Oxygen Isotope Thermometry of DaG 476 and SaU 008 Martian Meteorites: Implications for Their Origin
Abstract
:1. Introduction
2. Methodology
2.1. Samples
2.2. Oxygen Isotope Measurements
3. Results and Discussion
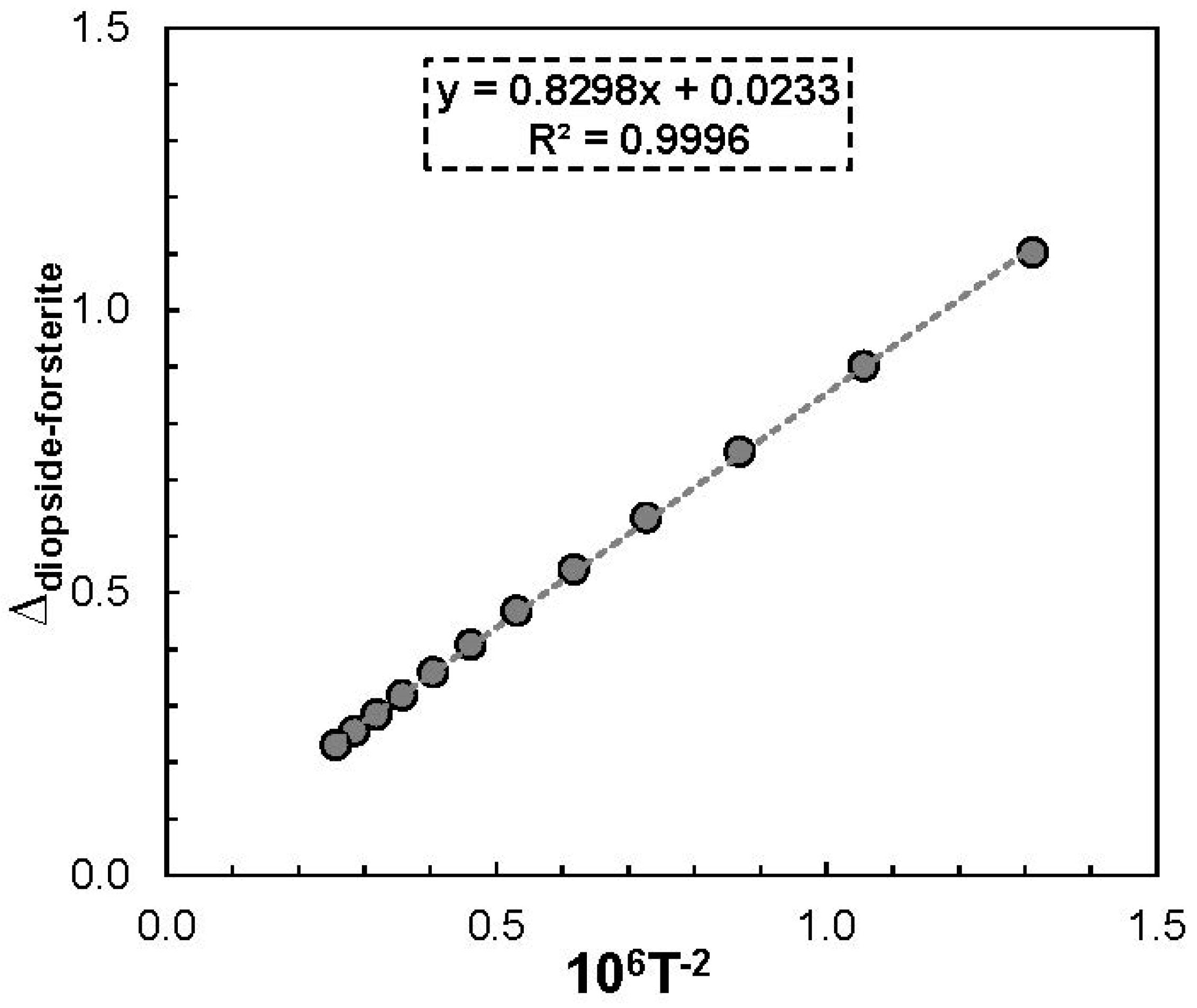
3.1. Oxygen Isotope Thermometry
3.2. Petrogenetic Relationship
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Neukum, G.; Jaumann, R.; Hoffmann, H.; Hauber, E.; Head, J.W.; Basilevsky, A.T.; Ivanov, B.A.; Werner, S.C.; van Gasselt, S.; Murray, J.B.; et al. Recent and episodic volcanic and glacial activity on Mars revealed by the High Resolution Stereo Camera. Nature 2004, 432, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Van Thienen, P.; Rivoldini, A.; Van Hoolst, T.; Lognonné, P. A top-down origin for martian mantle plumes. Icarus 2006, 185, 197–210. [Google Scholar] [CrossRef]
- Werner, S.C. The global martian volcanic evolutionary history. Icarus 2009, 201, 44–68. [Google Scholar] [CrossRef]
- Robbins, S.J.; Di Achille, G.; Hynek, B.M. The volcanic history of Mars: High resolution crater-based studies of the calderas of 20 volcanoes. Icarus 2011, 211, 1179–1203. [Google Scholar] [CrossRef]
- Bridges, J.C.; Warren, P.H. The SNC meteorites: Basaltic igneous processes on Mars. J. Geol. Soc. Lond. 2006, 163, 229–251. [Google Scholar] [CrossRef]
- Liu, Y.; Baziotis, I.P.; Asimow, P.D.; Bodnar, R.J.; Taylor, L.A. Mineral chemistry of the Tissint meteorite: Indications of two-stage crystallization in a closed system. Meteorit. Planet. Sci. 2016, 51, 2293–2315. [Google Scholar] [CrossRef]
- Borg, L.E.; Nyquist, L.E.; Wiesmann, H.; Shih, C.-Y. Constraints on martian differentiation processes from Rb–Sr and Sm–Nd isotopic analyses of the basaltic shergottite QUE 94201. Geochim. Cosmochim. Acta 1997, 61, 4915–4931. [Google Scholar] [CrossRef]
- Borg, L.E.; Draper, D.S. A petrogenetic model for the origin and compositional variation of the Martian basaltic meteorites. Meteorit. Planet. Sci. 2003, 38, 1713–1731. [Google Scholar] [CrossRef]
- Borg, L.E.; Brennecka, G.A.; Symes, S.J.K. Accretion timescale and impact history of Mars deduced from the isotopic systematics of martian meteorites. Geochim. Cosmochim. Acta 2016, 175, 150–167. [Google Scholar] [CrossRef]
- Borg, L.E.; Brennecka, G.A.; Marks, N.; Symes, S.J.K. Neodymium isotopic evolution of the solar system inferred from isochron studies of planetary materials. In Proceedings of the 44th Lunar Planetary Science Conference, The Woodlands, TX, USA, 18–22 March 2014. [Google Scholar]
- Bouvier, A.; Blichert-Toft, J.; Vervoort, J.D.; Albarède, F. The age of SNC meteorites and the antiquity of the Martian surface. Earth Planet. Sci. Lett. 2005, 240, 221–233. [Google Scholar] [CrossRef]
- Bouvier, A.; Blichert-Toft, J.; Vervoort, J.D.; Gillet, P.; Albarède, F. The case for old basaltic shergottites. Earth Planet. Sci. Lett. 2008, 266, 105–124. [Google Scholar] [CrossRef]
