Abstract
HEU-type zeolite-rich volcaniclastic tuff (Hellenic natural zeolite) is used as a raw material for the production of lighter mortars. The addition of natural zeolite in mortar mixtures of sand and Portland cement leads to a decrease of up to 18.35% unit weight. The increase of the natural zeolite proportions increases the porosity and water absorption of the mortar and, at the same time, decreases the uniaxial compressive strength. These variations in the mortar’s mechanical properties are due to the addition of natural zeolite, which causes incomplete hydration of C2S (2CaO.SiO2) and retardation of the mortar’s hardening.
1. Introduction
Lightweight added materials can be natural (pumice, perlite, diatomites, zeolites, clays, etc.) or artificial (expanded aggregates), the latter obtained from natural or artificial materials after a processing cycle able to create a cellular or highly porous structure. According to the European Union standard [1], lightweight aggregates must have a unit weight of particle lower than 2000 kg/m3 and a loose weight lower than 1200 kg/m3.
Lightweight mortars show a wide range of applications, especially when the need of decreasing the weight of the structures is crucial, e.g., historic masonry. Other advantages of lightweight mortars are thermal and sound insulation and fire resistance. Some natural zeolites display unique physical and chemical features and have a great variety of industrial, building, agricultural, aquacultural and environmental applications. Thus, natural zeolites are used as pozzolana, replacement material in cement production, raw material for lightweight aggregate, raw material for the production of lightweight expanded aggregate, anti-bacteria agent, fungus-resisting material and humidity-conditioning material [2,3,4,5,6,7,8,9].
Zeolites constitute a major class of crystalline hydrated aluminosilicate microporous minerals, including both natural and synthetic species. The crystalline frameworks of zeolites are based on three-dimensional networks of (Si,Al)O4 tetrahedra with extra-framework alkali and alkaline-earth cations. These ions are loosely bound to the anionic sites within the framework structure and can be exchanged for other cations, including H+. Zeolites of commercial interest are present in Greece as authigenic and alteration products in finely crystalline volcaniclastic sediments. HEU-type zeolites (Heulandite-Clinoptilolite group) are among the abundant zeolites found in Greece. Significant deposits of zeolite-rich volcaniclastic tuffs are located mainly in Evros District and Samos island, while some occurrences are located in the Aegean islands of Thira, Polyegos, Kimolos and Milos [9,10,11].
Portlant cement is a system composed of numerous minerals that react with water at different rates, giving hydration products of different composition and crystallinity, and influence the engineering properties of the final product (mortar or concrete). When a cement–water mixture comes in contact with a zeolite mineral, the aluminosilicate framework of the zeolite starts decomposing, under the attack of OH− in a high pH solution. Depolymerized species, such as [SiO(OH)3]− and [Al(OH)4]−, enter the solution and react with Ca2+, forming hydrated calcium silicate and calcium aluminate compounds, very similar to those formed during the hydration of cement [12]. The pozzolanic activity of zeolites depends on their chemical and mineralogical composition. The SiO2 and Al2O3 react with the Ca(OH)2, liberated during the hydration of cement, leading to the formation of C-S-H-gels and aluminates. As a result, the micro-structure of hardened cement mortar or concrete is improved, and the mortar or concrete becomes more impervious [13].
The main goals of this research are: (I) testing the use of HEU-type zeolite-rich volcaniclastic tuff (Hellenic natural zeolite) as a raw material for the production of lighter mortars with higher porosity; (II) the evaluation of the role of the natural zeolite in the properties of the produced mortars; and, (III) finding the best mixtures for the production of lighter mortars.
2. Materials and Methods
Portland cement type CEM IV/B (produced by HERACLES General Cement Co. S.A., a member of the Lafarge Group), sand from the Axios riverbed (Thessaloniki Prefecture, Sindos, Greece) and natural zeolite (grain size of sand) from Evros Prefecture, Greece, have been used in the present study. For all the measurements, specimens were prepared in plastic mould with 12 cubic posts. In this way, each mortar was formed in 12 cubic specimens with a mean edge of 5 cm. The proportion of cement:aggregates:water was 1:2.75:0.485 by weight, and the procedure of mixing was made according to the specifications of [14]. In these mortars, Aggregates used in the mortars were made of variable proportions of (river) sand and natural zeolite. Five mortars containing different proportions of natural zeolite as an aggregate were prepared in which the sand was replaced by natural zeolite from 0 (REF1) to 100% (REF2), as shown in Table 1.

Table 1.
