Andesite and CO2-Saturated Water Interaction at Different Temperatures and Flow Rates Using a Flow-Through Reactor
Abstract
1. Introduction
2. Materials and Methods
2.1. Rock Samples and Treatments
2.2. Flow-Through Reactor
2.3. Analysis of Water Quality and Rock Composition
2.4. Geochemical Modeling
3. Results
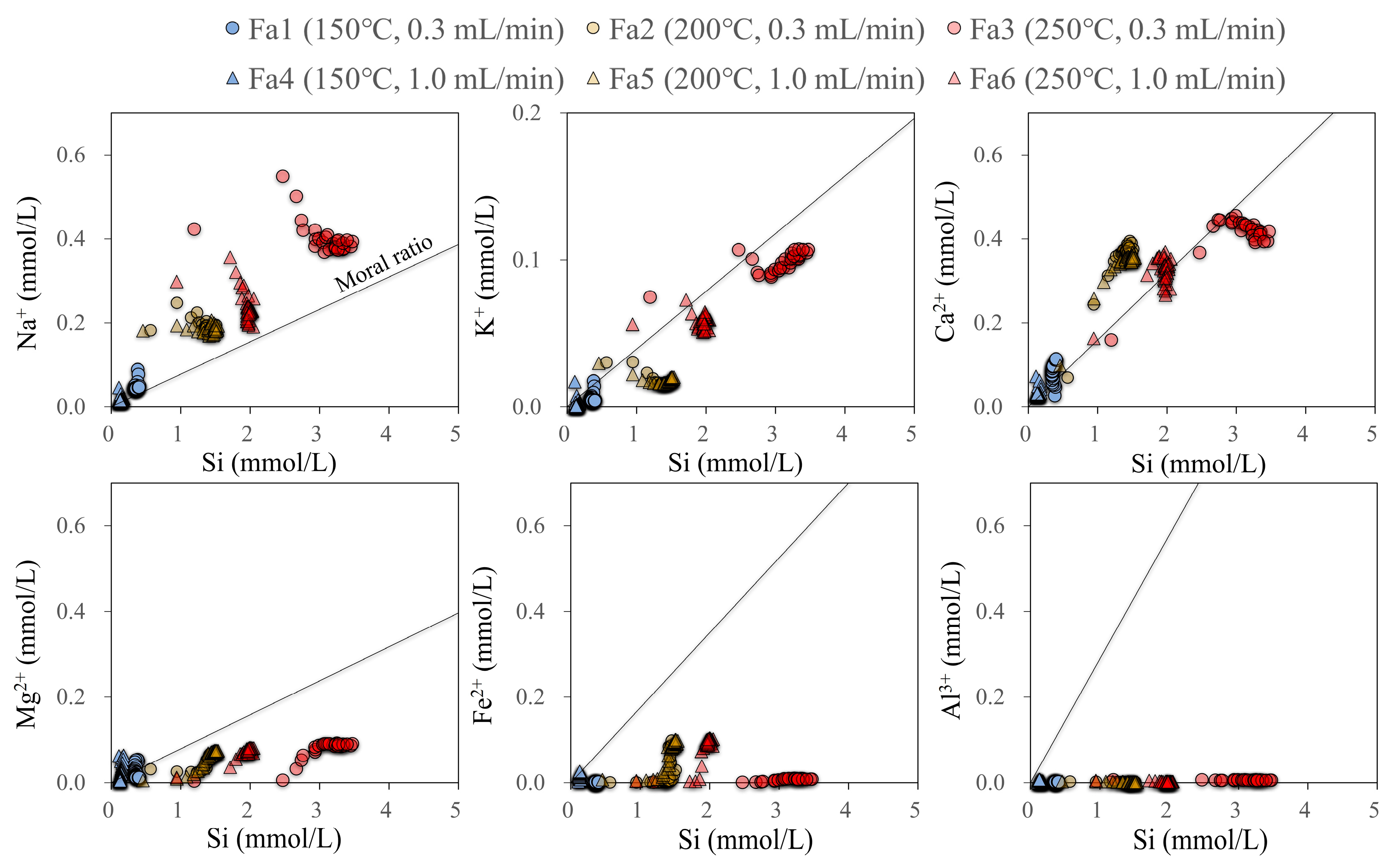
3.1. Dissolved Chemical Components
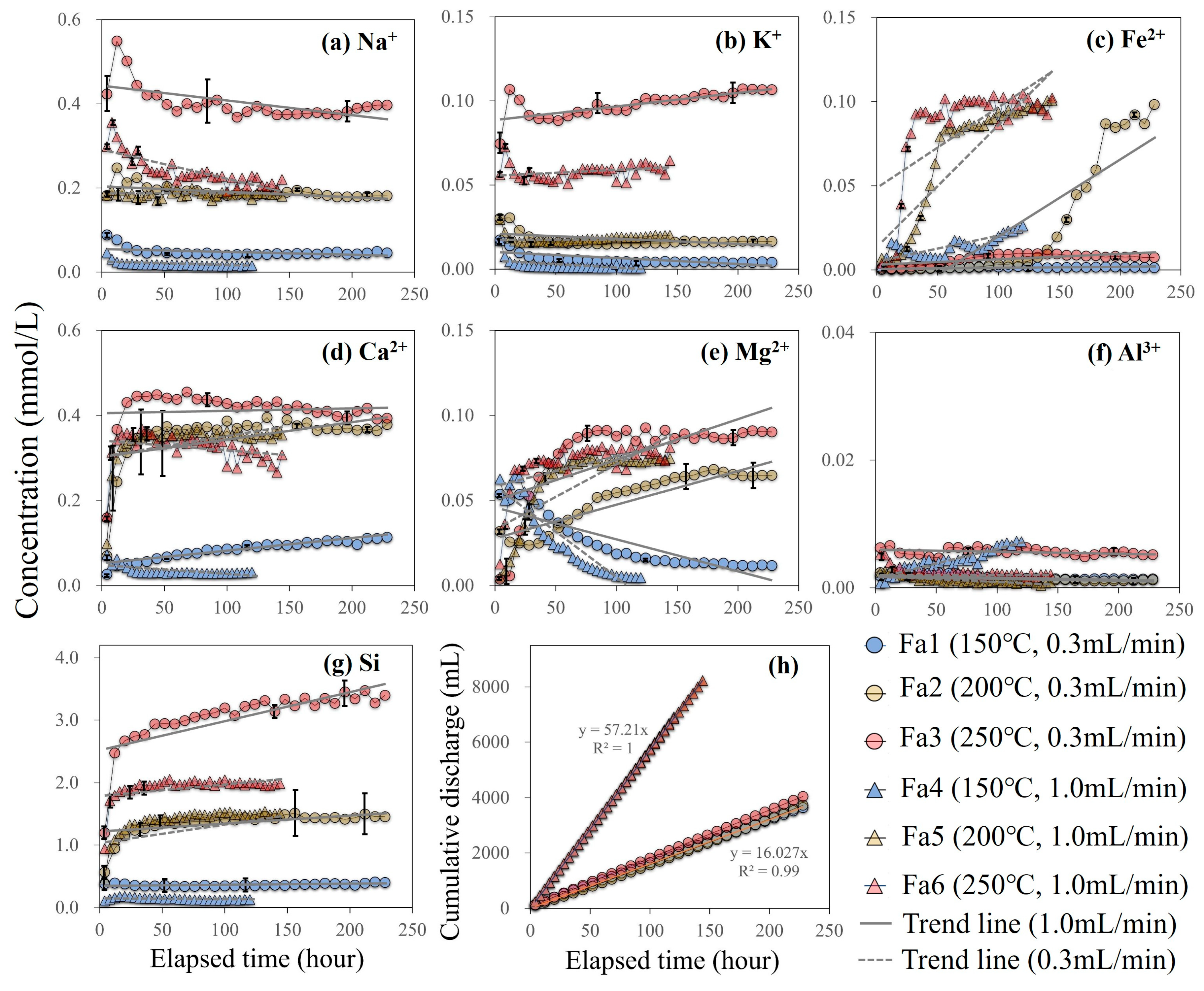
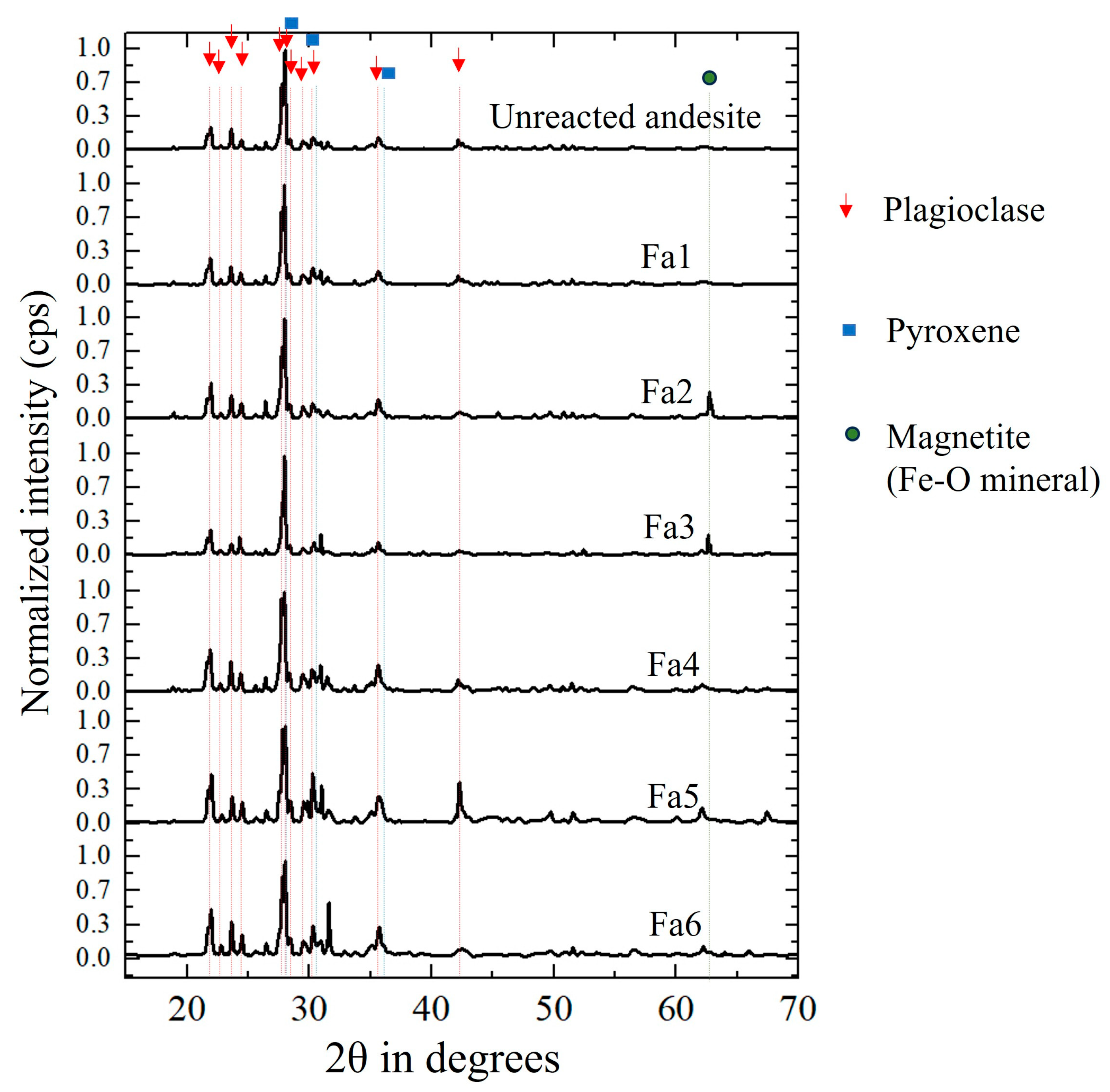
3.2. Rock Properties
3.2.1. Reacted Rock Samples and XRD
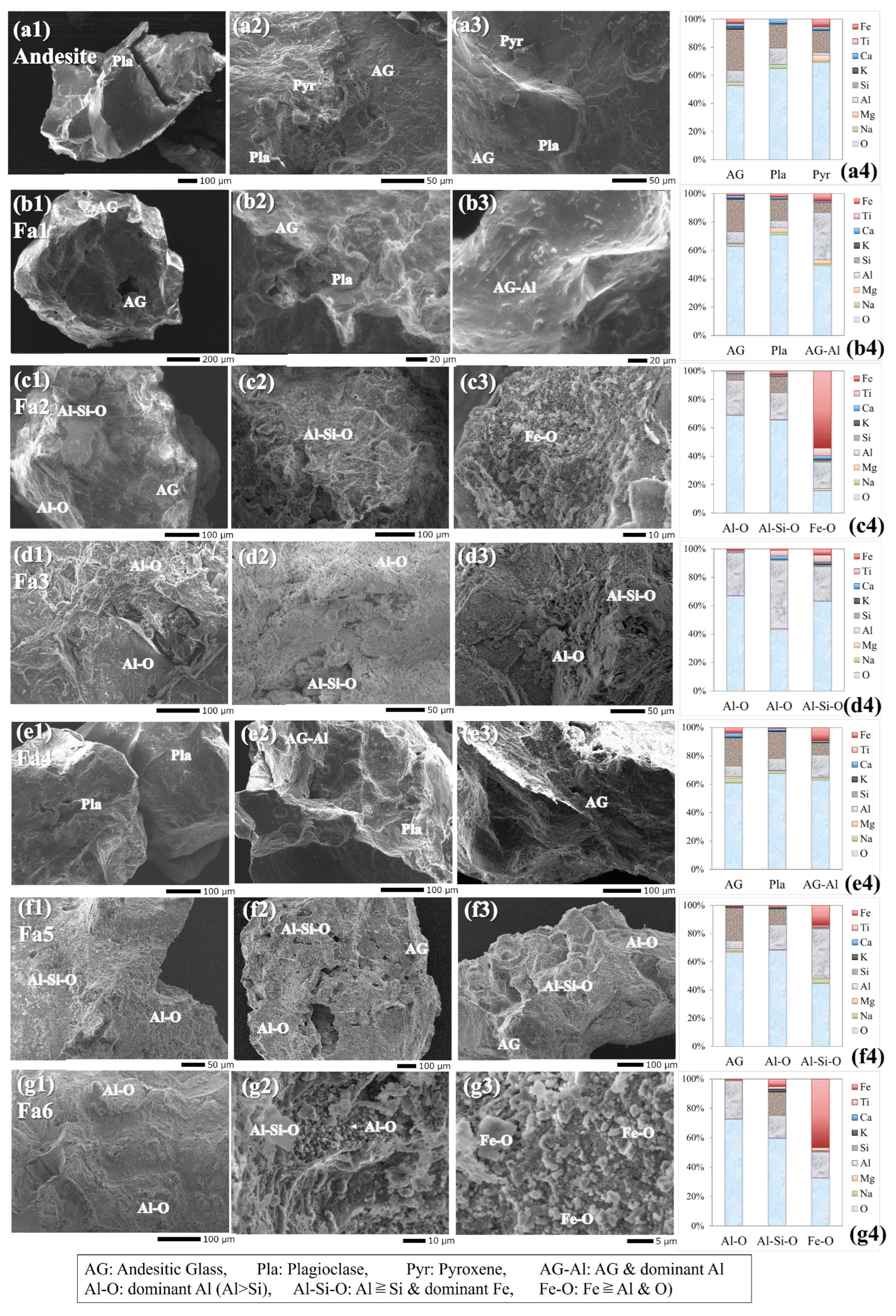
3.2.2. SEM-EDS
4. Discussion
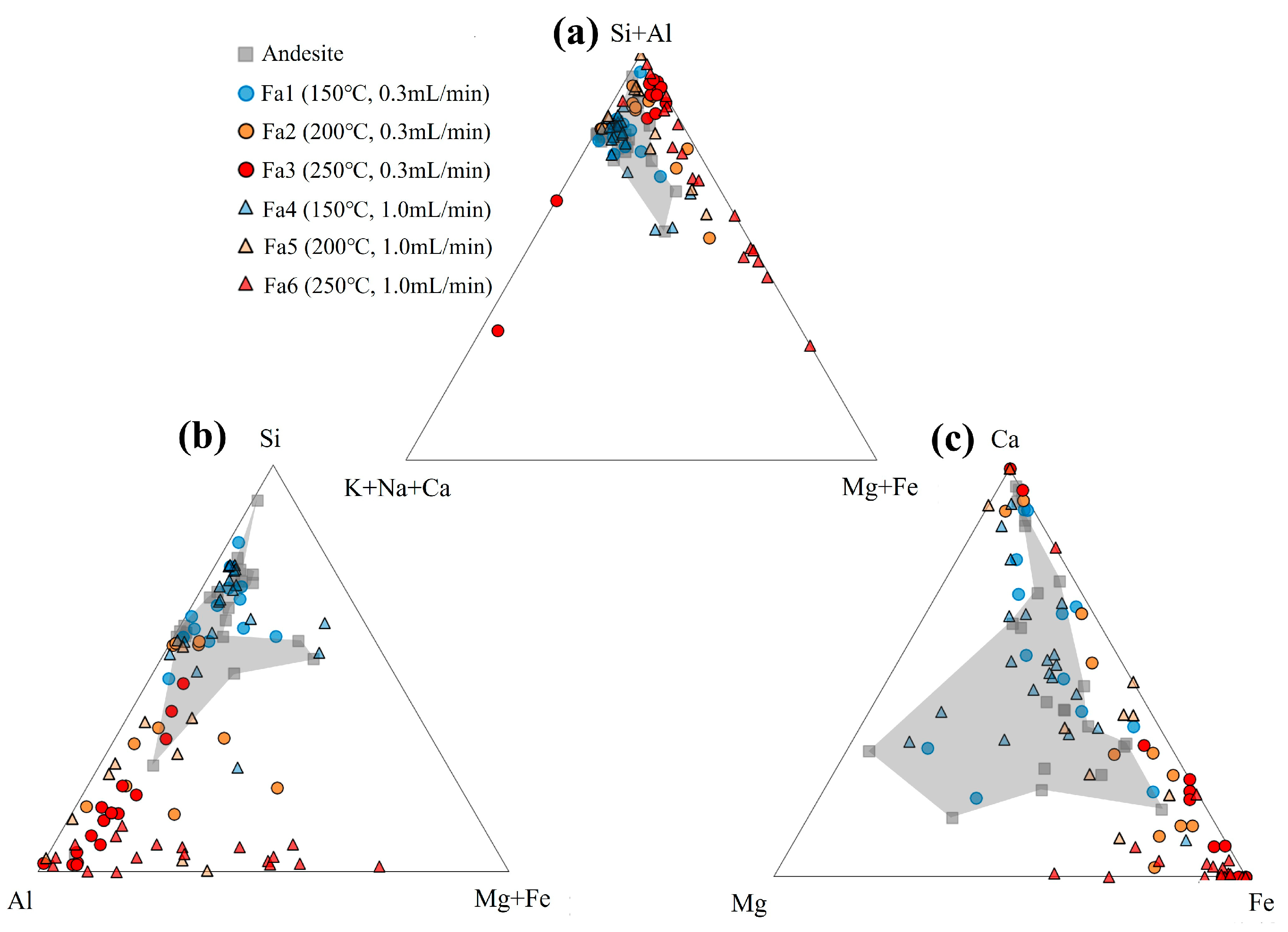
4.1. Dissolution of Andesite
4.2. Precipitation of Minerals
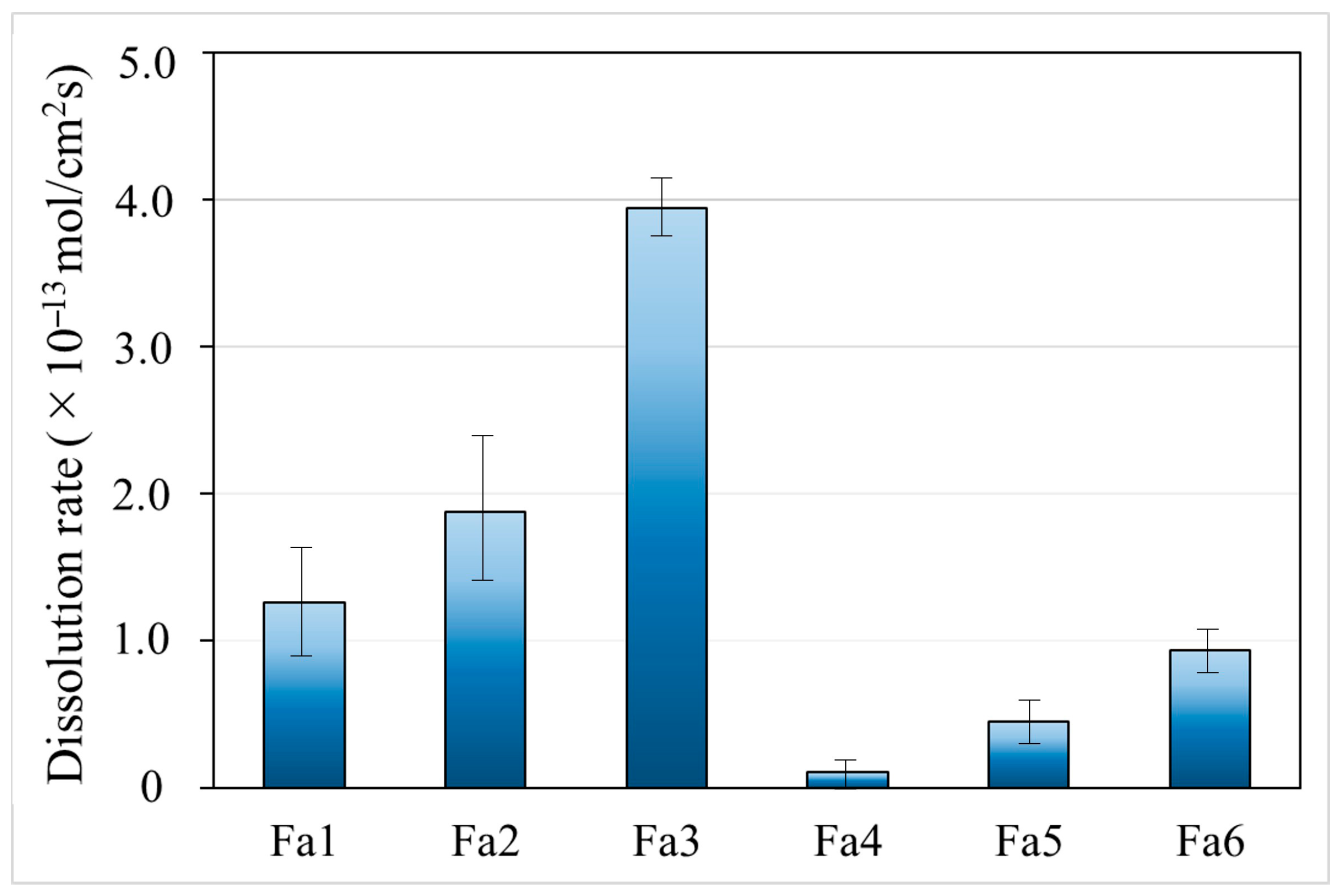
4.3. Difference of Flow Rate Using Si Dissolution Rates
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adams, H.D.; Barron-Gafford, G.A.; Minor, R.L.; Gardea, A.A.; Bentley, L.P.; Law, D.J.; Breshears, D.D.; McDowell, N.G.; Huxman, T.E. Temperature response surfaces for mortality risk of tree species with future drought. Environ. Res. Lett. 2017, 12, 115014. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, L.; Qiu, X.; Liu, B.; Chang, X.; Liu, L.; Zhang, X.; Cao, W.; Zhu, Y. Impacts of 1.5 and 2.0 degrees C global warming on rice production across China. Agric. For. Meteorol. 2020, 284, 107900. [Google Scholar] [CrossRef]
- Wu, Y.; Li, P. The potential of coupled carbon storage and geothermal extraction in a CO2-enhanced geothermal system: A review. Geotherm. Energy 2020, 8, 19. [Google Scholar] [CrossRef]
