Removal of Barium, Cobalt, Strontium, and Zinc from Solution by Natural and Synthetic Allophane Adsorbents
Abstract
1. Introduction
2. Materials, Experimental, and Methods
2.1. Materials
2.2. Adsorption Experiments
2.3. Analytical Methods
2.3.1. Fluid-Phase Characterization
2.3.2. Solid-Phase Characterization
3. Results and Discussion
3.1. Mineralogical Characterization of Allophane Adsorbents
3.2. Structural and Chemical Characterization of Allophane Adsorbents
3.3. Structural and Chemical Characterization of Allophane Adsorbents
3.3.1. Aqueous Speciation of Metal Ions Used as Adsorbates
3.3.2. Effect of Contact Time on the Adsorption Kinetics of Aqueous Metal Ions
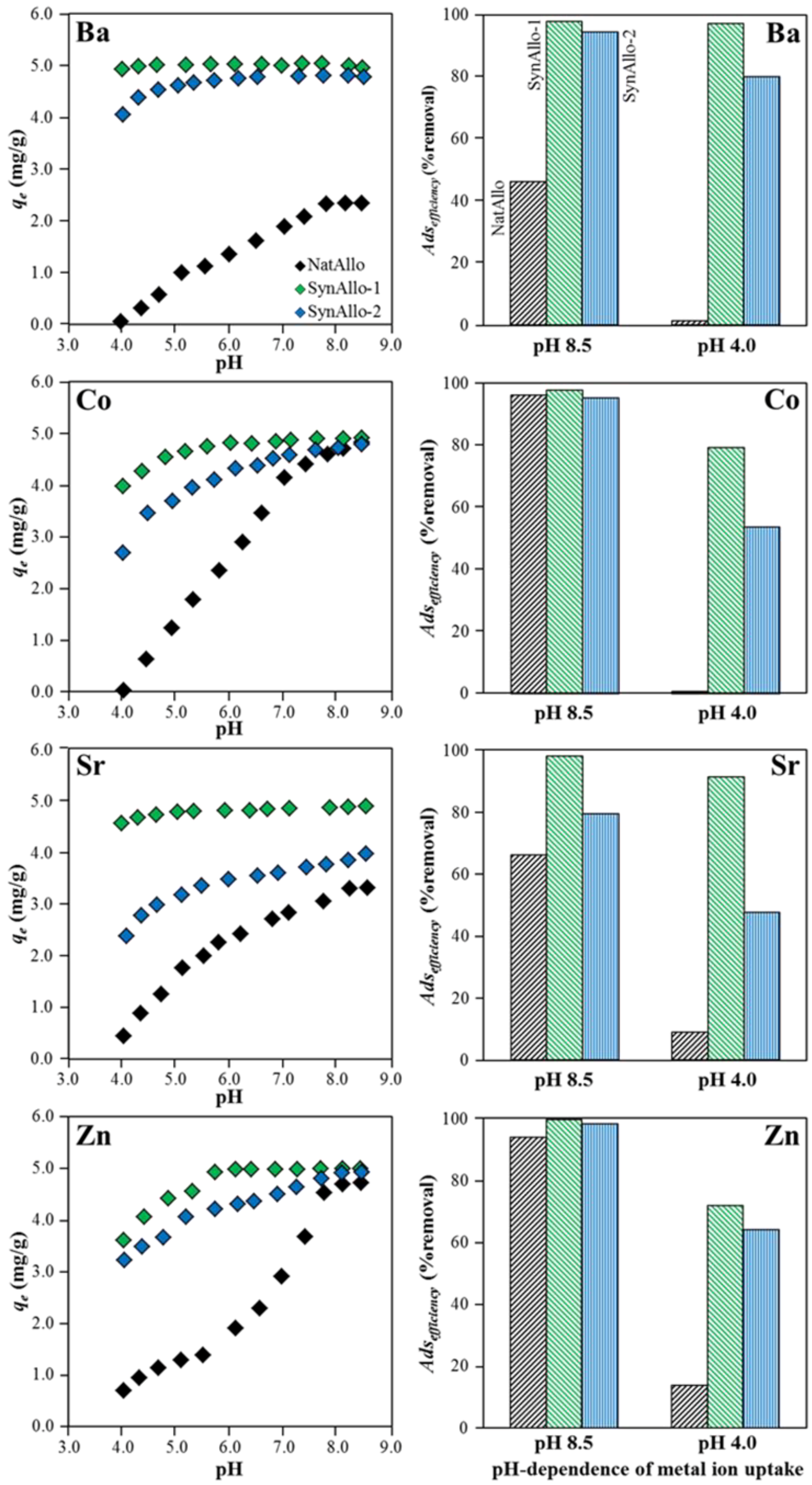
3.3.3. Effect of pH on Metal Ion Removal Efficiency
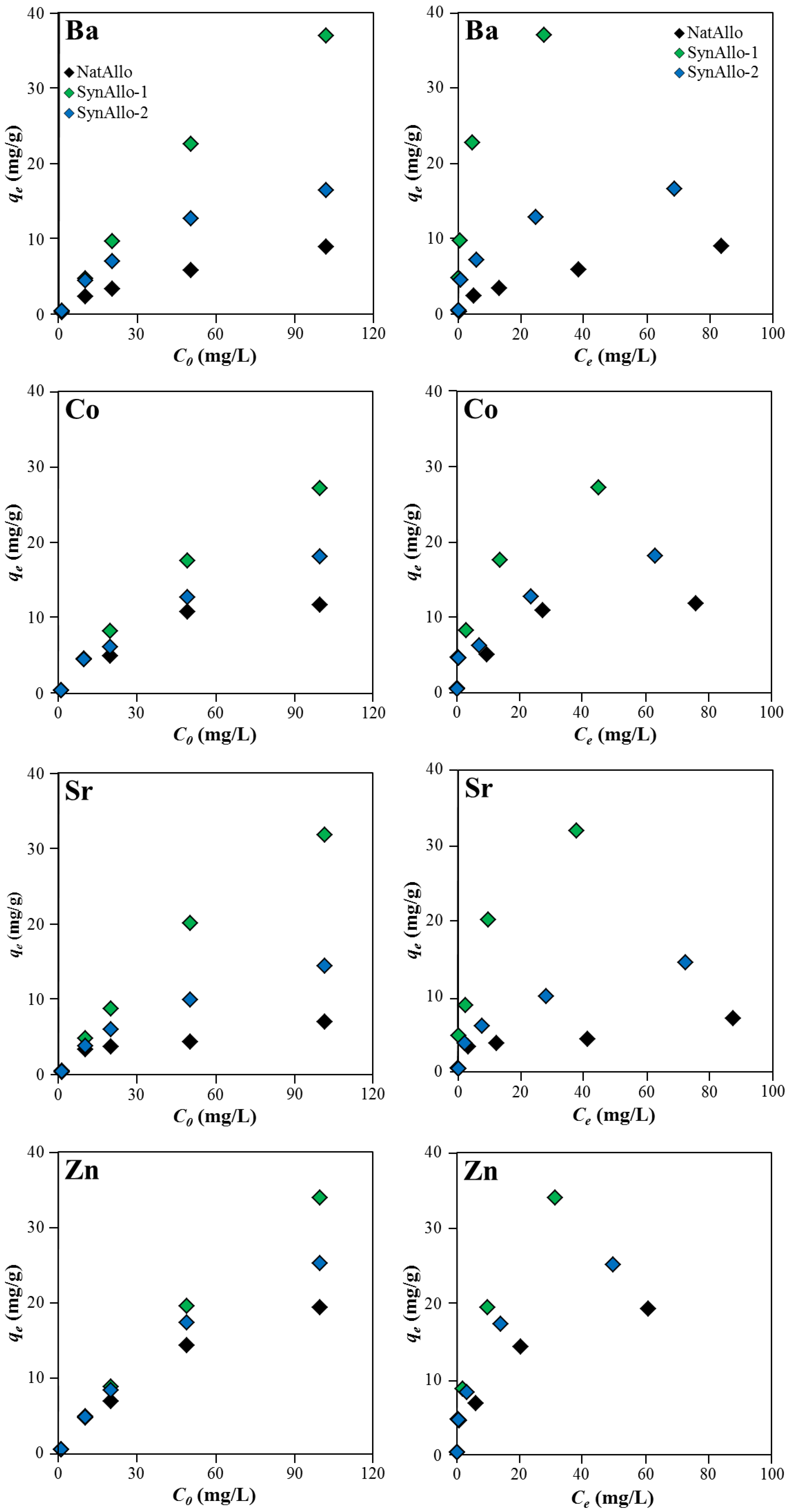
3.3.4. Effect of Metal Ion Concentration on the Adsorption Efficiency
3.3.5. Adsorption Isotherms and Adsorption Mechanism
3.4. Comparative Analysis of Sorption Capacities of Allophane and Other Materials
3.5. Application of Allophane in Water Processing Technologies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, M.A.H.; Islam, M.A.; Dampare, S.B.; Parvez, L.; Suzuki, S. Evaluation of hazardous metal pollution in irrigation and drinking water systems in the vicinity of a coal mine area of northwestern Bangladesh. J. Hazard. Mater. 2010, 179, 1065–1077. [Google Scholar] [CrossRef] [PubMed]
- Malik, Q.A.; Khan, M.S. Effect on Human Health due to Drinking Water Contaminated with Heavy Metals. J. Pollut. Eff. Cont. 2016, 5, 1000179. [Google Scholar] [CrossRef]
- Crévecoeur, S.; Debacker, V.; Joaquim-Justo, C.; Gobert, S.; Scippo, M.L.; Dejonghe, W.; Martin, P.; Thomé, J.P. Groundwater quality assessment of one former industrial site in Belgium using a TRIAD-like approach. Environ. Pollut. 2011, 159, 2461–2466. [Google Scholar] [CrossRef] [PubMed]
- Bacquart, T.; Frisbie, S.; Mitchell, E.; Grigg, L.; Cole, C.; Small, C.; Sarkar, B. Multiple inorganic toxic substances contaminating the groundwater of Myingyan Township, Myanmar: arsenic, manganese, fluoride, iron, and uranium. Sci. Total. Environ. 2015, 517, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Chabukdhara, M.; Gupta, S.K.; Kotecha, Y.; Nema, A.K. Groundwater quality in Ghaziabad district, Uttar Pradesh, India: Multivariate and health risk assessment. Chemosphere 2017, 179, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Calzadilla, A.; Rehdanz, K.; Tol, R.S.J. Water scarcity and the impact of improved irrigation management: a computable general equilibrium analysis. Agric. Econ. 2011, 42, 305–323. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Carolin, C.F.; Kumar, P.S.; Saravanan, A.; Joshiba, G.J.; Naushad, M. Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. J. Environ. Chem. Eng. 2017, 5, 2782–2799. [Google Scholar] [CrossRef]
