Contrasting Textural and Chemical Signatures of Chromitites in the Mesoarchaean Ulamertoq Peridotite Body, Southern West Greenland
Abstract
:1. Introduction
2. Geological Setting
3. Data and Results
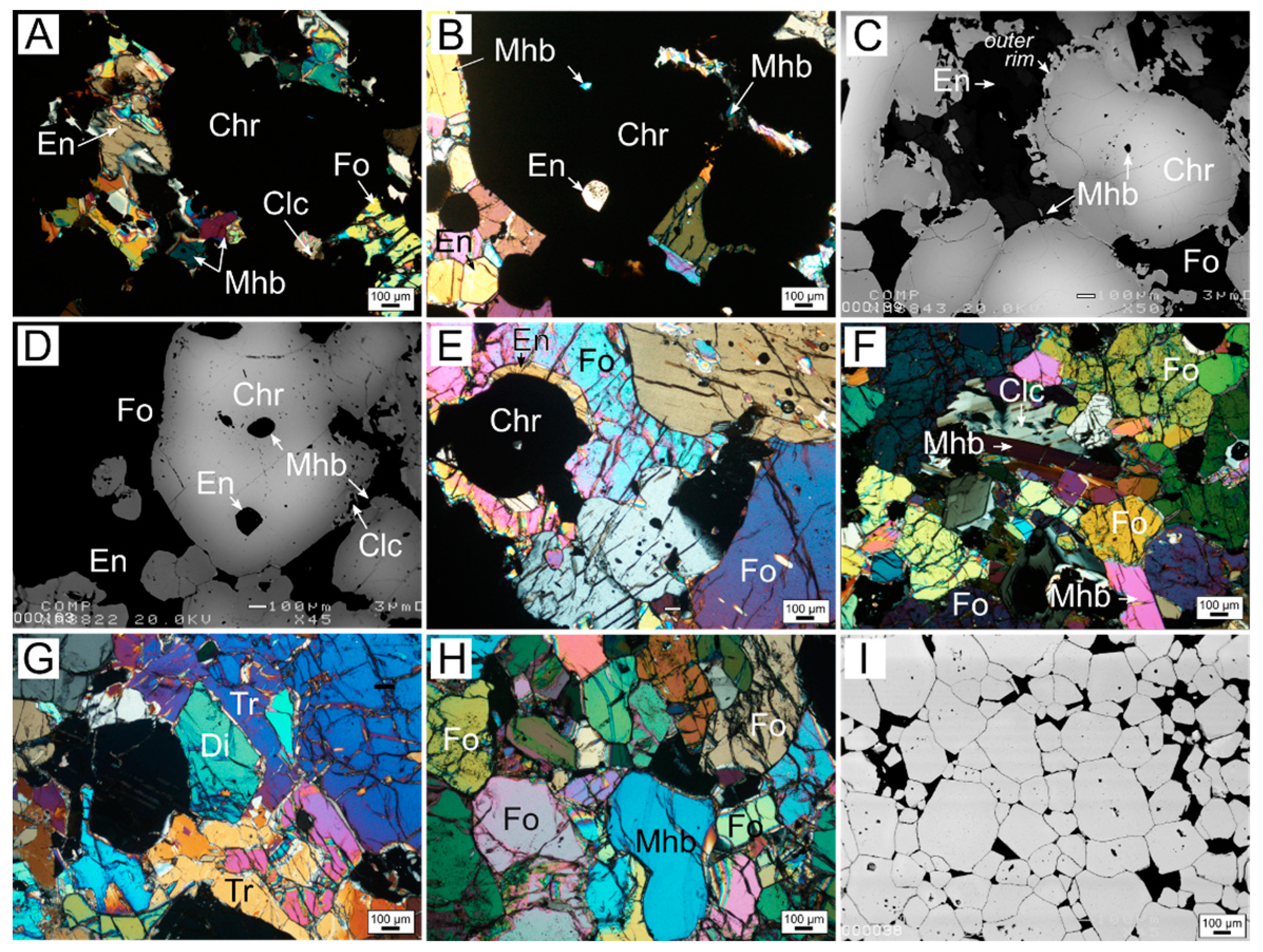
3.1. Petrography
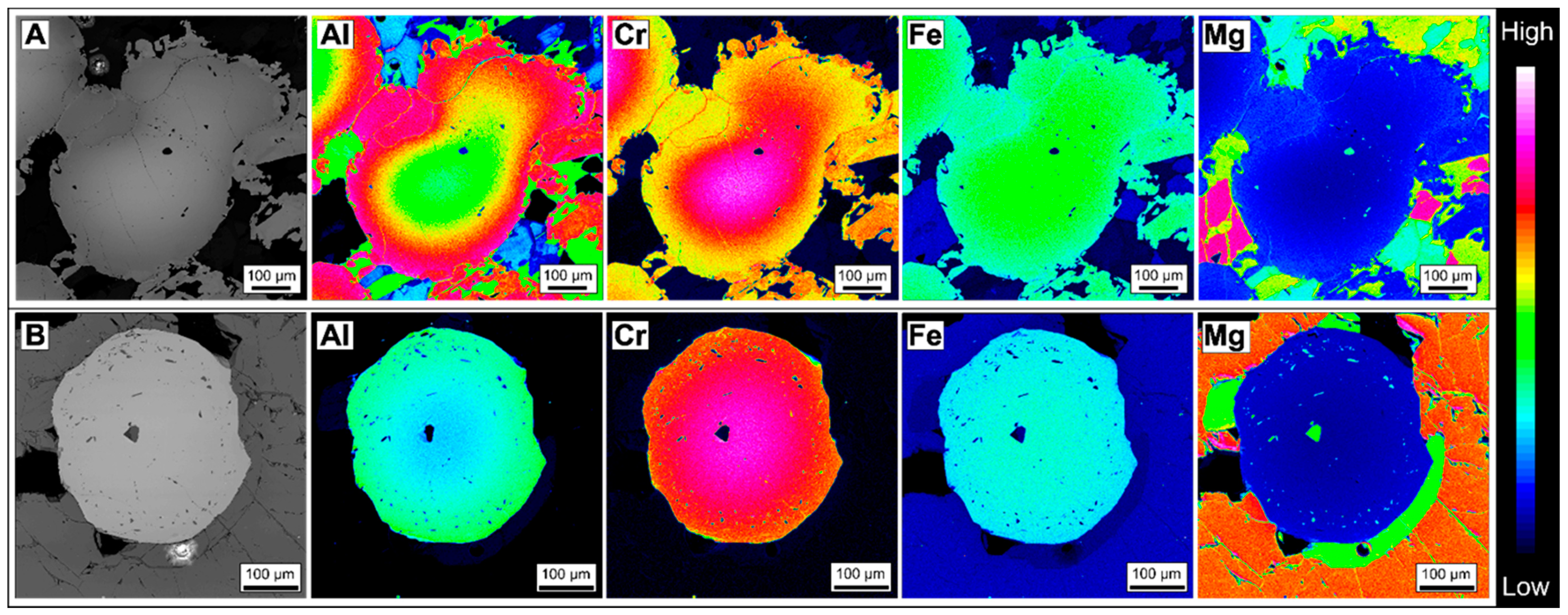
3.1.1. Zoned Chromite
3.1.2. Homogeneous Chromites
4. Mineral Chemistry
4.1. Analytical Methods
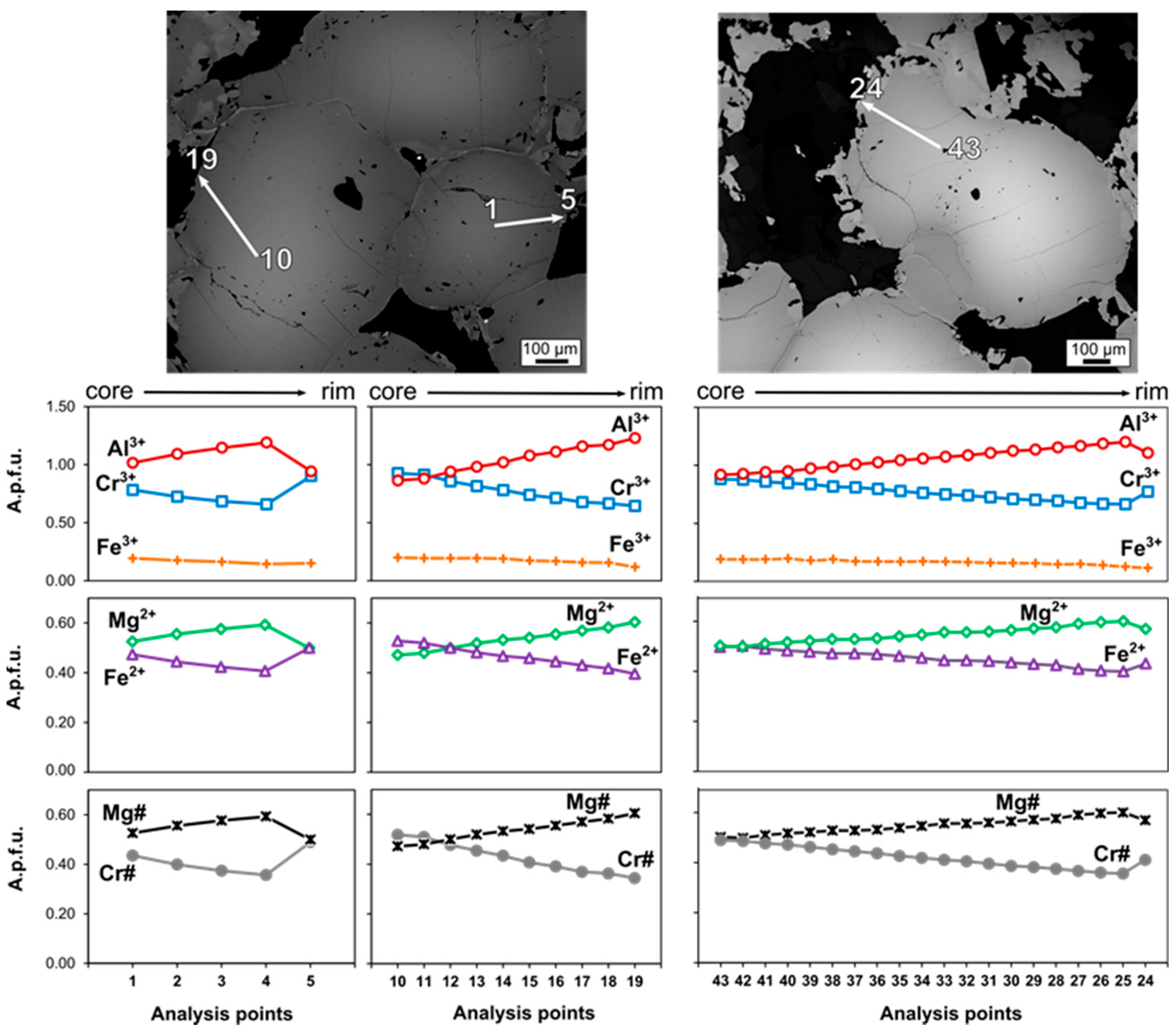
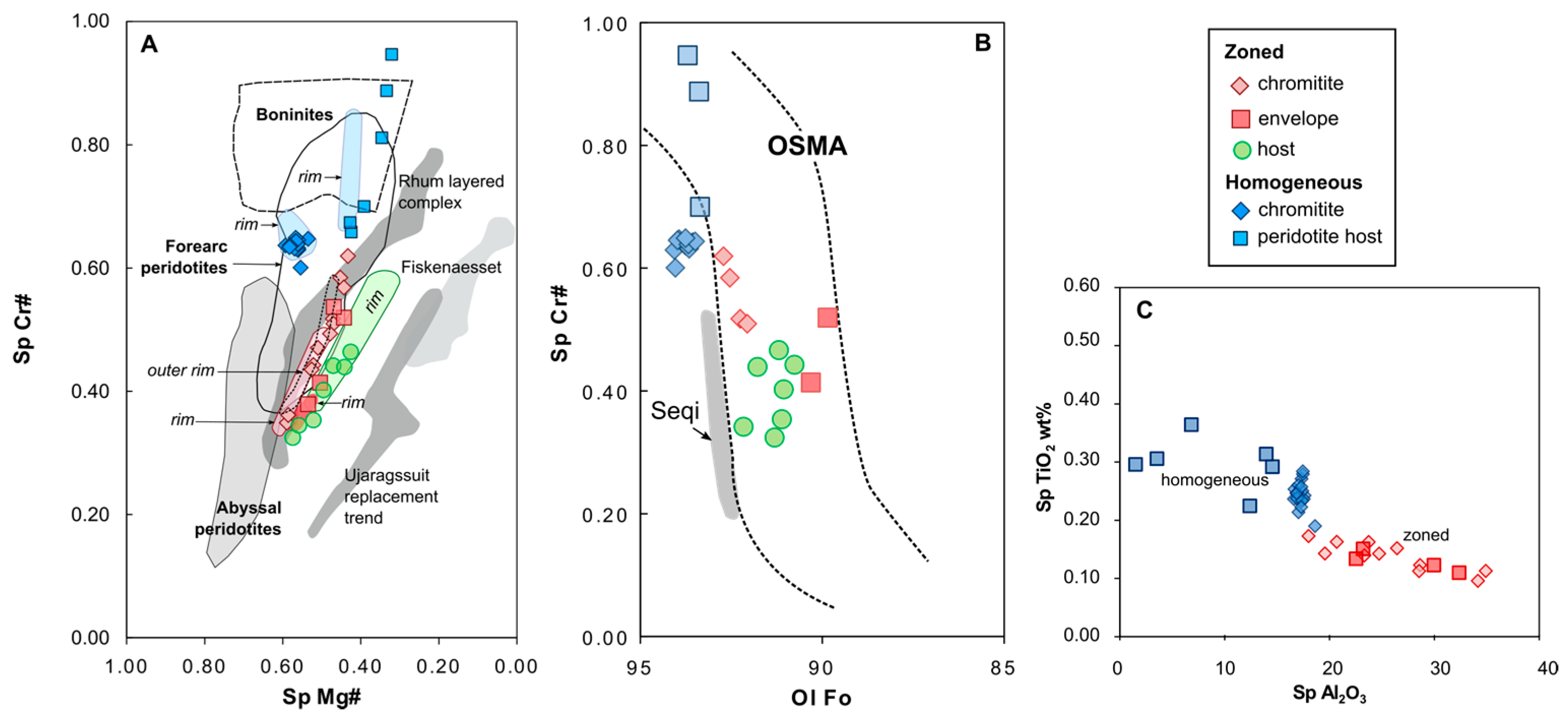
4.2. Chromite
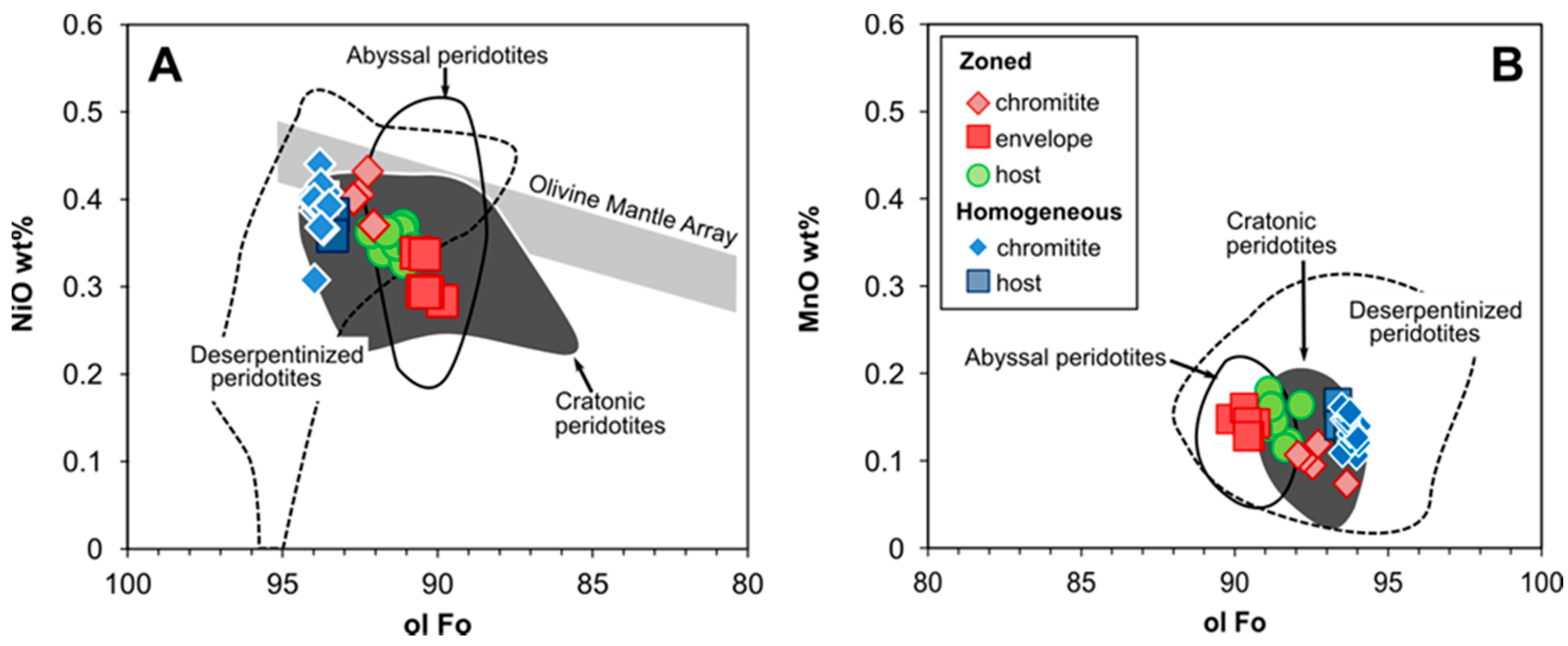
4.3. Olivine
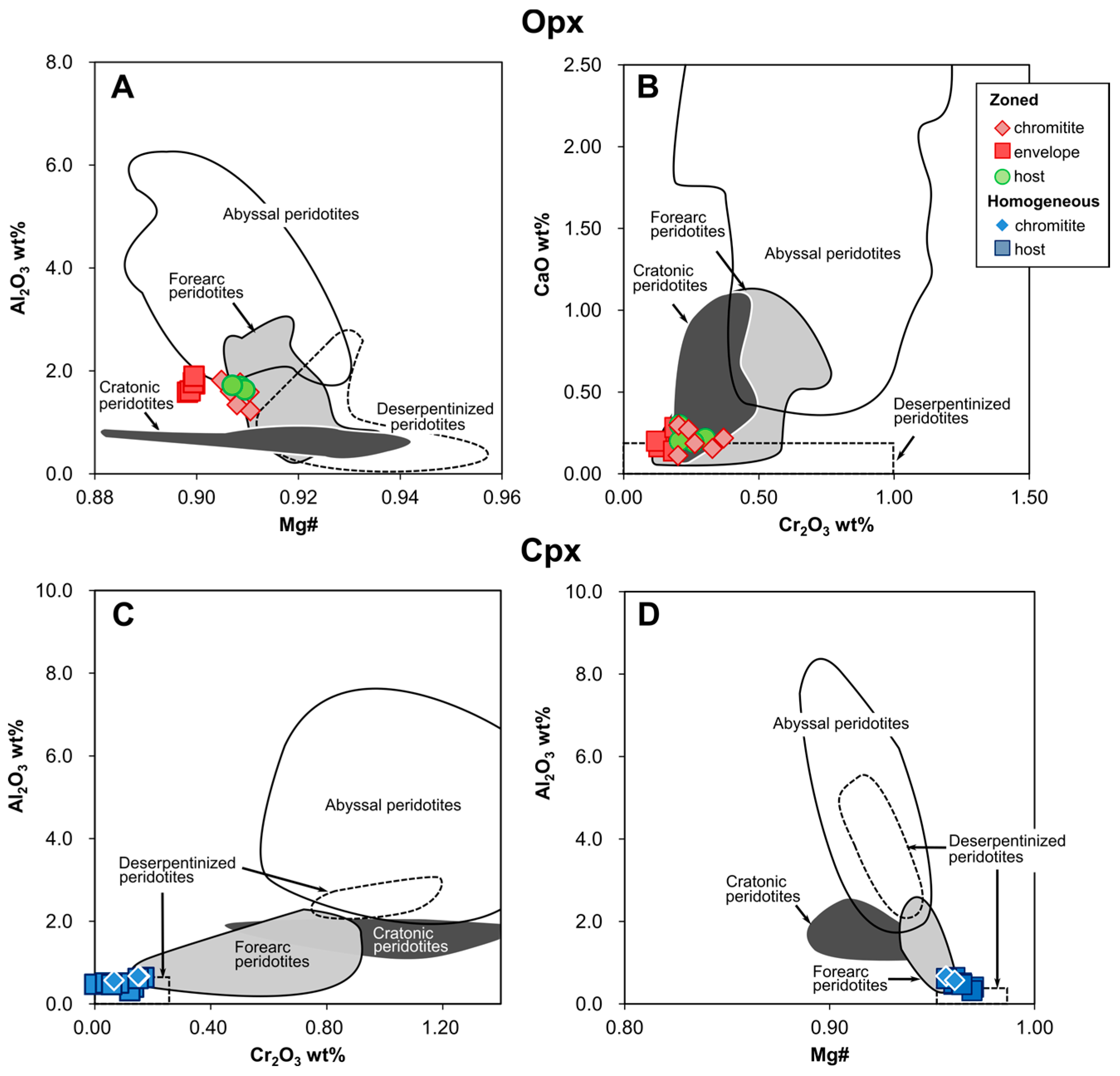
4.4. Pyroxenes
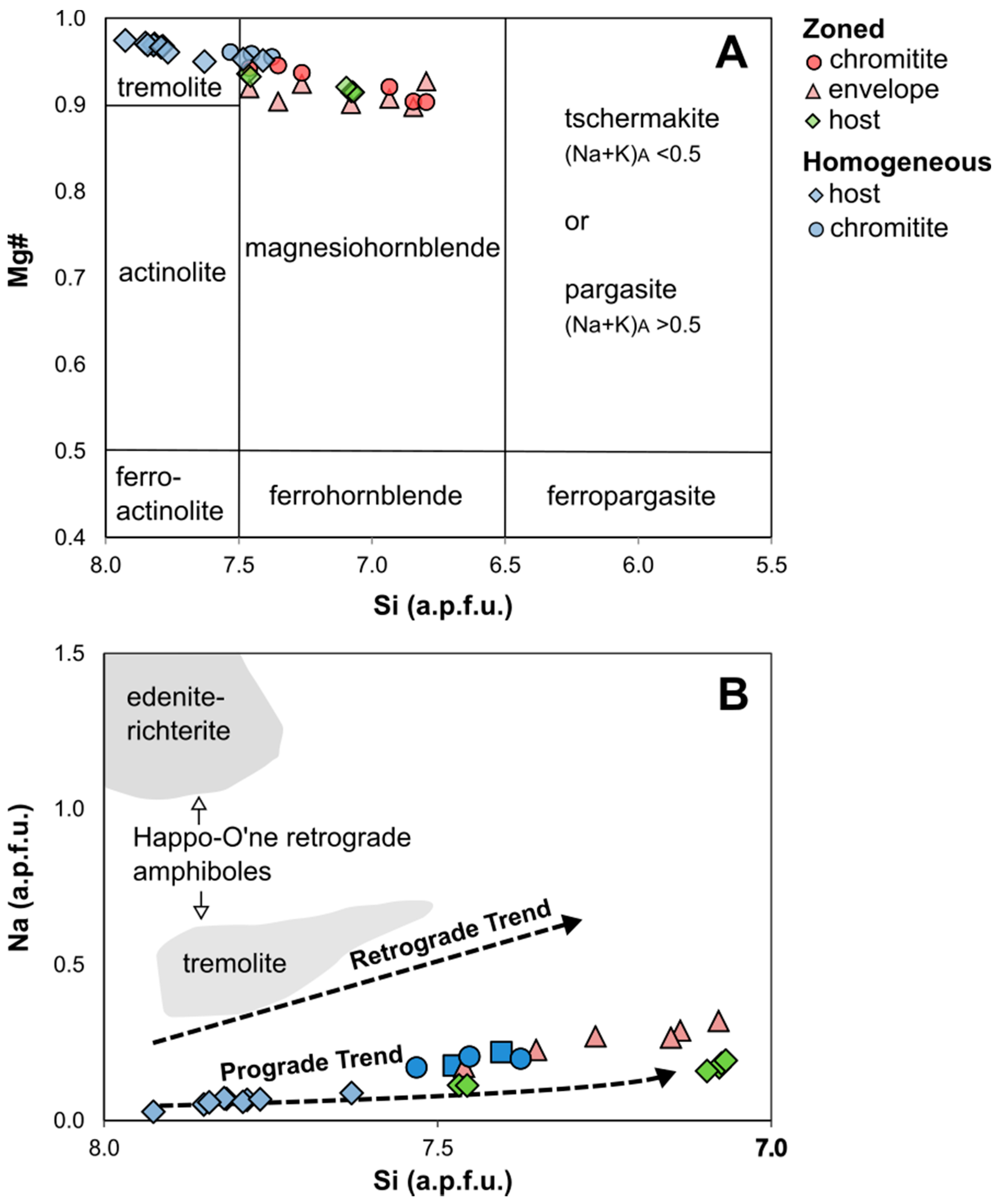
4.5. Amphibole
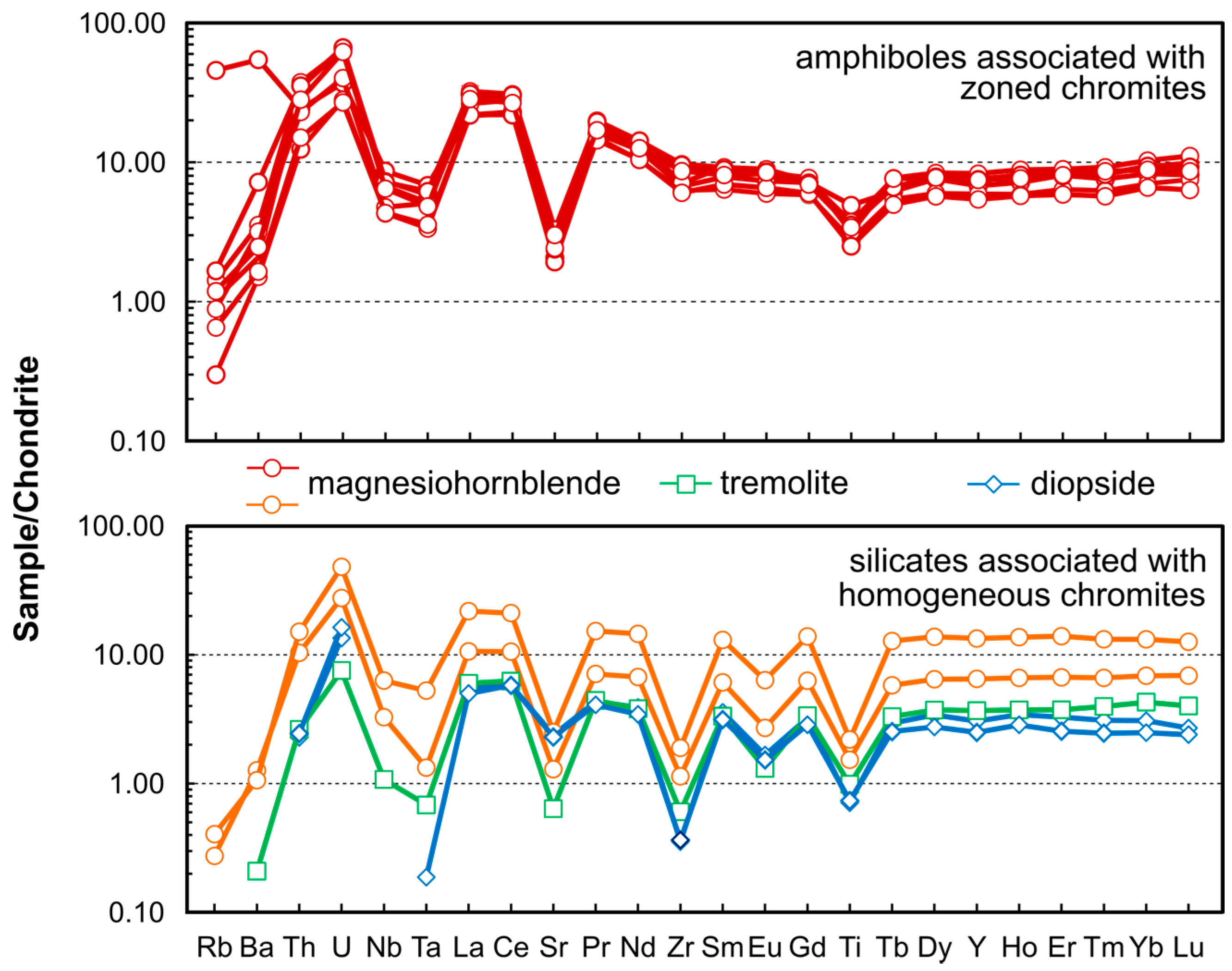
4.6. Trace Element Geochemistry of Amphiboles and Clinopyroxenes
5. Discussion
5.1. Original Composition of Zoned Chromites
5.1.1. Chromite Composition Variation
5.1.2. Metamorphism vs. Metasomatism
5.1.3. Metamorphism and Pressure-Temperature Path
5.1.4. Ulamertoq Peridotites: Mantle Residue or Cumulate?
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Szilas, K.; Kelemen, P.B.; Bernstein, S. Peridotite enclaves hosted by Mesoarchaean TTG-suite orthogneisses in the Fiskefjord region of Southern West Greenland. GeoResJ 2015, 7, 22–34. [Google Scholar] [CrossRef] [Green Version]
- Irvine, T.N. Chromian spinel as a petrogenetic indicator: Part 2. Petrologic applications. Can. J. Earth Sci. 1967, 4, 71–103. [Google Scholar] [CrossRef]
- Dick, H.J.B.; Bullen, T. Chromian spinel as a petrogenetic indicator in abyssal and alpine-type peridotites and spatially associated lavas. Contrib. Mineral. Petrol. 1984, 86, 54–76. [Google Scholar] [CrossRef]
- Arai, S. Characterization of spinel peridotites by olivine-spinel compositional relationships: Review and interpretation. Chem. Geol. 1994, 113, 191–204. [Google Scholar] [CrossRef]
- Barnes, S.J.; Roeder, P.L. The range of spinel compositions in terrestrial mafic and ultramafic rocks. J. Petrol. 2001, 42, 2279–2302. [Google Scholar] [CrossRef]
- Kamenestky, V. Factors Controlling Chemistry of Magmatic Spinel: An Empirical Study of Associated Olivine, Cr-spinel and Melt Inclusions from Primitive Rocks. J. Petrol. 2001, 42, 655–671. [Google Scholar] [CrossRef]
