Abstract
The geological containment of high-level radioactive waste has become widely accepted among international organizations, and it has been adopted by many countries as part of their national nuclear waste disposal plan. The multi-barrier system, including the compacted bentonite blocks or pellets serving as human-made containment or buffer media, is the key component of high-level radioactive waste disposal, which contains a waste canister that isolates the nuclear waste from a human being geosphere for one million years. The bentonite clay surrounding the nuclear waste capsule is subjected to prolonged exposure to elevated temperatures because of the continuous decay of radioactivity. Long-term heating at high temperatures could change the buffers’ microstructural characteristics and physicochemical and hydromechanical properties, which can influence their self-sealing ability. This paper offers a comprehensive overview of the current understanding of thermal effects on bentonite-based buffer systems. The thermal impact on the microstructure, Atterberg limits, and swelling pressure of bentonite clay are intensely reviewed, and the findings are summarized. This review paper highlights new insights into the design of multi-layered containment approaches for high-level radioactive waste isolation.
1. Introduction
Bentonite clay is a very fine and highly expansive clay, and it is quite popular in high-level radioactive waste sequestration due to its low permeability, high swelling pressure, high cation exchange, high radionuclide retention properties, and significant self-sealing ability. The SKB (Swedish Nuclear Fuel and Waste Management Company) method for the final high-level nuclear disposal consists of three parts: copper canisters, buffer, and bedrock. The canisters mainly hold the nuclear iron cast and are embedded in the bentonite clay in underground tunnels. All of these are finally placed in the bedrock. This kind of bentonite clay has been chosen as backfill material or engineered material by many countries (i.e., Finland, China, Spain, France, Switzerland, and Czechia) in their own country’s nuclear waste disposal design. For example, China plans to use GMZ bentonite, which is a local bentonite used in Beishan nuclear waste disposal etc. It is essential to evaluate the performance of the bentonite buffer under conditions representative of actual nuclear waste repositories. Among them, the SKB conducted a series of long-term buffer material (MX 80 bentonite) tests at the Äspö Hard Rock Laboratory (HRL). The test was performed in a 4 m-long vertical borehole and maintained 130 °C within the bottom section (2 m) of the borehole; the whole test lasted approximately 6 years.
A widely adopted layout of engineered barriers or backfill material with high-level nuclear waste disposal based on the SKB concept generally includes initially unsaturated bentonite. Bentonite, in the form of compacted blocks, usually serves as an intermediate barrier between the nuclear waste canisters and the host rocks; in the form of pellets, bentonite is used as backfill material for the sealing of the nuclear waste tunnels [1,2,3]. Once the initial unsaturated bentonite barrier materials are placed, the underground water from the surrounding host rock would ingress and cause the starting hydration. Hydration-induced swelling and swelling pressure can effectively seal structure gaps, limiting the transport of radionuclides [4]. However, the temperature caused by the high-level nuclear waste decay heat can increase up to high values (100–150 °C) and can sustain for tens or hundreds of years [5,6]. The increased temperature may evaporate the water in the near field of the canister and keep the bentonite at a very high temperature for the long term. Thus, the high-temperature effects on the bentonite sealing capacities must be well-understood and evaluated properly to ensure the safe operation of high-level nuclear waste disposal [1,2,3].
The increased temperature changes the water–clay interactions and clay microstructural properties. Interactions between water and clay minerals play a critical role in determining their mechanical stability and material properties. A comprehensive understanding of these complex processes is essential for assessing the performance of bentonite in high-level nuclear waste disposal. Temperature also greatly influences the viscosity and salinity of pore water and hence influences the swelling properties of the bentonite [7,8]. The mineral phase transition (convert the smectite into illite) will occur at high-temperature conditions [9]. The increased temperature affects the bentonite clay microstructure, such as the inter-layer spacing (d001 spacing), the thickness of diffuse double layers, the mineral compositions, the cation exchange capacity, the water retention properties, etc. [10,11,12,13,14]. The changes upon heating or at high temperatures deeply and irreversibly altered the bentonite Atterberg limits. The increased temperature can induce a significant decrease in the plastic limit, liquid limit, and plasticity index [15]. In the case of high-level radioactive waste sequestration, these effects seem to be negative because they make the bentonite clay less plastic.
Elevated temperatures can alter both the swelling pressure and hydraulic conductivity of bentonite, thereby casting doubt on the long-term sealing performance of the buffer material. Numerous experimental studies have been conducted to explore the effects of temperature on swelling pressure, which depends on many factors, such as bentonite compositions, over-consolidation ratio, initial water content, plasticity, etc.
In addition, the temperature may have either positive or negative effects on the swelling pressure. For example, the phenomena of heating–swelling, heating–contraction, heating–strengthening, and heating–softening have been observed by many researchers in laboratory measurements. A reduction in swelling pressure upon heating was observed in FEBEX bentonite [8], GMZ01 bentonite [16] and MX80 bentonite [17], while increases in swelling pressure were found in Kyungju bentonite [18], Bikaner bentonite [19], super clay, and Kunigel bentonite [20]. The authors attributed these findings to the different mineral compositions, initial states, and porewater chemicals. The swelling pressure is significantly affected by temperature, with its response largely determined by the material’s microstructure and environmental conditions.
The clays’ exposure to high temperatures not only occurred during high-level nuclear waste disposal but also in many geotechnical engineering or geo-energy applications, such as oil extraction, high voltage cables, energy piles, geopolymer cement, ceramic products, etc. The increased temperature changed the properties of bentonite, which contributed to the loss of its sealing abilities and endangered the engineered barrier systems in high-level radioactive waste sequestration [8]. The increased temperature-induced clay deformation may pose a threat to the structural stability of buildings constructed on the clays [21]. The results showed the high temperature exerts a considerable influence on the clay performance.
Due to the several factors related to the thermal aspect of clay behavior, this review paper provides a valuable assessment of bentonite clay properties, namely its microstructure, Atterberg limits, and swelling pressure in the context of high-level radioactive waste management at various temperatures. The influence of temperature on the bentonite clay reported by other researchers was reviewed, and controversial observations were also discussed. The review paper may contribute to a more profound understanding of the behavior of bentonite at elevated temperatures in high-level radioactive waste sequestration and create awareness of the potential hazards during the design of the multi-barrier systems considering high temperatures.
