Dissociation and Self-Preservation of Gas Hydrates in Permafrost
Abstract
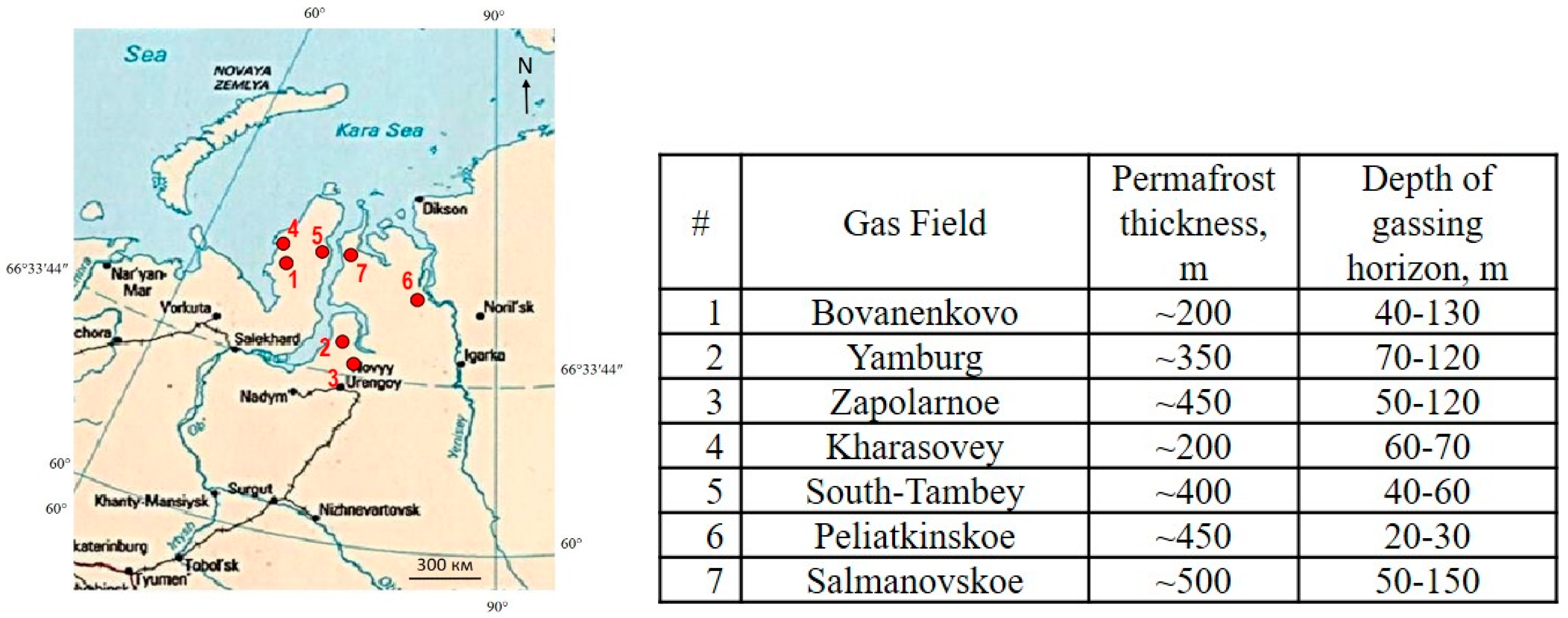
1. Introduction
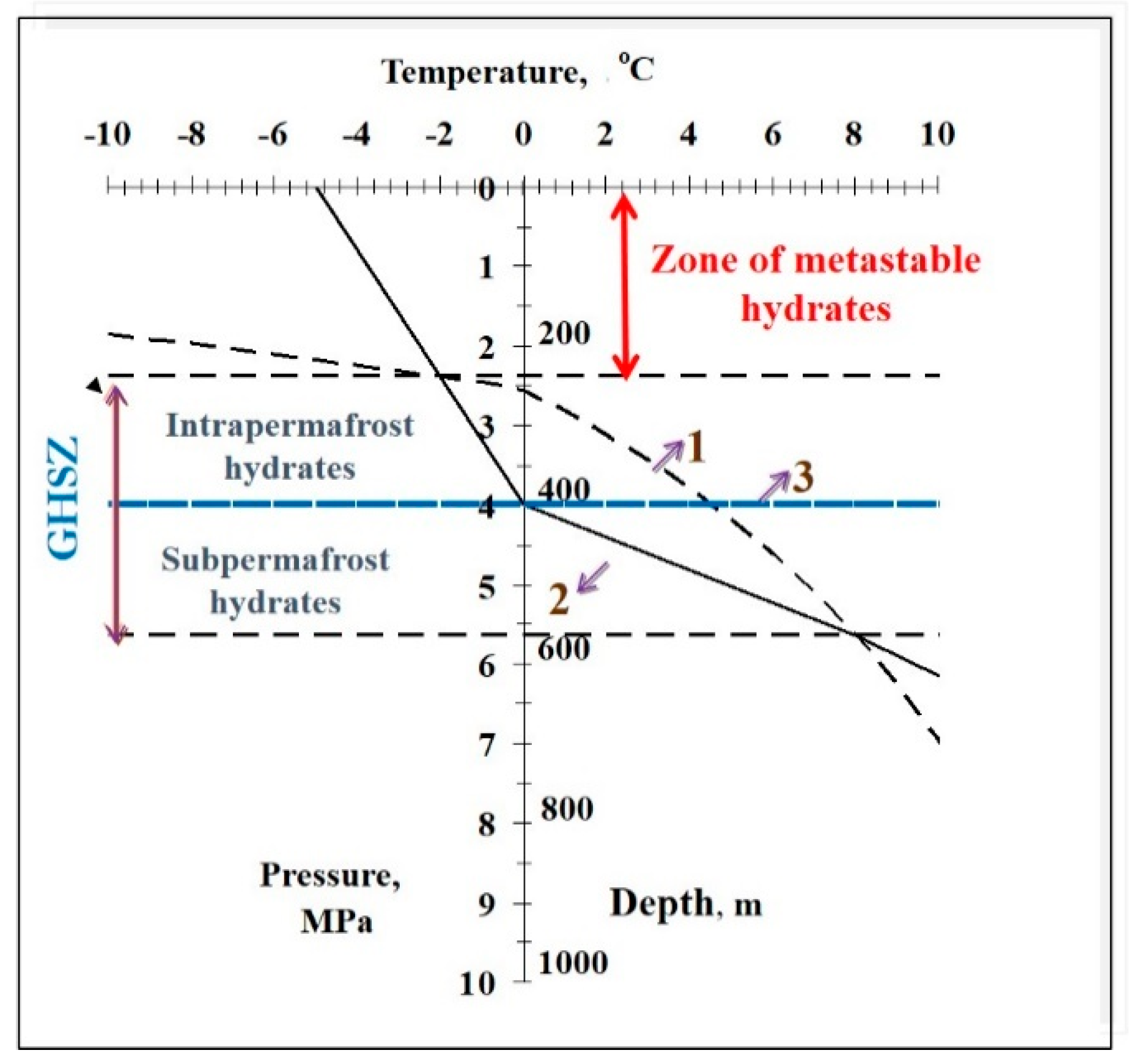
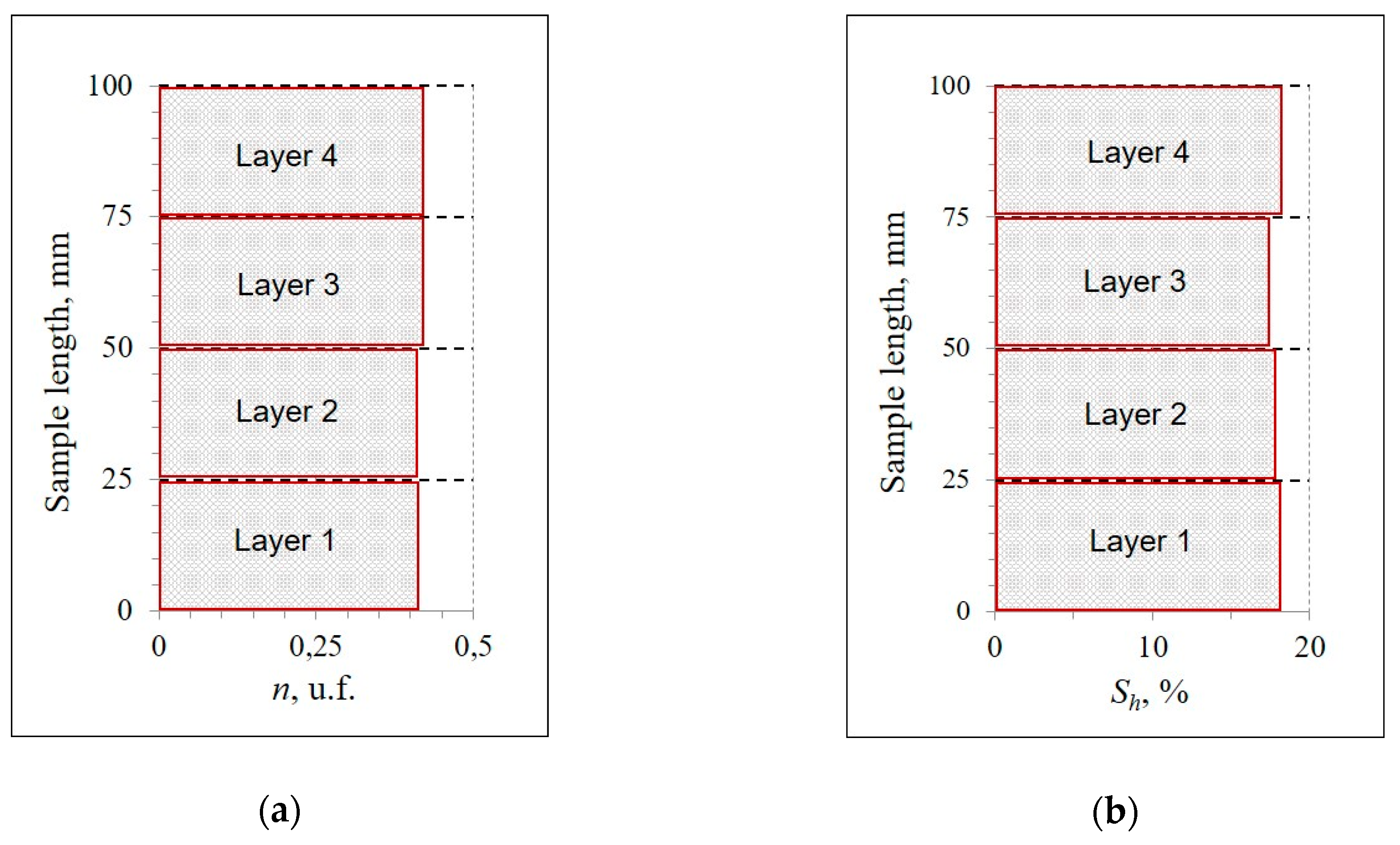
2. Methods
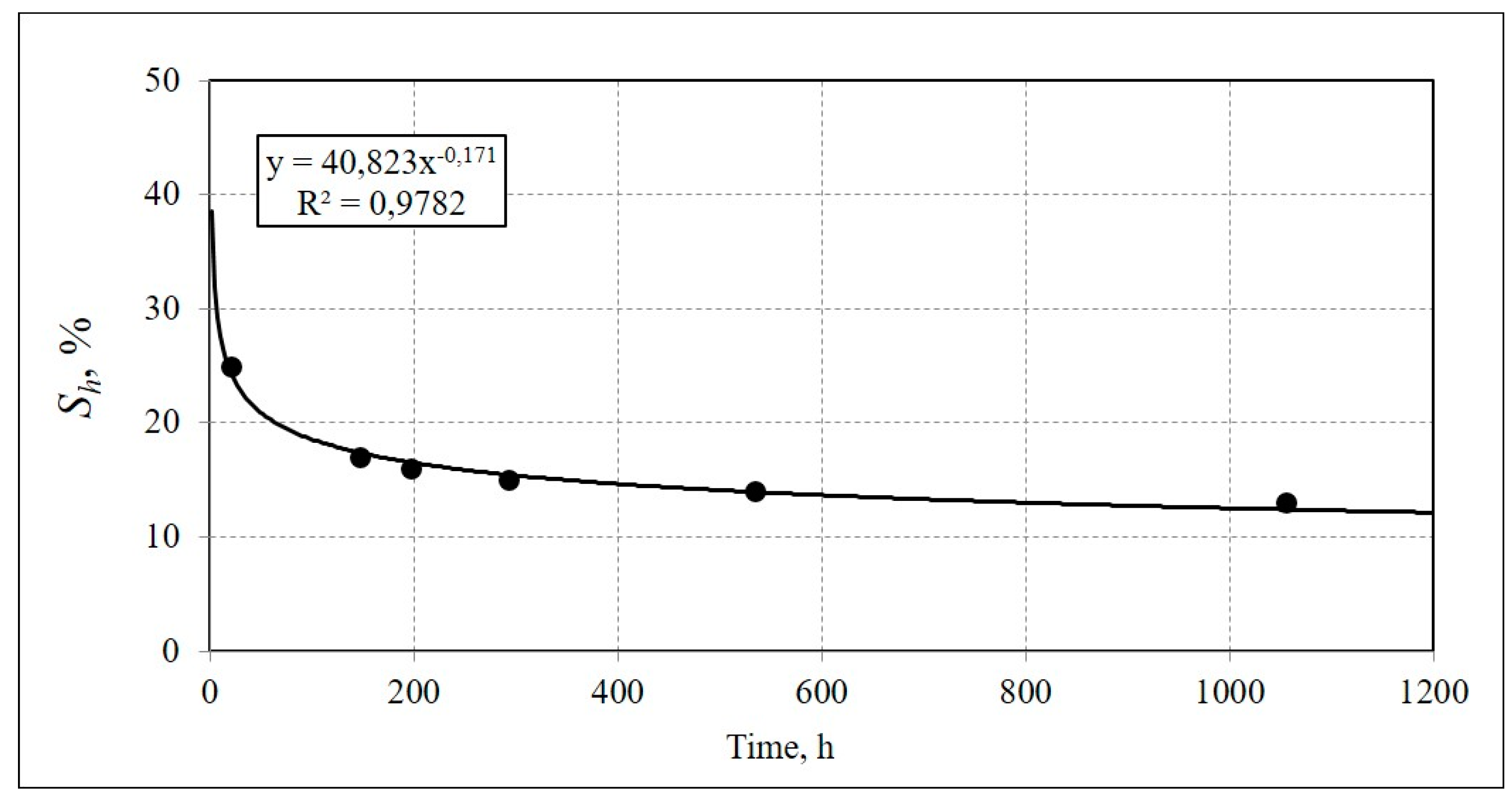
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Andersland, O.B.; Ladanyi, B. An Introduction to Frozen Ground Engineering; Springer Science & Business Media: Berlin, Germany, 1994. [Google Scholar]
- Yershov, E.D. General Geocryology; Cambridge University Press: Cambridge, UK, 1998; p. 580. [Google Scholar]
- Romanovskii, N.N.; Tumskoy, V.E. Retrospective approach to the estimation of the contemporary extension and structure of the shelf cryolithozone in East Arctic. J. Earth’s Cryosphere 2011, 15, 3–14. (in Russian). [Google Scholar]
- Badu, Y.B. The influence of gas bearing structures on the thickness of cryogenic strata of Jamal Peninsula. J. Earth’s Cryosphere 2014, 18, 11–22. (in Russian). [Google Scholar]