- Henderson, P.; Wood, R.J. Reaction relationships of chrome-spinels in igneous rocks—further evidence from the layered intrusions of Rhum and Mull, Inner Hebrides, Scotland. Contrib. Mineral. Petrol. 1982, 78, 225–229. [Google Scholar] [CrossRef]
- Roeder, P.L.; Campbell, I.H. The effect of postcumulus reactions on composition of chrome-spinels from the jimberlana intrusion. J. Petrol. 1985, 26, 763–786. [Google Scholar] [CrossRef]
- Evans, B.W.; Frost, B.R. Chrome-spinel in progressive metamorphism—A preliminary analysis. Geochim. Cosmochim. Acta 1975, 39, 959–972. [Google Scholar] [CrossRef]
- Proenza, J.A.; Ortega-Gutiérrez, F.; Camprubí, A.; Tritlla, J.; Elías-Herrera, M.; Reyes-Salas, M. Paleozoic serpentinite-enclosed chromitites from Tehuitzingo (Acatlán Complex, southern Mexico): A petrological and mineralogical study. J. South Am. Earth Sci. 2004, 16, 649–666. [Google Scholar] [CrossRef]
- Mellini, M.; Rumori, C.; Viti, C. Hydrothermally reset magmatic spinels in retrograde serpentinites: Formation of “ferritchromit” rims and chlorite aureoles. Contrib. Mineral. Petrol. 2005, 149, 266–275. [Google Scholar] [CrossRef]
- Merlini, A.; Grieco, G.; Diella, V. Ferritchromite and chromian-chlorite formation in mélange-hosted Kalkan chromitite (Southern Urals, Russia). Am. Mineral. 2009, 94, 1459–1467. [Google Scholar] [CrossRef]
- Gervilla, F.; Padrón-Navarta, J.A.; Kerestedjian, T.; Sergeeva, I.; González-Jiménez, J.M.; Fanlo, I. Formation of ferrian chromite in podiform chromitites from the Golyamo Kamenyane serpentinite, Eastern Rhodopes, SE Bulgaria: A two-stage process. Contrib. Mineral. Petrol. 2012, 164, 643–657. [Google Scholar] [CrossRef]
- Loomis, T.P. Reaction of zoning of garnet. Contrib. Mineral. Petrol. 1975, 52, 285–305. [Google Scholar] [CrossRef]
- Tracy, R.J. Compositional zoning and inclusions in metamorphic minerals. Rev. Mineral. Geochem. 1982, 10, 355–397. [Google Scholar]
- Ikeda, T. Compositional zoning patterns of garnet during prograde metamorphism from the Yanai district, Ryoke metamorphic belt, Southwest Japan. Lithos 1993, 30, 109–121. [Google Scholar] [CrossRef]
