Abstract
Composting the municipal organic fraction of waste results in a valuable product in the form of compost, which could be used instead of other forms of fertilisation. The organic waste stream may contain oxo-biodegradable and biodegradable plastics used for waste collection. Their components and decomposition residues may contaminate the compost chemically and physically. In this paper, the results of studies on the content of selected macro- and microelements in new and composted plastics have been analysed. Statistical analyses were carried out in order to determine the most characteristic components of plastics and to determine the character of chemical composition changes. The analysis of the test results showed that multidirectional changes in the content of macro- and microelements occur during composting, and they may be the source of contamination of the fertiliser produced. Contaminants in the form of microplastics may also be released into the environment, which may pose a threat to many elements of the environment, including animals and humans.
1. Introduction
Biodegradable and oxo-biodegradable plastics (bioplastics) are widely used, mainly for their specific properties. Due to their ability to degrade in biological processes (biodegradation) and the possibility of their production from renewable substrates, they replace petroleum-based plastics, which contribute to the improvement of the quality of the environment [1]. Bioplastics are groups of materials with different properties and applications. They can be produced in a natural way, such as cellulose and starch [2], but also through chemical modifications [3]. These materials include biodegradable and oxo-biodegradable plastics. Natural biodegradable plastics are made of a polymeric matrix from natural sources (e.g., polysaccharides–cellulose, starch, and their derivatives). Natural fibres from commonly grown plants, such as flax, jute, or hemp, are used to fill such materials [4,5]. Synthetic biodegradable polymers include: polycaprolactone—PCL, poly(vinyl alcohol), poly(ethylene oxide), polyesters obtained by fermentation polymerisation of polysaccharides, e.g. poly(hydroxybutyric acid)—PHB or poly(lactic acid)—PLA [6]. Oxo-biodegradable plastics differ from biodegradable plastics in their manufacturing process. They are produced with the use of additives accelerating the decomposition of plastics, so-called pro-degradants, or prooxidative additives. The most common additives on the market are d2w and TDPA (Totally Degradable Plastic Additives), usually produced from cobalt, manganese, and iron compounds [7]. Oxo-biodegradable plastics can be, e.g., high-density polyethylene—HDPE with a prooxidative additive, which is supposed to ensure its decomposition at the end of the product life. The decomposition of biodegradable polymers takes place with the participation of microorganisms such as bacteria and fungi. However, the decomposition of oxo-biodegradable polymers is more complex and has two stages. The first stage is the activation of the additives responsible for the oxidation of the material. This can occur, for example, under the influence of UV rays/heat [8,9,10]. The second stage is the degradation with the participation of microorganisms (proper biodegradation).
The studies presented in the scientific literature on the chemical composition of biodegradable and oxo-biodegradable plastics showed the presence of chlorine, phosphorus, and a number of heavy metals (zinc, cadmium, lead, nickel, copper, chromium, cobalt, iron, and manganese) [11,12,13,14,15,16]. The sources of metals such as zinc, lead, copper, and cadmium in the test materials can be additives such as dyes, fillers, antioxidants, stabilisers, or plasticisers used in the production of various types of plastics [12,17,18]. As confirmed by Rochman et al. [19], metals have the ability to accumulate in the environment, and their amount increases over time.
Bioplastics have been used in a number of applications, including organic waste disposal bags and food packaging bags. After being used, they are most often discharged into the organic waste stream, and then into composting plants or waste fermentation plants. It is, therefore, important that such materials are completely biodegradable. When they enter a composting plant, they become part of the waste manure (compost) and should not contaminate it.
The use of bioplastics on an increasing scale makes them the subject of research by many authors. Among them, research on decomposition in various conditions prevails, e.g., in soil, water, compost, with the use of isolated strains of bacteria or fungi. Often, studies on decomposition are conducted under laboratory conditions. The results obtained in this way cannot be translated into actual conditions, as these often differ significantly from those created artificially [20,21]. For example, Poonam et al. [22] studied the biodegradability of polyethylene by isolated bacteria and fungi under laboratory conditions, while Giacomucci et al. [23] studied the bacterial degradation of various plastics (polyethylene - PE, polypropylene - PP, polyvinyl chloride - PVC). The processes themselves (biodegradation and oxo-biodegradation) also take place in different ways. The additional problem is that the term “biodegradable” could be used in the name of the material, without any actual evidence of its biodegradability [24]. The physical and chemical structure of polymers, as well as the environment in which they are found, have a great influence on the course of decomposition processes [25,26,27]. It also happens that the results of tests of biodegradable and oxo-biodegradable plastics decomposition are misinterpreted—during the experiment, there are some changes in the structure of polymers, but there is no complete decomposition. Despite this, it is concluded that under these conditions, the complete decomposition of the material may occur [3]. Some studies are carried out in simulated conditions, which are to reflect the actual conditions, e.g., during composting [28,29]. It may also happen that only a part of polymers included in the plastic could be decomposed [30].
The prooxidative additives used in the processes do not always guarantee full decomposition of plastics, or their decomposition requires special conditions not found in the natural environment [31]. Such situations may lead to a problem with the remaining plastics in the environment, i.e., microplastics. The presence of bioplastics in the mass of organic waste may cause pollution of the compost produced from this waste. The use of such compost may lead to soil contamination. Unfortunately, it turns out that even bioplastics can be a source of microplastics that accumulate in the environment [32]. As Weithmann et al. [33] confirm, the processing of bio-waste together with plastics leads to the presence of microplastics up to 5 mm as well as <1 mm in the organic fertiliser produced.
The aim of this study was to assess the possibility of composting selected bioplastics (shopping bags, waste disposal bags) together with organic waste in real conditions in an industrial composting plant. The scope of the work included the analysis of changes in the chemical composition of the tested packaging, the selection of components that best characterise this composition (useful for monitoring the contamination of compost), preliminary assessment of the possibility of formation of physical contaminants (microplastics) during composting. In addition, an assessment of the possibility of using UV radiation to initiate oxo-biodegradation processes of plastics during the waste storage before filling the bioreactor was carried out.
2. Materials and Methods
Preliminary tests of selected 10 packages showed that the majority of composted samples of biodegradable and oxo-biodegradable plastics did not decompose or were only partially fragmented [34]. In order to obtain more data for statistical analysis, the number of samples was extended to 12, and three series of experiments on their composting were carried out. Table 1 presents the characteristics prepared on the basis of their own research and information from packaging manufacturers. All of them have been characterised as degradable in the composting process, biodegradable, oxo-biodegradable, or intended for organic waste. Samples of approximate 500 cm2 were cut from shopping bags and waste disposal bags. Figure 1 shows the appearance of the samples before composting (new bags).