Mortar proportions (wt %) used for the specimen’s preparation.
| Mixture | Cement | Sand | Zeolite |
|---|---|---|---|
| REF1 | 26.7 | 73.3 | 0.0 |
| M1 | 26.7 | 55.0 | 18.3 |
| M2 | 26.7 | 36.7 | 36.7 |
| M3 | 26.7 | 18.3 | 55.0 |
| REF2 | 26.7 | 0.0 | 73.3 |
Each mixture was tested at 3, 7, 28 and 90 days of hardening for bulk density [15], P-wave velocity [16], uniaxial compressive strength [14] and mineralogical composition (XRPD: X-Ray Powder Diffraction). For each P-wave velocity test, three cubic specimens were measured in all directions, X-Y-Z (in total, nine measurements for each mixture), and the reported results represent the average value of those three specimens. XRPD analysis was performed using a Phillips (PW1710: Phillips, Almelo, The Netherlands) diffractometer with Ni-filtered Cukα radiation. The samples were scanned over the 3–63° 2θ interval at a scanning speed of 1.2°/min. Quantitative estimates of the abundance of the mineral phases were derived from the XRPD data using the intensity of a certain reflection, the density and the mass absorption coefficient for Cukα radiation for the minerals present. Corrections were made using external standard mixtures of minerals. The detection limit of the method was ±2 wt %. Chemical analyses of major elements and sulfur were performed at Activation Laboratories Ltd., Ancaster, Ontario, Canada.
3. Results and Discussion
3.1. Chemical and Mineralogical Composition
The chemical analyses of the starting materials are shown in Table 2. Portland cement contains high amounts of CaO (49.36 wt %) and SiO2 (27.79 wt %) and minor amounts of Al2O3 (8.36 wt %) and Fe2O3T (3.70 wt %), whereas the other major oxides are below 2 wt %. Sand and natural zeolite contain high amounts of SiO2 (74.37 and 66.49 wt %, respectively) and minor amounts of Al2O3 (10.26 and 11.78 wt %, respectively), whereas the other major oxides is contained in lesser amounts. The presence of 89 wt % HEU-type zeolite (Table 3) explains the increased loss on ignition (12.81 wt %) of the zeolitic starting material.
The semi-quantitative mineralogical composition of the cement, sand and the Hellenic natural zeolite are shown in Table 3. Cement contains mainly di- and tri-calcium silicate and minor amounts of C4AF (4CaO.Αl2O3.Fe2O3), C3A (3CaO.Αl2O3), quartz, anhydrite, mica and amorphous phase. Sand contains high amounts of quartz and feldspars (89 wt % in total) and minor amounts of calcite, amphibole, mica and clays. The natural zeolite contains high amounts of HEU-type zeolite and minor amounts of feldspars, quartz, clays and mica.
The tested mortars showed significant mineralogical differences during their hardening. As shown in Table 4, the mineral phases C3A, C3S (3CaΟ.SiΟ2) and C4AF were below the detection limit in all tested samples; this implies that these phases became fully hydrated during the first three days.

Table 2.
Chemical analysis (wt %) of the starting materials.
| Starting material | SiO2 | TiO2 | Al2O3 | Fe2O3T | MnO | MgO | CaO | Na2O | K2O | P2O5 | SO3 | L.O.I. | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cement | 27.79 | 0.33 | 8.36 | 3.70 | 0.06 | 1.94 | 49.36 | 0.80 | 1.56 | 0.14 | 1.32 | 4.57 | 99.93 |
| Sand | 74.37 | 0.35 | 10.26 | 2.36 | 0.06 | 1.13 | 3.18 | 2.74 | 2.29 | 0.09 | - | 2.48 | 99.31 |
| HNZ | 66.49 | 0.20 | 11.78 | 1.30 | 0.04 | 0.98 | 2.96 | 0.80 | 1.94 | 0.03 | - | 12.81 | 99.33 |
Notes: HNZ: Hellenic natural zeolite; L.O.I.: Loss on ignition, 1050 °C/2h.

Table 3.
Semi-quantitative mineralogical composition (wt %) of the starting materials.
| Starting material | C2S | C3S | C4AF | C3A | Q | F | C | A | An | H | M | Cl | Am |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cement | 43 | 27 | 7 | 5 | 4 | - | - | - | 4 | - | 3 | - | 7 |
| Sand | - | - | - | - | 48 | 41 | 2 | 2 | - | - | 5 | 2 | - |
| HNZ | - | - | - | - | 2 | 6 | - | - | - | 89 | 1 | 2 | - |
Notes: HNZ: Hellenic natural zeolite; C2S: 2CaO.SiO2; C3S: 3CaΟ.SiΟ2; C4AF: 4CaO.Αl2O3.Fe2O3; C3A: 3CaO.Αl2O3; Q: Quartz; F: Feldspars; C: Calcite; A: Amphibole; An: Anhydrite; H: HEU-type zeolite; M: Micas; Cl: Clay minerals; Ch: Chlorite; Am: Amorphous phase; Detection limit 1 wt %.

