Challenges in Interpreting 40Ar/39Ar Age Spectra: Clues from Hydrothermally Altered Alkali Feldspars
Abstract
1. Introduction
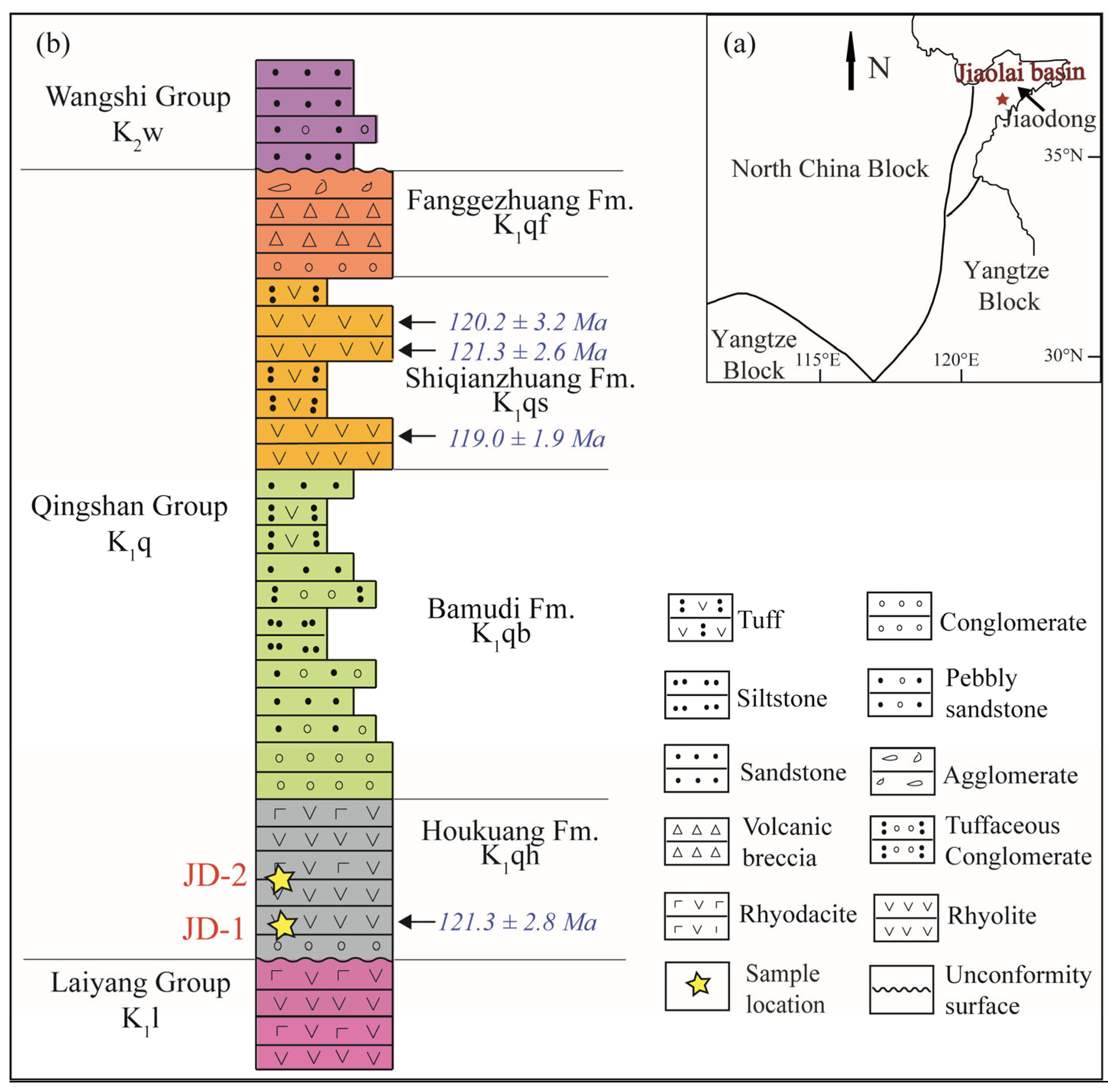
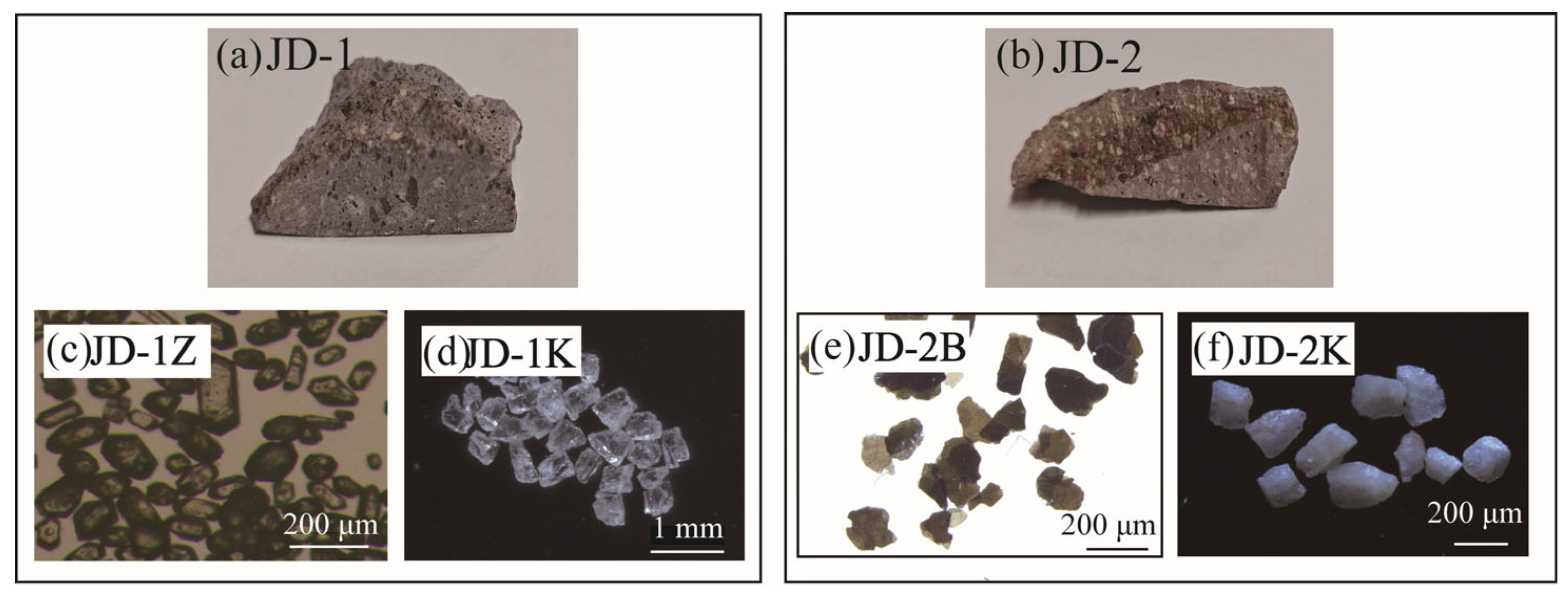
2. Sample Description
3. Methods
3.1. 40Ar/39Ar and U-Pb Analytical Processes
3.1.1. 40Ar/39Ar Dating
3.1.2. U-Pb Dating
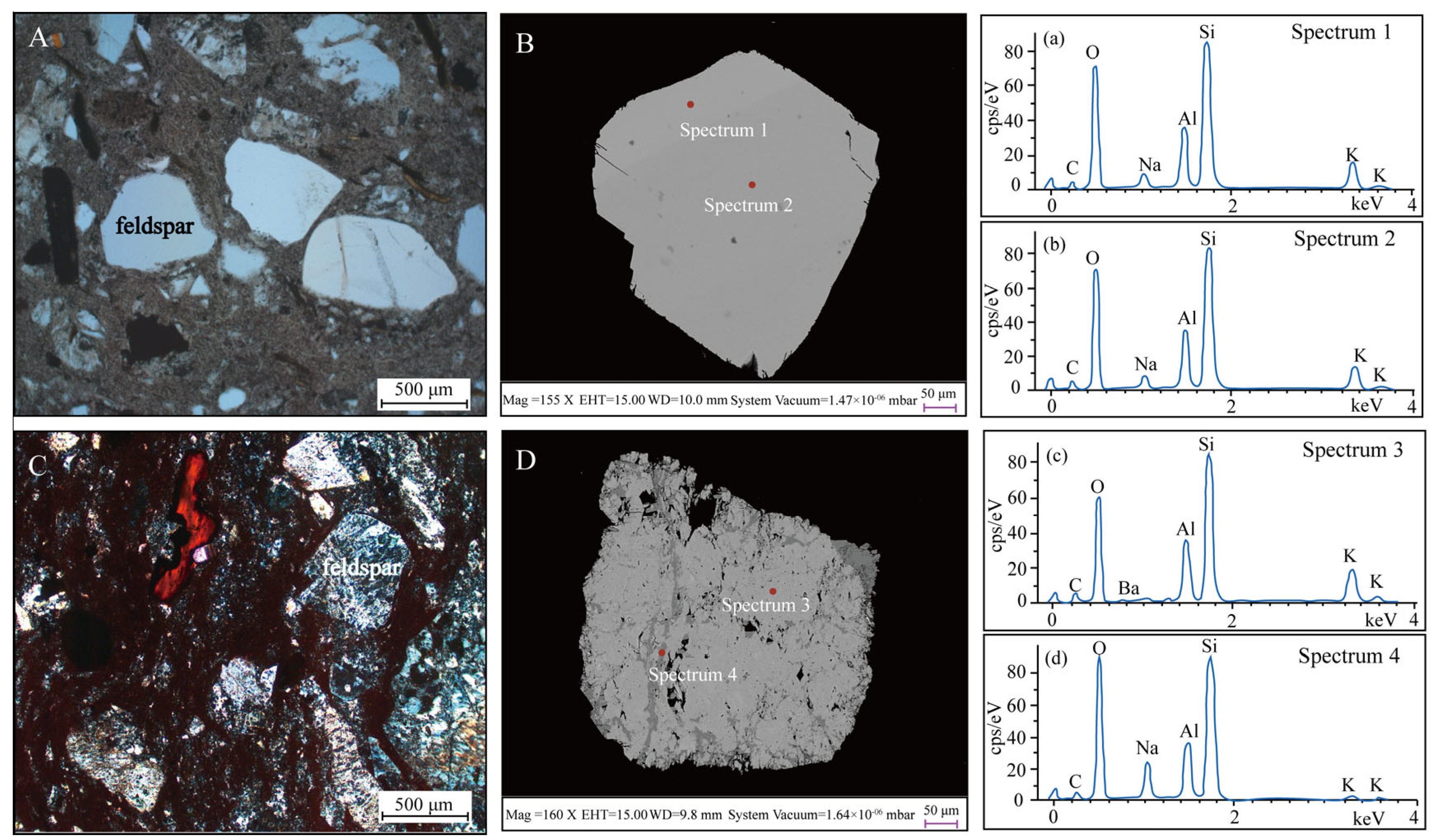
3.2. Backscattered Electron (BSE) Images and Energy-Dispersive X-Ray Spectroscopy (EDS)
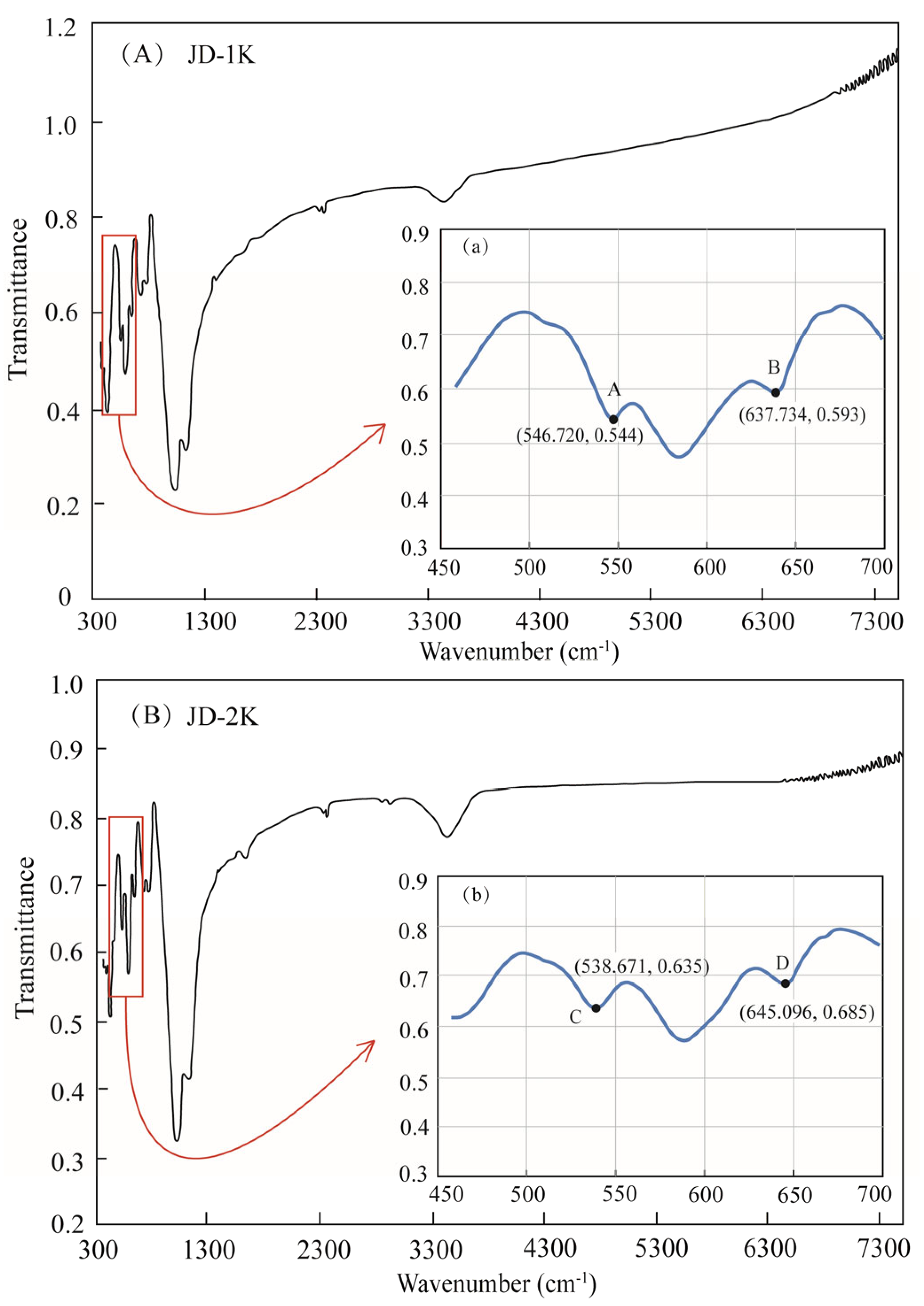
3.3. Fourier Transform Infrared Spectroscopy (FTIR)