- Abas, N.; Kalair, A.; Khan, N.; Kalair, A.R. Review of GHG emissions in Pakistan compared to SAARC countries. Renew. Sustain. Energy Rev. 2017, 80, 990–1016. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Summary for policymakers. In Global Warming of 1.5 °C; Masson-Delmotte, V., Zhai, P., Portner, H.O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2018; pp. 3–24. [Google Scholar]
- Brown, D. A hot dry rock geothermal energy concept utilizing supercritical CO2 instead of water. In Proceedings of the 24th Workshop on Geothermal Reservoir Engineering, Stanford University, Standford, CA, USA, 24–26 January 2000. [Google Scholar]
- Pruess, K. Enhanced Geothermal Systems (EGS) Using CO2 as working fluid—A novel approach for generating renewable energy with simultaneous sequestration of carbon. Geothermics 2006, 35, 351–367. [Google Scholar] [CrossRef]
- Wu, Y.; Li, P.; Hao, Y.; Wanniarachchi, A.; Zhang, Y.; Peng, S. Experimental research on carbon storage in a CO2-Based enhanced geothermal system. Renew. Energy 2021, 175, 68–79. [Google Scholar] [CrossRef]
- Li, P.; Hao, Y.; Wu, Y.; Wanniarachchi, A.; Zhang, H.; Cui, Z. Experimental study on the effect of CO2 storage on the reservoir permeability in a CO2-based enhanced geothermal system. Geotherm. Energy 2023, 11, 24. [Google Scholar] [CrossRef]
- Yang, H.; Satake, S.; Kuramitz, H.; Ueda, A.; Kusakabe, M.; Hoshino, Y.; Masuoka, K.; Enomoto, H.; Terai, A. Experimental study for basalt-CO2-water interaction with a high temperature and pressure flow reactor. In Proceedings of the WR-17 Y AIG-14, Sendai, Japan, 18–22 August 2023. [Google Scholar]
- Satake, S.; Yang, H.; Mori, K.; Hoshino, Y.; Ueda, A.; Kuramitz, H.; Masuoka, K.; Enomoto, H.; Terai, A. CO2 geothermal power generation: Laboratory experiment on the interaction between carbonated water and Rishiri Island basalt in the vicinity of injection wells. Energies 2025, 18, 2251. [Google Scholar] [CrossRef]