Table 4.
Semi-quantitative mineralogical composition of the tested mortars.
| Sample | Days | C2S | C3S | C4AF | C3A | F | TC | HEU | Am | Ch | Q | Po | C | A |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| REF1 | 3 | +++ | bdl | bdl | bdl | +++ | + | na | + | + | +++ | +++ | + | ++ |
| 7 | ++ | bdl | bdl | bdl | +++ | + | na | + | + | +++ | +++ | + | ++ | |
| 28 | + | bdl | bdl | bdl | +++ | + | na | + | + | +++ | ++ | + | ++ | |
| 90 | bdl | bdl | bdl | bdl | +++ | + | na | + | + | +++ | bdl | + | ++ | |
| M1 | 3 | +++ | bdl | bdl | bdl | +++ | + | ++ | + | + | +++ | +++ | + | ++ |
| 7 | ++ | bdl | bdl | bdl | +++ | + | ++ | + | + | +++ | +++ | + | ++ | |
| 28 | + | bdl | bdl | bdl | +++ | + | ++ | + | + | +++ | ++ | + | ++ | |
| 90 | bdl | bdl | bdl | bdl | +++ | + | ++ | + | + | +++ | + | + | ++ | |
| M2 | 3 | +++ | bdl | bdl | bdl | ++ | + | ++ | + | + | +++ | +++ | + | ++ |
| 7 | ++ | bdl | bdl | bdl | ++ | + | ++ | + | + | +++ | +++ | + | ++ | |
| 28 | + | bdl | bdl | bdl | ++ | + | ++ | + | + | +++ | ++ | + | ++ | |
| 90 | + | bdl | bdl | bdl | ++ | + | ++ | + | + | +++ | + | + | ++ | |
| M3 | 3 | +++ | bdl | bdl | bdl | ++ | + | +++ | + | + | +++ | +++ | + | ++ |
| 7 | +++ | bdl | bdl | bdl | ++ | + | +++ | + | + | +++ | ++ | + | ++ | |
| 28 | ++ | bdl | bdl | bdl | ++ | + | +++ | + | + | +++ | ++ | + | ++ | |
| 90 | ++ | bdl | bdl | bdl | ++ | + | +++ | + | + | +++ | + | + | ++ | |
| REF2 | 3 | +++ | bdl | bdl | bdl | ++ | + | +++ | nd | nd | ++ | ++ | + | ++ |
| 7 | +++ | bdl | bdl | bdl | ++ | + | +++ | nd | nd | ++ | + | + | ++ | |
| 28 | +++ | bdl | bdl | bdl | ++ | + | +++ | nd | nd | ++ | + | + | ++ | |
| 90 | ++ | bdl | bdl | bdl | ++ | + | +++ | nd | nd | ++ | bdl | + | ++ |
Notes: C2S: 2CaO.SiO2; C3S: 3CaΟ.SiΟ2; C4AF: 4CaO.Αl2O3.Fe2O3; C3A: 3CaO.Αl2O3; F: Feldspars; TC: Micas + Clay minerals; HEU: HEU-type zeolite; Am: Amphibole; Ch: Chlorite; Q: Quartz; Po: Portlandite; C: Calcite; A: Amorphous phase; na: not added; bdl: below detection limit (<3 wt %); nd: not detected; +++: main phase; ++: secondary phase; +: minor phase.
In particular, phase C3A, which is an initial constituent in small amounts of the Portland cement, hydrates quickly. The hydration reaction is: 2C3A + 21Η → C2AH8 + C4AH13. The products of this reaction are not stable, converting thus to more stable products: 2C3AH6 + 9Η.
Phase C4AF, the initial constituent of the Portland cement, is also contained in small amounts. It hydrates slower and has almost the same hydration reaction products with C3A. The hydration reaction is: C4AF + 16H → 2C2(A,F)H8 or C4AF + 16H → C4(A,F)H13 + (A,F)H3.