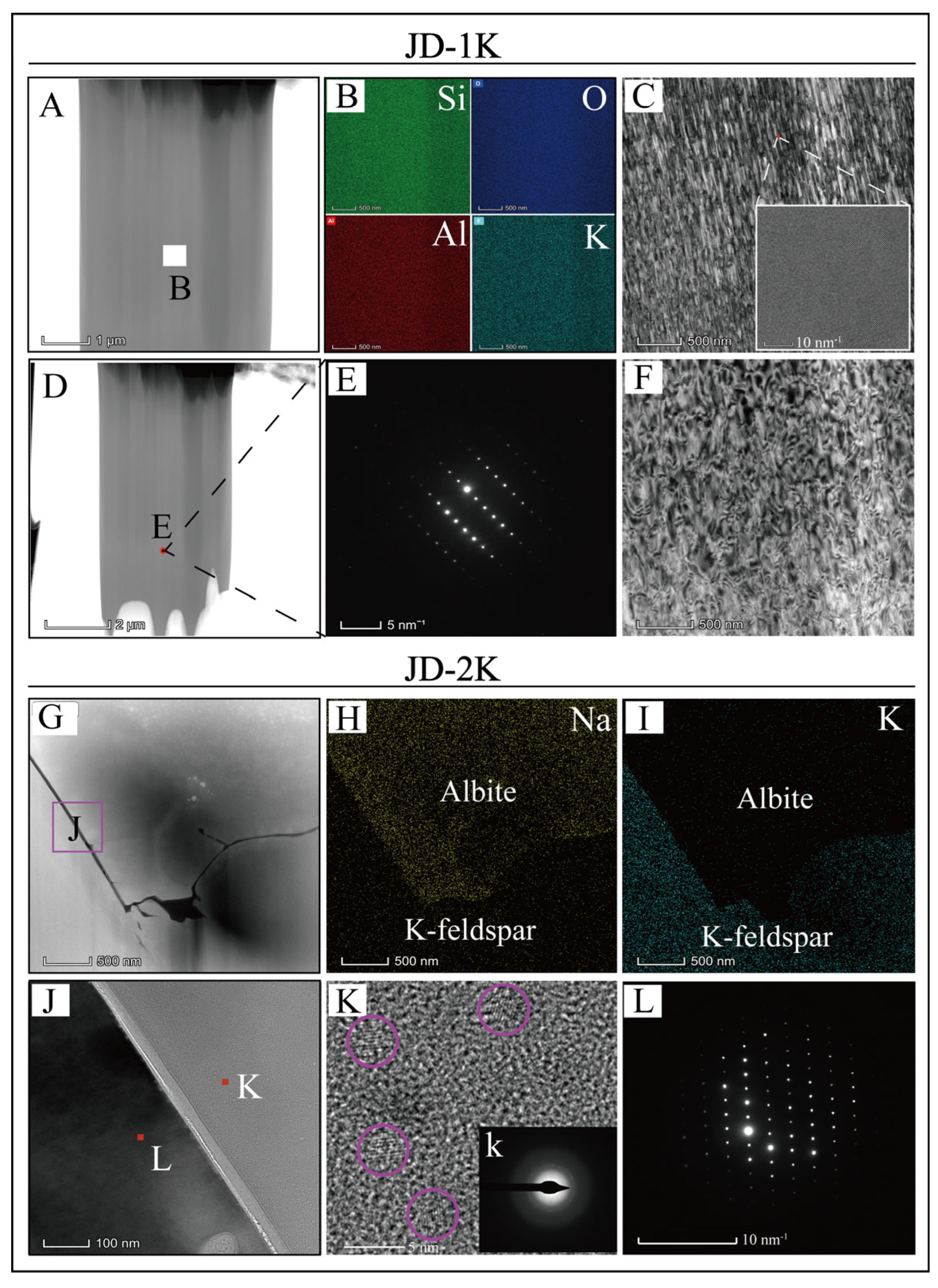
3.4. Transmission Electron Microscopy (TEM)
4. Age Results
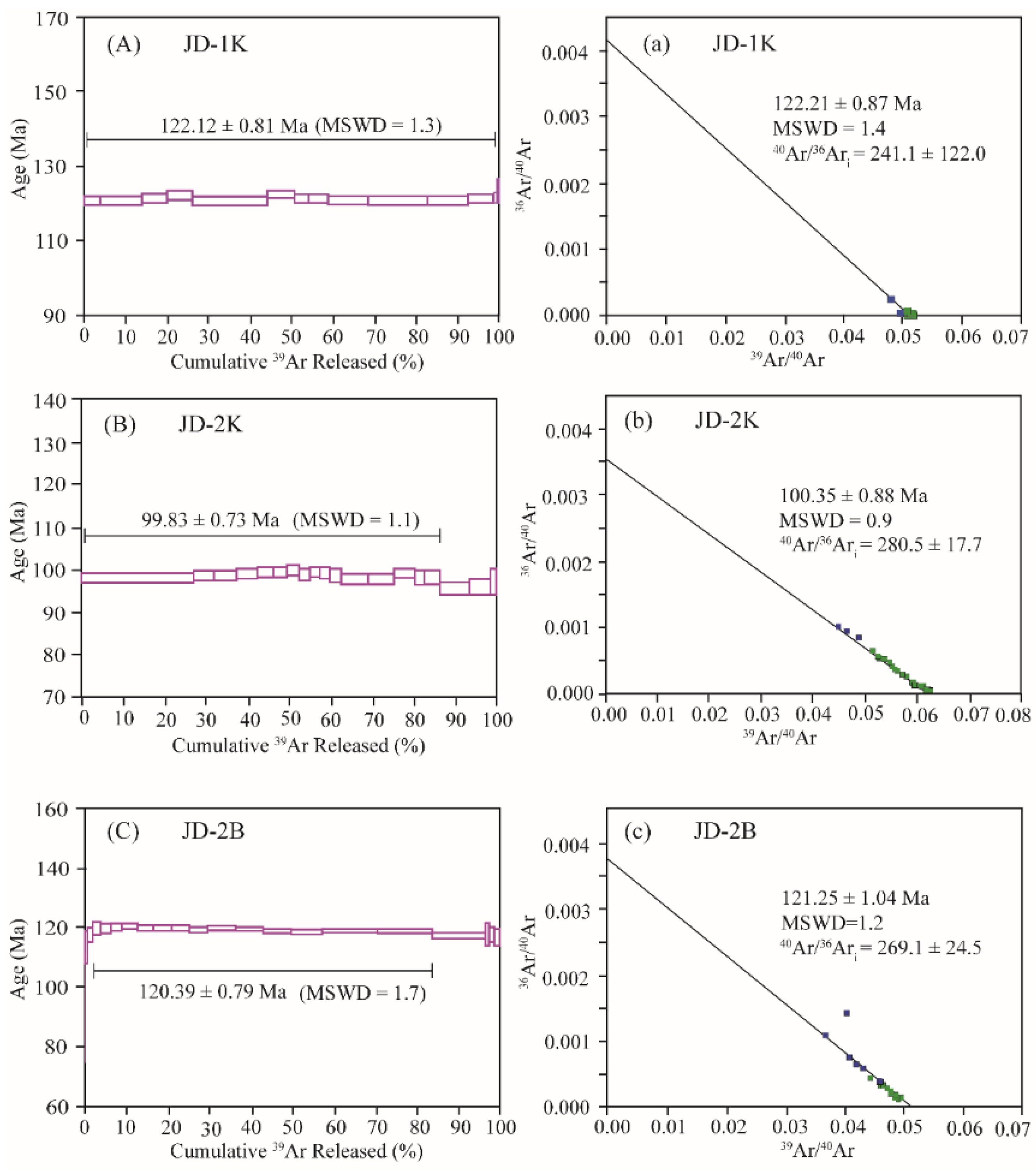
4.1. 40Ar/39Ar Ages
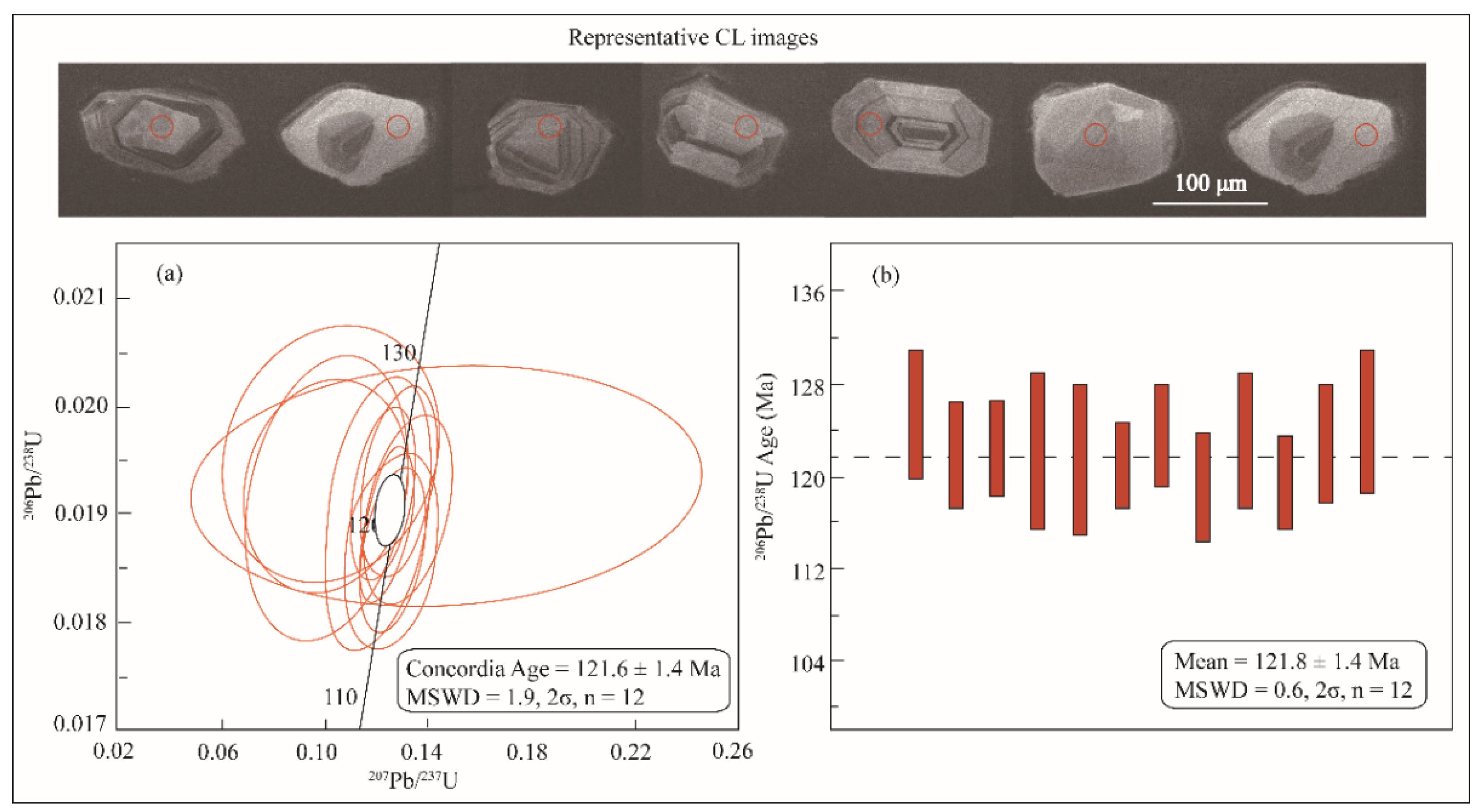
4.2. U-Pb Age
4.3. Age Comparison Between JD-1K and JD-2K
5. Composition and Structure of JD-1K and JD-2K
5.1. Optical Microscopy and SEM-EDS
5.2. FTIR
5.3. TEM
6. Discussion
6.1. Different Genesis of K-Feldspars JD-1K and JD-2K
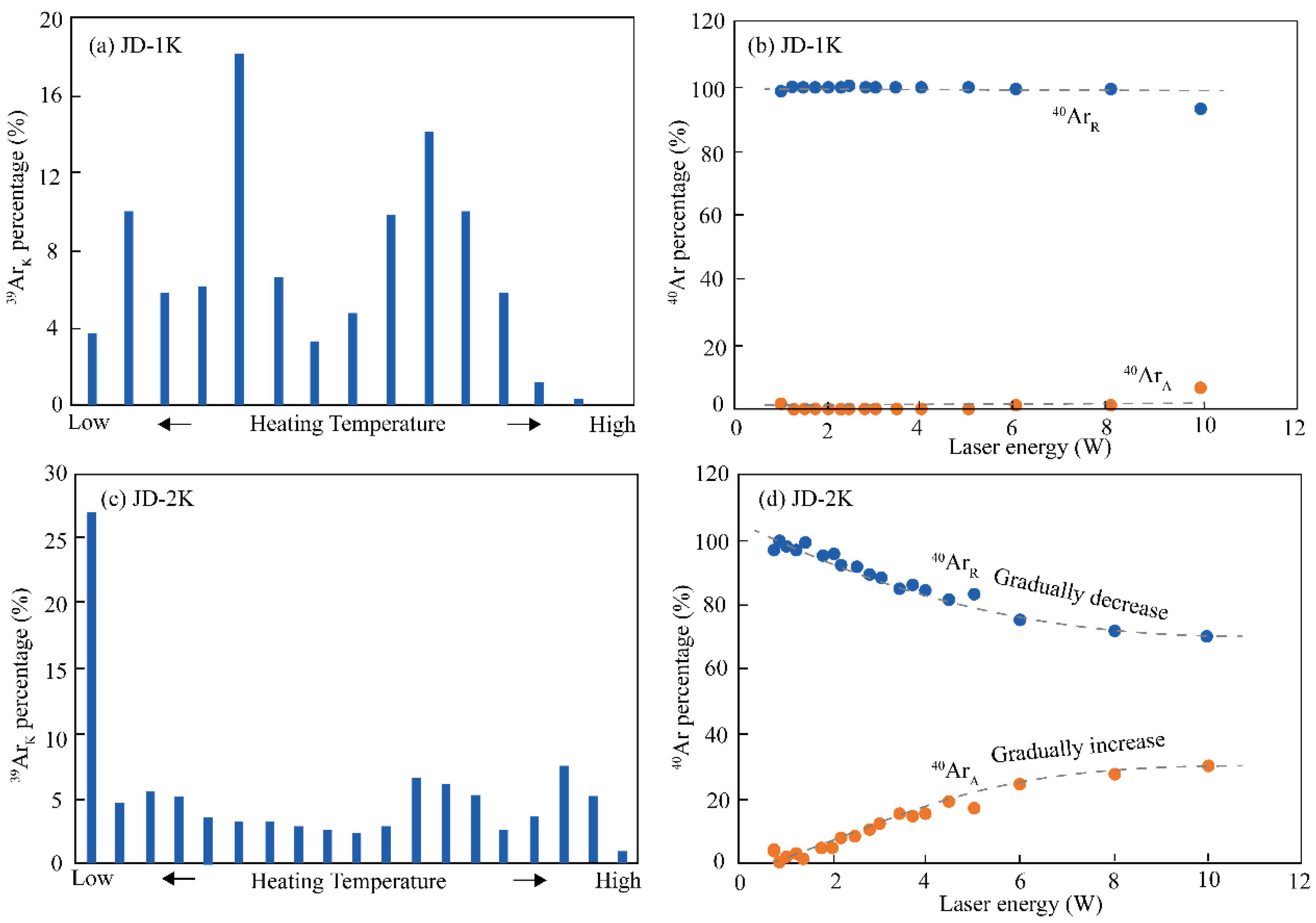
6.2. Differential Gas Release Patterns During Step-Heating