- Iyoda, F.; Hayashi, S.; Arakawa, S.; John, B.; Okamoto, M.; Hayashi, H.; Yuan, G. Synthesis and adsorption characteristics of hollow spherical allophane nano-particles. Appl. Clay Sci. 2012, 56, 77–83. [Google Scholar] [CrossRef]
- Yuan, G.; Wada, S.I. Allophane and imogolite nanoparticles in soil and their environmental applications. In Nature’s Nanostructures; Barnard, A.S., Guo, H.B., Eds.; Pan Stanford Publishing Pte Ltd.: Singapore, 2012; pp. 485–508. ISBN 9789814316828. [Google Scholar]
- Babel, S.; Kurniawan, T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, B97, 219–243. [Google Scholar] [CrossRef]
- Singha, A.S.; Guleria, A. Chemical modification of cellulosic biopolymer and its use in removal of heavy metal ions from wastewater. Int. J. Biol. Macromol. 2014, 67, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Ismadji, S.; Soetaredjo, F.E.; Ayucitra, A. Natural Clay Minerals as Environmental Cleaning Agents. In Clay Materials for Environmental Remediation; Ismadji, S., Soetaredjo, F.E., Ayucitra, A., Eds.; Springer Nature: Berlin, Germany, 2015; pp. 5–37. ISBN 978-3-319-16711-4. [Google Scholar]
- Mukai, H.; Hirose, A.; Motai, S.; Kikuchi, R.; Tanoi, K.; Nakanishi, T.M.; Yaita, T.; Kogure, T. Cesium adsorption/desorption behavior of clay minerals considering actual contamination conditions in Fukushima. Sci. Rep. 2016, 6, 21543. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.N.; You, S.-J.; Hosseini-Bandegharaei, A.; Chao, H.-P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water. Res. 2017, 120, 88–116. [Google Scholar] [CrossRef] [PubMed]
- Nishikiori, H.; Kobayashi, K.; Kubota, S.; Tanaka, N.; Fujii, T. Removal of detergents and fats from waste water using allophane. Appl. Clay Sci. 2010, 47, 325–329. [Google Scholar] [CrossRef]
- Kaufhold, S.; Dohrmann, R.; Abidin, Z.; Henmi, T.; Matsue, N.; Eichinger, L.; Kaufhold, A.; Jahn, R. Allophane compared with other sorbent minerals for the removal of fluoride from water with particular focus on a mineable Ecuadorian allophane. Appl. Clay Sci. 2015, 5, 25–33. [Google Scholar] [CrossRef]
- Parfitt, R.L. Allophane in New Zealand—A review. Aust. J. Soil Res. 1990, 28, 343–360. [Google Scholar] [CrossRef]