2. Thermal Effects on Clay Microstructure
The hydro-mechanical behavior of bentonite is quite important in engineering applications, especially in high-level radioactive waste sequestration. The swelling behavior mainly depends on its microstructure characteristics.
The typical unit layer structure of montmorillonite is shown in Figure 1. It contains two silica tetrahedral sheets sandwiched with one aluminum octahedral sheet. The alumina octahedron sheet contains an aluminum atom for particle coordination. The individual layers are primarily held together by weak van der Waals interactions that occur between adjacent molecular structures. The strong chemical bond is formed by atoms. Extensive isomorphous substitution takes place, wherein silicon and aluminum atoms are partially replaced by other cations, such as magnesium, iron, zinc, etc. The resulting charge imbalance is compensated by exchangeable cations located within the interlayer spaces of clay particles. The thickness of a single unit layer is approximately 9.6 Å. Because the surface of the clay platelets is negatively charged, ions and water molecules are always absorbed by the clay platelets. However, the cation concentrations were distributed higher on clay surfaces and lower further away from these surfaces; this diffuse tendency is counterbalanced by the electrostatic attraction between the cations and the negatively charged clay surface.
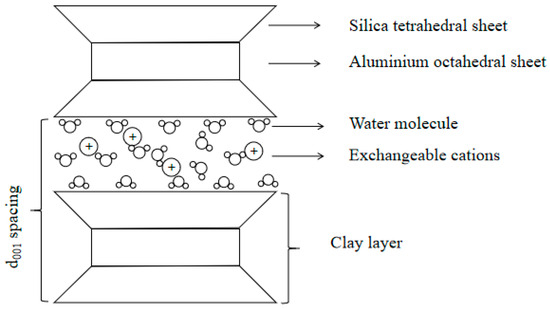
Figure 1.
Unit layer structure of montmorillonite.
The region of distributed charges near the clay surface is referred to as a diffuse double layer (DDL). This structure determines the swelling properties of montmorillonite. The repulsive forces and attractive electrostatic forces dominate platelet interactions—the balance between them is denoted as crystalline swelling [22,23]. Upon the continued hydration of clay platelets, interlayer cations become fully solvated, resulting in a localized ion concentration higher than that in the surrounding bulk solution. The resulting expansion, driven by this concentration gradient between the clay surface and the surrounding bulk fluid, is referred to as osmotic swelling or diffuse double-layer swelling [24]. The system is composed of negatively charged clay surfaces, associated cations distributed in the vicinity, and the adsorbed water molecules collectively constitute the diffuse double layer [24,25]. The d001 spacing (also basal spacing) denotes the distance from one unit layer bottom to the neighboring unit layer bottom.
The clay mineral compositions, clay–water structural changes and amount of absorbed water on the clay surface can be investigated in situ X-ray diffraction (XRD), which constitutes the most frequently utilized method in clay science [26]. XRD is a primary technique employed in the identification and structural analysis of compounds based on their diffraction responses. The XRD analysis is always conducted with an X-ray source of radiation. Crystalline materials exhibit long-range order, which manifests as sharp diffraction peaks in XRD patterns and can be precisely described by Bragg’s equation. Bragg’s equation relates the wavelength of the X-ray, the interplanar spacing in the crystal, and the glancing angle of incidence.
Temperature significantly affects the viscosity and salinity of pore water, thereby influencing the swelling behavior of bentonite. The continuous heating at high-temperature conditions can also slowly convert the smectite into illite (Na-smectite + K+ + Al3+ → illite + Si4+) [9]. This transformation is known as the illitization process, which is a thermally activated mineralogical alteration involving the gradual incorporation of potassium ions into the smectite interlayers, leading to a structural rearrangement and loss of expandability. This phenomenon causes smectite breakdown and the liberation of silicon, potentially leading to the precipitation of SiO2 cement. As a result, the illitization process not only reduces the swelling capacity and plasticity of the bentonite material but also may induce cementation effects that affect its long-term buffer performance in engineered barrier systems. Moreover, high temperatures have a significant influence on the phase transformation of clay types; for example, Figure 2 shows the transformation from goethite to hematite (α-FeOOH → α-Fe2O3) [27]. The same phenomenon was observed by Laufek et al. [11] for the heat treatment of Czech BCV 2017 bentonite. The XRD pattern reflects the disappearance of goethite in the material when it is subjected to long-term heating and its dehydration to hematite. An experimental study showed that the goethite–hematite transformation occurred at a temperature of 240–300 °C [27,28,29]. The sustained heating of BCV 2017 at 200 °C for 27 months effectively triggered the dehydration of goethite. A noticeable outcome of this transformation is the alteration in the sample’s color after prolonged thermal exposure, as depicted in Figure 3.
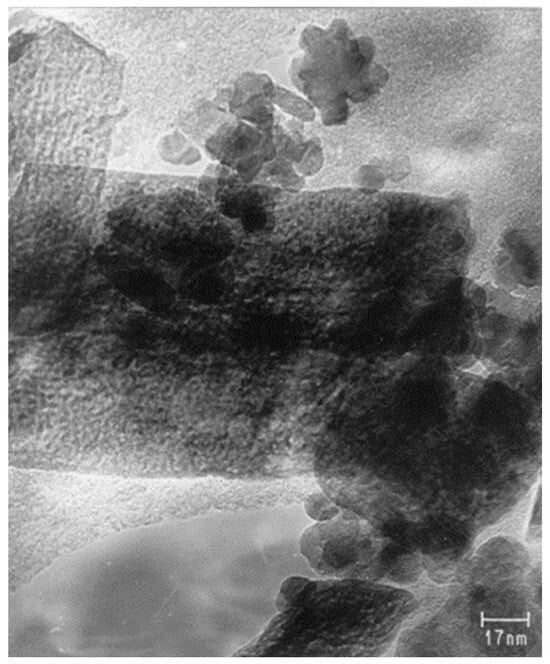
Figure 2.
Brightfield TEM images of goethite after 16 h of grinding. Elongated particles were identified by electron diffraction as goethite, and the small rounded particles were identified as hematite (González et al., 2000) [27].
