Is the Kornati National Park Still an Acceptable Reference Area for Environmental Studies?
Abstract
1. Introduction
2. Materials and Methods
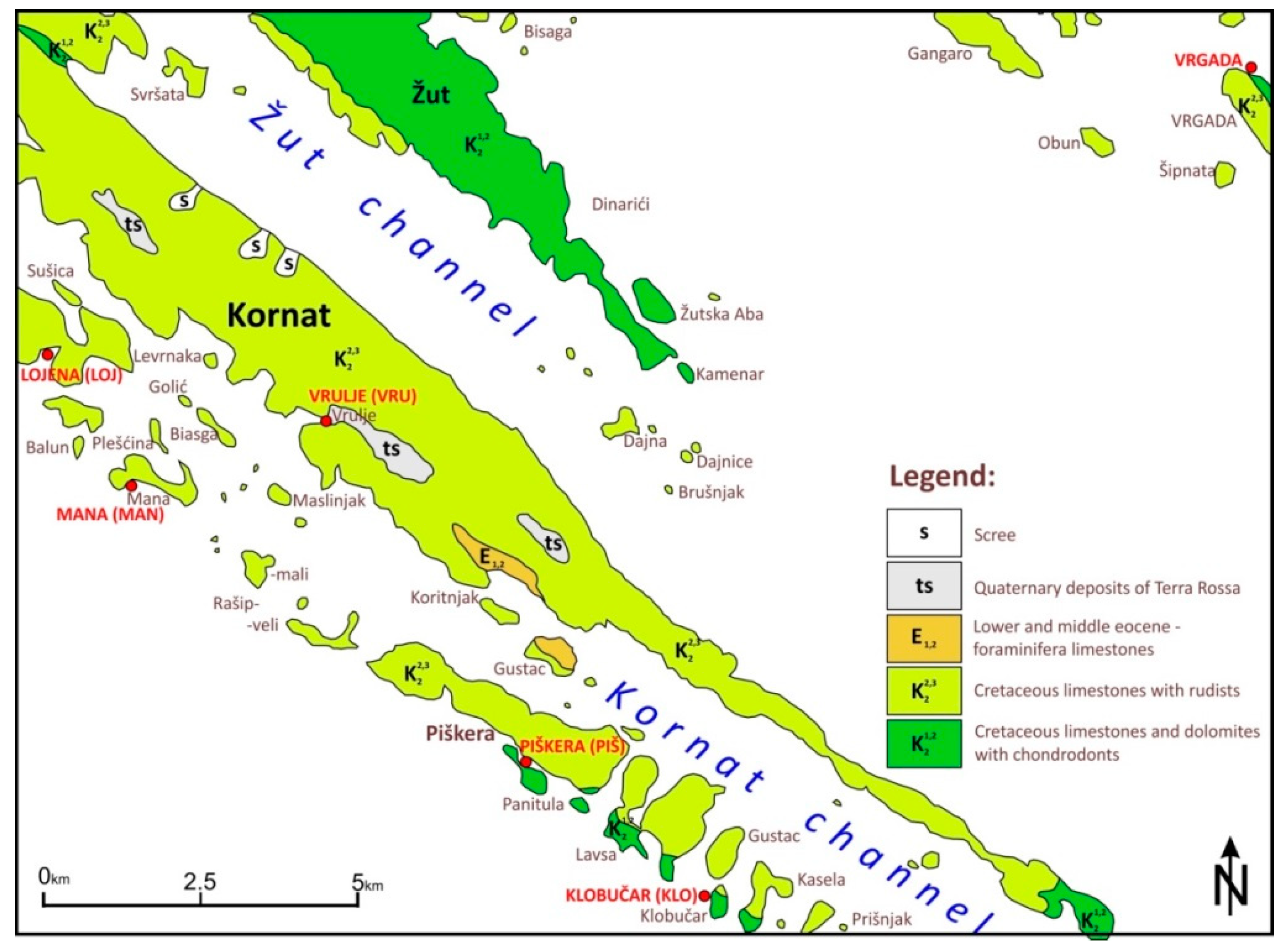
2.1. Environmental Setting
2.2. Sample Preparations and Methodology
3. Results
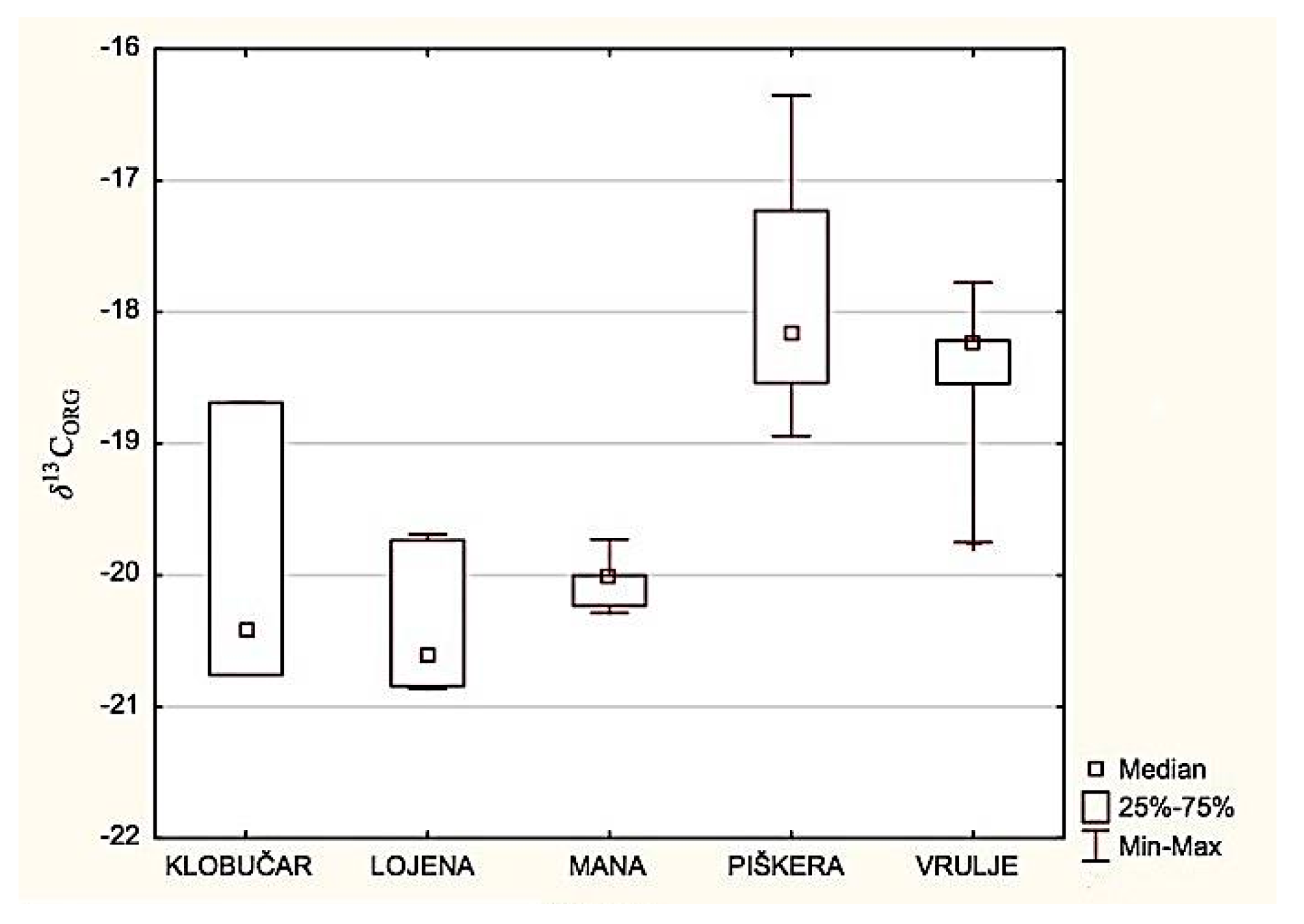
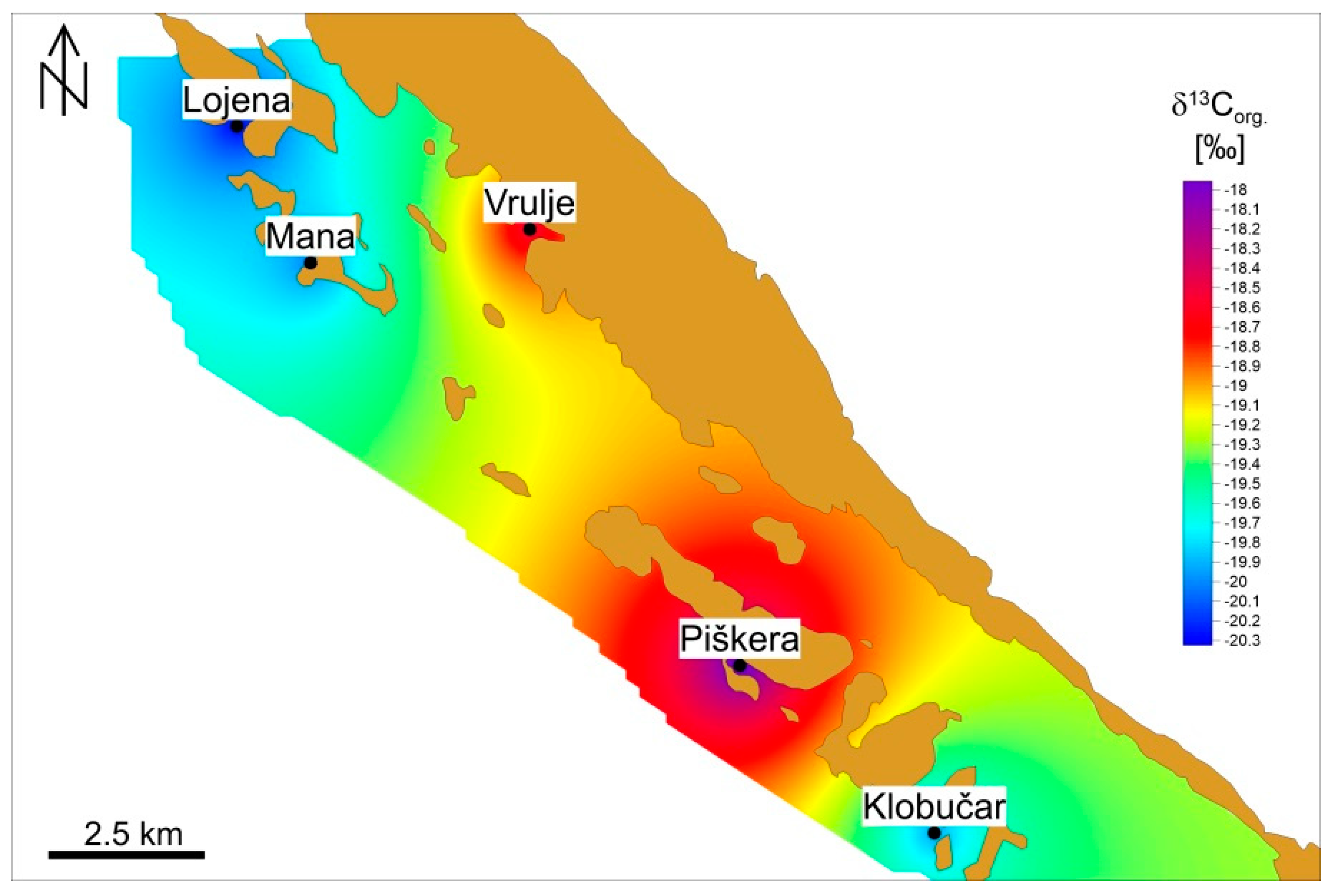
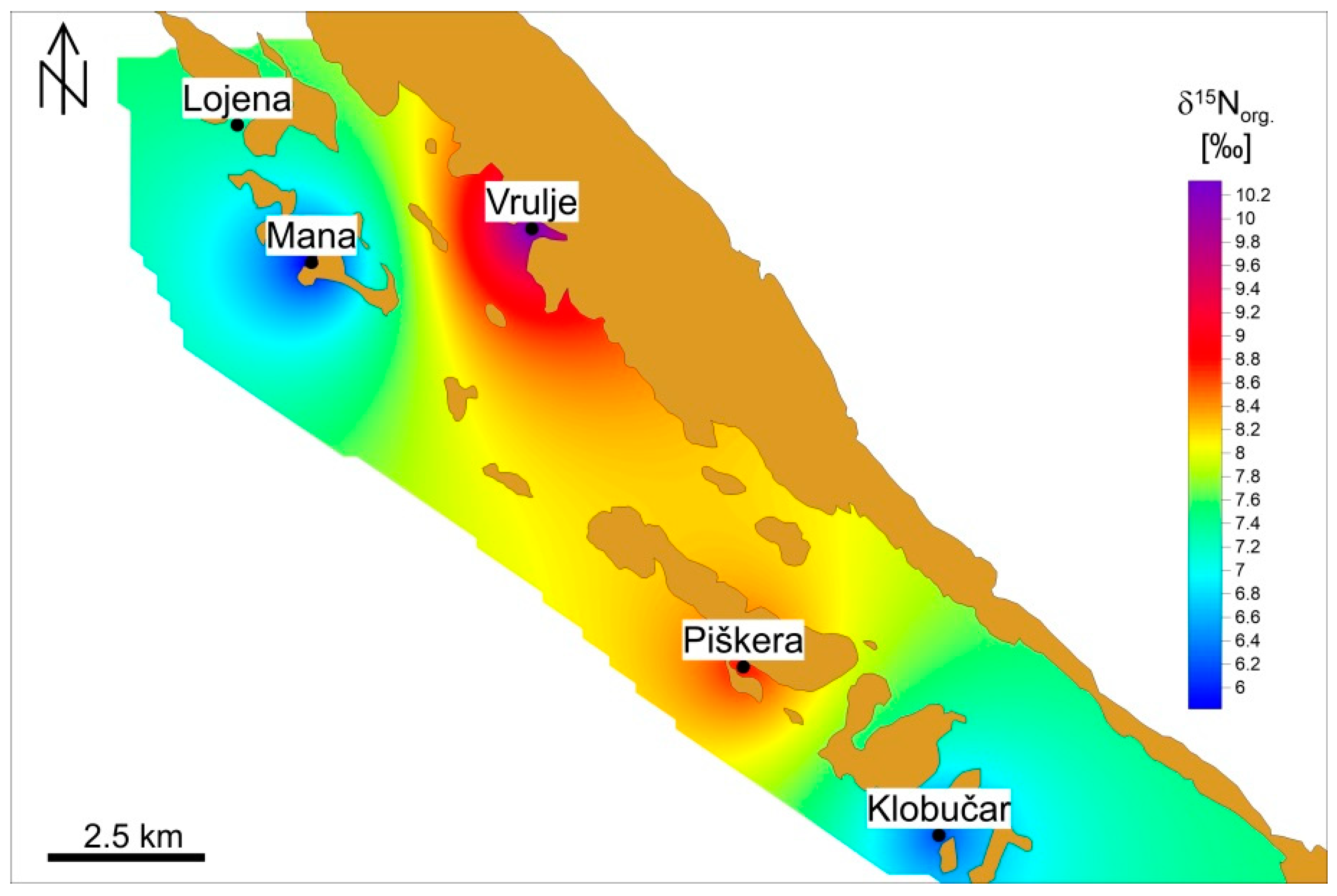
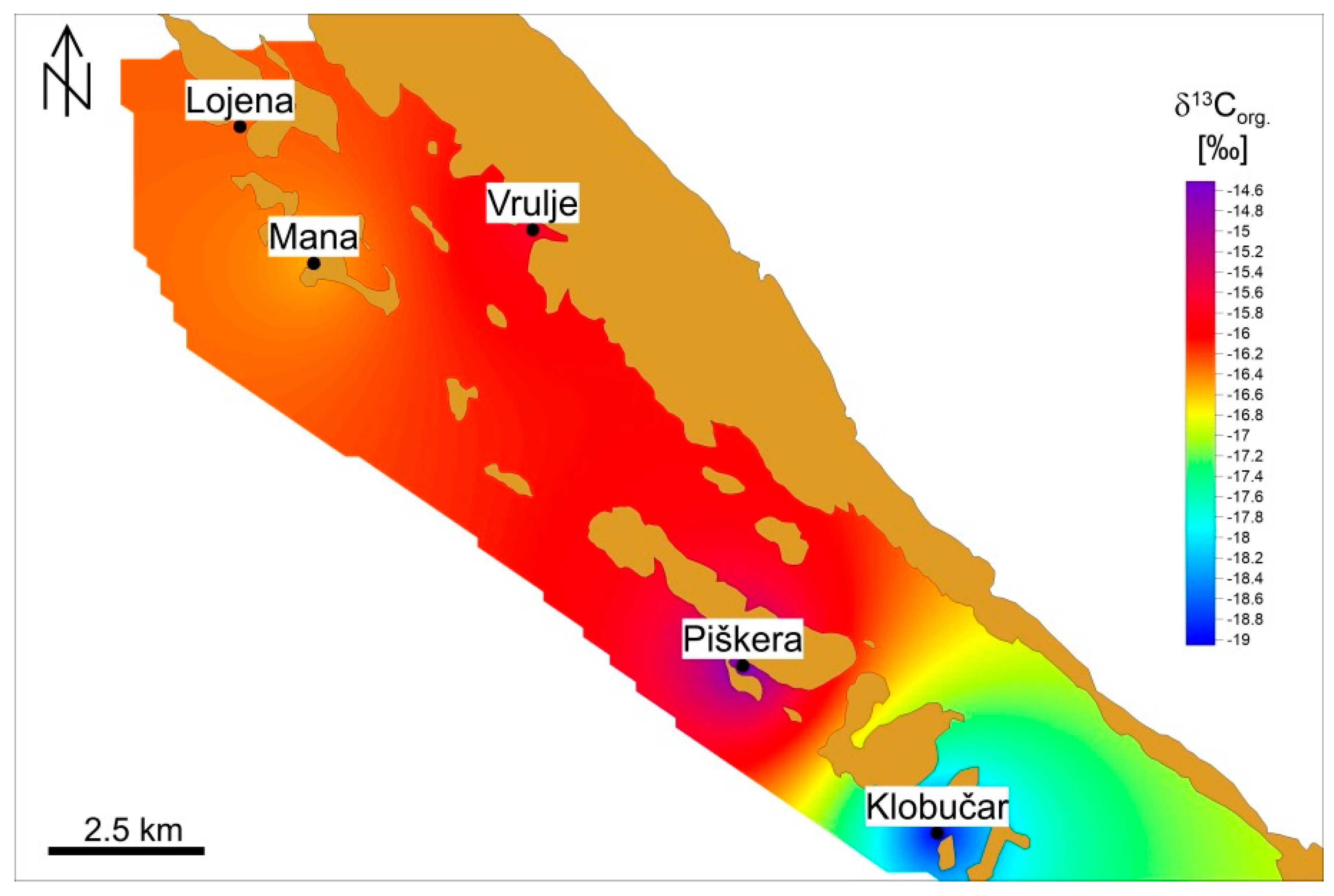
3.1. Isotopic Analysis of Organic Carbon in the Sediment
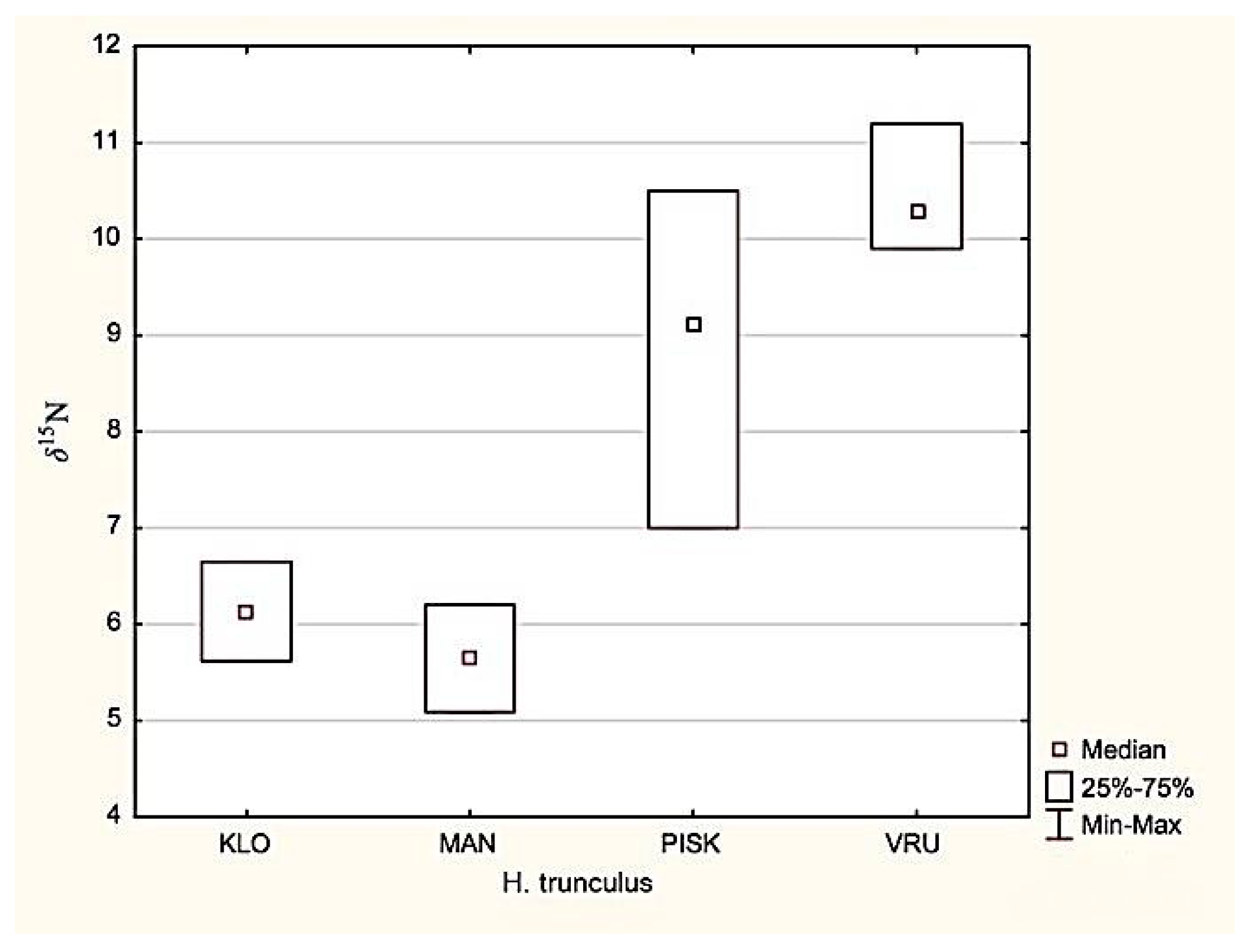
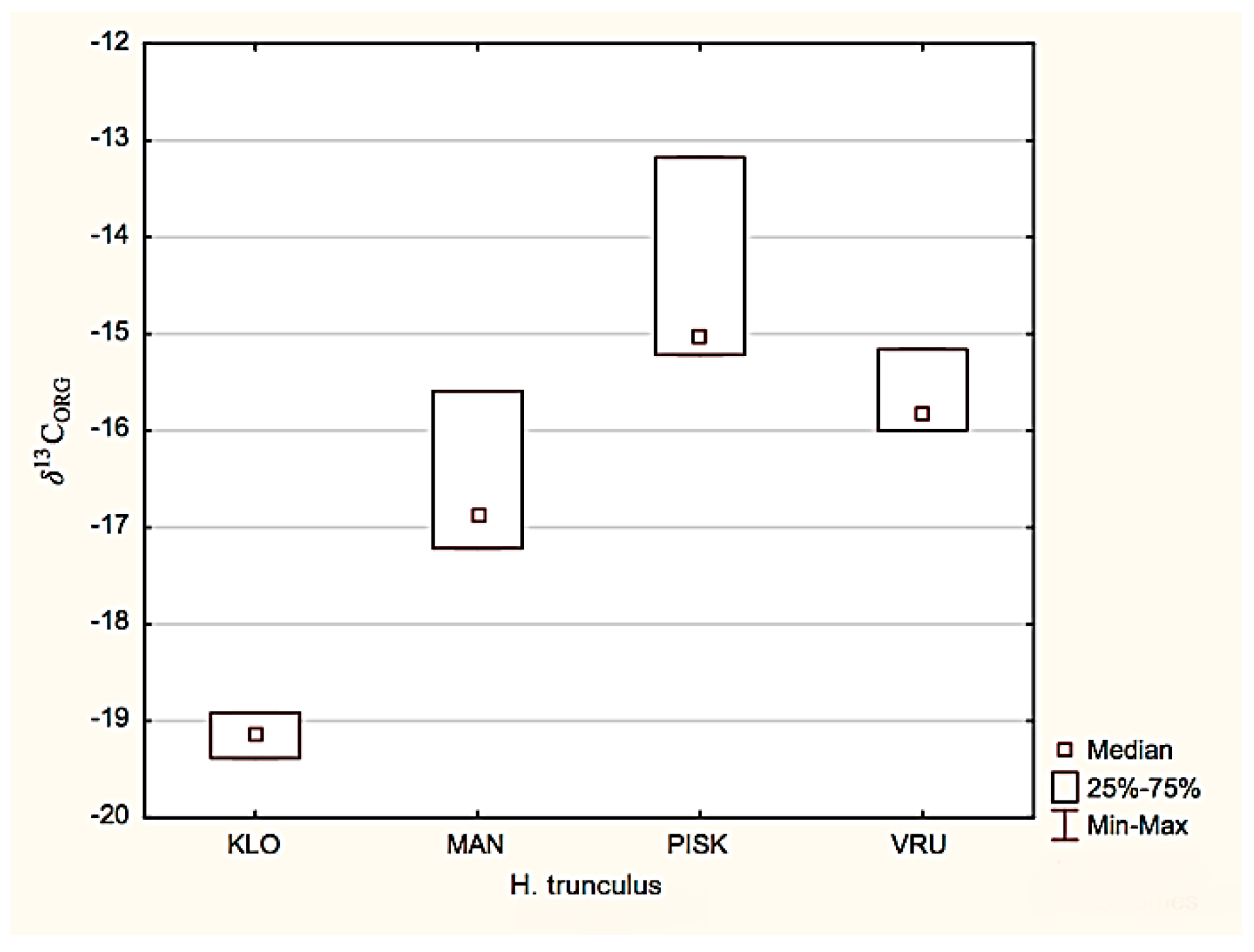
