Geochemical Characteristics of the Hida Granitoids in the Unazuki and Katakaigawa Areas, Central Japan
Abstract
1. Introduction
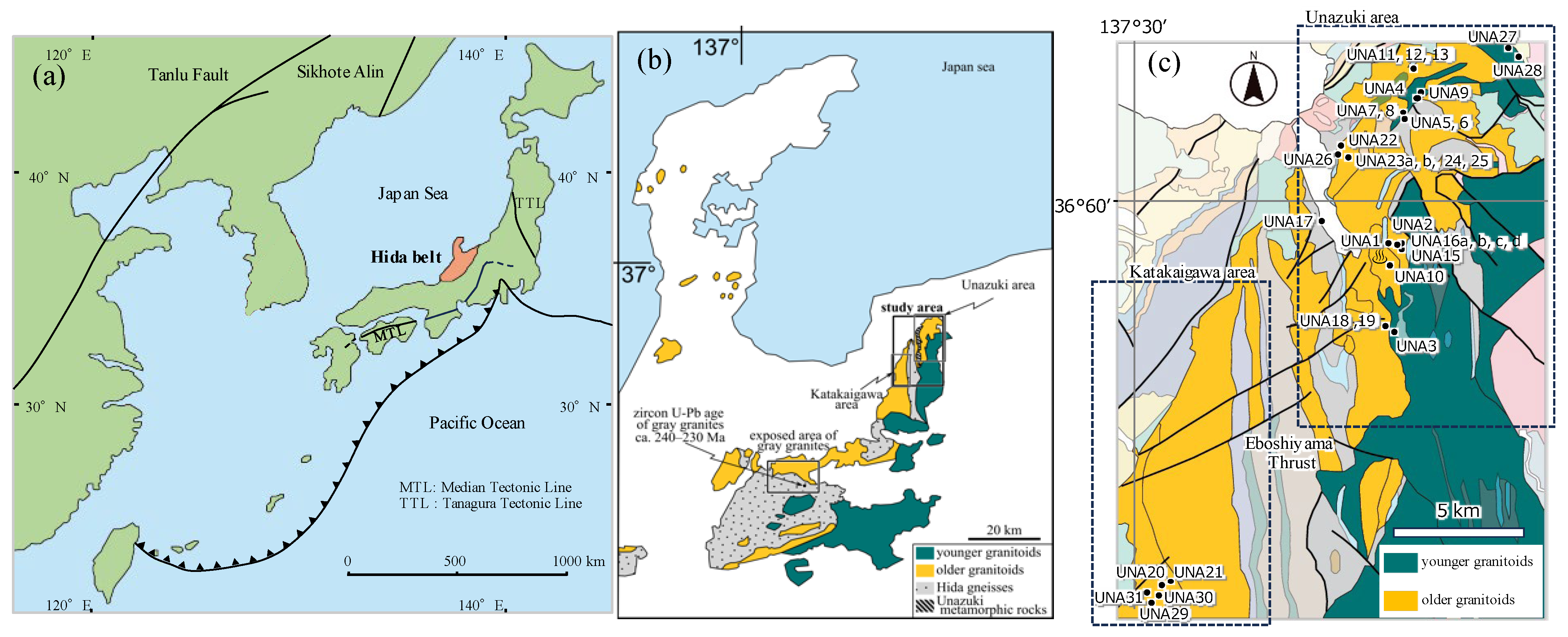
2. Geological Background
2.1. Basement Rocks
2.2. Hida Granitoids
3. Analytical Methods
3.1. Whole-Rock Chemical Analysis
3.2. Rb-Sr Isotope Analysis
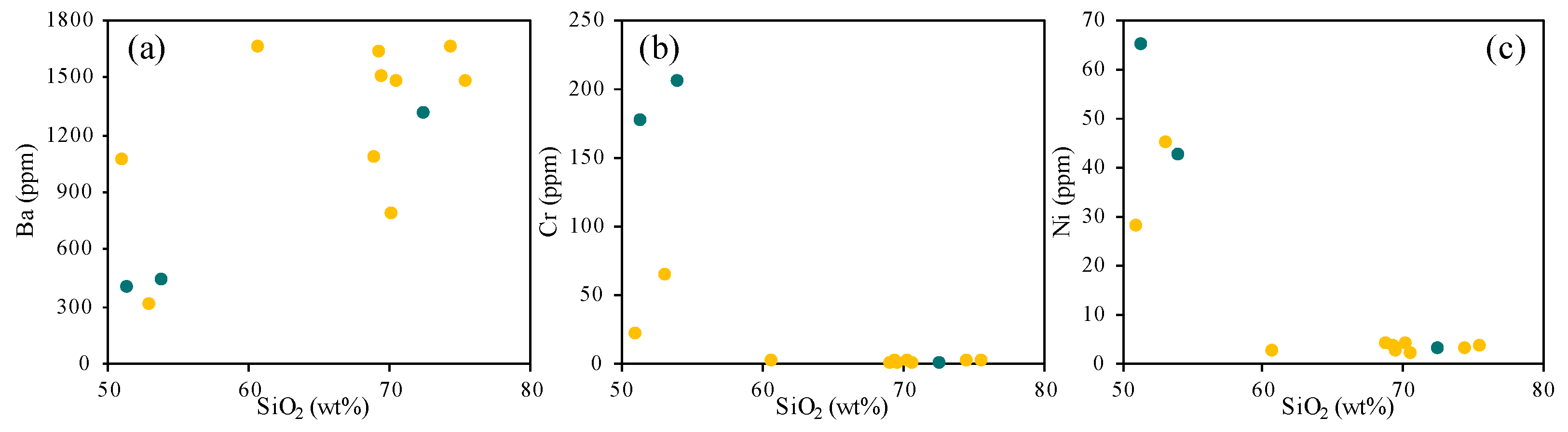
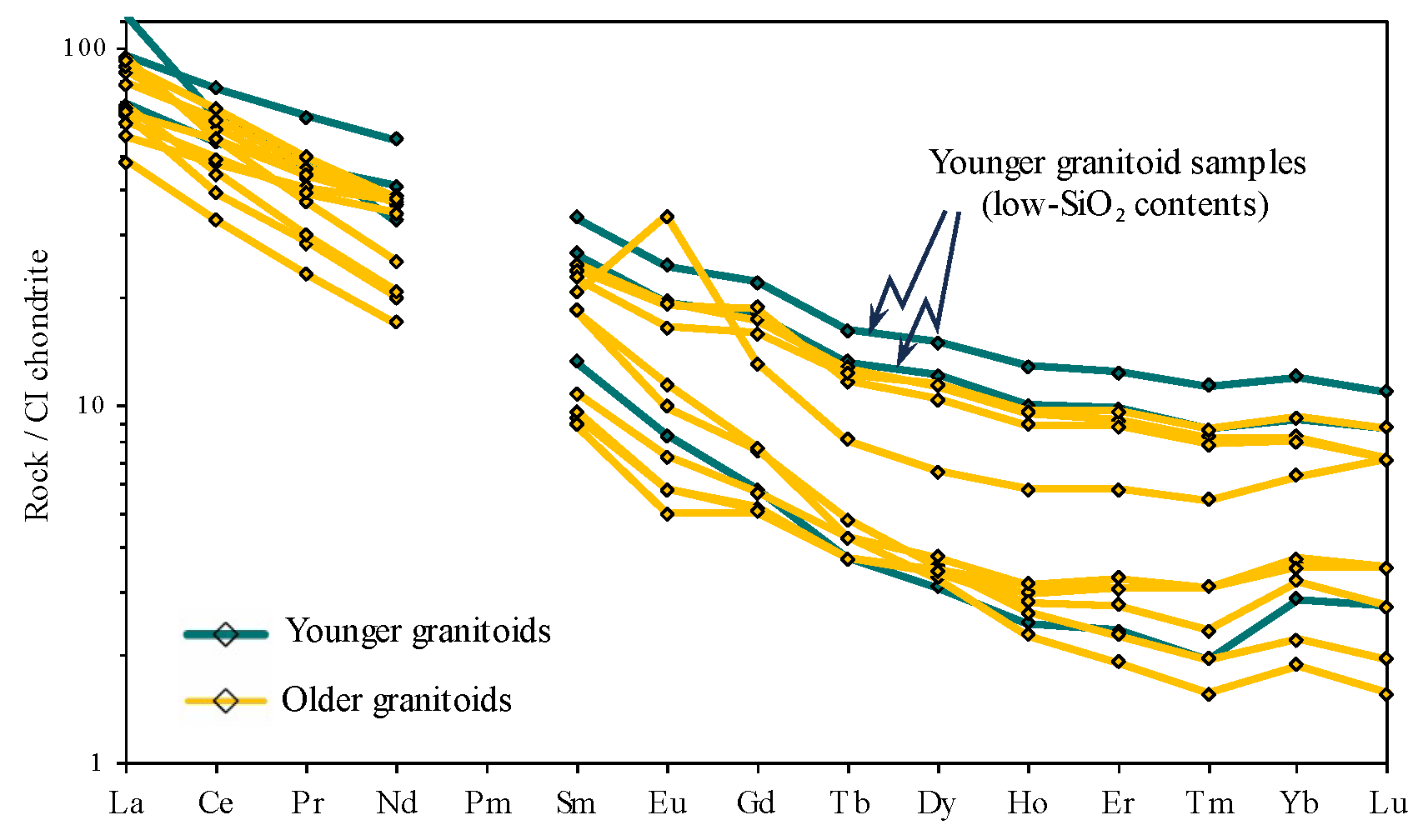
4. Results
4.1. Whole-Rock Chemical Analysis
| Rock Suite | Hida Younger Granitoids | |||||||||||
| Igneous Bodies | KG | YQD | YHQD | |||||||||
| Sample | UNA3 | UNA5 | UNA6 | UNA9 | UNA27 | UNA2 | UNA4 | UNA7 | UNA8 | UNA18 | UNA19 | UNA28 |
| Major oxides (wt%) | ||||||||||||
| SiO2 | 72.49 | 64.13 | 75.38 | 65.61 | 74.64 | 51.44 | 53.97 | 57.07 | 58.99 | 50.51 | 50.25 | 53.21 |
| TiO2 | 0.25 | 0.62 | 0.21 | 0.65 | 0.24 | 0.97 | 0.97 | 1.07 | 1.23 | 1.26 | 1.20 | 0.64 |
| Al2O3 | 15.06 | 15.85 | 12.71 | 18.43 | 14.07 | 14.77 | 15.45 | 16.50 | 16.86 | 20.06 | 18.57 | 16.09 |
| Fe2O3 | 1.61 | 5.51 | 1.20 | 2.18 | 1.67 | 10.55 | 9.17 | 8.22 | 6.52 | 7.19 | 8.97 | 6.88 |
| MnO | 0.04 | 0.11 | 0.02 | 0.08 | 0.04 | 0.17 | 0.16 | 0.14 | 0.10 | 0.14 | 0.16 | 0.15 |
| MgO | 0.43 | 1.18 | 0.61 | 0.45 | 0.27 | 7.26 | 6.15 | 3.94 | 2.31 | 4.13 | 3.16 | 2.34 |
| CaO | 1.17 | 2.53 | 1.57 | 3.39 | 1.04 | 8.93 | 7.58 | 6.51 | 3.34 | 9.21 | 9.60 | 5.86 |
| Na2O | 4.17 | 3.90 | 3.49 | 4.12 | 2.66 | 3.05 | 3.29 | 3.81 | 4.18 | 4.12 | 3.59 | 3.60 |
| K2O | 3.95 | 2.05 | 4.14 | 2.30 | 4.72 | 1.90 | 1.65 | 1.91 | 3.40 | 1.28 | 1.91 | 2.44 |
| P2O5 | 0.08 | 0.26 | 0.05 | 0.17 | 0.05 | 0.32 | 0.29 | 0.34 | 0.54 | 0.41 | 0.34 | 0.15 |
| Total | 99.25 | 96.15 | 99.39 | 97.37 | 99.41 | 99.37 | 98.68 | 99.50 | 97.47 | 98.30 | 97.75 | 91.35 |
| Trace elements (ppm) | ||||||||||||
| V | 24.3 | 27.5 | 27.4 | 35.8 | 17.5 | 242.9 | 205.8 | 172.8 | 82.1 | 162.3 | 212.6 | 50.4 |
| Cr | 0.9 | 0.3 | 0.3 | 13.0 | 2.1 | 177.8 | 205.8 | 35.9 | 2.6 | 12.6 | 1.1 | 133.9 |
| Co | 3.1 | 17.7 | 3.1 | 5.0 | 5.2 | 57.3 | 38.4 | 34.3 | 24.9 | 25.6 | 31.3 | 28.9 |
| Ni | 3.2 | 4.1 | 10.2 | 4.4 | 4.2 | 65.3 | 42.9 | 28.5 | 0.7 | 14.6 | 2.4 | 4.1 |
