Nutrients and Heavy Metals Contamination in an Urban Estuary of Northern New Jersey
Abstract
:1. Introduction
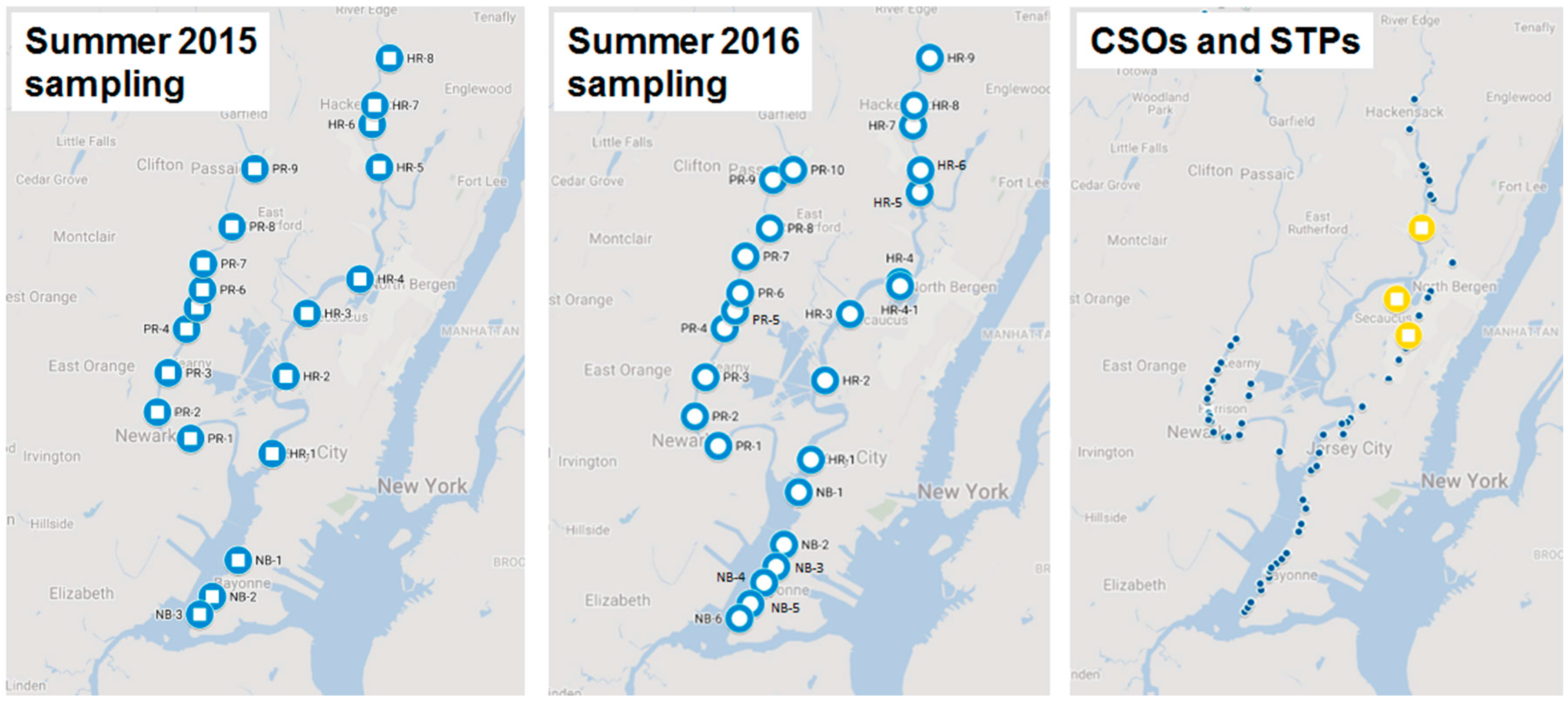
2. Materials and Methods
3. Results and Discussion
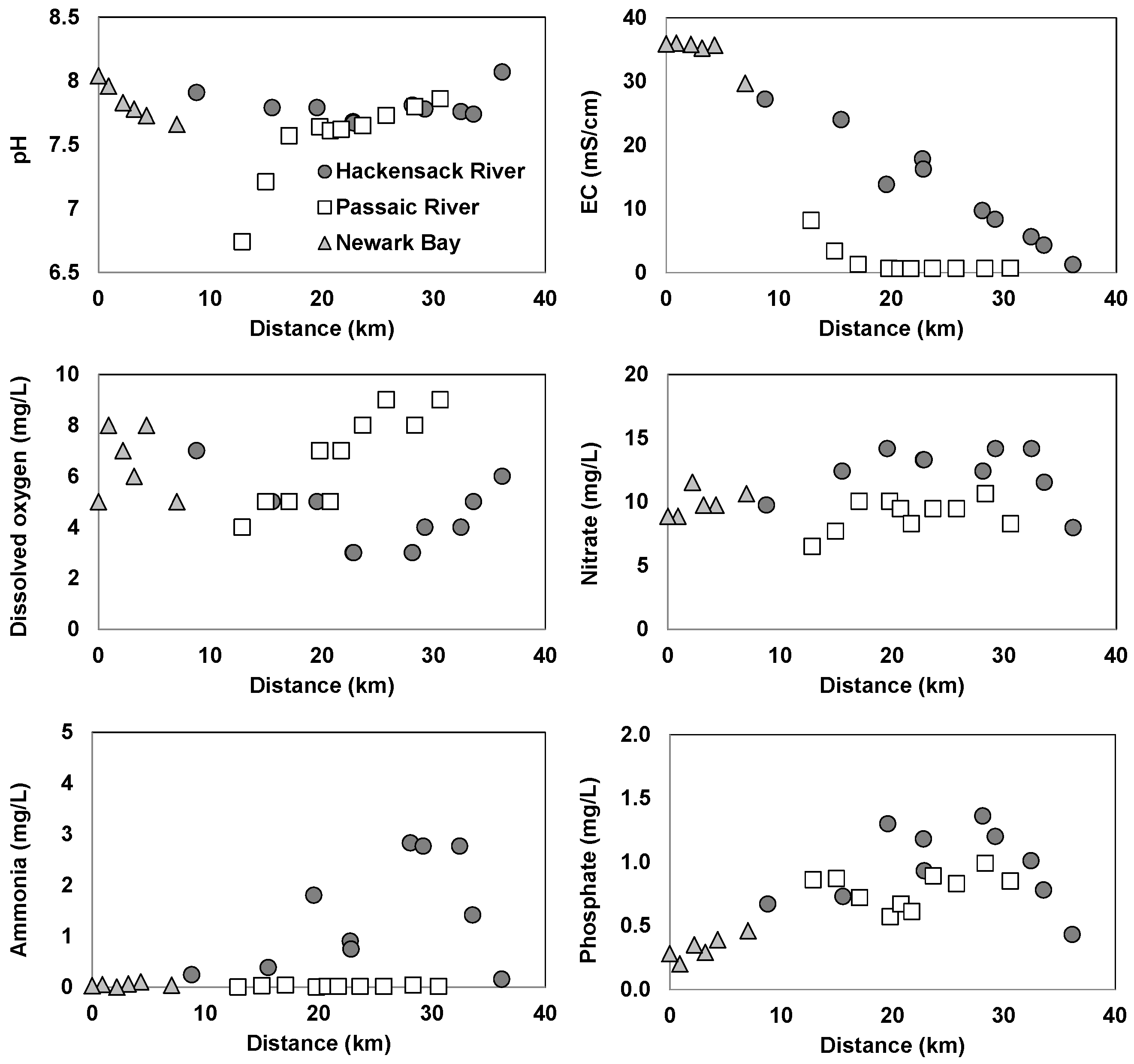
3.1. Spatial Variations of Water Quality in the Newark Bay Estuary
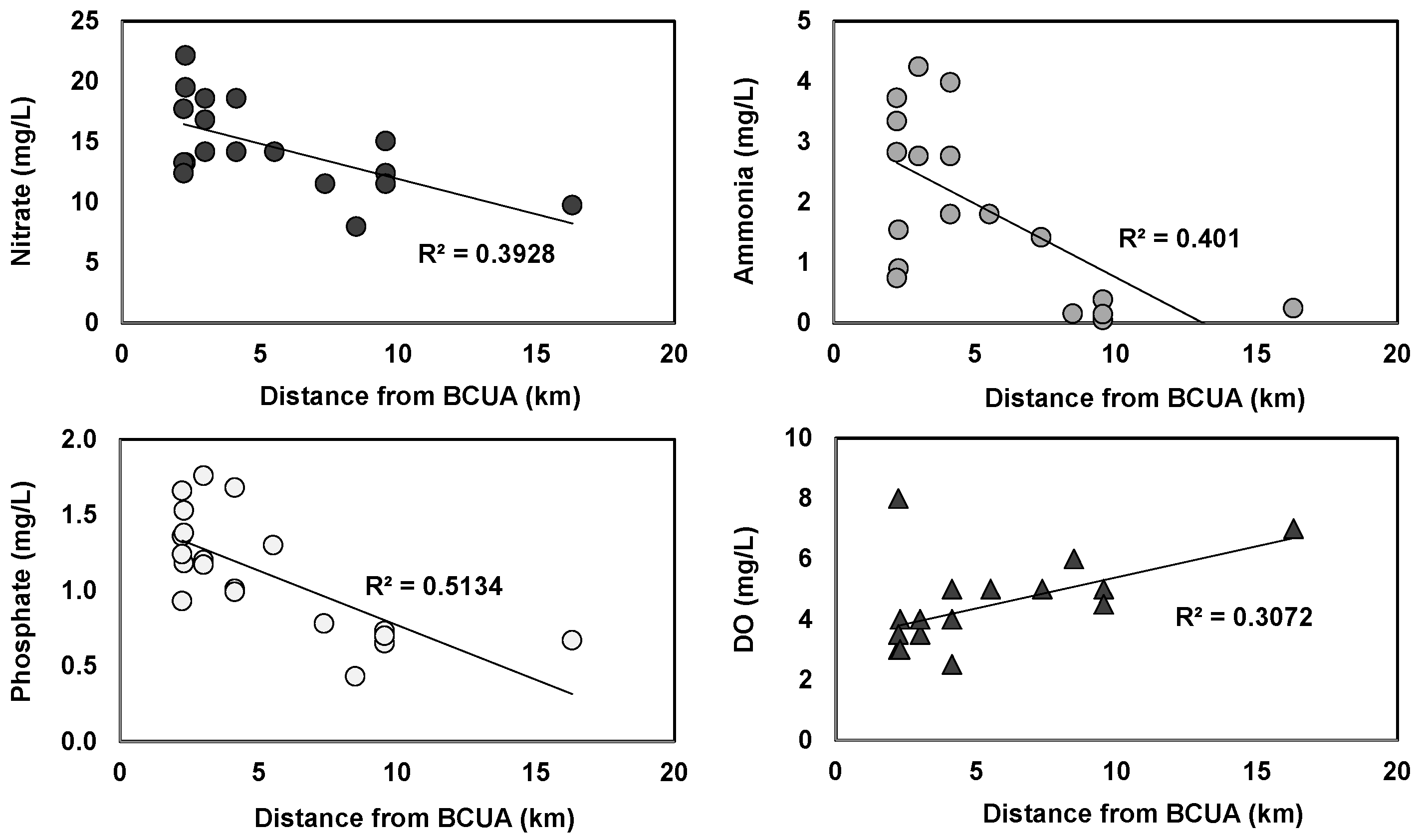
3.2. Effects of Tidal Cycle on Water Quality in the Newark Bay Estuary
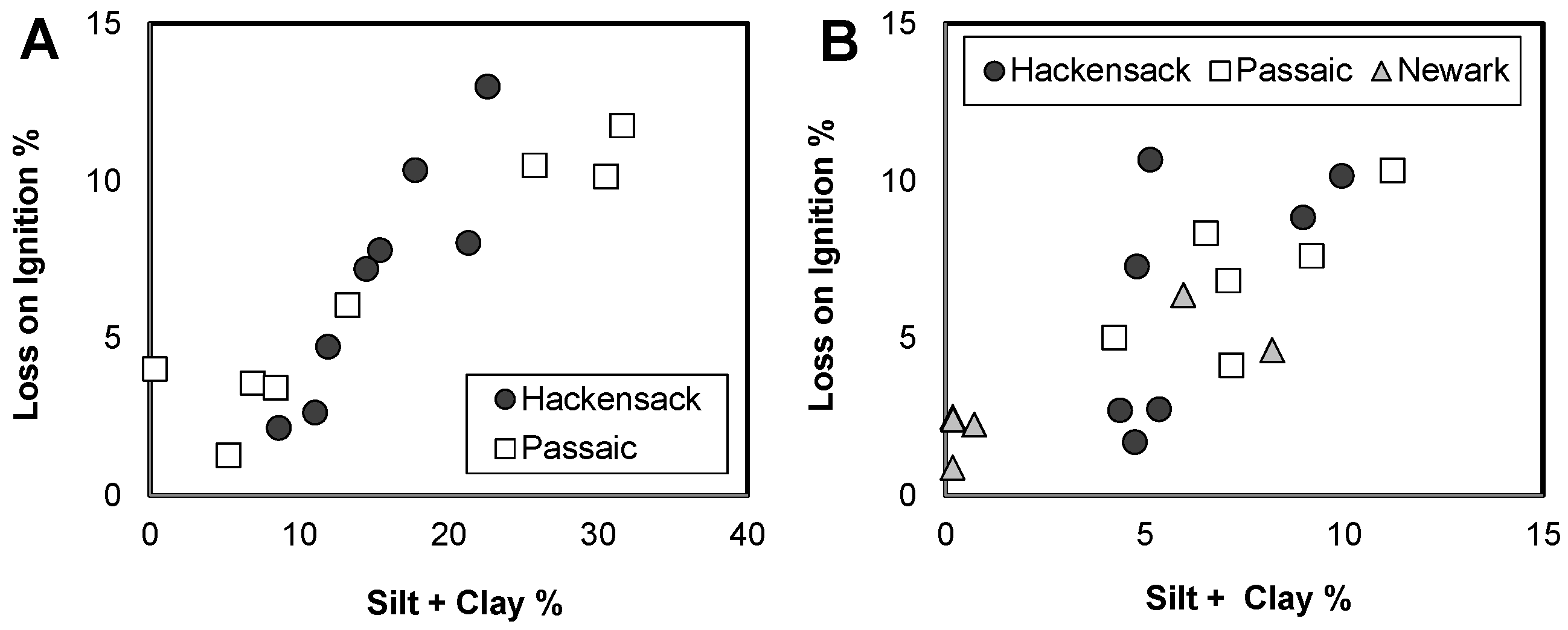
3.3. Spatial Variations of Sediment Quality in the Newark Bay Estuary
3.4. Geochemical Distribution of Heavy Metals in Sediments
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Day, J.W.; Hall, C.A.S.; Kemp, W.M.; Yañez-Arancibia, A. Estuarine Ecology, 2nd ed.; John Wiley and Sons: New York, NY, USA, 2012. [Google Scholar]
- Mitsch, W.J.; Gosselink, J.G. Wetlands, 2nd ed.; Van Nostrand Reinhold: New York, NY, USA, 1993. [Google Scholar]
- Wiegner, T.N.; Seitzinger, S.P.; Breitburg, D.L.; Sanders, J.G. The effects of multiple stressors on the balance between autotrophic and heterotrophic processes in an estuarine system. Estuaries 2003, 26, 352–364. [Google Scholar] [CrossRef]
- US Army Corps of Engineers. Hudson-Raritan Estuary Comprehensive Restoration Plan-Version 1; US Army Corps of Engineers: New York, NY, USA, 2016. [Google Scholar]
- Crawford, D.W.; Bonnevie, N.L.; Gillis, C.A.; Wenning, R.J. Historical changes in the ecological health of the Newark Bay Estuary, New Jersey. Ecotoxicol. Environ. Saf. 1994, 29, 276–303. [Google Scholar] [CrossRef]