Table 1.
Characteristics of selected samples [own study based on information from producers].

Figure 1.
Appearance of shopping bags and waste disposal bags selected for the study [photo F. Markowicz].
In order to initiate the process of decomposition of oxo-biodegradable plastics, the samples were exposed to a 36 W UV lamp. The cycles and exposure times were selected so as to be replicable under real conditions in the composting plant by irradiation for 2 × 10 h (for two consecutive days) or 5 × 10 h (for five consecutive days) during the waste storage, prior to loading the bioreactor. Non-irradiated sets of samples were also prepared for the tests. In this way, three sets of samples for composting were created:
- 12 non-irradiated samples,
- 12 samples irradiated for 20 h,
- 12 samples irradiated for 50 h.
In total, three series (replications) were subjected to composting during the study; each of them consisted of three sets of samples that were not exposed to light and were exposed to light for 20 and 50 h. The samples were placed between two layers of coated glass fibre mesh [35], resistant to heat and UV radiation. Figure 2 shows the image of the prepared sample. The meshes with the single samples were joined together with cable ties resistant to temperatures that occur during composting. During the filling of the bioreactor in the industrial composting plant, the sets of meshes with samples were placed horizontally, on previously arranged layers of waste, and then covered with successive layers. The research at the composting plant in Jarocin (Wielkopolskie Voivodeship, Poland) was carried out in 2017–2018. After the composting process, the samples were washed and prepared for laboratory analyses. The detailed course of this phase of research and the characteristics of the composting plant are presented in the paper by Markowicz et al. [34].
Figure 2.
The appearance of a sample sandwiched between two layers of mesh [photo F. Markowicz].
In the prepared samples (new and composted), the content of components that may pose a threat to the environment in case of leakage of plastic into the compost was determined. Laboratory analyses were carried out after dissolving (mineralisation) in a mixture of concentrated nitric(V), chloric(VII), and sulphuric(VI) acids (HNO3, HClO4, H2SO4), prepared in a ratio of 10:4:1. In the dissolved samples, the content of calcium (Ca), magnesium (Mg), potassium (K), sodium (Na), iron (Fe), and cadmium (Cd) was determined using Atomic Absorption Spectrometry (AAS) [36].
The results of plastics composition tests were subjected to statistical analysis using Statistica 13.1 (StatSoft Poland, StatSoft, Inc. USA). To assess the significance of chemical composition differences between the groups of new samples, samples composted without irradiation, samples composted after 20 h and 50 h irradiation, Student’s t-test (parametric test), and Mann–Whitney U test (nonparametric test) were used. The principal components analysis (PCA) was used to indicate which of the selected metals best characterised the composition of the tested plastic samples.
3. Results
3.1. Chemical Composition of Composted Bioplastics
Table 2 presents the content of analysed metals in the samples before composting, i.e., in new biodegradable and oxo-biodegradable plastics. The majority of the samples contained large amounts of calcium (Ca), magnesium (Mg), potassium (K), and iron (Fe). Lower contents of Ca, Mg, and Fe were found only in samples 2 and 11. The total content of macroelements (calcium, magnesium, potassium, sodium, iron, manganese) ranged from 10,230 mg/kg in sample 3 to 428 mg/kg in sample 11. It should be noted that all plastics selected for the study contained heavy metals—copper (Cu), zinc (Zn), chromium (Cr), lead (Pb), nickel (Ni), and cadmium (Cd). Their contents were differentiated, with the highest content of copper and zinc, the lowest in the case of nickel and cadmium. Samples that were presented as 100% oxo-biodegradable (No. 8) and compostable (No. 12) had the highest content of heavy metals (over 300 mg/kg). The slightly lower content of heavy metals (over 200 mg/kg) was found in sample 6 (biodegradable) and samples 5, 7, 9, and 10 (oxo-biodegradable). Their decomposition may cause an increase in the content of heavy metals, exceeding the levels safe for the environment. The lowest content of heavy metals (below 40 mg/kg) was found in samples 1 and 2. Negative effects may also occur in case of excessive content of macroelements in soil, e.g., death of plants in the case of excess calcium [37,38].

Table 2.
Contents of the analysed metals in samples before composting.
Figure 3, Figure 4 and Figure 5 present the results of the analysis of the content of twelve metals in all samples—new (3, 4, 5, 6, 7, 8, 9, 10, 12), composted without irradiation (A), composted after 20 h of irradiation (B), and composted after 50 h of irradiation (C). Roman numbers (I, II, III) indicate test/replicate series. The figures do not show the samples 1, 2, and 11, which were completely decomposed in all replicates. Samples 1 and 2 were marked by the manufacturers as compostable under industrial or domestic conditions (Table 1), sample 11 was made of HDPE (high-density polyethylene) and intended for the storage of organic waste. In other cases, only single fragments of plastics were decomposed, hence the missing results on the pictures.
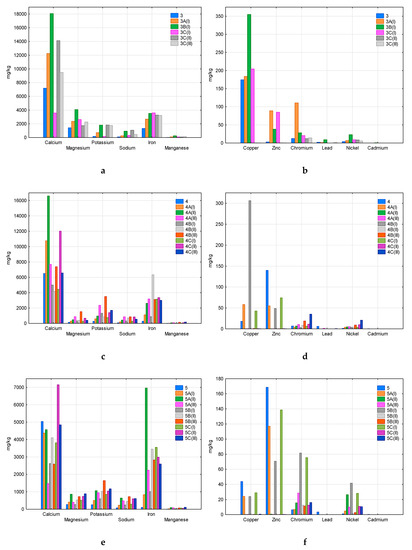
Figure 3.
Comparison of the content of analysed metals in new and composted samples 3 (a—macroelements, b—heavy metals), 4 (c—macroelements, d—heavy metals), and 5 (e—macroelements, f—heavy metals).
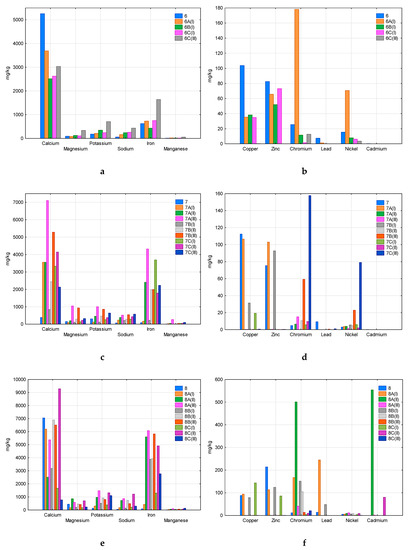
Figure 4.