- Chuvilin, E.M.; Bukhanov, B.A.; Tumskoy, V.E.; Shakhova, N.E.; Dudarev, O.V.; Semiletov, I.P. Thermal conductivity of bottom sediments in the region of Buor-Khaya Bay (shelf of the Laptev Sea). J. Earth’s Cryosphere 2013, 17, 32–40. (in Russian). [Google Scholar]
- Shakhova, N.; Semiletov, I.; Gustafsson, O.; Sergienko, V.; Lobkovsky, L.; Dudarev, O.; Tumskoy, V.; Grigoriev, M.; Mazurov, A.K.; Salyuk, A.; et al. Current rates and mechanisms of subsea permafrost degradation in the East Siberian Arctic Shelf. Nat. Commun. 2017, 8, 15872. [Google Scholar] [CrossRef] [PubMed]
- Max, D.M.; Johnson, A.H.; Dillon, W.P. Natural Gas Hydrate—Arctic Ocean Deepwater Resource Potential; Springer: New York, NY, USA, 2013; p. 113. [Google Scholar]
- Cherskiy, N.V.; Tsarev, V.P.; Nikitin, S.P. Investigation and prediction of conditions of accumulation of gas resources in gas-hydrate pools. Pet. Geol. 1985, 21, 65–89. [Google Scholar]
- Bird, K.J.; Magoon, L.B. Petroleum Geology of the Northern Part of the Arctic National Wildlife Refuge, Northeastern Alaska; US Government Printing Office: Washington, DC, USA, 1987; p. 329.
- Judge, A.S.; Smith, S.L.; Majorowicz, J.A. The current distribution and thermal stability of gas hydrates in the Canadian Polar Region. In Proceedings of the Fourth Offshore and Polar Engineering Conference, Osaka, Japan, 10–15 April 1994; pp. 307–313. [Google Scholar]
- Judge, A.S.; Majorowicz, J.A. Geothermal conditions for gas hydrate stability in the Beaufort-Mackenzie area: The global change aspect. Glob. Planet. Chang. 1992, 98, 251–263. [Google Scholar] [CrossRef]
- Dallimore, S.R.; Uchida, T.; Collett, T.S. Scientific Results from JAPEX/ JNOC/GSC Mallik 2L-38 Gas Hydrate Research Well, Mackenzie Delta, Northwest Territories, Canada. Geological Survey of Canada, Bulletin 544; US Government Printing Office: Washington, DC, USA, 1999; p. 403.