- Hashizume, H. Adsorption of nucleic acid bases, ribose, and phosphate by some clay minerals. Life 2015, 5, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Lee, C.-P.; Mimura, H.; Zhang, X.; Wei, Y. Stable solidification of silica-based ammonium molybdophosphate by allophane: Application to treatment of radioactive cesium in secondary solid wastes generated from fukushima. J. Hazard. Mater. 2018, 341, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.J.; McBride, M.B. Chemisorption of Cu(II) and Co(II) on allophane and imogolite. Clays Clay Miner. 1984, 32, 300–310. [Google Scholar] [CrossRef]
- Jara, A.A.; Violante, A.; Pigna, M.; Mora, M.L. Mutual interactions of sulfate, oxalate, citrate, and phosphate on synthetic and natural allophanes. Soil Sci. Soc. Am. J. 2006, 70, 337–346. [Google Scholar] [CrossRef]
- Kaufhold, S.; Kaufhold, A.; Jahn, R.; Brito, S.; Dohrmann, R.; Hoffmann, R.; Gliemann, H.; Weidler, P.; Frechen, M. A new massive deposit of allophane raw material from Ecuador. Clays Clay Miner. 2009, 57, 72–81. [Google Scholar] [CrossRef]
- Wada, S.-I.; Eto, A.; Wada, K. Synthetic allophane and imogolite. J. Soil Sci. 1979, 30, 347–355. [Google Scholar] [CrossRef]
- Baldermann, A.; Dohrmann, R.; Kaufhold, S.; Nickel, C.; Letofsky-Papst, I.; Dietzel, M. The Fe-Mg-saponite solid solution series—A hydrothermal synthesis study. Clay Miner. 2014, 49, 391–415. [Google Scholar] [CrossRef]
- Baldermann, A.; Warr, L.N.; Letofsky-Papst, I.; Mavromatis, V. Substantial iron sequestration during green-clay authigenesis in modern deep-sea sediments. Nat. Geosci. 2015, 8, 885–889. [Google Scholar] [CrossRef]
- Baldermann, A.; Dietzel, M.; Mavromatis, V.; Mittermayr, F.; Warr, L.N.; Wemmer, K. The role of Fe on the formation and diagenesis of interstratified glauconite-smectite and illite-smectite: A case study of Upper Cretaceous shallow-water carbonates. Chem. Geol. 2017, 453, 21–34. [Google Scholar] [CrossRef]
- Kaufhold, S.; Ufer, K.; Kaufhold, A.; Stucki, J.W.; Anastácio, A.S.; Jahn, R.; Dohrmann, R. Quantification of allophane from Ecuador. Clays Clay Miner. 2010, 58, 707–716. [Google Scholar] [CrossRef]
- Opiso, E.; Sato, T.; Yoneda, T. Adsorption and co-precipitation behavior of arsenate, chromate, selenate and boric acid with synthetic allophane-like materials. J. Hazard. Mater. 2009, 170, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Levard, C.; Doelsch, E.; Basile-Doelsch, I.; Abidin, Z.; Miche, H.; Masion, A.; Rose, J.; Borschneck, D.; Bottero, J.-Y. Structure and distribution of allophanes, imogolite and proto-imogolite in volcanic soils. Geoderma 2012, 183–184, 100–108. [Google Scholar] [CrossRef]