The hydration product of C3S at room temperature is a very low crystalline, almost amorphous phase of hydrated calcium silicate (C-S-H) [17] and calcium hydroxide (Po, Portlandite). According to Olson and Jennings [18], C-S-H is the main and most important component of the hydration, which tightens the hardened cement paste and contributes the most to the growth of the tensile strength. The hydration of the C3S phase can be attributed to the reaction: 2C3S + 6Η → C3S2H3 + 3CH.
The symbol C-S-H (or C3S2H3) for the calcium-silicate hydration products is not accurate, since the chemical compound produced is non-stoichiometric; actually more than one C-S-H structures is formed during hydration, depending on the preparation conditions and storage environment [17].
The calcite amount in all specimens did not show any significant change; this fact implies that the pozzolanic compounds of the cement halted the reaction between Ca(OH)2 (portlandite) and atmospheric CO2 towards the production of extra calcite.
Moreover, the mineral phases that showed particular interest were C2S (2CaO.SiO2) and Ca(OΗ)2. More specific, in specimen REF1 (mortar with 26.7 wt % cement and 73.3 wt % sand, Table 1), a gradual reduction of C2S presence is observed due to its hydration. As a result, after 90 days of hardening, C2S is not detected, implying that C2S was fully hydrated in specimen REF1. Also in REF1, the same observation was made for Ca(OΗ)2; a high amount during the first days of hydration, since most mineral phases of cement have Ca(OΗ)2 as a hydration product, and a gradual reduction of its presence until the 90th day of hardening, where Ca(OΗ)2 was not detected. From this observation, we can suppose that the reaction between cement pozzolanic components + Ca(OΗ)2 + water was complete and led to the production of C-S-H.
In contrast, in specimen REF2 (mortar with 26.7 wt % cement and 73.3 wt % natural zeolite, Table 1) C2S was the main phase at the 28th day of hardening and was present until the 90th day, which implies its partial hydration. As a result, small amounts of Ca(OΗ)2 were observed during hardening, except at the 90th day, where Ca(OH)2 is under the detection limit. Also, the partial hydration of C2S leads to small amounts of C-S-H phase, with the latter being responsible for the cement hardening.
The partial hydration of C2S is attributed exclusively to the natural zeolite, which acts competitively to C2S by adsorbing the water of the starting mixture for the preparation of mortar. As a consequence, water was not available for C2S hydration. In specimens M1, M2 and M3, intermediate conditions were observed: smaller amounts of natural zeolite in the mixture resulted in the formation of a higher amount of hydrated C2S, whereas bigger amounts of natural zeolite produced less hydrated C2S.
3.2. Physico-Mechanical Characteristics
The mean values and the standard deviation of the physico-mechanical properties of the studied mortars are summarized in Table 5.
3.2.1. Primary Wave Velocity
The mean values of the primary wave velocity (VP) in Table 5 range from 4213 m/s (REF1 at the third day of hardening) to 1965 m/s (REF2 at the ninetieth day of hardening). Experimental results by Matusinovic et al. [19] demonstrated that frequency shift decreases hydration progresses, indicating the change of microstructure and development of more dense structure with less defects and micro-cracks. Hernandez et al. [20] measured primary wave velocities of up to 4521 m/s in less than 24 h of hardening.

Table 5.
Physico-mechanical characteristics of the mortar-mixtures studied.
| Mixture | Days | Vp (m/s) | S.D. | Unit Weight (g/cm3) | S.D. | U.C.S. (MPa) | S.D. |
|---|---|---|---|---|---|---|---|
| REF1 | 3 | 4213 | 242 | 1.952 | 0.014 | 10.8 | 0.6 |
| 7 | 2797 | 69 | 1.920 | 0.035 | 13.3 | 1.7 | |
| 28 | 2833 | 66 | 1.880 | 0.019 | 14.2 | 1.0 | |
| 90 | 2663 | 62 | 1.869 | 0.015 | 14.6 | 0.6 | |
| Μ1 | 3 | 3913 | 258 | 1.845 | 0.018 | 8.1 | 0.4 |
| 7 | 2752 | 95 | 1.778 | 0.022 | 9.4 | 1.2 | |
| 28 | 2734 | 109 | 1.771 | 0.016 | 10.0 | 0.2 | |
| 90 | 2553 | 48 | 1.727 | 0.027 | 10.4 | 1.0 | |
| Μ2 | 3 | 3616 | 180 | 1.778 | 0.035 | 7.0 | 0.2 |
| 7 | 2488 | 45 | 1.698 | 0.016 | 8.7 | 0.1 | |
| 28 | 2524 | 137 | 1.667 | 0.007 | 9.2 | 0.7 | |
| 90 | 2317 | 130 | 1.657 | 0.011 | 9.0 | 0.9 | |
| Μ3 | 3 | 3320 | 176 | 1.674 | 0.008 | 4.1 | 0.5 |
| 7 | 2274 | 48 | 1.611 | 0.020 | 6.4 | 0.3 | |
| 28 | 2300 | 55 | 1.584 | 0.032 | 6.5 | 0.6 | |
| 90 | 2125 | 68 | 1.577 | 0.013 | 6.2 | 0.2 | |
| REF2 | 3 | 2589 | 114 | 1.594 | 0.008 | 3.6 | 0.8 |
| 7 | 2307 | 91 | 1.561 | 0.030 | 6.5 | 0.5 | |
| 28 | 2201 | 86 | 1.531 | 0.013 | 6.6 | 0.5 | |
| 90 | 1965 | 76 | 1.526 | 0.010 | 6.3 | 0.2 |
Notes: Vp = P-wave velocity; S.D. = Standard deviation; U.C.S. = Uniaxial Compressive Strength.