- Bienenfeld, A.L.; Golden, L.A.; Garland, J.E. Consumption of fish from polluted waters by WIC participants in East Harlem. J. Urban Health 2003, 80, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Crawford, D.W.; Bonnevie, N.L.; Wenning, R.J. Sources of pollution and sediment contamination in Newark Bay, New Jersey. Ecotoxicol. Environ. Saf. 1995, 30, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Ecology and Environment Inc. Lower Hackensack River Bergen and Hudson Counties New Jersey-Preliminary Assessment; Technical Direction Document Number: 15–03–0008; Ecology and Environment Inc.: Lancaster, NY, USA, 2015. [Google Scholar]
- Louis Berger Group. Remedial Investigation Report for the Focused Feasibility Study of the Lower Eight Miles of the Lower Passaic River; Louis Berger Group: Morristown, NJ, USA, 2014. [Google Scholar]
- Shear, N.M.; Schmidt, C.W.; Huntley, S.L.; Crawford, D.W.; Finley, B.L. Evaluation of the factors relating combined sewer overflows with sediment contamination of the lower Passaic River. Mar. Pollut. Bull. 1996, 32, 288–304. [Google Scholar] [CrossRef]
- US Fish and Wildlife Service. The Hackensack Meadowlands Initiative: Preliminary Conservation Planning for the Hackensack Meadowlands Hudson and Bergen Counties, New Jersey; US Fish and Wildlife Service: Falls Church, VA, USA, 2007.