3.2. Isotopic Analysis of Nitrogen and Organic Carbon in the Muscle Tissues of the Banded Dye-Murex H. Trunculus
4. Discussion
4.1. Sediment
4.2. Hexaplex trunculus
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Correction Statement
References
- National Research Council. Understanding Marine Biodiversity; National Academy Press: Washington, DC, USA, 1995. [Google Scholar]
- Cresson, P.; Ruitton, S.; Harmelin-Vivien, M. Artificial reefs do increase secondary biomass production: Mechanisms evidenced by stable isotopes. Mar. Ecol. Prog. Ser. 2014, 509, 15–26. [Google Scholar] [CrossRef]
- Michener, R.H.; Lajtha, K. Stable Isotopes in Ecology and Environmental Science; Blackwell Pub.: Malden, MA, USA, 2007. [Google Scholar]
- Malej, A.; Dolenec, T.; Lojen, S. Variability of 13C/12C and 15N/14N in different mussel tissues (Mytilus galloprovinvialis): Implications for food web studies. Rapp. Comm. int. Mer Medit. 1998, 35, 272–273. [Google Scholar]
- Žvab, R.P.; Dolenec, T.; Lojen, S.; Kniewald, G.; Dolenec, M. Use of stable isotope composition variability of particulate organic matter to assess the anthropogenic organic matter in