As shown in Figure 1, the mean values of the REF2 mortar are lower than those from the other mixtures. Even from the first days, the replacement of sand by natural zeolite led to smaller primary wave velocities. The mean values of the primary wave velocities for the mixtures M1, M2 and M3 are within the range of the mean values for samples REF1 and REF2. As the percentage of natural zeolite in the mortar increases, the mean value of P-wave velocity decreases. This behavior can be attributed to the special solid crystalline microporous structure of natural zeolites, to the micropores of the HEU-type zeolite and to the meso- and macro-pores of the natural zeolite (the rock), as well as to the water absorption capacity of the natural zeolite. There is general agreement that lower P-wave velocities are linked to higher porosity values [21]. Consequently the mortar’s porosity increases with higher content of natural zeolite. According to Janotka and Mojumdar [22], the presence of zeolite in mixtures with cement leads to an increase of the micro-pores, while Janotka [23] proposed that the higher content of zeolite in mortars results in higher porosity of the mortar.
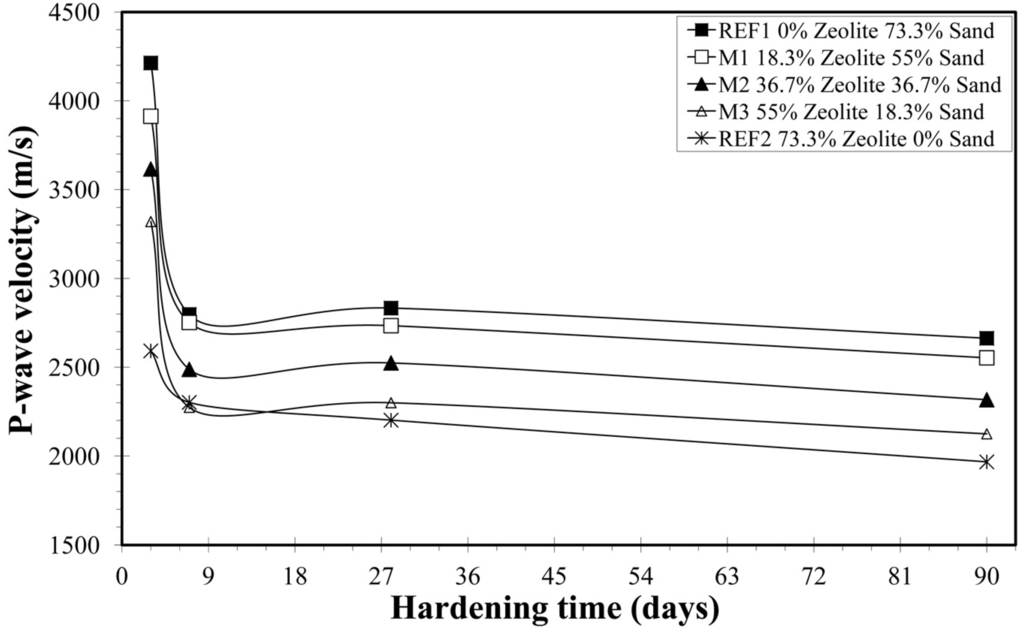
Figure 1.
Variation of P-wave velocity vs. days of hardening.
Figure 1.
Variation of P-wave velocity vs. days of hardening.
