A Geochemical Study of Near-Shore Sediment Cores from Utah Lake, UT, USA
Abstract
1. Introduction
1.1. Study Motivation
1.2. Background on Utah Lake
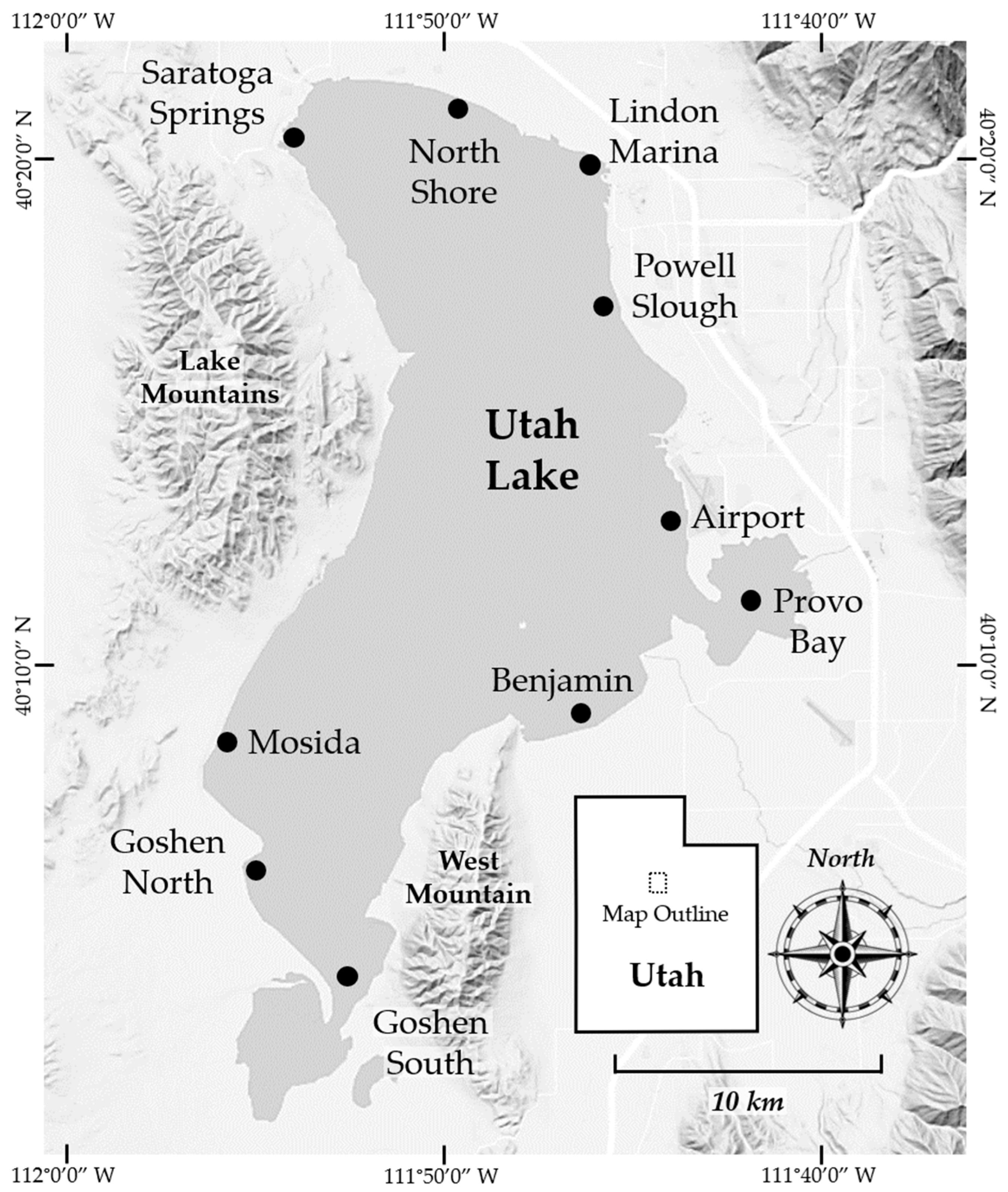
1.3. Core Collection Sites
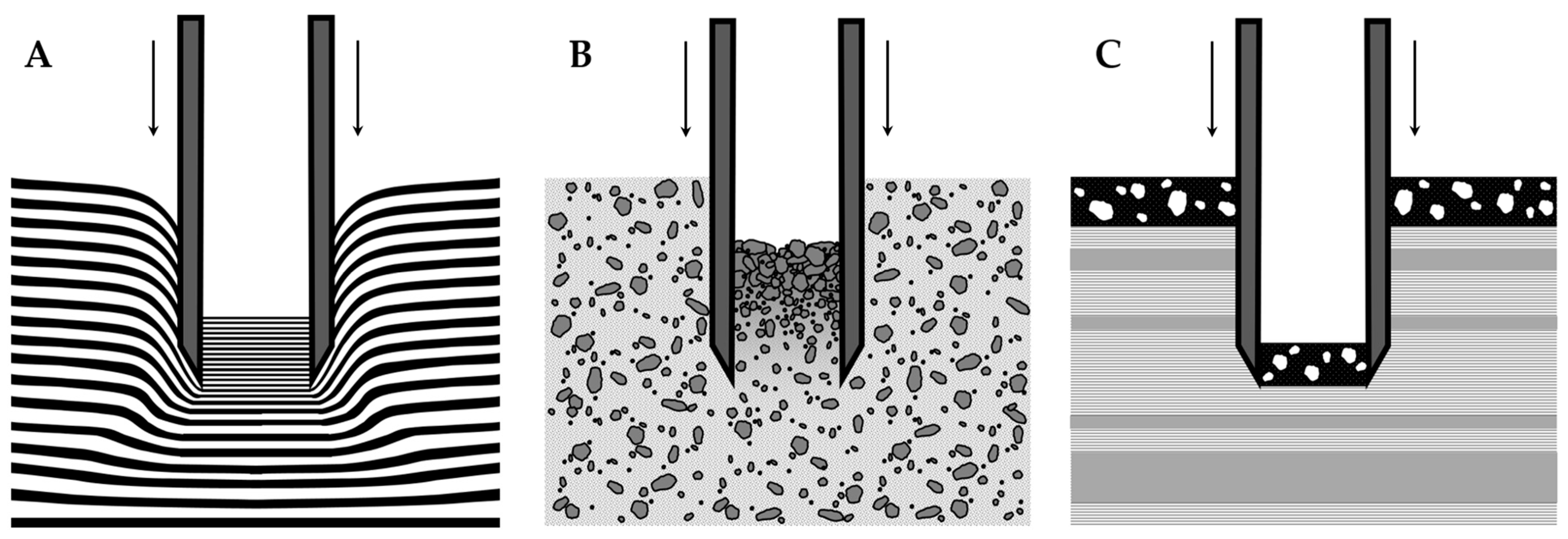
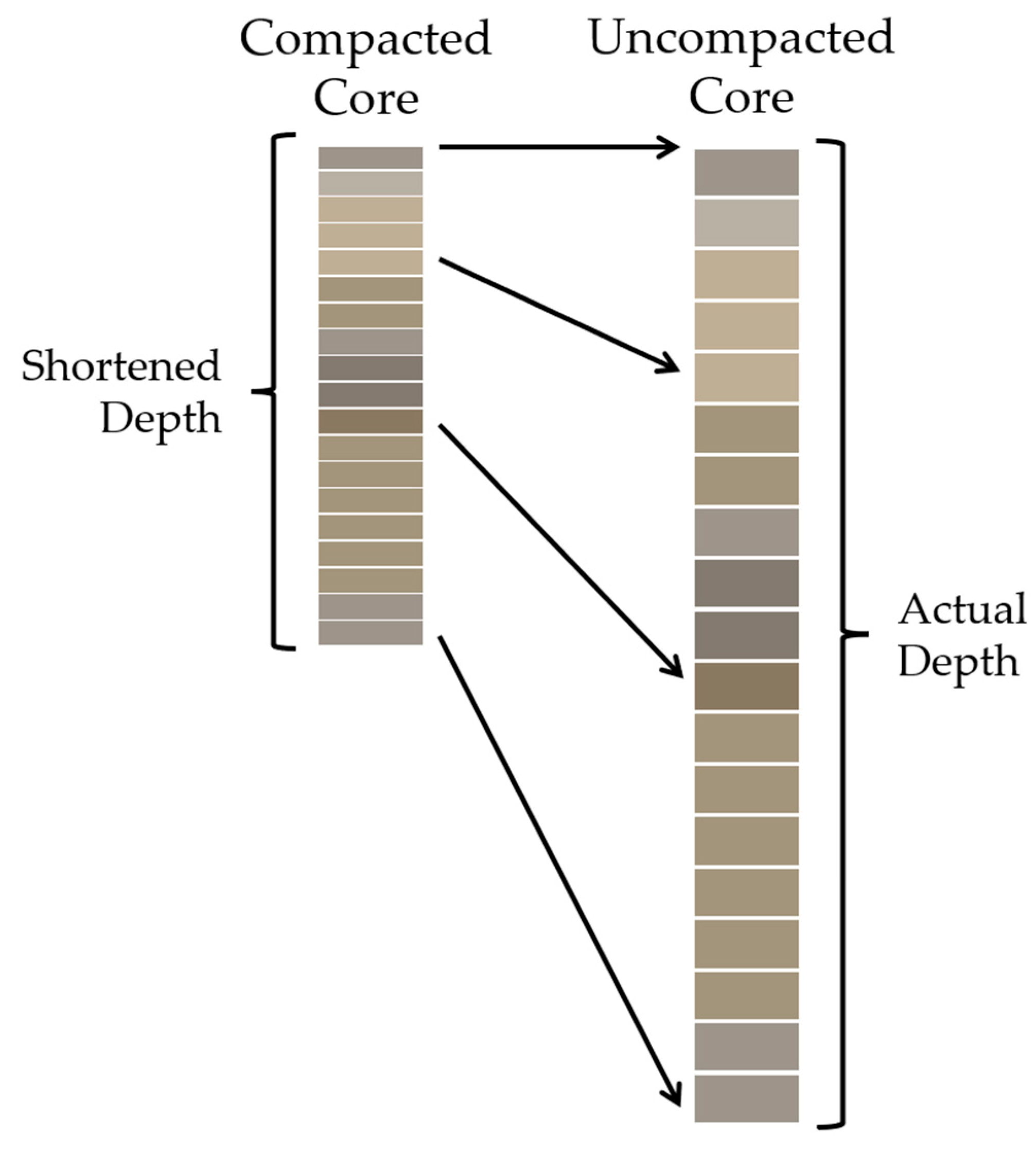
1.4. Core Shortening
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Microwave Digestion and ICP-OES
