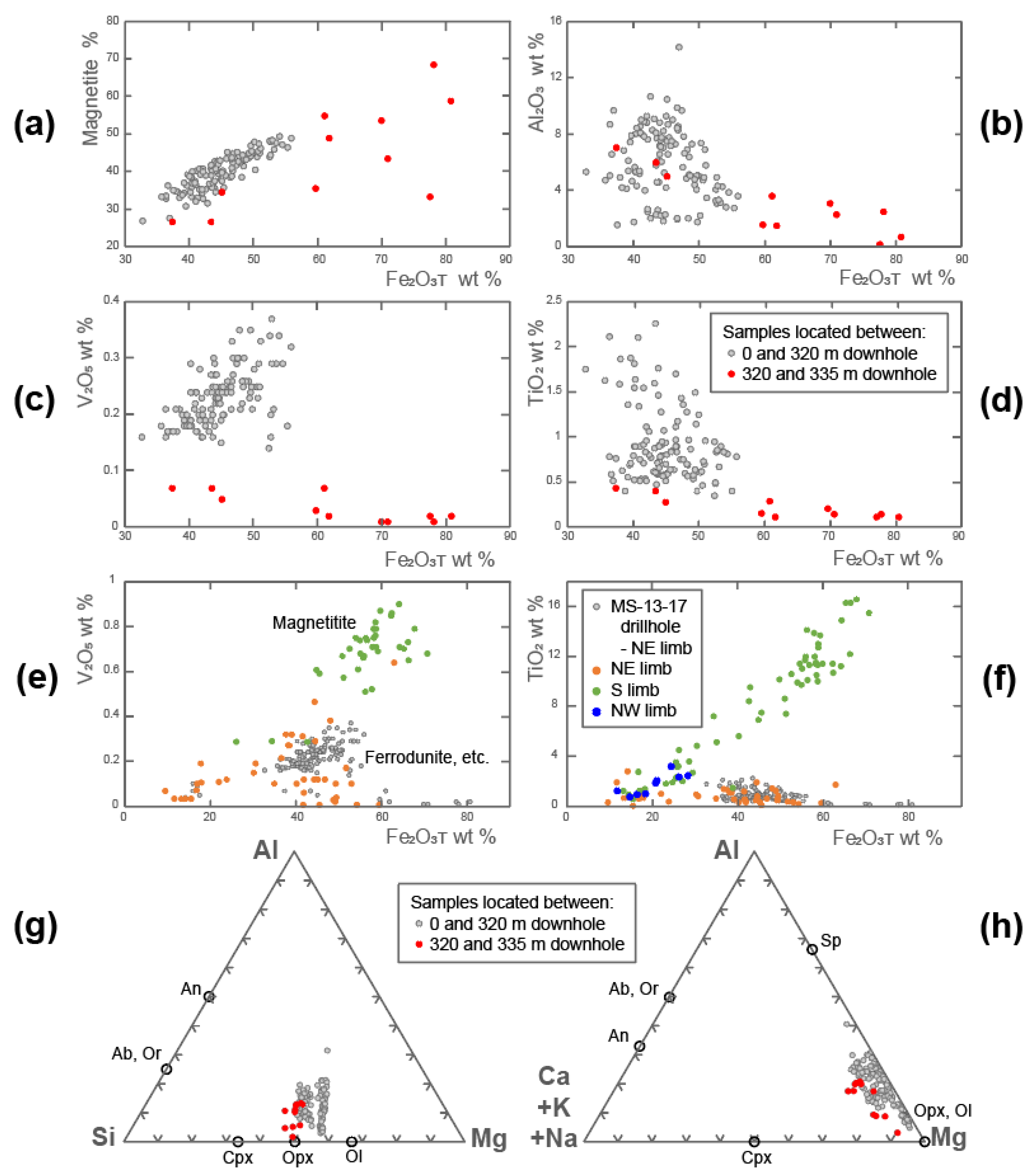
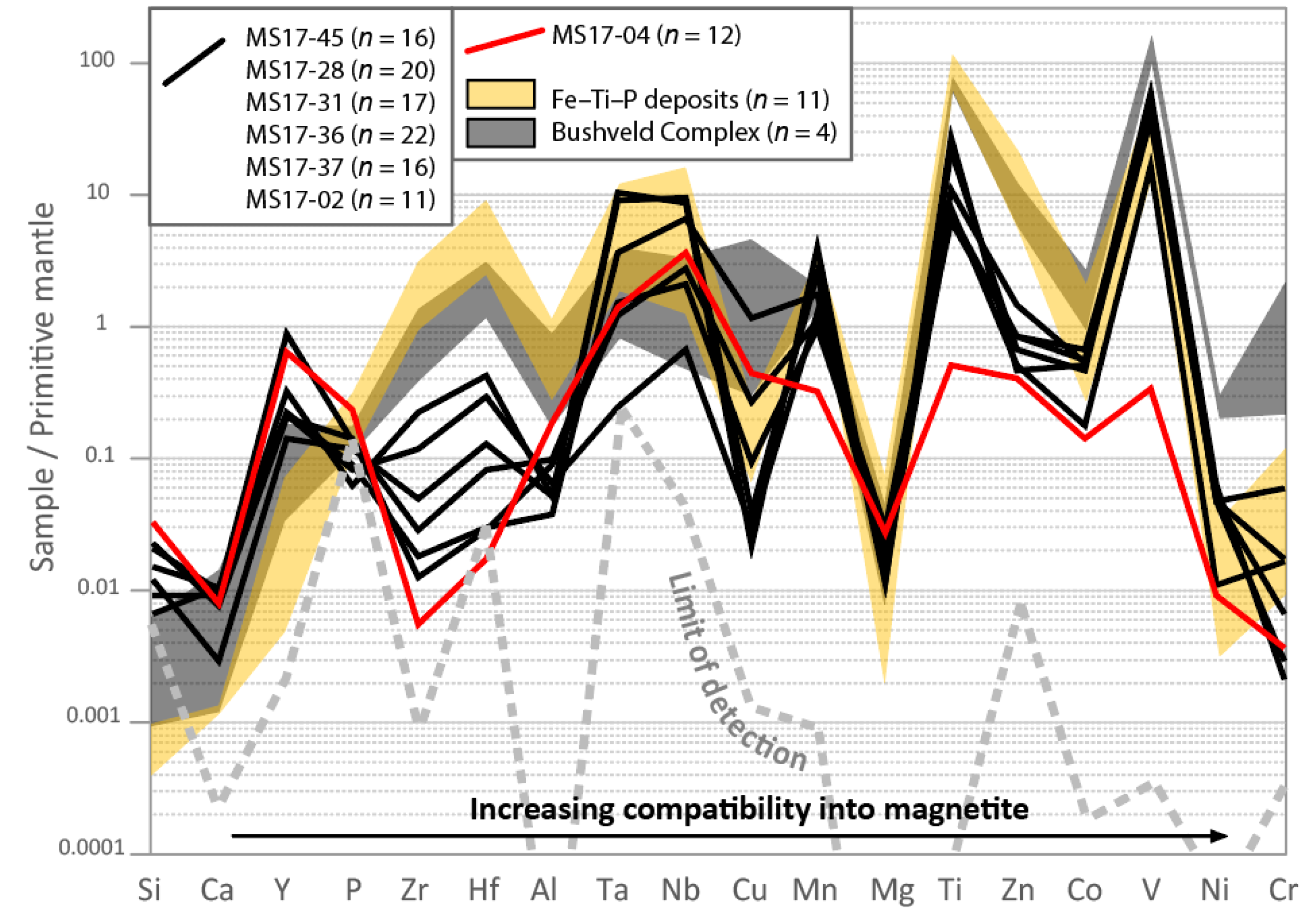
5.4. Whole Rock Chemical Analyses
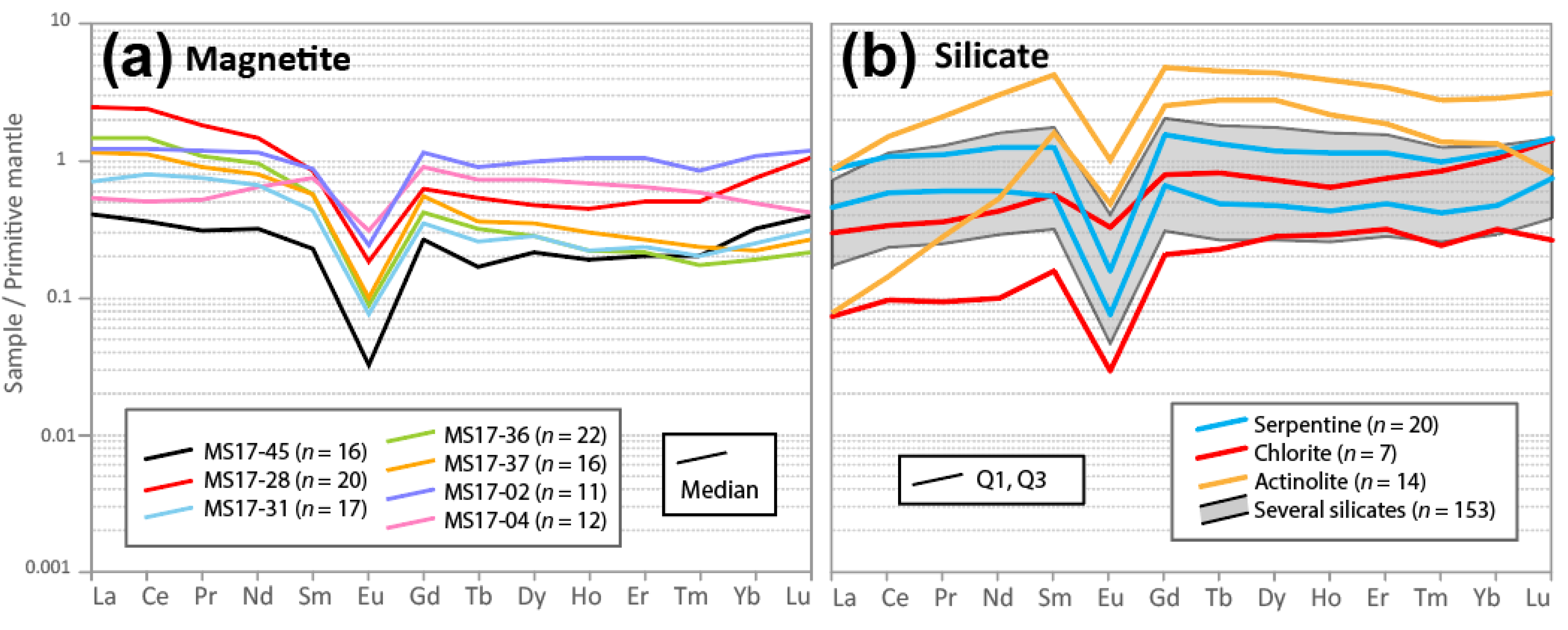
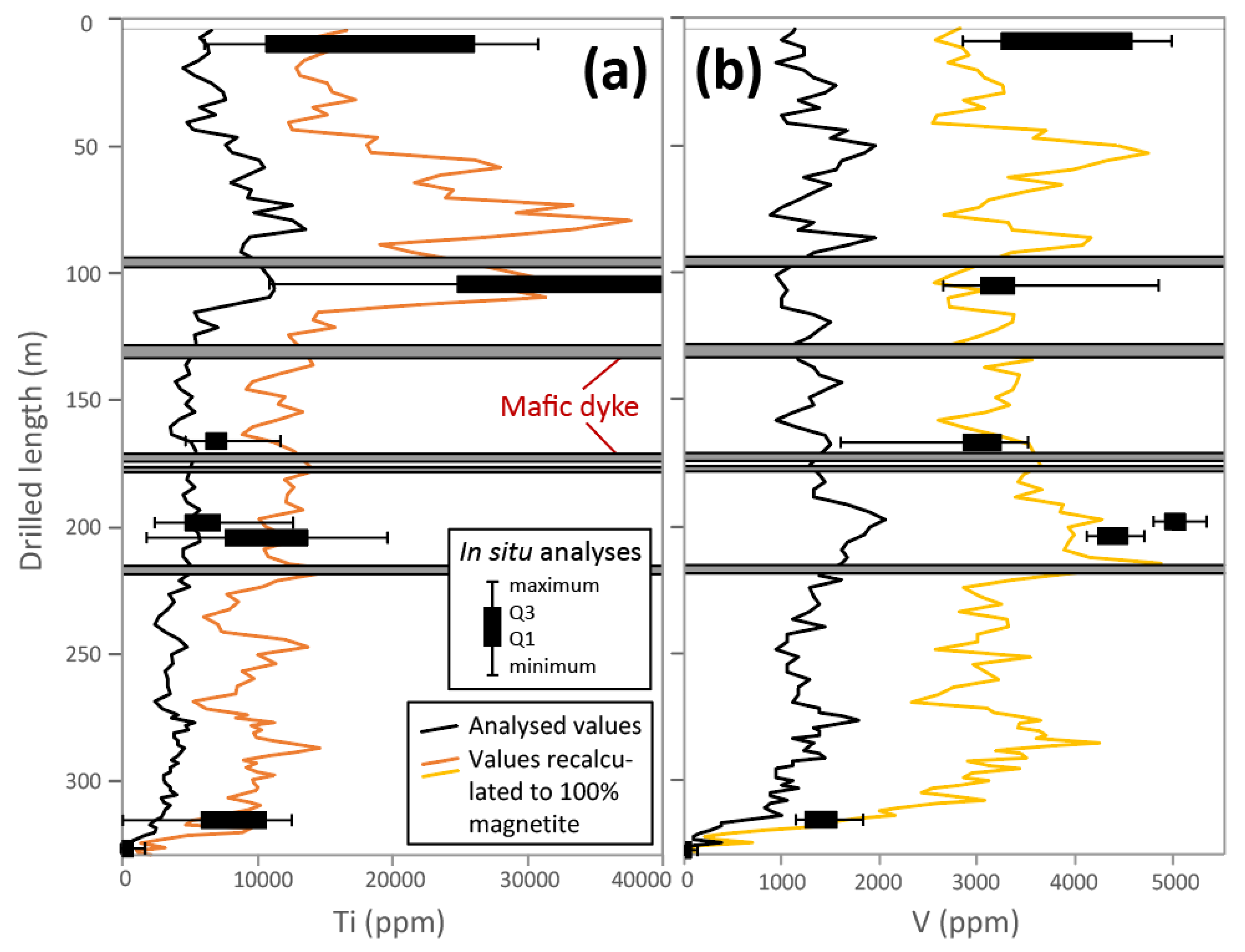
5.5. In Situ Chemical Analyses
6. Chemical Modelling
6.1. Modelling Procedure
6.2. Results of the Quantification of Hydrothermal Alteration
7. Discussion
7.1. Metamorphism
7.2. Deformation
7.3. Hydrothermal Alteration
7.4. Hosting Rocks
7.5. Magnetite Chemistry
7.6. V-Content of Magnetite
7.7. Origin of the Olivine–Magnetite-Enriched Rocks
- Evolved characteristics: apatite-bearing unit located in the upper portion of the LDC that contains Ni-poor olivine. The unit is Fe-V-rich and is depleted in Cr and Ti (with ilmenite that originate from primary crystallization only observed in the 50–120 m interval);
- Primitive characteristics: elevated Mg-content, low Si-content, and Fo82-90 olivine;
- Multi-injections of magmas that may have solidified at a faster rate than the magmas of the NW and S limbs layered zones.
8. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Allard, G.O. Doré Lake Complex and its Importance to Chibougamau Geology and Metallogeny, Québec; MERN report DP-386; Ministère des richesses naturelles: Peterboroyugh, Canada, 1976. [Google Scholar]
- Namur, O.; Charlier, B.; Toplis, M.J.; Higgins, M.D.; Liégeois, J.-P.; Vander Auwera, J. Crystallization sequence and magma chamber processes in the ferrobasaltic Sept Iles layered intrusion, Canada. J. Petrol. 2010, 51, 1203–1236. [Google Scholar] [CrossRef]
- Arguin, J.-P.; Pagé, P.; Barnes, S.-J.; Girard, R.; Duran, C. An Integrated Model for Ilmenite, Al-Spinel, and Corundum Exsolutions in Titanomagnetite from Oxide-Rich Layers of the Lac Doré Complex (Québec, Canada). Minerals 2018, 8, 476. [Google Scholar] [CrossRef]
- Dorr, A. Magnetite Deposits in the Northern Part of the Doré Lake Complex, Chibougamau District, Québec. Master’s thesis, McGill University, Montréal, QC, Canada, 1969. [Google Scholar]
- Lapallo, C.M. Petrographic and Geochemical Relations between the Rocks on the North and South Limbs of the Chibougamau Anticline: Assimilation of Roof Rocks, Crystallization and Residual Liquid Compositions in the Dore Lake Complex, Quebec, Canada. Master’s Thesis, University of Georgia, Athens, GA, USA, 1988. [Google Scholar]
- Pilote, P. Le camp minier de Chibougamau et le parautochtone grenvillien: Métallogénie, métamorphisme et aspects structuraux. In Proceedings of the Livret-guide d’excursion B1; Joined anual meeting, Geological Association of Canada—Mineralogical Association of Canada (GAC-MAC), Montréal, QC, Canada, 12–14 May 2006; p. 138. [Google Scholar]
- Pilote, P.; Robert, F.; Kirkham, R.; Daigneault, R.; Sinclair, W.D. Minéralisation de type porphyrique et filonienne dans le Complexe du lac Doré - le secteur du lac Clark et de l’île Merrill. In Géologie et Métallogénie du District Minier de Chapais-Chibougamau: Nouvelle Vision du Potentiel de Découverte; Pilote, P., Ed.; MERN report DV 98-03; Ministère des Ressources naturelles du Québec: Québec, QC, Canada, 1998; pp. 71–90. [Google Scholar]
- Daigneault, R.; Allard, G.O. Le Complexe du Lac Doré et Son Environnement Géologique (Région de Chibougamau-Sous-Province de l’Abitibi); MERN report MM-89-03; Ministère des Ressources naturelles du Québec: Québec, QC, Canada, 1990; ISBN 2551123313. [Google Scholar]
- Polat, A.; Frei, R.; Longstaffe, F.J.; Woods, R. Petrogenetic and geodynamic origin of the Neoarchean Doré Lake Complex, Abitibi subprovince, Superior Province, Canada. Int. J. Earth Sci. 2018, 107, 811–843. [Google Scholar] [CrossRef]
- Leclerc, F.; Roy, P.; Pilote, P.; Bédard, J.H.; Harris, L.B.; McNicoll, V.J.; van Breemen, O.; David, J.; Goulet, N. Géologie de la Région de Chibougamau; MERN report RG 2015-03; Ministère des Ressources naturelles du Québec: Québec, QC, Canada, 2017. [Google Scholar]
- Mercier-Langevin, P.; Lafrance, B.; Bécu, V.; Dubé, B.; Kjarsgaard, I.; Guha, J. The Lemoine auriferous volcanogenic massive sulfide deposit, Chibougamau camp, Abitibi greenstone belt, Quebec, Canada: Geology and genesis. Econ. Geol. 2014, 109, 231–269. [Google Scholar] [CrossRef]
- Mortensen, J.K. U–Pb geochronology of the eastern Abitibi subprovince. Part 1: Chibougamau–Matagami–Joutel region. Can. J. Earth Sci. 1993, 30, 11–28. [Google Scholar] [CrossRef]
- Moritz, H.W. Muscovite-Paragonite Relationships in Altered and Mineralized Meta-anorthosite at Chibougamau, Quebec. Master’s Thesis, University of Georgia, Athens, GA, USA, 1975. [Google Scholar]
- Rivers, T.; Martignole, J.; Gower, C.F.; Davidson, A. New tectonic divisions of the Grenville Province, southeast Canadian Shield. Tectonics 1989, 8, 63–84. [Google Scholar] [CrossRef]
- Allard, G.O.; Caty, J.L. Géologie du Quart Nordest et D’une Partie du Quart Sud-est du Canton de Lemoine, Comtés d’Abitibi-Est et de Roberval; MERN report RP-566; Ministère des Ressources naturelles du Québec: Québec, QC, Canada, 1969. [Google Scholar]
- Taner, M.F.; Ercit, T.S.; Gault, R.A. Vanadium-bearing magnetite from the Matagami and Chibougamau mining districts, Abitibi, Quebec, Canada. Explor. Min. Geol. 1998, 7, 299–311. [Google Scholar]
- Arguin, J.-P.; Pagé, P.; Girard, R.; Barnes, S.-J. The spatial variation of mineralogy in the vanadiferous magnetite deposit of the Lac Doré Complex (Chibougamau, Québec) and its implications on the chemical purity of magnetite concentrates. In Proceedings of the 14th SGA Biennial Meeting 2017, the Mineral Resources to Discover, Quebec, QC, Canada, 20–23 August 2017; Volume 4, pp. 247–250. [Google Scholar]
- Baskin, G.D. The Petrology and Chemistry of a Portion of the North Limb of the Dore Lake Complex, Chibougamau, Québec, Canada. Master’s Thesis, University of Georgia, Athens, GA, USA, 1975. [Google Scholar]
- Henry, R.L. The Lac Sauvage Volcanogenic Iron Formation near Chibougamau, Quebec, Canada: Its Petrology, Geochemistry, Stratigraphy, Structure, and Economic Significance. Master’s Thesis, University of Georgia, Athens, GA, USA, 1978. [Google Scholar]
- Leblanc, M.; Larouche, C.P. Project: Mont Sorcier—Report of the Limited Surface Diamond Drilling Campaign Carried Out during the Period of August 29th to October 30th, 2013; Report GM-68458; Mines Indépendantes Chibougamau Inc.: Chibougamau, QC, Canada, 2014. [Google Scholar]
- Gaucher, E.H. Rapport Préliminaire sur le Quart Sud-est du Canton de Barlow, District Électoral d’Abitibi-est; MERN report RP-425; Ministère des Ressources naturelles du Québec: Québec, QC, Canada, 1960. [Google Scholar]