- Enami, M. Pressure-temperature path of Sanbagawa prograde metamorphism deduced from grossular zoning of garnet. J. Metamorph. Geol. 1998, 16, 97–106. [Google Scholar] [CrossRef]
- Friend, C.R.L.; Nutman, A.P. New pieces to the Archaean terrane jigsaw puzzle in the Nuuk region, Southern West Greenland: Steps in transforming a simple insight into a complex regional tectonothermal model. J. Geol. Soc. Lond. 2005, 162, 147–162. [Google Scholar] [CrossRef]
- Mcgregor, V.R.; Friend, C.R.L.; Nutman, A.P. The late Archaean mobile belt through Godthåbsfjord, Southern West Greenland: a continent-continent collision zone? Bull. Geol. Soc. Denmark 1991, 39, 179–197. [Google Scholar]
- Komiya, T.; Maruyama, S.; Masuda, T.; Nohda, S.; Hayashi, M.; Okamoto, K. Plate Tectonics at 3.8–3.7 Ga: Field Evidence from the Isua Accretionary Complex, Southern West Greenland. J. Geol. 1999, 107, 515–554. [Google Scholar] [CrossRef] [PubMed]
- Garde, A.A. Accretion and Evolution of an Archaean High-Grade Grey Gneiss-Amphibolite Complex: The Fiskefjord Area, Southern West Greenland; Geological Survey of Denmark and Greenland, Ministry of Environment and Energy: Copenhagen, Denmark, 1997. [Google Scholar]
- Szilas, K.; van Hinsberg, V.; McDonald, I.; Næraa, T.; Rollinson, H.; Adetunji, J.; Bird, D. Highly refractory Archaean peridotite cumulates: Petrology and geochemistry of the Seqi Ultramafic Complex, SW Greenland. Geosci. Front. 2018, 9, 689–714. [Google Scholar] [CrossRef]
- Sinton, J.M. Equilibration History of the Basel Alpine-Type Peridotite, Red Mountain, New Zealand. J. Petrol. 1977, 18, 216–246. [Google Scholar] [CrossRef]
- Maurel, C.; Maurel, P. Étude expérimentale de la distribution de l’aluminium entre bain silicaté basique et spinelle chromifère. Implications pétrogénétiques: Teneur en chrome des spinelles. Bull. Minéral 1982, 105, 197–202. [Google Scholar]
- Pearce, N.J.G.; Perkins, W.T.; Westgate, J.A.; Gorton, M.P.; Jackson, S.E.; Neal, C.R.; Chenery, S.P. A compilation of new and published major and trace element data for NIST SRM 610 and NIST SRM 612 glass reference materials. Geostand. Geoanal. Res. 1997, 21, 115–144. [Google Scholar] [CrossRef]