- Baldermann, A.; Mavromatis, V.; Frick, P.M.; Dietzel, M. Effect of aqueous Si/Mg ratio and pH on the nucleation and growth of sepiolite at 25 °C. Geochim. Cosmochim. Acta 2018, 227, 211–226. [Google Scholar] [CrossRef]
- Usiyama, T.; Fukushi, K. Predictive model for Pb(II) adsorption on soil minerals (oxides and low-crystalline aluminum silicate) consistent with spectroscopic evidence. Geochim. Cosmochim. Acta 2016, 190, 134–155. [Google Scholar] [CrossRef]
- Lagergren, S. Zur theorie der sogenannten adsorption geloster stoffe. Kungliga Svenska Vetenskapsakademiens 1898, 24, 1–39. [Google Scholar]
- Okada, K.; Nishimuta, K.; Kameshima, Y.; Nakajima, A. Effect on uptake of heavy metal ions by phosphate grafting of allophane. J. Colloid Interface Sci. 2005, 286, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Kaufhold, A. Eigenschaften von Allophan aus Ecuador (Santo Domingo de los Colorados) und Anwendungspotential als Rohstoff; Hallenser Bodenwissenschaftliche Abhandlungen 16: Halle, Germany, 2011; pp. 1–175. ISBN 978-3-86247-172-0. (In German) [Google Scholar]
- Chávez, M.L.; de Pablo, L.; García, T.A. Adsorption of Ba2+ by Ca-exchange clinoptilolite tuff and montmorillonite clay. J. Hazard. Mater. 2010, 175, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Radushkevich, L.V. Equation of the characteristic curve of activated charcoal. Proc. Acad. Sci. USSR Phys. Chem. Sect. 1947, 55, 331–333. [Google Scholar]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sust. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef]
- Ghaemi, A.; Torab-Mostaedi, M.; Ghannadi-Maragheh, M. Characterizations of strontium(II) and barium(II) adsorption from aqueous solutions using dolomite powder. J. Hazard. Mater. 2011, 190, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Torab-Mostaedi, M.; Ghaemi, A.; Ghassabzadeh, H.; Ghannadi-Maragheh, M. Removal of strontium and barium from aqueous solutions by adsorption onto expanded Perlite. Can. J. Chem. Eng. 2011, 89, 1247–1254. [Google Scholar] [CrossRef]
- Manohar, D.M.; Noeline, B.F.; Anirudhan, T.S. Adsorption performance of Al-pillared bentonite clay for the removal of cobalt(II) from aqueous phase. Appl. Clay Sci. 2006, 31, 194–206. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Gupta, S.S. Kaolinite and montmorillonite as adsorbents for Fe(III), Co(II) and Ni(II) in aqueous medium. Appl. Clay Sci. 2008, 41, 1–9. [Google Scholar] [CrossRef]
- Al-Dwairi, R.A.; Al-Rawajfeh, A.E. Removal of cobalt and nickel from wastewater by using Jordan low-cost zeolite and bentonite. J. Univ. Chem. Technol. Metall. 2012, 47, 69–76. [Google Scholar]