Comparison of the content of analysed metals in new and composted samples 6 (a—macroelements, b—heavy metals), 7 (c—macroelements, d—heavy metals), and 8 (e—macroelements, f—heavy metals).
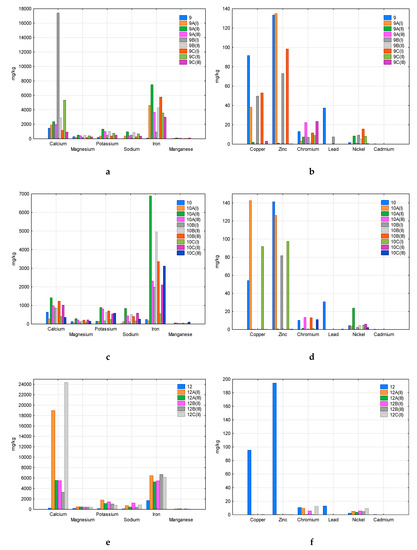
Figure 5.
Comparison of the content of analysed metals in new and composted samples 9 (a—macroelements, b—heavy metals), 10 (c—macroelements, d—heavy metals), and 12 (e—macroelements, f—heavy metals).
Among the analysed macroelements (Ca, Mg, K, Na, Fe, Mn), calcium was the main component of samples 3, 4, 5, 6, 7, and 8 (Figure 3a,c,e, Figure 4a,c,e). The second component was iron, which dominated in sample 10 (Figure 5c) and also had a significant share in samples 4, 5, 7, 8, 9, and 12 (Figure 3c,e, Figure 4c,e, Figure 5a,e). This concerned both new samples and those after composting. This may indicate the durability of the plastic components that contain these macroelements, which limits their transfer to the compost.
Among heavy metals, copper was found in the highest amounts in sample 3 (Figure 3a), and also in samples 4, 6, 7, 9 (Figure 3d, Figure 4b,d, Figure 5b). The studied materials also contained a lot of zinc and chromium–samples 3, 4, 5 (Figure 3b,d,f), 6, 7, 8 (Figure 4b,d,f), 9, 10 (Figure 5b,d). In samples 5, 6, 7, 9, and 10, significant nickel contents were also determined (Figure 3f, Figure 4b,d, Figure 5b,d). Heavy metal content fluctuated quite a lot, even in replications of the same sample. The increase in the content compared to the new material, visible in some cases, may indicate the durability of the plastics components (e.g., dyes, stabilisers), which limits their transfer to the composted mass (e.g., copper, zinc and chromium in sample 3, Figure 3b). There were also cases of a significant decrease in the heavy metal content of composted plastics due to susceptibility to degradation processes and the entry of the component into the compost (e.g., copper and zinc in Sample 6, Figure 4b). Please note that in the case of incomplete decomposition of bags, only part of their components was released into the compost, and the packaging itself was a physical contaminant. An example of the appearance of such samples was presented in Markowicz’s et al. work [34]. The total decomposition (which occurred in samples 1, 2, and 11) eliminates the problem of physical contamination; however, all the components of the packaging are mixed with the compost.
3.2. Influence of Irradiation on the Plastic Degradation Processes and Changes in their Composition
In order to determine the differences between the properties of the tested biodegradable and oxo-biodegradable plastics, the Student’s t-test was used. It is a parametric test used for data demonstrating compliance with the normal distribution. Due to the limited amount of analysed data (which usually is connected with a lack of compliance with the normal distribution), additionally, the nonparametric Mann–Whitney U test was used, which does not require meeting this condition [39]. The samples were divided into groups: a—new samples, b—composted samples without irradiation, c—composted samples irradiated for 20 h, d—composted samples irradiated for 50 h. Table 3, Table 4 and Table 5 show the results of tests carried out between the groups: a–b (Table 3), a–c (Table 4), a–d (Table 5). The highlighted results are significant with p < 0.05. The comparison of groups a and b (Table 3) showed statistically significant differences between the contents of potassium, sodium, iron, manganese, copper, and zinc. These differences were confirmed by both tests. Furthermore, the nonparametric test showed statistically significant differences between the lead, nickel and cadmium contents. None of the applied tests showed any differences between the studied groups in the case of calcium, magnesium, and chromium content. This was due to similar contents of these components in new and composted samples without irradiation.

Table 3.
Analysis of the differences between the composition of new (a) and composted samples without exposure to UV light (b)—the highlighted results are significant with p < 0.05.

Table 4.
Analysis of differences between the composition of new (a) and composted samples after 20 h irradiation (c)—the highlighted results are significant with p < 0.05.

Table 5.
Analysis of differences between the composition of new (a) and composted samples after 50 h irradiation (d)—the highlighted results are significant with p < 0.05.
Table 4 presents a comparison between the a and c groups, which showed statistically significant differences between potassium, sodium, iron, manganese, zinc, lead, and cadmium contents. These differences were confirmed by both tests. Furthermore, the nonparametric test showed statistically significant differences between copper and nickel contents. None of the tests showed any differences between the studied groups in the case of calcium, magnesium, and chromium content (as in the previous comparison).
Table 5 presents a comparison between the groups a and d, which showed statistically significant differences between potassium, sodium, iron, manganese, copper, zinc, and lead content. These differences were confirmed by both tests. Moreover, the nonparametric test showed statistically significant differences between nickel and cadmium contents. As in previous comparisons, none of the tests showed any differences between the studied groups in the case of calcium, magnesium, and chromium content.
Figure 6 and Figure 7 show descriptive statistics (mean, standard error) for potassium, iron, copper, and zinc content in the compared pairs of groups: a–b, a–c, a–d. In the case of potassium and iron (Figure 6), there is an increase in concentration in composted samples. These components were released to the composted mass of waste only to a small extent. The situation was different in the case of copper and zinc (Figure 7), whose contents in the samples after composting were lower. This could have been due to the release of some of these components into the compost.