2.3. Fractional Calcium Carbonate
2.4. Loss on Ignition
2.5. Color and Texture
2.6. Analysis Methods
3. Results and Discussion
3.1. Core Depth, Color, and Texture
3.2. Elemental Concentrations
3.2.1. Analysis and Detection
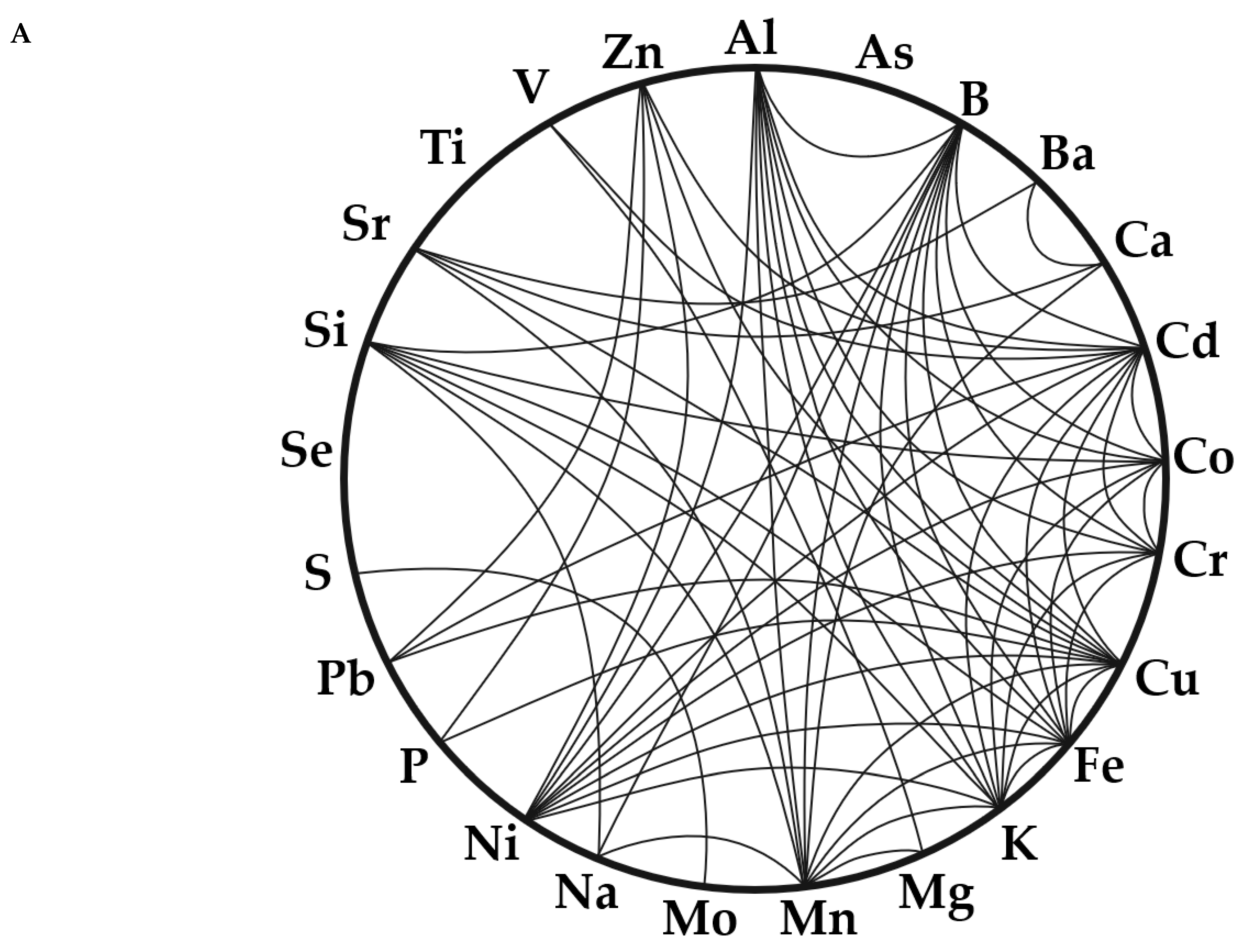
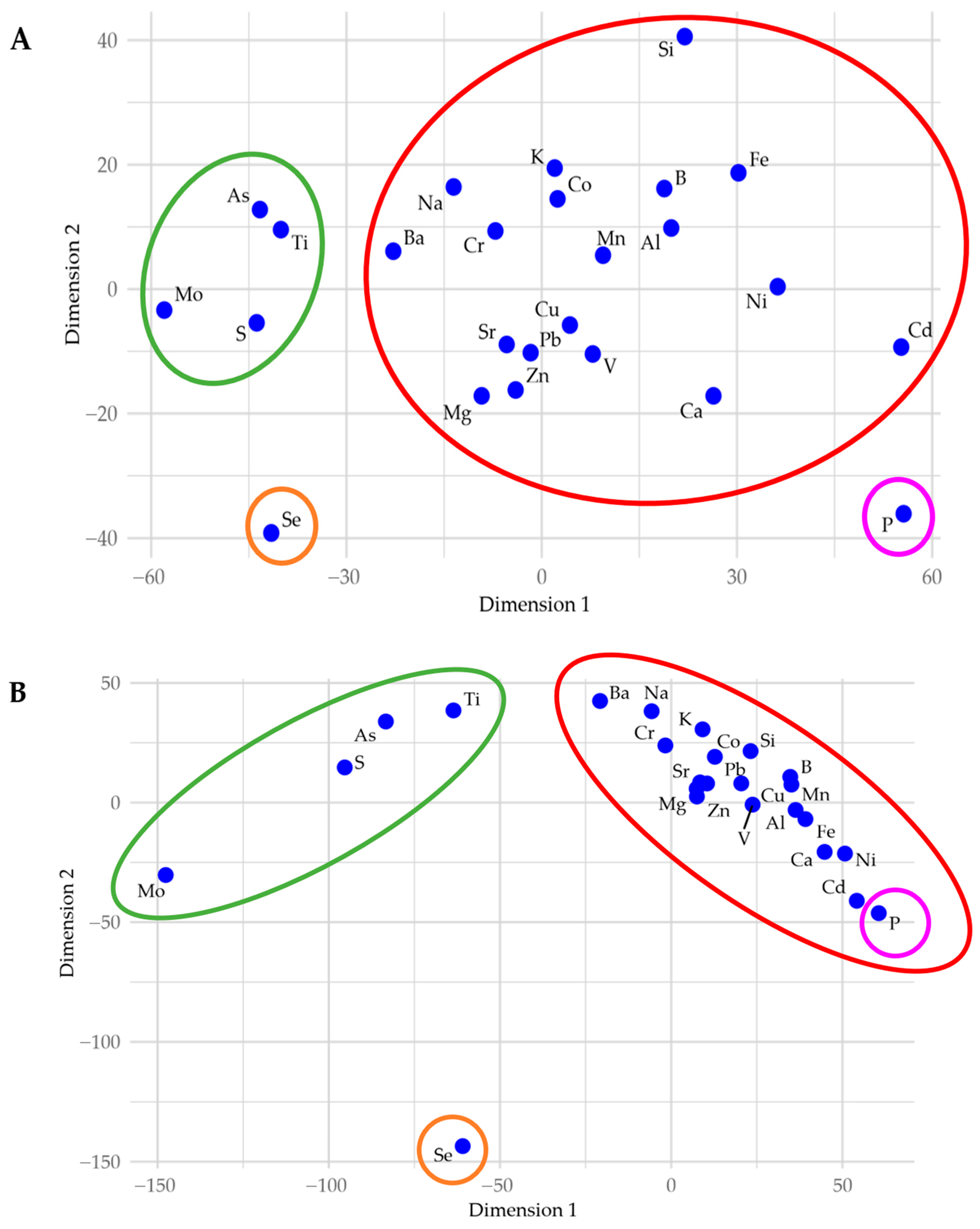
3.2.2. Relationship Between Elements
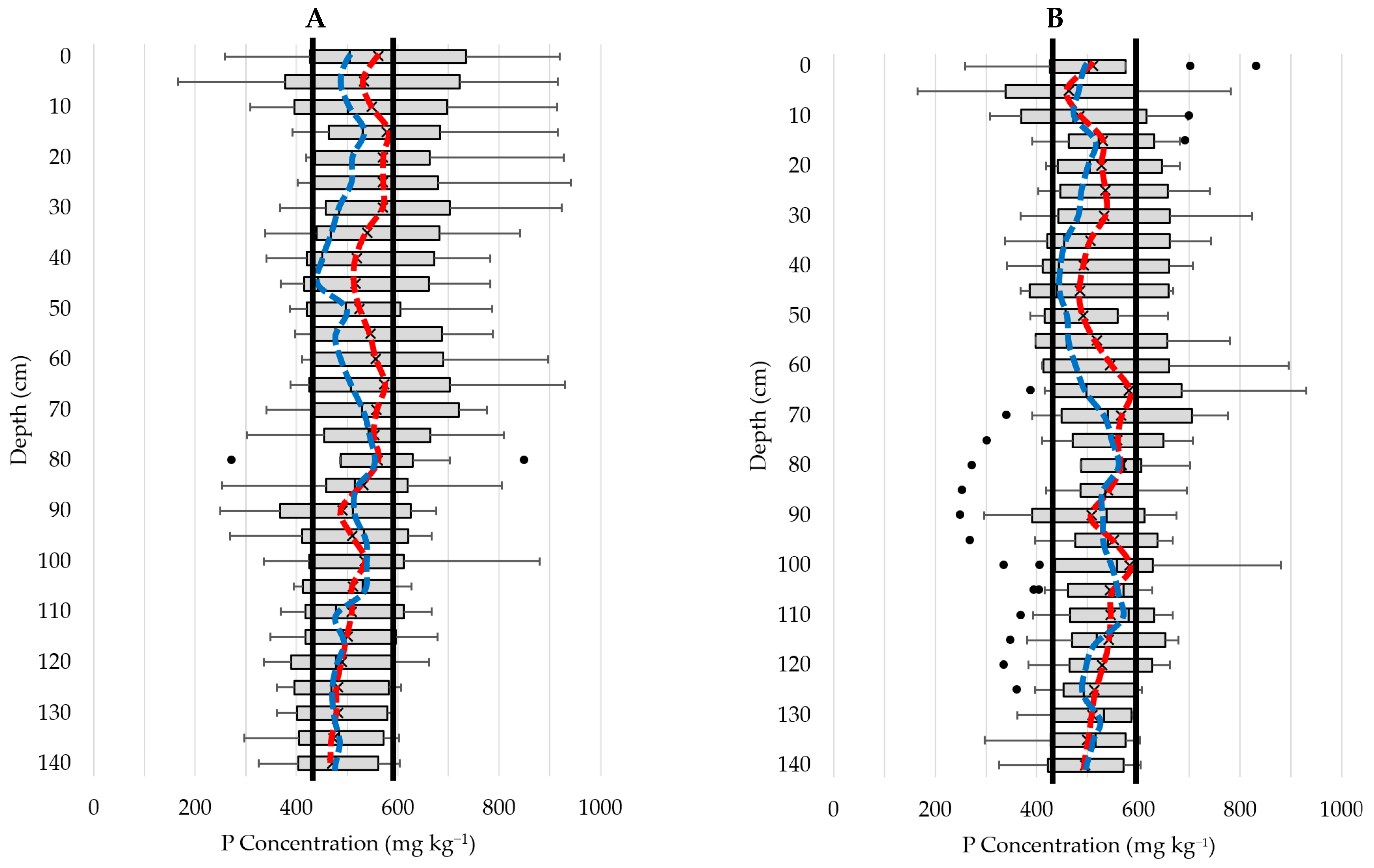
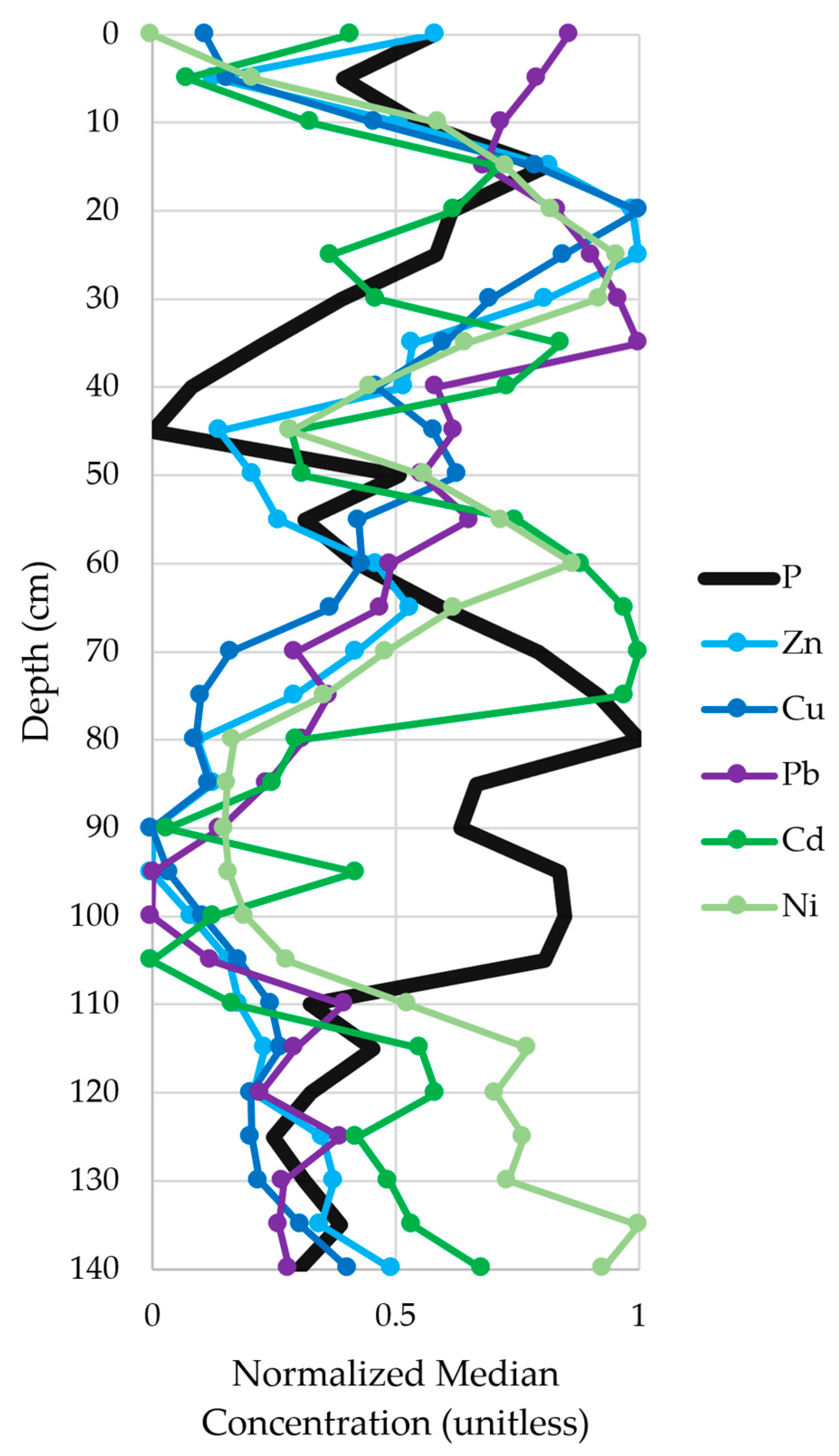
