1. Introduction
Coral skeletons, mainly composed of aragonite, have been used for the reconstruction of past sea-level and paleoclimate in the tropics. In particular, the annual banded skeletons of
Porites grow over hundreds of years at a high growth rate of 11–20 mm/year [
1]. Therefore, the remanent magnetization of coral has been an attractive topic because corals have enormous potential for high-resolution paleomagnetic records of pre-observatory times. However, most coral skeletons have shown extremely weak magnetization, and their magnetic origin is not well understood. Sato et al. (2014) [
2] succeeded in measuring sufficient magnetization of ceased coral tsunamigenic boulders washed up on the beach of Ishigaki Island, despite using a conventional fluxgate spinner magnetometer with a sensitivity of 5 × 10
−9 Am
2. Although many studies on the rock magnetism of shallow-water carbonates have been conducted, recently ceased coral skeletons are a fairly new material applied to rock magnetism. It is necessary to determine the characterization of magnetic assemblages in these coral skeletons to utilize them as new paleomagnetic recorders because paleomagnetic records are affected not only by past geomagnetic field variations but also by the lithologic factors of the samples, such as the mineralogy, grain size, and concentration of the magnetic phases [
3].
According to previous rock-magnetic studies carried out on late Cenozoic shallow-water carbonate platforms, two different sources of magnetic mineral assemblages have been reported: biogenic magnetite (magnetosome) and detrital titanomagnetite, which are the main components of remanence carriers in the Bahamas [
4] and Tahiti [
5], respectively. In the case of samples located under rare terrigenous influx conditions, such as pelagic marine carbonates, reef platforms, and red clay, magnetosome can contribute to paleomagnetic records as stable single-domain magnetic carriers, e.g., [
4,
6,
7]. In Japan, Sakai and Jige (2006) [
8] carried out the characterization of magnetic particles in Holocene and Pleistocene shallow-water carbonates in the Ryukyu Islands, determining the presence of fine-grained, single-domain magnetite/maghemite. Based on transmission electron microscope (TEM) observations, they concluded that very short lengths (40–140 nm) and characteristic morphological features (elongate chains, multi-grain clusters) of magnetite crystals are compatible with magnetite chains formed by magnetotactic bacteria.
Identification of magnetosomes in geological samples has been conducted using micro- and macro-analyses, such as electron microscopy, first-order reversal curve (FORC) diagrams, and ferromagnetic resonance (FMR) spectra, which have been suggested to be effective methods for the detection of magnetosomes, e.g., [
9,
10]. The distinctive characteristics of biogenic magnetite, for instance, a stable single-domain size, narrow shape distribution, and chain-like structure, enable the detection of magnetosomes in geological materials through the combined use of electron microscopy and magnetic methods. FORC diagrams, e.g., [
11,
12,
13], have been widely used to characterize the magnetic particles in natural samples such as lake or sea sediment, e.g., [
14,
15,
16]. Such diagrams provide information on the statistical distribution of coercivities and magnetostatic interaction fields for magnetic particles within a sample. Egli et al. (2010) [
13] showed that FORC diagrams of environmental samples with abundant magnetosomes are characterized by a central ridge feature, indicating no or negligible magnetostatic interaction [
12,
13,
15]. This is because intact magnetosome chains in matrixes act like a well-dispersed uniaxial stable single-domain magnetic grain [
17,
18,
19].