coastal environment (Istra Peninsula, Northern Adriatic). Environ. Earth Sci. 2014, 73, 3109–3118. [Google Scholar] [CrossRef]
- De Souza, B.J.R.; Costa, A.B.; de Azevedo, G.A.E.; dos Santos, R.T.H.; Spano, S.; Lentini, C.A.D.; Bonabamba, T.J.; Silva, R.O.; Novotny, E.H.; do Rosario-Zucchi, M. Carbon and nitrogen stable isotope compositions of organic matter in marine sediment cores from the Abrolhos region: Indicators of sources and preservation. Geochim. Brasiliensis 2013, 27, 13–23. [Google Scholar] [CrossRef]
- Fry, B. Stable Isotope Ecology; Springer: New York, NY, USA, 2006. [Google Scholar]
- Dolenec, T.; Lojen, S.; Dolenec, M.; Lambaša, Ž.; Dobnikar, M.; Rogan, N. 15N and 13C Enrichment in Balanus perforatus: Tracers of Municipal Particulate Waste in the Murter Sea (Central Adriatic, Croatia). Acta Chim. Slov. 2006, 53, 469–476. [Google Scholar]
- Fretter, V.; Graham, A. British Prosobranch Molluscs; The Ray Society: London, UK, 1962. [Google Scholar]
- Romeo, M.; Gharbi Bouraoui, S.; Gnassia Barelli, M.; Dellali, M.; Aissa, P. Responses of Hexaplex (Murex) trunculus to selected pollutants. Sci. Total Environ. 2006, 359, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Marzouk, Z.; Aurelle, D.; Said, K.; Chenuil, A. Cryptic lineages and high population genetic structure in the exploited marine snail Hexaplex trunculus (Gastropoda: Muricidae). Biol. J. Linn. Soc. 2017, 122, 411–428. [Google Scholar] [CrossRef]
- Pados, T. A Time-Lapse Camera Experiment on Benthic Reactions to Anoxia in the Northern Adriatic Sea. Master’s Thesis, Faculty of Life Sciences, University of Vienna, Vienna, Austria, 2010. Available online: http://othes.univie.ac.at/10322/ (accessed on 18 October 2018).