6.3. The Relationship Between Hydrothermal Processes and Age Spectra
7. Conclusions
- (1)
- The 40Ar/39Ar ages obtained for the K-feldspar JD-1K and the biotite JD-2B, along with the U-Pb age of the zircon JD-1Z, exhibit consistence within the error margins. In contrast, the 40Ar/39Ar age spectrum of the JD-2K sample displays a flat profile, and its determined age is more than 20 Ma younger than the aforementioned samples.
- (2)
- Detailed petrological analyses reveal that sample JD-1K exhibits an exceptionally uniform microstructure and chemical composition, consistent with magmatic crystallization under closed-system conditions. In marked contrast, JD-2K displays unambiguous evidence of hydrothermal alteration, including dissolution–reprecipitation textures, which have resulted in a complex microstructural hierarchy.
- (3)
- The 40Ar/39Ar age data for sample JD-2K highlight a critical limitation in interpreting flattened age spectra: such profiles should not be interpreted as definitive evidence of closed isotope systematics since crystallization.
- (4)
- Hydrothermally induced recrystallization can generate apparently undisturbed 40Ar/39Ar age plateaus even in samples undergoing significant alteration. This finding provides evidence that flat 40Ar/39Ar age spectra may result from fluid-assisted isotopic resetting rather than primary magmatic processes.
- (5)
- During step-heating experiments, it is necessary to pay close attention to the compositional ratios of radiogenic 40Ar, 39ArK generated by irradiation, and 40Ar derived from the atmosphere released from different stages. Different release patterns of Ar can serve as indicators for evaluating the characteristics of the microstructures within samples.