3.2.3. Relationship with Sample Depth
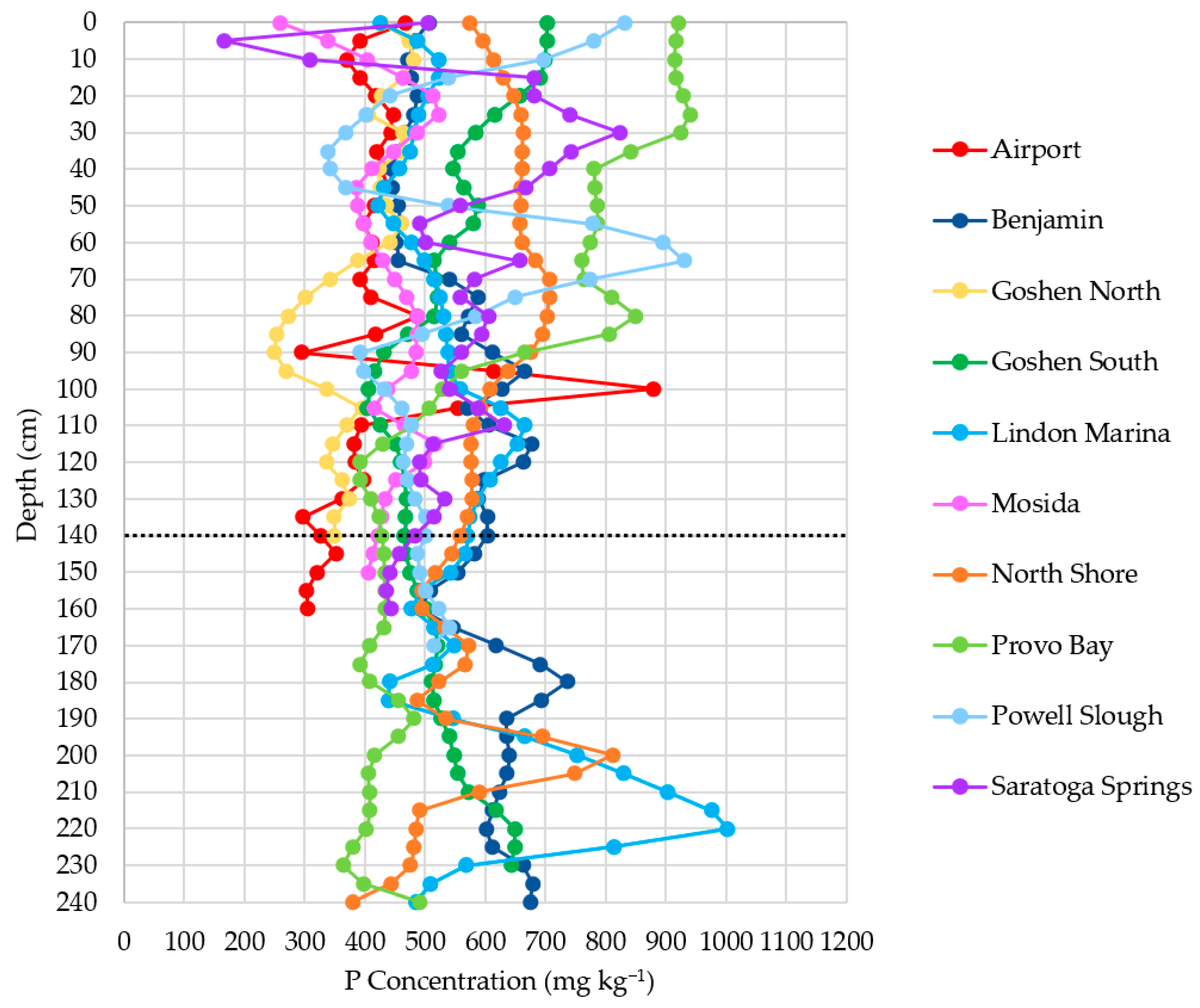
3.2.4. Relationship with Sample Location
3.3. Fractional Calcium Carbonate
3.4. Loss on Ignition
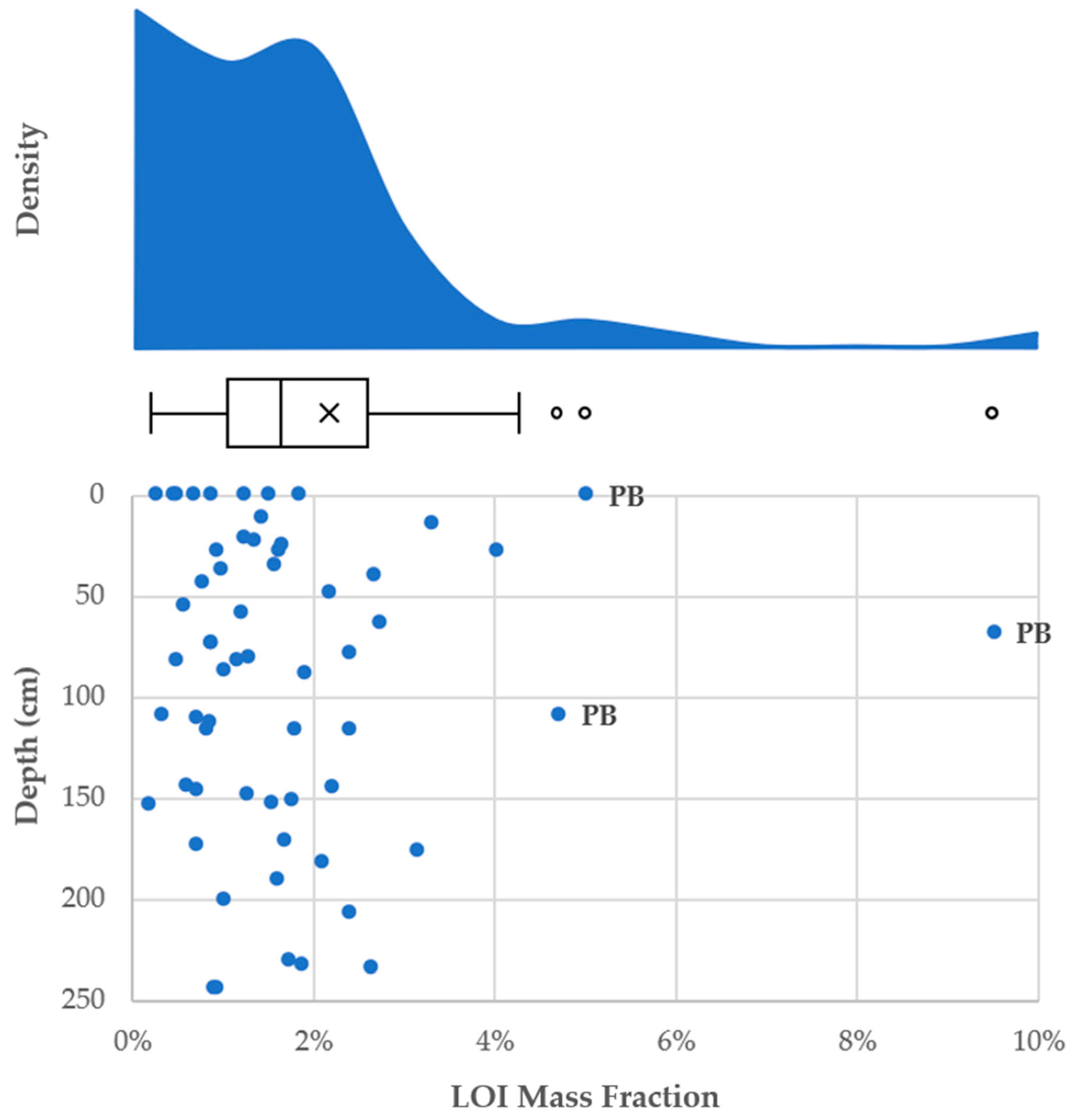
3.5. Sediment Phosphorus
3.5.1. The Use of Sediment Phosphorus for Paleolimnological Studies
3.5.2. Phosphorus Concentration-Depth Profiles
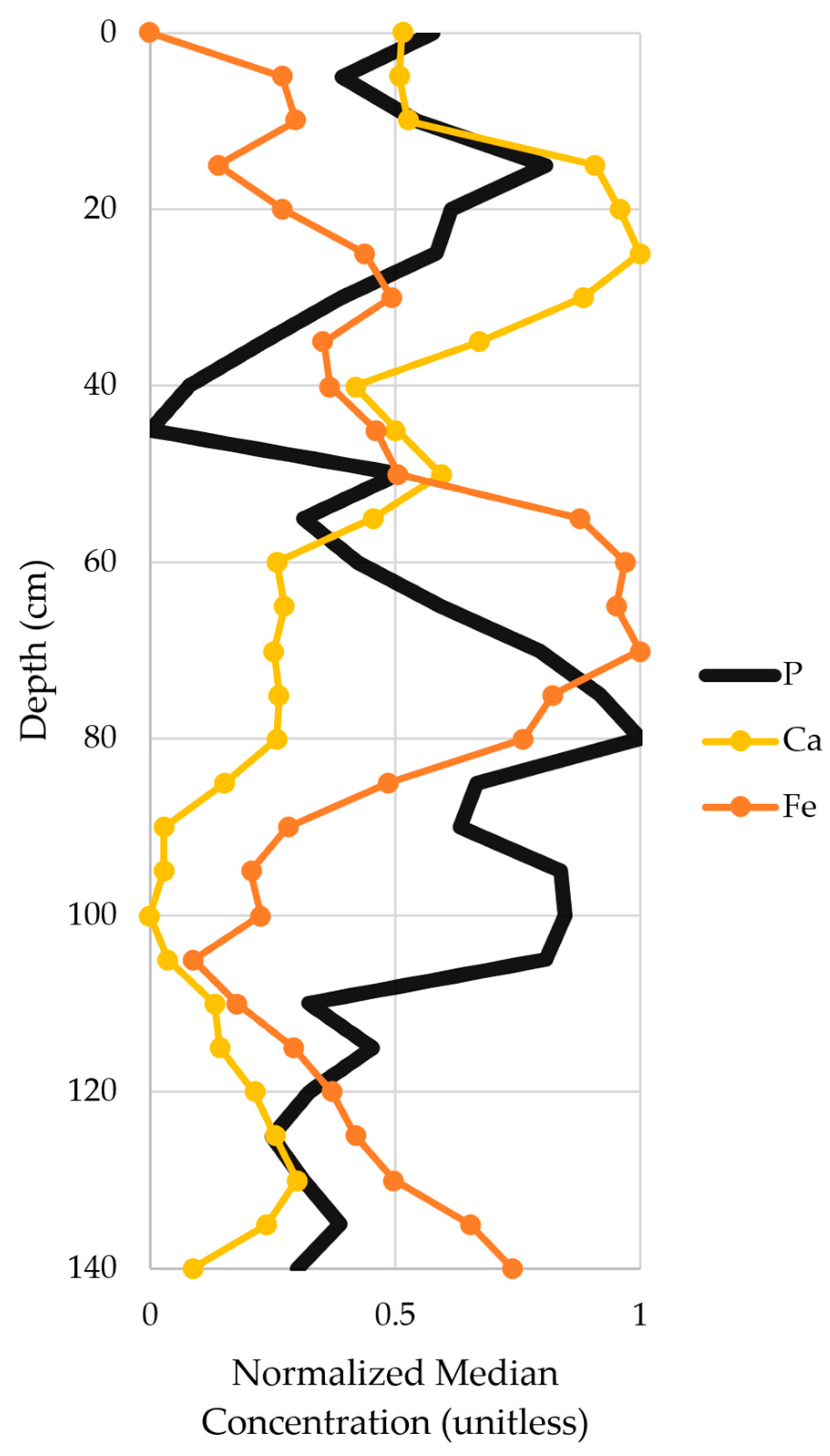
3.5.3. Connection to Calcium and Iron
- P concentrations are less skewed than the other elements and have fewer outliers (Figure 7).
- P has variable sediment depth trends, decreasing in concentration in some cores, remaining stable in others, and even increasing in some cases (Figure 14).
- P is geochemically isolated, as highlighted by Manhattan distances in a multidimensional scaling (MDS) plot of lake-wide elemental concentrations (Figure 8A).