3.2.2. Unit Weight
After 28 days of hardening values of the unit weight of all mixtures are much lower than those determined at the beginning of the tests (Table 5, Figure 2). The unit weight of the mortar-mixture REF2 (1.526 ± 0.010 g/cm3) measured at 90 days was 18% smaller (lighter mortar) than in the mortar-mixture REF1 (1.869 ± 0.015 g/cm3), while the mean values of the unit weight of mortar-mixtures M1, M2 and M3 are within the range of the mean values of REF1 and REF2. Since the unit weight of sand is 2.6–2.8 g/cm3, and that of natural zeolite is 2.0–2.2 g/cm3, the presence of natural zeolite in the mortars enlarges the micro-pores, resulting in higher porosity. Short and Kinniburgh [24] concluded that the reduction of the unit weight of a lightweight concrete can be achieved by the addition of a void system within the cementitious conglomerate.
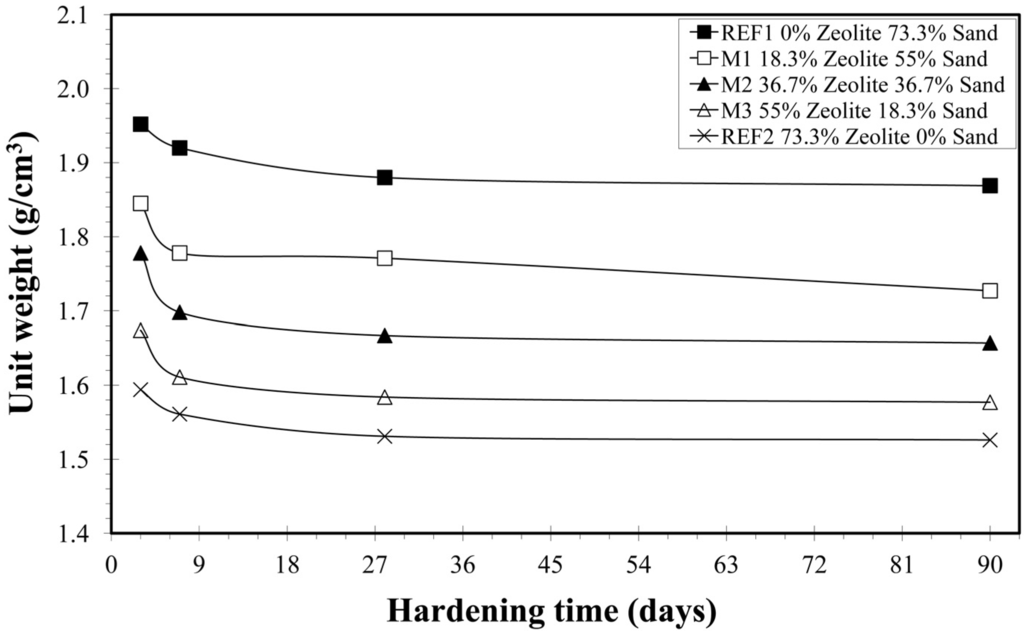
Figure 2.
Variation of unit weight vs. hardening time.
Figure 2.
Variation of unit weight vs. hardening time.
3.2.3. Uniaxial Compressive Strength
The mean value of the uniaxial compressive strength of the mortar sample REF1 varies from 10.8 ± 0.6 MPa at the third day of hardening to 14.6 ± 0.6 MPa at the ninetieth day of hardening (Table 5 and Figure 3). The mean value of the uniaxial compressive strength at the 28th day (14.2 ± 1 MPa) is according to the standard ASTM C 1329-04 [25] specifications for mortars types N and S. The mean values of mortar sample REF2 for uniaxial compressive strength vary from 3.6 ± 0.8 MPa to 6.3 ± 0.2 MPa, respectively, while mean values of the unit uniaxial compressive strength of the other samples (M1, M2 and M3) are between the mean values of samples REF1 and REF2. Consequently, the replacement of sand by natural zeolite in the mortar results in lesser uniaxial strength. Since the presence of natural zeolite in mortars increases porosity, it increases the air inside the mortar. After compressive strength tests, Park et al. [7] concluded that the increased air content in the mortar decreases the compressive strength. The difference of the mean values of the uniaxial compressive strength, between mortar samples REF1 and REF2, can also be explained by the mineralogical composition of the mortar samples. The mineral phase of major relevant interest is C2S, whose hydration products are responsible for mortar’s hardening [17]. At the mortar sample REF1, a gradual decrease of the presence of C2S was observed, and finally, at the ninetieth day of hardening, the C2S is not traceable. Consequently, the C2S was fully hydrated, and mortar sample REF1 shows the highest uniaxial compressive strength. On the contrary, in mortar sample REF2, the C2S is present even at the ninetieth day of hardening. This means that the C2S was not fully hydrated in the presence of natural zeolite, leading to lower uniaxial compressive strength, compared to sample REF1 (Figure 3).