Ferromagnetic resonance (FMR) spectroscopy has been used as a detector of the magnetic anisotropy effects derived from the mineralogy, composition, and crystal shape. Magnetic grains formed by magnetotactic bacteria are encapsulated in membranes and organized into chains. Because these magnetic grains in magnetosome chains are separated by only a few nanometers, each magnetosome should represent magnetic anisotropy like a single, elongated single-domain magnetic particle. Weiss et al. (2004) [
20] first showed that intact magnetotactic bacteria and well-dispersed intact chains of fossilized biogenic magnetite cause a distinguishable FMR spectrum, resulting primarily from the magnetic anisotropy of the magnetosomes, which can be readily distinguished in natural samples. Charilaou et al. (2011) [
21] presented a numerical simulation of the FMR spectra of randomly oriented magnetosome assemblies, which takes into account the magnetocrystalline and shape anisotropy field, and displayed very good correspondence with measured spectra of intact cells of magnetotactic bacteria. They modeled the linear particle chains of magnetosome as a prolate ellipsoid, which has its long axis parallel to the Miller index [111] crystallographic direction, the same as that of magnetosome. The magnetostatic interactions between the particles in the linear chain occur in a uniaxial shape anisotropy. Weiss et al. (2004) [
20] and Kopp et al. (2006) [
22] determined that intact magnetosome chains yield characteristic FMR spectra with secondary or multiple low-field absorption peaks and low-field extended asymmetry on the derivative spectra. They also reported that the FMR parameters of magnetosome-bearing samples are
geff < 2.12,
A < 1,
α < 0.30. They indicated that these traits are caused by the uniaxial-like anisotropy of the magnetosome chains [
21].
Although many magnetic techniques for magnetofossil detection have been developed, electron microscopic observations remain a powerful tool for detecting the distinctive morphology of magnetosomes. In the case of carbonate samples, such as pelagic limestones and stalagmites, the dissolving processes of a carbonate matrix are often conducted before a microscopic procedure used to concentrate the magnetic minerals. Freeman (1986) [
23] dissolved milled limestone rock samples with a buffered (pH 4) acetic acid solution, which does not alter the iron oxide or iron oxyhydroxide. This suggestion was applied to speleothems by Perkins (1996) [
24] to prepare samples for electron microscopy of the magnetic grains. Chang et al. (2014) [