- Allard, G.O. Géologie du Quart Nord-ouest du Canton de Rinfret, Comtés d’Abitibi-Est et de Roberval: Rapport Préliminaire; MERN report 567, Map 1649; Ministère des Ressources naturelles du Québec: Québec, QC, Canada, 1967. [Google Scholar]
- Caty, J.L. Petrographie et Petrologie du Flanc Sud-est du Complexe du Lac Dore. Master’s Thesis, Université de Montréal, Montreal, QC, Canada, 1970. [Google Scholar]
- Bédard, J.H.; Leclerc, F.; Harris, L.B.; Goulet, N. Intra-sill magmatic evolution in the Cummings Complex, Abitibi greenstone belt: Tholeiitic to calc-alkaline magmatism recorded in an Archaean subvolcanic conduit system. Lithos 2009, 111, 47–71. [Google Scholar] [CrossRef]
- Toplis, M.J.; Carroll, M.R. An experimental study of the influence of oxygen fugacity on Fe-Ti oxide stability, phase relations, and mineral-melt equilibria in ferro-basaltic systems. J. Petrol. 1995, 36, 1137–1170. [Google Scholar] [CrossRef]
- Thy, P.; Lesher, C.E.; Nielsen, T.F.D.; Brooks, C.K. Experimental constraints on the Skaergaard liquid line of descent. Lithos 2006, 92, 154–180. [Google Scholar] [CrossRef]
- Grove, T.L.; Baker, M.B. Phase equilibrium controls on the tholeiitic versus calc alkaline differentiation trends. J. Geophys. Res. Solid Earth 1984, 89, 3253–3274. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Song, X.-Y.; Chen, L.-M.; Xie, W.; Yu, S.-Y.; Zheng, W.-Q.; Deng, Y.-F.; Zhang, J.-F.; Gui, S.-G. Fractional crystallization and the formation of thick Fe–Ti–V oxide layers in the Baima layered intrusion, SW China. Ore Geol. Rev. 2012, 49, 96–108. [Google Scholar] [CrossRef]
- Irvine, T.N. Crystallization sequences in the Muskox intrusion and other layered intrusions-II. Origin of chromitite layers and similar deposits of other magmatic ores. In Chromium: Its Physicochemical Behavior and Petrologic Significance: Papers from a Carnegie Institution of Washington Conference, Geophysical Laboratory; Pergamon Press Ltd.: Oxford, UK, 1976; pp. 991–1020. [Google Scholar]
- Mungall, J.E.; Kamo, S.L.; McQuade, S. U-Pb geochronology documents out-of-sequence emplacement of ultramafic layers in the Bushveld Igneous Complex of South Africa. Nat. Commun. 2016, 7, 13385. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, M.A. Origin of the Granophyres within the Dore Lake Complex, Chibougamau, Quebec, Canada. Master’s Thesis, University of Georgia, Athens, GA, USA, 1990. [Google Scholar]
- Allard, G.O.; Whitney, J.A. Spatial and petrological relationships between granophyre and layering, and the type of country rock in the Dore Lake Complex, Chibougamau, Québec. In Proceedings of the 1977 Annual Meetings of GAC-MAC-SEG-CGU, Vancouver, Program with abstracts, Vancouver, BC, Canada, 15–19 May 1977; p. 4. [Google Scholar]
- Trépanier, S.; Mathieu, L.; Daigneault, R. CONSONORM-LG: New normative minerals and alteration indexes for low-grade metamorphic rocks. Econ. Geol. 2015, 110, 2127–2138. [Google Scholar] [CrossRef]
- Whitney, D.L.; Evans, B.W. Abbreviations for names of rock-forming minerals. Am. Mineral. 2010, 95, 185–187. [Google Scholar] [CrossRef]
- Jochum, K.P.; Willbold, M.; Raczek, I.; Stoll, B.; Herwig, K. Chemical Characterisation of the USGS Reference Glasses GSA 1G, GSC 1G, GSD 1G, GSE 1G, BCR 2G, BHVO 2G and BIR 1G Using EPMA, ID TIMS, ID ICP MS and LA ICP MS. Geostand. Geoanalytical Res. 2005, 29, 285–302. [Google Scholar] [CrossRef]
- Dare, S.A.S.; Barnes, S.-J.; Beaudoin, G. Variation in trace element content of magnetite crystallized from a fractionating sulfide liquid, Sudbury, Canada: Implications for provenance discrimination. Geochim. Cosmochim. Acta 2012, 88, 27–50. [Google Scholar] [CrossRef]
- Savard, D.; Bouchard-Boivin, B.; Barnes, S.-J.; Garbe-Schönberg, D. UQAC-FeS: A new series of base metal sulfide quality control reference material for LA-ICP-MS analysis. In Proceedings of the 10th International Conference on the Analysis of Geological and Environmental Materials, Sydney, Australia, 8–13 July 2018. [Google Scholar]
- Garbe-Schönberg, D.; Müller, S. Nano-particulate pressed powder tablets for LA-ICP-MS. J. Anal. At. Spectrom. 2014, 29, 990–1000. [Google Scholar] [CrossRef]
- Paton, C.; Hellstrom, J.; Paul, B.; Woodhead, J.; Hergt, J. Iolite: Freeware for the visualisation and processing of mass spectrometric data. J. Anal. At. Spectrom. 2011, 26, 2508–2518. [Google Scholar] [CrossRef]
- Buddington, A.F.; Lindsley, D.H. Iron-titanium oxide minerals and synthetic equivalents. J. Petrol. 1964, 5, 310–357. [Google Scholar] [CrossRef]
- Winchester, J.A.; Floyd, P.A. Geochemical discrimination of different magma series and their differentiation products using immobile elements. Chem. Geol. 1977, 20, 325–343. [Google Scholar] [CrossRef]
- Hofmann, A.W. Chemical differentiation of the Earth: the relationship between mantle, continental crust, and oceanic crust. Earth Planet. Sci. Lett. 1988, 90, 297–314. [Google Scholar] [CrossRef]
- McDonough, W.F.; Sun, S.-S. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Dare, S.A.S.; Barnes, S.-J.; Beaudoin, G.; Méric, J.; Boutroy, E.; Potvin-Doucet, C. Trace elements in magnetite as petrogenetic indicators. Miner. Depos. 2014, 49, 785–796. [Google Scholar] [CrossRef]
- Trépanier, S.; Mathieu, L.; Daigneault, R.; Faure, S. Precursors predicted by artificial neural networks for mass balance calculations: Quantifying hydrothermal alteration in volcanic rocks. Comput. Geosci. 2016, 89, 32–43. [Google Scholar] [CrossRef]
- Middlemost, E.A.K. Iron oxidation ratios, norms and the classification of volcanic rocks. Chem. Geol. 1989, 77, 19–26. [Google Scholar] [CrossRef]