- Shrestha, P.L.; Su, S.H.; James, S.C.; Shaller, P.J.; Doroudian, M.; Firstenberg, C.E.; Thompson, C.T. Conceptual Site Model for Newark Bay—Hydrodynamics and Sediment Transport. J. Mar. Sci. Eng. 2014, 2, 123–139. [Google Scholar] [CrossRef]
- DiLorenzo, J.L.; Filadelfo, R.J.; Surak, C.R.; Litwack, H.S.; Gunawardana, V.K.; Najarian, T.O. Tidal variability in the water quality of an urbanized estuary. Estuaries 2004, 27, 851–860. [Google Scholar] [CrossRef]
- Bonnevie, N.L.; Huntley, S.L.; Found, B.W.; Wenning, R.J. Trace metal contamination in surficial sediments from Newark Bay, New Jersey. Sci. Total Environ. 1994, 144, 1–16. [Google Scholar] [CrossRef]
- Lodge, J.; Landeck Miller, R.E.; Suszkowski, D.; Litten, S.; Douglas, S. Contaminant Assessment and Reduction Project Summary Report; Hudson River Foundation: New York, NY, USA, 2015. [Google Scholar]
- Squibb, K.S.; O’Connor, J.M.; Kneip, T.J. New York/New Jersey Harbor Estuary Program Module 3.1: Toxics Characterization Report; Institute of Environmental Medicine, New York University Medical Center: Tuxedo, NY, USA, 1991. [Google Scholar]
- Wenning, R.J.; Bonnevie, N.L.; Huntley, S.L. Accumulation of metals, polychlorinated-biphenyls, and polycyclic aromatic-hydrocarbons in sediments from the lower Passaic River, New Jesrey. Arch. Environ. Contam. Toxicol. 1994, 27, 64–81. [Google Scholar] [CrossRef]
- HDR-HydroQual. Summary of Progress on Completing a Dissolved Oxygen Management Plan/TMDL for the NY/NJ Harbor July 2010 to October 2012; HDR Inc.: Omaha, NE, USA, 2012. [Google Scholar]
- The New Jersey Harbor Dischargers Group. The New Jersey Harbor Dischargers Group 2010 Water Quality Report; Passaic Valley Sewerage Commission: Newark, NJ, USA, 2012. [Google Scholar]
- Shin, J.Y.; Artigas, F.; Hobble, C.; Lee, Y.S. Assessment of anthropogenic influences on surface water quality in urban estuary, northern New Jersey: Multivariate approach. Environ. Monit. Assess. 2013, 185, 2777–2794. [Google Scholar] [CrossRef] [PubMed]
- Howarth, R.W. Coastal nitrogen pollution: A review of sources and trends globally and regionally. Harmful Algae 2008, 8, 14–20. [Google Scholar] [CrossRef]
- Seitzinger, S.P.; Mayorga, E.; Bouwman, A.F.; Kroeze, C.; Beusen, A.H.W.; Billen, G.; Van Drecht, G.; Dumont, E.; Fekete, B.M.; Garnier, J.; et al. Global river nutrient export: A scenario analysis of past and future trends. Glob. Biogeochem. Cycles 2010, 24. [Google Scholar] [CrossRef]
- Van Drecht, G.; Bouwman, A.F.; Harrison, J.; Knoop, J.M. Global nitrogen and phosphate in urban wastewater for the period 1970 to 2050. Global Biogeochem. Cycles 2009, 23. [Google Scholar] [CrossRef]
- Alloway, B.J. Heavy Metals in Soils; Blackie and Son Ltd.: Glasgow, UK, 1995. [Google Scholar]
- Barbieri, M.; Sappa, G.; Vitale, S.; Parisse, B.; Battistel, M. Soil control of trace metals concentrations in landfills: A case study of the largest landfill in Europe, Malagrotta, Rome. J. Geochem. Explor. 2014, 143, 146–154. [Google Scholar] [CrossRef]
- D’Amore, J.J.; Al-Abed, S.R.; Scheckel, K.G.; Ryan, J.A. Methods for speciation of metals in soils: A review. J. Environ. Qual. 2005, 34, 1707–1745. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.B.; Yun, S.T.; Mayer, B.; Kim, S.O.; Park, S.S.; Lee, P.K. Transport and sediment-water partitioning of trace metals in acid mine drainage: An example from the abandoned Kwangyang Au-Ag mine area, South Korea. Environ. Geol. 2005, 48, 437–449. [Google Scholar] [CrossRef]
- Jung, H.B.; Zamora, F.; Duzgoren-Aydin, N.S. Water Quality Monitoring of an Urban Estuary and a Coastal Aquifer Using Field Kits and Meters: A Community-Based Environmental Research Project. J. Chem. Educ. 2017, 94, 1512–1516. [Google Scholar] [CrossRef]
- Heiri, O.; Lotter, A.F.; Lemcke, G. Loss on ignition as a method for estimating organic and carbonate content in sediments: Reproducibility and comparability of results. J. Paleolimnol. 2001, 25, 101–110. [Google Scholar] [CrossRef]
- Kiviat, E.; MacDonald, K. Hackensack Meadowlands, New Jersey, Biodiversity: A Review and Synthesis; Hudsonia Ltd.: Annandale, NY, USA, 2002. [Google Scholar]
- Tiner, R.W.; Bergquist, H.C. The Hackensack River Watershed, New Jersey/New York Wetland Characterization, Preliminary Assessment of Wetland Functions, and Remotely-sensed Assessment of Natural Habitat Integrity; U.S. Fish and Wildlife Service, National Wetlands Inventory, Ecological Services: Hadley, MA, USA, 2007.