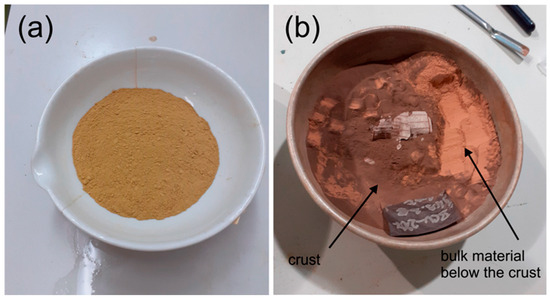
Figure 3.
Comparison of the original (a) and thermal-treated (b) BCV 2017 at 200 °C. Note the presence of a thin dark crust on the surface of the thermally treated material (Laufek et al., 2021) [11].
Temperature also exerts a substantial effect on the d001 spacing of the clay platelets. Clay platelets incorporating 0 to 4 layers of interlayer water molecules can be identified by characteristic d001 spacings of approximately 10.0, 12.5, 15.0, 17.5, and 20.0 Å, respectively [30]. The thickness of interlamellar hydrates depends on the hydration ions in montmorillonite clay; for example, for Na+ ions, the first layer hydrate is 3.03 Å, second layer hydrate is 3.23 Å, and third hydrate is 3.48 Å; while for Ca2+ ions, the first and second layer hydrate is 3.89 and 2.75 Å, respectively [12]. The bentonite treated at a very high temperature can decrease the d001 spacing from 20 Å to 10 Å.
Figure 4 shows the four fine fractions of montmorillonite isolated from four distinct bentonite samples from Kriva Palanka (KP, Republic of Macedonia), Sarigus (Sa, Republic of Armenia), Ivancice (Iv, Czech Republic), and Otay (Ot, USA). At a standard laboratory temperature of 20 °C, the d001 spacing of unheated KP, Sa, and Ot are all in the range 12.3–12.5 Å, while a slightly higher value obtained for Iv is connected with the highest Ca2+ content in this sample because divalent Ca2+ ions are more hydrated than mono valent Li+ ions (see above Pusch, 2015 [12]). The decrease in spacing decreased, and treatment temperature occurred in all four series. Between the temperature of 130–180 °C, the d001 spacing decreased sharply along with the temperature. It can be attributed to the evaporation of adsorbed water layers. At the temperature of 200 °C, the adsorbed water was probably removed and showed layer spacings of 9.6 Å, which corresponds to the complete collapse of montmorillonite interlayers.
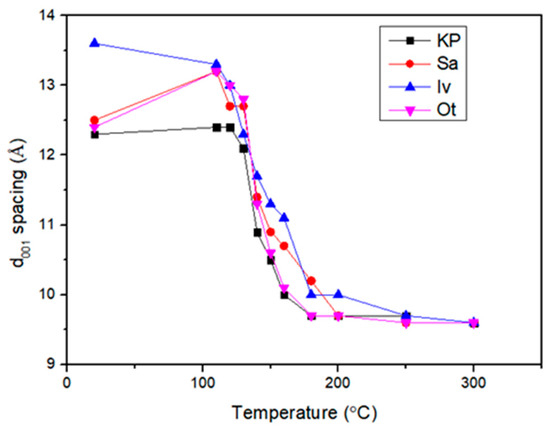
Figure 4.
The d001 spacing changes were replotted with heat treatment at different temperatures for four bentonites (from Hrobáriková et al., 2001) [31].
The same phenomenon was also observed by many researchers, such as FEBEX bentonite and MX80 bentonite [10], where the d001 spacing decreased from 18.5 Å near the hydration surface to 14.5 Å close to the heater. For Czech BCV bentonite [11], heating the material at 200 °C caused the complete removal of interlayer water, reducing the d001 spacing from 14.5 Å to 9.8 Å, which indicated a collapse of the montmorillonite structure. For the natural bentonite (deposit of locality Jelšový Potok, Slovakia) [32], the d001 spacing decreased from 15 Å of non-heated montmorillonite to 9.8 Å of a sample heated at 650 °C. The gradual heating of natural bentonite up to 500 °C initiated dehydration, which was fully achieved at the temperature of 650 °C. Differential Thermal Analysis (DTA) indicated that extended thermal treatment may result in the dehydroxylation of hydroxyl groups. The authors further confirmed that the mesoporous structure of bentonite remained intact post-heating, as evidenced by the adsorption-desorption hysteresis loop. However, the mesopore volume decreased, and new macropores emerged compared to the unheated sample based on pore size distribution analyses. Sun et al. [33] also discussed the effect of oven-drying (heating under 105 °C for 24 h) on the bentonite microstructure, which has a great influence on the largest pores due to the drying-wetting cycles.
The d001 spacing decreased with increasing temperature, which was observed by many researchers for different materials at higher temperatures. However, Morodome and Kawamura [34] showed the d001 spacing of the Japan Kunipa-F bentonite changed not only related to temperature but also depended on relative humidity (i.e., water content and suction) at temperatures lower than 150 °C. The Na- and Ca-montmorillonite showed different patterns. The increase in temperature seems to increase the d001 spacing at a higher relative humidity (RH) range (70–95%) and decrease at a lower RH range (20–40%) for Na-bentonite. For Ca-bentonite, with the increasing temperature, the d001 spacing decreased at a lower RH range (0–50%). The increasing temperature leads to the increase of d001 spacing, also captured by molecular dynamic modelers [35,36,37]. This phenomenon can be explained by the enhanced thermal agitation of the molecules within the interlayer region.
The d001 spacing related to temperature not only depends on temperature but also connects with the pore water compositions, exchangeable cations, and clay mineral surface properties. Further study is needed to explore this mechanism through various research methods that consider these factors.
3. Thermal Effects on Clay Index Characteristics—Atterberg Limits
The liquid limit is defined as the water content at which soil changes from the plastic state to the liquid state. Casagrande’s method and fall cone test are quite often used to determine the liquid limit. The plastic limit is defined as the moisture content at which fine-grained soil cannot be remolded without cracking. Usually, the rolling-out method was used to determine it. The liquid limit, plastic limit and plastic index are always referred to as Atterberg limits. The standard measurement methods for the Atterberg limits can be found in any standard references or books such as Jackson [38].