- Longerich, H.P.; Jackson, S.E.; Günther, D. Inter-laboratory note. Laser ablation inductively coupled plasma mass spectrometric transient signal data acquisition and analyte concentration calculation. J. Anal. At. Spectrom. 1996, 11, 899–904. [Google Scholar] [CrossRef]
- Morishita, T.; Arai, S.; Green, D.H. Evolution of Low-Al Orthopyroxene in the Horoman Peridotite, Japan: An Unusual Indicator of Metasomatizing Fluids. J. Petrol. 2003, 44, 1237–1246. [Google Scholar] [CrossRef]
- Morishita, T.; Ishida, Y.; Arai, S.; Shirasaka, M. Determination of Multiple Trace Element Compositions in Thin (<30 um) Layers of NIST SRM 614 and 616 Using Laser Ablation-Inductively Coupled Plasma-Mass Spectrometry (LA-ICP-MS). Geostand. Geoanal. Res. 2005, 29, 107–122. [Google Scholar] [CrossRef]
- Kelemen, P.B.; Hart, S.R.; Bernstein, S. Silica enrichment in the continental upper mantle via melt/rock reaction. Earth Planet. Sci. Lett. 1998, 164, 387–406. [Google Scholar] [CrossRef] [Green Version]
- Larsen, J.G. Mantle-derived dunite and lherzolite nodules from Ubekendt Ejland, West Greenland Tertiary province. Mineral. Mag. 1982, 46, 329–336. [Google Scholar] [CrossRef]
- Boyd, F.R.; Pokhilenko, N.P.; Pearson, D.G.; Mertzman, S.A.; Sobolev, N.V.; Finger, L.W. Composition of the Siberian cratonic mantle: evidence from Udachnaya peridotite xenoliths. Contrib. Mineral. Petrol. 1997, 128, 228–246. [Google Scholar] [CrossRef]
- Bernstein, S.; Kelemen, P.B.; Brooks, C.K. Depleted spinel harzburgite xenoliths in Tertiary dykes from East Greenland: Restites from high degree melting. Earth Planet. Sci. Lett. 1998, 154, 221–235. [Google Scholar] [CrossRef]
- Takahashi, E.; Uto, K.; Schilling, J.G. Primary Magma compositions and Mg/Fe ratios of their mantle residues along mid-atlantic ridge 29° N to 73° N. Tech. Rep. ISEI Okayama Univ. 1987, Ser. A, 1–14. [Google Scholar]
- Warren, J.M. Global variations in abyssal peridotite compositions. Lithos 2016, 248–251, 193–219. [Google Scholar] [CrossRef]
- Khedr, M.Z.; Arai, S. Petrology and geochemistry of prograde deserpentinized peridotites from Happo-O’ne, Japan: Evidence of element mobility during deserpentinization. J. Asian Earth Sci. 2012, 43, 150–163. [Google Scholar] [CrossRef]