- DeGraeve, M. New York-New Jersey Harbor Estuary Program: New Jersey Toxics Reduction Work Plan Study I-G Project Report; Great Lakes Environmental Center: Traverse City, MI, USA, 2008. [Google Scholar]
- McCormick, J.M.; Hires, R.I.; Luther, G.W.; Cheng, S.L. Partial recovery of Newark Bay, NJ, following pollution-abatement. Mar. Pollut. Bull. 1983, 14, 188–197. [Google Scholar] [CrossRef]
- Steinberg, N.; Suskowski, D.J.; Clark, L.; Way, J. Health of the Harbor: The First Comprehensive Look at the State of the NY/NJ harbor Estuary. A report to the NY/NJ Harbor Estuary Program; Hudson River Foundation: New York, NY, USA, 2004; 82p. [Google Scholar]
- Miller, D.C.; Poucher, S.L.; Coiro, L. Determination of lethal dissolved oxygen levels for selected marine and estuarine fishes, crustaceans, and a bivalve. Mar. Biol. 2002, 140, 287–296. [Google Scholar]
- Miskewitz, R.; Uchrin, C. In-Stream Dissolved Oxygen Impacts and Sediment Oxygen Demand Resulting from Combined Sewer Overflow Discharges. J. Environ. Eng. 2013, 139, 1307–1313. [Google Scholar] [CrossRef]
- Sturm, C.; Dickerson, N. The State of Water Infrasturcture in New Jersey Cities and Why it Matters; New Jersey Future: Trenton, NJ, USA, 2014. [Google Scholar]
- HydroQual. Assessment of Pollutant Loadings to New York-New Jersey Harbor; U.S. Environmental Protection Agency: Washington, DC, USA, 1991.
- Geyer, W.R.; Signell, R.P. A reassessment of the role of tidal dispersion in estuaries and bays. Estuaries 1992, 15, 97–108. [Google Scholar] [CrossRef]
- MacPherson, T.A.; Cahoon, L.B.; Mallin, M.A. Water column oxygen demand and sediment oxygen flux: Patterns of oxygen depletion in tidal creeks. Hydrobiologia 2007, 586, 235–248. [Google Scholar] [CrossRef]
- Huntley, S.L.; Iannuzzi, T.J.; Avantaggio, J.D.; Carlson-Lynch, H.; Schmidt, C.W.; Finley, B.L. Combined sewer overflows (CSOs) as sources of sediment contamination in the lower Passaic River, New Jersey. 2. Polychlorinated dibenzo-p-dioxins, polychlorinated dibenzofurans, and polychlorinated biphenyls. Chemosphere 1997, 34, 233–250. [Google Scholar] [CrossRef]
- Iannuzzi, T.J.; Huntley, S.L.; Schmidt, C.W.; Finley, B.L.; McNutt, R.P.; Burton, S.J. Combined sewer overflows (CSOs) as sources of sediment contamination in the lower Passaic River, New Jersey. 1. Priority pollutants and inorganic chemicals. Chemosphere 1997, 34, 213–231. [Google Scholar] [CrossRef]
- Walker, W.J.; McNutt, R.P.; Maslanka, C.K. The potential contribution of urban runoff to surface sediments of the Passaic River: Sources and chemical characteristics. Chemosphere 1999, 38, 363–377. [Google Scholar] [CrossRef]
- Long, E.R.; Macdonald, D.D.; Smith, S.L.; Calder, F.D. Incidence of adverse biological effects within ranges of chemical concentrations in marine and esturine sediments. Environ. Manag. 1995, 19, 81–97. [Google Scholar] [CrossRef]
- Horowitz, A.J.; Elrick, K.A. The relation of stream sediment surface area, grain size and composition to trace element chemistry. Appl. Geochem. 1987, 2, 437–451. [Google Scholar] [CrossRef]