Numerous studies have investigated the effect of temperature on the Atterberg limits of clay, as it is quite important in engineering applications. In this review paper, various clay types from different regions worldwide were selected to fully understand the temperature effects on the Atterberg limits. Table 1 lists the clays studied in this section. The studied clays are as follows: tropical black clay from Nigeria heating for 4 h reported by Gadzama et al. [39]; marine clay from India heated for 7 days reported by Nayak and Preetham [40]; Jordan clay reported by Abu-Zreig et al. [41]; tropical residual soil from Nigeria heated for 6 h reported by Attah and Etim [42]; commercially available Na-bentonite heated for 3 days reported by Estabragh et al. [15]; Olut clays from Turkey heated for 24 h reported by Tan et al. [43]; Kaolin, Illitic clay, Montmorillonitic, Montmorillonitic-Illitic clays from US heated for 3 h reported by Laguros JG [44]. All of these clays were heated at different temperatures from room temperature (20–25 °C) to 500 °C. The temperature used here means that the clay was heated at this temperature. However, it is quite hard to get the unified heating duration for all the clays; the detailed heating duration is listed in Table 1. It can be seen that the liquid limit varied from 32% to 349%, and the plastic limit varied from 15% to 55% for all the clays. The details of the clay information can be obtained from the reference list.

Table 1.
The basic properties of the clays (initial conditions at room temperature) used for Atterberg limit analysis at various temperatures.
Figure 5 and Figure 6 show the plastic limit and liquid limit changes with the temperature of all clays. In general, it can be seen that both decreased with the increase in temperature. However, the decrease trend is different from the others. The type of clay was classified by the plasticity chart; the initial clay type of each clay can be obtained in the upper right corner of the map in Figure 7. As observed, some clays changed their state during heating temperature (from lower temperature region point A to higher temperature region point B) in Figure 7. For example, the Jordan clay is classified as MV at room temperature; it is reclassified as CL after heating at 400 degrees. This almost happened to each kind of clay; they are almost transferred from higher plasticity zones to lower plasticity zones. The temperature significantly decreases the plasticity of clays and transfers the clays to lower or non-plasticity clays.
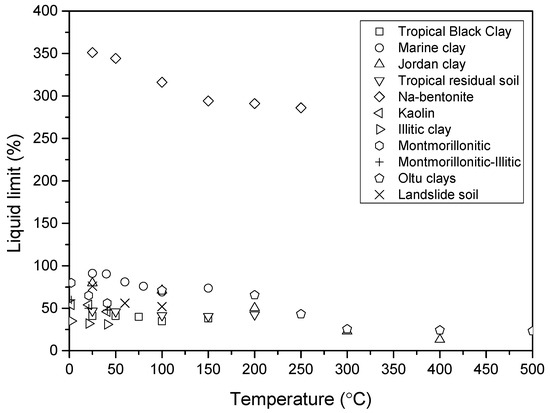
Figure 5.
The liquid limit changes with temperature for bentonite clays.
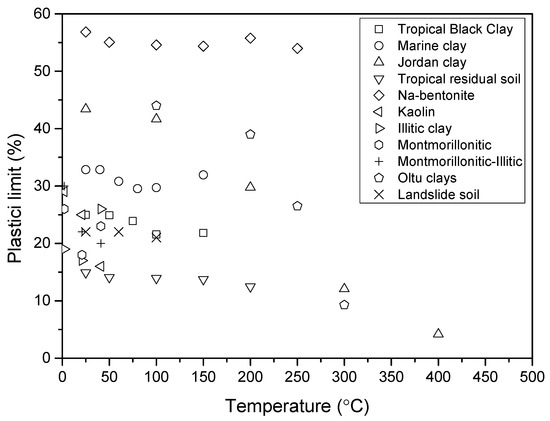
Figure 6.
The plastic limit changes with the temperature of bentonite clays.
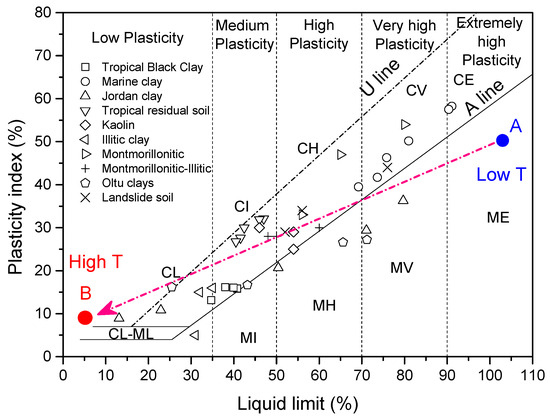
Figure 7.
The plastic chart for all kinds of clays at various temperatures.
The plastic limit, liquid limit and plastic index are related to many factors such as heating time, mineral compositions, specific surface area, microstructural properties (i.e., d001 spacing discussed before) and solid particle size (or clay fractions) [21,45].
Heating time: The plastic limit, liquid limit, and plasticity index are heating time-dependent; the longer the time is spent heating, the lower the Atterberg limits are. This phenomenon was observed by Estabragh et al. [15] for bentonite clay heating for 3, 7, 14 and 30 days. The lowest Atterberg limits were the clay heated for 30 days, the longest heating time.
Mineral compositions: As discussed in the previous section, the clay minerals and aggregates were deeply altered by heating (especially at very high temperatures), which could result in phase transition. The phase transformation of goethite into hematite was observed at approximately 250 °C. The transformation from smectite to the illitization process reduces the plasticity of the bentonite clay. The increasing temperature also facilitated the formation of cementing agents such as iron oxide (Fe2O3), carbonates (CaCO3) and organic matter. The heating can induce the agglomeration of clay particles, which further leads to the formation of bulk particles [46]. The heating can also break down its particles, changing its mineralogical compositions [47,48]. At a temperature of 200 °C, the de-hydroxylation of clay minerals occurred, followed by the assembly and sintering of the particles [45]. Additionally, the aggregation of fine particles in clays was easily formed at higher porewater salt concentration conditions [8,45]. The mineral compositions changed by temperature could lead to a decrease in Atterberg limits.