| Cu | 0.8 | 32.4 | 21.6 | 8.9 | 4.2 | 87.3 | 42.0 | 42.6 | 29.4 | 36.8 | 25.4 | 12.8 |
| Zn | 39.0 | 83.8 | 12.4 | 34.1 | 38.2 | 93.8 | 62.0 | 79.5 | 77.9 | 63.7 | 78.1 | 108.8 |
| Rb | 68.4 | 72.2 | 79.4 | 83.7 | 145.9 | 53.4 | 43.7 | 50.2 | 112.3 | 37.6 | 48.7 | 103.1 |
| Sr | 460.8 | 196.9 | 192.4 | 289.9 | 147.5 | 1066.1 | 764.7 | 694.8 | 869.0 | 1158.2 | 980.9 | 380.5 |
| Y | 13.5 | 26.8 | 21.1 | 22.2 | 26.6 | 16.7 | 17.3 | 22.2 | 19.9 | 25.3 | 23.6 | 19.3 |
| Zr | 145.2 | 327.2 | 138.4 | 174.2 | 163.9 | 93.5 | 124.2 | 80.5 | 76.8 | 140.3 | 168.8 | 150.2 |
| Nb | 2.6 | 5.6 | 3.9 | 7.5 | 4.1 | 1.0 | 1.6 | 4.6 | 4.8 | 3.5 | 1.5 | 4.1 |
| Ba | 1314.7 | 664.1 | 718.4 | 593.7 | 742.5 | 409.6 | 447.2 | 468.5 | 780.6 | 358.3 | 743.5 | 533.6 |
| Pb | 16.5 | 9.6 | 9.5 | 14.2 | 29.3 | 10.0 | 5.0 | 6.7 | 9.6 | 8.7 | 15.8 | 19.9 |
| Th | 11.5 | 5.2 | 13.7 | 11.5 | 11.9 | 11.8 | 3.3 | 7.2 | 5.7 | 0.3 | 0.1 | 4.1 |
| Rare-earth elements (ppm) | ||||||||||||
| La | 29.25 | 22.46 | 16.50 | |||||||||
| Ce | 38.87 | 47.16 | 33.40 | |||||||||
| Pr | 4.71 | 6.09 | 4.41 | |||||||||
| Nd | 15.49 | 25.97 | 19.23 | |||||||||
| Sm | 2.04 | 5.16 | 4.07 | |||||||||
| Eu | 0.48 | 1.44 | 1.13 | |||||||||
| Gd | 1.20 | 4.58 | 3.72 | |||||||||
| Tb | 0.14 | 0.61 | 0.50 | |||||||||
| Dy | 0.79 | 3.82 | 3.10 | |||||||||
| Ho | 0.14 | 0.73 | 0.57 | |||||||||
| Er | 0.39 | 2.06 | 1.63 | |||||||||
| Tm | 0.05 | 0.29 | 0.22 | |||||||||
| Yb | 0.49 | 2.06 | 1.56 | |||||||||
| Lu | 0.07 | 0.28 | 0.22 | |||||||||
| ASI | 1.13 | 1.20 | 0.97 | 1.19 | 1.24 | 0.63 | 0.74 | 0.82 | 1.01 | 0.81 | 0.73 | 0.84 |
| Mg# | 18.8 | 15.6 | 30.5 | 15.1 | 12.2 | 37.2 | 36.6 | 29.2 | 23.4 | 33.1 | 23.3 | 22.7 |
| Sr/Y | 34.2 | 7.3 | 9.1 | 13.1 | 5.5 | 63.7 | 44.1 | 31.3 | 43.6 | 45.8 | 41.6 | 19.7 |
| (Eu/Eu*)N | 0.94 | 0.91 | 0.89 | |||||||||
| Rock suite | Hida Older granitoids | |||||||||||
| Igneous bodies | UG | FG | ||||||||||
| Sample | UNA11 | UNA12 | UNA13 | UNA17 | UNA22 | UNA23a | UNA26 | UNA1 | UNA10 | UNA15 | UNA16a | UNA16b |
| Major oxides (wt%) | ||||||||||||