- Muraoka, H.; Sakaguchi, K.; Komazawa, M.; Sasaki, S. Assessment of Hydrothermal Resource Potentials in Japan. In Proceedings of the Geothermal Research Society of Japan, Kanazawa, Japan, 30 October–1 November 2008. [Google Scholar]
- Xu, T. Numerical studies of fluid-rock interactions in Enhanced Geothermal Systems (EGS) with CO2 as working fluid. In Proceedings of the Thirty-Third Workshop on Geothermal Reservoir Engineering, Stanford University, Standford, CA, USA, 28–30 January 2008. [Google Scholar]
- Mitiku, A.B.; Li, D.; Bauer, S.; Beyer, C. Geochemical modelling of CO2–water–rock interactions in a potential storage formation of the North German sedimentary basin. Appl. Geochem. 2013, 36, 168–186. [Google Scholar] [CrossRef]
- Ueda, A.; Ajima, S.; Yamamoto, M. Isotopic study of carbonate minerals from the Sumikawa geothermal area and its application to water movement. J. Geotherm. Res. Soc. 2001, 23, 181–196. (In Japanese) [Google Scholar]
- Xu, T.; Feng, G.; Shi, Y. On fluid-rock chemical interaction in CO2-based geothermal systems. J. Geochem. Explor. 2014, 144, 179–193. [Google Scholar] [CrossRef]
- Utomo, G.P.; Güleç, N. Preliminary geochemical investigation of a possible CO2 injection in the Ungaran geothermal field, Indonesia: Equilibrium and kinetic modeling. Greenh. Gases Sci. Technol. 2021, 11, 3–18. [Google Scholar] [CrossRef]
- Gislason, S.R.; Wolff-Boenisch, D.; Stefansson, A.; Oelkers, E.H.; Gunnlaugsson, E.; Sigurdardottir, H.; Sigfusson, B.; Broecker, W.S.; Matter, J.M.; Stute, M.; et al. Mineral sequestration of carbon dioxide in basalt: A preinjection overview of the CarbFix project. Int. J. Greenh. Gas Control 2010, 4, 537545. [Google Scholar] [CrossRef]
