Cl-Bearing Mineral Microinclusions in Arc Lavas: An Overview of Recent Findings with Some Metallogenic Implications
Abstract
1. Introduction
2. Geologic Background
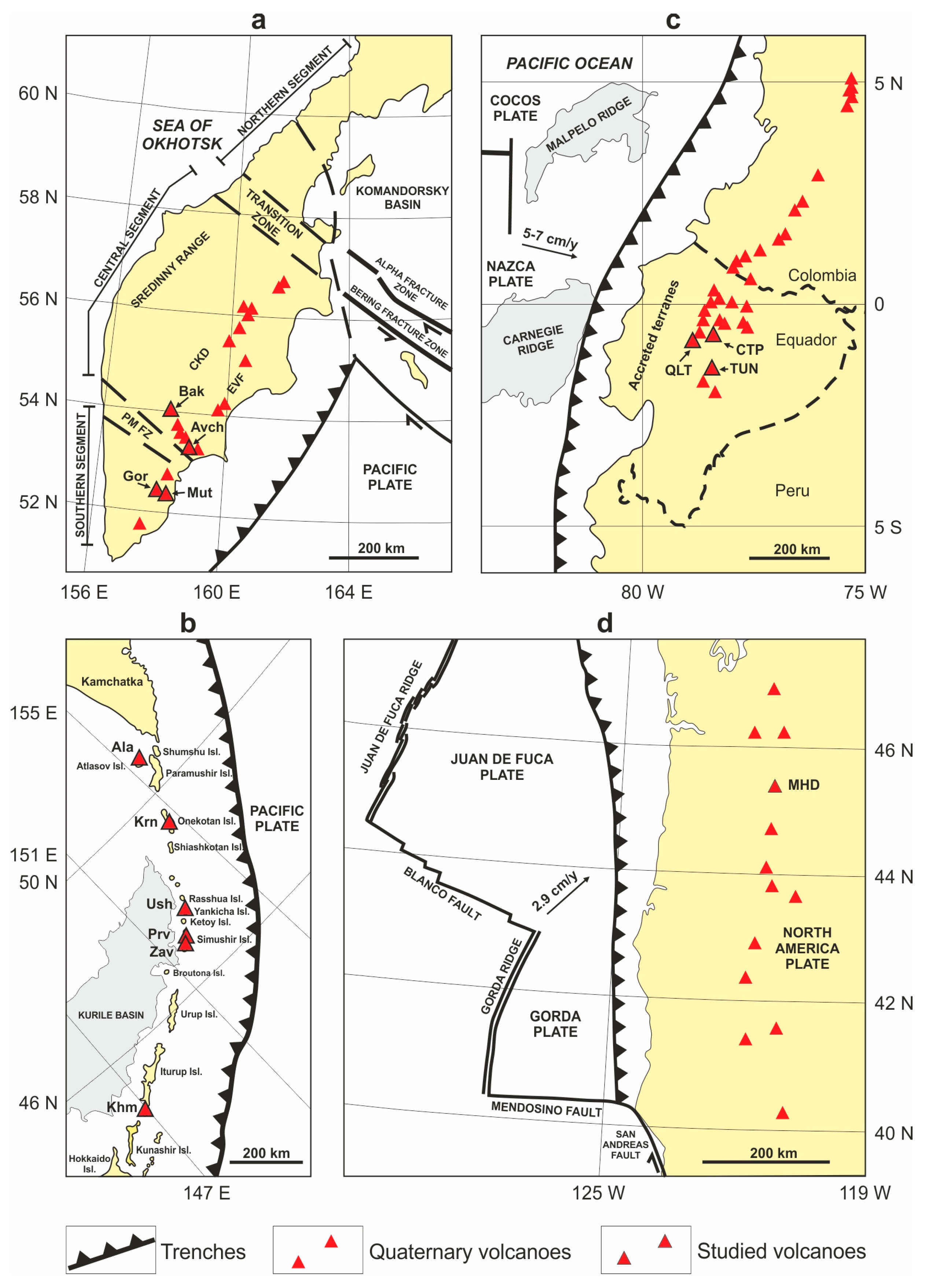
2.1. Kamchatka
2.2. The Kuriles
2.3. Ecuador
2.4. The Cascades
3. Samples and Methods
4. Results
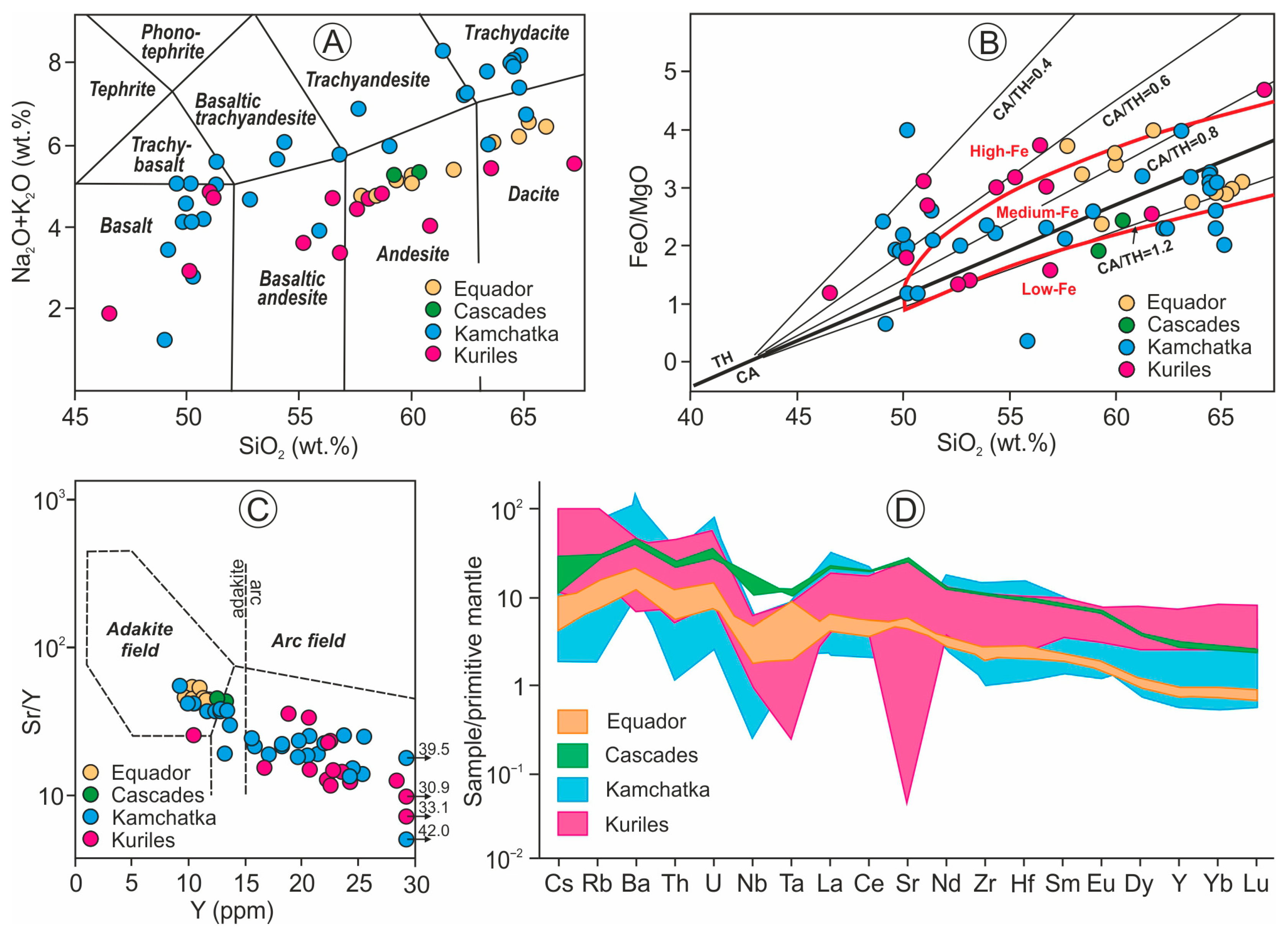
4.1. Petrology and Geochemistry of Host Arc Lavas
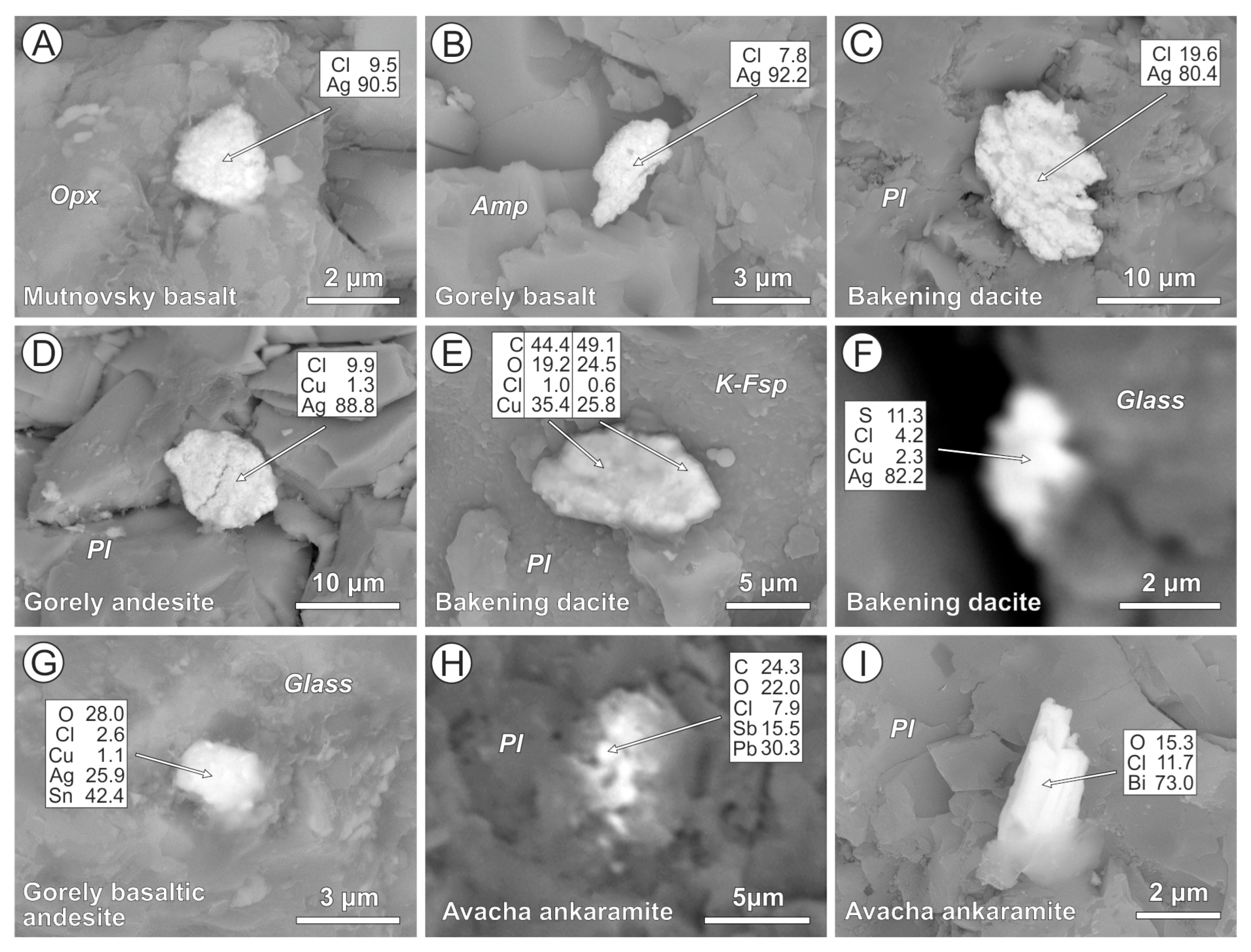
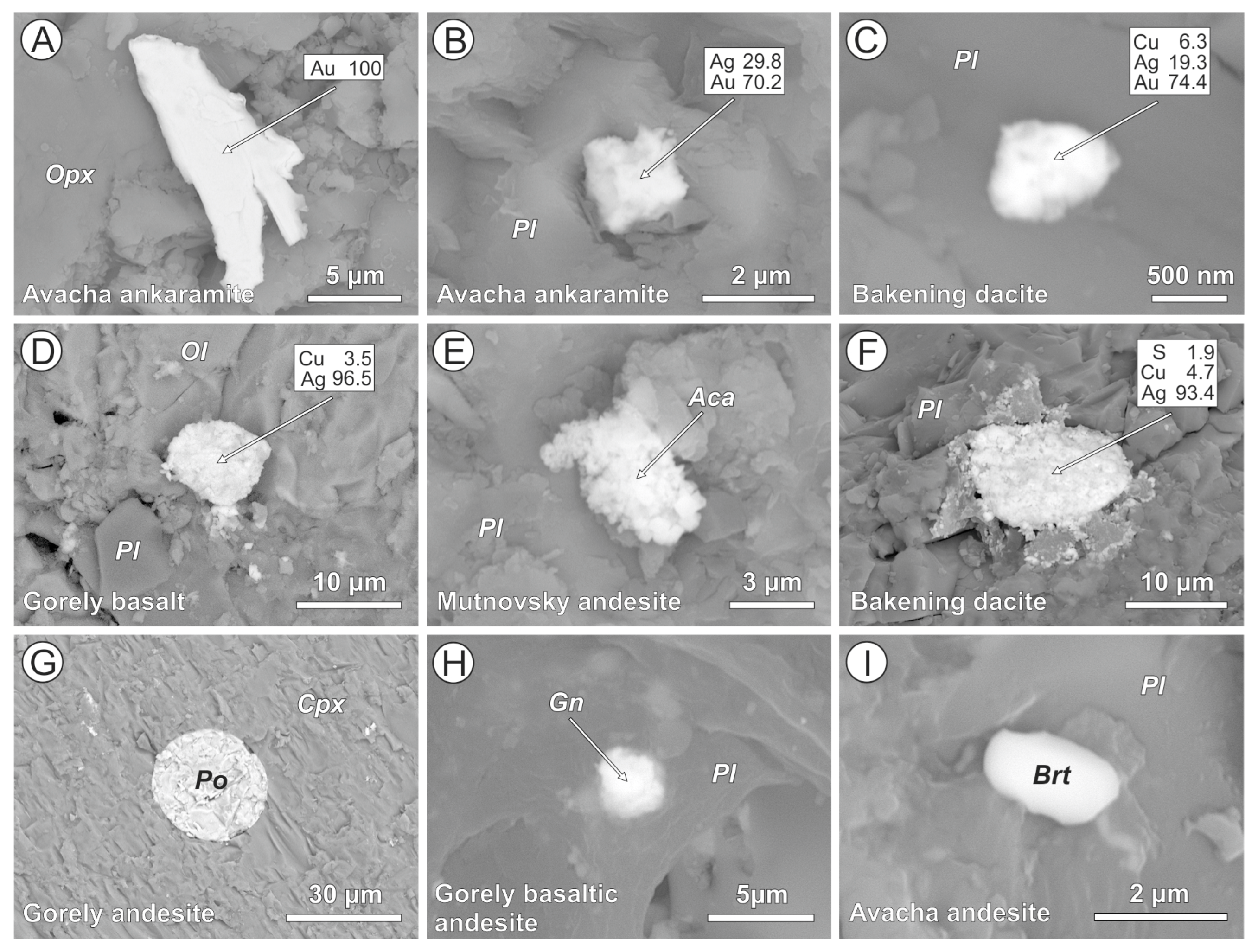
4.2. Cl-Bearing and Associated Mineral Microinclusions in Arc Lavas
4.2.1. Kamchatka
4.2.2. Kuriles
4.2.3. Ecuador
4.2.4. Cascades
5. Discussion
5.1. Cl-Bearing Solid Microinclusions in Magmatic Minerals
5.2. Post-Magmatic Cl-Bearing Mineral Microinclusions
5.3. Comparisons with Arc-Related Epithermal and Porphyry Mineralization
5.4. Implications for Ore Element Mobility in Volcanic Arcs
6. Conclusions
- 1.
- Arc ankaramite, basalt, basaltic andesite, andesite, and dacite in the Kamchatka, the Kuriles, Ecuador, and the Cascades contain microinclusions of chlorine-bearing minerals represented primarily by chalcophile metal chlorides, oxychlorides and their composites with native metals, alloys, and sulfides, as well as apatite with variable amounts of chlorine and fluorine.
- 2.
- The Cl-bearing microminerals are commonly associated with microinclusions of precious and base metals, their alloys, sulfides (pyrrhotite, chalcopyrite, chalcocite, covellite, bornite, galena, and other composite chalcophile sulfides), oxides (titanomagnetite, ilmenite, rutile, and cassiterite), and sulfates (barite).
- 3.
- Some Cl-bearing microinclusions are enclosed in magmatic rock-forming minerals such as olivine, ortho- and clinopyroxenes, amphibole, plagioclase, anorthoclase, and quartz and could have crystallized from the immiscible Cl-rich melts exsolved from volcanic arc magmas during their differentiation in the sub-arc crust. Alternatively, specific microinclusions with Ag-S-Cl compositions may record the oxidation and decomposition of earlier magmatic sulfides in the presence of Cl-bearing magmatic volatiles.
- 4.
- Post-magmatic Cl-bearing microminerals and associated metal, sulfide, oxide, and sulfate microinclusions are observed in secondary fractures and cracks, pores and voids, and intergranular contacts and spaces, as well as in groundmass volcanic glass. These post-magmatic microinclusion assemblages most likely precipitated from hydrothermal fluids that accompanied emplacement and post-eruption transformations of host volcanic rocks.
- 5.
- Assemblages of Cl-bearing and associated microinclusions in arc lavas in many aspects resemble ore mineral associations in subduction-related, magmatic-hydrothermal Cu-Ag-Au deposits, suggesting that the former may possibly record the initial stages of epithermal and porphyry ore formation beneath arc volcanoes.
- 6.