- Nyquist, L.E.; Bogard, D.D.; Shih, C.-Y.; Greshake, A.; Stöffler, D.; Eugster, O. Ages and histories of martian meteorites. In Chronology and Evolution of Mars; Kallenbach, R., Geiss, J., Hartmann, W.K., Eds.; Springer: Berlin, Germany, 2001; pp. 105–164. [Google Scholar]
- Shih, C.-Y.; Nyquist, L.E.; Wiesmann, H.; Reese, Y.; Misawa, K. Rb–Sr and Sm–Nd dating of olivine-phyric shergottites Y980459: Petrogenesis of depleted shergottites. Antarct. Meteorit. Res. 2005, 18, 46–65. [Google Scholar]
- Symes, S.J.K.; Borg, L.E.; Shearer, C.K.; Irving, A.J. The age of the martian meteorite Northwest Africa 1195 and the differentiation history of the shergottites. Geochim. Cosmochim. Acta 2008, 72, 1696–1710. [Google Scholar] [CrossRef]
- Lapen, T.J.; Righter, M.; Brandon, A.D.; Debaille, V.; Beard, B.L.; Shafer, J.T.; Peslier, A.H. A young age for ALH84001 and its geochemical link to shergottite sources in Mars. Science 2010, 328, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Lapen, T.J.; Righter, M.; Andreasen, R.; Irving, A.J.; Satkoski, A.M.; Beard, B.L.; Nishiizumi, K.; Jull, A.J.T.; Caffee, M.W. Two billion years of magmatism recorded from a single Mars meteorite ejection site. Sci. Adv. 2017, 3, e1600922. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, W.K.; Berman, D.C. Elysium Planitia lava flows: Crater count chronology and geological implications. J. Geophys. Res. 2000, 105, 15011–15026. [Google Scholar] [CrossRef]
- Stolper, E.; McSween, H.Y. Petrology and origin of the shergottite meteorites. Geochim. Cosmochim. Acta 1979, 43, 1475–1498. [Google Scholar] [CrossRef]
- Herd, C.D.K.; Borg, L.E.; Jones, J.H.; Papike, J.J. Oxygen fugacity and geochemical variations in the martian basalts: Implications for martian basalt petrogenesis and the oxidation state of the upper mantle of Mars. Geochim. Cosmochim. Acta 2002, 66, 2025–2036. [Google Scholar] [CrossRef]
- Wadhwa, M. Redox state of Mars’ upper mantle and crust from Eu anomalies in shergottite pyroxenes. Science 2001, 291, 1527–1530. [Google Scholar] [CrossRef] [PubMed]
- Brennecka, G.A.; Borg, L.E.; Wadhwa, M. Insights into the martian mantle: The age and isotopics of the meteorite fall Tissint. Meteorit. Planet. Sci. 2014, 49, 412–418. [Google Scholar] [CrossRef]
- Balta, J.B.; McSween, H.Y. Water and the composition of Martian magmas. Geology 2013, 41, 1115–1118. [Google Scholar] [CrossRef]
- Tornabene, L.L.; Moersch, J.E.; McSween, H.Y.; McEwen, A.S.; Piatek, J.L.; Milam, K.A.; Christensen, P.R. Identification of large (2–10 km) rayed craters on Mars in THEMIS thermal infrared images: Implications for possible Martian meteorite source regions. J. Geophys. Res. 2006, 111, E10006. [Google Scholar] [CrossRef]
- Lang, N.P.; Tornabene, L.L.; McSween, H.Y.; Christensen, P.R. Tharsis-sourced relatively dust-free lavas and their possible relationship to martian meteorites. J. Volcanol. Geotherm. Res. 2009, 185, 103–115. [Google Scholar] [CrossRef]
- French, B.M.; Short, N.M. (Eds.) Shock Metamorphism of Natural Materials; Mono Book Corporation: Baltimore, MD, USA, 1968. [Google Scholar]