- Gislason, S.R.; Oelkers, E.H. Carbon storage in basalt. Science 2014, 344, 373374. [Google Scholar] [CrossRef]
- Gysi, A.P.; Stefánsson, A. CO2-water-basalt interaction. Numerical simulation of low temperature CO2 sequestration into basalts. Geochim. Cosmochim. Acta 2011, 75, 4728–4751. [Google Scholar] [CrossRef]
- Gysi, A.P.; Stefansson, A. Mineralogical aspects of CO2 sequestration during hydrothermal basalt alteration—An experimental study at 75 to 250 °C and elevated pCO2. Chem. Geol. 2012, 306, 146–159. [Google Scholar] [CrossRef]
- Gysi, A.P.; Stefansson, A. Experiments and geochemical modeling of CO2 sequestration during hydrothermal basalt alteration. Chem. Geol. 2012, 306–307, 10–28. [Google Scholar] [CrossRef]
- Matter, J.M.; Takahashi, T.; Goldberg, D. Experimental evaluation of in situ CO2 water rock reactions during CO2 injection in basaltic rocks: Implications for geological CO2 sequestration. Geochem. Geophys. Geosyst. 2007, 8, Q02001. [Google Scholar] [CrossRef]
- McGrail, B.P.; Schaef, H.T.; Spane, F.A.; Cliff, J.B.; Qafoku, O.; Horner, J.A.; Thompson, C.J.; Owen, A.T.; Sullivan, C.E. Field validation of supercritical CO2 reactivity with basalts. Environ. Sci. Technol. Lett. 2017, 4, 6–10. [Google Scholar] [CrossRef]
- Belshaw, G.E.; Steer, E.; Ji, Y.; Azis, H.; Sapiie, B.; Muljadi, B.; Vandeginste, V. Fluid-rock interaction experiments with andesite at 100 °C for potential carbon storage in geothermal reservoirs. Deep Undergr. Sci. Eng. 2024, 3, 369–382. [Google Scholar] [CrossRef]