| SiO2 | 72.01 | 74.66 | 72.70 | 74.58 | 74.48 | 75.53 | 70.62 | 64.13 | 60.72 | 70.27 | 65.74 | 65.92 |
| TiO2 | 0.24 | 0.23 | 0.24 | 0.20 | 0.22 | 0.14 | 0.35 | 0.33 | 0.80 | 0.42 | 0.26 | 0.21 |
| Al2O3 | 14.01 | 13.80 | 14.37 | 13.74 | 14.13 | 13.39 | 15.18 | 17.99 | 19.54 | 16.09 | 16.94 | 17.26 |
| Fe2O3 | 2.51 | 3.23 | 2.22 | 1.24 | 1.48 | 1.13 | 2.14 | 5.91 | 4.48 | 2.32 | 3.99 | 3.75 |
| MnO | 0.01 | 0.01 | 0.01 | 0.04 | 0.04 | 0.01 | 0.05 | 0.12 | 0.08 | 0.05 | 0.07 | 0.08 |
| MgO | 0.40 | 0.41 | 0.42 | 0.17 | 0.22 | 0.16 | 0.13 | 0.51 | 1.23 | 0.54 | 0.42 | 0.42 |
| CaO | 1.23 | 1.10 | 1.30 | 0.67 | 0.58 | 0.42 | 0.68 | 2.66 | 2.59 | 1.77 | 1.65 | 1.85 |
| Na2O | 2.52 | 2.45 | 2.72 | 3.97 | 3.73 | 3.21 | 4.71 | 5.97 | 6.18 | 5.39 | 6.11 | 6.77 |
| K2O | 4.72 | 4.72 | 4.70 | 4.25 | 4.85 | 5.16 | 4.43 | 1.81 | 3.12 | 2.44 | 2.93 | 2.43 |
| P2O5 | 0.05 | 0.05 | 0.02 | 0.05 | 0.05 | 0.02 | 0.09 | 0.07 | 0.24 | 0.15 | 0.08 | 0.07 |
| Total | 97.70 | 100.66 | 98.69 | 98.89 | 99.79 | 99.18 | 98.37 | 99.50 | 98.98 | 99.44 | 98.19 | 98.78 |
| Trace elements (ppm) | ||||||||||||
| V | 23.9 | 22.3 | 25.4 | 16.7 | 17.3 | 19.0 | 21.5 | 6.1 | 33.0 | 29.4 | 6.7 | 11.2 |
| Cr | 1.3 | 7.1 | 0.5 | 1.9 | 2.1 | 2.2 | 3.3 | 2.4 | 1.9 | 1.6 | 1.2 | 3.1 |
| Co | 6.7 | 9.5 | 6.3 | 2.9 | 3.1 | 3.1 | 4.3 | 15.7 | 10.0 | 3.9 | 10.8 | 10.8 |
| Ni | 2.8 | 2.6 | 3.6 | 4.6 | 3.3 | 3.7 | 5.2 | 2.2 | 2.8 | 4.4 | 2.9 | 3.8 |
| Cu | 10.6 | 1.3 | 6.7 | 1.1 | 0.4 | 0.4 | 1.2 | 2.3 | 4.3 | 14.7 | 8.2 | 25.9 |
| Zn | 38.6 | 35.4 | 35.5 | 41.8 | 48.9 | 53.3 | 24.8 | 108.6 | 47.8 | 72.0 | 43.5 | 40.7 |
| Rb | 146.1 | 150.5 | 144.8 | 95.3 | 68.5 | 56.4 | 161.6 | 52.6 | 44.5 | 42.9 | 93.4 | 90.6 |
| Sr | 148.6 | 140.1 | 162.1 | 189.1 | 669.0 | 663.7 | 172.2 | 407.2 | 680.3 | 700.4 | 385.8 | 305.1 |
| Y | 29.6 | 25.1 | 28.4 | 14.0 | 14.7 | 14.7 | 26.3 | 19.7 | 15.3 | 12.1 | 23.7 | 21.8 |
| Zr | 166.8 | 161.0 | 161.6 | 120.3 | 175.1 | 203.2 | 152.9 | 479.7 | 619.9 | 174.5 | 418.9 | 310.5 |
| Nb | 1.9 | 3.0 | 3.3 | 1.6 | 0.9 | 0.2 | 4.5 | 2.6 | 0.7 | 0.4 | 1.6 | 0.3 |
| Ba | 875.9 | 798.1 | 805.2 | 582.2 | 1668.6 | 1484.5 | 693.4 | 694.8 | 1657.8 | 784.6 | 806.0 | 613.7 |