- Yakushev, V.S.; Chuvilin, E.M. Natural gas and hydrate accumulation within permafrost in Russia. Cold Reg. Sci. Technol. 2000, 149, 46–50. [Google Scholar] [CrossRef]
- Liang, Y.P.; Li, X.S.; Li, B. Assessment of gas production potential from hydrate reservoir in Qilian mountain permafrost using five-spot horizontal well system. Energies 2015, 8, 10796–10817. [Google Scholar] [CrossRef]
- Ruppel, C. Permafrost-associated gas hydrate: Is it really approximately 1% of the global system? J. Chem. Eng. Data 2015, 60, 429–436. [Google Scholar] [CrossRef]
- Henninges, J.; Schrötter, J.; Erbas, K.; Huenges, E. Temperature field of the Mallik gas hydrate occurrence —Implications on phase changes and thermal properties. In Scientific Results from the Mallik 2002 Gas Hydrate Production Research WellProgram, Mackenzie Delta, Northwest Territories, Canada. Geological Survey of Canada, Bulletin 585; Dallimore, S.R., Collett, T.S., Eds.; US Government Printing Office: Washington, DC, USA, 2005. [Google Scholar]
- Collett, T.S.; Lee, M.W.; Agena, W.F.; Miller, J.J.; Lewis, K.A.; Zyrianova, M.V.; Boswell, R.; Inks, T.L. Permafrost associated natural gas hydrate occurrences on the Alaskan North Slope. Mar. Petr. Geol. 2011, 28, 279–294. [Google Scholar] [CrossRef]
- Fang, H.; Xu, M.; Lin, Z.; Zhong, Q.; Bai, D.; Liu, J.; Pei, F.; He, M. Geophysical characteristics of gas hydrate in the Muli area, Qinghai province. J. Nat. Gas Sci. Eng. 2017, 37, 539–550. [Google Scholar] [CrossRef]
- Dallimore, S.R.; Chuvilin, E.M.; Yakushev, V.S.; Grechischev, S.E.; Ponomarev, V.; Pavlov, A. Field and laboratory characterization of interpermafrost gas hydrates, Mackenzie Delta, N.W.T., Canada. In Proceedings of the 2nd International Conference on Natural Gas Hydrates, Toulouse, France, 2–6 June 1996; pp. 525–531. [Google Scholar]
- Yershov, E.D.; Lebedenko, Y.P.; Chuvilin, E.M.; Istomin, V.A.; Yakushev, V.S. Features of gas hydrate occurrence in permafrost. USSR Acad. Sci. 1991, 321, 788–791. (in Russian). [Google Scholar]
- Chuvilin, E.M.; Yakushev, V.S.; Perlova, E.V. Gas and gas hydrates in the permafrost of Bovanenkovo gas field, Yamal Peninsula, West Siberia. Polarforschung 2000, 68, 215–219. [Google Scholar]
- Kuhs, W.F.; Genov, G.; Staykova, D.K.; Hansen, T. Ice perfection and onset of anomalous preservation of gas hydrates. Phys. Chem. Chem. Phys. 2004, 6, 4917–4920. [Google Scholar] [CrossRef]
- Istomin, V.A.; Yakushev, V.S.; Mokhonina, N.A.; Kwon, V.G.; Chuvilin, E.M. Self-preservation phenomenon of gas hydrate. Gas Ind. Russ. 2006, 4, 16–27. (in Russian). [Google Scholar]