10] conducted FMR and FORC analyses with the assistance of TEM observations on various types of sediments, and compared their advantages and limitations. They concluded that a combination of FMR and FORC measurements is efficient for the discrimination of many types of samples, although some inorganic materials can have FMR characteristics and a FORC central ridge similar to those of intact magnetosomes.
In this study, we characterized the magnetic mineral assemblages of corals in Ishigaki Island using FMR spectroscopy, FORC measurements, and petrologic observations with a field-emission type scanning electron microscope (FE-SEM) to reveal why the corals in Ishigaki possess such measurable magnetic remanence. Owing to its low NRM intensities and low concentration of magnetic minerals, acid treatment (e.g., Freeman (1986) [
23], Perkins (1996) [
24]) was also conducted on powdered corals, not only for FE-SEM observations but also for FORC and FMR measurements.
2. Materials and Methods
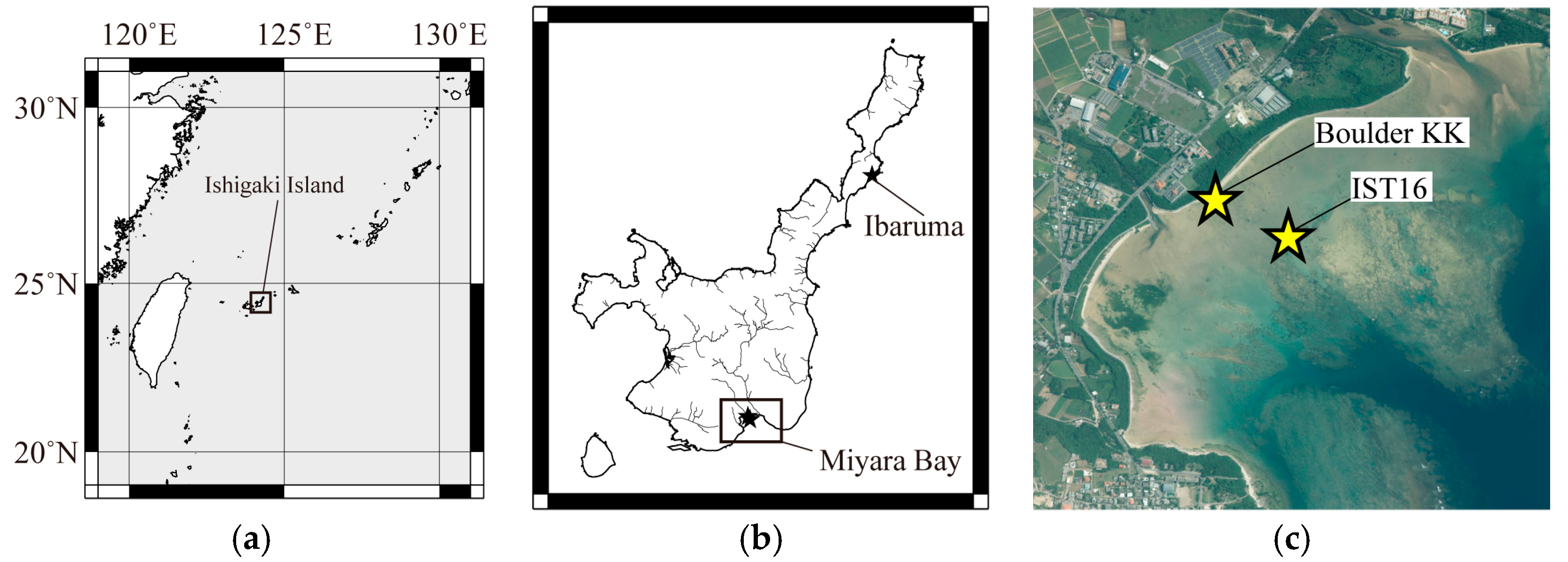
Ishigaki Island is rimmed by coral reefs grown on the bedrock of Ryukyu limestone and Jurassic schist. Large tsunamis transport colonies of
Porites and coral boulders from the shallow lagoon and reef edge, and throw them up onto the reef flat and lowlands. This study focuses on four recently ceased coral tsunamigenic boulders collected from Ishigaki Island (
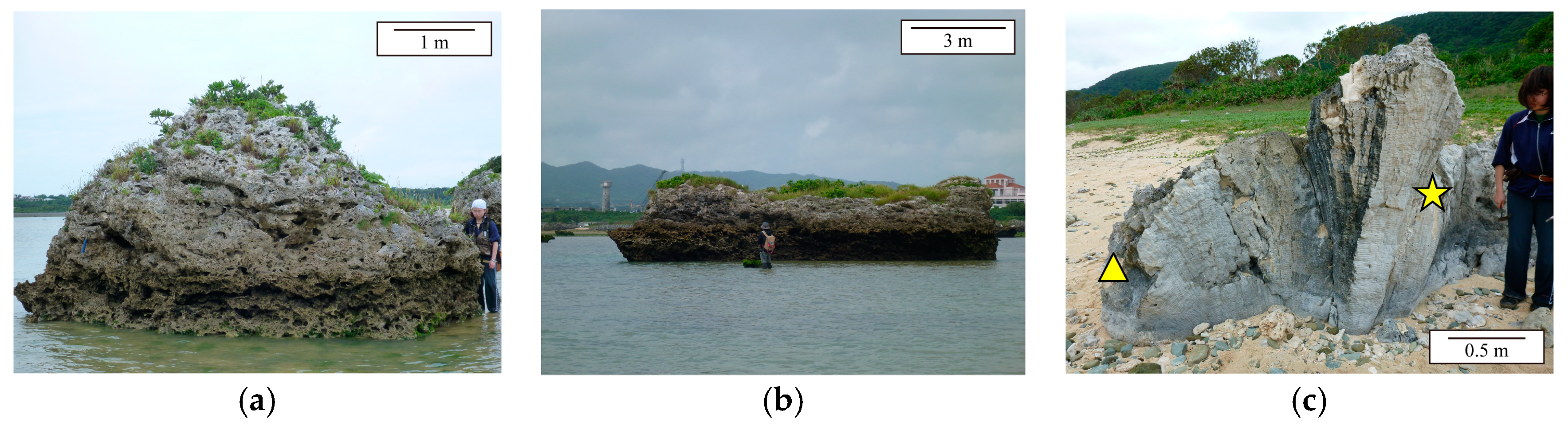
Figure 1). Two of the samples were collected from two coral tsunami boulders at Miyara Bay, on the southeastern shoreline of the island. The first, Boulder KK (24°21′15.91″ N, 124°12′18.28″ E, named in [
25]), is an approximately 40-ton reef boulder near the shoreline (
Figure 2a). The second, IST16, was collected from a roughly cylindrical reef boulder (24°21′07.75″ N, 124°12′24.31″ E), which has a diameter of 10 m and a height of ~3 m (
Figure 2b). These two tsunami boulders composed of small coral colonies and fragments (e.g., massive and tabular corals [
26]). The natural remanent magnetizations of these boulders were reported in [
2]. In addition, two samples were collected from single-colony
Porites at Ibaruma (24°31′22.65″ N, 124°18′05.98″ E), on the northeastern coast of the island, and named K09 and IB02, respectively (
Figure 2c). The top and bottom of the
Porites colony were dated from A.D. 1298–1621 and A.D. 1360–1715, respectively, using
14C. The natural remanent magnetizations of these subsamples are represented in the
Appendix (
Figure A1 and
Figure A2).