- Elevated ore metal contents in some volcanic rocks from Kamchatka, Kuriles, Ecuador, and the Cascades may indicate the enhanced mobility of chalcophile metals in the presence of Cl-S fluids in subduction zones.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wyllie, P.J. Magmas and volatile components. Am. Mineral. 1979, 64, 469–500. [Google Scholar]
- Hedenquist, J.W.; Lowenstern, J.B. The role of magmas in the formation of hydrothermal ore deposits. Nature 1994, 370, 519–527. [Google Scholar] [CrossRef]
- Audétat, A. The metal content of magmatic-hydrothermal fluids and its relationships to mineralization potential. Econ. Geol. 2019, 114, 1033–1056. [Google Scholar] [CrossRef]
- Stock, M.J.; Humphreys, M.C.S.; Smith, V.C.; Isaia, R.; Pyle, D.M. Late-stage volatile saturation as a potential trigger for explosive volcanic eruptions. Nat. Geosci. 2016, 9, 249–254. [Google Scholar] [CrossRef]
- Harris, D.M.; Anderson, A.T., Jr. Volatiles H2O2, CO2, and Cl in a subduction related basalt. Contrib. Mineral. Petrol. 1984, 87, 120–128. [Google Scholar] [CrossRef]
- Keppler, H. Fluids and trace element transport in subduction zones. Am. Mineral. 2017, 102, 5–20. [Google Scholar] [CrossRef]
- Ferrando, S.; Petrelli, M.; Frezzotti, M.L. Gradual and selective trace-element enrichment in slab-released fluids at sub-arc depths. Sci. Rep. 2019, 9, 16393. [Google Scholar] [CrossRef]
- Spandler, C.; Pirard, C. Element recycling from subducting slabs to arc crust: A review. Lithos 2013, 170, 208–223. [Google Scholar] [CrossRef]
- Richards, J.R. Magmatic to hydrothermal metal fluxes in convergent and collided magmas. Ore Geol. Rev. 2011, 40, 1–26. [Google Scholar] [CrossRef]
- Cox, D.; Watt, S.F.L.; Jenner, F.E.; Hastie, A.R.; Hammond, S.J.; Kunz, B.E. Elevated magma fluxes deliver high-Cu magmas to the upper crust. Geology 2020, 48, 957–960. [Google Scholar] [CrossRef]
- Schettino, E.; González-Jiménez, J.M.; Marchesi, C.; Palozza, F.; Blanco-Quintero, I.F.; Gervilla, F.; Braga, R.; Garrido, C.J.; Fiorentini, M. Mantle-to-crust metal transfer by nanomelts. Commun. Earth Environ. 2023, 4, 256. [Google Scholar] [CrossRef]
- Pokrovski, G.S.; Borisova, A.Y.; Bychkov, A.Y. Speciation and transport of metals and metalloids in geological vapors. Rev. Mineral. Geochem. 2013, 76, 165–218. [Google Scholar] [CrossRef]
- Zhong, R.; Brugger, J.; Chen, Y.; Li, W. Contrasting regimes of Cu, Zn and Pb transport in ore-forming hydrothermal fluids. Chem. Geol. 2015, 395, 154–164. [Google Scholar] [CrossRef]
- Guan, Q.; Mei, Y.; Liu, W.; Brugger, J. Different metal coordination in sub- and super-critical fluids: Do molybdenum(IV) chloride complexes contribute to mass transfer in magmatic systems? Geochim. Cosmochim. Acta 2023, 354, 240–251. [Google Scholar] [CrossRef]
- Webster, J.D.; Kinzler, R.J.; Mathez, E.A. Chloride and water solubility in basalt and andesite melts and implications for magmatic degassing. Geochim. Cosmochim. Acta 1999, 63, 729–738. [Google Scholar] [CrossRef]
- Thomas, R.W.; Wood, B.J. The effect of composition on chlorine solubility and behavior in silicate melts. Am. Mineral. 2023, 108, 814–825. [Google Scholar] [CrossRef]
- Frank, M.R.; Candela, P.A.; Piccoli, P.M.; Glascock, M.D. Gold solubility, speciation, and partitioning as a function of HCl in the brine-silicate melt-metallic gold system at 800 °C and 100 MPa. Geochim. Cosmochim. Acta 2002, 66, 3719–3732. [Google Scholar] [CrossRef]
- Yin, Y.; Zajacz, Z. The solubility of silver in magmatic fluids: Implications for silver transfer to the magmatic-hydrothermal ore-forming environment. Geochim. Cosmochim. Acta 2018, 238, 235–251. [Google Scholar] [CrossRef]
- Tattitch, B.; Chelle-Michou, C.; Blundy, J.; Loucks, R.R. Chemical feedbacks during magma degassing control chlorine partitioning and metal extraction in volcanic arcs. Nat. Commun. 2021, 12, 1774. [Google Scholar] [CrossRef]
- Webster, J.D. Chloride solubility in felsic melts and the role of chloride in magmatic degassing. J. Petrol. 1997, 38, 1793–1807. [Google Scholar] [CrossRef]
- Michael, P.J.; Schilling, J.-G. Chlorine in mid-ocean ridge magmas: Evidence for assimilation of seawater-influenced components. Geochim. Cosmochim. Acta 1989, 53, 3131–3143. [Google Scholar] [CrossRef]
- Kent, A.J.R.; Peate, D.W.; Newman, S.; Stolper, E.M.; Pearce, J. Chlorine in submarine glasses from the Lau Basin: Seawater contamination and constraints on the composition of slab-derived fluids. Earth Planet. Sci. Lett. 2002, 202, 361–377. [Google Scholar] [CrossRef]
- Stroncik, N.A.; Haase, K.M. Chlorine in oceanic intraplate basalts: Constraints on mantle sources and recycling processes. Geology 2004, 32, 945–948. [Google Scholar] [CrossRef]
- Straub, S.M.; Layne, G.D. The systematics of chlorine, fluorine, and water in Izu arc front volcanic rocks: Implications for volatile recycling in subduction zones. Geochim. Cosmochim. Acta 2003, 67, 4179–4203. [Google Scholar] [CrossRef]
- Portnyagin, M.; Hoernle, K.; Plechov, P.; Mironov, N.; Khubunaya, S. Constraints on mantle melting and composition and nature of slab components in volcanic arcs from volatiles (H2O, S, Cl, F) and trace elements in melt inclusions from the Kamchatka Arc. Earth Planet. Sci. Lett. 2007, 255, 53–69. [Google Scholar] [CrossRef]
- Brahm, R.; Coulthard, D., Jr.; Zellmer, G.; Kuritani, T.; Sakamoto, N.; Taniuchi, H.; Yurimoto, H.; Nakagawa, M.; Sato, E. Olivine-hosted melt inclusions track progressive dehydration reactions in subducting slabs across volcanic arcs. J. Petrol. 2024, 65, egae017. [Google Scholar] [CrossRef]