- Arai, S. Contact metamorphosed dunite-harzburgite complex in the Chugoku district, Western Japan. Contrib. Mineral. Petrol. 1975, 52, 1–16. [Google Scholar] [CrossRef]
- Nozaka, T.; Shibata, T. Mineral paragenesis in thermally metamorphosed serpentinites, Ohsa-yama, Okayama Prefecture. Okayama Univ. Earth Sci. Rep. 1995, 2, 1–11. [Google Scholar]
- Sand, K.K.; Waight, T.E.; Pearson, D.G.; Nielsen, T.F.D.; Makovicky, E.; Hutchison, M.T. The lithospheric mantle below southern West Greenland: A geothermobarometric approach to diamond potential and mantle stratigraphy. Lithos 2009, 112, 1155–1166. [Google Scholar] [CrossRef]
- Leake, B.E.; Woolley, A.R.; Arps, C.E.S.; Birch, W.D.; Gilbert, M.C.; Grice, J.D.; Hawthorne, Ec.; Kato, A.; Kisch, H.J.; Krivovichev, V.G.; et al. Nomenclature of amphiboles: report of the Subcommittee on Amphiboles of the International Mineralogical Association, Commission on New Minerals and Mineral Names. Eur. J. Mineral. 1997, 9, 623–651. [Google Scholar] [CrossRef]
- Nozaka, T. Metamorphic history of serpentinite mylonites from the Happo ultramafic complex, Central Japan. J. Metamorph. Geol. 2005, 23, 711–723. [Google Scholar] [CrossRef]
- Sun, S.-S.; McDonough, W.F. Chemical and isotopic systematics of oceanic basalts: Implications for mantle composition and processes. Geol. Soc. Lond. Spec. Publ. 1989, 42, 313–345. [Google Scholar] [CrossRef]
- Bai, Y.; Su, B.-X.; Xiao, Y.; Lenaz, D.; Asamoah Sakyi, P.; Liang, Z.; Chen, C.; Yang, S.-H. Origin of Reverse Zoned Cr-Spinels from the Paleoproterozoic Yanmenguan Mafic-Ultramafic Complex in the North China Craton. Minerals 2018, 8, 62. [Google Scholar] [CrossRef]
- Purvis, A.C.; Nesbitt, R.W.; Hallberg, J.A. The Geology of Part of the Carr Boyd Rocks Complex and Its Associated Nickel Mineralization, Western Australia. Econ. Geol. 1972, 67, 1093–1113. [Google Scholar] [CrossRef]
- De Freitas Suita, M.T.; Strieder, A.J. Cr-Spinels from Brazilian Mafic-Ultramafic Complexes: Metamorphic Modifications. Int. Geol. Rev. 1996, 38, 245–267. [Google Scholar] [CrossRef]