To concentrate the magnetic minerals, the following procedure for dissolution of the carbonate matrix was conducted prior to the FE-SEM observations (Boulder KK and K09), FORC measurements (IST16, K09, and IB02), and FMR spectroscopy (IST16, K09, and IB02). Magnetic extraction from the resulting residue was also conducted only for the microscopic observations. Coral skeleton specimens were subsampled using a nonmagnetic diamond saw and crushed with a wooden hammer to prevent contamination of trace metals from the cutting processes, after which the subsamples were sonicated in water and dried at room temperature. Each group of subsamples was then additionally ground using an agate mortar and pestle. The powdered coral skeleton was added to a 500 mL beaker and dissolved in an aqueous acetic acid solution. During the dissolution, the beaker was left for 7–14 days, and the pH was controlled from 4 to 5.5. For the FE-SEM (JEOL 7001F FE-SEM at the Department of Earth Science, Tohoku University) observations, the remaining skeleton and iron oxide residue were collected using a permanent neodymium (NdFeB) magnet inserted within a test tube and pipette, and transferred onto a glass slide after the dissolution. The last residue was dried in an oven for subsequent FMR and FORC measurements.
FMR spectra were obtained using a JEOL JES-RE2X ESR spectrometer at the Department of Chemistry, Tohoku University. In the FMR experiments, a sample is exposed to a change in the direct current (DC) magnetic fields using a fixed microwave. The magnetic moments in a sample absorb microwave energy at an amount that depends on the strength of the external and any internal magnetic fields, and sustain the Larmor precessional motion in an applied field. This absorption occurs when the precession frequency of the magnetization, changed by a static DC magnetic field
B, corresponds to the perpendicularly applied microwave frequency
ν. The resonance condition can be described as follows:
where ħ is Plank’s constant,
ν is the frequency of a microwave,
g is Landé’s splitting factor, μ
B is Bohr magneton, and
B is the applied magnetic field.
According to pioneering studies (e.g., [
20,
22,
27]), some parameters have been defined for an analysis of the FMR spectra, namely,
geff,
Blow,
Bhigh, Δ
BFWHM, and
A. The effective
g-factor (Landé factor) is given as follows:
where
Beff, which is the effective magnetic field, is defined as the magnetic field of maximum absorption, or synonymously, the zero crossing field of the derivative spectrum. In addition,
Blow and
Bhigh are the positions of half maximum absorption at the low- and high-field ends of the absorption peak, respectively (e.g., [
20,
22,
27]), and Δ
Blow and Δ
Bhigh are given by Δ
Blow =
Beff −
Blow and Δ
Bhigh =
Bhigh −
Beff, respectively. Their sum provides the full width at half maximum Δ
BFWHM. The asymmetry ratio
A is ascertained through the following equation:
An empirical discrimination parameter that combines
A and Δ
BFWHM is indicated by
α, and is defined as
in [
22].