- Mohapatra, M.; Mohapatra, L.; Singh, S.; Anand, S.; Mishra, B.K. A comparative study on Pb(II), Cd(II), Cu(II), Co(II) adsorption from single and binary aqueous solutions on additive assisted nano-structured goethite. Int. J. Eng. Sci. Technol. 2010, 2, 89–103. [Google Scholar] [CrossRef]
- Bhatnagara, A.; Minocha, A.K.; Sillanpää, M. Adsorptive removal of cobalt from aqueous solution by utilizing lemon peel as biosorbent. Biochem. Eng. J. 2010, 48, 181–186. [Google Scholar] [CrossRef]
- Chegrouche, S.; Mellah, A.; Barkat, M. Removal of strontium from aqueous solutions by adsorption onto activated carbon: kinetic and thermodynamic studies. Desalination 2009, 235, 306–318. [Google Scholar] [CrossRef]
- Ahmadpour, A.; Zabihi, M.; Tahmasbi, M.; Rohani Bastami, T. Effect of adsorbents and chemical treatments on the removal of strontium from aqueous solutions. J. Hazard. Mater. 2010, 182, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, W.; Feng, Q.; Dong, F. Preparation of magnetic clinoptilolite/CoFe2O4 composites for removal of Sr2+ from aqueous solutions: Kinetic, equilibrium, and thermodynamic studies. J. Saudi Chem. Soc. 2017, 21, 58–66. [Google Scholar] [CrossRef]
- Elouear, Z.; Bouzid, J.; Boujelben, N.; Feki, M.; Jamoussi, F.; Montiel, A. Heavy metal removal from aqueous solutions by activated phosphate rock. J. Hazard. Mater. 2008, 156, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; Siafaka, P.I.; Pavlidou, E.G.; Chrissafis, K.J.; Bikiaris, D.N. Synthesis and adsorption application of succinyl-grafted chitosan for the simultaneous removal of zinc and cationic dye from binary hazardous mixtures. Chem. Eng. J. 2015, 259, 438–448. [Google Scholar] [CrossRef]
- Stojakovic, D.; Hrenovic, J.; Mazaj, M.; Rajic, N. On the zinc sorption by the Serbian natural clinoptilolite and the disinfecting ability and phosphate affinity of the exhausted sorbent. J. Hazard. Mater. 2011, 185, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Shahmohammadi-Kalalagh, S.; Babazadeh, H.; Nazemi, A.H.; Manshouri, M. Isotherm and kinetic studies on adsorption of Pb, Zn and Cu by kaolinite. Caspian J. Environ. Sci. 2011, 9, 243–255. [Google Scholar]
- Jain, R.; Jordan, N.; Schild, D.; van Hullebusch, E.D.; Weiss, S.; Franzen, C.; Farges, F.; Hübner, R.; Lens, P.N.L. Adsorption of zinc by biogenic elemental selenium nanoparticles. Chem. Eng. J. 2015, 260, 855–863. [Google Scholar] [CrossRef]
- Richoz, S.; Baldermann, A.; Frauwallner, A.; Harzhauser, M.; Daxner-Höck, G.; Klammer, D.; Piller, W.E. Geochemistry and mineralogy of the Oligo-Miocene sediments of the Valley of Lakes, Mongolia. Palaeobio. Palaeoenv. 2017, 97, 233–258. [Google Scholar] [CrossRef] [PubMed]