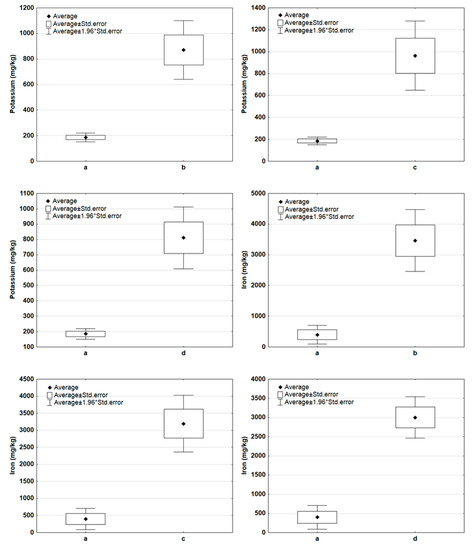
Figure 6.
Comparison of basic statistics (mean, standard error) for potassium and iron content in the tested sample groups (a—new samples, b—composted samples without irradiation, c—composted samples irradiated for 20 h, d—composted samples irradiated for 50 h).
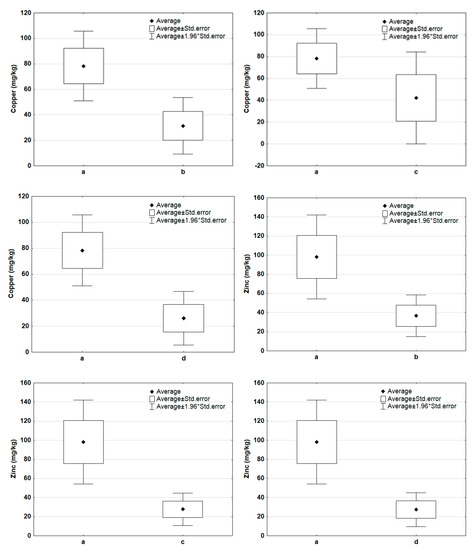
Figure 7.
Comparison of basic statistics (mean, standard error) for copper and zinc content in the tested sample groups (a—new samples, b—composted samples without irradiation, c—composted samples irradiated for 20 h, d—composted samples irradiated for 50 h).
Table 6 summarises the results of all comparisons carried out between groups a, b, c, and d. Statistically significant differences occurred only in the case of comparison of new (a) and composted samples (b, c, and d). No statistically significant differences were found between the samples subjected to composting (with and without irradiation), which means that the irradiation did not affect the decomposition process of the samples, as they were not completely decomposed and their chemical composition did not change significantly. The applied tests (parametric and nonparametric) showed significant differences in the case of groups:

Table 6.
Summary of the results of the analysis of differences in composition between the tested sample groups (a—new samples, b—composted samples without irradiation, c—composted samples irradiated for 20 h, d—composted samples irradiated for 50 h)—the contents of the mentioned metals in the groups differed significantly.
- a–b: K, Na, Fe, Mn, Cu, Zn,
- a–c: K, Na, Fe, Mn, Zn, Pb, Cd,
- a–d: K, Na, Fe, Mn, Cu, Zn, Pb.
The metals whose contents differed significantly in all the comparisons (in which such differences occurred) were: potassium, sodium, iron, manganese, and zinc.
3.3. Selection of Ingredients that Best Characterise the Biodegradable and Oxo-biodegradable Plastics Analysed
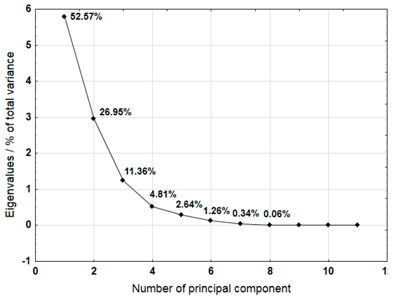
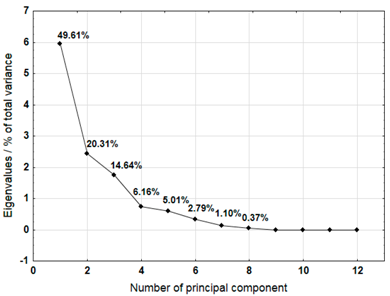
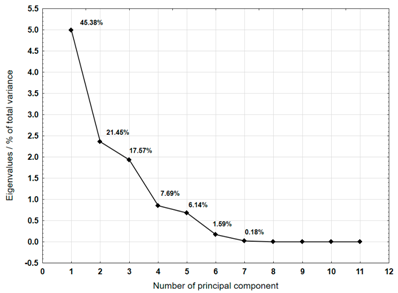
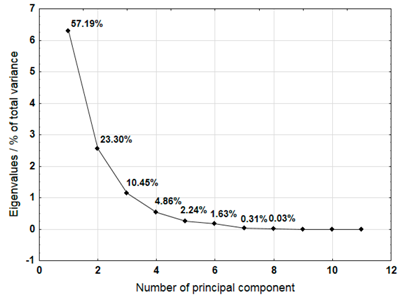
The Principal Component Analysis (PCA) method was used to select the variables (metals) that best characterise the properties of the plastic bags selected for testing. This method reduces the number of variables, enabling a process or phenomenon to be described with a maximum amount of information [40]. The analysis was carried out for samples 3, 4, 5, 6, 7, 8, 9, 10, and 12, which were not completely decomposed during composting in an industrial composting plant.
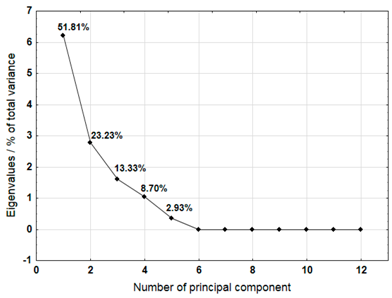
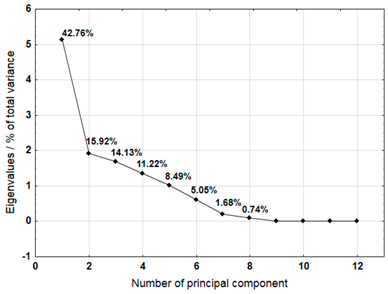
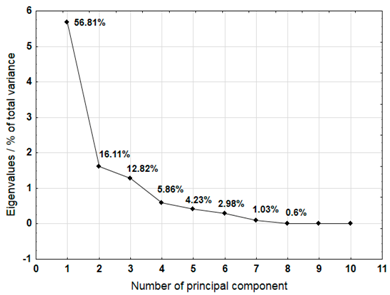
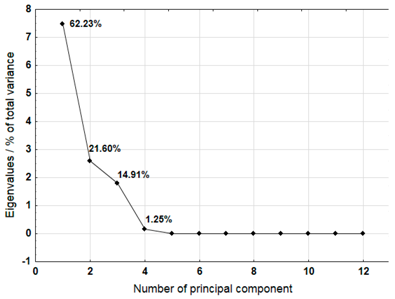
For each material, a graph of the own values of the main components and the percentage of variance explained by them is presented. For the first three components (for sample 4 there are four components), which explained the largest part of the variance, the input values of individual variables, determined on the basis of the correlation between the variables and components, were presented. The marked values correspond to strong correlation (correlation coefficient 0.7–0.89) and very strong correlation (correlation coefficient 0.9–1.0) [41].