- Rice, K.C. Trace-element concentrations in streambed sediment across the conterminous United States. Environ. Sci. Technol. 1999, 33, 2499–2504. [Google Scholar] [CrossRef]
- Lin, J.G.; Chen, S.Y. The relationship between adsorption of heavy metal and organic matter in river sediments. Environ. Int. 1998, 24, 345–352. [Google Scholar] [CrossRef]
- Wallin, J.M.; Hattersley, M.D.; Ludwig, D.F.; Iannuzzi, T.J. Historical assessment of the impacts of chemical contaminants in sediments on benthic invertebrates in the Tidal Passaic River, New Jersey. Hum. Ecol. Risk Assess. 2002, 8, 1155–1176. [Google Scholar] [CrossRef]
- Stern, A.H.; Gochfeld, M.; Lioy, P.J. Two decades of exposure assessment studies on chromate production waste in Jersey City, New Jersey-what we have learned about exposure characterization and its value to public health and remediation. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Martello, L.; Fuchsman, P.; Sorensen, M.; Magar, V.; Wenning, R.J. Chromium geochemistry and bioaccumulation in sediments from the lower Hackensack River, New Jersey. Arch. Environ. Contam. Toxicol. 2007, 53, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Pais, I.; Benton Jones, J., Jr. The Handbook of Trace Elements; St. Lucie Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Feng, H.; Cochran, J.K.; Lwiza, H.; Brownawell, B.J.; Hirschberg, D.J. Distribution of heavy metal and PCB contaminants in the sediments of an urban estuary: The Hudson River. Mar. Environ. Res. 1998, 45, 69–88. [Google Scholar] [CrossRef]
- Adams, D.A.; O’Connor, J.S.; Weisberg, S.B. Sediment Quality of the NY/NJ Harbor System: An Investigation under the Regional Environmental Monitoring and Assessment Program (R-EMAP); U.S. Environmental Protection Agency—Region 2: Edison, NJ, USA, 1998.
- Fu, Q.S.; Barkovskii, A.L.; Adriaens, P. Dioxin cycling in aquatic sediments: The Passaic River Estuary. Chemosphere 2001, 43, 643–648. [Google Scholar] [CrossRef]
- Feng, H.; Cochran, J.K.; Hirschberg, D.J. Transport and sources of metal contaminants over the course of tidal cycle in the turbidity maximum zone of the Hudson River estuary. Water Res. 2002, 36, 733–743. [Google Scholar] [CrossRef]
- Williams, T.P.; Bubb, J.M.; Lester, J.N. Metal accumulation within salt-marsh environments—A review. Mar. Pollut. Bull. 1994, 28, 277–290. [Google Scholar] [CrossRef]
- Miskewitz, R.; Su, T.L.; Hires, R.I.; Dimou, K.; Korfiatis, G.P. Tidal variations of heavy metal concentrations in the Hackensack River, New Jersey, USA. J. Mar. Environ. Eng. 2005, 7, 241–248. [Google Scholar]
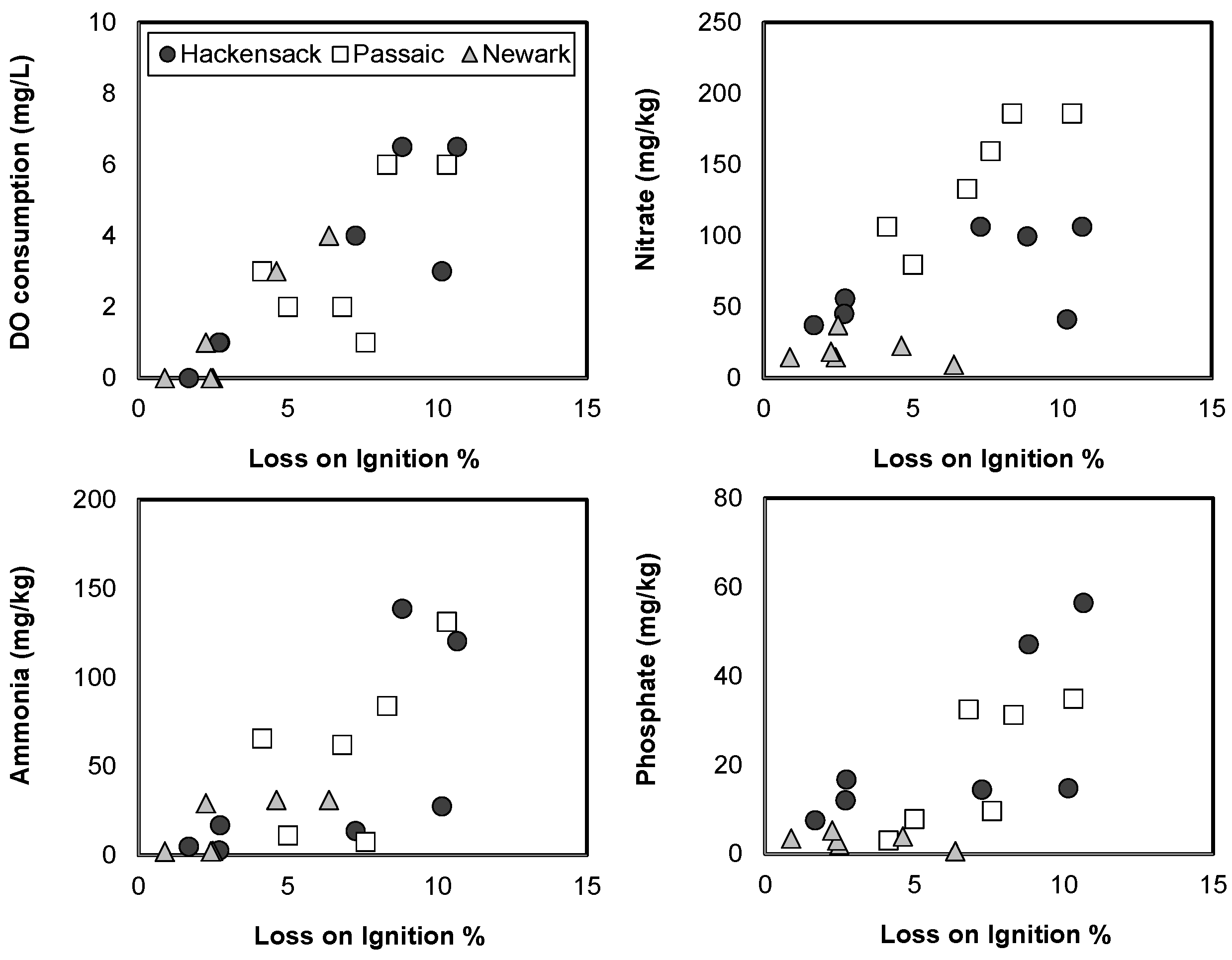
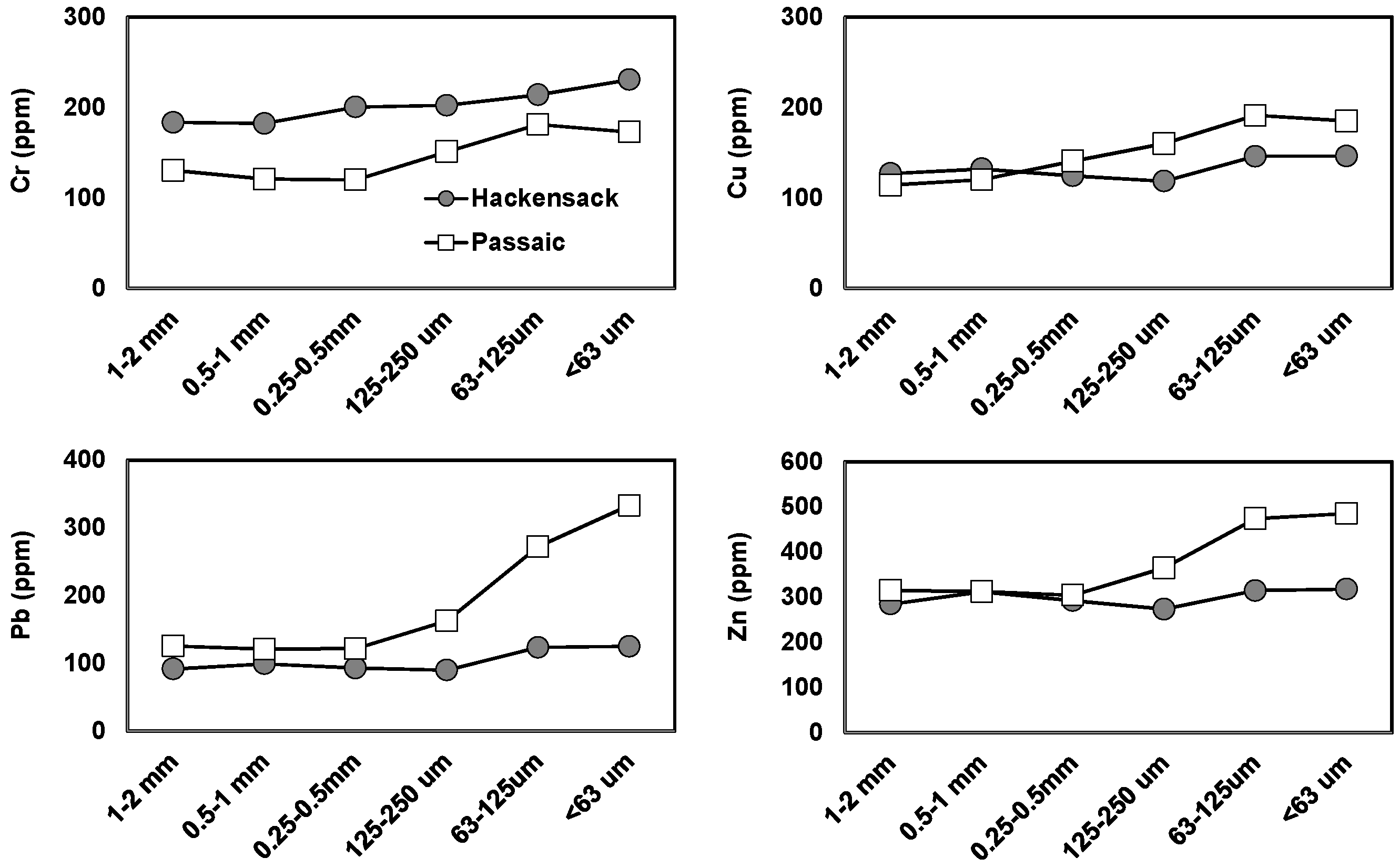
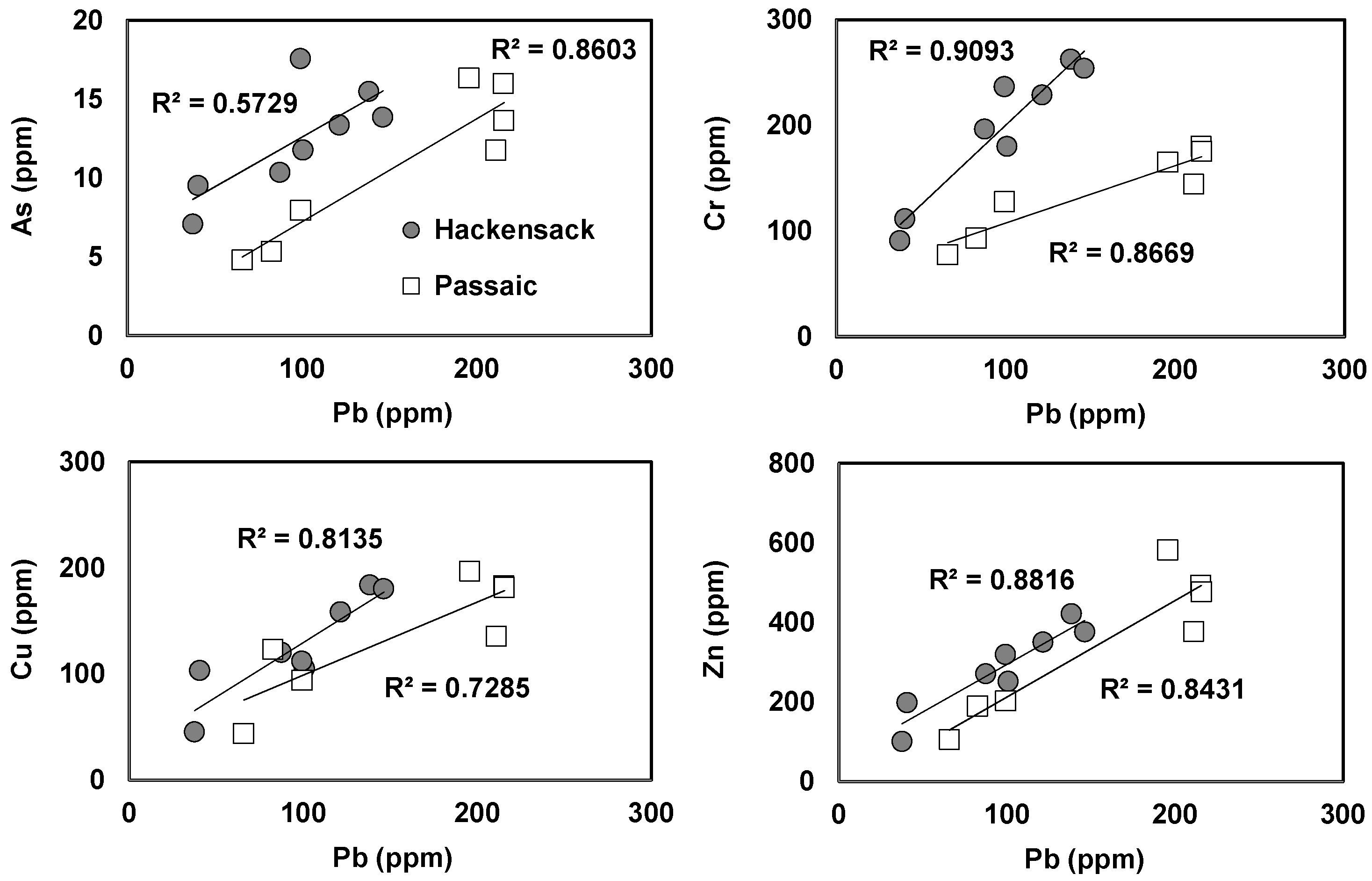
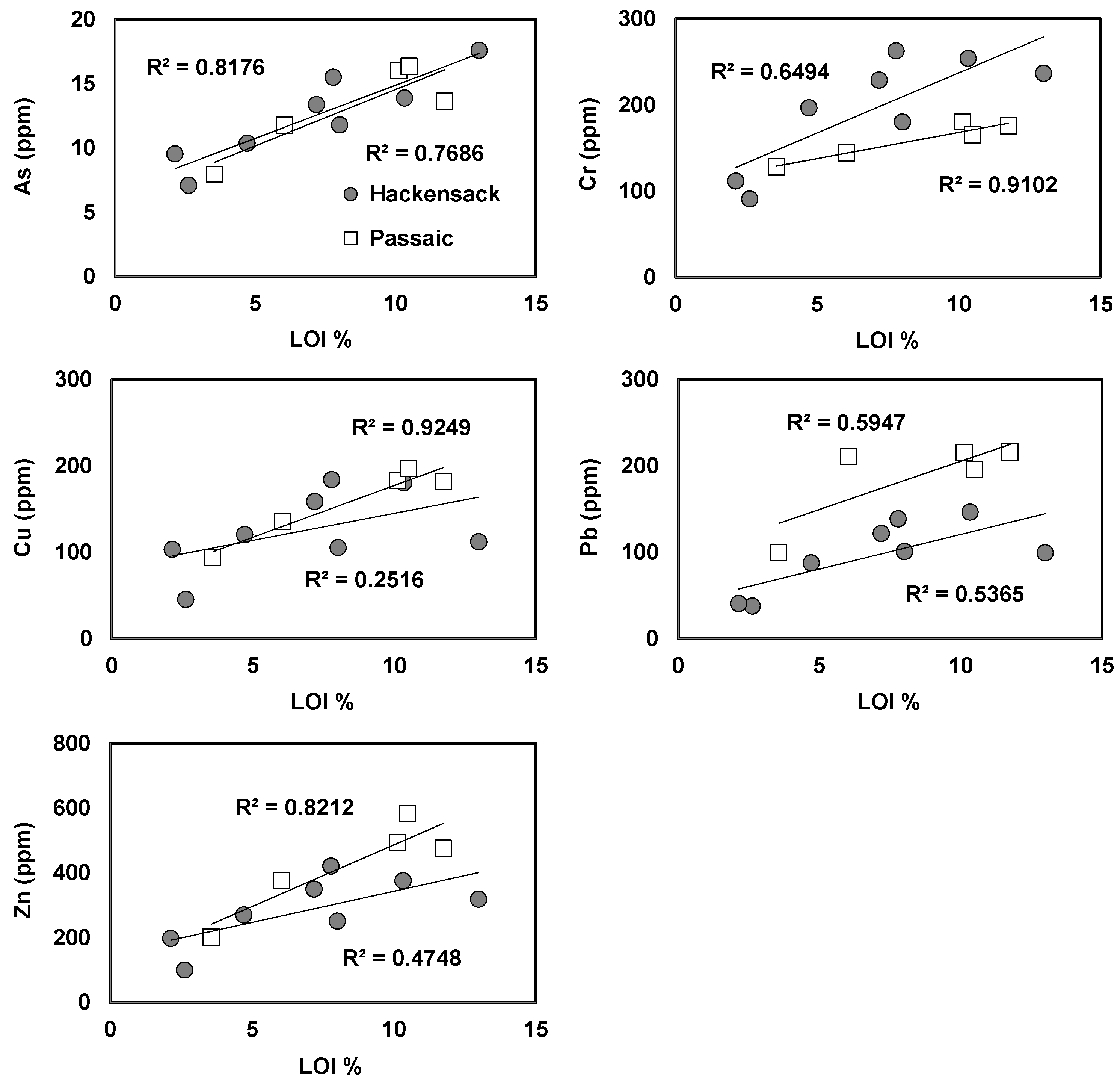
| Sample ID | LAT | LONG | Distance (km) | pH | EC (mS/cm) | DO (mg/L) | Nitrate (mg/L) | Ammonia (mg/L) | Phosphate (mg/L) | Water Level (m) |
|---|---|---|---|---|---|---|---|---|---|---|
| HR-1 | 40.727 | 74.096 | 8.8 | 7.91 | 27.20 | 7 | 9.7 | 0.24 | 0.67 | 1.92 |
| HR-2 | 40.763 | 74.088 | 15.6 | 7.79 | 24.00 | 5 | 12.4 | 0.39 | 0.73 | 1.86 |