- Mathieu, L. Quantifying hydrothermal alteration: a review of methods. Geosciences 2018, 8, 245. [Google Scholar] [CrossRef]
- Le Bas, M.J.; Le Maitre, R.W.; Woolley, A.R. The construction of the total alkali-silica chemical classification of volcanic rocks. Mineral. Petrol. 1992, 46, 1–22. [Google Scholar] [CrossRef]
- Spear, F.S. Metamorphic Phase Equilibria and Pressure-Temperature-Time Paths; Monograph series; Mineralogical Society of America: Washington, DC, USA, 1993; Volume 1. [Google Scholar]
- Frost, B.R. Magnetic petrology; factors that control the occurrence of magnetite in crustal rocks. In Oxide Minerals: Petrologic and Magnetic Significance; Lindsley, D.H., Ed.; Mineralogical Society of America Reviews in Mineralogy Reviews in Mineralogy and Geochemistry: Mclean, Virginia, 1991; Volume 25, pp. 489–509. [Google Scholar]
- Hunter, R.H. Texture development in cumulate rocks. In Developments in Petrology; Cawthorn, R.G., Ed.; Springer: Berlin, Germany, 1996; Volume 15, pp. 77–101. ISBN 0167-2894. [Google Scholar]
- Higgins, M.D. Quantitative textural analysis of rocks in layered mafic intrusions. In Layered Intrusions; Charlier, B., Namur, O., Latypov, R., Tegner, C., Eds.; Springer Geology; Springer: Dordrecht, The Netherlands, 2015; pp. 153–181. [Google Scholar]
- Herzberg, C.; Asimow, P.D.; Ionov, D.A.; Vidito, C.; Jackson, M.G.; Geist, D. Nickel and helium evidence for melt above the core-mantle boundary. Nature 2013, 493, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Toplis, M.J.; Libourel, G.; Carroll, M.R. The role of phosphorus in crystallisation processes of basalt: an experimental study. Geochim. Cosmochim. Acta 1994, 58, 797–810. [Google Scholar] [CrossRef]
- Jenner, F.E.; O’neill, H.S.C.; Arculus, R.J.; Mavrogenes, J.A. The magnetite crisis in the evolution of arc-related magmas and the initial concentration of Au, Ag and Cu. J. Petrol. 2010, 51, 2445–2464. [Google Scholar] [CrossRef]
- Kaufmann, F.E.D.; Vukmanovic, Z.; Holness, M.B.; Hecht, L. Orthopyroxene oikocrysts in the MG1 chromitite layer of the Bushveld Complex: implications for cumulate formation and recrystallisation. Contrib. to Mineral. Petrol. 2018, 173, 17. [Google Scholar] [CrossRef]
- Barnes, S.J.; Mole, D.R.; Le Vaillant, M.; Campbell, M.J.; Verrall, M.R.; Roberts, M.P.; Evans, N.J. Poikilitic textures, heteradcumulates and zoned orthopyroxenes in the Ntaka Ultramafic Complex, Tanzania: implications for crystallization mechanisms of oikocrysts. J. Petrol. 2016, 57, 1171–1198. [Google Scholar] [CrossRef]
- B Barnes, S.-J.; Lightfoot, P.C. Formation of magmatic nickel-sulfide ore deposits and processses affecting their copper and platinum-group element contents. In Economic Geology 100th Anniversary Volume: 1905–2005; Society of Economic Geologists: Littelton, CO, USA, 2005; pp. 179–213. [Google Scholar]
- Franklin, J.M.; Gibson, H.L.; Jonasson, I.R.; Galley, A.G. Volcanogenic Massive Sulfide Deposits. In One Hundredth Anniversary Volume; Hedenquist, J.W., Thompson, J.F.H., Goldfarb, R.J., Richards, J.P., Eds.; Society of Economic Geologists: Littelton, CO, USA, 2005; pp. 523–560. ISBN 9781887483018. [Google Scholar]
- Galley, A.G.; Hannington, M.D.; Jonasson, I.R. Volcanogenic massive sulphide deposits. In Mineral Deposits of Canada: A Synthesis of Major Deposit-Types, District Metallogeny, the Evolution of Geological Provinces, and Exploration Methods; Geological Association of Canada, Mineral Deposits Division: St. John’s, NL, USA, 2007; Volume 5, pp. 141–161. [Google Scholar]
- Hannington, M.D.; de Ronde, C.D.J.; Petersen, S. Sea-floor tectonics and submarine hydrothermal systems. In One Hundredth Anniversary Volume; Hedenquist, J.W., Thompson, J.F.H., Goldfarb, R.J., Richards, J.P., Eds.; Society of Economic Geologists: Littelton, CO, USA, 2005; pp. 111–141. [Google Scholar]
- Mathieu, L.; Bouchard, R.-A.; Pearson, V.; Daigneault, R. The Coulon deposit: Quantifying alteration in volcanogenic massive sulphide systems modified by amphibolite-facies metamorphism. Can. J. Earth Sci. 2016, 53, 1443–1457. [Google Scholar] [CrossRef]
- McCarthy, T.S.; Cawthorn, R.G. The geochemistry of vanadiferous magnetite in the Bushveld Complex: implications for crystallization mechanisms in layered complexes. Miner. Depos. 1983, 18, 505–518. [Google Scholar] [CrossRef]
- Tegner, C.; Cawthorn, R.G.; Kruger, F.J. Cyclicity in the Main and Upper Zones of the Bushveld Complex, South Africa: crystallization from a zoned magma sheet. J. Petrol. 2006, 47, 2257–2279. [Google Scholar] [CrossRef]
- Song, X.Y.; Qi, H.W.; Hu, R.Z.; Chen, L.M.; Yu, S.Y.; Zhang, J.F. Formation of thick stratiform Fe Ti oxide layers in layered intrusion and frequent replenishment of fractionated mafic magma: evidence from the Panzhihua intrusion, SW China. Geochem. Geophys. Geosystems 2013, 14, 712–732. [Google Scholar] [CrossRef]
- Tan, W.; Liu, P.; He, H.; Wang, C.Y.; Liang, X. Mineralogy and origin of exsolution in Ti-rich magnetite from different magmatic Fe-Ti oxide-bearing intrusions. Can. Mineral. 2017, 54, 539–553. [Google Scholar] [CrossRef]
- Wallace, P.; Carmichael, I.S.E. Sulfur in basaltic magmas. Geochim. Cosmochim. Acta 1992, 56, 1863–1874. [Google Scholar] [CrossRef]
- Humphris, S.E.; Thompson, G. Trace element mobility during hydrothermal alteration of oceanic basalts. Geochim. Cosmochim. Acta 1978, 42, 127–136. [Google Scholar] [CrossRef]
- Polivchuk, M. The Formation of Vanadium Deposits in the Archean Rivière Bell Complex, Quebec: Insights from Fe-Ti Oxide Chemistry. Master’s Thesis, University of Ottawa, Ottawa, Canada, 2017. [Google Scholar]