Specific surface area: The heating temperature also influences the interparticle contact area and their motions on the microstructural scale. The water layer is removed in two stages: in the first stage, the adsorbed water can be evaporated by the temperature of 100–110 °C; in the second stage, the chemically bound OH- group depletion above the temperature of 500 °C as stated in the previous section. It can cause the attenuation or complete loss of diffuse of a double layer surrounding the clay platelets, as discussed in Zihms et al. [48]. Heating can induce the collapse of large pores, leading the initial loose structure to a dense packing structure. The higher the temperature, the higher the capillary stress. This increases the interparticle attraction and facilitates fine particle aggregation [49], thereby promoting the formation of compact particle assemblages characterized by strong Van der Waal force and coulombic bonds, which are not easily separable [46]. This mechanism can be explained as follows: the increase in temperature promotes the adhesion of clay particles, leading to the formation of larger aggregates. The aluminum and iron can be liberated from the clay when the temperature is high and can be rapidly oxidized and precipitated around the clay surface and cement the clay particles to form large aggregates, which resulted in decreasing the fine content of the clay [50]. Peter G. Fookes [51] pointed out that dehydrated iron oxides form strong interparticle bonds among clay particles, thereby inhibiting water penetration. This transformation is irreversible by rewetting. It may result in a marked enlargement of particle size and a notable decline in the plasticity of the soil. The increase in temperature decreases the value of the specific surface area of clay, which can also be indirectly reflected by the decrease in the water retention properties of clay [14,52]. Thus, the loss of adsorbed water and the decrease in specific surface area at elevated temperatures reduce the water retention capacity of particles, thereby lowering the Atterberg limits [21].
4. Thermal Effects on Clay Swelling Pressure
The determination of swelling pressure was usually performed using laboratory tests. Generally, bentonite powder at a constant water content was employed in the preparation of compacted specimens. The bentonite powder was compacted uniaxially and statically in a compaction device. The diameters of the compaction device vary from 30 to 100 mm, and the initial dry density is determined by the compaction force, hence different final heights of compacted samples. The oedometer devices were frequently employed to determine the swelling pressure. During the measurement, the sample volume is not allowed to change; it is also called the constant volume swelling pressure, which differs from the swell-consolidation swelling pressure [2]. Measuring the swelling pressure at room temperature, once the compacted samples were ready, the distilled water infiltrated from the bottom to the top of the samples. A load cell was mounted on the loading frame to measure the pressure generated by the swelling of clay. As for the high temperature, the oedometer ring system was connected to some thermostatic bath (i.e., oil or water) to keep the constant temperature around the specimen. The thermal condition of the specimen was monitored by some thermal sensors (i.e., Pt100) once it reached the stabilized conditions, following the same procedures as presented at room temperature. The swelling pressure developed rapidly at the early starting stage (i.e., 200–400 min) and then reached a nearly constant state after several days. Both sodium and calcium bentonite showed the same pattern. The evolution of swelling pressure is associated with moisture redistribution and particle rearrangements within clay. The final degree of saturation exceeded 95%, confirming that the samples were fully saturated, and the data were reliable. Details of the sample preparations and the laboratory measurement procedures can be found in the reference lists in Table 2.

Table 2.
The basic properties of bentonite clays (initial conditions at room temperature) used for swelling pressure at various temperatures.
In this section, the swelling pressure refers to the constant volume of swelling pressure. The effect of temperature on the swelling pressure of bentonite clay has been extensively investigated due to its significance in engineering applications, especially in nuclear waste disposal. Generally, there are two kinds of bentonite—namely sodium bentonite and calcium bentonite—which are determined by the dominated cations. Table 2 lists the bentonite clays in this section. The clays are as follows: FEBEX bentonite from Spain, as reported by Villar and Lloret [8]; GMZ01 bentonite from China, as reported by Ye et al. [16]; Kyungju bentonite, as reported by Cho et al. [18]; Bikaner bentonite from India as reported by Bag and Rabbani [19]; super clay and Kunigel sodium bentonite from Japan, as reported by Shirazi et al. [20]; and commercially available MX80 bentonite clay, as reported by Tripathy et al. [17]. It must be mentioned the observed variability in swelling pressure data can be primarily ascribed to differences in dry density, given its high sensitivity to even slight density changes, particularly at higher initial values. The dominated cation, initial states and dimensions of the bentonite are listed in Table 2.
Here, we try to obtain the unified initial dry density of 1.60 g/cm3 in order to directly compare the swelling pressure at different temperatures. The temperature used here means that the clay was hydrated at this temperature for several days until there was no change in recorded swelling pressure data. The details of the bentonite clay information can be obtained from the reference list in Table 2.
Swelling pressure is a function of temperature. The value of swelling pressure depends on the content of montmorillonite [53]. The swelling pressure also depends on the type of dominant cation it possesses, i.e., sodium or calcium. The increase in temperature can decrease the swelling pressure, as shown in Figure 8a for FEBEX bentonite, GMZ01 bentonite and MX80 bentonite, or increase the swelling pressure, as shown in Figure 8b for Kyungju bentonite, Bikaner bentonite, super clay, and Kunigel bentonite.
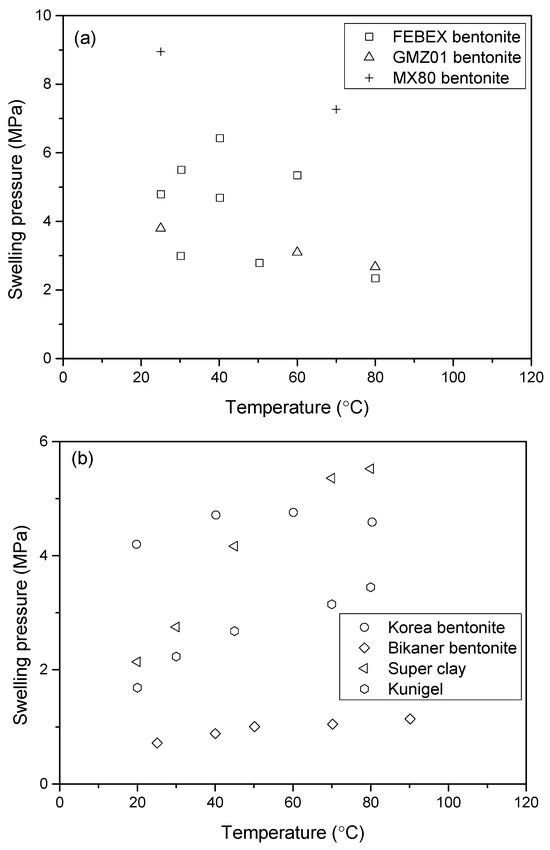
Figure 8.
Swelling pressure changes with temperature for various bentonite clays.