- Takeya, S.; Ripmeester, J.A. Anomalous Preservation of CH4 hydrate and its dependence on the morphology of hexagonal ice. Phys. Chem. Chem. Phys. 2010, 11, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Davidson, D.W.; Garg, S.K.; Gough, S.R.; Handa, Y.P.; Ratcliffe, C.I.; Ripmeester, J.A.; Tse, J.S.; Lawson, W.F. Laboratory analysis of a naturally occurring gas hydrate from sediment of the Gulf of Mexico. Geochim. Cosmochim. Acta 1986, 50, 619–623. [Google Scholar] [CrossRef]
- Handa, Y.P. Calorimetric determinations of the composition, enthalpies of dissociation and heat capacities in the range 85 to 270 К for clathrate hydrates of xenon and krypton. J. Chem. Thermodyn. 1986, 18, 891–902. [Google Scholar] [CrossRef]
- Yershov, E.D.; Lebedenko, Y.P.; Chuvilin, E.M.; Yakushev, V.S. Microstructure of an ice—methane hydrate agglomerate: An experimental study. Eng. Geol. 1990, 3, 38–44. (in Russian). [Google Scholar]
- Ershov, E.D.; Yakushev, V.S. Experimental research on gas hydrate decomposition in frozen rocks. Cold Reg. Sci. Technol. 1992, 20, 147–156. [Google Scholar] [CrossRef]
- Yakushev, V.S.; Istomin, V.A. Gas-hydrates self-preservation effect. In Physics and Chemistry of Ice; Maeno, N., Hondoh, T., Eds.; Hokkaido University Press: Sapporo, Japan, 1992; pp. 136–139. [Google Scholar]
- Stern, L.; Circone, S.; Kirby, S.H.; Durham, W. Temperature, pressure, and compositional effects on anomalous or “self” preservation of gas hydrates. Can. J. Phys. 2003, 81, 271–283. [Google Scholar] [CrossRef]
- Stern, L.; Circone, S.; Kirby, S.H.; Durham, W. Anomalous preservation of pure methane hydrates at 1 atm. J. Phys. Chem. 2001, 105, 537–542. [Google Scholar] [CrossRef]
- Takeya, S.; Ebinuma, T.; Uchida, T.; Nagao, J.; Narita, H. Self-preservation effect and dissociation rates of CH4 hydrate. J. Cryst. Growth 2002, 237, 379–382. [Google Scholar] [CrossRef]
- Takeya, S.; Uchida, T.; Nagao, J.; Ohmura, R.; Shimada, W.; Kamata, Y.; Ebinuma, H.; Narita, H. Particle size effect of CH4 hydrate for self-preservation. Chem. Eng. Sci. 2005, 60, 1383–1387. [Google Scholar] [CrossRef]
- Takeya, S.; Muromachi, S.; Yamamoto, Y.; Umeda, H.; Matsuo, S. Preservation of CO2 hydrate under different atmospheric conditions. Fluid Phase Equilib. 2016, 413, 137–141. [Google Scholar] [CrossRef]
- Takeya, S.; Mimachi, H.; Murayama, T. Methane storage in water frameworks: Self-preservation of methane hydrate pellets formed from NaCl solutions. Appl. Energy 2018, 230, 86–93. [Google Scholar] [CrossRef]
- Shimada, W.; Takeya, S.; Kamata, Y.; Uchida, T.; Nagao, J.; Ebinuma, T.; Narita, H. Mechanism of self-preservation during dissociation of methane clathrate hydrate. In Proceedings of the 5th International Conference on Gas Hydrate, Trondheim, Norway, 13–16 June 2005; Volume 1, pp. 208–212. [Google Scholar]