| Pb | 24.1 | 19.2 | 17.7 | 25.4 | 19.1 | 15.8 | 18.6 | 13.1 | 11.5 | 26.2 | 11.5 | 11.7 |
| Th | 9.8 | 10.4 | 11.4 | 13.5 | 3.2 | 3.0 | 21.4 | 35.5 | 1.6 | 2.8 | 29.8 | 23.8 |
| Rare-earth elements (ppm) | ||||||||||||
| La | 20.28 | 18.90 | 21.21 | |||||||||
| Ce | 36.36 | 38.76 | 41.36 | |||||||||
| Pr | 4.16 | 4.39 | 4.69 | |||||||||
| Nd | 17.49 | 17.21 | 18.01 | |||||||||
| Sm | 3.19 | 2.84 | 2.83 | |||||||||
| Eu | 1.97 | 0.58 | 0.67 | |||||||||
| Gd | 2.70 | 1.55 | 1.58 | |||||||||
| Tb | 0.30 | 0.18 | 0.16 | |||||||||
| Dy | 1.67 | 0.91 | 0.84 | |||||||||
| Ho | 0.33 | 0.15 | 0.13 | |||||||||
| Er | 0.97 | 0.38 | 0.32 | |||||||||
| Tm | 0.14 | 0.05 | 0.04 | |||||||||
| Yb | 1.09 | 0.38 | 0.32 | |||||||||
| Lu | 0.18 | 0.05 | 0.04 | |||||||||
| ASI | 1.22 | 1.24 | 1.21 | 1.11 | 1.13 | 1.15 | 1.10 | 1.08 | 1.07 | 1.09 | 1.04 | 1.00 |
| Mg# | 12.2 | 9.9 | 14.2 | 10.6 | 11.5 | 11.2 | 4.9 | 6.9 | 19.2 | 16.8 | 8.4 | 8.8 |
| Sr/Y | 5.0 | 5.6 | 5.7 | 13.5 | 45.7 | 45.3 | 6.5 | 20.7 | 44.4 | 57.7 | 16.3 | 14.0 |
| (Eu/Eu*)N | 2.05 | 0.85 | 0.97 | |||||||||
| Rock suite | Hida older granitoids | |||||||||||
| Igneous bodies | FG | HG | AGM | OG | ||||||||
| Sample | UNA16c | UNA16d | UNA20 | UNA21 | UNA29 | UNA31 | UNA30 | UNA14 | UNA23b | UNA24 | UNA25 | |
| Major oxides (wt%) | ||||||||||||
| SiO2 | 68.95 | 67.30 | 70.92 | 69.56 | 69.49 | 69.36 | 70.60 | 48.70 | 49.37 | 51.00 | 53.09 | |
| TiO2 | 0.43 | 0.53 | 0.17 | 0.21 | 0.21 | 0.16 | 0.17 | 0.74 | 1.33 | 1.34 | 0.91 | |
| Al2O3 | 16.04 | 13.51 | 16.65 | 17.18 | 16.98 | 16.77 | 16.58 | 11.62 | 16.54 | 18.33 | 15.84 | |
| Fe2O3 | 2.18 | 4.46 | 1.63 | 1.61 | 1.71 | 1.28 | 1.51 | 10.21 | 10.79 | 10.31 | 7.89 | |
| MnO | 0.04 | 0.18 | 0.04 | 0.03 | 0.04 | 0.03 | 0.04 | 0.20 | 0.16 | 0.19 | 0.17 | |
| MgO | 0.57 | 2.34 | 0.29 | 0.26 | 0.15 | 0.14 | 0.29 | 12.27 | 6.21 | 4.67 | 5.71 | |
| CaO | 2.17 | 4.39 | 1.52 | 1.68 | 1.92 | 1.50 | 0.46 | 12.56 | 8.20 | 5.13 | 7.90 | |
| Na2O | 5.09 | 3.74 | 5.27 | 5.69 | 5.42 | 5.38 | 5.55 | 1.19 | 3.70 | 4.18 | 3.42 | |
| K2O | 3.23 | 3.58 | 3.59 | 3.20 | 3.26 | 3.42 | 3.77 | 0.82 | 1.27 | 1.84 | 2.37 | |