- Sharp, Z.D.; Barnes, J.D. Water-soluble chlorides in massive seafloor serpentinites: A source of chloride in subduction zones. Earth Planet. Sci. Lett. 2004, 226, 243–254. [Google Scholar] [CrossRef]
- Manzini, M.; Bouvier, A.-S.; Barnes, J.D.; Bonifacie, M.; Rose-Koga, E.F.; Ulmer, P.; Métrich, N.; Bardoux, G.; Williams, J.; Layne, G.D.; et al. SIMS chlorine isotope analyses in melt inclusions from arc settings. Chem. Geol. 2017, 449, 112–122. [Google Scholar] [CrossRef]
- Ransom, B.; Spivack, A.J.; Kastner, M. Stable Cl isotopes in subduction-zone pore waters: Implications for fluid-rock reactions and the cycling of chlorine. Geology 1995, 23, 715–718. [Google Scholar] [CrossRef]
- Castillo, P.R. Arc magmatism and porphyry-ore deposition are primarily controlled by chlorine from seawater. Chem. Geol. 2022, 589, 120683. [Google Scholar] [CrossRef]
- Piccoli, P.M.; Candela, P.A. Apatite in igneous systems. Rev. Mineral. Geochem. 2002, 48, 255–292. [Google Scholar] [CrossRef]
- Jenkins, D.M. The incorporation of chlorine into calcium amphibole. Am. Mineral. 2019, 104, 514–524. [Google Scholar] [CrossRef]
- Mernagh, T.P.; Mavrogenes, J. Significance of high temperature fluids and melts in the Grasberg porphyry copper-gold deposit. Chem. Geol. 2019, 508, 210–224. [Google Scholar] [CrossRef]
- Xu, X.; Bain, W.M.; Tornos, F.; Hanchar, J.M.; Lamadrid, H.; Lehmann, B.; Xu, X.; Steadman, J.A.; Bottrill, R.S.; Soleymani, M.; et al. Magnetite-apatite ores record widespread involvement of molten salts. Geology 2024, 52, 417–422. [Google Scholar] [CrossRef]
- Kepezhinskas, N.; Kjarsgaard, B.A.; Sarkar, C.; Luo, Y.; Locock, A.J.; Pearson, D.G. Petrology, geochemistry, and geochronology of the Mel Kimberlites, Nunavut, Canada and their relationship to Neoproterozoic to Cambrian magmatism in North America. Mineral. Petrol. 2024, 119, 1023–1042. [Google Scholar] [CrossRef]
- Kepezhinskas, N.; Kjarsgaard, B.A.; Sarkar, C.; Luo, Y.; Locock, A.J.; Pearson, D.G. Petrology, geochemistry, and Triassic eruption age of the Dharma kimberlite, Northwest Territories. Mineral. Petrol. 2025, 119, 987–1004. [Google Scholar] [CrossRef]
- Golovin, A.V. Unusual mineralogy of kimberlites: Alkali carbonates, sulfates, and chlorides among groundmass minerals from unserpentinized coherent kimberlite of the Udachnaya-East pipe, Siberian craton. Minerals 2025, 15, 586. [Google Scholar] [CrossRef]
- Nesbitt, B.E.; Kelly, W.C. Magmatic and hydrothermal inclusions in carbonatite of the Magnet Cove complex, Arkansas. Contrib. Mineral. Petrol. 1977, 63, 271–294. [Google Scholar] [CrossRef]
- Prokopyev, I.; Doroshkevich, A.; Redina, A. Brine-melts and fluids of the Fe-F-P-(Ba)-(Sr)-REE Central Asian carbonatite province (Southern Siberia and Mongolia): The petrogenetic aspects. Minerals 2023, 13, 573. [Google Scholar] [CrossRef]
- Hanley, J.J.; Mungall, J.E.; Pettke, T.; Spooner, E.T.C.; Bray, C.J. Fluid and halide melt inclusions of magmatic origin in the Ultramafic and Lower Banded Series, Stillwater Complex, Montana, USA. J. Petrol. 2008, 49, 1133–1160. [Google Scholar] [CrossRef]
- Webster, J.D. Fluid-melt interactions involving Cl-rich granites: Experimental study from 2 to 8 kbar. Geochim. Cosmochim. Acta 1992, 56, 659–678. [Google Scholar] [CrossRef]
- Webster, J.D. The exsolution of magmatic hydrosaline chloride liquids. Chem. Geol. 2004, 210, 33–48. [Google Scholar] [CrossRef]
- Lira, R.; Poklepovic, M.F.; Darais, M.J. Solid inclusions of magmatic halite and sylvite in felsic granitoids, Sierra Norte, Córdoba, Argentina. Lithos 2007, 99, 363–384. [Google Scholar] [CrossRef]
- Kepezhinskas, P.K.; Kepezhinskas, N.P.; Berdnikov, N.V.; Krutikova, V.O. Native metals and intermetallic compounds in subduction-related ultramafic rocks from the Stanovoy mobile belt (Russian Far East): Implications for redox heterogeneity in subduction zones. Ore Geol. Rev. 2020, 127, 103800. [Google Scholar] [CrossRef]
- Kepezhinskas, P.; Berdnikov, N.; Kepezhinskas, N.; Krutikova, V.; Astapov, I. Magmatic-hydrothermal transport of metals at arc plutonic roots: Insights from the Ildeus mafic-ultramafic complex, Stanovoy suture zone (Russian Far East). Minerals 2023, 13, 878. [Google Scholar] [CrossRef]
- Berdnikov, N.; Kepezhinskas, P.; Nevstruev, V.; Krutikova, V.; Konovalova, N. Explosive ultramafic volcanism in Phanerozoic accreted terranes: A case study of the Taragai peridotite complex (Russian Far East). J. Asian Earth Sci. 2025, 281, 106503. [Google Scholar] [CrossRef]
- Kepezhinskas, P.; Berdnikov, N.; Kepezhinskas, N.; Konovalova, N. Adakites, high-Nb basalts and copper-gold deposits in magmatic arcs and collisional orogens: An overview. Geosciences 2022, 12, 29. [Google Scholar] [CrossRef]
- Erlich, E.N.; Gorshkov, G.S. Quaternary volcanism and tectonics in Kamchatka. Bull. Volcanol. 1979, 42, 13–43. [Google Scholar]
- Kepezhinskas, P.; McDermott, F.; Defant, M.J.; Hochstaedter, A.; Drummond, M.S.; Hawkesworth, C.J.; Koloskov, A.; Maury, R.; Bellon, H. Trace element and Sr-Nd-Pb isotopic constraints on a three-component model of Kamchatka Arc petrogenesis. Geochim. Cosmochim. Acta 1997, 60, 577–600. [Google Scholar] [CrossRef]
- Martynov, Y.A.; Khanchuk, A.I.; Kimura, J.I.; Rybin, A.V.; Martynov, A.Y. Geochemistry and petrogenesis of volcanic rocks in the Kuril Island Arc. Petrology 2010, 18, 489–513. [Google Scholar] [CrossRef]
- Santamaria, S.; Quidelleur, X.; Samaniego, P.; Audin, L.; Le Pennec, J.-L.; Hidalgo, S.; Liorzou, C.; Guillou, H. Timing of Quaternary volcanism and its relationship with tectonics in the central segment of the Ecuadorian Andes. J. Volcanol. Geotherm. Res. 2023, 442, 107895. [Google Scholar] [CrossRef]