For each measurement, ~100–150 mg of air-dried coral powder sample was loaded into glass tubes and exposed to microwaves at a frequency of ~9.4 GHz (X-band) with power of 1 mW. The range of the applied magnetic field was from zero to 800 mT. FORC measurement for Boulder KK was conducted using a vibrating sample magnetometer (VSM) (Model 29/3902, Princeton Measurements) at the Japan Agency for Marine-Earth Science and Technology (JAMSTEC) on dry chip subsample. FORC diagram for Boulder KK was obtained by stacking 10 measured FORC diagrams because of weak magnetization. The other FORC measurements were conducted using an alternating gradient magnetometer (AGM) (MicroMag 2900 series AGM, PMC) at the National Institute of Advanced Industrial Science and Technology (AIST) on powdered subsamples (See
Table 1 for sample mass and magnetic concentration for FMR and FORC measurements). FORCinel v.3.06 software [
28] was used for the data processing. Key parameters for FORCinel calculations (see [
29] for details) were: s
c,0 = 3, s
c,1 = 5, s
b,0 = 3, s
b,1 = 5 and λ = 0.1.
FE-SEM observations were conducted on two subsamples (Boulder KK and K09). The samples were prepared for SEM observation by allowing approximately 80 μL of the suspension of the dissolved coral skeleton residue to dry on a glass slide with a carbon coating. Energy dispersive spectroscopy (EDS) was conducted using an x-act (Oxford Instruments, Abingdon, Oxfordshire, UK) for an elemental analysis.
3. Results
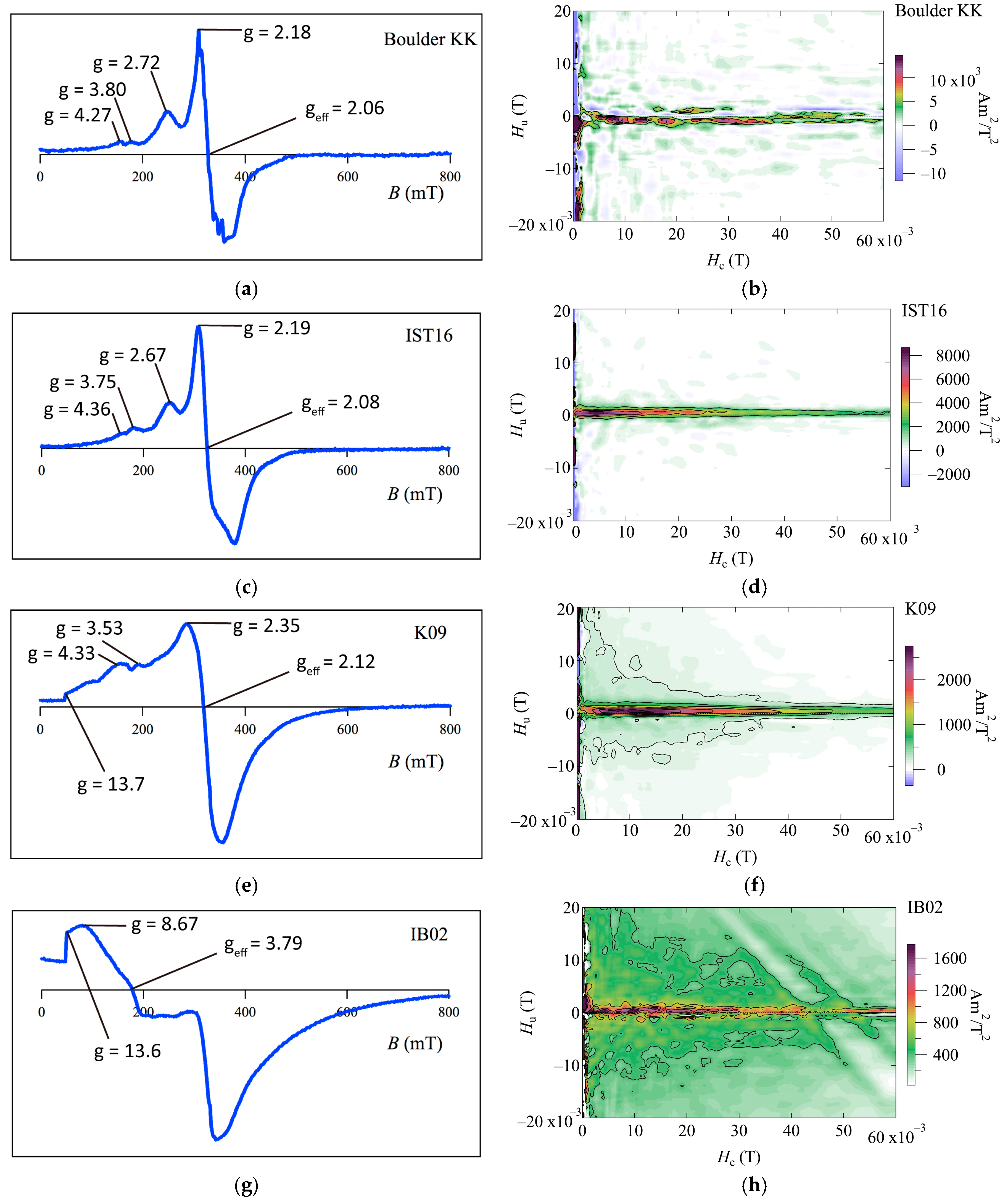
Most of the FMR derivative spectra of the coral skeleton samples, with the exception of IB02, indicate multiple derivative maxima and extended low-field absorption, with
A between 0.56 and 0.95,
geff between 2.06 and 2.11, and Δ
BFWHM between 111 and 163 mT (
Figure 3 and
Table 2). Following the criteria used in [
20,
22] (
A < 1,
geff < 2.12, and