| P2O5 | 0.15 | 0.15 | 0.03 | 0.04 | 0.04 | 0.03 | 0.03 | 0.12 | 0.37 | 0.37 | 0.27 | |
| Total | 98.85 | 100.18 | 100.12 | 99.48 | 99.23 | 98.07 | 99.01 | 98.44 | 97.95 | 97.36 | 97.58 | |
| Trace elements (ppm) | ||||||||||||
| V | 32.3 | 73.7 | 14.3 | 20.5 | 15.1 | 16.8 | 16.4 | 197.6 | 252.2 | 171.4 | 168.7 | |
| Cr | 0.6 | 4.6 | 5.3 | 2.9 | 1.1 | 1.7 | 1.0 | 674.6 | 138.4 | 22.0 | 64.2 | |
| Co | 4.4 | 14.1 | 4.2 | 4.8 | 3.3 | 3.1 | 2.6 | 47.3 | 59.6 | 51.8 | 39.2 | |
| Ni | 4.0 | 3.5 | 4.6 | 4.0 | 2.5 | 3.6 | 2.4 | 123.2 | 50.8 | 28.4 | 45.2 | |
| Cu | 3.6 | 7.0 | 0.1 | 1.8 | 0.9 | 1.5 | 1.5 | 32.9 | 23.3 | 46.3 | 43.3 | |
| Zn | 89.9 | 98.6 | 8.2 | 23.9 | 47.9 | 46.4 | 36.4 | 118.1 | 94.5 | 141.7 | 90.4 | |
| Rb | 49.7 | 94.3 | 178.4 | 96.3 | 48.5 | 84.3 | 60.1 | 20.9 | 41.2 | 55.4 | 87.7 | |
| Sr | 1084.6 | 340.5 | 120.5 | 224.3 | 709.5 | 681.8 | 564.1 | 430.3 | 740.5 | 793.7 | 734.7 | |
| Y | 11.4 | 22.4 | 23.1 | 28.8 | 13.9 | 14.5 | 14.0 | 14.8 | 18.3 | 17.5 | 18.1 | |
| Zr | 183.5 | 207.7 | 113.0 | 296.1 | 195.1 | 152.4 | 171.2 | 55.9 | 98.9 | 81.2 | 128.7 | |
| Nb | 0.7 | 3.3 | 3.3 | 6.3 | 0.9 | 1.6 | 0.3 | 0.5 | 0.8 | 3.4 | 1.3 | |
| Ba | 1088.2 | 879.7 | 590.0 | 835.9 | 1503.2 | 1636.3 | 1481.8 | 177.1 | 324.3 | 1073.9 | 312.7 | |
| Pb | 35.4 | 13.8 | 16.6 | 15.8 | 16.5 | 20.5 | 16.7 | 12.4 | 6.5 | 11.7 | 11.1 | |
| Th | 3.9 | 7.3 | 27.4 | 15.8 | 0.8 | 0.1 | 1.5 | 4.8 | 0.8 | 4.1 | 2.6 | |
| Rare-earth elements (ppm) | ||||||||||||
| La | 15.35 | 21.94 | 13.43 | 16.17 | 11.45 | 13.43 | 15.73 | 14.69 | ||||
| Ce | 24.07 | 33.94 | 29.18 | 27.34 | 20.14 | 29.18 | 34.11 | 29.96 | ||||
| Pr | 2.71 | 3.52 | 3.89 | 2.85 | 2.22 | 3.89 | 4.19 | 3.72 | ||||
| Nd | 9.44 | 11.83 | 17.46 | 9.75 | 8.02 | 17.46 | 17.61 | 16.20 | ||||
| Sm | 1.43 | 1.66 | 3.82 | 1.48 | 1.36 | 3.82 | 3.68 | 3.51 | ||||
| Eu | 0.34 | 0.42 | 1.14 | 0.34 | 0.29 | 1.14 | 1.11 | 0.96 | ||||
| Gd | 1.07 | 1.18 | 3.57 | 1.05 | 1.04 | 3.57 | 3.86 | 3.28 | ||||
| Tb | 0.14 | 0.16 | 0.48 | 0.14 | 0.14 | 0.48 | 0.44 | 0.46 | ||||
| Dy | 0.89 | 0.96 | 2.90 | 0.88 | 0.87 | 2.90 | 2.66 | 2.93 | ||||