- Humphreys, E.D.; Grunder, A.L. Tectonic controls on the origin and segmentation of the Cascade Arc, USA. Bull. Volcanol. 2022, 84, 102. [Google Scholar] [CrossRef]
- Viccaro, M.; Giuffrida, M.; Nicotra, E.; Ozerov, A.Y. Magma storage, ascent and recharge history prior to the 1991 eruption at Avachinsky Volcano, Kamchatka, Russia: Inferences on the plumbing system geometry. Lithos 2012, 140–141, 11–24. [Google Scholar] [CrossRef]
- Dorendorf, F.; Churikova, T.; Koloskov, A.; Wörner, G. Late Pleistocene to Holocene activity at Bakening volcano and surrounding monogenetic centers (Kamchatka): Volcanic geology and geochemical evolution. J. Volcanol. Geotherm. Res. 2000, 104, 131–151. [Google Scholar] [CrossRef]
- Simon, A.; Yogodzinski, G.M.; Robertson, K.; Smith, E.; Selyangin, O.; Kiryukhin, A.; Mulcahy, S.R.; Walker, J.D. Evolution and genesis of volcanic rocks from Mutnovsky Volcano, Kamchatka. J. Volcanol. Geotherm. Res. 2014, 286, 116–137. [Google Scholar] [CrossRef]
- Gavrilenko, M.; Ozerov, A.; Kyle, P.R.; Carr, M.J.; Nikulin, A.; Vidito, C.; Danyushevsky, L. Abrupt transition from fractional crystallization to magma mixing at Gorely volcano (Kamchatka) after caldera collapse. Bull. Volcanol. 2016, 78, 47. [Google Scholar] [CrossRef]
- Duggen, S.; Portnyagin, M.; Baker, J.; Ulfbeck, D.; Hoernle, K.; Garbe-Schönberg, D.; Grassineau, N. Drastic shifts in lava geochemistry in the volcanic-front to rear-arc region of the Southern Kamchatkan subduction zone: Evidence for the transition from slab surface dehydration to sediment melting. Geochim. Cosmochim. Acta 2007, 71, 452–480. [Google Scholar] [CrossRef]
- Taran, Y.A. Geochemistry of volcanic and hydrothermal fluids and volatile budget of the Kamchatka-Kuril subduction zone. Geochim. Cosmochim. Acta 2009, 73, 1067–1094. [Google Scholar] [CrossRef]
- Zelenski, M.; Taran, Y. Geochemistry of volcanic and hydrothermal gases of Mutnovsky volcano, Kamchatka: Evidence for mantle, slab and atmosphere contributions to fluids of a typical arc volcano. Bull. Volcanol. 2011, 73, 373–394. [Google Scholar] [CrossRef]
- Aiuppa, A.; Giudice, G.; Liuzzo, M.; Tamburello, G.; Allard, P.; Calabrese, S.; Chaplygin, I.; McGonigle, A.J.S.; Taran, Y. First volatile inventory for Gorely volcano, Kamchatka. Geophys. Res. Lett. 2012, 39. [Google Scholar] [CrossRef]
- Zelenski, M.; Bortnikova, S. Sublimate speciation at Mutnovsky volcano, Kamchatka. Eur. J. Mineral. 2005, 17, 107–118. [Google Scholar] [CrossRef]
- Pekov, I.V.; Agakhanov, A.A.; Zubkova, N.V.; Koshlyakova, N.N.; Shchipalkina, N.V.; Sandalov, F.D.; Yapaskurt, V.O.; Turchkova, A.G.; Sidorov, E.G. Oxidizing-type fumaroles of the Tolbachik volcano, a mineralogical and geochemical unique. Russ. Geol. Geophys. 2020, 61, 675–688. [Google Scholar] [CrossRef]
- Plutakhina, E.Y.; Malik, N.A.; Nikolaeva, I.Y. The chemical and phase composition of high temperature sublimates: Avachinsky volcano, Kamchatka. J. Volcanol. Seismol. 2025, 19, 319–336. [Google Scholar] [CrossRef]
- Zlobin, T.K.; Piskunov, B.N.; Frolova, T.I. New data on the Earth’s crust structure in the central part of the Kurile Island arc. Dokl. Akad. Nauk. SSSR 1987, 293, 185–187. [Google Scholar]
- Werner, R.; Baranov, B.; Hoernle, K.; van der Bogaard, P.; Hauff, F.; Tararin, I. Discovery of ancient volcanoes in the Okhotsk Sea (Russia): New constraints on the opening history of the Kurile back-arc basin. Geosciences 2020, 10, 442. [Google Scholar] [CrossRef]
- Bailey, J.C.; Frolova, T.I.; Burikova, I.A. Geochemistry and petrogenesis of Kuril island-arc basalts. Contrib. Mineral. Petrol. 1989, 102, 265–280. [Google Scholar] [CrossRef]
- Gorshkov, G.S. Volcanism of the Kurile Island Arc; Nauka Publishers: Moscow, Russia, 1967; pp. 1–183. (In Russian) [Google Scholar]
- Taran, Y.; Kalacheva, E. Seawater hydrothermal system in the middle of the Kuril Arc: Yankich Island, Ushishir Archipelago. J. Volcanol. Geotherm. Res. 2023, 436, 107784. [Google Scholar] [CrossRef]
- Didenko, A.N.; Rashidov, V.A.; Markov, G.P.; Trusenko, M.S.; Petrova, V.V.; Anikin, L.P. Petromagnetic and geochemical descriptions of volcanics discharged by Alaid volcano, Kuril Islands, in 2015–2016. J. Volcanol. Seismol. 2021, 15, 1–18. [Google Scholar] [CrossRef]
- Taran, Y.; Zelenski, M.; Chaplygin, I.; Malik, N.; Campion, R.; Inguaggiato, S.; Pokrovsky, B.; Kalacheva, E.; Melnikov, D.; Kazahaya, R.; et al. Gas emissions from volcanoes of the Kuril island arc (NW Pacific): Geochemistry and fluxes. Geochem. Geophys. Geosys 2018, 19, 1859–1880. [Google Scholar] [CrossRef]
- Petrova, V.V.; Rashidov, V.A.; Perepelov, A.B.; Silaev, V.I.; Anikin, L.P.; Gorkova, N.V.; Mikheev, V.V. Ore elements and minerals in the sublimates of Alaid volcano, Kuril island arc. J. Volcanol. Seismol. 2024, 18, 180–200. [Google Scholar] [CrossRef]
- Yudovskaya, M.A.; Distler, V.V.; Chaplygin, I.V.; Mokhov, A.V.; Trubkin, N.V.; Gorbacheva, S.A. Gaseous transport and deposition of gold in magmatic fluid: Evidence from the active Kudryavy volcano, Kurile Islands. Miner. Depos. 2006, 40, 828–848. [Google Scholar] [CrossRef]
- Jaillard, E.; Lapierre, H.; Ordoñez, M.; Toro Álava, J.; Amórtegui, A.; Vanmelle, J. Accreted oceanic terranes in Ecuador: Southern edge of the Caribbean Plate? Geol. Soc. Lond. Spec. Publ. 2009, 328, 469–485. [Google Scholar] [CrossRef]