The effect of temperature on the hydro-mechanical response of clay depends on many factors, such as the over-consolidation ratio, initial water content, plasticity, etc. The temperature may have either positive or negative effects, such as heating–swelling, heating–contraction, heating–strengthening, and heating–softening. For example, the normally consolidated clay showed contract, while the over-consolidated clay showed expansion upon heating [54].
The compacted bentonite clearly shows two pore families, the macropores and micropores [14,33]. Water moisture is redistributed within these two pore families, and particle rearrangements within the clay are rearranged upon heating. The transfer of intra-aggregate water to inter-aggregate water at high temperatures reduces the water molecule layer on the surface of the clay platelets, leading to decreased hydration pressure and then decreased swelling pressure [8]. This transfer phenomenon is also reflected by the water retention capacity decreased upon heating [14].
As discussed in Section 3 for the Atterberg limits. Heating can also induce the formation of cement in the clay particles caused by precipitation of dissolved silica/aluminum in the hydration phase [55], thus decreasing swelling pressure.
The swelling pressure of sodium-dominated bentonite increased with the increase in temperature and decreased for calcium-dominated bentonite [56], which contributed to the different dominated cations of bentonite. There are two main mechanisms to control the development of swelling pressure upon heating: the lattice contraction due to the dehydration of the interlayer space and osmotic stress due to the stack contacts (salt concentration increase), which are different for the two types of clay. Heating contracts the lattice structure or increases the electrical potentials at the Stern plane, changing the diffuse double layer [17], which was discussed in Section 2 with the d001 spacing at different temperatures. Heating contracts the dominant lattice structure in calcium bentonite, leading to a reduction in the swelling capacity of the lattice structure (interlayers), while the osmotic pressure increased dominated in sodium bentonite. These could explain the decreased swelling pressure upon heating. However, the real laboratory test results from calcium Bikaner bentonite showed an increase in swelling pressure upon heating. The reason behind the heating invoking the swelling mechanism is complex.
The increase in temperature reduces the thickness of the diffuse double layer, leading to increases in osmotic pressure, pore water pressure, thermal energy, and the diffusion of ions, changing the system’s pressure and pore fluid properties [18], thereby resulting in a rise in swelling pressure. According to Ye et al. [16], the rise in temperature contributes to enhanced swelling pressure, attributed to the incorporation of more water molecules into the interlayer spaces of expansive minerals and strengthened electrostatic repulsion between double layers. The swelling pressure changes with temperature were also captured by the molecule dynamic modeling [35]. Moreover, the non-swelling compositions in bentonite clay can convert to swelling clay during heating, which would contribute to the increase in swelling pressure [57].
Swelling pressure exhibits a complex dependency on temperature; there are many factors affecting the swelling pressure, such as mineral compositions, chemical compositions, experimental conditions, etc. The impacts of temperature increasing or decreasing swelling pressure should be studied further to focus on the most significant factors.
5. Conclusions
This paper presents an in-depth analysis of the literature on the thermal effects on the microstructure, Atterberg limits and swelling pressure of bentonite clay. The study was motivated by the need for the safe long-term containment of high-level radioactive waste, especially the design of multi-engineered barrier systems. By synthesizing and comparing findings from multiple experimental studies, the paper highlights how elevated temperatures can alter the key physical and hydro-mechanical behaviors of bentonite. These insights contribute to the state of the art by clarifying the temperature-dependent behavior of bentonite in long-term geological storage conditions. In addition, the findings may serve as a valuable reference for researchers and engineers involved in the safety assessment and material design of engineered barriers under thermal loads. Some useful concluding remarks are as follows:
- Continuous heating at high-temperature conditions can slowly convert the bentonite/smectite into illite, and at higher temperatures, the transformation from goethite to hematite can occur. Heating of the natural bentonite up to 500 °C caused its dehydration, which was completed at 650 °C. The d001 spacing decreased sharply along with the increasing temperature; however, it is also related to many factors, such as porewater compositions, exchangeable cations, and clay mineral surface properties.
- The increase in temperature significantly decreases the plasticity of bentonite clays and can transfer the bentonite clays to lower or non-plasticity clays. The extent of temperature influence on the value of Atterberg limits depends on many factors such as heating time, mineral compositions, specific surface area, microstructural properties, and solid particle size.
- The increase in temperature can either increase or decrease the swelling pressure of bentonite clays, which was determined by the initial state of bentonite, such as montmorillonite content, dominated cations, mineral compositions, pore water chemical compositions, etc.
Author Contributions
Conceptualization, L.L. (Lingling Li) and H.S.; methodology, L.L. (Lingling Li); investigation, L.L. (Lingling Li), X.F. and L.L. (Liangliang Lu); resources, H.S.; data curation, L.L. (Lingling Li), X.F. and L.L. (Liangliang Lu); writing—original draft preparation, L.L. (Lingling Li) and H.S.; writing—review and editing, H.S.; supervision, H.S.; project administration, H.S.; funding acquisition, H.S. All authors have read and agreed to the published version of the manuscript..
Funding
This research was funded by the National Sciences Foundation of China NO. 42207170, NanHaiXinXing project Grant NO. NHXXRCXM202363, Supported by the Hainan Province Science and Technology Special Fund, Grant NO: ZDYF2023GXJS011, and Research Startup Funding from the Hainan Institute of Zhejiang University (NO. 0206-6602-A12202).