| HR-3 | 40.792 | 74.073 | 19.6 | 7.79 | 13.81 | 5 | 14.2 | 1.80 | 1.30 | 1.62 |
| HR-4 | 40.806 | 74.044 | 22.8 | 7.68 | 17.85 | 3 | 13.3 | 0.90 | 1.18 | 1.37 |
| HR-4-1 | 40.805 | 74.043 | 22.9 | 7.67 | 16.24 | 3 | 13.3 | 0.75 | 0.93 | 1.34 |
| HR-5 | 40.847 | 74.032 | 22.9 | 7.81 | 9.72 | 3 | 12.4 | 2.83 | 1.36 | 1.08 |
| HR-6 | 40.856 | 74.031 | 28.1 | 7.78 | 8.33 | 4 | 14.2 | 2.76 | 1.20 | 0.93 |
| HR-7 | 40.876 | 74.036 | 29.2 | 7.76 | 5.60 | 4 | 14.2 | 2.76 | 1.01 | 0.74 |
| HR-8 | 40.885 | 74.035 | 32.5 | 7.74 | 4.30 | 5 | 11.5 | 1.41 | 0.78 | 0.60 |
| HR-9 | 40.907 | 74.026 | 33.6 | 8.07 | 1.23 | 6 | 8.0 | 0.15 | 0.43 | 0.45 |
| Min | 7.67 | 1.23 | 3 | 8.0 | 0.15 | 0.43 | ||||
| Max | 8.07 | 27.20 | 7 | 14.2 | 2.83 | 1.36 | ||||
| AVG | 7.80 | 12.83 | 5 | 12.3 | 1.40 | 0.96 | ||||
| PR-1 | 40.733 | 74.151 | 12.9 | 6.74 | 8.15 | 4 | 6.5 | <0.02 | 0.86 | 0.37 |
| PR-2 | 40.746 | 74.165 | 15.0 | 7.21 | 3.36 | 5 | 7.7 | 0.03 | 0.87 | 0.49 |
| PR-3 | 40.764 | 74.159 | 17.1 | 7.57 | 1.28 | 5 | 10.0 | 0.04 | 0.72 | 0.66 |
| PR-4 | 40.786 | 74.147 | 19.8 | 7.64 | 0.64 | 7 | 10.0 | <0.02 | 0.57 | 0.78 |
| PR-5 | 40.794 | 74.141 | 20.8 | 7.61 | 0.62 | 5 | 9.4 | <0.02 | 0.67 | 0.85 |
| PR-6 | 40.802 | 74.138 | 21.7 | 7.62 | 0.60 | 7 | 8.3 | <0.02 | 0.61 | 0.94 |
| PR-7 | 40.818 | 74.135 | 23.7 | 7.65 | 0.62 | 8 | 9.4 | <0.02 | 0.89 | 1.18 |
| PR-8 | 40.830 | 74.121 | 25.8 | 7.73 | 0.63 | 9 | 9.4 | <0.02 | 0.83 | 1.27 |
| PR-9 | 40.852 | 74.119 | 28.3 | 7.80 | 0.62 | 8 | 10.6 | 0.04 | 0.99 | 1.40 |
| PR-10 | 40.857 | 74.107 | 30.6 | 7.86 | 0.65 | 9 | 8.3 | <0.02 | 0.85 | 1.48 |
| Min | 6.74 | 0.60 | 4 | 6.5 | 0.03 | 0.57 | ||||
| Max | 7.86 | 8.15 | 9 | 10.6 | 0.04 | 0.99 | ||||
| AVG | 7.54 | 1.72 | 7 | 9.0 | 0.02 | 0.79 | ||||
| NB-1 | 40.712 | 74.104 | 7.0 | 7.66 | 29.7 | 5 | 10.6 | 0.04 | 0.46 | 1.31 |
| NB-2 | 40.689 | 74.112 | 4.3 | 7.73 | 35.7 | 8 | 9.7 | 0.10 | 0.39 | 1.14 |
| NB-3 | 40.679 | 74.117 | 3.2 | 7.78 | 35.2 | 6 | 9.7 | 0.06 | 0.29 | 0.99 |
| NB-4 | 40.672 | 74.124 | 2.2 | 7.83 | 35.8 | 7 | 11.5 | <0.02 | 0.35 | 0.74 |
| NB-5 | 40.662 | 74.132 | 0.9 | 7.96 | 36.0 | 8 | 8.9 | 0.05 | 0.2 | 0.66 |
| NB-6 | 40.656 | 74.139 | 0.0 | 8.04 | 35.9 | 5 | 8.9 | 0.03 | 0.28 | 0.55 |
| Min | 7.66 | 29.7 | 5 | 8.9 | 0.03 | 0.20 | ||||
| Max | 8.04 | 36.0 | 8 | 11.5 | 0.10 | 0.46 | ||||
| AVG | 7.83 | 34.7 | 7 | 9.9 | 0.05 | 0.33 |
| Water Body | Sample ID | LAT | LONG | Distance | Chloride | Sulfate | Water Level |
|---|---|---|---|---|---|---|---|
| (km) | mg/L | m | |||||
| HR-1 | 40.7273 | 74.0963 | 8.8 | 7674 | 1014 | 0.33 | |
| HR-2 | 40.7625 | 74.0881 | 15.6 | 3868 | 531 | 0.41 | |
| Hackensack | HR-4 | 40.8065 | 74.0436 | 22.8 | 2666 | 317 | 0.95 |
| River | HR-5 | 40.8572 | 74.0319 | 28.1 | 1014 | 117 | 1.27 |
| HR-6 | 40.8766 | 74.0361 | 29.2 | 605 | 69 | 1.44 | |
| HR-7 | 40.8853 | 74.0347 | 32.5 | 554 | 60 | 1.49 | |
| HR-8 | 40.9067 | 74.0257 | 33.6 | 276 | 29 | 1.54 | |
| PR-1 | 40.7342 | 74.1453 | 12.9 | 402 | 56 | 0.13 | |
| PR-2 | 40.7462 | 74.1652 | 15.0 | 157 | 28 | 0.05 | |