- Lindstrom, D.J. Experimental Study of the Partitioning of the Transition Metals between Clinopyroxene and Coexisting Silicate Liquids. Ph.D. Thesis, University of Oregon, Eugene, OR, USA, 1976. [Google Scholar]
- Barnes, S.J. The effect of trapped liquid crystallization on cumulus mineral compositions in layered intrusions. Contrib. Mineral. Petrol. 1986, 93, 524–531. [Google Scholar] [CrossRef]
- Toplis, M.J.; Corgne, A. An experimental study of element partitioning between magnetite, clinopyroxene and iron-bearing silicate liquids with particular emphasis on vanadium. Contrib. to Mineral. Petrol. 2002, 144, 22–37. [Google Scholar] [CrossRef]
- Canil, D. Vanadium partitioning and the oxidation state of Archaean komatiite magmas. Nature 1997, 389, 842. [Google Scholar] [CrossRef]
- Cawthorn, R.G.; Ashwal, L.D. Origin of anorthosite and magnetitite layers in the Bushveld Complex, constrained by major element compositions of plagioclase. J. Petrol. 2009, 50, 1607–1637. [Google Scholar] [CrossRef]
- Maier, W.D.; Barnes, S.-J.; Groves, D.I. The Bushveld Complex, South Africa: formation of platinum-palladium, chrome-and vanadium-rich layers via hydrodynamic sorting of a mobilized cumulate slurry in a large, relatively slowly cooling, subsiding magma chamber. Miner. Depos. 2013, 48, 1–56. [Google Scholar] [CrossRef]
- Wilson, A.H. The earliest stages of emplacement of the eastern Bushveld Complex: development of the Lower Zone, Marginal Zone and Basal Ultramafic Sequence. J. Petrol. 2015, 56, 347–388. [Google Scholar] [CrossRef]
- Pang, K.-N.; Zhou, M.-F.; Lindsley, D.; Zhao, D.; Malpas, J. Origin of Fe-Ti oxide ores in mafic intrusions: evidence from the Panzhihua intrusion, SW China. J. Petrol. 2007, 49, 295–313. [Google Scholar] [CrossRef]
- Namur, O.; Abily, B.; Boudreau, A.E.; Blanchette, F.; Bush, J.W.M.; Ceuleneer, G.; Charlier, B.; Donaldson, C.H.; Duchesne, J.-C.; Higgins, M.D. Igneous layering in basaltic magma chambers. In Layered Intrusions; Charlier, B., Namur, O., Latypov, R., Tegner, C., Eds.; Springer Geology; Springer: Dordrecht, The Netherlands, 2015; pp. 75–152. [Google Scholar]
- Pang, K.-N.; Shellnutt, J.G.; Zhou, M.-F. The panzhihua intrusion, SW China. In Layered Intrusions; Springer: Berlin, Germany, 2015; pp. 435–463. [Google Scholar]
- Presnall, D.C. The join forsterite-diopside-iron oxide and its bearing on the crystallization of basaltic and ultramafic magmas. Am. J. Sci. 1966, 264, 753–809. [Google Scholar] [CrossRef]
- Muan, A.; Osborn, E. Phase equilibria at liquidus temperatures in the system MgO-FeO-Fe2O3-SiO2. J. Am. Ceram. Soc. 1956, 39, 121–140. [Google Scholar] [CrossRef]
- Roeder, P.L.; Osborn, E.F. Experimental data for the system MgO-FeO-Fe2O3-CaAl2Si2O8-SiO2 and their petrologic implications. Am. J. Sci. 1966, 264, 428–480. [Google Scholar] [CrossRef]
- Lepage, L.D. ILMAT: an excel worksheet for ilmenite--magnetite geothermometry and geobarometry. Comput. Geosci. 2003, 29, 673–678. [Google Scholar] [CrossRef]
- Spencer, K.J.; Lindsley, D.H. A solution model for coexisting iron-titanium oxides. Am. Mineral. 1981, 66, 1189–1201. [Google Scholar]
- Powell, R.; Powell, M. Geothermometry and oxygen barometry using coexisting iron-titanium oxides: A reappraisal. Mineral. Mag. 1977, 41, 257–263. [Google Scholar] [CrossRef]
- Andersen, D.J.; Lindsley, D.H. New (and final!) models for the Ti-magnetite/ilmenite geothermometer and oxygen barometer. In Proceedings of the Abstracts of American Geophysical Union 1985 Spring Meeting, Baltimore, MD, USA, 27–31 May 1985. [Google Scholar]
- Nabil, H. Genèse des dépôts de Fe-Ti-P Associés Aux Intrusions Litées (Exemples: L’intrusion Mafique de Sept-Iles, au Québec; Complexe de Duluth aux États-Unis). Ph.D. Thesis, Université du Québec à Montréal, Montreal, QC, Canada, 2003. [Google Scholar]
- McBirney, A.R.; Noyes, R.M. Crystallization and layering of the Skaergaard intrusion. J. Petrol. 1979, 20, 487–554. [Google Scholar] [CrossRef]
- Boudreau, A.E. Investigations of the Stillwater Complex; 4, The role of volatiles in the petrogenesis of the JM Reef, Minneapolis adit section. Can. Mineral. 1988, 26, 193–208. [Google Scholar]
- Marsh, B.D. On some fundamentals of igneous petrology. Contrib. to Mineral. Petrol. 2013, 166, 665–690. [Google Scholar] [CrossRef]
- Giuffrida, M.; Holtz, F.; Vetere, F.; Viccaro, M. Effects of CO2 flushing on crystal textures and compositions: experimental evidence from recent K-trachybasalts erupted at Mt. Etna. Contrib. to Mineral. Petrol. 2017, 172, 90. [Google Scholar] [CrossRef]
- Mollo, S.; Gaeta, M.; Freda, C.; Di Rocco, T.; Misiti, V.; Scarlato, P. Carbonate assimilation in magmas: a reappraisal based on experimental petrology. Lithos 2010, 114, 503–514. [Google Scholar] [CrossRef]
- Ariskin, A.A.; Konnikov, E.G.; Danyushevsky, L.V.; Kislov, E.V.; Nikolaev, G.S.; Orsoev, D.A.; Barmina, G.S.; Bychkov, K.A. The Dovyren intrusive complex: Problems of petrology and Ni sulfide mineralization. Geochem. Int. 2009, 47, 425. [Google Scholar] [CrossRef]
- Iacono-Marziano, G.; Gaillard, F.; Pichavant, M. Limestone assimilation and the origin of CO2 emissions at the Alban Hills (Central Italy): Constraints from experimental petrology. J. Volcanol. Geotherm. Res. 2007, 166, 91–105. [Google Scholar] [CrossRef]
- Ariskin, A.A.; Fomin, I.S.; Zharkova, E.V.; Kadik, A.A.; Nikolaev, G.S. Redox conditions during crystallization of ultramafic and gabbroic rocks of the Yoko-Dovyren massif (Based on the results of measurements of intrinsic oxygen fugacity of olivine). Geoche. Int. 2017, 55, 595–607. [Google Scholar] [CrossRef]