- Takeya, M.; Matsui, R.; Kobayashi, Y. Evaluating the potential for CO2 mineralization in andesite: Experiments and geochemical modeling. In Proceedings of the 17th International Conference on Greenhouse Gas Control Technologies, Calgary, AB, Canada, 20–24 October 2024. [Google Scholar]
- Delerce, S.; Bénézeth, P.; Schott, J.; Oelkers, E.H. The dissolution rates of naturally altered basalts at pH 3 and 120 °C: Implications for the in-situ mineralization of CO2 injected into the subsurface. Chem. Geol. 2023, 621, 121353. [Google Scholar] [CrossRef]
- Sato, J.; Nakamura, T.; Sugawara, S.; Takahashi, H.; Sato, K. Elemental composition of the 1783 eruption products from Asama volcano. Volcanol. Soc. Jpn. 1989, 1, 19–39. [Google Scholar]
- Nomura, M.; Kakubuchi, S.; Iijima, S.; Kanazawa, Y. The volcanic rocks of 5 active volcanos in Gunma prefecture, Japan–Disciptions under the polarizing microscope and chemical composition. Bull. Gunma Mus. Nat. Hist. 2005, 9, 109–119. [Google Scholar]
- Kumagai, N.; Tanaka, T.; Kitao, K. Characterization of geothermal fluid flows at Sumikawa geothermal area, Japan, using two types of tracers and an improved multi-path model. Geothermics 2004, 33, 257–275. [Google Scholar] [CrossRef]
- Hossain, S.; Hosono, T.; Yang, H.; Shimada, J. Geochemical processes controlling fluoride enrichment in groundwater at the western part of Kumamoto area, Japan. Water Air Soil Pollut. 2016, 227, 385. [Google Scholar] [CrossRef]