| Ho | 0.17 | 0.18 | 0.54 | 0.18 | 0.16 | 0.54 | 0.50 | 0.55 | ||||
| Er | 0.51 | 0.55 | 1.51 | 0.51 | 0.46 | 1.51 | 1.45 | 1.60 | ||||
| Tm | 0.08 | 0.08 | 0.21 | 0.08 | 0.06 | 0.21 | 0.20 | 0.22 | ||||
| Yb | 0.62 | 0.64 | 1.39 | 0.60 | 0.55 | 1.39 | 1.35 | 1.58 | ||||
| Lu | 0.09 | 0.09 | 0.18 | 0.09 | 0.07 | 0.18 | 0.18 | 0.22 | ||||
| ASI | 1.01 | 0.75 | 1.08 | 1.08 | 1.06 | 1.10 | 1.18 | 0.45 | 0.74 | 1.01 | 0.70 | |
| Mg# | 18.4 | 31.1 | 13.3 | 12.3 | 7.2 | 8.5 | 14.2 | 50.9 | 33.2 | 28.1 | 38.4 | |
| Sr/Y | 95.0 | 15.2 | 5.2 | 7.8 | 50.9 | 47.1 | 40.4 | 29.0 | 40.5 | 45.3 | 40.6 | |
| (Eu/Eu*)N | 0.84 | 0.92 | 0.94 | 0.83 | 0.75 | 0.94 | 0.90 | 0.86 | ||||
| Area | Igneous Suite | Igneous Bodies | Sample | 87Rb/86Sr | 87Sr/86Sr | SrI |
|---|---|---|---|---|---|---|
| Unazuki | Hida younger granitoids | YQD | UNA2 | 0.1449 | 0.70485 | 0.70446 |
| YHQD | UNA4 | 0.1651 | 0.70493 | 0.70447 | ||
| Hida older granitoids | FG | UNA10 | 0.1892 | 0.70511 | 0.70447 | |
| UNA15 | 0.1770 | 0.70528 | 0.70468 | |||
| UNA16c | 0.1325 | 0.70516 | 0.70471 | |||
| OG | UNA24 | 0.2018 | 0.70540 | 0.70469 | ||
| UNA25 | 0.3451 | 0.70551 | 0.70455 | |||
| Katakaigawa | Hida older granitoids | HG | UNA20 | 0.2960 | 0.70740 | 0.70645 |
| UNA21 | 0.2457 | 0.70717 | 0.70639 | |||
| UNA29 | 0.1979 | 0.70700 | 0.70637 | |||
| AGM | UNA30 | 0.3575 | 0.70758 | 0.70633 |

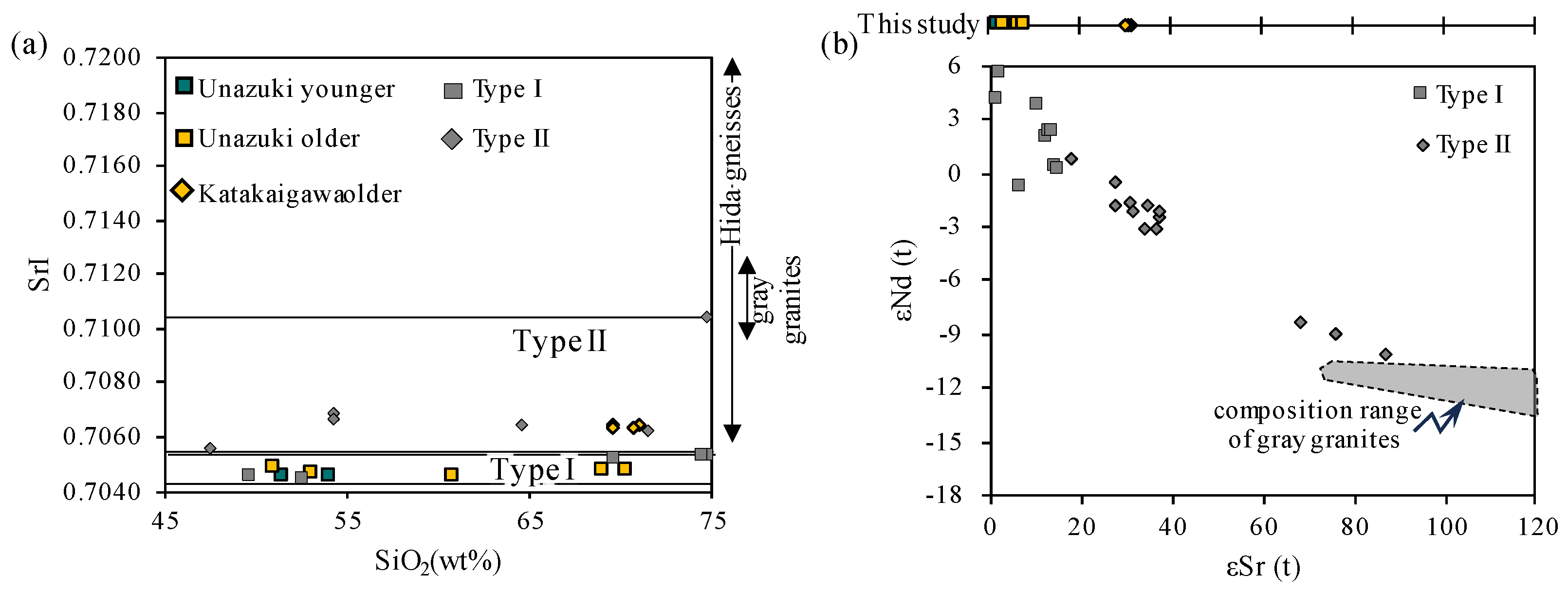
4.2. Rb-Sr Isotope Analysis
5. Discussion
6. Conclusions
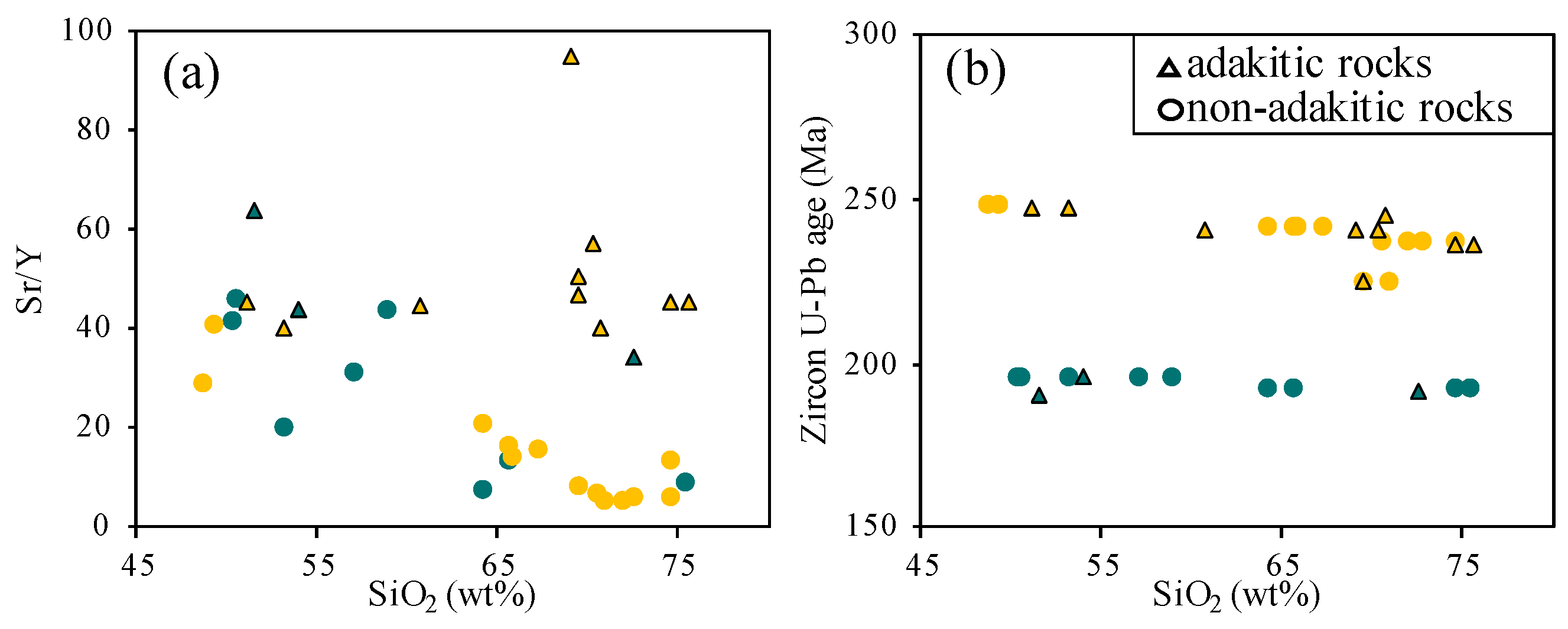
- This study revealed the geochemical characteristics of the Hida granitoids in the Unazuki and Katakaigawa areas. Trace element data identified 13 adakitic rocks among 35 samples. These were found in both older and younger granitoids, suggesting adakitic features are not time-restricted.
- SrI distinguished regional differences: Katakaigawa older granitoids match Type II signatures, implying crustal assimilation, while both older and younger granitoids in Unazuki show low SrI, fitting Type I characteristics. These results suggest crustal composition, rather than age, played a key role in magma evolution.