| Element | LOD 1 | MS17-14 | MS17-20 | MS17-28 | MS17-36 | MS17-37 | MS17-45 |
|---|---|---|---|---|---|---|---|
| Volcanic Rocks | Upper Fe-rich Zone of the LDC | ||||||
| SiO2 (wt %) | 0.01 | 47.4 | 50.6 | 28.2 | 14.65 | 20.7 | 23.3 |
| Al2O3 | 0.01 | 12.3 | 13.25 | 5.06 | 2.94 | 4.79 | 2.95 |
| Fe2O3T | 0.01 | 19.8 | 15.55 | 36.3 | 63.8 | 46.3 | 45.8 |
| CaO | 0.01 | 5.24 | 3.27 | 2.65 | 0.18 | 0.2 | 0.08 |
| MgO | 0.01 | 8.08 | 7.99 | 20.5 | 14.45 | 20.8 | 22.7 |
| Na2O | 0.01 | 3.03 | 3.21 | 0.03 | 0.03 | 0.03 | 0.03 |
| K2O | 0.01 | 0.44 | 0.15 | 0.05 | 0.03 | 0.04 | 0.03 |
| TiO2 | 0.01 | 1.65 | 2.13 | 1.69 | 0.96 | 0.92 | 1.16 |
| MnO | 0.01 | 0.45 | 0.19 | 0.28 | 0.15 | 0.15 | 0.15 |
| P2O5 | 0.01 | 0.11 | 0.18 | 0.12 | 0.1 | 0.11 | 0.02 |
| LOI | 0.01 | 1.42 | 4.77 | 4.89 | 2.95 | 5.79 | 5.62 |
| Total | 0.01 | 99.95 | 101.29 | 99.77 | 100.24 | 99.83 | 101.85 |
| Zr (ppm) | 2 | 99 | 154 | 13 | 27 | 25 | 41 |
| Cr | 10 | 80 | 40 | 20 | n.d. 2 | 30 | 70 |
| Th | 0.05 | 0.53 | 0.76 | 0.36 | 0.56 | 0.45 | 0.58 |
| Nb | 0.2 | 4.9 | 6.9 | 2.8 | 2.8 | 2.8 | 2.2 |
| Y | 0.1 | 30.6 | 50.9 | 8.2 | 7.3 | 6.8 | 7.2 |
| V | 5 | 437 | 446 | 1150 | 3620 | 2450 | 1670 |
| La | 0.1 | 8 | 13.1 | 2 | 1.2 | 1.3 | 1 |
| Ce | 0.1 | 20.1 | 30.9 | 6.5 | 4.2 | 4.1 | 2.8 |
| Pr | 0.03 | 2.83 | 4.48 | 1.03 | 0.68 | 0.64 | 0.42 |
| Nd | 0.1 | 13.2 | 21.5 | 5.1 | 3.3 | 3 | 2.1 |
| Sm | 0.03 | 3.92 | 7.2 | 1.24 | 0.93 | 0.8 | 0.83 |
| Eu | 0.03 | 1.27 | 1.31 | 0.12 | 0.06 | 0.04 | 0.03 |
| Gd | 0.05 | 4.63 | 8.06 | 1.58 | 1.2 | 1.14 | 0.93 |
| Tb | 0.01 | 0.81 | 1.38 | 0.23 | 0.21 | 0.14 | 0.18 |
| Dy | 0.05 | 5.3 | 9.26 | 1.48 | 1.13 | 0.99 | 1.05 |
| Ho | 0.01 | 1.12 | 1.91 | 0.29 | 0.25 | 0.22 | 0.22 |
| Er | 0.03 | 3.46 | 5.62 | 0.83 | 0.69 | 0.66 | 0.66 |
| Tm | 0.01 | 0.52 | 0.79 | 0.11 | 0.1 | 0.08 | 0.09 |
| Yb | 0.03 | 3.33 | 4.99 | 0.71 | 0.73 | 0.63 | 0.83 |
| Lu | 0.01 | 0.53 | 0.81 | 0.12 | 0.15 | 0.14 | 0.14 |
| Alteration Indices of the CONSONORM_LG Method (Dimensionless Values Comprised between 0 and 100) | |||||||
| Modelled Fe2O3/Fe2O3T | 0.2 | 0.2 | 0.6 | 0.6 | 0.6 | 0.6 | |
| ALT_CARBS index | 0 | 3.97 | 0.78 | 0.51 | 1.17 | 0.02 | |
| Element | Isotope | LOD 1 | MS17-45 (n = 16) | MS17-28 (n = 20) | MS17-31 (n = 17) | MS17-36 (n = 22) | MS17-37 (n = 16) | MS17-02 (n = 11) | MS17-04 (n = 12) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | std. 2 | Median | std. | Median | std. | Median | std. | Median | std. | Median | std. | Median | std. | |||
| Si (ppm) | 28 | 1500 | 2872.5 | 3598.83 | 2115.0 | 2642.30 | 1550.0 | 3515.74 | 5362.5 | 4327.02 | 3567.5 | 3802.46 | 4850.0 | 8029.09 | 7572.5 | 6135.76 |
| Ca | 44 | 5.0 | 59.50 | 108.18 | 195.00 | 943.42 | 214.50 | 604.75 | 159.00 | 173.69 | 223.25 | 115.08 | 207.00 | 1716.62 | 163.50 | 574.53 |
| Y | 89 | 0.01 | 0.618 | 0.348 | 1.425 | 0.805 | 0.912 | 1.235 | 0.883 | 0.216 | 0.988 | 0.328 | 3.905 | 2.712 | 2.733 | 0.397 |
| P | 31 | 15 | 10.83 | 6.06 | 5.78 | 3.30 | 7.65 | 82.03 | 13.08 | 6.33 | 8.48 | 42.72 | 12.30 | 13.16 | 21.48 | 7.36 |
| Zr | 90 | 0.01 | 0.520 | 0.254 | 2.425 | 2.662 | 1.260 | 179.073 | 0.134 | 0.262 | 0.194 | 0.074 | 0.309 | 0.860 | 0.057 | 0.310 |
| Hf | 178 | 0.01 | 0.037 | 0.020 | 0.121 | 0.106 | 0.084 | 4.457 | 0.008 | 0.005 | 0.009 | 0.006 | 0.023 | 0.044 | 0.005 | 0.006 |
| Al | 27 | 0.2 | 1250.5 | 1915.16 | 1262.5 | 1525.45 | 1550.0 | 1714.15 | 2092.5 | 1613.37 | 876.25 | 1468.22 | 2350.0 | 2609.27 | 4487.5 | 2989.17 |
| Ta | 181 | 0.01 | 0.350 | 0.171 | 0.400 | 0.169 | 0.009 | 0.132 | 0.045 | 0.031 | 0.136 | 0.086 | 0.059 | 0.062 | 0.053 | 0.022 |
| Nb | 93 | 0.03 | 6.510 | 3.627 | 5.930 | 2.645 | 0.445 | 2.375 | 1.868 | 0.725 | 4.323 | 2.992 | 1.380 | 0.888 | 2.428 | 0.516 |
| Cu | 63 | 0.04 | 1.21 | 1.89 | 0.67 | 4.48 | 0.90 | 7.23 | 8.05 | 78.60 | 36.00 | 92.51 | 2.85 | 17.00 | 13.35 | 325.22 |
| Mn | 55 | 1.0 | 2702.5 | 857.59 | 4247.5 | 1558.88 | 1376.5 | 248.24 | 1182.5 | 268.75 | 1845.8 | 842.72 | 1036.0 | 248.07 | 339.75 | 79.96 |
| Mg | 24 | 0.1 | 2467.5 | 4823.66 | 2460.0 | 3184.89 | 3450.0 | 3985.70 | 6055.0 | 5710.81 | 4192.5 | 4835.59 | 6550.0 | 7470.53 | 5932.5 | 4752.36 |
| Ti | 47 | 0.1 | 21,137 | 7261.64 | 27075 | 11,093.60 | 6980 | 1548.27 | 6225 | 1657.05 | 10,635 | 5873.54 | 7690.0 | 2372.93 | 502.50 | 291.58 |
| Zn | 66 | 0.5 | 36.78 | 142.71 | 26.53 | 232.92 | 48.00 | 90.57 | 48.05 | 50.63 | 80.75 | 39.59 | 28.25 | 8.48 | 22.85 | 16.03 |
| Co | 59 | 0.02 | 48.97 | 5.53 | 55.40 | 3.22 | 72.40 | 3.88 | 58.40 | 4.50 | 57.28 | 2.72 | 18.75 | 124.20 | 14.93 | 121.69 |
| V | 51 | 0.03 | 4025.8 | 461.75 | 3142.5 | 420.24 | 3186.5 | 361.71 | 5009.0 | 120.77 | 4322.3 | 146.35 | 1360.50 | 276.28 | 27.78 | 6.28 |
| Ni | 60 | 0.1 | 95.90 | 31.37 | 98.05 | 20.60 | 95.00 | 8.09 | 126.13 | 13.87 | 94.25 | 9.62 | 21.80 | 465.95 | 18.13 | 236.53 |
| Cr | 52 | 1.0 | 157.43 | 86.44 | 17.26 | 28.63 | 7.78 | 8.39 | 5.61 | 2.26 | 46.38 | 19.90 | 43.70 | 230.74 | 9.70 | 2.40 |
| La | 139 | 0.01 | 0.246 | 0.188 | 1.505 | 1.088 | 0.429 | 0.188 | 0.903 | 0.392 | 0.704 | 0.287 | 0.749 | 0.350 | 0.330 | 0.097 |
| Ce | 140 | 0.01 | 0.573 | 0.387 | 3.853 | 1.955 | 1.281 | 0.571 | 2.328 | 0.838 | 1.755 | 0.635 | 1.930 | 0.849 | 0.805 | 0.224 |
| Pr | 141 | 0.01 | 0.075 | 0.052 | 0.438 | 0.210 | 0.183 | 0.068 | 0.261 | 0.085 | 0.219 | 0.073 | 0.284 | 0.115 | 0.126 | 0.030 |
| Nd | 146 | 0.01 | 0.374 | 0.234 | 1.760 | 0.743 | 0.795 | 0.350 | 1.135 | 0.346 | 0.950 | 0.329 | 1.360 | 0.565 | 0.760 | 0.149 |
| Sm | 152 | 0.02 | 0.087 | 0.069 | 0.326 | 0.117 | 0.166 | 0.095 | 0.217 | 0.053 | 0.221 | 0.083 | 0.340 | 0.225 | 0.286 | 0.072 |