Table 7 shows the results of PCA analysis for samples 3, 4, and 5. For sample 3, the first three components explained 88.37% of the variance. Strong and very strong correlations were found between components 1 and 2 and most of the analysed variables (metals). Only sodium, iron, and chromium did not show such correlations. In the case of sample 4, the first four components explained 84.04% of the variance. Strong and very strong correlations with the main components were not found for iron, copper, lead, and nickel. The first three main components identified for sample 5 explained 85.74% of the variance. The analysis did not take into account the content of lead and cadmium, which did not show variability. Among other variables, only iron did not show strong or very strong correlations with the components.

Table 7.
The results of the Principal Component Analysis (PCA) for samples 3, 4, and 5.
Table 8 shows the results of PCA analysis for samples 6, 7, and 8. For sample 6, the first three components explained 98.75% of the variance. Strong and very strong correlations occurred between the components and most of the analysed variables (metals). Only calcium did not show such correlations. In the case of sample 7, the first three components explained 90.89% of the variance. The analysis did not take into account the content of cadmium, which did not show any variability. Strong and very strong correlations with the main components were not found for calcium and copper. The first three main components identified for sample 8 explained 84.57% of the variance. Among the remaining variables, manganese, lead, and nickel did not show strong or very strong correlations with the components.

Table 8.
The results of the Principal Component Analysis (PCA) for samples 6, 7, and 8.
Table 9 shows the results of PCA analysis for samples 9, 10, and 12. For sample 9, the first three components explained 84.39% of the variance. The analysis did not take into account the content of cadmium which did not show any variability. Among the remaining variables, strong and very strong correlations with the components were not found for sodium, chromium, lead, and nickel. In the case of sample 10, the first three components explained 90.94% of the variance. The analysis did not include cadmium content, which showed no variation. Strong and very strong correlations with the main components were not found in the case of manganese and nickel. The first three main components identified for sample 12 explained 95.54% of the variance. Among the remaining variables, manganese and nickel did not show strong or very strong correlations with the components.

Table 9.
The results of the Principal Component Analysis (PCA)for samples 9, 10, and 12.
3.4. Biodegradable and Oxo-biodegradable Plastics As a Source of Microplastics
Some of the bioplastics (especially those containing added plastics) may not be completely biodegradable but only fragmented, which may lead to the formation of microplastics. Microplastics are small polymer particles with a size ranging from 1 μm to 5 mm. There are also smaller particles (<1 μm), which are referred to as nanoplastics [42]. The materials tested are also a potential source of microplastics. Some have been fragmented into particles up to 5 mm (sample 3), others have become more brittle, which results in the division into smaller fragments even under the influence of a small force (sample 9). Figure 8 shows the appearance of samples 3 and 9 after composting.
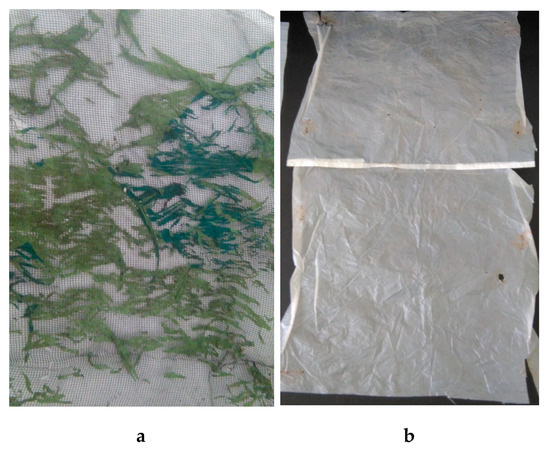
Figure 8.
The appearance of the samples after composting: (a) biodegradable plastic from corn and potato starch (Sample 3), (b) oxo-biodegradable plastic (Sample 9) [photo F. Markowicz].
Plastic decomposition was studied under conditions of industrial composting. After completion of the process, the compost is mixed and screened on a drum screen to separate the contaminants from the finished compost. Under these conditions, plastics that are susceptible to mechanical impact will burst and fragment, resulting in compost that will then be used as fertiliser (some technologies also involve grinding compost) [33]. The regulations setting requirements for compost quality often do not take into account the occurrence of physical impurities, which are micro and nanoplastics. For example, in Poland, where the research was conducted, the requirements for the quality of organic fertilisers (including compost) focus on the content of nutrients, heavy metals, and microbiological contaminants [43,44]. It is not required to conduct analyses of physical contamination, which may include micro and nanoplastics.
4. Discussion
The scientific literature provides many examples of how plastics decompose in the composting of organic waste processes. However, many of these experiments were carried out under controlled composting conditions (similar to actual conditions in a composting plant but created artificially) or the process took place in the presence of bacteria or fungi isolated from the compost. Zhao et al. [45] studied the decomposition under composting conditions; however, it consisted of the isolation of selected strains of bacteria from the compost and then inoculating them on the studied materials. Our research on the decomposition of packaging was carried out in real conditions in an industrial composting plant. Selected plastics were composted together with bio-waste processed in the installation. The process conditions were similar to those prevailing in many industrial composting plants, where biodegradable and oxo-biodegradable plastics mixed with organic waste are deposited. Similar studies were carried out by Vaverkova [46] and Adamcova [47] in a windrow composting plant. The tested shopping bags were taken out for examination every several days. The entire composting process took place in a windrow area exposed to weather conditions. Zafar et al. [48] also analysed the decomposition of plastics in the conditions of windrow composting; however, the studies included the decomposition of only one polymer and changes in the number of microorganisms. Our research in the industrial composting plant was carried out in two stages. The first one took place in closed bioreactors and then on a windrow area, which ensured higher temperatures of the process. Furthermore, the samples were not removed from the bioreactor after being placed in the mass, and the decomposition process was not interfered with until its completion. Removal of samples from a high-temperature windrow can cause the death of microorganisms present on the plastic surface due to a sudden change in temperature. Providing stable temperature during composting promotes an optimal process that can ensure the best possible decomposition of the packaging under these conditions.