- Surowiec, I.; Nowik, W.; Moritz, T. Mass spetrometric identification of new minor indigoids in shellfish purple dye. Dyes Pigments 2012, 94, 363–369. [Google Scholar] [CrossRef]
- Abramovič, M. Regionalna Geografija Kornatov. Bachelor’s Thesis, University of Ljubljana, Faculty of Arts, Ljubljana, Slovenia, 2009. Available online: https://repozitorij.uni-lj.si/IzpisGradiva.php?id=23459&lang=eng&prip=dkum:19556:d3 (accessed on 18 October 2018).
- Kalušič, S. Kornatska otočna skupina. Geografski Glasnik 1965, 27, 215–245. Available online: https://hrcak.srce.hr/56136 (accessed on 18 October 2018). (In Croatian).
- Mamužić, P.; Nedela-Devide, D. Basic geological map of SFRY [ca 1:100.000]. Tolmač list Zadar (K 33-7); Zvezni Geološki Zavod: Beograd, Serbia, 1963. [Google Scholar]
- Cigrovski-Detelić, B.; Bonaca, J.; Bučo, I. National Parks of Croatia. In Proceedings of the “GIS ODYSSEY 2010”, Geographic Information Systems Conference and Exhibition, Pula, Croatia, 3–9 September 2010. [Google Scholar]
- Public Institution National Park Kornati. Management Plan (2014–2023). Available online: http://www.np-kornati.hr/images/plan_upravljanja/Plan_upravljanja_NP_Kornati_tekst_%20i_zone.pdf (accessed on 18 October 2018).
- Tourism in Figures. 2016. Available online: https://www.htz.hr/sites/default/files/2017-06/Tourism_in_figures_ENG_2016.pdf (accessed on 18 October 2018).
- Sustainable Tourism Development Strategy for the Broader Kornati National Park Area. Available online: http://np-kornati.hr/images/novosti/sustainable-tourism-development.pdf (accessed on 18 October 2018).
- Kennedy, H.; Beggins, J.; Duarte, M.C.; Fourqurean, J.W.; Holmer, M.; Marba, N.; Middelburg, J.J. Seagrass sediments as a global carbon sink: Isotopic constraints. Glob. Biogeochem. Cycles 2010, 24, GB4026. [Google Scholar] [CrossRef]
- Davis, J.C. Statistics and Data Analysis in Geology; John Wiley and Sons: New York, NY, USA, 1986. [Google Scholar]
- Franke, R. Scattered Data Interpolation: Test of Some Methods. Math. Comput. 1982, 33, 181–200. [Google Scholar]
- Ilenič, A. Geochemical and Isotopic Study of Surface Sediments and Banded Dye-Murex Muscles from Selected Locations in the National Park Kornati—Croatia. Bachelor’s Thesis, University of Ljubljana, Faculty of Natural Science, Ljubljana, Slovenia, 2017. Available online: https://repozitorij.uni-lj.si/IzpisGradiva.php?id=95579&lang=slv (accessed on 18 October 2018).
- Villarreal, J.S.; Lovelock, C.E.; Saunders, M.I.; Roelfsema, C.; Mumby, P.J. Organic carbon in seagrass sediments is influenced by seagrass canopy complexity, turbidity, wave height and water depth. Limnol. Oceanogr. 2016, 61, 938–952. [Google Scholar] [CrossRef]
- Lepoint, G.; Dauby, P.; Fontaine, M.; Bouquegneau, J.M.; Gobert, S. Carbon and Nitrogen Isotopic Ratios of the Seagrass Posidonia oceanica: Depth-related Variations. Bot. Mar. 2003, 46, 555–561. [Google Scholar] [CrossRef]
- Gacia, E.; Duarte, C.M.; Middelburg, J.J. Carbon and nutrient deposition in a Mediterranean seagrass (Posidonia oceanica) meadow. Limnol. Oceanogr. 2001, 47, 23–32. [Google Scholar] [CrossRef]
- Dolenec, T.; Lojen, S.; Lambaša, Ž.; Dolenec, M. Effects of fish farm loading on sea grass Posidonia oceanica at Vrgada Island (Central Adriatic): A nitrogen stable isotope study. Isot. Environ. Health Stud. 2006, 42, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Mancinelli, G.; Glamuzina, B.; Petrić, M.; Carrozzo, L.; Glamuzina, L.; Zotti, M.; Raho, D.; Vizzini, S. The trophic position of the Atlantic blue crab Callinectes sapidus (Rathbun, 1896) in the food web of Paila Lagoon (South Eastern Adriatic, Croatia): A first assessment using stable isotopes. Mediterr. Mar. Sci. 2016, 3, 634–643. [Google Scholar] [CrossRef]
- Peharda, M.; Morton, B. Experimental prey species preferences of Hexaplex trunclus (Gastropoda: Muricidae) and predator-prey interactions with the Black mussel Mytilus galloprovincialis (Bivalvia: Mytilidae). Mar. Biol. 2006, 148, 1011–1019. [Google Scholar] [CrossRef]
- Erdelez, A. Volak Hexaplex Trunculus (Linnaeus, 1758) Kao Bioindikator Onečišćenja Tributilkositrom u Jadranu. Ph.D. Thesis, University of Dubrovnik, Split, Croatia, 2017. Available online: https://repozitorij.more.unist.hr/islandora/object/morest%3A17/datastream/PDF/view (accessed on 18 October 2018).