| Eu | 153 | 0.01 | 0.005 | 0.003 | 0.027 | 0.010 | 0.011 | 0.008 | 0.013 | 0.008 | 0.014 | 0.006 | 0.036 | 0.019 | 0.045 | 0.011 |
| Gd | 157 | 0.03 | 0.136 | 0.081 | 0.320 | 0.123 | 0.178 | 0.128 | 0.215 | 0.070 | 0.283 | 0.074 | 0.580 | 0.331 | 0.466 | 0.067 |
| Tb | 159 | 0.01 | 0.016 | 0.013 | 0.050 | 0.027 | 0.024 | 0.021 | 0.029 | 0.006 | 0.034 | 0.015 | 0.085 | 0.064 | 0.069 | 0.011 |
| Dy | 163 | 0.02 | 0.137 | 0.086 | 0.304 | 0.190 | 0.179 | 0.170 | 0.178 | 0.038 | 0.223 | 0.071 | 0.630 | 0.472 | 0.463 | 0.097 |
| Ho | 165 | 0.01 | 0.027 | 0.015 | 0.064 | 0.039 | 0.031 | 0.043 | 0.032 | 0.010 | 0.042 | 0.014 | 0.149 | 0.098 | 0.098 | 0.017 |
| Er | 166 | 0.02 | 0.085 | 0.044 | 0.210 | 0.152 | 0.098 | 0.211 | 0.089 | 0.022 | 0.111 | 0.039 | 0.437 | 0.284 | 0.269 | 0.052 |
| Tm | 169 | 0.01 | 0.013 | 0.006 | 0.032 | 0.030 | 0.013 | 0.038 | 0.011 | 0.003 | 0.015 | 0.005 | 0.054 | 0.038 | 0.038 | 0.007 |
| Yb | 172 | 0.01 | 0.133 | 0.041 | 0.306 | 0.284 | 0.104 | 0.325 | 0.078 | 0.019 | 0.090 | 0.027 | 0.448 | 0.229 | 0.201 | 0.044 |
| Lu | 175 | 0.01 | 0.025 | 0.007 | 0.067 | 0.070 | 0.019 | 0.072 | 0.014 | 0.004 | 0.017 | 0.004 | 0.076 | 0.039 | 0.027 | 0.006 |
| Element | Isotope | LOD 4 | Chlorite 1 n = 6 | Serpentine ± Talc, Brucite 2 n = 19 | Actinolite 3 n = 13 | |||
|---|---|---|---|---|---|---|---|---|
| Median | std. 5 | Median | std. | Median | std. | |||
| SiO2 (wt %) | 28 | 1500 | 30.70 | 4.88 | 42.98 | 2.53 | 53.07 | 8.76 |
| TiO2 | 47 | 0.1 | 0.03 | 0.01 | 0.01 | 0.00 | 0.02 | 0.02 |
| Al2O3 | 27 | 0.2 | 17.570 | 1.001 | 0.927 | 0.429 | 0.207 | 0.851 |
| CaO | 44 | 5.0 | 0.10 | 0.28 | 0.03 | 0.21 | 12.66 | 4.83 |
| FeO | 56 | 1.0 | 9.746 | 9.210 | 6.088 | 2.823 | 6.270 | 3.788 |
| MgO | 24 | 0.1 | 29.222 | 3.818 | 37.749 | 2.101 | 22.971 | 2.151 |
| Na2O | 23 | 0.7 | 0.00 | 0.00 | 0.00 | 0.14 | 0.04 | 0.05 |
| K2O | 39 | 2.0 | 0.016 | 0.006 | 0.001 | 0.010 | 0.016 | 0.007 |
| P2O5 | 31 | 15 | 0.005 | 0.005 | 0.016 | 0.047 | 0.007 | 0.009 |
| MnO | 55 | 1.0 | 0.08 | 0.09 | 0.07 | 0.02 | 0.24 | 0.15 |
| Mg# | 0.75 | 0.15 | 0.86 | 0.06 | 0.78 | 0.09 | ||
| V (ppm) | 51 | 0.03 | 29.54 | 15.95 | 86.58 | 69.45 | 8.53 | 61.81 |
| Cr | 52 | 1.0a | 1.51 | 1.09 | 0.79 | 2.42 | 0.46 | 0.74 |
| Ni | 58 | 0.1 | 31.28 | 4.75 | 30.16 | 101.58 | 14.25 | 10.66 |
| La | 139 | 0.01 | 0.11 | 0.06 | 0.42 | 0.15 | 0.31 | 2.28 |
| Ce | 140 | 0.01 | 0.32 | 0.16 | 1.30 | 0.53 | 1.41 | 8.97 |
| Pr | 141 | 0.01 | 0.05 | 0.03 | 0.21 | 0.08 | 0.37 | 1.74 |
| Nd | 146 | 0.01 | 0.30 | 0.18 | 1.17 | 0.43 | 2.62 | 5.66 |
| Sm | 152 | 0.02 | 0.13 | 0.08 | 0.38 | 0.14 | 1.09 | 0.74 |
| Eu | 153 | 0.01 | 0.021 | 0.019 | 0.015 | 0.006 | 0.127 | 0.045 |
| Gd | 157 | 0.03 | 0.222 | 0.147 | 0.603 | 0.220 | 1.748 | 0.717 |
| Tb | 159 | 0.01 | 0.032 | 0.028 | 0.102 | 0.035 | 0.312 | 0.136 |
| Dy | 163 | 0.02 | 0.263 | 0.151 | 0.627 | 0.216 | 1.928 | 0.643 |
| Ho | 165 | 0.01 | 0.060 | 0.032 | 0.133 | 0.046 | 0.393 | 0.154 |
| Er | 166 | 0.02 | 0.180 | 0.090 | 0.384 | 0.126 | 1.161 | 0.469 |
| Tm | 169 | 0.01 | 0.021 | 0.018 | 0.057 | 0.019 | 0.109 | 0.070 |
| Yb | 172 | 0.01 | 0.161 | 0.142 | 0.417 | 0.134 | 0.657 | 0.746 |
| Lu | 175 | 0.01 | 0.030 | 0.032 | 0.087 | 0.026 | 0.083 | 0.143 |
| Sample | Unit | Srp 1 | Act | Chl | Ab, Ep | Mag | Ttn | Ap | Sulphide | Alteration Minerals |
|---|---|---|---|---|---|---|---|---|---|---|
| MS17-45 | LDC | 30% | 20% | 40% | <1% | 1% (Py, ±Ccp) | ||||
| MS17-28 | LDC | 5% | 20% | 35% | 36%, 4% (Ilm) | <1% | ||||
| MS17-31 | LDC | 50% | 47% | <1% | <1% (Po, ±Ccp) | 2% (Amp, Cal) | ||||
| MS17-36 | LDC | 20% | 30% | 50% | <1% | <1% (Po) | ||||
| MS17-37 | LDC | 15% | 35% | 50% | <1% | <1% (Py) | <1% (Cal vein) | |||
| MS17-38 | mafic dyke | 34% | 30% | 30% | 5% | <1% (Po, ±Ccp) | 1% (Ep, Fsp, Chl, Cal) | |||
| MS17-02 | LDC | 20% | 35% | 30% | 5% | 10% (Po, ±Ccp) | 1% (Ms-vein) | |||
| MS17-04 | LDC | 10% | 20% | 50% | 20% (Po, ±Ccp) | Act | ||||
| MS17-07 | volc. 2 | 2% | 25% | 3% | 60% (Po, ±Ccp) | 10% Qz | ||||
| MS17-08 | volc. | 28% | 22% | 2% (Ilm) | 38% (Po, ±Ccp) | 10% Qz | ||||
| MS17-14 | volc. | 30% | 30% | 30% | 9% (Mag) | 1% (Py) | ||||
| MS17-16 | volc. | 20% | 37% | 25% | 5% (Ilm) | 8% (Po, Py, ±Ccp) | 5% (Amp) | |||
| MS17-20 | volc. | 10% | 50% | 10% | 5% | 10% (Po, Py) | 5% (Cal, Amp) |
| MS17-14 | MS17-20 | |||
|---|---|---|---|---|
| Precursor 1 | BM-ABS 2 | Precursor | BM-ABS | |
| SiO2 | 49.85 | 6.87 | 49.48 | 4.74 |
| FeOT | 12.37 | 8.95 | 13.00 | 1.99 |
| MgO | 5.87 | 3.80 | 4.33 | 4.23 |
| CaO | 10.45 | −4.18 | 8.77 | −5.26 |
| Na2O | 2.49 | 1.13 | 2.72 | 0.72 |
| K2O | 0.37 | 0.16 | 0.72 | −0.56 |
| Global mass change | 19.61 | 8.53 | ||
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathieu, L. Origin of the Vanadiferous Serpentine–Magnetite Rocks of the Mt. Sorcerer Area, Lac Doré Layered Intrusion, Chibougamau, Québec. Geosciences 2019, 9, 110. https://doi.org/10.3390/geosciences9030110
Mathieu L. Origin of the Vanadiferous Serpentine–Magnetite Rocks of the Mt. Sorcerer Area, Lac Doré Layered Intrusion, Chibougamau, Québec. Geosciences. 2019; 9(3):110. https://doi.org/10.3390/geosciences9030110
Chicago/Turabian StyleMathieu, Lucie. 2019. "Origin of the Vanadiferous Serpentine–Magnetite Rocks of the Mt. Sorcerer Area, Lac Doré Layered Intrusion, Chibougamau, Québec" Geosciences 9, no. 3: 110. https://doi.org/10.3390/geosciences9030110
APA StyleMathieu, L. (2019). Origin of the Vanadiferous Serpentine–Magnetite Rocks of the Mt. Sorcerer Area, Lac Doré Layered Intrusion, Chibougamau, Québec. Geosciences, 9(3), 110. https://doi.org/10.3390/geosciences9030110