The contents of the analysed components in the examined bioplastics were very diverse. In most new and composted samples the concentrations of heavy metals did not exceed the limit values for compost (Cr: 100 mg/kg dm, Cd: 5 mg/kg dm, Ni: 60 mg/kg dm, Pb: 140 mg/kg dm) [44]. There was only one case of exceeding the limit value for cadmium. The content of heavy metals in the remaining samples was relatively low and did not exceed the amounts observed in the Acosta–Coley’s and Alam‘s studies [13,49]. Their source could have been additives used in the production of plastics. Stabilisers, plasticisers, or dyes can pose a threat to the environment, causing heavy metals to accumulate in the soil or move further, including into water [15,19]. Printing on packaging can also hinder decomposition and compost production, restricting the access of bacteria and fungi to plastics [50].
Oxo-biodegradable plastics constituted a large part of the studied packaging (samples 4, 5, 7, 8, 9, and 10). However, no significant influence of UV radiation on their degradation and on composition changes in the composting process was found. Significant differences in chemical composition occurred only between the new and composted samples (regardless of the irradiation and its time). The metals that showed the highest variability were potassium, sodium, iron, manganese, and zinc. However, no significant differences were found in the content of metals between the composted samples exposed and those not exposed to UV radiation. Also, single samples showed a large variation in chemical properties. The use of PCA analysis to select the most characteristic components showed the lowest usefulness of cadmium, which in some samples was present in very low amounts and showed no variability. Individual samples were described by different sets of variables (metals), among which the most frequent were calcium, magnesium, potassium, sodium. The characteristic heavy metals for the analysed plastics were: zinc, copper, chromium, and lead. The changes in the quantities of the macroelements and heavy metals during composting which involved their concentration (for components resistant to degradation processes) or decrease in the content for components entering the compost. With a small share of plastics in the composted waste, their decomposition will not cause a significant deterioration in the quality of compost; however, the number of packaging delivered to the composting plant in the organic waste stream should be controlled.
The problem in composting biodegradable and oxo-biodegradable plastics may also be the presence of their residues in the produced compost. Some authors [51] have shown in their research that the presence of biodegradable plastics does not affect the quality of compost, but did not consider the possibility of accumulation of contaminants in soil. In addition, the requirements for organic fertilisers limit only some ingredients, allowing relatively high contents, and the use of such compost could lead to the accumulation of contaminants [15].
Bioplastics can be a solution to the problems of increasing amounts of plastics in landfills, but they have to be properly processed [52]. In addition, it is necessary to eliminate from the market the bioplastics which, despite the declarations of the producers, do not decompose at all or only partially decompose. Such products will be a source of microplastics in the environment, posing a risk to the health and life of animals and humans due to their heavy metal content and their potential to accumulate in the environment [49,53].
Microplastics are also a source of contaminants that can enter living organisms [54]. According to Bradney, [11] microplastics can be absorbed by fish, for example, and contaminants from plastic particles can accumulate. Therefore, if fish absorb a certain amount of microplastics, their components can accumulate in their bodies. Ultimately, contaminants could also be absorbed by humans after consuming contaminated fish. Many authors have identified the problem of the presence of microplastics in sea and ocean waters [55], but few have pointed to the accumulation of microplastics on land and in soil.
5. Conclusions
The content of macroelements (calcium, magnesium, potassium, sodium, iron, manganese) in the tested samples varied from 10,230 mg/kg to 428 mg/kg. All the studied materials contained heavy metals (copper, zinc, chromium, lead, nickel, and cadmium). The highest contents were recorded for copper and zinc, the lowest for nickel and cadmium. The highest contents of heavy metals reached over 300 mg/kg and the lowest was below 40 mg/kg. The changes in composition, resulting from composting, involved an increase in the concentration of components (related to components resistant to degradation) or its decrease in the case of components susceptible to decomposition. The biggest differences in the composition of the tested packages were observed in the case of potassium, sodium, iron, manganese, and zinc. No significant effect of UV radiation on the degree of decomposition and on the composition of oxo-biodegradable plastics was observed.
The use of PCA for the selection of variables (metals) that best characterise the properties of the plastic bags selected for testing showed the lowest usefulness of cadmium, which in some samples was very low in content and not variable. Individual samples were described by different sets of variables (metals), among which the most frequent were calcium, magnesium, potassium, and sodium. The most characteristic heavy metals for the analysed plastics were: zinc, copper, chromium, and lead.
The conducted research also proved that there is a problem of contamination in the form of micro and nanoplastics, which are formed as a result of fragmentation of biodegradable and oxo-biodegradable plastics. Their small size facilitates their displacement in the environment and even their penetration into living organisms.
Author Contributions
Conceptualisation—F.M. and A.S-P.; methodology—F.M. and A.S-P.; software—A.S-P.; validation—F.M. and A.S-P.; formal analysis—F.M.; investigation—F.M.; resources—F.M.; data curation, —A.S-P.; writing—original draft preparation—F.M.; writing—review and editing—A.S-P.; visualisation—A.S-P.; supervision—A.S-P.; project administration—F.M.; funding acquisition—F.M.
Funding
The research was carried out as part of a targeted subsidy for research and development of young scientists and PhD students at the Faculty of Environmental Engineering and Geodesy of the Wrocław University of Environmental and Life Sciences–contract no. B030/0111/18.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Thakur, S.; Chaudhary, J.; Sharma, B.; Verma, A.; Tamulevicius, S.; Thakur, V.K. Sustainability of bioplastics: Opportunities and challenges. Curr. Opin. Green Sustain. Chem. 2018, 13, 68–75. [Google Scholar] [CrossRef]
- Christopher, B. Biopolymers: Biodegradable Alternatives to Traditional Plastics. In Green Chemistry: An Inclusive Approach; Béla, T., Timothy, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 753–770. [Google Scholar]
- Da Luz, J.M.R.; Paes, S.A.; Nunes, M.D.; Silva, M.D.C.S.D.; Kasuya, M.C.M. Degradation of Oxo-Biodegradable Plastic by Pleurotus ostreatus. PLOS ONE 2013, 8, 69386. [Google Scholar] [CrossRef] [PubMed]
- Bismarck, A.; Aranberri-Askargorta, I.; Lampke, T.; Wielage, B.; Stamboulis, A.; Shenderovich, I.; Limbach, H.-H. Surface characterization of flax, hemp and cellulose fibers; Surface properties and the water uptake behavior. Polym. Compos. 2002, 23, 872–894. [Google Scholar] [CrossRef]
- Kaisangsri, N.; Kerdchoechuen, O.; Laohakunjit, N. Biodegradable foam tray from cassava starch blended with natural fiber and chitosan. Ind. Crop. Prod. 2012, 37, 542–545. [Google Scholar] [CrossRef]
- What Makes Green Plastics Green? Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=11&ved=2ahUKEwijy43spLflAhVUMd4KHU1gDQcQFjAKegQIBBAC&url=https%3A%2F%2Fpdfs.semanticscholar.org%2F00f0%2Fd5de50ede924c1f1973a73eab40af466e0f7.pdf&usg=AOvVaw3Pye84bHWTIOkwFc3xHj7z (accessed on 25 October 2019).