3.5.4. Connection to Immobile Pollutants
4. Conclusion
4.1. Key Findings
4.2. Study Limitations and Suggestions for Future Research
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A | Airport |
| B | Benjamin |
| GN | Goshen North |
| GS | Goshen South |
| LM | Lindon Marina |
| M | Mosida |
| NS | North Shore |
| PB | Provo Bay |
| PS | Powell Slough |
| SS | Saratoga Springs |
References
- Last, W.M.; Smol, J.P. Tracking Environmental Change Using Lake Sediments: Basin Analysis, Coring, and Chronological Techniques; Springer Science & Business Media: London, UK, 2002; Volume 1. [Google Scholar]
- Last, W.M.; Smol, J.P. Tracking Environmental Change Using Lake Sediments: Physical and Geochemical Methods; Springer Science & Business Media: London, UK, 2002; Volume 2. [Google Scholar]
- Bissell, H.J. Preliminary Study of the Bottom Sediments of Utah Lake, Utah. In Report of the Committee on Sedimentation, 1940-41; National Research Council, Division of Geology and Geography: Washington, DC, USA, 1941; pp. 62–69. [Google Scholar]
- Bingham, C.C. Recent Sedimentation Trends in Utah Lake. Master’s Thesis, Brigham Young University, Provo, UT, USA, 1974. [Google Scholar]
- Bolland, R.F. Paleoecological Interpretation of the Diatom Succession in the Recent Sediments of Utah Lake, Utah. Ph.D. Thesis, The University of Utah, Salt Lake City, UT, USA, 1974. [Google Scholar]
- Brimhall, W.H.; Bassett, I.G.; Merritt, L.B. Reconnaissance Study of Deep-Water Springs and Strata of Utah Lake; Eyring Research Institute: Provo, UT, USA, 1976; pp. 1–21. [Google Scholar]
- Bushman, J.R. The Rate of Sedimentation in Utah Lake and the Use of Pollen as an Indicator of Time in the Sediments; 0068-1016, Brigham Young University Geology Studies; Brigham Young University: Provo, UT, USA, 1980; pp. 35–43. [Google Scholar]
- Brimhall, W.H.; Merritt, L.B. Geology of Utah Lake: Implications for resource management. Great Basin Nat. Mem. 1981, 5, 24–42. [Google Scholar]
- Macharia, A.N. Reconstructing Paleoenvironments Using a Mass-Energy Flux Framework. Ph.D. Thesis, The University of Utah, Salt Lake City, UT, USA, 2012. [Google Scholar]
- Brothers, S.; King, L.; Klein, A.; Brahney, J. Final Report to the Utah Division of Water Quality: Littoral-Benthic Primary Production in Utah Lake; Department of Watershed Sciences and Ecology Center, Utah State University: Logan, UT, USA, 2021. [Google Scholar]
- Williams, R.R.R. Determining the Anthropogenic Effects on Eutrophication of Utah Lake Since European Settlement Using Multiple Geochemical Approaches. Master’s Thesis, Brigham Young University, Provo, UT, USA, 2021. [Google Scholar]
- Devey, M.R. Comparing Multiple Approaches to Reconstructing the Phosphorus History of Marl Lakes: A Utah Lake Case Study. Master’s Thesis, Utah State University, Logan, UT, USA, 2022. [Google Scholar]
- Williams, R.; Nelson, S.; Rushforth, S.; Rey, K.; Carling, G.; Bickmore, B.; Heathcote, A.; Miller, T.; Meyers, L. Human-Driven Trophic Changes in a Large, Shallow Urban Lake: Changes in Utah Lake, Utah from Pre-European Settlement to the Present. Water Air Soil Pollut. 2023, 234, 218. [Google Scholar] [CrossRef]
- Brahney, J.; Powers, M.; King, L.; Devey, M.; Carter, M.; Carling, G.; Brothers, S.; Provard, A.; Young, B.; West, R. Utah Lake Paleoecology Study Report to DEQ; Watershed Sciences Faculty Publications; Utah State University: Logan, UT, USA, 2024; Volume 1163, p. 44. [Google Scholar] [CrossRef]
- King, L.; Devey, M.; Leavitt, P.R.; Power, M.J.; Brothers, S.; Brahney, J. Anthropogenic forcing leads to an abrupt shift to phytoplankton dominance in a shallow eutrophic lake. Freshw. Biol. 2024, 69, 335–350. [Google Scholar] [CrossRef]
- Abbott, B.W.; Errigo, I.M.; Follett, A.; Lawson, G.; Meyer, M.M.; Moon, H.; Shurtleff, K.; LeMonte, J.J.; Proteau, M.; Davis, K.; et al. Getting to Know the Utah Lake Ecosystem; Brigham Young University: Provo, UT, USA, 2021. [Google Scholar]
- Abu-Hmeidan, H.Y.; Williams, G.P.; Miller, A.W. Characterizing total phosphorus in current and geologic utah lake sediments: Implications for water quality management issues. Hydrology 2018, 5, 8. [Google Scholar] [CrossRef]
- Randall, M.C.; Carling, G.T.; Dastrup, D.B.; Miller, T.; Nelson, S.T.; Rey, K.A.; Hansen, N.C.; Bickmore, B.R.; Aanderud, Z.T. Sediment potentially controls in-lake phosphorus cycling and harmful cyanobacteria in shallow, eutrophic Utah Lake. PLoS ONE 2019, 14, e0212238. [Google Scholar] [CrossRef]
- Taggart, J.B.; Ryan, R.L.; Williams, G.P.; Miller, A.W.; Valek, R.A.; Tanner, K.B.; Cardall, A.C. Historical Phosphorus Mass and Concentrations in Utah Lake: A Case Study with Implications for Nutrient Load Management in a Sorption-Dominated Shallow Lake. Water 2024, 16, 933. [Google Scholar] [CrossRef]
- Taggart, J.B. Inorganic Phosphorus Chemistry of Utah Lake’s Effluent Mixing Zones. Master’s Thesis, Colorado School of Mines, Golden, CO, USA, 2021. [Google Scholar]
- Valek, R.A.; Tanner, K.B.; Taggart, J.B.; Ryan, R.L.; Cardall, A.C.; Woodland, L.M.; Oxborrow, M.J.; Williams, G.P.; Miller, A.W.; Sowby, R.B. Regulated Inductively Coupled Plasma–Optical Emission Spectrometry Detectible Elements in Utah Lake: Characterization and Discussion. Water 2024, 16, 2170. [Google Scholar] [CrossRef]
- Tanner, K.B.; Cardall, A.C.; Williams, G.P. A spatial long-term trend analysis of estimated chlorophyll-a concentrations in Utah Lake using Earth observation data. Remote Sens. 2022, 14, 3664. [Google Scholar] [CrossRef]
- Carter, R.D. Utah Lake: Legacy. In June Sucker Recovery Implementation Program; Utah Department of Environmental Quality: Salt Lake City, UT, USA, 2002. [Google Scholar]
- Hooton, L.W., Jr. Utah Lake & Jordan River Water Rights & Management Plan; The Salt Lake City Department of Public Utilities: Salt Lake City, UT, USA, 1989. [Google Scholar]
- Department of the Interior, Fish and Wildlife Service. Endangered and Threatened Wildlife and Plants: Reclassification of the Endangered June Sucker to Threatened with a Section 4(d) Rule. In Federal Register; Department of the Interior, Fish and Wildlife Service: Washington, DC, USA, 2021; Volume 86, pp. 192–212. [Google Scholar]
- Utah Lake Wetland Preserve. Available online: https://www.mitigationcommission.gov/wetlands/wetlands_ulwp.html (accessed on 10 October 2024).
- Hongve, D.; Erlandsen, A.H. Shortening of Surface Sediment Cores During Sampling. Hydrobiologia 1979, 65, 283–287. [Google Scholar] [CrossRef]
- Morton, R.A.; White, W.A. Characteristics of and Corrections for Core Shortening in Unconsolidated Sediments. J. Coast. Res. 1997, 13, 761–769. [Google Scholar]
- Zheng, G.; Takano, B.; Matsuo, M.; Tanaka, Y. Compaction of modern soft sediments during core sampling—An in situ investigation at an estuary site. Environ. Geosci. 2002, 9, 109–114. [Google Scholar] [CrossRef]
- Ramirez, S.G.; Hales, R.C.; Williams, G.P.; Jones, N.L. Extending SC-PDSI-PM with neural network regression using GLDAS data and Permutation Feature Importance. Environ. Model. Softw. 2022, 157, 105475. [Google Scholar] [CrossRef]
- Evans, S.; Williams, G.P.; Jones, N.L.; Ames, D.P.; Nelson, E.J. Exploiting earth observation data to impute groundwater level measurements with an extreme learning machine. Remote Sens. 2020, 12, 2044. [Google Scholar] [CrossRef]
- Wrath, W.F. Contamination and compaction in core sampling. Science 1936, 84, 537–538. [Google Scholar] [CrossRef]
- EPA. Method 3051A (SW-846): Microwave Assisted Acid Digestion of Sediments, Sludges, and Oils; EPA: Washington, DC, USA, 2007.
- Gavlak, R.; Horneck, D.; Miller, R.O.; Kotuby-Amacher, J. Soil, Plant and Water Reference Methods for the Western Region, 3rd ed.; Western Region Extension Publication (WREP 125); Colorado State University: Fort Collins, CO, USA, 2003; pp. 1–207. [Google Scholar]
- Munsell. Munsell Soil Color Charts; Munsell Color: New Windsor, NY, USA, 2000. [Google Scholar]
- Jackson, E.K.; Roberts, W.; Nelsen, B.; Williams, G.P.; Nelson, E.J.; Ames, D.P. Introductory overview: Error metrics for hydrologic modelling—A review of common practices and an open source library to facilitate use and adoption. Environ. Model. Softw. 2019, 119, 32–48. [Google Scholar] [CrossRef]
- Roberts, W.; Williams, G.P.; Jackson, E.; Nelson, E.J.; Ames, D.P. Hydrostats: A Python package for characterizing errors between observed and predicted time series. Hydrology 2018, 5, 66. [Google Scholar] [CrossRef]
- Carey, C.C.; Rydin, E. Lake trophic status can be determined by the depth distribution of sediment phosphorus. Limnol. Oceanogr. 2011, 56, 2051–2063. [Google Scholar] [CrossRef]
- Carignan, R.; Flett, R. Postdepositional mobility of phosphorus in lake sediments. Limnol. Oceanogr. 1981, 26, 361–366. [Google Scholar] [CrossRef]
- Wang, L.; Liang, T. Distribution characteristics of phosphorus in the sediments and overlying water of Poyang Lake. PLoS ONE 2015, 10, e0125859. [Google Scholar] [CrossRef] [PubMed]
- Kenney, W.F.; Brenner, M.; Curtis, J.H.; Arnold, T.E.; Schelske, C.L. A holocene sediment record of phosphorus accumulation in shallow Lake Harris, Florida (USA) offers new perspectives on recent cultural eutrophication. PLoS ONE 2016, 11, e0147331. [Google Scholar] [CrossRef][Green Version]
- Casbeer, W.; Williams, G.P.; Borup, M.B. Phosphorus distribution in delta sediments: A unique data set from deer creek reservoir. Hydrology 2018, 5, 58. [Google Scholar] [CrossRef]
- Taggart, J.; Miller, T.; Navarre-Sitchler, A.; Carling, G. Mineral Precipitation In Utah Lake And Its Effluent Mixing Zones. In Proceedings of the 2022 Intermountain Engineering, Technology and Computing (IETC), Orem, UT, USA, 13–14 May 2022; pp. 1–5. [Google Scholar]
- He, J.; Liu, G.; Zhu, D.; Cai, J.; Zhou, W.; Guo, W. Sequential extraction of calcium in lake sediments for investigating the cycle of phosphorus in water environment. Int. J. Environ. Sci. Technol. 2015, 12, 1123–1136. [Google Scholar] [CrossRef]
- Bertrand, S.; Tjallingii, R.; Kylander, M.E.; Wilhelm, B.; Roberts, S.J.; Arnaud, F.; Brown, E.; Bindler, R. Inorganic geochemistry of lake sediments: A review of analytical techniques and guidelines for data interpretation. Earth-Sci. Rev. 2024, 249, 104639. [Google Scholar] [CrossRef]
- Kovarki, B. A Century of Tragedy: How the Car and Gas Industry Knew About the Health Risks of Leaded Fuel but Sold It for 100 Years Anyway. Available online: https://theconversation.com/a-century-of-tragedy-how-the-car-and-gas-industry-knew-about-the-health-risks-of-leaded-fuel-but-sold-it-for-100-years-anyway-173395 (accessed on 27 March 2025).