- Stöffler, D. Progressive metamorphism and classification of shocked and brecciated crystalline rocks at impact craters. J. Geophys. Res. 1971, 76, 5541–5551. [Google Scholar] [CrossRef]
- Melosh, H.J. Impact Cratering: A Geologic Process; Oxford University Press: New York, NY, USA, 1989; p. 253. [Google Scholar]
- Osinski, G.R.; Pierazzo, E. Impact Cratering: Processes and Products; Wiley-Blackwell: Oxford, UK, 2013; p. 316. [Google Scholar]
- Jaret, S.J.; Woerner, W.R.; Phillips, B.L.; Ehm, L.; Nekvasil, H.; Wright, S.P.; Glotch, T.D. Maskelynite formation via solid-state transformation: Evidence of infrared and X-ray anisotropy. J. Geophys. Res. Planets 2015, 120, 570–587. [Google Scholar] [CrossRef]
- Walton, E.L.; Herd, C.D.K. Localized shock melting in lherzolitic shergottite Northwest Africa 1950: Comparison with Allan Hills 77005. Meteorit. Planet. Sci. 2007, 42, 63–80. [Google Scholar] [CrossRef]
- Chatzitheodoridis, E.; Haigh, S.; Lyon, I. A conspicuous clay ovoid in Nakhla: Evidence for subsurface hydrothermal alteration on Mars with implications for astrobiology. Astrobiology 2014, 14, 651–693. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, J.; Scherer, P.; Spettel, B.; Schultz, L. Petrology and chemistry of the new shergottite Dar al Gani 476. Meteorit. Planet. Sci. 2000, 35, 95–106. [Google Scholar] [CrossRef]
- Fritz, J.; Artemieva, N.; Greshake, A. Ejection of Martian meteorites. Meteorit. Planet. Sci. 2005, 40, 1393–1411. [Google Scholar] [CrossRef]
- Pickersgill, A.E. Shock Metamorphic Effects in Lunar and Terrestrial Plagioclase Feldspar Investigated by Optical Petrography and Micro-X-ray Diffraction. Electronic Thesis and Dissertation Repository. Master’s Thesis, University of Western Ontario, London, ON, Canada, 2014. [Google Scholar]
- Ostertag, R. Shock experiments on feldspar crystals. J. Geophys. Res. 1983, 88, B364–B376. [Google Scholar] [CrossRef]
- Kieffer, S.W. Numerical models of caldera-scale volcanic eruptions on Earth, Venus, and Mars. Science 1995, 269, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- Glaze, L.S.; Baloga, S.M.; Wimert, J. Explosive volcanic eruptions from linear vents on Earth, Venus, and Mars: Comparisons with circular vent eruptions. J. Geophys. Res. 2011, 116. [Google Scholar] [CrossRef]
- Usui, T.; McSween, H.Y., Jr.; Floss, C. Petrogenesis of olivine-phyric shergottite Yamato 980459, revisited. Geochim. Cosmochim. Acta 2008, 72, 1711–1730. [Google Scholar] [CrossRef]
- Blichert-Toft, J.; Gleason, J.D.; Télouk, P.; Albarède, F. The Lu–Hf isotope geochemistry of shergottites and the evolution of the Martian mantle-crust system. Earth Planet. Sci. Lett. 1999, 173, 25–39. [Google Scholar] [CrossRef]
- Elkin-Tanton, L.T.; Parmentier, E.M.; Hess, P.C. Magma ocean fractional crystallization and cumulate overturn in terrestrial planets: Implications for Mars. Meteorit. Planet. Sci. 2003, 38, 1753–1771. [Google Scholar] [CrossRef]
- Jones, J.H. Isotopic relationships among the shergottites, the nakhlites and Chassigny. In Proceedings of the 19th Lunar and Planetary Science Conference, Houston, TX, USA, 14–18 March 1988; pp. 465–474. [Google Scholar]