- Falenty, A.; Kuhs, W.F. “Self-Preservation” of CO2 Gas HydratessSurface Microstructure and Ice Perfection. J. Phys. Chem. B 2009, 113, 15975–15988. [Google Scholar] [CrossRef] [PubMed]
- Melnikov, V.P.; Nesterov, A.N.; Reshetnikov, A.M.; Zavodovsky, A.G. Evidence of liquid water formation during methane hydrates dissociation below the ice point. Chem. Eng. Sci. 2009, 64, 1160–1166. [Google Scholar] [CrossRef]
- Melnikov, V.P.; Nesterov, A.N.; Reshetnikov, A.M.; Istomin, V.A. Metastable states during dissociation of carbon dioxide hydrates below 273 K. Chem. Eng. Sci. 2011, 66, 73–77. [Google Scholar] [CrossRef]
- Ohno, H.; Nishimura, O.; Suzuki, K.; Narita, H.; Nagao, J. Morphological and compositional characterization of self-preserved gas hydrates by low-vacuum scanning electron microscopy. ChemPhysChem 2011, 12, 1661–1665. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T.; Kida, M.; Nagao, J. Dissociation termination of methane–ethane hydrates in temperature-ramping tests at atmospheric pressure below the melting point of ice. ChemPhysChem 2011, 12, 1652–1656. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.-R.; Zeng, X.-Y.; Zhou, F.-H.; Ran, Q.-D.; Sun, C.-Y.; Zhong, R.-Q.; Yang, L.-Y.; Chen, G.-J.; Koh, C.A. Self-preservation and structural transition of gas hydrates during dissociation below the ice point: An in situ study using Raman spectroscopy. Sci. Rep. 2016, 6, 38855. [Google Scholar] [CrossRef] [PubMed]
- Dou, B.; Jiang, G.; Wu, X.; Zhang, L.; Ning, F. Research methane hydrate meta-stable property for application to natural gas storage and transportation. In Proceedings of the Asia Pacific Oil and Gas Conference & Exhibition, Jakarta, Indonesia, 4–6 August 2009. [Google Scholar] [CrossRef]
- Takeya, S.; Yoneyama, A.; Ueda, K.; Mimachi, H.; Takahashi, M.; Sano, K.; Hyodo, K.; Takeda, T.; Gotoh, Y. Anomalously preserved clathrate hydrate of natural gas in pellet form at 253 K. J. Phys. Chem. C 2012, 116, 13842–13848. [Google Scholar] [CrossRef]
- Falenty, A.; Kuhs, W.F.; Glockzin, M.; Rehder, G. "Self-preservation" of CH4 hydrates for gas transport technology: Pressure-temperature dependence and ice microstructures. Energy Fuels 2014, 10, 6275–6283. [Google Scholar] [CrossRef]
- Mimachi, H.; Takeya, S.; Yoneyama, A.; Hyodo, K.; Takeda, T.; Gotoh, Y.; Murayama, T. Natural gas storage and transportation within gas hydrate of smaller particle: Size dependence of self-preservation phenomenon of natural gas hydrate. Chem. Eng. Sci. 2014, 118, 208–213. [Google Scholar] [CrossRef]
- Chuvilin, E.M.; Kozlova, E.V. Experimental estimation of hydrate-bearing sediments stability. In Proceedings of the 5th International Conference on Gas Hydrate, Trondheim, Norway, 13–16 June 2005; Volume 3, pp. 1506–1514. [Google Scholar]