- U.S. Energy Information Administration. Leaded Gasoline Was Gradually Taken off the U.S. Market. Available online: https://www.eia.gov/energyexplained/gasoline/gasoline-and-the-environment-leaded-gasoline.php (accessed on 27 March 2025).
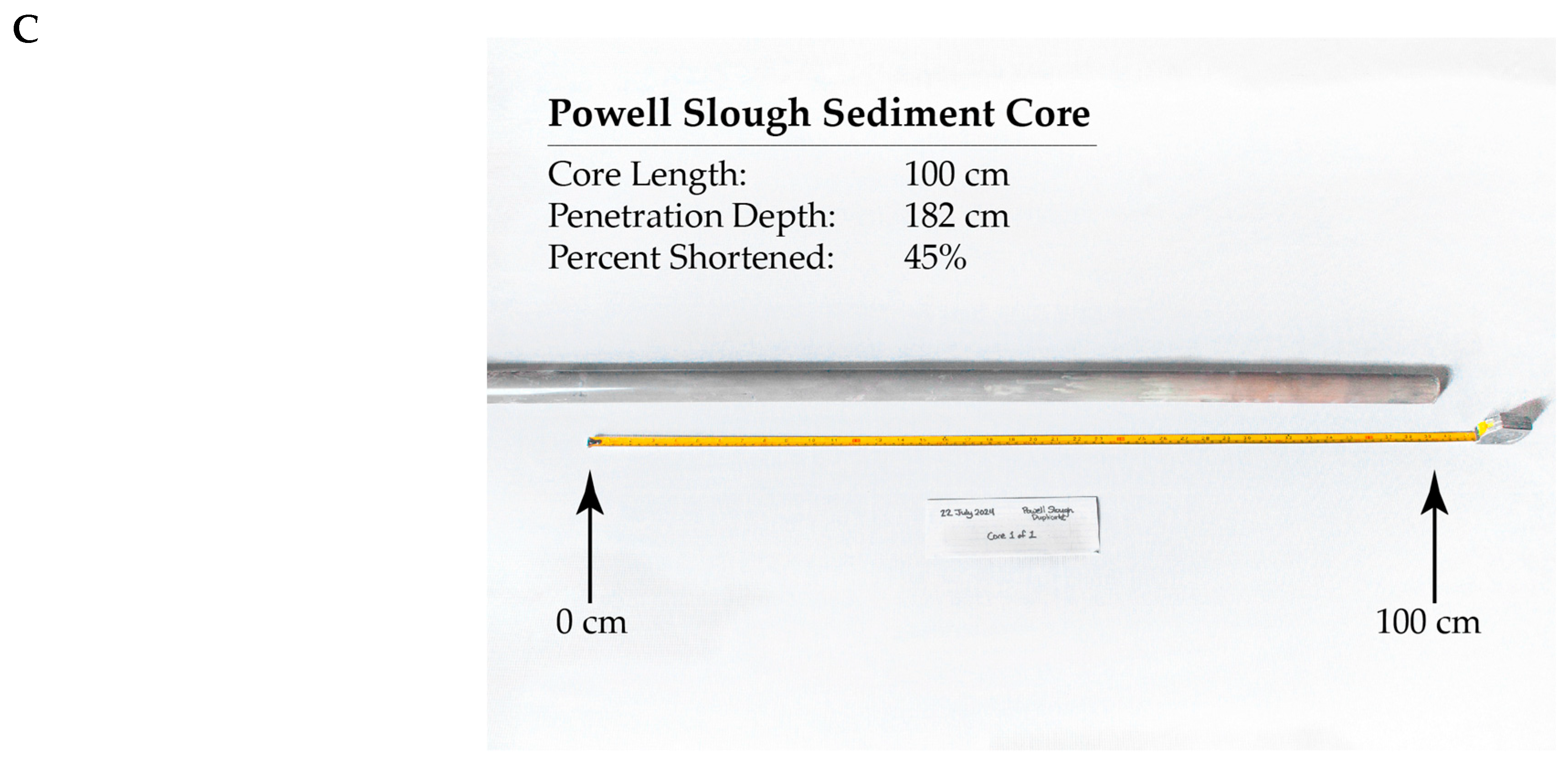
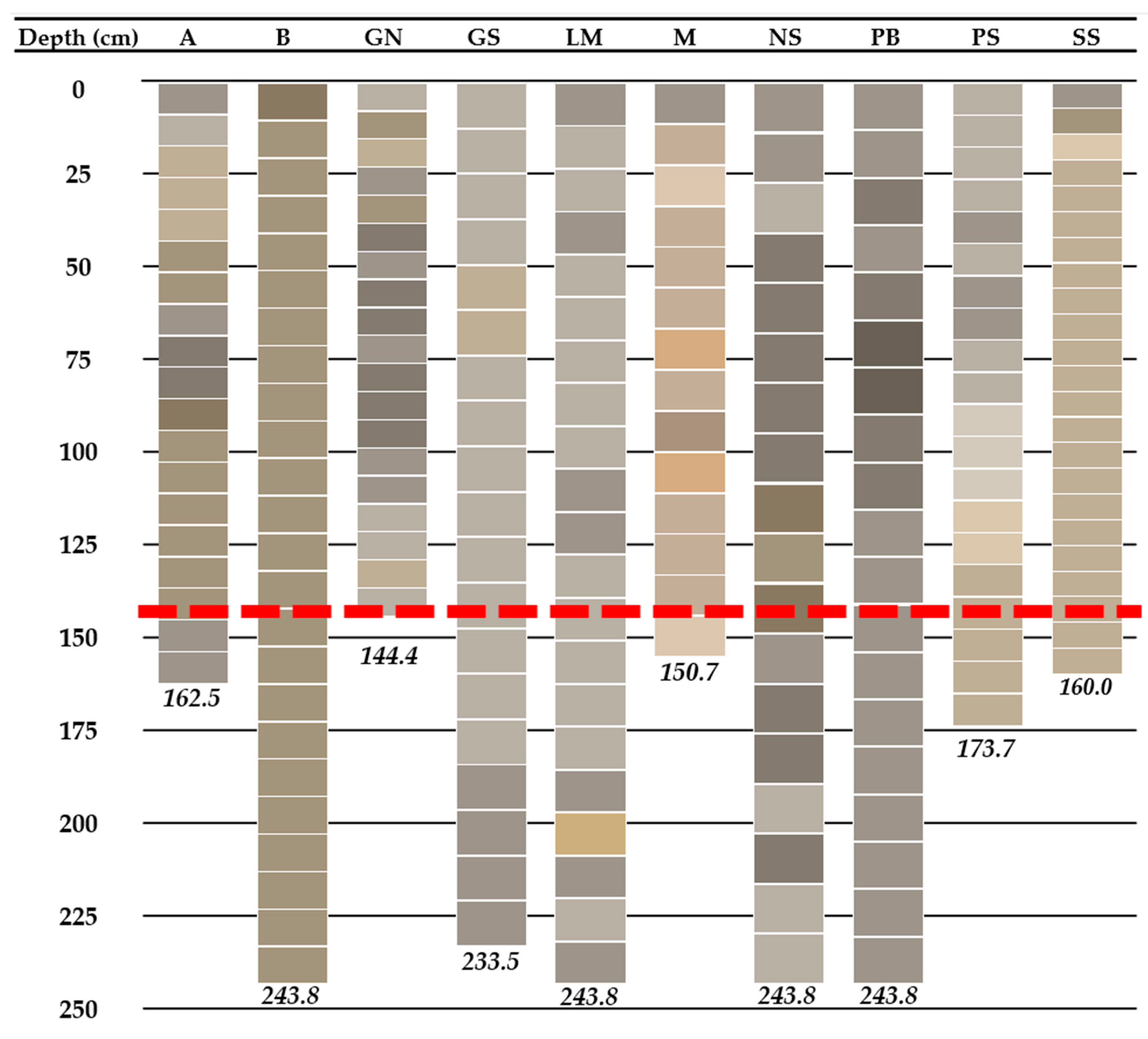


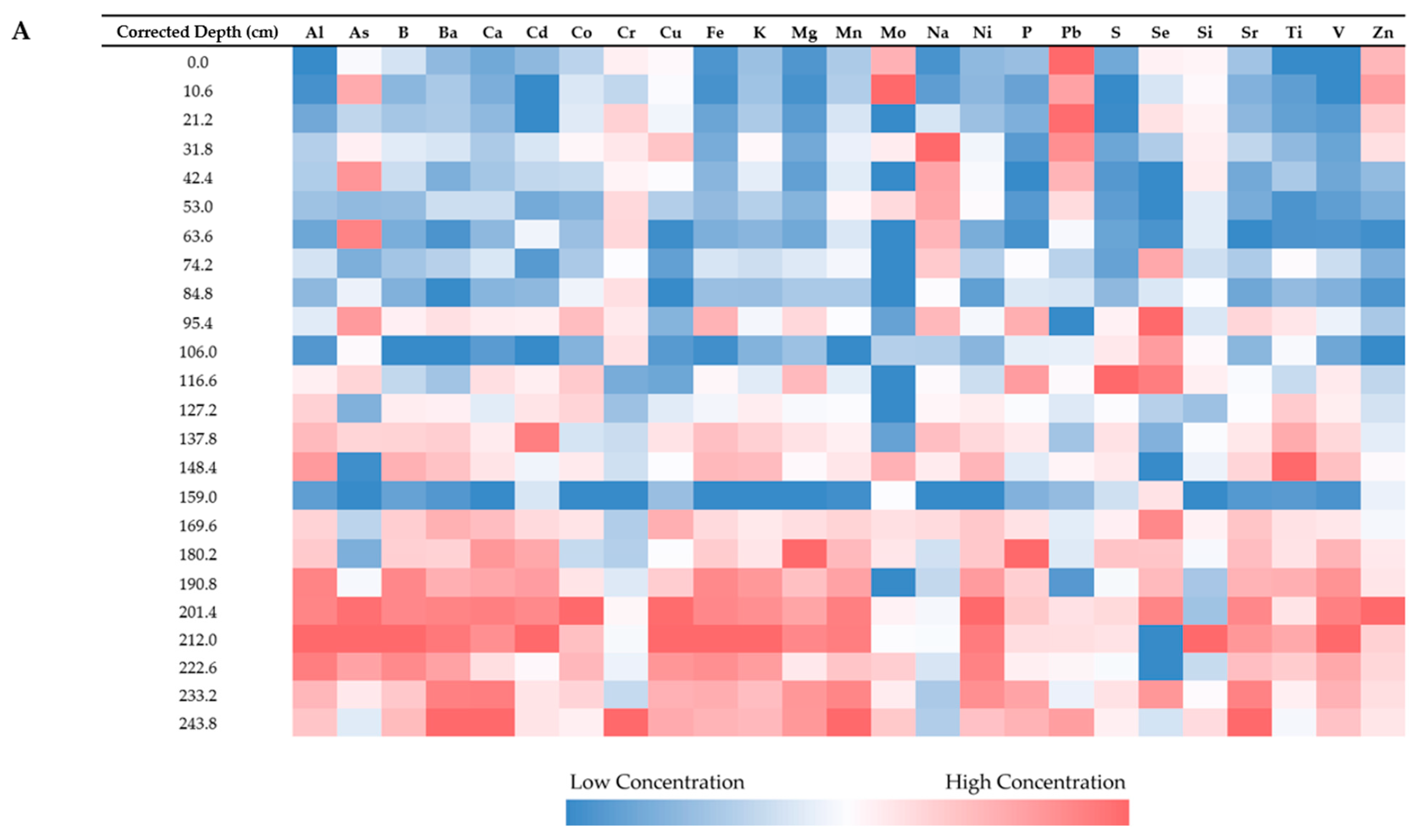
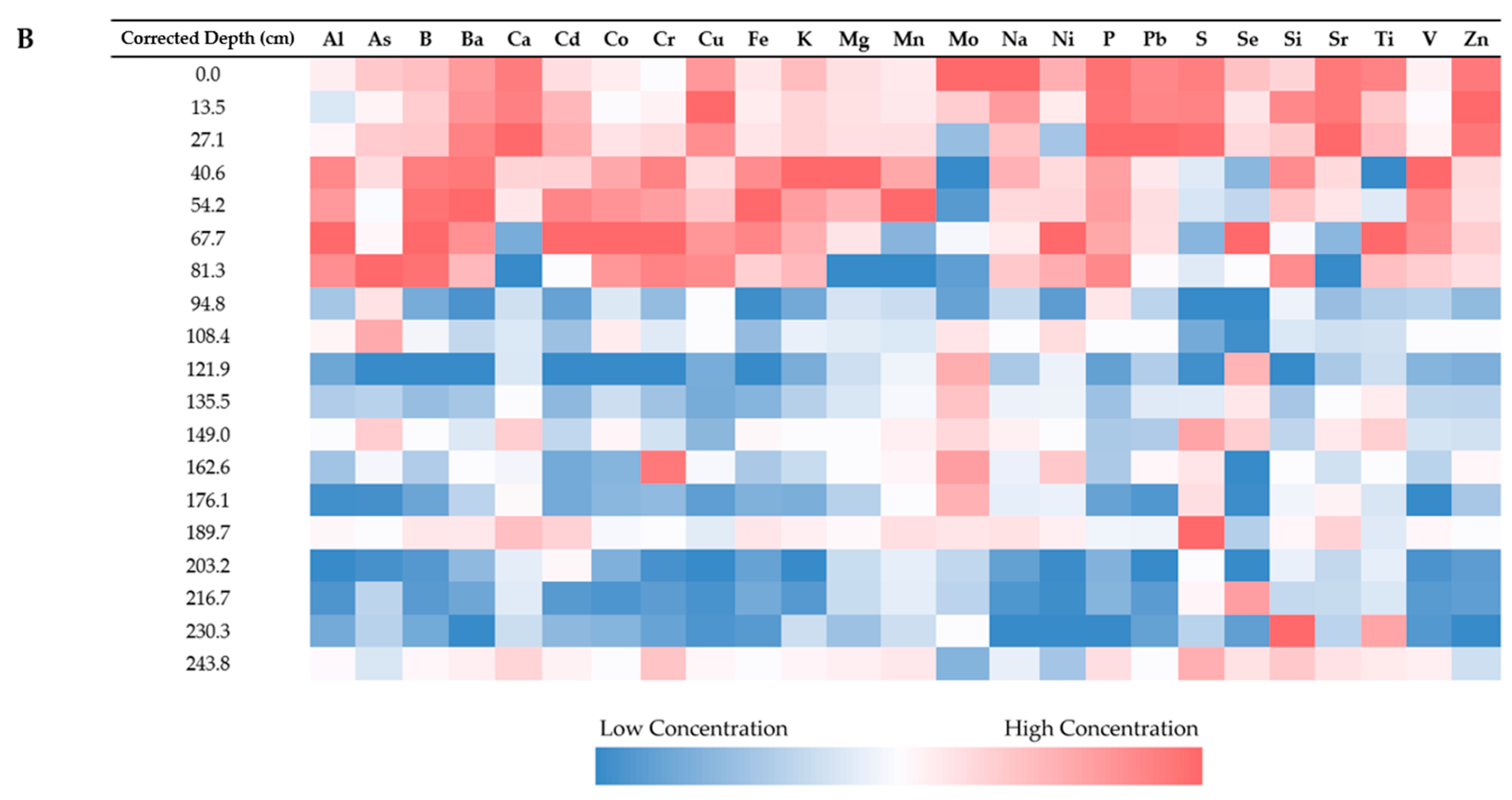
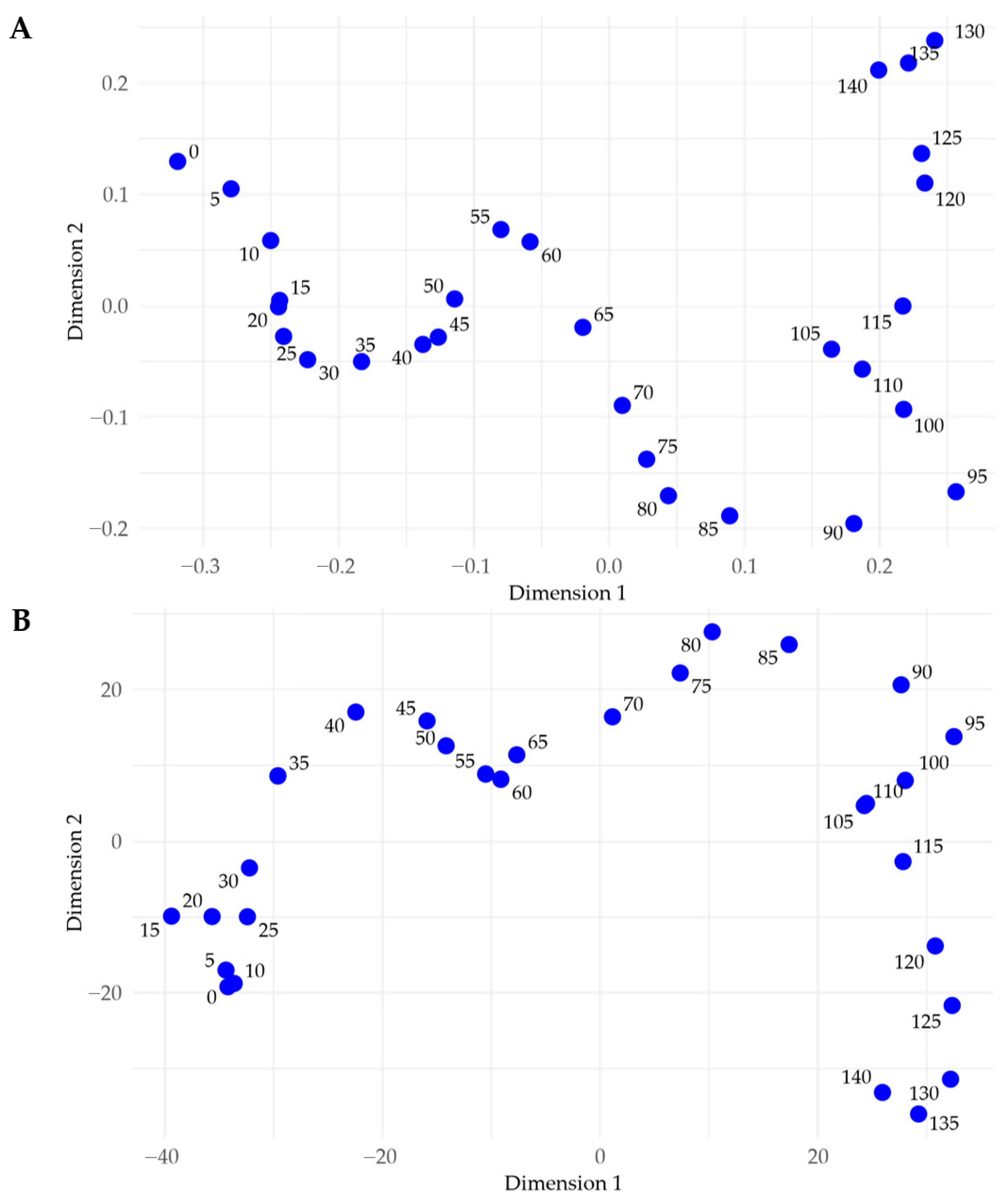

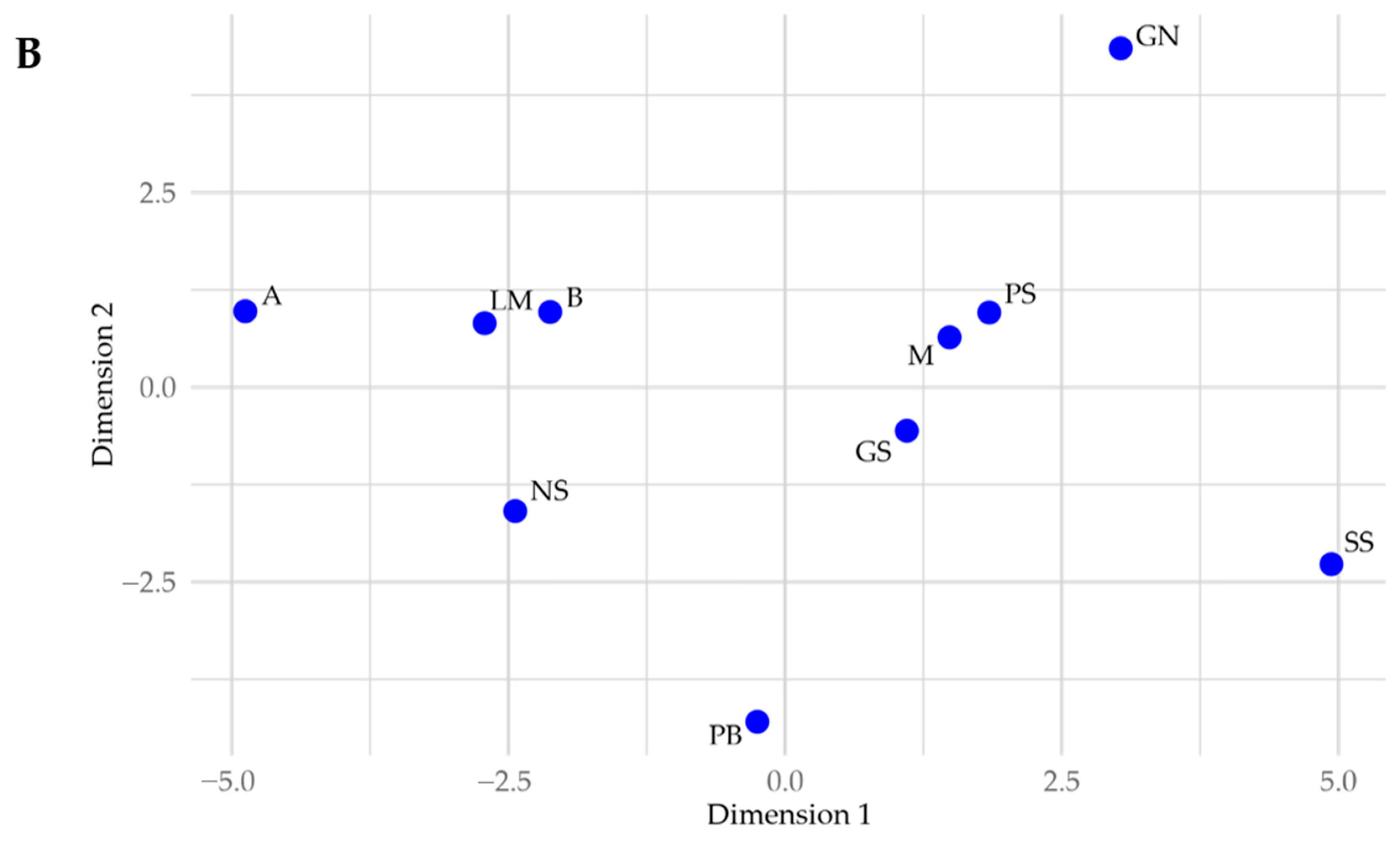


| Site Name | Water Depth | Penetration Depth | Core Length | % Shortened |
|---|---|---|---|---|
| Airport (A) | 2.33 m/7.66 ft | 1.62 m/5.33 ft | 0.90 m/2.95 ft | 45% |
| Benjamin (B) | 1.80 m/5.91 ft | 2.44 m/8.00 ft | 1.15 m/3.77 ft | 53% |
| Goshen North (GN) | 1.73 m/5.67 ft | 1.52 m/5.00 ft | 0.95 m/3.12 ft | 38% |
| Goshen South (GS) | 1.75 m/5.75 ft | 2.44 m/8.00 ft | 0.94 m/3.08 ft | 61% |
| Lindon Marina (LM) | 1.72 m/5.63 ft | 2.44 m/8.00 ft | 1.00 m/3.28 ft | 59% |
| Mosida (M) | 1.73 m/5.67 ft | 1.60 m/5.25 ft | 0.69 m/2.26 ft | 57% |
| North Shore (NS) | 1.17 m/3.83 ft | 2.44 m/8.00 ft | 0.85 m/2.79 ft | 65% |
| Powell Slough (PS) | 1.65 m/5.42 ft | 1.83 m/6.00 ft | 1.00 m/3.28 ft | 45% |
| Provo Bay (PB) | 2.18 m/7.16 ft | 2.44 m/8.00 ft | 0.90 m/2.95 ft | 63% |
| Saratoga Springs (SS) | 1.74 m/5.72 ft | 1.60 m/5.25 ft | 1.10 m/3.61 ft | 31% |
| Airport Core | Benjamin Core | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Length (cm) | Depth (cm) | Texture | Munsell | Color | Length (cm) | Depth (cm) | Texture | Munsell | Color | |
| Notation | Notation | |||||||||
| 0 | 0.00 | Sandy | 2.5Y 6/1 | 0 | 0.00 | Silty | 2.5Y 5/2 | |||