- Leshin, L.A.; Epstein, S.; Stolper, E.M. Hydrogen isotope geochemistry of SNC meteorites. Geochim. Cosmochim. Acta 1996, 60, 2635–2650. [Google Scholar] [CrossRef]
- Basu Sarbadhikari, A.; Babu, E.V.S.S.K.; Vijaya Kumar, T. Chemical layering in the upper mantle of Mars: Evidence from olivine-hosted melt inclusions in Tissint. Meteorit. Planet. Sci. 2016, 51, 251–267. [Google Scholar] [CrossRef]
- Filiberto, J.; Gross, J.; McCubbin, F.M. Constraints on the water, chlorine, and fluorine content of the Martian mantle. Meteorit. Planet. Sci. 2016, 51, 2023–2035. [Google Scholar] [CrossRef]
- McCubbin, F.M.; Boyce, J.W.; Srinivasan, P.; Santos, A.R.; Elardo, S.M.; Filiberto, J.; Steele, A.; Shearer, C.K. Heterogeneous distribution of H2O in the Martian interior: Implications for the abundances of H2O in depleted and enriched mantle sources. Meteorit. Planet. Sci. 2016, 51, 2036–2060. [Google Scholar] [CrossRef]
- Herd, C.D.K. The oxygen fugacity of olivine-phyric martian basalts and the components within the mantle and crust of Mars. Meteorit. Planet. Sci. 2003, 38, 1793–1805. [Google Scholar] [CrossRef]
- Watson, L.L.; Hutcheon, I.D.; Epstein, S.; Stolper, E.M. Water on Mars: Clues from deuterium/hydrogen and water contents of hydrous phases in SNC meteorites. Science 1994, 265, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Leshin, L.A. Insights into martian water reservoirs from analyses of Martian meteorite QUE94201. Geophys. Res. Lett. 2000, 27, 2017–2020. [Google Scholar] [CrossRef]
- Greenwood, J.P.; Itoh, S.; Sakamoto, N.; Vicenzi, E.P.; Yurimoto, H. Hydrogen isotope evidence for loss of water from mars through time. Geophys. Res. Lett. 2008, 35. [Google Scholar] [CrossRef]
- McCubbin, F.M.; Smirnov, A.; Nekvasil, H.; Wang, J.; Hauri, E.; Lindsley, D.H. Hydrous magmatism on Mars: A source of water for the surface and subsurface during the Amazonian. Earth Planet. Sci. Lett. 2010, 292, 132–138. [Google Scholar] [CrossRef]
- McCubbin, F.M.; Hauri, E.; Elardo, S.M.; Vander Kaaden, K.E.; Wang, J.; Shearer, C.K. Hydrous melting of the Martian mantle produced both depleted and enriched shergottites. Geology 2012, 40, 683–686. [Google Scholar] [CrossRef]
- Usui, T.; Alexander, C.M.O.D.; Wang, J.; Simon, J.I.; Jones, J.H. Origin of water and mantle-crust interactions on Mars inferred from hydrogen isotopes and volatile element abundances of olivine-hosted melt inclusions of primitive shergottites. Earth Planet. Sci. Lett. 2012, 357–358, 119–129. [Google Scholar] [CrossRef]
- Usui, T.; Alexander, C.M.O.D.; Wang, J.; Simon, J.I.; Jones, J.H. Meteoritic evidence for a previously unrecognized hydrogen reservoir on Mars. Earth Planet. Sci. Lett. 2015, 410, 140–151. [Google Scholar] [CrossRef]
- Gross, J.; Filiberto, J.; Herd, C.D.K.; Melwani Daswani, M.; Schwenzer, S.P.; Treiman, A.H. Petrography, mineral chemistry, and crystallization history of olivine-phyric shergottite NWA 6234: A new melt composition. Meteorit. Planet. Sci. 2013, 48, 854–871. [Google Scholar] [CrossRef]