- Beate, B.; Monzier, M.; Spikings, R.; Cotton, J.; Silva, J.; Bourdon, E.; Eissen, J.P. Mio-Pliocene adakite generation related to flat subduction in southern Ecuador: The Quimsacocha volcanic center. Earth Planet. Sci. Lett. 2001, 192, 561–570. [Google Scholar] [CrossRef]
- Hall, M.L.; Mothes, P.A. Quilotoa volcano—Ecuador: An overview of young dacitic volcanism in a lake-filled caldera. J. Geotherm. Volcanol. Res. 2008, 176, 44–55. [Google Scholar] [CrossRef]
- Garrison, J.M.; Davidson, J.P.; Hall, M.; Mothes, P. Geochemistry and petrology of the most recent deposits from Cotopaxi volcano, Northern Volcanic Zone, Ecuador. J. Petrol. 2011, 52, 1641–1678. [Google Scholar] [CrossRef]
- Hidalgo, S.; Battaglia, J.; Arellano, S.; Sierra, D.; Bernard, B.; Parra, R.; Kelly, P.; Dinger, F.; Barrington, C.; Samaniego, P. Evolution of the 2015 Cotopaxi eruption revealed by combined geochemical and seismic observations. Geochem. Geophys. Geosys. 2018, 19, 2087–2108. [Google Scholar] [CrossRef]
- Abdújar, J.; Martel, C.; Pichavant, M.; Samaniego, P.; Scaillet, B.; Molina, I. Structure of the plumbing system at Tungurahua volcano, Ecuador: Insights from phase equilibrium experiments on July-August 2006 eruption products. J. Petrol. 2017, 58, 1249–1278. [Google Scholar] [CrossRef]
- Warnach, S.; Bobrowski, N.; Hidalgo, S.; Arellano, S.; Sihler, H.; Dinger, F.; Lübcke, P.; Battaglia, J.; Steele, A.; Galle, B.; et al. Variation of the BrO/SO2 molar ratio in the plume of Tungurahua volcano between 2007 and 2017 and its relationships to volcanic activity. Front. Earth Sci. 2019, 7, 132. [Google Scholar] [CrossRef]
- Leeman, W.P.; Smith, D.R.; Hildreth, W.; Palacz, Z.; Rogers, N. Compositional diversity of Late Cenozoic basalts in a transect across the southern Washington Cascades: Implications for subduction zone magmatism. J. Geophys. Res. 1990, 95, 19561–19582. [Google Scholar] [CrossRef]
- Defant, M.J.; Drummond, M.S. Mount St. Helens: Potential example of the partial melting of the subducted lithosphere in a volcanic arc. Geology 1993, 21, 547–550. [Google Scholar] [CrossRef]
- Scott, W.E.; Gardner, C.A.; Tilling, R.I.; Lanphere, M.A. Geologic History of Mount Hood Volcano, Oregon—A Field-Trip Guidebook; (NO. 97-263) USGS Open-File Reports; US Geological Survey: Reston, VA, USA, 1997; pp. 1–40.
- Koleszar, A.M.; Kent, A.J.R.; Wallace, P.J.; Scott, W.E. Controls on long-term low explosivity at andesitic arc volcanoes: Insights from Mount Hood, Oregon. J. Volcanol. Geotherm. Res. 2012, 219–220, 1–14. [Google Scholar] [CrossRef]
- Krutikova, V.O.; Berdnikov, N.V.; Kepezhinskas, P.K. Microminerals as complimentary guides into metallogeny and the ore-forming potential of igneous rocks: Evidence from the Stanovoy superterrane (Russian Far East). Minerals 2025, 15, 504. [Google Scholar] [CrossRef]
- Le Bas, M.J.; Le Maitre, R.W.; Streckeisen, A.; Zanettin, B. A chemical classification of volcanic rocks based on the total alkalie-silica diagram. J. Petrol. 1986, 27, 745–750. [Google Scholar] [CrossRef]
- Miyashiro, A. Volcanic rock series in island arcs and active continental margins. Am. J. Sci. 1974, 274, 321–355. [Google Scholar] [CrossRef]
- Arculus, R.J. Use and abuse of the terms calcalkaline and calcalkalic. J. Petrol. 2003, 44, 929–935. [Google Scholar] [CrossRef]
- Hora, J.M.; Singer, B.S.; Wörner, G.; Beard, B.L.; Jicha, B.R.; Johnson, C.M. Shallow and deep crustal control on differentiation of calc-alkaline and tholeiitic magma. Earth Planet. Sci. Lett. 2009, 285, 75–86. [Google Scholar] [CrossRef]
- McDonough, W.F.; Sun, S.-s. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- de Seave, V.; Languille, M.-A.; Kociak, M.; Beling, S.; Ablett, J.; Andraud, C.; Stéphan, O.; Rueff, J.-P.; Fonda, E.; Lavedrine, B. Spectroscopies and electron microscopies unravel the origin of the first color photographs. Angew. Chem. 2020, 59, 9113–9119. [Google Scholar] [CrossRef]
- Kepezhinskas, P.; Berdnikov, N.; Krutikova, V.; Kozhemyako, N. Iron-titanium oxide-apatite-sulfide-sulfate microinclusions in gabbro and adakite from the Russian Far East indicate possible magmatic links to iron oxide-apatite and iron oxide-copper-gold deposits. Minerals 2024, 14, 188. [Google Scholar] [CrossRef]
- Migdisov, A.A.; Williams-Jones, A.E.; Suleimenov, O.M. Solubility of chlorargyrite (AgCl) in water vapor at elevated temperatures and pressures. Geochim. Cosmochim. Acta 1999, 63, 3817–3827. [Google Scholar] [CrossRef]
- Webster, J.D.; De Vivo, B. Experimental and modeled solubility of chlorine in aluminosilicate melts, consequences of magma evolution, and implications for exsolution of hydrous chloride melt at Somma-Vesuvius. Am. Mineral. 2002, 87, 1046–1061. [Google Scholar] [CrossRef]
- Webster, J.D.; Vetere, F.; Botcharnikov, R.E.; Goldoff, B.; McBirney, A.; Doherty, A.L. Experimental and modeled chlorine solubilities in aluminosilicate melts at 1 to 7000 bars and 700 to 1250 °C: Applications to magmas of Augustine Volcano, Alaska. Am. Mineral. 2015, 100, 522–535. [Google Scholar] [CrossRef]
- Renno, A.D.; Franz, L.; Witzke, T.; Herzig, P.M. The coexistence of melts of hydrous copper chloride, sulfide and silicate compositions in a magnesiohornblende cumulate, TUBAF seamount, Papua New Guinea. Can. Mineral. 2004, 42, 1–16. [Google Scholar] [CrossRef][Green Version]
- Alex, A.; Zajacz, Z. The solubility of Cu, Ag and Au in magmatic sulfur-bearing fluids as a function of oxygen fugacity. Geochim. Cosmochim. Acta 2022, 330, 93–115. [Google Scholar] [CrossRef]
- Georgatou, A.; Chiaradia, M.; Rezeau, H.; Walle, M. Magmatic sulphides in Quaternary Ecuadorian arc magmas. Lithos 2018, 296–299, 580–599. [Google Scholar] [CrossRef]