| 5 | 9.03 | Silty Sand | 2.5Y 7/1 | 5 | 10.6 | Silty | 10YR 6/2 | |||
| 10 | 18.05 | Silty | 2.5Y 7/2 | 10 | 21.2 | Silty | 10YR 6/2 | |||
| 15 | 27.08 | Silty | 2.5Y 7/2 | 15 | 31.81 | Silty | 10YR 6/2 | |||
| 20 | 36.1 | Silty | 2.5Y 7/2 | 20 | 42.41 | Silty | 10YR 6/2 | |||
| 25 | 45.13 | Silty Sand | 2.5Y 6/2 | 25 | 53.01 | Silty | 10YR 6/2 | |||
| 30 | 54.15 | Silty Sand | 2.5Y 6/2 | 30 | 63.61 | Silty | 10YR 6/2 | |||
| 35 | 63.18 | Silty Sand | 2.5Y 6/1 | 35 | 74.21 | Silty | 10YR 6/2 | |||
| 40 | 72.2 | Sandy | 2.5Y 5/1 | 40 | 84.81 | Silty | 10YR 6/2 | |||
| 45 | 81.23 | Sandy | 2.5Y 5/1 | 45 | 95.42 | Silty | 10YR 6/2 | |||
| 50 | 90.25 | Sandy | 2.5Y 5/2 | 50 | 106.02 | Silty | 10YR 6/2 | |||
| 55 | 99.28 | Sandy | 2.5Y 6/2 | 55 | 116.62 | Silty | 10YR 6/2 | |||
| 60 | 108.31 | Sandy | 2.5Y 6/2 | 60 | 127.22 | Silty | 10YR 6/2 | |||
| 65 | 117.33 | Sandy | 2.5Y 6/2 | 65 | 137.82 | Silty | 10YR 6/2 | |||
| 70 | 126.36 | Sandy | 2.5Y 6/2 | 70 | 148.42 | Silty | 10YR 6/2 | |||
| 75 | 135.38 | Sandy | 2.5Y 6/2 | 75 | 159.03 | Silty | 10YR 6/2 | |||
| 80 | 144.41 | Sandy | 2.5Y 6/2 | 80 | 169.63 | Silty | 10YR 6/2 | |||
| 85 | 153.43 | Sandy | 2.5Y 6/1 | 85 | 180.23 | Silty | 10YR 6/2 | |||
| 90 | 162.46 | Sandy | 2.5Y 6/1 | 90 | 190.83 | Silty | 10YR 6/2 | |||
| 95 | 201.43 | Silty | 10YR 6/2 | |||||||
| 100 | 212.03 | Silty | 10YR 6/2 | |||||||
| 105 | 222.64 | Silty | 10YR 6/2 | |||||||
| 110 | 233.24 | Silty | 10YR 6/2 | |||||||
| 115 | 243.84 | Silty | 10YR 6/2 | |||||||
| Element | Mean | Median | Standard Deviation | Skewness | Element | Mean | Median | Standard Deviation | Skewness |
|---|---|---|---|---|---|---|---|---|---|