- Chuvilin, E.M.; Guryeva, O.M. Experimental study of self-preservation effect of gas hydrates in frozen sediments. In Proceedings of the 9th International Conference on Permafrost, Fairbanks, Alaska, 23 June–3 July 2008; pp. 263–267. [Google Scholar]
- Kwon, T.-H.; Cho, G.-C.; Santamarina, J.C. Gas hydrate dissociation in sediments: Pressure-temperature evolution. Geochem. Geophys. Geosyst. 2008, 9, Q03019. [Google Scholar] [CrossRef]
- Hachikubo, A.; Takeya, S.; Chuvilin, E.; Istomin, V. Preservation phenomena of methane hydrate in pore spaces. Phys. Chem. Chem. Phys. 2011, 13, 17449–17452. [Google Scholar] [CrossRef] [PubMed]
- Takeya, S.; Fujihisa, H.; Gotoh, Y.; Istomin, V.; Chuvilin, E.; Sakagami, H.; Hachikubo, A. Methane clathrate hydrates formed within hydrophilic and hydrophobic porous media: kinetics of dissociation and distortion of host structure. J. Phys. Chem. C 2013, 117, 7081–7085. [Google Scholar] [CrossRef]
- Chuvilin, E.M.; Lupachik, M.V.; Guryeva, O.M. Kinetics research of ice transition into gas hydrate in porous media. In Physics and Chemistry of Ice; Furukawa, Y., Sazaki, G., Uchida, T., Watanabe, N., Eds.; Hokkaido University Press: Sapporo, Japan, 2011; pp. 127–132. [Google Scholar]
- Podenko, L.S.; Drachuk, A.O.; Molokitina, N.S.; Nesterov, A.N. Effect of silica nanoparticles on dry water gas hydrate formation and self-preservation efficiency. Russ. J. Phys. Chem. A 2018, 92, 255–261. [Google Scholar] [CrossRef]
- Yakushev, V.S.; Semenov, A.P.; Bogoyavlensky, V.I.; Medvedev, V.I.; Bogoyavlensky, I.V. Experimental modeling of methane release from intrapermafrost relic gas hydrates when sediment temperature change. Cold Reg. Sci. Technol. 2018, 31, 189–197. [Google Scholar] [CrossRef]
- Chuvilin, E.M.; Ebinuma, T.; Kamata, Y.; Uchida, T.; Takeya, S.; Nagao, J.; Narita, H. Effects of temperature cycling on the phase transition of water in gas-saturated sediments. Can. J. Phys. 2003, 81, 343–350. [Google Scholar] [CrossRef]
- Chuvilin, E.M.; Bukhanov, B.A. Effect of hydrate accumulation conditions on thermal conductivity of gas-saturated soils. Energy Fuels 2017, 31, 5246–5254. [Google Scholar] [CrossRef]
- Bukhanov, B.A.; Chuvilin, E.M.; Guryeva, O.M.; Kotov, P.I. Experimental study of the thermal conductivity of the frozen sediments containing gas hydrate. In Proceedings of the 9th International Conference on Permafrost, Fairbanks, Alaska, 23 June–3 July 2008; pp. 205–209. [Google Scholar]
- Chuvilin, E.M.; Bukhanov, B.A. Experimental study of the thermal conductivity of frozen hydrate-saturated sediments at atmospheric pressure. J. Earth’s Cryosphere 2013, 17, 69–79. (in Russian). [Google Scholar]