α < 0.3), these features are all consistent with the unusual feature of intact biogenic magnetite chains and magnetosome-bearing carbonates, although these samples show FMR spectra broadening at low fields. This broadening is due to inclusions of an isotropic magnetite component [
10]. The FMR spectrum for IB02 from the
Porites colony shows complex characteristics, particularly at low fields (
Figure 3g). The FMR parameters for IB02 are as follows:
geff = 3.79, Δ
BFWHM = 237.8 mT,
A = 1.45 > 1, and
α = 0.48 > 0.3. The spectrum formation and parameters resemble those of the FMR spectrum of continental margin marine sediment samples from core MD01-2421, off the shore of central Japan, in the northwest Pacific [
10].
Some of our FORC diagrams of the coralline boulders (Boulder KK and IST16) show a dominant central ridge feature with negligible vertical spread (
Figure 3b,d). The results from one of the
Porites colony samples (K09) show a central ridge feature with a vertical spread (
Figure 3f), which indicates the existence of coarser-grained detrital component particles [
12,
30,
31]. These narrow ridges have a peak within an
Hc range of ~0–20 mT, which is slightly different from the typical range for the coercivity of SD magnetite, at 20–30 mT [
14]. This is likely due to the contribution of collapsed magnetosome chains, which will depress the peak coercivity [
10]. As discussed in Ludwig et al. (2013) [
16], this can be due to an admixture of isolated SD or PSD magnetite particles either of authigenic/pedogenic origin, or in the form of inclusions in silicates of terrigenous origin. Therefore, FORC diagrams indicate that the magnetization of these coral skeletons is mainly carried out through intact magnetosomes and mixtures of grains from collapsed magnetosomes, pseudo-single domain grains, and multi-domain grains such as magnetite grain and magnetic inclusions in silicate, which have terrigenous origin. For the
Porites colony sample IB02 (
Figure 3h), the diagram shows a FORC distribution located at
Hu,
Hc = 0.