| Al | 3577 | 3024 | 1921 | 1.3 | Mo | 1.9 | 0.77 | 3.5 | 3.1 |
| As | 7.0 | 4.6 | 9.2 | 3.2 | Na | 537 | 463 | 303 | 1.8 |
| B | 13 | 12 | 6.1 | 0.8 | Ni | 8.3 | 7.4 | 3.6 | 0.6 |
| Ba | 112.5 | 85 | 86 | 2.5 | P | 530 | 498 | 143 | 0.7 |
| Ca | 89,543 | 84,941 | 43,819 | 0.5 | Pb | 6.9 | 5.9 | 4.6 | 1.8 |
| Cd | 0.71 | 0.66 | 0.3 | 0.5 | S | 1462 | 578 | 1879 | 2.1 |
| Co | 3.2 | 2.9 | 1.4 | 1.2 | Se | 0.98 | 0.31 | 1.3 | 1.5 |
| Cr | 26.3 | 25 | 11 | 1.1 | Si | 372 | 378 | 202 | 0.3 |
| Cu | 7.7 | 6.2 | 4.6 | 0.8 | Sr | 280 | 217 | 212 | 1.4 |
| Fe | 6906 | 6309 | 2992 | 0.7 | Ti | 121 | 102 | 84 | 4.1 |
| K | 1491 | 1096 | 1115 | 1.7 | V | 31 | 28 | 14 | 1.2 |
| Mg | 9484 | 8600 | 4979 | 1.8 | Zn | 33 | 28 | 18 | 2.1 |
| Mn | 387 | 333 | 193 | 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taggart, J.B.; Woodland, L.M.; Tanner, K.B.; Williams, G.P. A Geochemical Study of Near-Shore Sediment Cores from Utah Lake, UT, USA. Geosciences 2025, 15, 363. https://doi.org/10.3390/geosciences15090363
Taggart JB, Woodland LM, Tanner KB, Williams GP. A Geochemical Study of Near-Shore Sediment Cores from Utah Lake, UT, USA. Geosciences. 2025; 15(9):363. https://doi.org/10.3390/geosciences15090363
Chicago/Turabian StyleTaggart, Jacob B., Lauren M. Woodland, Kaylee B. Tanner, and Gustavious P. Williams. 2025. "A Geochemical Study of Near-Shore Sediment Cores from Utah Lake, UT, USA" Geosciences 15, no. 9: 363. https://doi.org/10.3390/geosciences15090363
APA StyleTaggart, J. B., Woodland, L. M., Tanner, K. B., & Williams, G. P. (2025). A Geochemical Study of Near-Shore Sediment Cores from Utah Lake, UT, USA. Geosciences, 15(9), 363. https://doi.org/10.3390/geosciences15090363