- Li, S.; Chu, F.; Zhu, J.; Ding, Y.; Zhu, Z.; Liu, J.; Wang, H.; Li, X.; Dong, Y.; Zhao, D. Magmatic sulfide formation and oxidative dissolution in the SW Okinawa Trough: A precursor to metal-bearing magmatic fluid. Geochim. Cosmochim. Acta 2019, 258, 138–155. [Google Scholar] [CrossRef]
- Kondratieva, L.A.; Anisimova, G.S.; Kardashevskaia, V.N. Ore mineralogy and typomorphism of native gold of the Spokoininsky cluster of the Aldan-Stanovoy gold province. Minerals 2023, 13, 543. [Google Scholar] [CrossRef]
- Testa, F.J.; Cooke, D.R.; Zhang, L.; Mas, G.R. Bismoclite (BiOCl) in the San Francisco de los Andes Bi-Cu-Au deposit, Argentina. First occurrence of a bismuth oxychloride in a magmatic-hydrothermal breccia pipe and its usefulness as an indicator phase in mineral exploration. Minerals 2016, 6, 62. [Google Scholar] [CrossRef]
- Pazout, R.; Sejkora, J. Nadorite, PbSb3+O2Cl, from ore veins in the Kutná Hora ore district—The first occurrence in Czech Republic. Bull. Mineral. Petrolog. Odd Nár. Muz. 2015, 23, 214–217. (In Czech) [Google Scholar]
- Bortnikov, N.S.; Tolstykh, N.D. Epithermal deposits of Kamchatka, Russia. Geol. Ore Depos. 2023, 65, 124–152. [Google Scholar] [CrossRef]
- Leary, S.; Sillitoe, R.H.; Stewart, P.W.; Roa, R.J.; Nicolson, B.E. Discovery, geology, and origin of the Frute del Norte epithermal gold-silver deposit, Southeastern Ecuador. Econ. Geol. 2016, 111, 1043–1072. [Google Scholar] [CrossRef]
- Schütte, P.; Chiaradia, M.; Barra, F.; Villagómez, D.; Beate, B. Metallogenic features of Miocene porphyry Cu and porphyry-related mineral deposits in Ecuador revealed by Re-Os, 40Ar/39Ar, and U-Pb geochronology. Miner. Depos. 2012, 47, 383–410. [Google Scholar] [CrossRef]
- So, C.-S.; Dunchenko, V.Y.; Yun, S.-T.; Park, M.-E.; Choi, S.-G.; Shelton, K.L. Te- and Se-bearing epithermal Au-Ag mineralization, Prasolovskoye, Kunashir Island, Kuril island arc. Econ. Geol. 1995, 90, 105–117. [Google Scholar] [CrossRef]
- Du Bray, E.A.; John, D.A. Petrologic, tectonics, and metallogenic evolution of the Ancestral Cascades magmatic arc, Washington, Oregon, and northern California. Geosphere 2011, 7, 1102–1133. [Google Scholar] [CrossRef]
- Bargar, K.E.; Keith, T.E.C.; Beeson, M.H. Hydrothermal alteration in the Mount Hood area, Oregon. U.S. Geol. Surv. Bull. 1993, 2054, 1–70. [Google Scholar]
- Li, H.; Xiao, Y.; Sun, H.; Tong, F.; Heuser, A.; Churikova, T.; Wörner, G. Trace element and Li isotope compositions across the Kamchatka arc: Constraints on slab-derived fluid sources. J. Geophys. Res. 2021, 125, e2019JB019237. [Google Scholar] [CrossRef]
- Kepezhinskas, P.K.; Potapova, N.V.; Berdnikov, N.V. Microminerals as evidence for metallogenic specialization and ore potential of Quaternary lavas from the Gorely volcano (Kamchatka). J. Volcanol. Seismol. 2025, 5, 72–88. [Google Scholar] [CrossRef]
- Kepezhinskas, P.; Berdnikov, N.; Kepezhinskas, N.; Konovalova, N. Metals in Avachinsky peridotite xenoliths with implications for redox heterogeneity and metal enrichment in the Kamchatka mantle wedge. Lithos 2022, 412–413, 106610. [Google Scholar] [CrossRef]
- Kepezhinskas, P.; Berdnikov, N.; Konovalova, N.; Kepezhinskas, N.; Krutikova, V.; Kirichenko, E. Native metals and alloys in trachytes and shoshonite from the continental United States and high-K dacite from the Bolivian Andes: Magmatic origins of ore metals in convergent and within-plate tectonic settings. Rus. J. Pacific Geol. 2023, 16, 405–426. [Google Scholar] [CrossRef]
- Keith, M.; Haase, K.M.; Klemd, R.; Schwarz-Schampera, U.; Franke, H. Systematic variations in magmatic sulphide chemistry from mid-ocean ridges, back-arc basins and island arcs. Chem. Geol. 2017, 451, 67–77. [Google Scholar] [CrossRef]

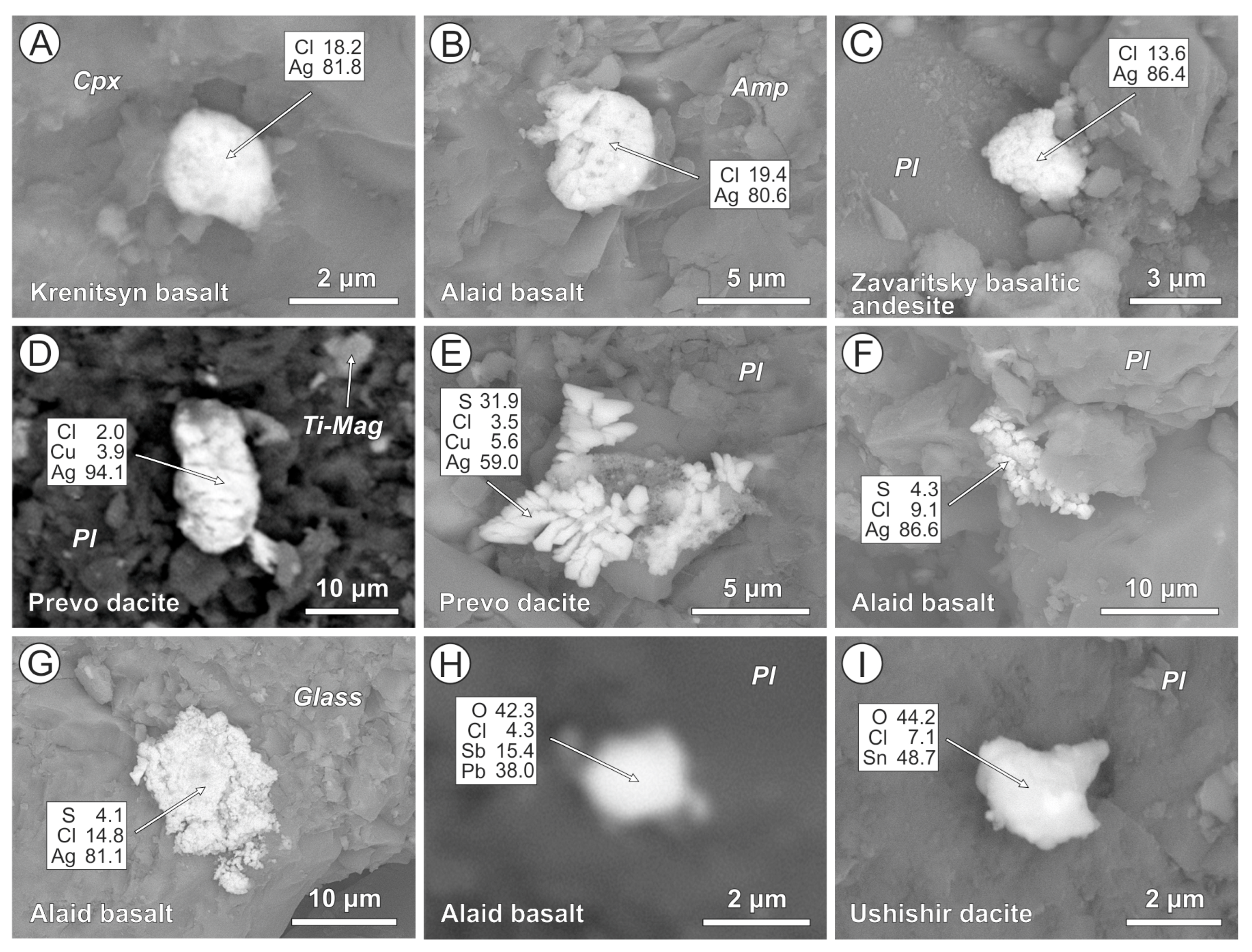
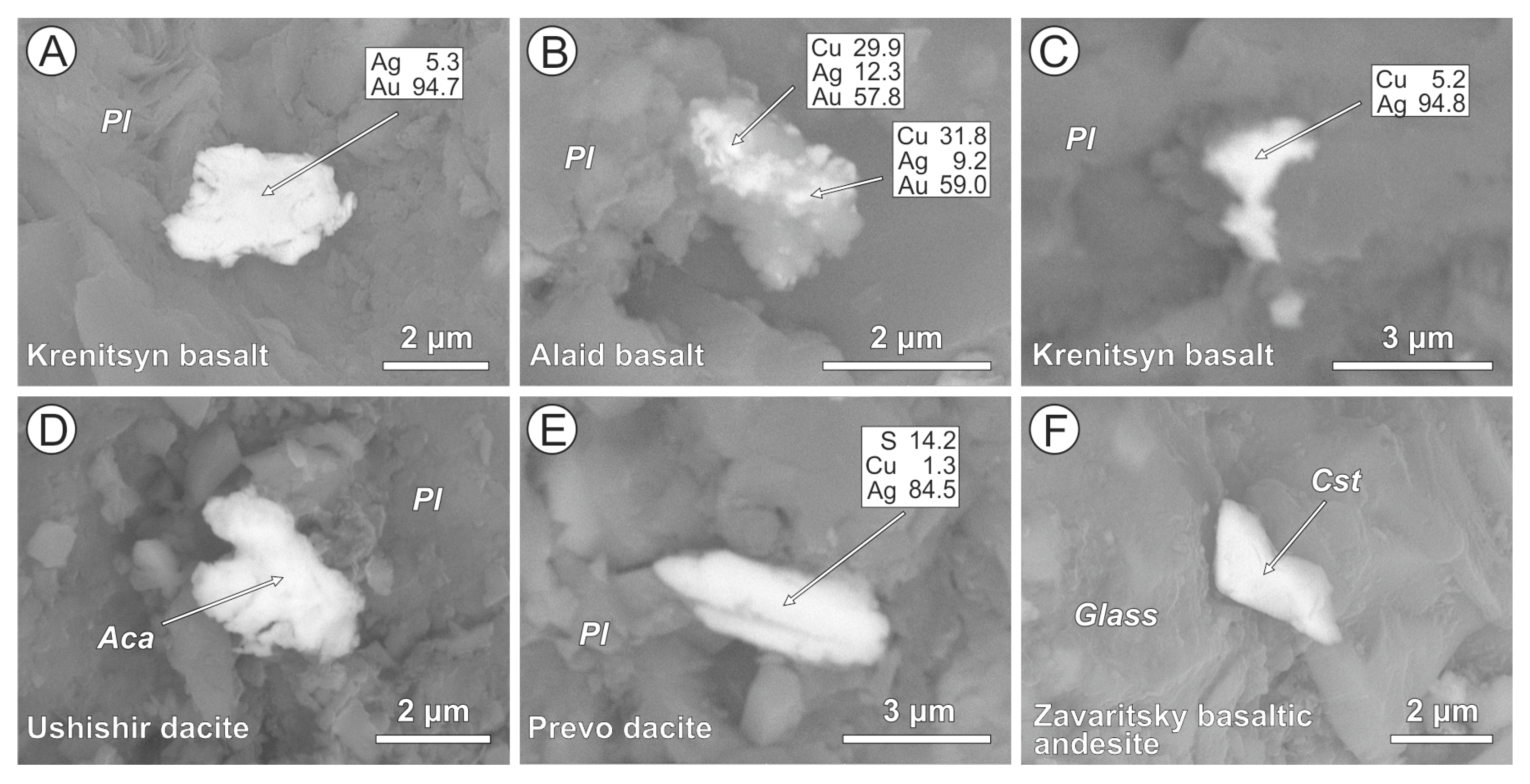

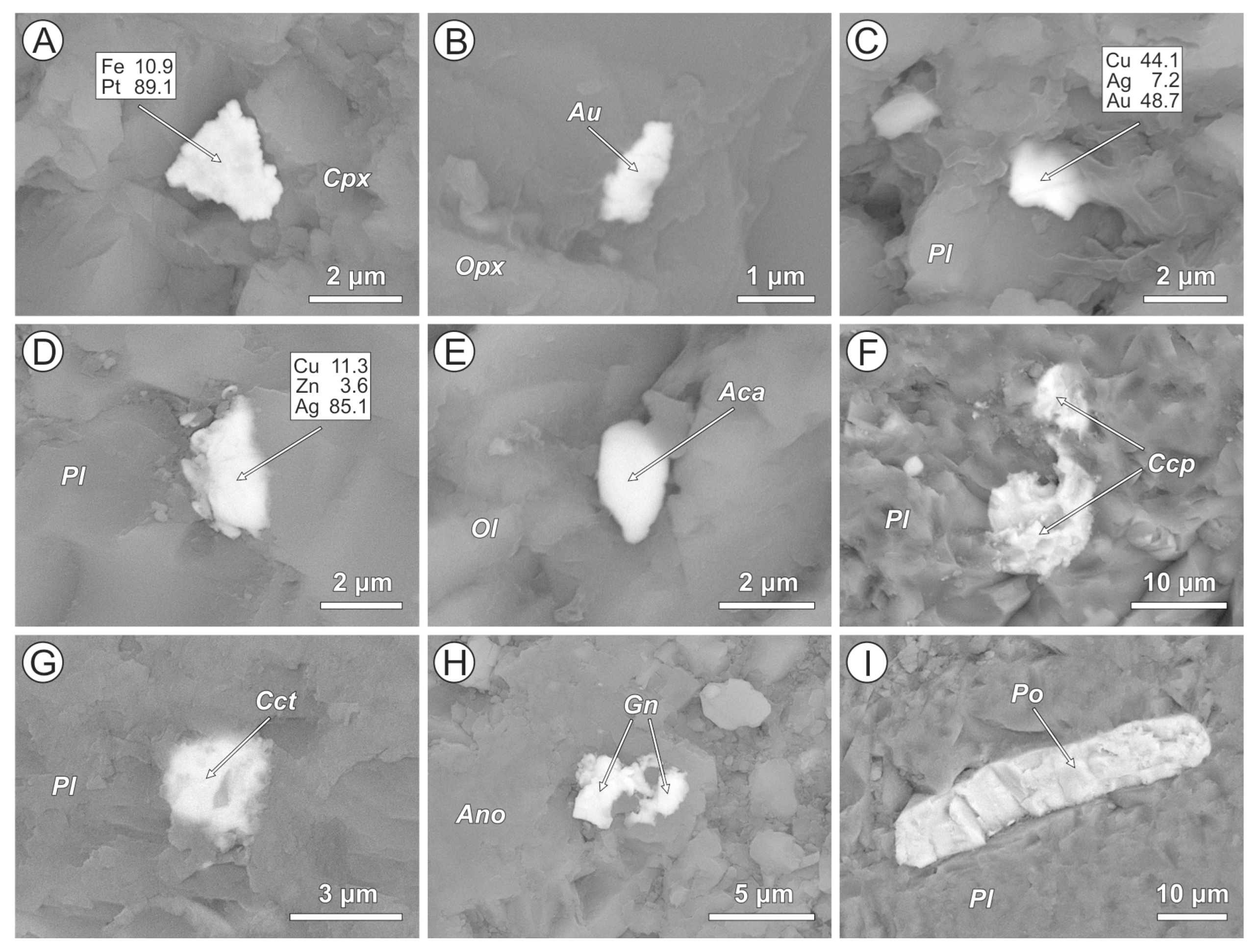

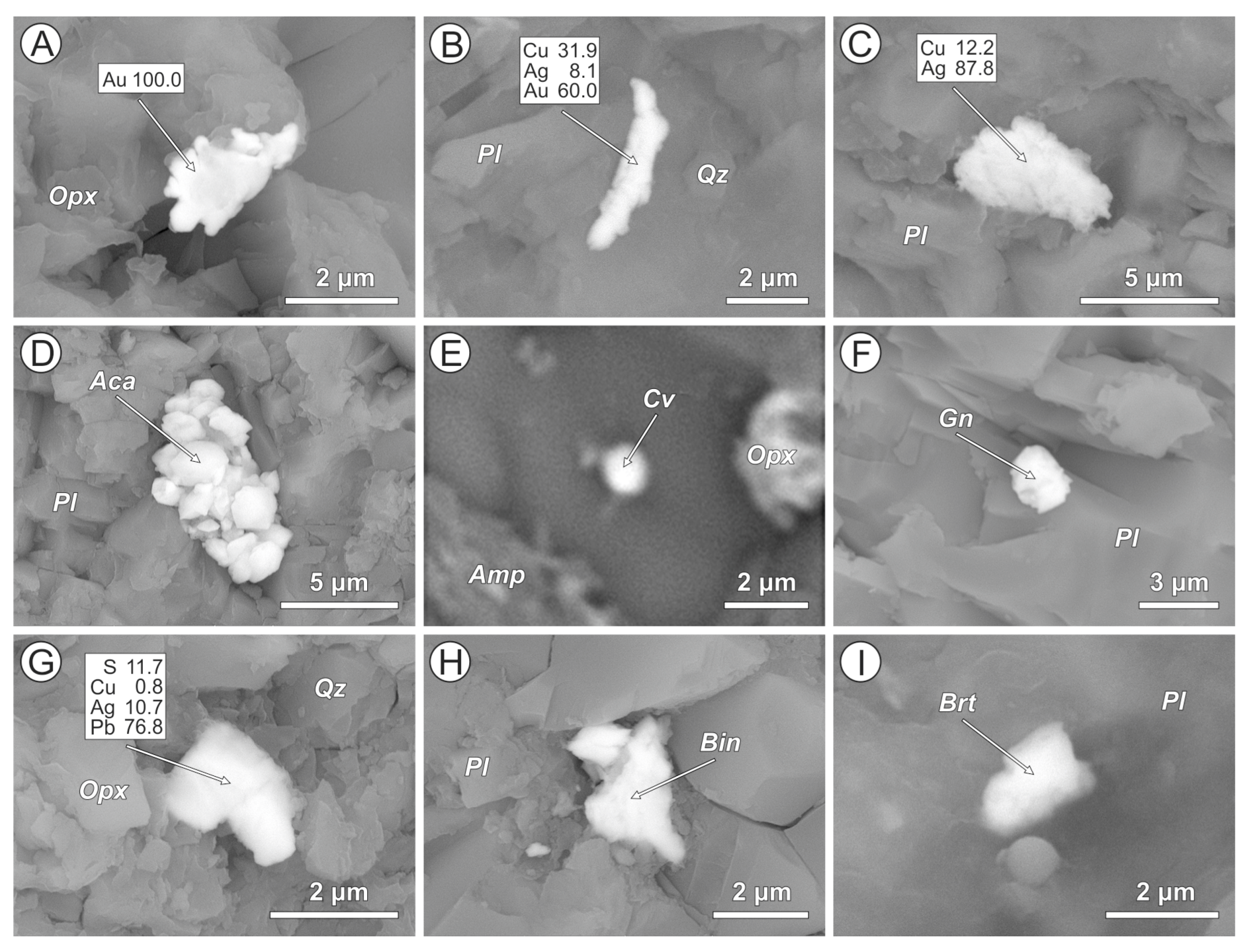
| Arc | Volcano | Rock Type | Cl-Bearing Minerals | Host Phases | Associated Metal and Mineral Microinclusions |
|---|---|---|---|---|---|
| Kamchatka | Avachinsky | Ankaramite | AgCl (1), bismoclite, Pb-Sb-oxychloride, and Cl-F-apatite | Clinopyroxene, orthopyroxene, and plagioclase | Native Pt (2) and Au, Ag-Au, Ag-Cu-Au, and Sn-Cu alloys, bismite, cassiterite, acanthite, Cu-Ag-sulfide, arsenopyrite, Zn-Pb-Sb-Cu-sulfide, barite, and monazite |
| Andesite | AgCl, Cl-apatite | Orthopyroxene, plagioclase, and volcanic glass | Cupriferous Ag, Fe-W and Cu-Ag-Au alloys, sphalerite, smithsonite, cassiterite, bismite, Pb and Sb oxides, and barite | ||
| Bakening | Dacite | Ag-Cl, Cu-Ag-Cl composite, AgCl + Cu-sulfide and malachite + atakamite (?) composites, Sr-Bi-oxychloride, and Cl-apatite | Amphibole, plagioclase, anorthoclase, K-feldspar, quartz, and volcanic glass | Cupriferous Ag, Ag-Au, Cu-Ag-Au, Co-W, and Sn-Cu alloys, acanthite, Cu-Ag-sulfide, pyrrhotite, pyrite, chalcopyrite, covellite, zincite, bismite, cassiterite, barite, and monazite | |
| Mutnovsky | Basalt | AgCl, Cu-Ag-Cl and zincite + AgCl composites | Orthopyroxene, amphibole, and plagioclase | Cu-Sn alloy, acanthite, Cu-Ag-sulfide, and barite | |
| Basaltic andesite | AgCl, Cu-Ag-Cl, and Zn-Cu-Ag-Cl and AgCl + Cu-sulfide composites | Orthopyroxene, amphibole, plagioclase, titanomagnetite, and volcanic glass | Cupriferous Ag, Zn-Cu-Ag alloy, Cu-Ag-sulfide, acanthite, and barite | ||
| Andesite | AgCl | Plagioclase | Cu-Ag-sulfide | ||
| Dacite | AgCl | Amphibole, anorthoclase, and volcanic glass | Cu-Zn alloy, acanthite, Cu-Ag-sulfide, bismite, and Cu-Zn-Ag-sulfide | ||