- Chuvilin, E.M.; Bukhanov, B.A.; Grebenkin, S.I.; Doroshin, V.V.; Iospa, A.V. Shear strength of frozen sand with dissociating pore methane hydrate: An experimental study. Cold Reg. Sci. Technol. 2018, 153, 101–105. [Google Scholar] [CrossRef]
- Chuvilin, E.; Bukhanov, B.; Cheverev, V.; Motenko, R.; Grechishcheva, E. Effect of ice and hydrate formation on thermal conductivity of sediments. In Impact of Thermal Conductivity on Energy Technologies; Shahzad, A., Ed.; IntechOpen: London, UK, 2018; pp. 115–132. [Google Scholar]
- Chuvilin, E.M.; Grebenkin, S.I. Dissociation of gas hydrates in frozen sands: effect on gas permeability. J. Earth’s Cryosphere 2018, 22, 41–45. (in Russian). [Google Scholar] [CrossRef]
- Takeya, S.; Uchida, T.; Kamata, Y.; Nagao, J.; Kida, M.; Minami, H.; Sakagami, H.; Hachikubo, A.; Takahashi, N.; Shoji, H.; et al. Lattice Expansion of Clathrate Hydrates of Methane Mixtures and Natural Gas. Angew. Chem. Int. Ed. 2005, 44, 6928–6931. [Google Scholar] [CrossRef] [PubMed]

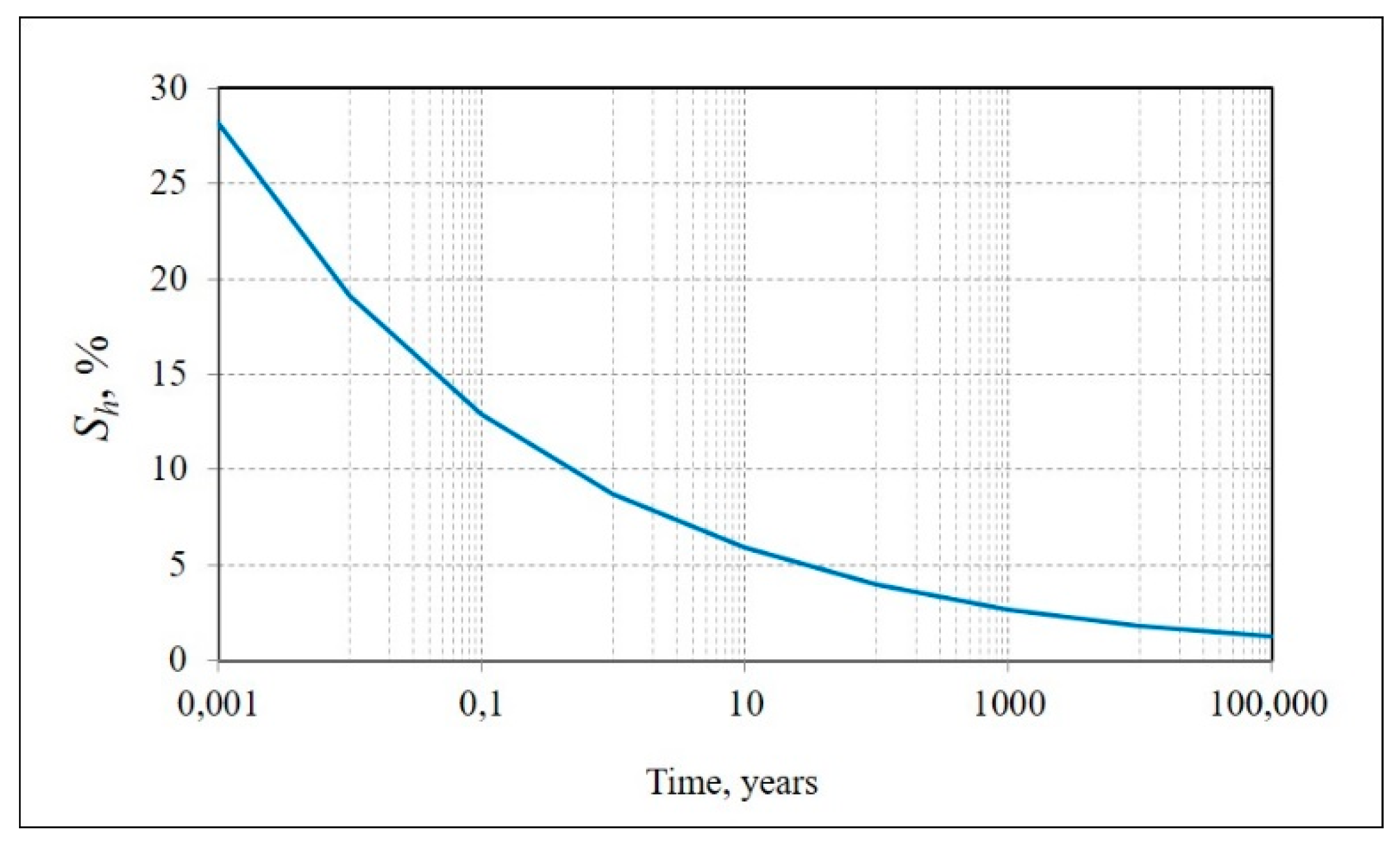
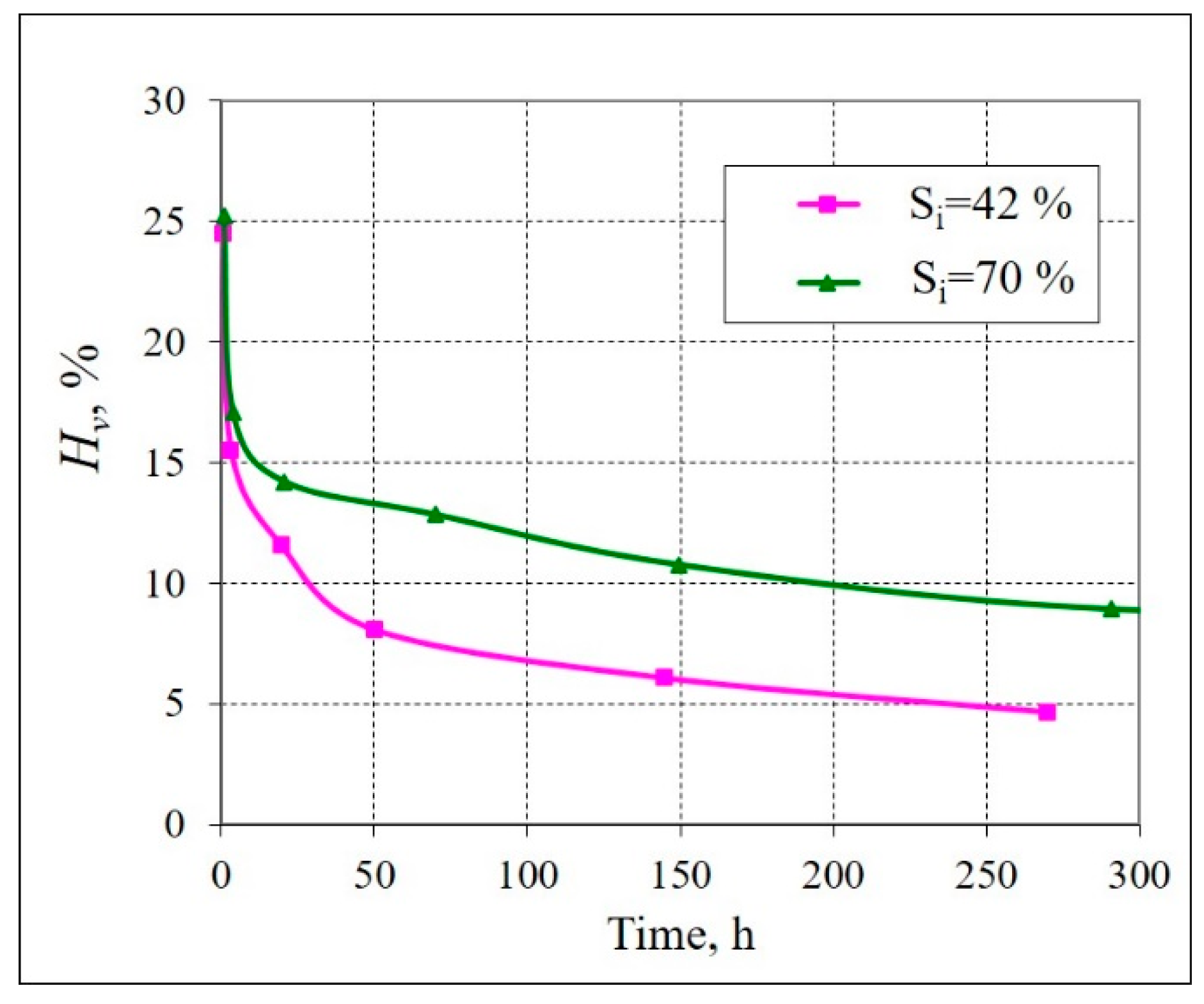
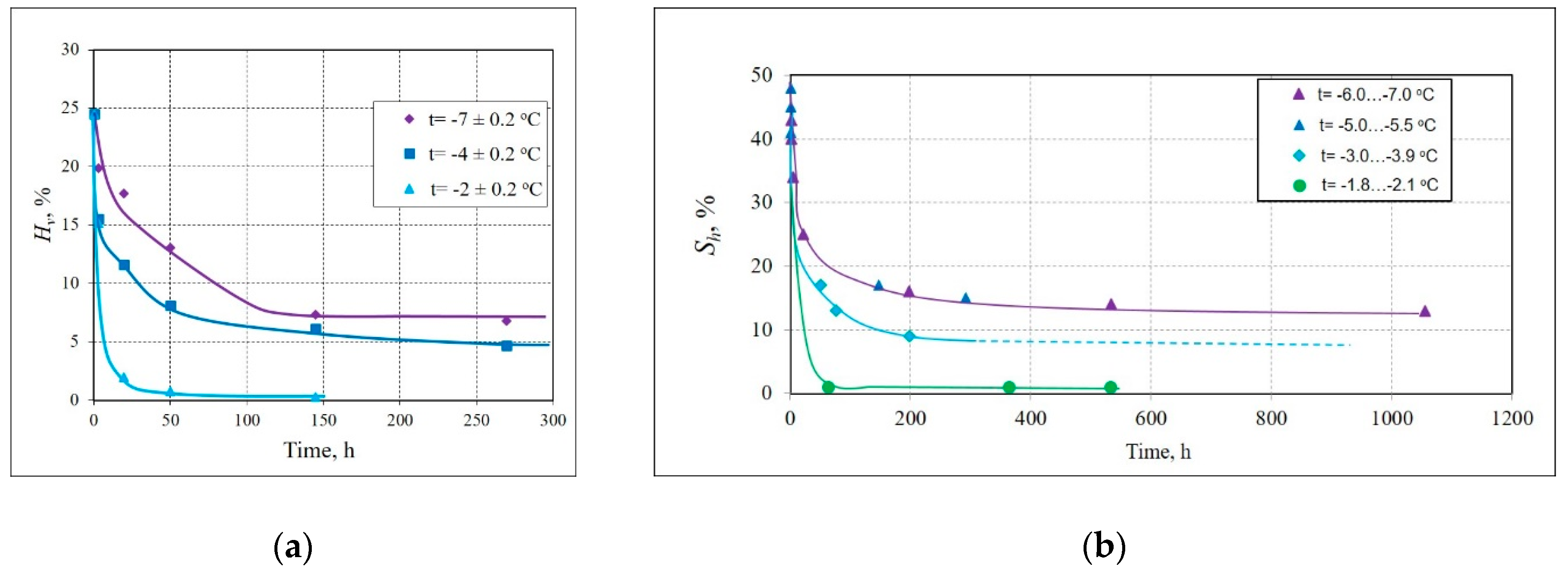
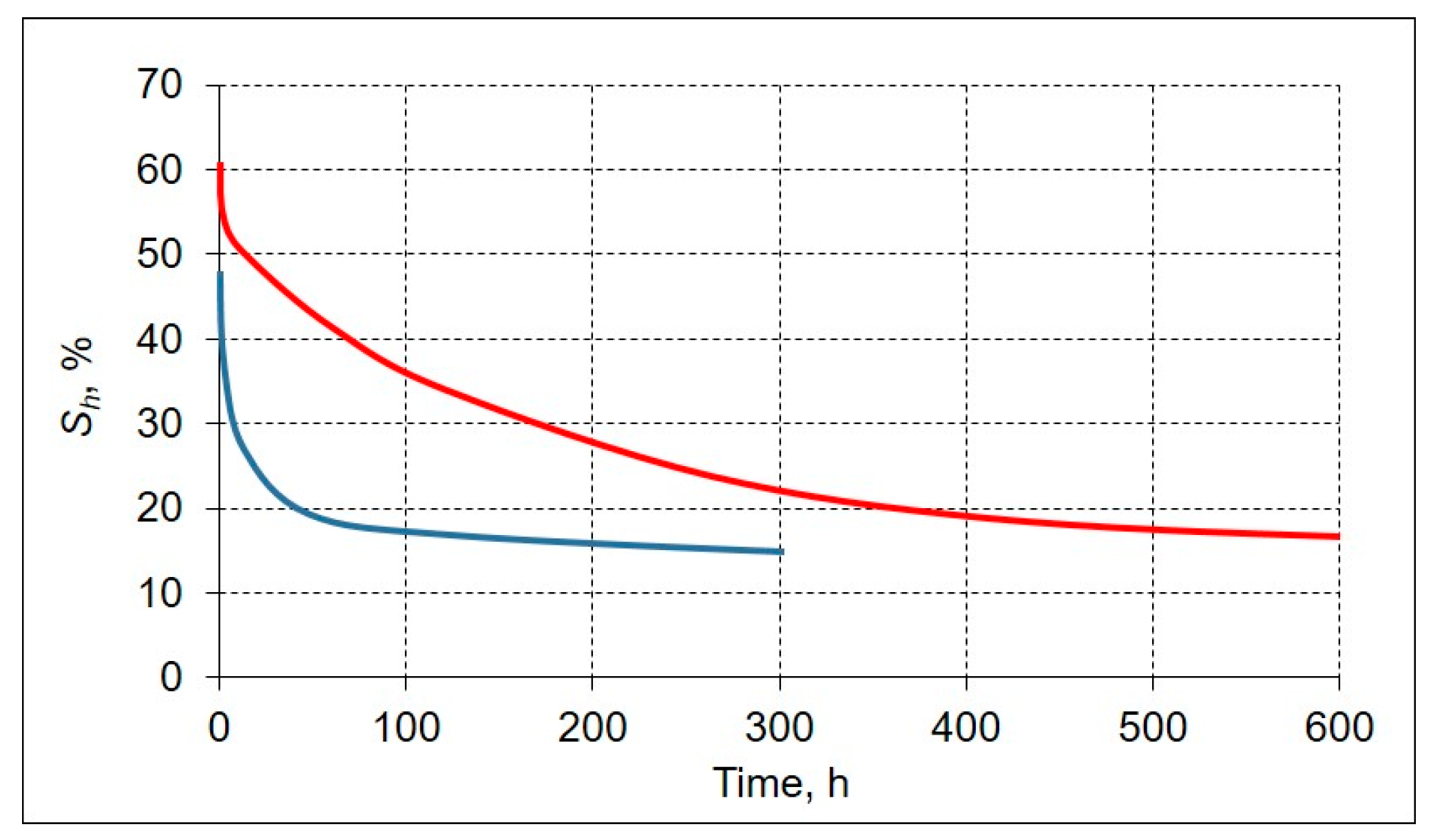
| Type of Sediment | Location and Core Depth | Particle Size Distribution, % | Mineralogy | Salinity, % | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1–0.5 mm | 0.5–0.25 mm | 0.25–0.1 mm | 0.1–0.05 mm | 0.05–0.001 mm | <0.001 mm | ||||
| Fine sand-1 | - | 6.5 | 6.5 | 79.6 | 2.2 | 3.1 | 2.1 | >90% quartz | 0.01 |
| Fine sand-2 | Yamburg gas field (~64 m) | 1.2 | 7.5 | 47.3 | 28.3 | 14.4 | 2.4 | 38% quartz 45% microcline + albite 9% illite 5% kaolinite + chlorite | 0.09 |
| Fine sand-3 | South Tambey gas field (36–46 m) | 0.1 | 12.5 | 62.9 | 21.9 | 1.6 | 1.0 | Quartz >90% | 0.11 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuvilin, E.; Bukhanov, B.; Davletshina, D.; Grebenkin, S.; Istomin, V. Dissociation and Self-Preservation of Gas Hydrates in Permafrost. Geosciences 2018, 8, 431. https://doi.org/10.3390/geosciences8120431
Chuvilin E, Bukhanov B, Davletshina D, Grebenkin S, Istomin V. Dissociation and Self-Preservation of Gas Hydrates in Permafrost. Geosciences. 2018; 8(12):431. https://doi.org/10.3390/geosciences8120431
Chicago/Turabian StyleChuvilin, Evgeny, Boris Bukhanov, Dinara Davletshina, Sergey Grebenkin, and Vladimir Istomin. 2018. "Dissociation and Self-Preservation of Gas Hydrates in Permafrost" Geosciences 8, no. 12: 431. https://doi.org/10.3390/geosciences8120431
APA StyleChuvilin, E., Bukhanov, B., Davletshina, D., Grebenkin, S., & Istomin, V. (2018). Dissociation and Self-Preservation of Gas Hydrates in Permafrost. Geosciences, 8(12), 431. https://doi.org/10.3390/geosciences8120431