| PR-3 | 40.7640 | 74.1588 | 17.1 | 161 | 24 | 0.06 | |
| Passaic | PR-4 | 40.7839 | 74.1478 | 19.8 | 141 | 23 | 0.12 |
| River | PR-5 | 40.7935 | 74.1412 | 20.8 | 140 | 23 | 0.29 |
| PR-6 | 40.8017 | 74.1381 | 21.7 | 132 | 21 | 0.40 | |
| PR-7 | 40.8134 | 74.1377 | 23.7 | 123 | 22 | 0.58 | |
| PR-8 | 40.8303 | 74.1205 | 25.8 | 124 | 22 | 0.83 | |
| PR-9 | 40.8565 | 74.1067 | 28.3 | 86 | 17 | 1.10 | |
| Newark | NB-1 | 40.6789 | 74.1167 | 3.2 | 9460 | 1274 | 1.64 |
| Bay | NB-2 | 40.6624 | 74.1323 | 0.9 | 9898 | 1268 | 1.70 |
| NB-3 | 40.6543 | 74.1398 | 0.0 | 10,261 | 1329 | 1.73 | |
| Water Body | Sample ID | Distance (km) | pH | EC (mS/cm) | DO (mg/L) | Nitrate (mg/L) | Ammonia (mg/L) | Phosphate (mg/L) | Water Level (m) |
|---|---|---|---|---|---|---|---|---|---|
| HR-2 | 15.6 | 6.76 | 26.70 | 5 | 11.5 | 0.05 | 0.65 | 1.58 | |
| HR-4 | 22.8 | 7.30 | 15.20 | 4 | 22.1 | 1.54 | 1.53 | 1.55 | |
| HR-5 | 22.9 | 7.39 | 10.85 | 3.5 | 17.7 | 3.73 | 1.66 | 1.44 | |
| HR-6 | 28.1 | 7.50 | 10.13 | 3.5 | 16.8 | 4.24 | 1.76 | 1.25 | |
| Hackensack | HR-7 | 29.2 | 7.63 | 8.30 | 2.5 | 18.6 | 3.99 | 1.68 | 1.17 |
| River | HR-2-1 | 15.6 | 7.32 | 25.40 | 4.5 | 15.1 | 0.14 | 0.70 | 0.89 |
| HR-4-1 | 22.8 | 7.56 | 15.30 | 3 | 19.5 | 1.54 | 1.38 | 0.67 | |
| HR-5-1 | 22.9 | 7.69 | 11.24 | 8 | 17.7 | 3.34 | 1.24 | 0.56 | |
| HR-6-1 | 28.1 | 7.76 | 8.64 | 4 | 18.6 | 4.24 | 1.17 | 0.48 | |
| HR-7-1 | 29.2 | 7.78 | 5.75 | 5 | 14.2 | 1.80 | 0.99 | 0.40 | |
| PR-1 | 12.9 | 7.00 | 18.69 | 4 | 7.7 | 0.03 | 0.68 | 0.67 | |
| PR-2 | 15.0 | 7.35 | 10.91 | 5 | 5.9 | <0.02 | 0.74 | 0.58 | |
| Passaic | PR-3 | 17.1 | 7.59 | 5.92 | 6 | 7.7 | 0.03 | 0.59 | 0.48 |
| River | PR-1-1 | 12.9 | 7.55 | 11.12 | 5 | 6.5 | <0.02 | 0.75 | 0.17 |
| PR-2-1 | 15.0 | 7.79 | 5.74 | 6 | 7.7 | <0.02 | 0.88 | 0.15 | |
| PR-3-1 | 17.1 | 8.01 | 2.02 | 9 | 8.3 | <0.02 | 0.68 | 0.12 | |
| NB-1 | 7.0 | 7.66 | 29.7 | 5 | 10.6 | 0.04 | 0.46 | 1.31 | |
| NB-2 | 4.3 | 7.73 | 35.7 | 8 | 9.7 | 0.10 | 0.39 | 1.14 | |
| Newark | NB-3 | 3.2 | 7.78 | 35.2 | 6 | 9.7 | 0.06 | 0.29 | 0.99 |
| Bay | NB-1-1 | 7.0 | 8.00 | 35.9 | 6 | 8.9 | 0.04 | 0.30 | 0.21 |
| NB-2-1 | 4.3 | 7.99 | 35.5 | 6 | 9.7 | <0.02 | 0.48 | 0.34 | |
| NB-3-1 | 3.2 | 7.96 | 35.9 | 9 | 9.7 | <0.02 | 0.24 | 0.42 |
| Sample ID | Distance | Coarse Sand | Medium Sand | Fine Sand | Silt + Clay | ||
|---|---|---|---|---|---|---|---|
| km | 1–2 mm | 0.5–1 mm | 0.25–0.5 mm | 125–250 μm | 63–125 μm | <63 μm | |
| HR-1 | 9 | 1 | 3 | 9 | 60 | 16 | 11 |
| HR-2 | 15 | 17 | 13 | 33 | 19 | 9 | 9 |
| HR-3 | 20 | 21 | 15 | 15 | 16 | 11 | 21 |
| HR-4 | 23 | 17 | 16 | 17 | 14 | 13 | 23 |
| HR-5 | 29 | 27 | 19 | 16 | 11 | 12 | 15 |
| HR-6 | 32 | 25 | 19 | 15 | 11 | 13 | 18 |
| HR-7 | 33 | 17 | 19 | 24 | 18 | 9 | 12 |
| HR-8 | 36 | 20 | 18 | 19 | 18 | 11 | 15 |
| AVG | 18 | 15 | 18 | 21 | 12 | 15 | |
| PR-1 | 13 | 14 | 19 | 15 | 11 | 11 | 31 |
| PR-2 | 15 | 22 | 19 | 12 | 10 | 11 | 26 |
| PR-3 | 18 | 32 | 25 | 17 | 8 | 5 | 13 |
| PR-4 | 20 | 12 | 17 | 15 | 13 | 11 | 32 |
| PR-6 | 21 | 13 | 18 | 36 | 19 | 7 | 7 |