- Adakitic rocks fall into two subtypes: high Cr–Ni types in younger granitoids indicate interaction with mantle material, while Ba-rich types in older granitoids suggest alkali-rich melt involvement. These differences imply varied magma genesis and ascent processes.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Omori, S.; Isozaki, Y. Paleozoic Japan and the Eastern Extension of the Collisional Suture between the North and South China Cratons. J. Geogr. 2011, 120, 40–51. [Google Scholar] [CrossRef]
- Horie, K.; Tsutsumi, Y.; Takehara, M.; Hidaka, H. Timing and duration of regional metamorphism in the Kagasawa and Unazuki regions, Hida metamorphic complex, southwest Japan. Chem. Geol. 2018, 484, 148–167. [Google Scholar] [CrossRef]
- Wallis, S.R.; Yamaoka, K.; Mori, H.; Ishiwatari, A.; Miyazaki, K.; Ueda, H. The basement geology of Japan from A to Z. Isl. Arc 2020, 29, e12339. [Google Scholar] [CrossRef]
- Suzuki, K.; Adachi, M. Middle Precambrian detrital monazite and zircon from the Hida gneiss on Oki-Dogo Island, Japan: Their origin and implications for the correlation of basement gneiss of Southwest Japan and Korea. Tectonophysics 1994, 235, 277–292. [Google Scholar] [CrossRef]
- Sano, Y.; Hidaka, H.; Terada, K.; Shimizu, H.; Suzuki, M. Ion microprobe U-Pb zircon geochronology of the Hida gneiss: Finding of the oldest minerals in Japan. Geochem. J. 2000, 34, 135–153. [Google Scholar] [CrossRef]
- Kunugiza, K. The Hida metamorphic belt developed near the triple junction among the Sino-Korea, Yangtze and Proto-Pacific plates. Proc. Mineral. Soc. Korea Conf. 2002, 1, 1–3. [Google Scholar]
- Tsutsumi, Y.; Yokoyama, K.; Horie, K.; Terada, K.; Hidaka, H. SHRIMP U-Pb dating of detrital zircons in paragneiss from Oki-Dogo Island, western Japan. J. Mineral. Petrol. Sci. 2006, 101, 289–298. [Google Scholar] [CrossRef]
- Horie, K.; Yamashita, M.; Hayasaka, Y.; Katoh, Y.; Tsutsumi, Y.; Katsube, A.; Hidaka, H.; Kim, H.; Cho, M. Eoarchean-Paleoproterozoic zircon inheritance in Japanese Permo-Triassic Granites (Unazuki region, Hida Metamorphic Complex): Unearthing more old crust and identifying source terranes. Precambrian Res. 2010, 183, 145–157. [Google Scholar] [CrossRef]
- Maruyama, S. Classification of Orogenic Belts: Constraints on the Reconstruction of Paleo-geotectonic Environments. J. Geogr. 2012, 121, 1090–1106. [Google Scholar] [CrossRef]
- Kano, T. Intrusive relation of the Okumayama granitic mass (Shimonomoto type) into the Iori granite mass (Funatsu type) in the Hayatsukigawa area; reexamination of the sub-division for early Mesozoic granites (Funatsu granites) in the Hida region. J. Geol. Soc. Jpn. 1990, 96, 379–388. [Google Scholar] [CrossRef]
- Kano, T. Granitic rocks in the Hida complex, central Japan. Min. Geol. 1990, 40, 397–413. [Google Scholar] [CrossRef]
- Arakawa, Y. Two contrasting types of Rb-Sr isotopes systems for the Funatsu granitic rocks in the northwestern part of the Hida belt, central Japan. J. Min. Petr. Econ. Geol. 1988, 83, 374–387. [Google Scholar] [CrossRef]
- Arakawa, Y. Two types of granitic intrusions in the Hida belt, Japan: Sr isotopic and chemical characteristics of the Mesozoic Funatsu granitic rocks. Chem. Geol. 1990, 85, 101–117. [Google Scholar] [CrossRef]
- Arakawa, Y.; Shinmura, T. Nd Sr isotopic and geochemical characteristics of two contrasting types of calc-alkaline plutons in the Hida belt, Japan. Chem. Geol. 1995, 124, 217–232. [Google Scholar] [CrossRef]
- Takahashi, Y.; Cho, D.L.; Kee, W.S. Timing of mylonitization in the Funatsu Shear Zone within Hida Belt of southwest Japan: Implications for correlation with the shear zones around the Ogcheon Belt in the Korean Peninsula. Gondwana Res. 2010, 17, 102–115. [Google Scholar] [CrossRef]
- Takahashi, Y.; Cho, D.L.; Mao, J.; Zhao, X.; Yi, K. SHRIMP U-Pb zircon ages of the Hida metamorphic and plutonic rocks, Japan: Implications for late Paleozoic to Mesozoic tectonics around the Korean Peninsula. Isl. Arc 2018, 27, e12220. [Google Scholar] [CrossRef]
- Tsuchiya, N.; Takeda, T.; Adachi, T.; Nakano, N.; Osanai, Y.; Adachi, Y. Early Cretaceous adakitic magmatism and tectonics in the Kitakami Mountains, Japan. Jpn. Mag. Mineral. Petrol. Sci. 2015, 44, 69–90. [Google Scholar] [CrossRef]
- Martin, H. Effect of steeper Archean geothermal gradient on geochemistry of subduction-zone magmas. Geology 1986, 14, 753–756. [Google Scholar] [CrossRef]
- Defant, M.J.; Drummond, M.S. Derivation of some modern arc magmas by melting of young subducted lithosphere. Nature 1990, 347, 662–665. [Google Scholar] [CrossRef]
- Rapp, R.P.; Shimizu, N.; Norman, M.D. Growth of early continental crust by partial melting of eclogite. Nature 2003, 425, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Atherton, M.P.; Petford, N. Generation of sodium-rich magmas from newly underplated basaltic crust. Nature 1993, 362, 144–146. [Google Scholar] [CrossRef]
- Uematsu, M.; Suido, K.; Kagami, H. Genesis of the tholeiitic basalt, high magnesian andesite, blonzite andesite and adakite-like andesite of the Late Oligocene Anamizu Formation, northern Noto Peninsula, Japan. Mem. Geol. Soc. Jpn. 1995, 44, 101–124. [Google Scholar]
- Macpherson, C.G.; Dreher, S.T.; Thirlwall, M.F. Adakites without slab melting: High pressure differentiation of island arc magma, Mindanao, the Philippines. Earth Planet. Sci. Lett. 2006, 243, 581–593. [Google Scholar] [CrossRef]
- Yamada, R.; Sawada, H.; Aoyama, S.; Ouchi, W.; Niki, S.; Nagata, M.; Takahashi, T.; Hirata, T. Zircon U-Pb ages and whole-rock geochemistry from the Hida Granites: Implications for the geotectonic history and the origin of Mesozoic Granites in the Hida belt, Japan. J. Mineral. Petrol. Sci. 2021, 116, 61–66. [Google Scholar] [CrossRef]
- Takeuchi, M.; Nagamori, H.; Furukawa, R.; Oikawa, T.; Sakano, Y.; Miyagawa, A. 1:200,000 geological map of Japan 1:200,000. In Toyama, 2nd ed.; Geological Survey of Japan: Tsukuba, Japan, 2023. [Google Scholar]
- Takeuchi, M.; Shibata, K.; Jia, S.; Yamamoto, K. U-Pb zircon ages of granitic rocks from Kagasawa, Hida Mountains. J. Geol. Soc. Jpn. 2019, 125, 453–459. [Google Scholar] [CrossRef]
- Hiroi, Y. Geology of the Unazuki District in the Hida Metamorphic Terrain, central Japan. J. Geol. Soc. Jpn. 1978, 84, 521–530. [Google Scholar] [CrossRef]