- Morita, M.; Yamaguchi, A.; Koyama, S.; Motoda, S. Method for imitating magnesium silicate scale formed at the geothermal power plant in Obama Hot Spring, Japan. Geothermics 2021, 9, 102203. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C.A.J. Description of input and examples for PHREEQC version 3-A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. In US Geological Survey Techniques and Methods; Chapter A43; U.S. Geological Survey: Drive Reston, VA, USA, 2013; Volume 6, p. 497. [Google Scholar]
- Lu, P.; Zhang, G.; Apps, J.; Zhu, C. Comparison of thermodynamic data files for PHREEQC. Earth-Sci. Rev. 2022, 225, 103888. [Google Scholar] [CrossRef]
- Voigt, M.; Marieni, C.; Clark, D.E.; Gíslason, S.R.; Oelkers, E.H. Evaluation and refinement of thermodynamic databases for mineral carbonation. Energy Procedia 2018, 146, 81–91. [Google Scholar] [CrossRef]
- Suto, Y.; Liu, L.; Yamasaki, N.; Hashida, T. Initial behavior of granite in response to injection of CO2-saturated fluid. Appl. Geochem. 2007, 22, 202–218. [Google Scholar] [CrossRef]
- Hangx, S.J.T.; Spiers, C.J. Reaction of plagioclase feldspars with CO2 under hydrothermal conditions. Chem. Geol. 2009, 265, 88–98. [Google Scholar] [CrossRef]
- Browne, P.R.L. Hydrothermal alteration in active geothermal fields. Annu. Rev. Earth Planet. Sci. 1978, 6, 229–250. [Google Scholar] [CrossRef]
- Stefánsson, A.; Gíslason, S.R. Chemical weathering of basalts. SW Iceland. Effect of rock crystallinity and secondary minerals on chemical fluxes to the ocean. Am. J. Sci. 2001, 301, 513–556. [Google Scholar] [CrossRef]
- Schiffman, P.; Fridleifsson, G.O. The smectite–chlorite transition in drillhole NJ-15, Nesjavellir geothermal field, Iceland: XRD, BSE and electron microprobe investigations. J. Metamorph. Geol. 1991, 9, 679–696. [Google Scholar] [CrossRef]
- Barnhisel, R.I.; Rich, C.I. Gibbsite, bayerite, and nordstrandite formation as affected by anions, pH, and mineral surfaces. Soil Sci. Soc. Am. J. 1965, 29, 531–534. [Google Scholar] [CrossRef]