- McSween, H.Y.; Harvey, R.P. Outgassed water on Mars: Constraints from melt inclusions in SNC meteorites. Science 1993, 259, 1890–1892. [Google Scholar] [CrossRef] [PubMed]
- Giesting, P.A.; Schwenzer, S.P.; Filiberto, J.; Starkey, N.A.; Franchi, I.A.; Trieman, A.H.; Tindle, A.G.; Grady, M.M. Igneous and shock processes affecting chassignite amphibole evaluated using chlorine/water partitioning and hydrogen isotopes. Meteorit. Planet. Sci. 2015, 50, 433–460. [Google Scholar] [CrossRef]
- Dann, J.C.; Holzheid, A.H.; Grove, T.L.; McSween, H.Y., Jr. Phase equilibria of the Shergotty meteorite: Constraints on pre-eruptive water contents of martian magmas and fractional crystallization under hydrous conditions. Meteorit. Planet. Sci. 2001, 36, 793–806. [Google Scholar] [CrossRef]
- Clayton, R.N.; Epstein, S. The use of oxygen isotopes in high temperature geological thermometry. J. Geol. 1961, 69, 447–452. [Google Scholar] [CrossRef]
- Onuma, N.; Clayton, R.N.; Mayeda, T.K. Apollo 11 rocks: Oxygen isotope fractionation between minerals and an estimate of the temperature of formation. Geochim. Cosmochim. Acta 1970, 1, 1429–1434. [Google Scholar]
- Clayton, R.N.; Onuma, N.; Mayeda, T.K. Oxygen isotope fractionation in Apollo 12 rocks and soils. In Proceedings of the 2nd Lunar and Planetary Science Conference, Houston, TX, USA, 11–14 January 1971; MIT Press: Cambridge, MA, USA; pp. 1417–1420. [Google Scholar]
- Clayton, R.N. Oxygen isotope composition of Luna 16 soil. Earth Planet. Sci. Lett. 1972, 13, 455–456. [Google Scholar] [CrossRef]
- Ali, A.; Jabeen, I.; Gregory, D.; Verish, R.; Banerjee, N.R. New triple oxygen isotope data of bulk and separated fractions from SNC meteorites: Evidence for mantle homogeneity of Mars. Meteorit. Planet. Sci. 2016, 51, 981–995. [Google Scholar] [CrossRef]
- Clayton, R.N.; Mayeda, T.K. Oxygen isotopes in Shergotty. Geochim. Cosmochim. Acta 1986, 50, 979–982. [Google Scholar] [CrossRef]
- Goodrich, C.A. Olivine-phyric martian basalts: A new type of shergottite. Meteorit. Planet. Sci. 2002, 37, S12. [Google Scholar] [CrossRef]
- Ali, A.; Nasir, S.; Jabeen, I.; Al Rawas, A. Review of the Sayh al Uhaymir (SaU) 005, plus pairings, Martian meteorite from Al Wusta, Oman. Sult. Qaboos Univ. J. Sci. 2017, 22, 29–39. [Google Scholar] [CrossRef]
- Folco, L.; Franchi, I.A.; D’Orazio, M.; Rocchi, S.; Schultz, L. A new martian meteorite from the Sahara: The shergottite Dar al Gani 489. Meteorit. Planet. Sci. 2000, 35, 827–839. [Google Scholar] [CrossRef]
- Wadhwa, M.; Lentz, R.C.F.; McSween, H.Y.; Crozaz, G. A petrologic and trace element study of Dar al Gani 476 and Dar al Gani 489: Twin meteorites with affinities to basaltic and lherzolitic shergottites. Meteorit. Planet. Sci. 2001, 36, 195–208. [Google Scholar] [CrossRef]
- Walton, E.L.; Spray, J.G.; Bartoschewitz, R. A new Martian meteorite from Oman: Mineralogy, petrology, and shock metamorphism of olivine-phyric basaltic shergottite Sayh al Uhaymir 150. Meteorit. Planet. Sci. 2005, 40, 1195–1214. [Google Scholar] [CrossRef]