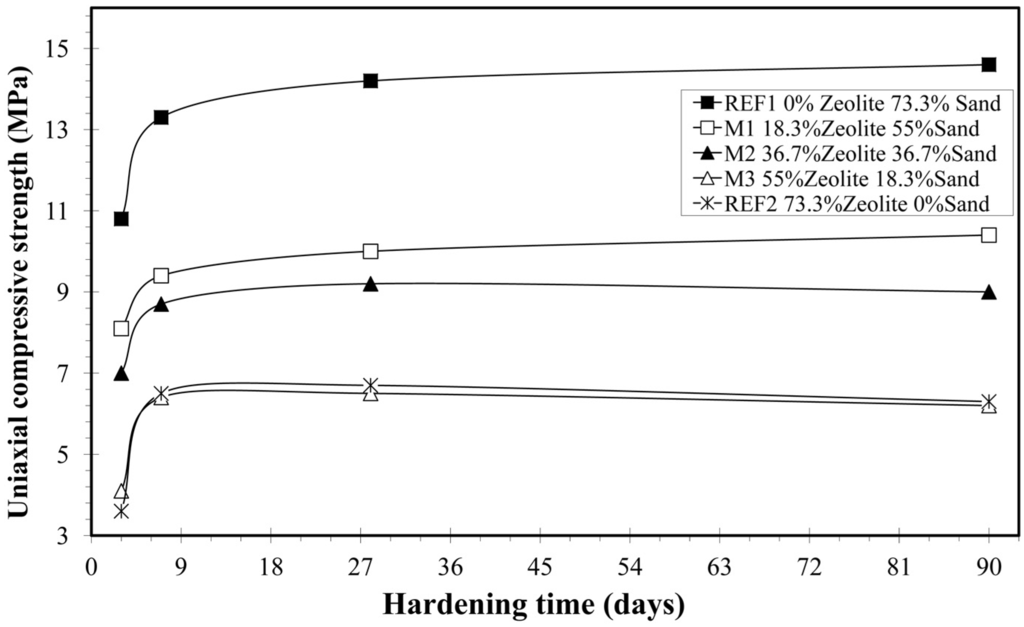
Figure 3.
Variation of uniaxial compressive strength vs. hardening time.
Figure 3.
Variation of uniaxial compressive strength vs. hardening time.
4. Conclusions
The replacement of sand by Hellenic natural zeolite led to a decrease of the unit weight. The unit weight of natural zeolitic mortar (sample REF2) is 18.35% lighter than that of normal mortar (sample REF1).
The gradual increase of the percentage of natural zeolite in mortar mixtures decreases the P-wave velocity, which is closely related to an increase of the porosity and the water absorption caused by the structure of the zeolite.
Considering the uniaxial compressive strength at the 28th day, the sample mortar REF1 can be classified either as type N or S. The replacement of sand by natural zeolite resulted in lesser uniaxial compressive strength, which is due to the incomplete hydration of the C2S phase caused by the presence of zeolite, and also resulted in a loss of hardening.
Acknowledgments
The authors express their thanks to GEOVET N. Alexandridis and Co O.E. for supplying the Hellenic natural zeolite. Also, we appreciate the four anonymous reviewers for their comments.
References
- Lightweight Aggregates—Part 1: Lightweight Aggregates for Concrete, Mortar and Grout; SS-EN 13055-1; European Standards (EN): Pilsen, Czech Republic, 2002.