- Lasaga, A.C. Kinetic Theory in the Earth Science; Princeton University Press: Princeton, NJ, USA, 1998; pp. 220–307. [Google Scholar]
- Nakata, E.; Watanabe, M. Dissolution and precipitation between pH 3.8, CO2 dissolved-solution and anorthite rich tuffaceous sandstone by using high temperature flow-through reactor. Jpn. Assoc. Mineral. Sci. 2009, 38, 161–174. (In Japanese) [Google Scholar]
- Carroll, S.A.; Knauss, K.G. Dependence of labradorite dissolution kinetics on CO2(aq), Al(aq), and temperature. Chem. Geol. 2005, 217, 213–225. [Google Scholar] [CrossRef]
- Gislason, S.R.; Oelkers, E.H. Mechanism, rates, and consequences of basaltic glass dissolution: II. An experimental study of the dissolution rates of basaltic glass as a function of pH and temperature. Geochim. Cosmochim. Acta 2003, 67, 3817–3832. [Google Scholar] [CrossRef]
- Luhmann, A.J.; Tutolo, B.M.; Bagley, B.C.; Mildner, D.F.R.; Seyfried, W.E., Jr.; Saar, M.O. Permeability, porosity, and mineral surface area changes in basalt cores induced by reactive transport of CO2-rich brine. Water Resour. Res. 2017, 53, 1908–1927. [Google Scholar] [CrossRef]



| Component | Sato et al. [28] (wt.%) | Nomura et al. [29] (wt.%) |
|---|---|---|
| SiO2 | 61.64 | 62.79 |
| Al2O3 | 16.00 | 15.74 |
| Total iron as Fe2O3 | 6.57 | 6.52 |
| CaO | 6.60 | 6.35 |
| K2O | 1.37 | 1.38 |
| Na2O | 2.91 | 3.16 |
| P2O3 | 0.18 | 0.12 |
| MgO | 3.98 | 3.67 |
| TiO2 | 0.61 | 0.68 |
| Total | 99.86 | 100.41 |
| CO2 Pressure | Temperature | Back Pressure | Flow Rate | Running Time | Total Discharge | Darcy Velocity | Particle Size | Surface Area | |