- Hamlyn, P.R.; Keays, R.R. Origin of chromite compositional variation in the Panton Sill, Western Australia. Contrib. Mineral. Petrol. 1979, 69, 75–82. [Google Scholar] [CrossRef]
- Henderson, P.; Suddaby, P. The nature and origin of the chrome-spinel of the Rhum layered intrusion. Contrib. Mineral. Petrol. 1971, 33, 21–31. [Google Scholar] [CrossRef]
- Henderson, P. Reaction trends shown by chrome-spinels of the Rhum layered intrusion. Geochim. Cosmochim. Acta 1975, 39, 1035–1044. [Google Scholar] [CrossRef]
- Cameron, E.N. Postcumulus and subsolidus equilibration of chomite and coexisting silicates in the Eastern Bushveld Complex. Geochim. Cosmochim. Acta 1975, 39, 1021–1033. [Google Scholar] [CrossRef]
- Hulbert, L.J.; Von Gruenewaldt, G. Textural and compositional features of chromite in the lower and critical zones of the Bushveld Complex south of Potgietersrus. Econ. Geol. 1985, 80, 872–895. [Google Scholar] [CrossRef]
- Merinero, R.; Lunar, R.; Ortega, L.; Piña, R.; Monterrubio, S.; Gervilla, F. Zoned chromite records multiple metamorphic episodes in the Calzadilla de los Barros ultramafic bodies (SW Iberian peninsula). Eur. J. Mineral. 2014, 26, 757–770. [Google Scholar] [CrossRef]
- Roeder, P.L. Chromite; from the fiery rain of chondrules to the Kilauea Iki lava lake. Can. Mineral. 1994, 32, 729–746. [Google Scholar]
- Abzalov, M.Z. Chrome–spinels in gabbro–wehrlite intrusions of the Pechenga area, Kola Peninsula, Russia: Emphasis on alteration features. Lithos 1998, 43, 109–134. [Google Scholar] [CrossRef]
- Barnes, S.J. Chromite in komatiites, II. Modification during greenschist to mid-amphibolite facies metamorphism. J. Petrol. 2000, 41, 387–409. [Google Scholar] [CrossRef]
- Nozaka, T. Compositional heterogeneity of olivine in thermally metamorphosed serpentinite from Southwest Japan. Am. Mineral. 2003, 88, 1377–1384. [Google Scholar] [CrossRef]
- Nozaka, T. A note on compositional variation of olivine and pyroxene in thermally metamorphosed ultramafic complexes from SW Japan. Earth Sci. Rep. Okayama Univ. 2010, 17, 1–5. [Google Scholar]
- Arai, S.; Kida, M. Origin of fine-grained peridotite xenoliths from Iraya volcano of Batan Island, Philippines: Deserpentinization or metasomatism at the wedge mantle beneath an incipient arc? Isl. Arc 2000, 9, 458–471. [Google Scholar] [CrossRef]