- Marić, M. Non-Indigenous Species in the Mediterranean Marine Protected Areas: Diversity, Distribution and Impacts. Ph.D. Thesis, Klaipeda University, Klaipeda, Lithuania, 2016. [Google Scholar]
- Dolenec, T.; Lojen, S.; Kniewald, G.; Dolenec, M.; Rogan, N. Nitrogen stable isotope composition as a tracer of fish farming in invertebrates Aplysina aerophoba, Balanus perforatus and Anemonia sulcata in central Adriatic. Aquaculture 2007, 262, 237–249. [Google Scholar] [CrossRef]
- Dolenec, M.; Žvab, P.; Mihelčić, G.; Lambaša Belak, Ž.; Lojen, S.; Kniewald, G.; Dolenec, T.; Rogan Šmuc, N. Use of stable nitrogen isotope signatures of anthropogenic organic matter in the coastal environment: A case study of the Kosirna Bay (Murter Island, Croatia). Geol. Croat. 2011, 64, 143–152. [Google Scholar] [CrossRef]
- Spies, R.B.; Kruger, H.; Ireland, R.; Rice, D.W. Stable isotope ratios and contaminant concentrations in a sewage—Distorted food web. Mar. Ecol. Prog. Ser. 1989, 54, 157–170. [Google Scholar] [CrossRef]
| δ13Corg [‰] | |
|---|---|
| KLO (n = 3) | −19.96 |
| LOJ (n = 5) | −20.35 |
| MAN (n = 5) | −20.05 |
| PIŠ (n = 5) | −17.84 |
| VRU (n = 5) | −18.50 |
| KLO | LOJ | MAN | PIŠ | VRU | |
|---|---|---|---|---|---|
| KLO | - | NSD | NSD | * | NSD |
| LOJ | NSD | - | NSD | * | * |
| MAN | NSD | NSD | - | * | * |
| PIŠ | * | * | * | - | NSD |
| VRU | NSD | * | * | NSD | - |
| δ15N [‰] | |
|---|---|
| KLO (n = 2) | +6.13 |
| MAN (n = 3) | +5.65 |
| PIŠ (n = 3) | +8.87 |
| VRU (n = 3) | +10.4 |
| δ13C [‰] | |
|---|---|
| KLO (n = 2) | −19.15 |
| MAN (n = 3) | −16.55 |
| PIŠ (n = 3) | −14.47 |
| VRU (n = 3) | −15.66 |
| KLO | MAN | PIŠ | VRU | |
|---|---|---|---|---|
| KLO | - | NSD | NSD | * |
| MAN | NSD | - | * | * |
| PIŠ | NSD | * | - | NSD |
| VRU | * | * | NSD | - |
| KLO | MAN | PIŠ | VRU | |
|---|---|---|---|---|
| KLO | - | * | * | * |
| MAN | * | - | NSD | NSD |
| PIŠ | * | NSD | - | NSD |
| VRU | * | NSD | NSD | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilenič, A.; Lojen, S.; Župan, I.; Šarić, T.; Šikić, Z.; Vrhovnik, P.; Dolenec, M. Is the Kornati National Park Still an Acceptable Reference Area for Environmental Studies? Geosciences 2018, 8, 385. https://doi.org/10.3390/geosciences8110385
Ilenič A, Lojen S, Župan I, Šarić T, Šikić Z, Vrhovnik P, Dolenec M. Is the Kornati National Park Still an Acceptable Reference Area for Environmental Studies? Geosciences. 2018; 8(11):385. https://doi.org/10.3390/geosciences8110385
Chicago/Turabian StyleIlenič, Anja, Sonja Lojen, Ivan Župan, Tomislav Šarić, Zoran Šikić, Petra Vrhovnik, and Matej Dolenec. 2018. "Is the Kornati National Park Still an Acceptable Reference Area for Environmental Studies?" Geosciences 8, no. 11: 385. https://doi.org/10.3390/geosciences8110385
APA StyleIlenič, A., Lojen, S., Župan, I., Šarić, T., Šikić, Z., Vrhovnik, P., & Dolenec, M. (2018). Is the Kornati National Park Still an Acceptable Reference Area for Environmental Studies? Geosciences, 8(11), 385. https://doi.org/10.3390/geosciences8110385