- Crozaz, G.; Wadhwa, M. The terrestrial alteration of Saharan shergottites Dar al Gani 476 and 489: A case study of weathering in a hot desert environment. Geochim. Cosmochim. Acta 2001, 65, 971–978. [Google Scholar] [CrossRef]
- Nishiizumi, K.; Caffee, M.W.; Jull, A.J.T.; Klandrud, S.E. Exposure history of Shergottites Dar al Gani 476/489/670/735 and Sayh al Uhaymir 005. In Proceedings of the 32nd Lunar and Planetary Science Conference, Houston, TX, USA, 12–16 March 2001. [Google Scholar]
- Dreibus, G.; Spettel, B.; Haubold, R.; Jochum, K.P.; Palme, H.; Zipfel, J. Chemistry of a new shergottite: Sayh al Uhaymir 005. Meteorit. Planet. Sci. 2000, 35, A49. [Google Scholar]
- Barrat, J.A.; Blichert-Toft, J.; Nesbitt, R.W.; Keller, F. Bulk chemistry of Saharan shergottite Dar al Gani 476. Meteorit. Planet. Sci. 2001, 34, 91–97. [Google Scholar] [CrossRef]
- Crozaz, G.; Floss, C.; Wadhwa, M. Chemical alteration and REE mobilization in meteorites from hot and cold deserts. Geochim. Cosmochim. Acta 2003, 67, 4727–4741. [Google Scholar] [CrossRef]
- Baertchi, P. Absolute 18O content of standard mean ocean water. Earth Planet. Sci. Lett. 1976, 31, 341–344. [Google Scholar] [CrossRef]
- Quinn, R.J.; Kitajima, K.; Nakashima, D.; Spicuzza, M.J.; Valley, J.W. Oxygen isotope thermometry using quartz inclusions in garnet. J. Metamorph. Geol. 2017, 35, 231–252. [Google Scholar] [CrossRef]
- Eiler, J.M.; Baumgartner, L.P.; Valley, J.W. Intercrystalline stable isotope diffusion—A fast grain-boundary model. Contrib. Mineral. Petrol. 1992, 112, 543–557. [Google Scholar] [CrossRef]
- Eiler, J.M.; Baumgartner, L.P.; Valley, J.W. Fast grain-boundary—A Fortran-77 program for calculating the effects of retrograde interdiffuion of stable isotopes. Comput. Geosci. 1994, 20, 1415–1434. [Google Scholar] [CrossRef]
- Dodson, M.H. Closure temperature in cooling geochronological and petrological systems. Contrib. Mineral. Petrol. 1973, 40, 259–274. [Google Scholar] [CrossRef]
- Clayton, R.N.; Kieffer, S.W. Oxygen isotopic thermometer calibrations. In Stable Isotope Geochemistry: A Tribute to Samuel Epstein; Special Publication No. 3; Taylor, H.P., O’Neil, J.R., Kaplan, I.R., Eds.; The Geochemical Society: Washington, DC, USA, 1991; pp. 3–10. [Google Scholar]
- Chiba, H.; Chacko, T.; Clayton, R.N.; Goldsmith, J.R. Oxygen isotope fractionations involving diopside, forsterite, magnetite, and calcite: Application to geothermometry. Geochim. Cosmochim. Acta 1989, 53, 2985–2995. [Google Scholar] [CrossRef]
- Steele, A.; McCubbin, F.M.; Fries, M.; Kater, L.; Boctor, N.Z.; Fogel, M.L.; Conrad, P.G.; Glamoclija, M.; Spencer, M.; Morrow, A.L.; et al. A reduced organic carbon component in Martian basalts. Science 2012, 337, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-F. Prediction of high-temperature oxygen isotope fractionation factors between mantle minerals. Phys. Chem. Miner. 1997, 24, 356–364. [Google Scholar] [CrossRef]