- Hiroi, Y. Progressive metamorphism of the Unazuki Pelitic Schists in the Hida Terrane, central Japan. Contrib. Mineral. Petrol. 1983, 82, 334–350. [Google Scholar] [CrossRef]
- Shibata, K.; Kano, T.; Asano, M. Isotopic ages of the Gray granite from the upper Kubusu River area, Hida Mountains. J. Min. Petr. Econ. Geol. 1989, 84, 243–251. [Google Scholar] [CrossRef]
- Ishihara, S. Source diversity of the older and early Mesozoic granitoids in the Hida Belt, central Japan. Bull. Geol. Surv. Jpn. 2005, 56, 117–126. [Google Scholar] [CrossRef]
- Takeuchi, M.; Jia, S.; Shimura, Y. Zircon U-Pb ages on plutonic rocks in the eastern area of 1:200,000 quadrangle, Toyama, central Japan. Bull. Geol. Surv. Jpn. 2021, 72, 41–64. [Google Scholar] [CrossRef]
- Ohta, M. The syntexite zone situated in the Eastern area of Unazuki in the Kurobe-gawa Region. J. Geol. Soc. Jpn. 1961, 67, 451–462. [Google Scholar] [CrossRef]
- Ohta, M. Geological and petrographical studies on the metamorphic and plutonic rocks in the northeastern part of the Hida metamorphic region Part 1: The Distribution and the Lithofacies of the rocks. Earth Sci. 1961, 57, 2435. [Google Scholar]
- Harayama, S.; Takahashi, Y.; Nakano, S.; Kariya, Y.; Komazawa, M. Geology of the Tateyama district. In Quadrangle Series, 1:50,000; Geological Survey of Japan: Tsukuba, Japan, 2000; pp. 1–218. [Google Scholar]
- Zhao, X.; Mao, J.; Ye, H.; Liu, K.; Takahashi, Y. New SHRIMP U-Pb zircon ages of granitic rocks in the Hida Belt, Japan: Implications for tectonic correlation with Jiamushi massif. Isl. Arc 2013, 22, 508–521. [Google Scholar] [CrossRef]
- Takeuchi, M.; Furukawa, R.; Nagamori, H.; Oikawa, T. Geology of the Tomari District. In Quadrangle Series, 1:50,000; Geological Survey of Japan: Tsukuba, Japan, 2017; pp. 1–121. [Google Scholar]
- Horie, K.; Takehara, M.; Suda, Y.; Hidaka, H. Potential Mesozoic reference zircon from the Unazuki plutonic complex: Geochronological and geochemical characterization. Isl. Arc 2013, 22, 292–305. [Google Scholar] [CrossRef]
- Nakazaki, M.; Tsuboi, M.; Kanagawa, K.; Kato, T.; Suzuki, K. Quantitative chemical analysis of rocks with X-ray fluorescence analyzer XRF-1800. Bull. Nagoya Univ. Mus. 2004, 20, 79–91. [Google Scholar]
- Koga, K.; Tsuboi, M. Petrogenesis of Granitic Rocks in the Hisakajima Island, Goto Archipelago, Southwestern Japan: A Geochemical Study. Minerals 2021, 11, 248. [Google Scholar] [CrossRef]
- Imai, N.; Terashima, S.; Itoh, S.; Ando, A. 1994 compilation values for GSJ reference samples, “Igneous rock series”. Geochem. J. 1995, 29, 91–95. [Google Scholar] [CrossRef]
- Xue, H.; Shimooka, K.; Tsuboi, M. Geochemical Study of the Osumi Granodiorite, southwestern Japan. Minerals 2024, 14, 680. [Google Scholar] [CrossRef]
- Steiger, R.; Jäger, E. Subcommision on geochronology: Convention on the use of decay constants in geo- and cosmo-chronology. Earth Planet. Sci. Lett. 1977, 36, 359–362. [Google Scholar] [CrossRef]
- Defant, M.J.; Richerson, P.M.; De Boer, J.Z.; Stewart, R.H.; Maury, R.C.; Bellon, H.; Drummond, M.S.; Feigenson, M.D.; Jackson, T.E. Dacite genesis via both slab melting and differentiation: Petrogenesis of La Yeguada volcanic complex, Panama. J. Petrol. 1991, 32, 1101–1142. [Google Scholar] [CrossRef]
- Sun, S.S.; McDonough, W.F. Chemical and isotopic systematics of oceanic basalts; implications for mantle composition and processes. Geol. Soc. Lond. Spec. Publ. 1989, 42, 313–345. [Google Scholar] [CrossRef]
- Peccerillo, A.; Taylor, S.R. Geochemistry of Eocene calc-alkaline volcanic rocks from the Kastamonu area, northern Turkey. Contrib. Mineral. Petrol. 1976, 58, 63–81. [Google Scholar] [CrossRef]
- Shand, S.J. The Eruptive Rocks; John Wiley Publishers: New York, NY, USA, 1943; p. 444. [Google Scholar]
- Takahashi, T.; Shuto, K. Genesis of adakitic andesite, high-magnesian andesite, calk-alkaline andesite and tholeiitic andesite in the Miocene Iwaine Formation, southern part of Toyama Prefecture, Japan. J. Geol. Soc. Jpn. 1999, 105, 789–809. [Google Scholar] [CrossRef]
- Arth, J.G. Behavior of trace elements during magmatic processes a summary of theoretical models and their applications. J. Res. U.S. Geol. Surv. 1976, 4, 41–47. [Google Scholar]
- Nash, W.P.; Crecraft, H.R. Partition coefficients for trace elements in silicic magmas. Geochim. Cosmochim. Acta 1985, 49, 2309–2322. [Google Scholar] [CrossRef]

| Igneous Bodies | Rock Type | Zircon U-Pb Age (Ma) | |
|---|---|---|---|
| Hida younger | Kekachidake granite (KG) | Biotite granite to quartz diorite | 192.0 ± 2.4 a |
| granitoids | Yatazodani quartz diorite (YQD) | Quartz diorite | 191.1 ± 0.3 b |
| Yatazodani hornblende-quartz diorite (YHQD) | Hornblende quartz diorite | 195.6 ± 2.0 a | |
| Hida older | Unazuki granite (UG) | Biotite granite, slightly mylonaitized | 236.5 ± 3.1 a |
| granitoids | Funakawa granite (FG) | Biotite granite, slightly mylonaitized | 240.7 ± 4.1 a |
| Hayatsukigawa granite (HG) | Biotite granite to granodiorite | 224.8 ± 1.7 a | |
| Augen granite mylonite (AGM) | Granitic mylonite | 245 ± 2 c | |
| Otodani gabbro (OG) | Hornblende gabbro, slightly mylonaitized | 247.7 ± 3.7 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oishi, K.; Kuwahara, R.; Shimooka, K.; Tsuboi, M. Geochemical Characteristics of the Hida Granitoids in the Unazuki and Katakaigawa Areas, Central Japan. Geosciences 2025, 15, 285. https://doi.org/10.3390/geosciences15080285
Oishi K, Kuwahara R, Shimooka K, Tsuboi M. Geochemical Characteristics of the Hida Granitoids in the Unazuki and Katakaigawa Areas, Central Japan. Geosciences. 2025; 15(8):285. https://doi.org/10.3390/geosciences15080285
Chicago/Turabian StyleOishi, Kazuki, Rui Kuwahara, Kazuya Shimooka, and Motohiro Tsuboi. 2025. "Geochemical Characteristics of the Hida Granitoids in the Unazuki and Katakaigawa Areas, Central Japan" Geosciences 15, no. 8: 285. https://doi.org/10.3390/geosciences15080285
APA StyleOishi, K., Kuwahara, R., Shimooka, K., & Tsuboi, M. (2025). Geochemical Characteristics of the Hida Granitoids in the Unazuki and Katakaigawa Areas, Central Japan. Geosciences, 15(8), 285. https://doi.org/10.3390/geosciences15080285