- Purgstaller, B.; Dietzel, M.; Baldermann, A.; Mavromatis, V. Control of temperature and aqueous Mg2+/Ca2+ ratio on the (trans-)formation of ikaite. Geochim. Cosmochim. Acta 2017, 217, 128–143. [Google Scholar] [CrossRef]
- Mittermayr, F.; Baldermann, A.; Baldermann, C.; Grathoff, G.H.; Klammer, D.; Köhler, S.J.; Leis, A.; Warr, L.N.; Dietzel, M. Environmental controls and reaction pathways of coupled de-dolomitization and thaumasite formation. Cem. Concr. Res. 2017, 95, 282–293. [Google Scholar] [CrossRef]
- Khan, H. Water Adsorption and Surface Acidity of Nano-Ball Allophane as Affected by Heat Treatment. J. Environ. Sci. Technol. 2009, 2, 22–30. [Google Scholar] [CrossRef]
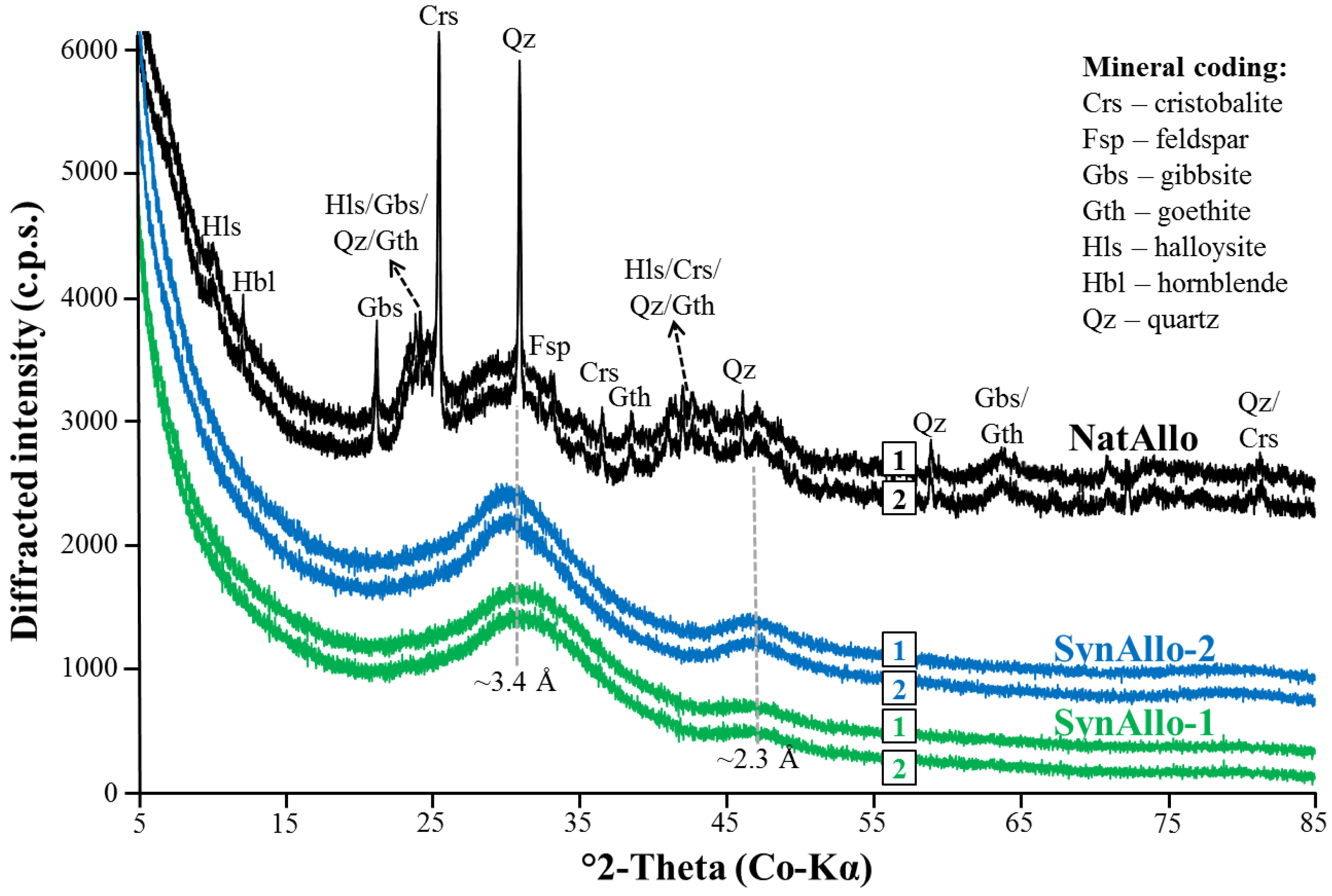
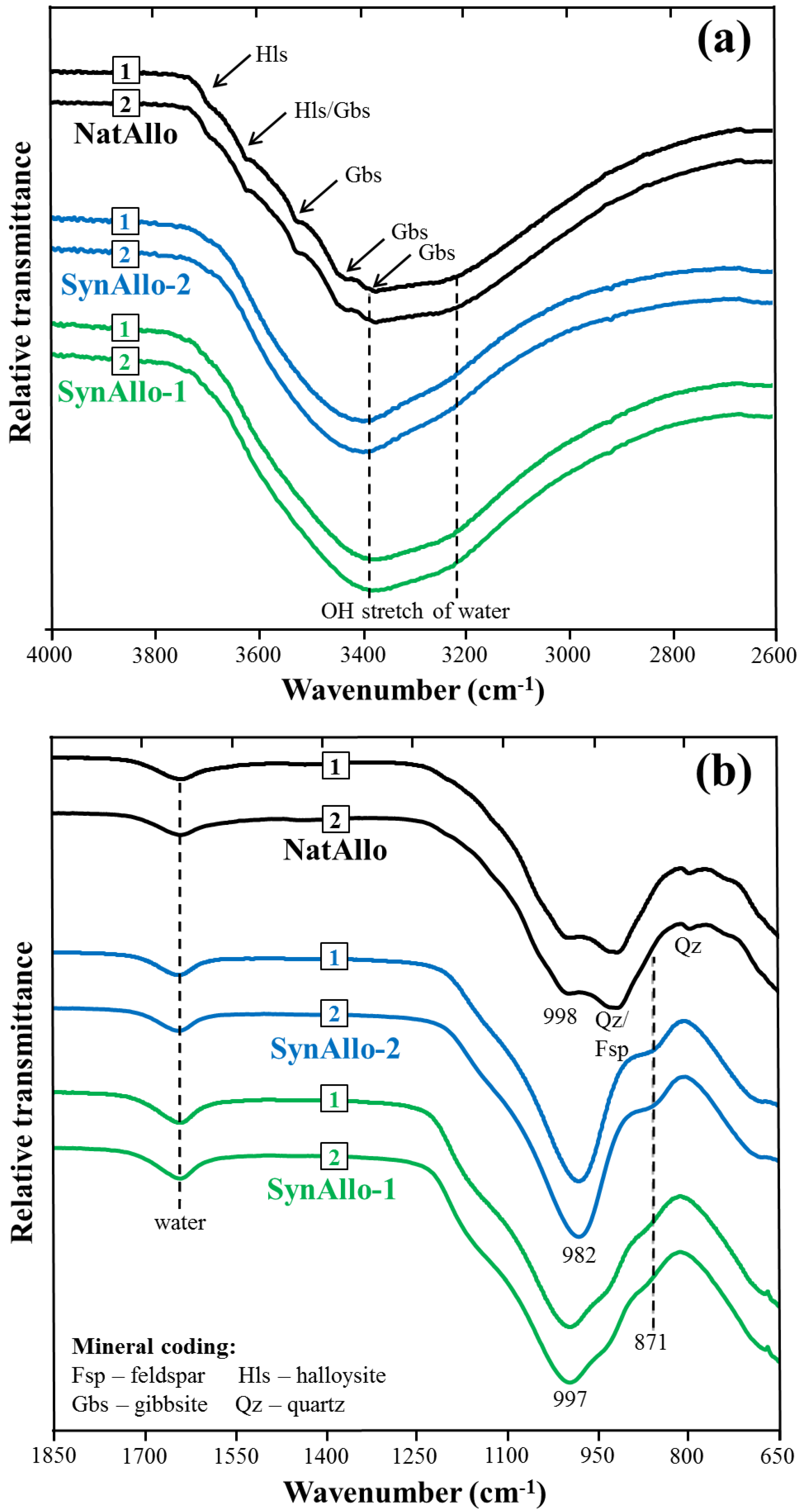
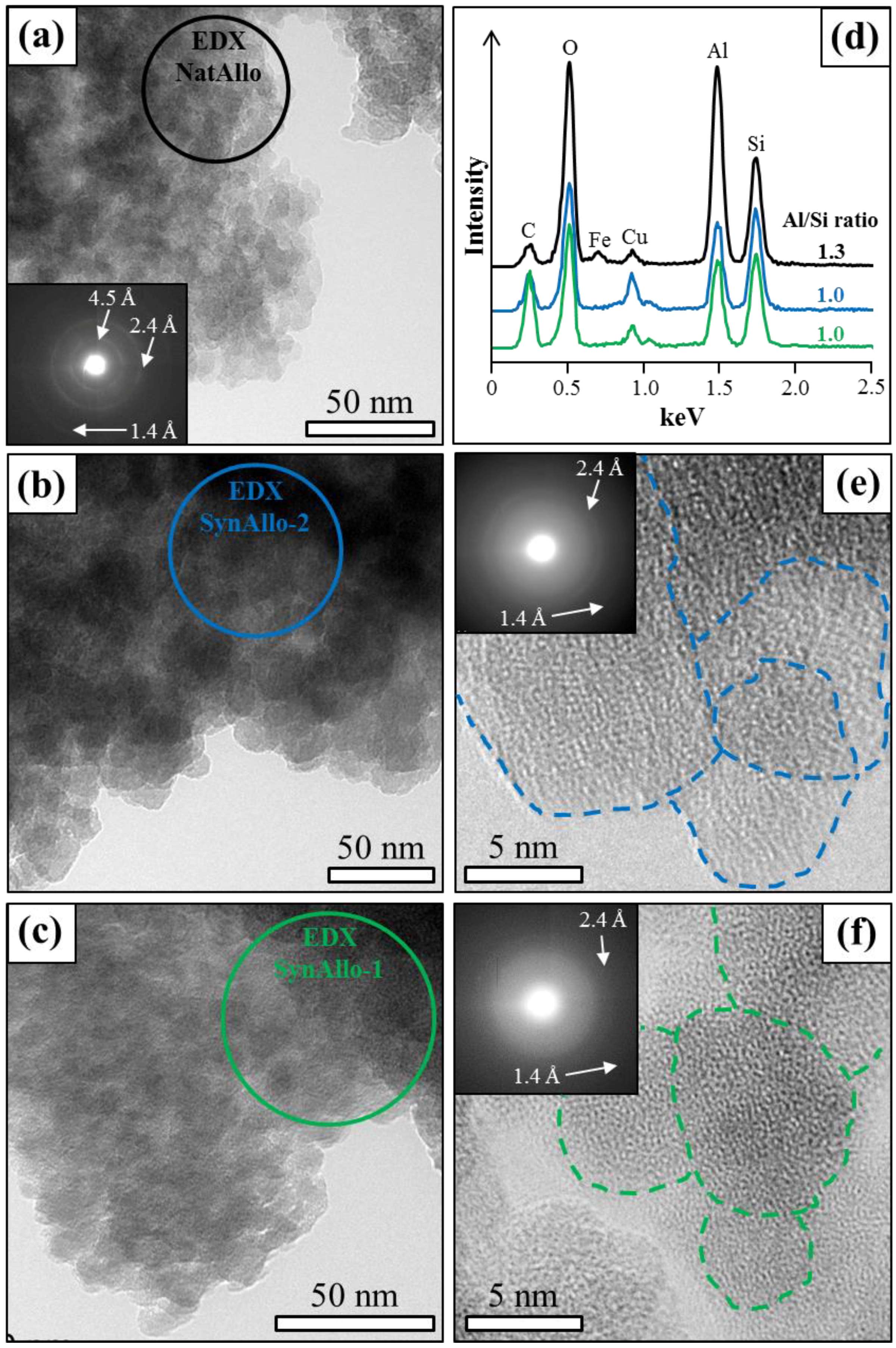
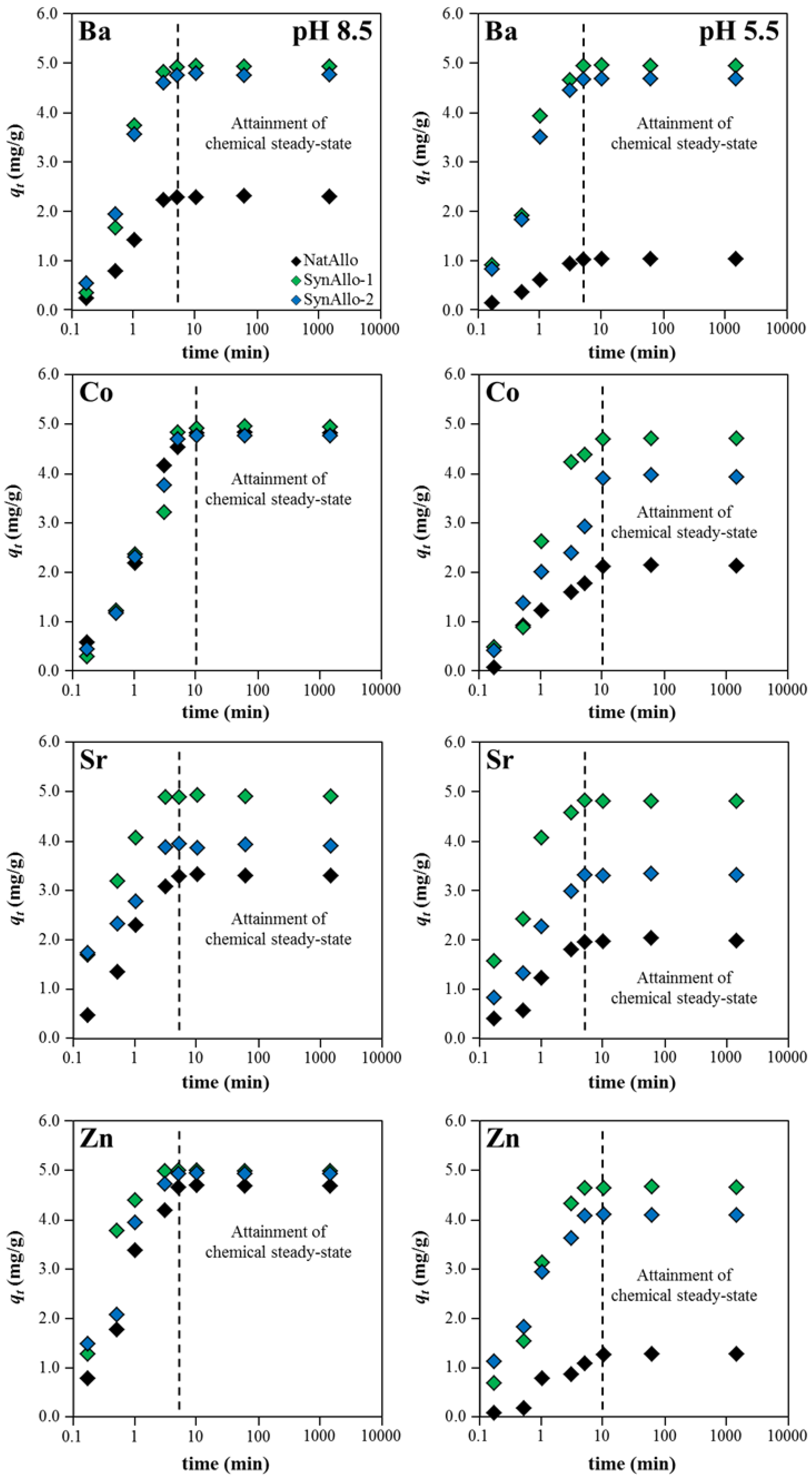
| Adsorbent | Adsorbate at pH 8.5 | Equil. Time (min) | Removal Rate (% Removal) | Pseudo-First Order Parameters | |||
|---|---|---|---|---|---|---|---|
| K (1/min) | R² (−) | ||||||
| NatAllo | Ba2+ | 5 | 2.3 | 45.2 | 1.090 | 2.5 | 0.996 |
| [HCoO2]− | 10 | 4.8 | 95.3 | 0.573 | 4.6 | 0.988 | |
| Sr2+ | 5 | 3.3 | 65.8 | 0.948 | 3.1 | 0.994 | |
| Zn2+ | 5 | 4.7 | 93.5 | 0.953 | 4.9 | 0.953 | |
| SynAllo-1 | Ba2+ | 5 | 4.9 | 97.1 | 1.179 | 4.9 | 0.992 |
| [HCoO2]− | 10 | 4.9 | 97.6 | 0.580 | 4.9 | 0.942 | |
| Sr2+ | 3 | 4.9 | 97.9 | 2.003 | 5.0 | 0.996 | |
| Zn2+ | 3 | 5.0 | 99.8 | 2.291 | 4.9 | 0.996 | |
| SynAllo-2 | Ba2+ | 5 | 4.8 | 93.9 | 1.150 | 4.8 | 0.997 |
| [HCoO2]− | 10 | 4.8 | 94.2 | 0.676 | 4.8 | 0.977 | |
| Sr2+ | 3 | 3.9 | 77.9 | 1.667 | 3.8 | 0.978 | |
| Zn2+ | 5 | 4.9 | 98.6 | 1.271 | 4.8 | 0.977 | |
| Adsorbent | Adsorbate at pH 5.5 | Equil. Time (min) | (mg/g) | Removal Rate (% removal) | Pseudo-First-Order Parameters | ||
| K (1/min) | (mg/g) | R² (−) | |||||
| NatAllo | Ba2+ | 5 | 1.0 | 20.4 | 0.953 | 1.1 | 0.989 |
| Co2+ | 10 | 2.1 | 42.3 | 0.475 | 2.0 | 0.957 | |
| Sr2+ | 5 | 2.0 | 39.8 | 0.875 | 2.0 | 0.994 | |
| Zn2+ | 10 | 1.3 | 25.4 | 0.462 | 1.3 | 0.965 | |
| SynAllo-1 | Ba2+ | 5 | 5.0 | 97.4 | 1.134 | 4.8 | 0.971 |
| Co2+ | 10 | 4.7 | 93.3 | 0.635 | 4.6 | 0.984 | |
| Sr2+ | 5 | 4.8 | 96.4 | 1.348 | 4.6 | 0.942 | |
| Zn2+ | 5 | 4.7 | 93.0 | 0.986 | 4.9 | 0.990 | |
| SynAllo-2 | Ba2+ | 5 | 4.68 | 92.1 | 1.162 | 4.81 | 0.986 |
| Co2+ | 10 | 3.93 | 78.0 | 0.437 | 4.12 | 0.925 | |
| Sr2+ | 5 | 3.32 | 66.4 | 1.046 | 3.51 | 0.952 | |
| Zn2+ | 5 | 4.09 | 81.7 | 1.014 | 3.98 | 0.940 | |
| Adsorbent | Adsorbate at pH 8.5 | Langmuir Parameters | RL (−) | ||