- Ojeda, T.F.; Dalmolin, E.; Forte, M.M.; Jacques, R.J.; Bento, F.M.; Camargo, F.A. Abiotic and biotic degradation of oxo-biodegradable polyethylenes. Polym. Degrad. Stab. 2009, 94, 965–970. [Google Scholar] [CrossRef]
- Yashchuk, O.; Portillo, F.; Hermida, É.B. Degradation of Polyethylene Film Samples Containing Oxo-Degradable Additives. Procedia Mater. Sci. 2012, 1, 439–445. [Google Scholar] [CrossRef]
- Contat-Rodrigo, L. Thermal characterization of the oxo-degradation of polypropylene containing a pro-oxidant/pro-degradant additive. Polym. Degrad. Stab. 2013, 98, 2117–2124. [Google Scholar] [CrossRef]
- Jolanta, W.-K.; Tomasz, R.; Gabriel, B.; Mieczysław, S.; Tomasz, K.; Vijay, K.T. Recent progress in biodegradable polymers and nanocomposite-based packaging materials for sustainable environment. Int. J. Polym. Anal. Ch. 2018, 23, 383–395. [Google Scholar]
- Bradney, L.; Wijesekara, H.; Palansooriya, K.N.; Obadamudalige, N.; Bolan, N.S.; Ok, Y.S.; Rinklebe, J.; Kim, K.-H.; Kirkham, M. Particulate plastics as a vector for toxic trace-element uptake by aquatic and terrestrial organisms and human health risk. Environ. Int. 2019, 131, 104937. [Google Scholar] [CrossRef]
- Alam, O.; Billah, M.; Yajie, D. Characteristics of plastic bags and their potential environmental hazards. Resour. Conserv. Recycl. 2018, 132, 121–129. [Google Scholar] [CrossRef]
- Alam, O.; Wang, S.; Lu, W. Heavy metals dispersion during thermal treatment of plastic bags and its recovery. J. Environ. Manag. 2018, 212, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Pivnenko, K.; Eriksen, M.; Martín-Fernández, J.; Eriksson, E.; Astrup, T.F. Recycling of plastic waste: Presence of phthalates in plastics from households and industry. Waste Manag. 2016, 54, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Zhang, L.; Huang, Q.; Yang, Y.; Nie, Z.; Cheng, J.; Yang, J.; Wang, Y.; Chai, M. Contamination and risk of heavy metals in soils and sediments from a typical plastic waste recycling area in North China. Ecotoxicol. Environ. Saf. 2015, 122, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M.; Browne, M.A.; Halpern, B.S.; Hentschel, B.T.; Hoh, E.; Karapanagioti, H.K.; Rios-Mendoza, L.M.; Takada, H.; Teh, S.; Thompson, R.C. Classify plastic waste as hazardous. Nature 2013, 494, 169–171. [Google Scholar] [CrossRef]
- Munier, B.; Bendell, L.I. Macro and micro plastics sorb and desorb metals and act as a point source of trace metals to coastal ecosystems. PLOS ONE 2018, 13, e0191759. [Google Scholar] [CrossRef]
- Jundong, W.; Jinping, P.; Zhi, T.; Yifan, G.; Zhiwei, Z.; Qiuqiang, C.; Liqi, C. Microplastics in the surface sediments from the Beijiang River litteroal zone: Composition, abundance, surface textures and interaction with heavy metals. Chemosphere 2017, 171, 248–258. [Google Scholar]
- Rochman, C.M.; Hentschel, B.T.; Teh, S.J. Long-Term Sorption of Metals Is Similar among Plastic Types: Implications for Plastic Debris in Aquatic Environments. PLOS ONE 2014, 9, e85433. [Google Scholar] [CrossRef]
- Ruggero, F.; Gori, R.; Lubello, C. Methodologies to assess biodegradation of bioplastics during aerobic composting and anaerobic digestion: A review. Waste Manag. Res. 2019, 37, 959–975. [Google Scholar] [CrossRef]
- Harrison, J.P.; Boardman, C.; O’Callaghan, K.; Delort, A.-M.; Song, J. Biodegradability standards for carrier bags and plastic films in aquatic environments: A critical review. R. Soc. Open Sci. 2018, 5, 171792. [Google Scholar] [CrossRef]
- Poonam, K. Diversity of plastic degrading microorganisms and their appraisal on biodegradable plastic. Appl. Ecol. Environ. Res. 2013, 11, 441–449. [Google Scholar] [CrossRef]
- Giacomucci, L.; Raddadi, N.; Soccio, M.; Lotti, N.; Fava, F. Polyvinyl chloride biodegradation by Pseudomonas citronellolis and Bacillus flexus. New Biotechnol. 2019, 52, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Tobias, P.H.; Carolin, V.; Johanna, K.; Katharina, L.; Frederik, R.W. Plastics of the Future? The Impact of Biodegradable Polymers on the Environment and on Society. Angew. Chem. Int. Edit. 2019, 58, 50–62. [Google Scholar]
- Oluranti, A.; Rotimi, S.; Touhami, M.; Ismael, A.; Odunayo, I. Polyolefins and the environment. In Polyolefin Fibres: Structure, Properties and Industrial Applications, 2nd ed.; Samuel, C.O.U., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 89–133. [Google Scholar]
- Ann, -C.A.; Minna, H. Designed to degrade. Science 2017, 358, 872–873. [Google Scholar]
- Emadian, S.M.; Onay, T.T.; Demirel, B. Biodegradation of bioplastics in natural environments. Waste Manag. 2017, 59, 526–536. [Google Scholar] [CrossRef]
- Du, Y.-L.; Cao, Y.; Lu, F.; Li, F.; Cao, Y.; Wang, X.-L.; Wang, Y.-Z. Biodegradation behaviors of thermoplastic starch (TPS) and thermoplastic dialdehyde starch (TPDAS) under controlled composting conditions. Polym. Test. 2008, 27, 924–930. [Google Scholar] [CrossRef]
- Kalita, N.K.; Nagar, M.K.; Mudenur, C.; Kalamdhad, A.; Katiyar, V. Biodegradation of modified Poly (lactic acid) based biocomposite films under thermophilic composting conditions. Polym. Test. 2019, 76, 522–536. [Google Scholar] [CrossRef]
- Eubeler, J.P.; Bernhard, M.; Knepper, T.P. Environmental biodegradation of synthetic polymers II. Biodegradation of different polymer groups. TrAC Trends Anal. Chem. 2010, 29, 84–100. [Google Scholar] [CrossRef]