- Canpolat, F.; Yilmaz, K.; Kose, M.M.; Sumer, M.; Yurdusev, M.A. Use of zeolite, coal bottom ash and fly ash as replacement materials in cement production. Cem. Concr. Res. 2004, 34, 731–736. [Google Scholar] [CrossRef]
- De’ Gennaro, R.; Cappelletti, P.; Cerri, G.; de’ Gennaro, M.; Dondi, M.; Langella, A. Zeolitic tuffs as raw materials for lightweight aggregates. Appl. Clay Sci. 2004, 25, 71–81. [Google Scholar] [CrossRef]
- De’ Gennaro, R.; Langella, A.; D’Amore, M.; Dondi, M.; Colella, A.; Cappelletti, P.; de’ Gennaro, M. Use of zeolite-rich rocks and waste materials for the production of structural lightweight concretes. Appl. Clay Sci. 2008, 41, 61–72. [Google Scholar] [CrossRef]
- Feng, N.Q.; Peng, G.F. Applications of natural zeolite to construction and building materials in China. Constr. Build. Mater. 2005, 19, 579–584. [Google Scholar] [CrossRef]
- Filippidis, A.; Kantiranis, N. Experimental neutralization of lake and stream waters from N. Greece using domestic HEU-type rich natural zeolitic material. Desalination 2007, 213, 47–55. [Google Scholar] [CrossRef]
- Park, S.-K.; Kim, J.J.-H.; Nam, J.-W.; Phan, H.D.; Kim, J.-K. Development of anti-fungal mortar and concrete using zeolite and zeocarbon microcapsules. Cem. Concr. Compos. 2009, 31, 447–453. [Google Scholar] [CrossRef]
- Perraki, T.; Kontori, E.; Tsivilis, S.; Kakali, G. The effect of zeolite on the properties and hydration of blended cements. Cem. Concr. Compos. 2010, 32, 128–133. [Google Scholar] [CrossRef]
- Stamatakis, M.G.; Hall, A.; Hein, J.R. The zeolite deposits of Greece. Miner. Deposita. 1996, 31, 473–481. [Google Scholar] [CrossRef]
- Filippidis, A. Environmental industrial and agricultural applications of Hellenic natural zeolite. Hell. J. Geosci. 2010, 45, 91–100. [Google Scholar]
- Kantiranis, N.; Chrissafis, C.; Filippidis, A.; Paraskevopoulos, K.M. Thermal distinction of HEU-type mineral phases contained in Greek zeolite-rich volcaniclastic tuffs. Eur. J. Mineral. 2006, 18, 509–516. [Google Scholar] [CrossRef]
- Shi, C.; Day, R. Pozzolanic reaction in the presence of chemical activators: Part II—Reaction products and mechanism. Cem. Concr. Res. 2000, 30, 607–613. [Google Scholar] [CrossRef]
- Chan, S.; Ji, X. Comparative study of the initial surface absorption and chloride diffusion of high performance zeolite, silica fume and PFA concretes. Cem. Concr. Compos. 1999, 21, 293–300. [Google Scholar] [CrossRef]
- ASTM C109/C109Μ-02 Standard Test Method for Compressive Strength of Hydraulic Cement Mortars Using 2-in. or [50-mm] Cube Specimens. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2002; Volume 04.01.
- ASTM C29/C29M-09 Standard Test Method for Bulk Density (“Unit Weight”) and Voids in Aggregate. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2009; Volume 04.02.
- NF B10-505-1973 Quarry Products. Limestones. Measurement of the Speed of Sound Propagation (Longitudinal Waves), AFNOR Group: Paris, France, 1973.
- Ramachandran, V.S.; Beaudoin, J.J. Concrete science. In Handbook of Analytical Techniques in Concrete Science and Technology: Principles, Techniques and Applications; William Andrew Publishing: Norwich, NY, USA, 2001; pp. 1–55. [Google Scholar]
- Olson, R.A.; Jennings, H.M. Estimation of C-S-H content in a blended cement paste using water adsorption. Cem. Concr. Res. 2001, 31, 351–356. [Google Scholar] [CrossRef]
- Matusinovic, T.; Kurajica, S.; Sipusic, J. The correlation between compressive strength and ultrasonic parameters of calcium aluminate cement materials. Cem. Concr. Res. 2004, 34, 1451–1457. [Google Scholar] [CrossRef]
- Hernandez, M.G.; Anaya, J.J.; Sanchez, T.; Segura, I. Porosity estimation of aged mortar using a micromechanical model. Ultrasonics 2006, 44, 1007–1011. [Google Scholar] [CrossRef]
- Wyllie, M.R.J.; Gregory, A.R.; Gardner, G.H.F. An experimental investigation of factors affecting elastic wave velocities in porous media. Geophysics 1958, 23, 459–493. [Google Scholar] [CrossRef]
- Janotka, I.; Mojumbar, S.C. Hydration of Portland cement, natural zeolite mortar in water and sulphate solution. Solid State Phenom. 2003, 90-91, 309–316. [Google Scholar] [CrossRef]
- Janotka, I. The influence of zeolitic cement and sand on resistance of mortar subjected to hydrochloric acid solution attack. Ceramics 1999, 43, 61–66. [Google Scholar]
- Short, A.; Kinniburgh, W. Lightweight Concrete, 3rd ed; Applied Science Publishers: London, UK, 1978. [Google Scholar]
- ASTM C 1329-04 Standard Specification for Mortar Cement. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2002; Volume 04.01.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