|---|---|---|---|---|---|
| Q0max (mg/g) | KL (L/mg) | R² (−) | |||
| NatAllo | Ba2+ | 10.6 | 0.049 | 0.947 | 0.17–0.95 |
| [HCoO2]− | 12.4 | 0.213 | 0.974 | 0.05–0.82 | |
| Sr2+ | 7.2 | 0.105 | 0.927 | 0.09–0.91 | |
| Zn2+ | 20.9 | 0.163 | 0.971 | 0.06–0.85 | |
| SynAllo-1 | Ba2+ | 38.6 | 0.676 | 0.994 | 0.01–0.60 |
| [HCoO2]− | 29.0 | 0.226 | 0.968 | 0.04–0.81 | |
| Sr2+ | 34.4 | 0.265 | 0.970 | 0.02–0.58 | |
| Zn2+ | 36.9 | 0.240 | 0.985 | 0.04–0.80 | |
| SynAllo-2 | Ba2+ | 17.2 | 0.219 | 0.985 | 0.04–0.82 |
| [HCoO2]− | 19.3 | 0.147 | 0.948 | 0.06–0.87 | |
| Sr2+ | 15.9 | 0.103 | 0.977 | 0.09–0.91 | |
| Zn2+ | 26.9 | 0.237 | 0.983 | 0.04–0.80 | |
| Adsorbent | Adsorbate at pH 8.5 | Dubinin–Radushkevich Parameters | E (kJ/mol) | ||
|---|---|---|---|---|---|
| qDR (mg/g) | KDR (mol²/kJ²) | R² (−) | |||
| NatAllo | Ba2+ | 7.4 | 1.480 × 10−8 | 0.979 | 5.8 |
| [HCoO2]− | 10.5 | 9.115 × 10−9 | 0.929 | 7.4 | |
| Sr2+ | 6.2 | 1.315 × 10−8 | 0.962 | 6.2 | |
| Zn2+ | 16.3 | 1.143 × 10−8 | 0.966 | 6.6 | |
| SynAllo-1 | Ba2+ | 44.5 | 7.914 × 10−9 | 0.996 | 7.9 |
| [HCoO2]− | 21.8 | 1.008 × 10−8 | 0.971 | 7.0 | |
| Sr2+ | 26.5 | 8.593 × 10−9 | 0.977 | 7.6 | |
| Zn2+ | 27.4 | 1.012 × 10−8 | 0.979 | 7.0 | |
| SynAllo-2 | Ba2+ | 15.1 | 7.892 × 10−9 | 0.988 | 8.0 |
| [HCoO2]− | 14.2 | 1.009 × 10−8 | 0.948 | 7.0 | |
| Sr2+ | 12.4 | 1.384 × 10−8 | 0.990 | 6.0 | |
| Zn2+ | 21.2 | 1.026 × 10−8 | 0.983 | 7.0 | |
| Adsorbate | Adsorbent | Q0max (mg/g) | Surface Area Particle Size | Conc. Range of Pollutant | Contact Time | T (°C) | pH | Ref. |
|---|---|---|---|---|---|---|---|---|
| Ba2+ | Ca-Cpt | 15.3 | <75 µm | 0.005–0.1 N | 168 h | - | - | [37] |
| Ca-Mnt | 15.3 | <75 µm | 0.005–0.1 N | 48 h | - | - | [37] | |
| Dolomite | 4.0 | 20 µm | 10–50 mg/L | 6 h | 20–50 | 2.5–8.5 | [41] | |
| Exp. perlite | 2.5 | 1.9 m²/g | 5–50 mg/dm³ | 6 h | 20–50 | 2.0–7.0 | [42] | |
| NatAllo | 10.6 | 294.0 m²/g | 1–100 mg/L | 2 h | 25 | 8.5 | TS | |
| SynAllo-1 | 38.6 | 358.0 m²/g | 1–100 mg/L | 2 h | 25 | 8.5 | TS | |
| Syn-Allo-2 | 17.2 | 370.0 m²/g | 1–100 mg/L | 2 h | 25 | 8.5 | TS | |
| Co2+/HCoO2− | Al-pillared bent. | 38.6 | 227.0 m²/g | 10–400 mg/L | 24 h | 30–60 | 2.0–9.0 | [43] |
| Kaolinite | 11.0 | 11.6 m²/g | 10–250 mg/L | 4 h | 30 | 5.8 | [44] | |
| Bent./Cpt mix | 2.7 | 300–1000 µm | 5–50 mg/L | 12 h | - | - | [45] | |
| Goethite | 86.6 | 2–10 nm | 5–500 mg/L | 1 h | 30 | 5.0 | [46] | |
| Lemon peel | 22.0 | 150–200 µm | 0–1000 mg/L | 10 h | 25 | 6.0 | [47] | |
| NatAllo | 12.4 | 294.0 m²/g | 1–100 mg/L | 2 h | 25 | 8.5 | TS | |