| PR-7 | 22 | 16 | 29 | 33 | 9 | 4 | 8 |
| PR-8 | 24 | 12 | 22 | 32 | 22 | 7 | 5 |
| AVG | 17 | 21 | 23 | 13 | 8 | 17 | |
| Sample ID | Distance | Coarse Sand | Medium Sand | Fine Sand | Silt + Clay | ||
|---|---|---|---|---|---|---|---|
| km | 1–2 mm | 0.5–1 mm | 0.25–0.5 mm | 125–250 μm | 63–125 μm | <63 μm | |
| HR-1 | 9 | 4 | 16 | 38 | 29 | 9 | 5 |
| HR-2 | 16 | 36 | 31 | 17 | 8 | 4 | 5 |
| HR-4 | 23 | 28 | 32 | 24 | 8 | 4 | 5 |
| HR-5 | 28 | 12 | 23 | 32 | 21 | 8 | 4 |
| HR-6 | 29 | 38 | 23 | 14 | 9 | 5 | 10 |
| HR-8 | 34 | 35 | 26 | 16 | 9 | 5 | 9 |
| HR-9 | 36 | 45 | 26 | 13 | 7 | 3 | 5 |
| AVG | 28 | 25 | 22 | 13 | 5 | 6 | |
| PR-1 | 13 | 28 | 26 | 22 | 12 | 6 | 7 |
| PR-3 | 17 | 25 | 30 | 23 | 10 | 5 | 7 |
| PR-5 | 21 | 23 | 29 | 27 | 13 | 5 | 4 |
| PR-6 | 22 | 24 | 21 | 19 | 16 | 9 | 11 |
| PR-7 | 24 | 5 | 10 | 23 | 37 | 18 | 7 |
| PR-8 | 26 | 8 | 17 | 31 | 24 | 11 | 9 |
| AVG | 21 | 22 | 22 | 18 | 10 | 7 | |
| NB-1 | 7 | 8 | 11 | 26 | 39 | 9 | 8 |
| NB-2 | 4 | 26 | 32 | 30 | 13 | 0 | 0 |
| NB-3 | 3 | 15 | 35 | 43 | 6 | 0 | 0 |
| NB-4 | 2 | 22 | 32 | 39 | 6 | 0 | 0 |
| NB-5 | 1 | 5 | 19 | 66 | 9 | 1 | 1 |
| NB-6 | 0 | 10 | 18 | 21 | 29 | 16 | 6 |
| AVG | 14 | 24 | 38 | 17 | 4 | 3 | |
| Element | River | Sediment Grain Size | Bulk <2 mm | ERL | ERM | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1–2 mm | 0.5–1 mm | 0.25–0.5 mm | 125–250 μm | 63–125 μm | <63 μm | |||||
| As | Hackensack | 13 | 14 | 12 | 12 | 14 | 14 | 12 | 8 | 70 |
| Passaic | 14 | 11 | 12 | 10 | 10 | 6 | 11 | |||
| Cr | Hackensack | 183 | 182 | 201 | 202 | 214 | 231 | 195 | 81 | 370 |
| Passaic | 130 | 121 | 120 | 151 | 181 | 173 | 137 | |||
| Cu | Hackensack | 127 | 132 | 124 | 118 | 146 | 146 | 126 | 34 | 270 |
| Passaic | 114 | 120 | 141 | 160 | 191 | 185 | 137 | |||
| Fe | Hackensack | 47605 | 41690 | 38258 | 37914 | 38810 | 38743 | 39467 | ||
| Passaic | 34940 | 31298 | 29249 | 35083 | 44363 | 39849 | 32433 | |||
| Mn | Hackensack | 729 | 817 | 779 | 901 | 773 | 703 | 756 | ||
| Passaic | 579 | 504 | 508 | 600 | 754 | 725 | 555 | |||
| Pb | Hackensack | 92 | 99 | 93 | 90 | 123 | 125 | 96 | 47 | 218 |
| Passaic | 126 | 121 | 122 | 163 | 272 | 333 | 155 | |||
| Zn | Hackensack | 284 | 312 | 292 | 273 | 314 | 318 | 286 | 150 | 410 |
| Passaic | 315 | 312 | 304 | 364 | 474 | 485 | 346 | |||
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, H.B. Nutrients and Heavy Metals Contamination in an Urban Estuary of Northern New Jersey. Geosciences 2017, 7, 108. https://doi.org/10.3390/geosciences7040108
Jung HB. Nutrients and Heavy Metals Contamination in an Urban Estuary of Northern New Jersey. Geosciences. 2017; 7(4):108. https://doi.org/10.3390/geosciences7040108
Chicago/Turabian StyleJung, Hun Bok. 2017. "Nutrients and Heavy Metals Contamination in an Urban Estuary of Northern New Jersey" Geosciences 7, no. 4: 108. https://doi.org/10.3390/geosciences7040108
APA StyleJung, H. B. (2017). Nutrients and Heavy Metals Contamination in an Urban Estuary of Northern New Jersey. Geosciences, 7(4), 108. https://doi.org/10.3390/geosciences7040108