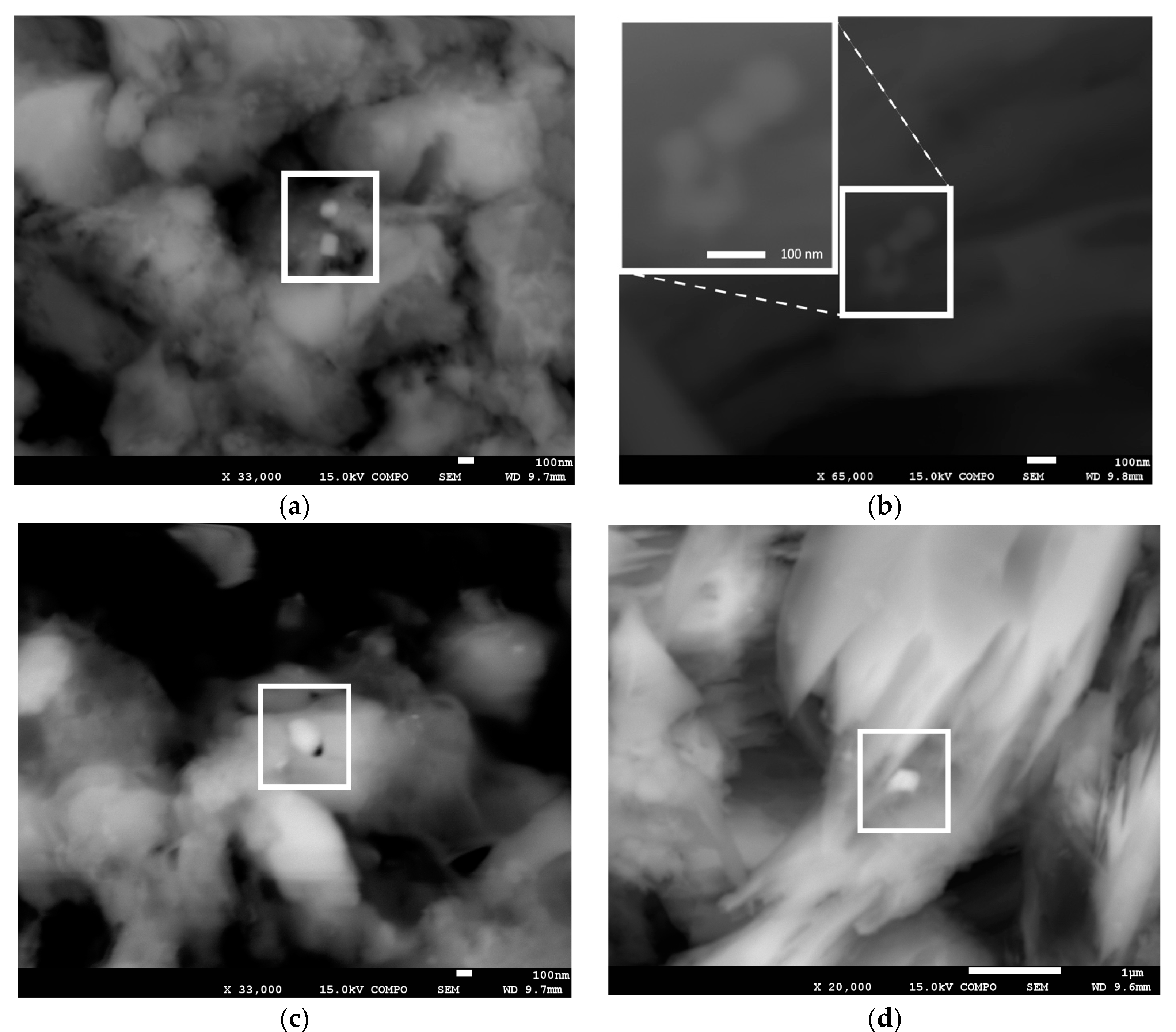
FE-SEM observations of Boulder KK and K09 indicate the existence of rectangular-shaped iron oxide grains in the coral skeletons (
Figure 4a). The sizes of the grains are around 100 nm, with most of the grains composing magnetosomes having grain sizes of between ~40 and ~150 nm [
32]. The existence of a very short chain of iron oxide grains was also observed in K09 (
Figure 4b), suggesting the origin of biogenic magnetite. On the other hand, some iron oxides are observed that represent neither rectangular-shaped morphology nor a chain-like structure (
Figure 4c,d). Therefore, the results of FE-SEM observations suggest that the magnetic carriers of coral skeletons on Ishigaki Island are composed of biogenic fine-grained iron oxides that have different (e.g., detrital) origin.