| SynAllo-1 | 29.0 | 358.0 m²/g | 1–100 mg/L | 2 h | 25 | 8.5 | TS | |
| Syn-Allo-2 | 19.3 | 370.0 m²/g | 1–100 mg/L | 2 h | 25 | 8.5 | TS | |
| Sr2+ | Act. carbon | 44.4 | >1200 m²/g | 10–200 mg/L | 7–8 h | 20–60 | 2.0–8.0 | [48] |
| AGH | 116.3 | <88 µm | 161–635 mg/L | <0.1 h | 25 | 7.0 | [49] | |
| Dolomite | 1.2 | 20 µm | 10–50 mg/L | 6 h | 20–50 | 2.5–8.5 | [42] | |
| Exp. perlite | 1.1 | 1.9 m²/g | 5–50 mg/dm³ | 6 h | 20–50 | 2.0–7.0 | [43] | |
| Cpt/CoFe2O4 | 20.6 | 200–250 µm | 20–400 mg/L | 19 h | 25–65 | 2.0–10.0 | [50] | |
| NatAllo | 7.2 | 294.0 m²/g | 1–100 mg/L | 2 h | 25 | 8.5 | TS | |
| SynAllo-1 | 34.4 | 358.0 m²/g | 1–100 mg/L | 2 h | 25 | 8.5 | TS | |
| Syn-Allo-2 | 15.9 | 370.0 m²/g | 1–100 mg/L | 2 h | 25 | 8.5 | TS | |
| Zn2+ | Act. phosphate | 12.3 | 22.5 m²/g | 10–500 mg/L | 2 h | 10–40 | 5.0 | [51] |
| Chitosan | 290.0 | 4.9 m²/g | 0–1000 mg/L | 24 h | 25 | 5.0 | [52] | |
| Cpt | 16.8 | 63–100 µm | 100–600 mg/dm³ | 24 h | 25–65 | 6.0 | [53] | |
| Kaolinite | 4.9 | 12.8 m²/g | 50–2000 mg/L | 120 h | 20 | 4.5 | [54] | |
| Nano-Se | 62.1 | 80–260 nm | 6–215 mg/L | 16 h | 30 | 6.5 | [55] | |
| NatAllo | 20.9 | 294.0 m²/g | 1–100 mg/L | 2 h | 25 | 8.5 | TS | |
| SynAllo-1 | 36.9 | 358.0 m²/g | 1–100 mg/L | 2 h | 25 | 8.5 | TS | |
| Syn-Allo-2 | 26.9 | 370.0 m²/g | 1–100 mg/L | 2 h | 25 | 8.5 | TS |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldermann, A.; Grießbacher, A.C.; Baldermann, C.; Purgstaller, B.; Letofsky-Papst, I.; Kaufhold, S.; Dietzel, M. Removal of Barium, Cobalt, Strontium, and Zinc from Solution by Natural and Synthetic Allophane Adsorbents. Geosciences 2018, 8, 309. https://doi.org/10.3390/geosciences8090309
Baldermann A, Grießbacher AC, Baldermann C, Purgstaller B, Letofsky-Papst I, Kaufhold S, Dietzel M. Removal of Barium, Cobalt, Strontium, and Zinc from Solution by Natural and Synthetic Allophane Adsorbents. Geosciences. 2018; 8(9):309. https://doi.org/10.3390/geosciences8090309
Chicago/Turabian StyleBaldermann, Andre, Andrea Cäcilia Grießbacher, Claudia Baldermann, Bettina Purgstaller, Ilse Letofsky-Papst, Stephan Kaufhold, and Martin Dietzel. 2018. "Removal of Barium, Cobalt, Strontium, and Zinc from Solution by Natural and Synthetic Allophane Adsorbents" Geosciences 8, no. 9: 309. https://doi.org/10.3390/geosciences8090309
APA StyleBaldermann, A., Grießbacher, A. C., Baldermann, C., Purgstaller, B., Letofsky-Papst, I., Kaufhold, S., & Dietzel, M. (2018). Removal of Barium, Cobalt, Strontium, and Zinc from Solution by Natural and Synthetic Allophane Adsorbents. Geosciences, 8(9), 309. https://doi.org/10.3390/geosciences8090309