| Gorely | Basalt | AgCl, Cu-Ag-Cl composite | Olivine, orthopyroxene, clinopyroxene, amphibole, plagioclase, K-feldspar, and volcanic glass | Cupriferous Ag, Cu-Ag-sulfide, acanthite, Fe-W alloy, and cassiterite | |
| Basaltic andesite | AgCl, AgCl + cassiterite composite | Plagioclase, volcanic glass | Native Au, Sn-Cu alloy, acanthite, Cu-Ag-sulfide, galena, and barite | ||
| Andesite | AgCl, AgCl + Cu-sulfide composite | Anorthoclase, volcanic glass | Native Ag, cupriferous Ag, Cu-Ag-sulfide, Sn-Cu alloy, and galena | ||
| Dacite | AgCl, Cl-apatite | Amphibole, anortoclase, K-feldspar, and titanomagnetite | Cupriferous Ag, native Fe, and pyrrhotite | ||
| Kuriles | Ushishir | Dacite | Ag-Cl-S and Cu-Ag-Cl-S, Sn-oxychloride | Plagioclase | Ni-Cu-Au alloy, cupriferous Ag, acanthite, and Cu-Ag-sulfide |
| Zavaritsky | Basaltic andesite | AgCl, Cu-Ag-Cl-S | Plagioclase, volcanic glass | Native Pt (2), cupriferous Ag, Cu-Ag-sulfide, and cassiterite | |
| Prevo | Dacite | Cu-Ag-Cl, Ag-Cl-S, and Cu-Ag-Cl-S | Plagioclase | Cu-Ag-sulfide | |
| Krenitsyn | Basalt | AgCl | Clinopyroxene, plagioclase | Native Pt (2), Ag-Au, Cu-Ag-Au, Ni-Cu-Ag-Au, Rh-Cu-Au and Rh-Zn-Ni-Cu-Au alloys, Fe-W and Sn-Cu alloys, acanthite, Cu-Ag-sulfide, chalcocite, and chalcopyrite | |
| Basaltic andesite | AgCl | Plagioclase | Acanthite, Cu-Ag-sulfide, and sphalerite | ||
| Alaid | Basalt | AgCl, Ag-Cl-S, Cu-Ag-Cl-S, and Pb-Sb-oxychloride | Plagioclase, volcanic glass | Native Pt, Cu-Ag-U and Pb-Sb (2) alloys, acanthite, Cu-Ag-sulfide, cassiterite, and W-carbide | |
| Ecuador | Quilotoa | Andesite | AgCl | Clinopyroxene, plagioclase | Cu-Pt, Fe-Pt, native Au, cupriferous Ag, Zn-Cu-Ag alloy, Cu-Ag-sulfide, and chalcocite |
| Cotopaxi | Andesite | AgCl, Cu-Ag-Cl | Amphibole, plagioclase, and volcanic glass | Native Pt, Ag-Au, Zn-Cu-Ag and Sn-Cu alloys, cupriferous Ag, Cu-Ag-sulfide, pyrrhotite (±Cu), chalcopyrite, and chalcocite | |
| Tungurahua | Dacite | Cu-Ag-Cl, Cl- and Cl-F-apatite | Plagioclase, anorthoclase, and titanomagnetite | Cu-Ni, Cu-Zn-Ag and Cu-Ag-Au alloys, cupriferous Ag, Cu-Ag-sulfide, monazite, galena, chalcopyrite, and pyrrhotite | |
| Cascades | Mt. Hood | Andesite | AgCl, Cu-Ag-Cl | Orthopyroxene, plagioclase, anorthoclase, titanomagnetite, and quartz | Native Au, Fe-W, Cu-Zn, Cu-Au, Ni-Cu-Au, Ni-Cu-Ag-Au and Cu-Ag-Au alloys, cupriferous Ag, acanthite, Cu-Ag- and Ag-Pb sulfides, galena, bismuthinite, covellite, barite, cassiterite, monazite, and W-carbide |
| Arc | Rock Types | Microinclusions in Lavas | Epithermal Mineralization | Porphyry Mineralization |
|---|---|---|---|---|
| Kamchatka | Ankaramite, basalt, basaltic andesite, andesite, and dacite | Cag, Cu-Ag-Cl, Cu-Ag-S-Cl, Cu-O-C-Cl, Sr-Bi-O-Cl, Zn-O-Cl, Zn-Cu-Ag-Cl, Sn-Ag-O-Cl, Bmc, Pb-Sb-oxychloride, Pt, Au, Ag, Cu-Ag, Cu-Zn, Zn-Cu-Ag, Fe-W, Co-W, Sn-Cu, Cu-Ag-Au, Cu-Ag-sulfide, Aca, Cu-Zn-Ag-sulfide, Gn, Sp, Apy, Zn-Pb-Sb-Cu-sulfide, Py, Po, Ccp, Cv, Cst, Mnz, Pb-Sb oxides, Brt, and Clap | Au, Ag, Cu-Ag, Cu-Zn, Ag-Au, Cu-Ag-Au alloys, Cag, Aca, Plb, Gn, Sp, Ccp, Cv, Apy, Ttr, Ttn, Pyg, Alt, Hes, Sb-sulfides, Py, Ag-sulfosalts, tellurides and selenides, Sn, Sb and As sulfosalts, Ag-Au-Pb bismutides and antimonides, Cin, Cst, Brt, and Clap | Au (±Cu, Pd), Pt, Ccp, Bn, Cct, Po, Gn, Sp, Mol, Dg, Azu, Mla, and Brt |
| Kuriles | Basalt, basaltic andesite, andesite, and dacite | Cag, Cu-Ag-Cl, Ag-Cl-S, Cu-Ag-Cl-S, Sn-oxychloride, Pb-S-oxychloride, Pt, Ag-Au, Cu-Ag, Ni-Cu-Ag-Au, Rh-Cu-Au, Rh-Zn-Ni-Cu-Au, Cu-Ag-Au, Fe-W, Sn-Cu alloys, Aca, Cu-Ag-sulfide, Ccp, Cct, Sp, Cst, and W-carbide | Ag, Cu-Ag, Hem, Cag, Aca, Cu, Py, Sp, Gn, Cct, Cv, Apy, Ttr, Ttn, Bin, Bis, Bit, Alt, Hes, Gf, Prs, Plb, Knn Stn, Cst, and Brt | |
| Ecuador | Andesite, dacite | Cag, Cu-Ag-Cl, Au, Ag, Pt (± Fe, Cu), Ag-Au, Cu-Ag, Cu-Ni, Sn-Cu, Cu-Zn-Ag, Cu-Ag-Au alloys, Po (± Cu), Cu-Ag-sulfide, Ccp, Cct, Gn, Mnz, and Cl-F Ap | Au, Ag, Ag-Au alloys, Py, Po, Ccp, Sp, Gn, Aca, Brt, Hem, Ttr, Ag-sulfosalts, Prs, Pyg, Fb, Ja, Bou, Cin, and Sbn | Au, Py, Po, Ccp, Bn, Cv, Eng, Sp, Gn, Mol, Ttr, Tnt, Aca, Brt, and Luz |
| Cascades | Andesite | Ag, Au, Cag, Cu-Ag-Cl, Fe-W, Cu-Zn, Cu-Au, Ni-Cu-Ag-Au, Cu-Ag-Au alloys, Cu-Ag, Aca, Cu-Ag and Ag-Pb-sulfides, Gn, Bin, Cv, Cst, Brt, Mnz, and W-carbides | Ag, Cu, Py, Ccp, Gn, Sp, and Ccl |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Kepezhinskas, P.; Berdnikov, N.; Voinova, I.; Kepezhinskas, N.; Potapova, N.; Krutikova, V. Cl-Bearing Mineral Microinclusions in Arc Lavas: An Overview of Recent Findings with Some Metallogenic Implications. Geosciences 2026, 16, 40. https://doi.org/10.3390/geosciences16010040
Kepezhinskas P, Berdnikov N, Voinova I, Kepezhinskas N, Potapova N, Krutikova V. Cl-Bearing Mineral Microinclusions in Arc Lavas: An Overview of Recent Findings with Some Metallogenic Implications. Geosciences. 2026; 16(1):40. https://doi.org/10.3390/geosciences16010040
Chicago/Turabian StyleKepezhinskas, Pavel, Nikolai Berdnikov, Irina Voinova, Nikita Kepezhinskas, Nadezhda Potapova, and Valeria Krutikova. 2026. "Cl-Bearing Mineral Microinclusions in Arc Lavas: An Overview of Recent Findings with Some Metallogenic Implications" Geosciences 16, no. 1: 40. https://doi.org/10.3390/geosciences16010040
APA StyleKepezhinskas, P., Berdnikov, N., Voinova, I., Kepezhinskas, N., Potapova, N., & Krutikova, V. (2026). Cl-Bearing Mineral Microinclusions in Arc Lavas: An Overview of Recent Findings with Some Metallogenic Implications. Geosciences, 16(1), 40. https://doi.org/10.3390/geosciences16010040