|---|---|---|---|---|---|---|---|---|---|
| MPa | °C | MPa | mL/min | hour | mL | m/s | mm | cm2 | |
| Fa1 | 2 | 150 | 10 | 0.3 | 228 | 3640 | 2.8 × 10−5 | 0.14 1.0 | 5565 770 |
| Fa2 | 2 | 200 | 10 | 0.3 | 228 | 3720 | 2.8 × 10−5 | 0.14 1.0 | 5565 770 |
| Fa3 | 2 | 250 | 10 | 0.3 | 228 | 4040 | 2.8 × 10−5 | 0.14 1.0 | 5565 770 |
| Fa4 | 2 | 150 | 10 | 1 | 120 | 6920 | 9.4 × 10−5 | 0.14 1.0 | 5565 770 |
| Fa5 | 2 | 200 | 10 | 1 | 144 | 8230 | 9.4 × 10−5 | 0.14 1.0 | 5565 770 |
| Fa6 | 2 | 250 | 10 | 1 | 144 | 8230 | 9.4 × 10−5 | 0.14 1.0 | 5565 770 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Ueda, A.; Kuramitz, H.; Satake, S.; Masuoka, K.; Terai, A. Andesite and CO2-Saturated Water Interaction at Different Temperatures and Flow Rates Using a Flow-Through Reactor. Geosciences 2025, 15, 351. https://doi.org/10.3390/geosciences15090351
Yang H, Ueda A, Kuramitz H, Satake S, Masuoka K, Terai A. Andesite and CO2-Saturated Water Interaction at Different Temperatures and Flow Rates Using a Flow-Through Reactor. Geosciences. 2025; 15(9):351. https://doi.org/10.3390/geosciences15090351
Chicago/Turabian StyleYang, Heejun, Akira Ueda, Hideki Kuramitz, Sakurako Satake, Kentaro Masuoka, and Amane Terai. 2025. "Andesite and CO2-Saturated Water Interaction at Different Temperatures and Flow Rates Using a Flow-Through Reactor" Geosciences 15, no. 9: 351. https://doi.org/10.3390/geosciences15090351
APA StyleYang, H., Ueda, A., Kuramitz, H., Satake, S., Masuoka, K., & Terai, A. (2025). Andesite and CO2-Saturated Water Interaction at Different Temperatures and Flow Rates Using a Flow-Through Reactor. Geosciences, 15(9), 351. https://doi.org/10.3390/geosciences15090351