- Selke, S.; Auras, R.; Nguyen, T.A.; Aguirre, E.C.; Cheruvathur, R.; Liu, Y. Evaluation of Biodegradation-Promoting Additives for Plastics. Environ. Sci. Technol. 2015, 49, 3769–3777. [Google Scholar] [CrossRef]
- Shruti, V.C.; Gurusamy, K.-M. Bioplastics: Missing link in the era of Microplastics. Sci. Total Environ. 2019, 697, 134139. [Google Scholar] [CrossRef]
- Weithmann, N.; Möller, J.N.; Löder, M.G.J.; Piehl, S.; Laforsch, C.; Freitag, R. Organic fertilizer as a vehicle for the entry of microplastic into the environment. Sci. Adv. 2018, 4, eaap8060. [Google Scholar] [CrossRef]
- Markowicz, F.; Król, G.; Szymańska-Pulikowska, A. Biodegradable Package – Innovative Purpose or Source of the Problem. J. Ecol. Eng. 2019, 20, 228–237. [Google Scholar] [CrossRef]
- Dana, A.; Magdalena, D.V. Biodegradation of Degradable/Biodegradable Plastic Material in Controlled Composting Environment. Pol. J. Environ. Stud. 2014, 23, 1465–1474. [Google Scholar]
- Baranowska, I. (Ed.) Handbook of Trace analysis. Fundamentals and Applications; Springer International Publishing: Cham, Switzerland, 2016; p. 453. [Google Scholar]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Warne, R.W. The Micro and Macro of Nutrients across Biological Scales. Integr. Comp. Boil. 2014, 54, 864–872. [Google Scholar] [CrossRef]
- Treister, R.; Nielsen, C.S.; Stubhaug, A.; Farrar, J.T.; Pud, D.; Sawilowsky, S.; Oaklander, A.L. Experimental comparison of parametric versus nonparametric analyses of data from the cold pressor test. J. Pain 2015, 16, 537–548. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, H.; Wang, M.; Guo, X.; Lei, Y.; Jin, G.; Zhu, A.S.; Chen, B.H.; Wang, C.M.; Guo, D.X.; et al. Plastic solid waste identification system based on near infrared spectroscopy in combination with support vector machine. Adv. Ind. Eng. Polym. Res. 2019, 2, 77–81. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Frias, J.; Nash, R. Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef]
- Act of 10 July 2007 on fertilizers and fertilization. Journal of Laws of the Republic of Poland 2018, item 1259 (with changes) [in Polish]. Available online: http://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20180001259/O/D20181259.pdf (accessed on 26 October 2019).
- Regulation of the Minister of Agriculture and Rural Development of 18 June 2008 regarding the implementation of certain provisions of the Act on fertilizers and fertilization. Journal of Laws of the Republic of Poland 119/2008, item 765 [in Polish]. Available online: http://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20081190765/O/D20080765.pdf (accessed on 26 October 2019).
- Zhao, J.-H.; Wang, X.-Q.; Zeng, J.; Yang, G.; Shi, F.-H.; Yan, Q. Biodegradation of poly(butylene succinate) in compost. J. Appl. Polym. Sci. 2005, 97, 2273–2278. [Google Scholar] [CrossRef]
- Magdalena, V.; Dana, A.; Jana, K.; František, T. Evaluation of biodegradability of plastics bags in composting conditions. Ecol. Chem. Eng. S 2014, 21, 45–57. [Google Scholar]
- Dana, A.; Maja, R.; Jan, Z.; Helena, D.; Jakub, E.; Jindrich, K.; Martin, B.; Magdalena, D.V. SEM Analysis and Degradation Behavior of Conventional and Bio-Based Plastics During Composting. Acta Univ. Agric. Silvic. Mendelianae Brun. 2018, 66, 349–356. [Google Scholar]
- Zafar, U.; Nzeram, P.; Langarica-Fuentes, A.; Houlden, A.; Heyworth, A.; Saiani, A.; Robson, G.D. Biodegradation of polyester polyurethane during commercial composting and analysis of associated fungal communities. Bioresour. Technol. 2014, 158, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Coley, I.; Mendez-Cuadro, D.; Rodriguez-Cavallo, E.; De La Rosa, J.; Olivero-Verbel, J. Trace elements in microplastics in Cartagena: A hotspot for plastic pollution at the Caribbean. Mar. Pollut. Bull. 2019, 139, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Carlos, J.; Judith, S.; Isabel, C.; Angel, F. Study of the Biodisintegration on a Painted Bioplastic Material Waste. Mater. Plast. 2015, 52, 116–121. [Google Scholar]
- Unmar, G.; Mohee, R. Assessing the effect of biodegradable and degradable plastics on the composting of green wastes and compost quality. Bioresour. Technol. 2008, 99, 6738–6744. [Google Scholar] [CrossRef]
- Dilkes-Hoffman, L.; Pratt, S.; Lant, P.; Laycock, B. The Role of Biodegradable Plastic in Solving Plastic Solid Waste Accumulation. In Plastics to Energy; Al-Salem, S.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 469–505. [Google Scholar]
- Brennecke, D.; Duarte, B.; Paiva, F.; Caçador, I.; Clode, J.C. Microplastics as vector for heavy metal contamination from the marine environment. Estuarine, Coast. Shelf Sci. 2016, 178, 189–195. [Google Scholar] [CrossRef]
- Laskar, N.; Kumar, U. Plastics and microplastics: A threat to environment. Environ. Technol. Innov. 2019, 14, 100352. [Google Scholar] [CrossRef]
- Gallo, F.; Fossi, C.; Weber, R.; Santillo, D.; Sousa, J.; Ingram, I.; Nadal, A.; Romano, D. Marine litter plastics and microplastics and their toxic chemicals components: The need for urgent preventive measures. Environ. Sci. Eur. 2018, 30, 13. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).