4. Discussion
FORC diagrams for Boulder KK and the cylindrical reef boulder (IST16) have a narrow ridge FORC distribution along the
Hc axis with a lack of vertical spread (
Figure 3a,c), which is called a central ridge feature, e.g., [
13]. FMR derivative spectra for these coral boulders contain a low-field secondary peak, and the FMR parameters fall within the
geff < 2.12,
A < 1, and
α < 0.3 regions (
Figure 3a,c and
Table 2). These signatures are all consistent with the presence of intact magnetosome chains, as based on [
13,
20,
22]. Therefore, we propose that magnetic carriers in two coral boulders in Miyara Bay are composed mainly of biogenic magnetite chains.
The FORC diagrams and FMR spectra showed distinct features for the K09 and IB02 subsamples from the
Porites colony at Ibaruma beach. For K09, the FMR spectrum contains extended low-field absorption lines similar to a near-edge absorption, with the FMR parameters satisfying the criteria of
geff < 2.12,
A < 1, and
α < 0.3 (
Figure 3e and
Table 2). Magnetostatic interactions within concentrated magnetic particle assemblages may cause the FMR spectra broadening at low fields. To clarify this, nominal magnetite contents are calculated comparing saturation magnetization of samples with one for magnetite of 92 Am
2/kg [
33] (
Table 1). The values range from 1~8 wt %, suggesting that most magnetic particles were originally attached to non-carbonate constituents and remained in-place during the extraction procedure. Therefore, the extraction procedure remains some chains of magnetosome.
The FORC diagram shows a central ridge feature with a vertical spread and lower coercivity (
Figure 3f). According to [
31], these traits indicate the presence of both magnetosome and coarser-grained detrital components. It is also supported by FE-SEM observations, which indicate the presence of iron oxides from biogenic and different origin (
Figure 4b,d). For another subsample, IB02, the FORC diagram shows weak central ridge, vertical spread feature, and a FORC distribution around the origin of the diagram (
Figure 3h). The latter may suggest the presence of magnetic grains at or just below the superparamagnetic threshold volume [
12]. The FMR spectrum shows different forms and parameters from the magnetosome bearing samples, which resemble those of a continental margin marine sediment sample from off the shore of central Japan, as measured in [
10] (
Figure 3g). These traits were interpreted in [
10] as possible contributions from mixtures of the domain states and magnetic minerals, which have a detrital origin. Our results indicate that the main magnetic carriers of the
Porites colony are from both biogenic and detrital fine-grained magnetite, the balance of which might vary depending on the part of the coral skeleton considered. FORC and FMR measurements, and petrologic observations using FE-SEM, were conducted to characterize the magnetic mineral assemblages of coral boulders on Ishigaki Island. Combined analyses of a FORC diagram and FMR spectroscopy confirmed the presence of magnetosomes in the coralline boulders from Miyara Bay. They also indicated that the main magnetic carriers of the
Porites colony on the Ibaruma coastline are both magnetosomes and other magnetic minerals, the balance of which might vary dramatically from one part of the
Porites skeleton to another. The coral skeletons in Ishigaki Island have the potential to provide a role as a new paleomagnetic recorder if some attention is paid to their origin of magnetic remanence.