- Channon, M.B.; Stolper, E.M.; Eiler, J.M. Oxygen isotope compositions of mineral separates from SNC meteorites: Constraints on SNC parental magmas. In Proceedings of the 41st Lunar and Planetary Science Conference, Houston, TX, USA, 13–17 March 2010. [Google Scholar]
- Treiman, A.H.; Filiberto, J. Geochemical diversity of shergottite basalts: Mixing and fractionation, and their relation to mars surface basalts. Meteorit. Planet. Sci. 2015, 50, 632–648. [Google Scholar] [CrossRef]
- Goodrich, C.A. Petrogenesis of olivine-phyric shergottites Sayh al Uhaymir 005 and Elephant Moraine A79001 lithology A. Geochim. Cosmochim. Acta 2003, 67, 3735–3771. [Google Scholar] [CrossRef]
- Balta, J.B.; Sanborn, M.E.; Udry, A.; Wadhwa, M.; McSween, H.Y. Petrology and trace element geochemistry of Tissint, the newest shergottite fall. Meteorit. Planet. Sci. 2015, 50, 63–85. [Google Scholar] [CrossRef]
- French, W.J.; Cameron, E.P. Calculation of the temperature of crystallization of silicates from basaltic melts. Mineral. Mag. 1981, 44, 19–26. [Google Scholar] [CrossRef]
- Leshin, L.A.; Rubin, A.E.; McKeegan, K.D. The oxygen isotopic composition of olivine and pyroxene from CI chondrites. Geochim. Cosmochim. Acta 1997, 61, 835–845. [Google Scholar] [CrossRef]
- Grossman, J.N. The Meteoritical Bulletin, No. 83, 1999 July. Meteorit. Planet. Sci. 1999, 34, 169–186. [Google Scholar] [CrossRef]
- Nekvasil, H.; Filiberto, J.; McCubbin, F.M.; Lindsley, D.H. Alkalic parental magmas for the chassignites? Meteorit. Planet. Sci. 2007, 42, 979–992. [Google Scholar] [CrossRef]
- Nekvasil, H.; McCubbin, F.M.; Harrington, A.D.; Elardo, S.M.; Lindsley, D.H. Linking the Chassigny meteorite and the Martian surface rock Backstay: Insights into igneous crustal differentiation processes on Mars. Meteorit. Planet. Sci. 2009, 44, 853–869. [Google Scholar] [CrossRef]
- Filiberto, J. Experimental constraints on the parental liquid of the Chassigny meteorite: A possible link between the Chassigny meteorite and a Gusev basalt. Geochim. Cosmochim. Acta 2008, 72, 690–701. [Google Scholar] [CrossRef]
- Boctor, N.Z.; Alexander, C.M.O.D.; Wang, J.; Hauri, E. The sources of water in Martian meteorites: Clues from hydrogen isotopes. Geochim. Cosmochim. Acta 2003, 67, 3971–3989. [Google Scholar] [CrossRef]
- Filiberto, J.; Wood, J.; Dasgupta, R.; Shimizu, N.; Le, L.; Treiman, A.H. Effect of fluorine on near-liquidus phase equilibria of an Fe–Mg rich basalt. Chem. Geol. 2012, 312–313, 118–126. [Google Scholar] [CrossRef]
- Filiberto, J.; Dasgupta, R.; Gross, J.; Treiman, A.H. Effect of chlorine on near-liquidus phase equilibria of a Fe-Mg-rich tholeiitic basalt. Contrib. Mineral. Petrol. 2014, 168, 1–13. [Google Scholar] [CrossRef]
- Giehl, C.; Marks, M.W.; Nowak, M. An experimental study on the influence of fluorine and chlorine on phase relations in peralkaline phonolitic melts. Contrib. Mineral. Petrol. 2014, 167, 977. [Google Scholar] [CrossRef]