Acknowledgments
The authors are grateful to the reviewers for their valuable suggestions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gens, A. Soil–environment interactions in geotechnical engineering. Géotechnique 2010, 60, 3–74. [Google Scholar] [CrossRef]
- Sun, H.; Scaringi, G.; Mašín, D.; Najser, J. An experimental investigation on the swelling behavior of compacted B75 bentonite. Eng. Geol. 2022, 296, 106452. [Google Scholar] [CrossRef]
- Tsang, C.-F.; Neretnieks, I.; Tsang, Y. Hydrologic issues associated with nuclear waste repositories. Water Resour. Res. 2015, 51, 6923–6972. [Google Scholar] [CrossRef]
- Delage, P.; Cui, Y.J.; Tang, A.M. Clays in radioactive waste disposal. J. Rock Mech. Geotech. Eng. 2010, 2, 111–123. [Google Scholar] [CrossRef]
- Johnson, L.; Niemeyer, M.; Klubertanz, G.; Siegel, P.; Gribi, P. Calculations of the Temperature Evolution of a Repository for Spent Fuel, Vitrified High-Level Waste and Intermediate Level Waste in Opalinus Clay; Colenco Power Engineering AG: Bade, Switzerland, 2002; pp. 1015–2636. [Google Scholar]
- Landolt, D.; Davenport, A.; Payer, J.; Shoesmith, D. ChemInform Abstract: A Review of Materials and Corrosion Issues Regarding Canisters for Disposal of Spent Fuel and High-Level Waste in Opalinus Clay. ChemInform 2011, 42. [Google Scholar] [CrossRef]
- Villar, M. MX-80 Bentonite. Thermo-Hydro-Mechanical Characterisation Performed at CIEMAT in the Context of the Prototype Project; Report number: Informes Técnicos CIEMAT 1053; CIEMAT: Madrid, Spain, 2005. [Google Scholar]
- Villar, M.V.; Lloret, A. Influence of temperature on the hydro-mechanical behaviour of a compacted bentonite. Appl. Clay Sci. 2004, 26, 337–350. [Google Scholar] [CrossRef]
- Wersin, P.; Johnson, L.; McKinley, I. Performance of the bentonite barrier at temperatures beyond 100 C: A critical review. Phys. Chem. Earth Parts A/B/C 2007, 32, 780–788. [Google Scholar] [CrossRef]
- Gómez-Espina, R.; Villar, M.V. Geochemical and mineralogical changes in compacted MX-80 bentonite submitted to heat and water gradients. Appl. Clay Sci. 2010, 47, 400–408. [Google Scholar] [CrossRef]
- Laufek, F.; Hanusová, I.; Svoboda, J.; Vašíček, R.; Najser, J.; Koubová, M.; Čurda, M.; Pticen, F.; Vaculíková, L.; Sun, H. Mineralogical, geochemical and geotechnical study of BCV 2017 bentonite—The initial state and the state following thermal treatment at 200 C. Minerals 2021, 11, 871. [Google Scholar] [CrossRef]
- Pusch, R. Bentonite Clay: Environmental Properties and Applications; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Sun, H.; Mašín, D.; Najser, J.; Neděla, V.; Navrátilová, E. Fractal characteristics of pore structure of compacted bentonite studied by ESEM and MIP methods. Acta Geotech. 2020, 15, 1655–1671. [Google Scholar] [CrossRef]
- Sun, H.; Mašín, D.; Najser, J.; Scaringi, G. Water retention of a bentonite for deep geological radioactive waste repositories: High-temperature experiments and thermodynamic modeling. Eng. Geol. 2020, 269, 105549. [Google Scholar] [CrossRef]
- Estabragh, A.; Khosravi, F.; Javadi, A. Effect of thermal history on the properties of bentonite. Environ. Earth Sci. 2016, 75, 657. [Google Scholar] [CrossRef]
- Ye, W.-M.; Zheng, Z.; Chen, B.; Chen, Y.-G.; Cui, Y.-J.; Wang, J. Effects of pH and temperature on the swelling pressure and hydraulic conductivity of compacted GMZ01 bentonite. Appl. Clay Sci. 2014, 101, 192–198. [Google Scholar] [CrossRef]
- Tripathy, S.; Bag, R.; Thomas, H. Enhanced Isothermal Effect on Swelling Pressure of Compacted MX80 Bentonite; Springer International Publishing: Cham, Switzerland, 2015; pp. 537–539. [Google Scholar] [CrossRef]
- Cho, W.-J.; Lee, J.-O.; Kang, C.-H. Influence of temperature elevation on the sealing performance of a potential buffer material for a high-level radioactive waste repository. Ann. Nucl. Energy 2000, 27, 1271–1284. [Google Scholar] [CrossRef]
- Bag, R.; Rabbani, A. Effect of temperature on swelling pressure and compressibility characteristics of soil. Appl. Clay Sci. 2017, 136, 1–7. [Google Scholar] [CrossRef]
- Shirazi, S.M.; Kazama, H.; Kuwano, J.; Rashid, M. The influence of temperature on swelling characteristics of compacted bentonite for waste disposal. Environ. Asia 2010, 3, 60–64. [Google Scholar]
- Chen, Z.; Zhu, H.; Yan, Z.; Zhao, L.; Shen, Y.; Misra, A. Experimental study on physical properties of soft soil after high temperature exposure. Eng. Geol. 2016, 204, 14–22. [Google Scholar] [CrossRef]
- Kittrick, J. Interlayer forces in montmorillonite and vermiculite. Soil Sci. Soc. Am. J. 1969, 33, 217–222. [Google Scholar] [CrossRef]
- Norrish, K. Crystalline swelling of montmorillonite: Manner of swelling of montmorillonite. Nature 1954, 173, 256–257. [Google Scholar] [CrossRef]
- Olphen, H.v. An Introduction to Clay Colloid Chemistry: For Clay Technologists, Geologists and Soil Scientists; Wiley: Hoboken, NJ, USA, 1963. [Google Scholar]
- Bolt, G. Physico-chemical analysis of the compressibility of pure clays. Geotechnique 1956, 6, 86–93. [Google Scholar] [CrossRef]
- Reynolds, R.C. Modern Powder Diffraction; David, L.B., Jeffrey, E.P., Eds.; De Gruyter: Berlin, Germany; Boston, MA, USA, 1989; pp. 145–182. [Google Scholar]
- González, G.; Sagarzazu, A.; Villalba, R. Study of the mechano-chemical transformation of goethite to hematite by TEM and XRD. Mater. Res. Bull. 2000, 35, 2295–2308. [Google Scholar] [CrossRef]
- de Faria, D.L.A.; Lopes, F.N. Heated goethite and natural hematite: Can Raman spectroscopy be used to differentiate them? Vib. Spectrosc. 2007, 45, 117–121. [Google Scholar] [CrossRef]