- Nakamura, Y.; Kushiro, I. Composition of the gas phase in Mg2SiO4SiO2–H2O at high pressures. In Washington Yearbook; Carnegie Institution of Washington: Washington, DC, USA, 1974; Volume 73, pp. 266–268. [Google Scholar]
- Spear, F.S. Metamorphic Phase Equilibria and Pressure-Temperature-Time Paths; Mineralogical Society of America: Washington, DC, USA, 1993. [Google Scholar]
- Arai, S.; Ishimaru, S. Insights into petrological characteristics of the lithosphere of mantle wedge beneath arcs through peridotite xenoliths: A review. J. Petrol. 2008, 49, 665–695. [Google Scholar] [CrossRef]
- Payot, B.D.; Arai, S.; Tamayo, R.A.; Yumul, G.P. What underlies the Philippine island arc? Clues from the Calaton Hill, Tablas island, Romblon (Central Philippines). J. Asian Earth Sci. 2009, 36, 371–389. [Google Scholar] [CrossRef]
- Green, D.H.; Ringwood, A.E. The genesis of basaltic magmas. Contrib. Mineral. Petrol. 1967, 15, 103–190. [Google Scholar] [CrossRef]
- Presnall, D.C.; Dixon, S.A.; Dixon, J.R.; O’donnell, T.H.; Brenner, N.L.; Schrock, R.L.; Dycus, D.W. Liquidus phase relations on the join diopside-forsterite-anorthite from 1 atm to 20 kbar: Their bearing on the generation and crystallization of basaltic magma. Contrib. Mineral. Petrol. 1978, 66, 203–220. [Google Scholar] [CrossRef]
- Borghini, G.; Fumagalli, P.; Rampone, E. The geobarometric significance of plagioclase in mantle peridotites: A link between nature and experiments. Lithos 2011, 126, 42–53. [Google Scholar] [CrossRef]
- Milholland, C.S.; Presnall, D.C. Liquidus phase relations in the CaO-MgO-Al2O3-SiO2 system at 3.0 GPa: The aluminous pyroxene thermal divide and high-pressure fractionation of picritic and komatiitic magmas. J. Petrol. 1998, 39, 3–27. [Google Scholar] [CrossRef]
- Klemme, S.; O’Neill, H.S. The near-solidus transition from garnet lherzolite to spinel lherzolite. Contrib. Mineral. Petrol. 2000, 138, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Berman, R.G.; Engi, M.; Greenwood, H.J.; Brown, T.H. Derivation of Internally-Consistent Thermodynamic Data by the Technique of Mathematical Programming: A Review with Application the System MgO-SiO2-H2O. J. Petrol. 1986, 27, 1331–1364. [Google Scholar] [CrossRef]