- Ford, C.E.; Russell, D.G.; Craven, J.A.; Fisk, M.R. Olivine-liquid equilibria: Temperature, pressure, and composition dependence of the crystal/liquid cation partition coefficients for Mg, Fe2+, Ca and Mn. J. Petrol. 1983, 24, 256–265. [Google Scholar] [CrossRef]
- Falloon, T.J.; Danyushevsky, L.V.; Arsikin, A.; Green, D.H.; Ford, C.E. The application of olivine geothermometry to infer crystallization temperatures of parental liquids: Implications for the temperature of MORB magmas. Chem. Geol. 2007, 241, 207–233. [Google Scholar] [CrossRef]
- Draper, D.S. Water-undersaturated near-liquidus phase relations of Yamato 980459: Preliminary results. In Proceedings of the 38th Lunar and Planetary Science Conference, League City, TX, USA, 12–16 March 2007. [Google Scholar]
- Gnos, E.; Hofmann, B.; Franchi, I.A.; Al-Kathiri, A.; Hauser, M.; Moser, L. Say al Uhaymir 094: A new martian meteorite from the Oman desert. Meteorit. Planet. Sci. 2002, 37, 835–854. [Google Scholar] [CrossRef]
- Shih, C.; Nyquist, L.E.; Reese, Y. Rb–Sr and Sm–Nd isotopic studies of martian depleted shergottites SaU 094/005. In Proceedings of the 38th Lunar and Planetary Science Conference, League City, TX, USA, 12–16 March 2007. [Google Scholar]



| Sample | Mineral | δ18O (‰) ± SE | N | ∆Di-Fo | T (K) † | 1σ | T (°C) † | T (K) ‡ | Data Source |
|---|---|---|---|---|---|---|---|---|---|
| DaG 476 | px (Di) | 4.708 ± 0.045 | 2 | 105 | [63] | ||||
| DaG 476 | ol (Fo) | 4.301 ± 0.024 | 3 | 0.407 ± 0.05 | 1470 | −90 | 1200 | 1480 | [63] |
| SaU 008 | px (Di) | 4.533 ± 0.045 | 2 | 220 | [63] | ||||
| SaU 008 | ol (Fo) | 4.223 ± 0.045 | 2 | 0.310 ± 0.06 | 1700 | −155 | 1430 | 1720 | [63] |
| NWA 2046 | px (Di) | 4.62 ± 0.09 | 2 | 330 | [84] | ||||
| NWA 2046 | ol (Fo) | 4.27 ± 0.17 | 2 | 0.35 ± 0.19 | 1595 | −890 | 1320 | 1620 | [84] |
| ALHA 77005 * | px (Di) | 4.72 ± 0.07 | 1 | 130 | [84] | ||||
| ALHA 77005 * | ol (Fo) | 4.28 ± 0.05 | 6 | 0.44 ± 0.09 | 1410 | −175 | 1140 | 1445 | [84] |
| NWA 1950 * | px (Di) | 4.62 ± 0.05 | 4 | 165 | [84] | ||||
| NWA 1950 * | ol (Fo) | 4.33 ± 0.03 | 6 | 0.29 ± 0.06 | 1765 | −230 | 1490 | 1780 | [84] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.; Jabeen, I.; Nasir, S.J.; Banerjee, N.R. Oxygen Isotope Thermometry of DaG 476 and SaU 008 Martian Meteorites: Implications for Their Origin. Geosciences 2018, 8, 15. https://doi.org/10.3390/geosciences8010015
Ali A, Jabeen I, Nasir SJ, Banerjee NR. Oxygen Isotope Thermometry of DaG 476 and SaU 008 Martian Meteorites: Implications for Their Origin. Geosciences. 2018; 8(1):15. https://doi.org/10.3390/geosciences8010015
Chicago/Turabian StyleAli, Arshad, Iffat Jabeen, Sobhi J. Nasir, and Neil R. Banerjee. 2018. "Oxygen Isotope Thermometry of DaG 476 and SaU 008 Martian Meteorites: Implications for Their Origin" Geosciences 8, no. 1: 15. https://doi.org/10.3390/geosciences8010015
APA StyleAli, A., Jabeen, I., Nasir, S. J., & Banerjee, N. R. (2018). Oxygen Isotope Thermometry of DaG 476 and SaU 008 Martian Meteorites: Implications for Their Origin. Geosciences, 8(1), 15. https://doi.org/10.3390/geosciences8010015