- Ruan, H.; Frost, R.; Kloprogge, J.T. The behavior of hydroxyl units of synthetic goethite and its dehydroxylated product hematite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2001, 57, 2575–2586. [Google Scholar] [CrossRef]
- Laird, D.A. Influence of layer charge on swelling of smectites. Appl. Clay Sci. 2006, 34, 74–87. [Google Scholar] [CrossRef]
- Hrobáriková, J.; Madejová, J.; Komadel, P. Effect of heating temperature on Li-fixation, layer charge and properties of fine fractions of bentonites. J. Mater. Chem. 2001, 11, 1452–1457. [Google Scholar] [CrossRef]
- Orolínová, Z.; Mockovčiaková, A.; Dolinská, S.; Briančin, J. Uticaj Termičke Obrade na Bentonitska Svojstva. Arh. Teh. Nauk. 2012, 7, 49–56. [Google Scholar]
- Sun, H.; Mašín, D.; Najser, J.; Neděla, V.; Navrátilová, E. Bentonite microstructure and saturation evolution in wetting–drying cycles evaluated using ESEM, MIP and WRC measurements. Géotechnique 2019, 69, 713–726. [Google Scholar] [CrossRef]
- Morodome, S.; Kawamura, K. Swelling behavior of Na-and Ca-montmorillonite up to 150° C by in situ X-ray diffraction experiments. Clays Clay Miner. 2009, 57, 150–160. [Google Scholar] [CrossRef]
- Akinwunmi, B.; Sun, L.; Hirvi, J.T.; Kasa, S.; Pakkanen, T.A. Influence of temperature on the swelling pressure of bentonite clay. Chem. Phys. 2019, 516, 177–181. [Google Scholar] [CrossRef]
- Teich-McGoldrick, S.L.; Greathouse, J.A.; Jove-Colon, C.F.; Cygan, R.T. Swelling properties of montmorillonite and beidellite clay minerals from molecular simulation: Comparison of temperature, interlayer cation, and charge location effects. J. Phys. Chem. C 2015, 119, 20880–20891. [Google Scholar] [CrossRef]
- Zheng, Y.; Zaoui, A.; Shahrour, I. Evolution of the interlayer space of hydrated montmorillonite as a function of temperature. Am. Mineral. 2010, 95, 1493–1499. [Google Scholar] [CrossRef]
- Jackson, R. Manual of soil laboratory testing. Environ. Eng. Geosci. 2015, 21, 247–248. [Google Scholar] [CrossRef]
- Gadzama, E.; Nuhu, I.; Yohanna, P. Influence of temperature on the engineering properties of selected tropical black clays. Arab. J. Sci. Eng. 2017, 42, 3829–3838. [Google Scholar] [CrossRef]
- Nayak, S.; Preetham, H. Effect of drying temperature and rewetting on the engineering properties of marine clay. Transp. Infrastruct. Geotechnol. 2020, 7, 517–534. [Google Scholar] [CrossRef]
- Abu-Zreig, M.M.; Al-Akhras, N.M.; Attom, M.F. Influence of heat treatment on the behavior of clayey soils. Appl. Clay Sci. 2001, 20, 129–135. [Google Scholar] [CrossRef]
- Attah, I.C.; Etim, R.K. Experimental investigation on the effects of elevated temperature on geotechnical behaviour of tropical residual soils. SN Appl. Sci. 2020, 2, 370. [Google Scholar] [CrossRef]
- Tan, Ö.; Yılmaz, L.; Zaimoğlu, A.S. Variation of some engineering properties of clays with heat treatment. Mater. Lett. 2004, 58, 1176–1179. [Google Scholar] [CrossRef]
- Laguros, J.G. Effect of temperature on some engineering properties of clay soils. In Highway Research Board Special Report; National Academy Press: Washington, DC, USA, 1969. [Google Scholar]
- Aldaeef, A.; Rayhani, M.T. Hydraulic performance of Compacted Clay Liners (CCLs) under combined temperature and leachate exposures. Waste Manag. 2014, 34, 2548–2560. [Google Scholar] [CrossRef]
- Pandian, N.; Nagaraj, T.; Sivakumar Babu, G. Effects of drying on the engineering behaviour of Cochin marine clays. Geotechnique 1991, 41, 143–147. [Google Scholar] [CrossRef]
- Certini, G. Effects of fire on properties of forest soils: A review. Oecologia 2005, 143, 1–10. [Google Scholar] [CrossRef]
- Zihms, S.; Switzer, C.; Tarantino, A.; Karstunen, M. Understanding the effects of high temperature processes on the engineering properties of soils. In Proceedings of the 18th International Conference on Soil Mechanics and Geotechnical Engineering, Paris, France, 2–6 September 2013; pp. 3427–3430. [Google Scholar]
- Rao, S.M.; Sridharan, A.; Chandrakaran, S. Engineering behavior of uplifted Smectite-rich Cochin and Mangalore marine clays. Mar. Georesources Geotechnol. 1990, 9, 243–259. [Google Scholar] [CrossRef]
- White, R.E. Principles and Practice of Soil Science: The Soil as a Natural Resource; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Fookes, P.G.; Geological Society of London Engineering Group Working Party. Tropical Residual Soils: A Geological Society Engineering Group Working Party Revised Report; Geological Society: London, UK, 1997. [Google Scholar]
- Akin, I.D.; Likos, W.J. Specific surface area of clay using water vapor and EGME sorption methods. Geotech. Test. J. 2014, 37, 1016–1027. [Google Scholar] [CrossRef]
- Sun, H. A new method to predict swelling pressure of compacted bentonites based on diffuse double layer theory. Geomech. Eng. 2018, 16, 71–83. [Google Scholar]
- Cekerevac, C.; Laloui, L. Experimental study of thermal effects on the mechanical behaviour of a clay. Int. J. Numer. Anal. Methods Geomech. 2004, 28, 209–228. [Google Scholar] [CrossRef]
- Pusch, R.; Bluemling, P.; Johnson, L. Performance of strongly compressed MX-80 pellets under repository-like conditions. Appl. Clay Sci. 2003, 23, 239–244. [Google Scholar] [CrossRef]
- Pusch, R.; Karnland, O.; Hökmark, H. GMM-a General Microstructural Model for Qualitative and Quantitative Studies of Smectite Clays; Swedish Nuclear Fuel and Waste Management Co.: Stockholm, Sweden, 1990. [Google Scholar]
- Hansen, E.L.; Hemmen, H.; Fonseca, D.d.M.; Coutant, C.; Knudsen, K.; Plivelic, T.; Bonn, D.; Fossum, J.O. Swelling transition of a clay induced by heating. Sci. Rep. 2012, 2, 618. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).