- Bucher, K.; Frey, M. Definition, Conditions and Types of Metamorphism. In Petrogenesis of Metamorphic Rocks; Springer: Berlin/Heidelberg, Germany, 2002; pp. 3–15. [Google Scholar]
- Pearson, D.G.; Canil, D.; Shirey, S.B. Mantle samples included in volcanic rocks: Xenoliths and diamonds. Treatise Geochem. 2003, 2, 171–275. [Google Scholar]
- Wittig, N.; Pearson, D.G.; Webb, M.; Ottley, C.J.; Irvine, G.J.; Kopylova, M.; Jensen, S.M.; Nowell, G.M. Origin of cratonic lithospheric mantle roots: A geochemical study of peridotites from the North Atlantic Craton, West Greenland. Earth Planet. Sci. Lett. 2008, 274, 24–33. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guotana, J.M.; Morishita, T.; Yamaguchi, R.; Nishio, I.; Tamura, A.; Tani, K.; Harigane, Y.; Szilas, K.; Pearson, D.G. Contrasting Textural and Chemical Signatures of Chromitites in the Mesoarchaean Ulamertoq Peridotite Body, Southern West Greenland. Geosciences 2018, 8, 328. https://doi.org/10.3390/geosciences8090328
Guotana JM, Morishita T, Yamaguchi R, Nishio I, Tamura A, Tani K, Harigane Y, Szilas K, Pearson DG. Contrasting Textural and Chemical Signatures of Chromitites in the Mesoarchaean Ulamertoq Peridotite Body, Southern West Greenland. Geosciences. 2018; 8(9):328. https://doi.org/10.3390/geosciences8090328
Chicago/Turabian StyleGuotana, Juan Miguel, Tomoaki Morishita, Ryoko Yamaguchi, Ikuya Nishio, Akihiro Tamura, Kenichiro Tani, Yumiko Harigane, Kristoffer Szilas, and D. Graham Pearson. 2018. "Contrasting Textural and Chemical Signatures of Chromitites in the Mesoarchaean Ulamertoq Peridotite Body, Southern West Greenland" Geosciences 8, no. 9: 328. https://doi.org/10.3390/geosciences8090328
APA StyleGuotana, J. M., Morishita, T., Yamaguchi, R., Nishio, I., Tamura, A., Tani, K., Harigane, Y., Szilas, K., & Pearson, D. G. (2018). Contrasting Textural and Chemical Signatures of Chromitites in the Mesoarchaean Ulamertoq Peridotite Body, Southern West Greenland. Geosciences, 8(9), 328. https://doi.org/10.3390/geosciences8090328