- (6)
- Moreover, prudence must be exercised when deciphering the geological implications of 40Ar/39Ar age spectra. Comprehensive mineralogical analysis and cross-verification using multiple dating techniques are of paramount importance.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alexandrov, P.; Ruffet, G.; Cheilletz, A. Muscovite recrystallization and saddle-shaped 40Ar/39Ar age spectra: Example from the Blond granite (Massif Central, France). Geochim. Cosmochim. Acta 2002, 66, 1793–1807. [Google Scholar] [CrossRef]
- Forster, M.A.; Lister, G.S. The interpretation of 40Ar/39Ar apparent age spectra produced by mixing: Application of the method of asymptotes and limits. J. Struct. Geol. 2004, 26, 287–305. [Google Scholar] [CrossRef]
- Jourdan, F.; Renne, P.R.; Reimold, W.U. The problem of inherited 40Ar* in dating impact glass by the 40Ar/39Ar method: Evidence from the Tswaing impact crater (South Africa). Geochim. Cosmochim. Acta 2007, 71, 1214–1231. [Google Scholar] [CrossRef]
- Kula, J.; Spell, T.L.; Zanetti, K.A. 40Ar/39Ar analyses of artificially mixed micas and the treatment of complex age spectra from samples with multiple mica populations. Chem. Geol. 2010, 275, 67–77. [Google Scholar] [CrossRef]
- Boehnke, P.; Harrison, T.M.; Heizler, M.T.; Warren, P.H. A model for meteoritic and lunar 40Ar/39Ar age spectra: Addressing the conundrum of multi-activation energies. Earth Planet. Sci. Lett. 2016, 453, 267–275. [Google Scholar] [CrossRef]
- Ware, B.; Jourdan, F. 40Ar/39Ar geochronology of terrestrial pyroxene. Geochim. Cosmochim. Acta 2018, 230, 112–136. [Google Scholar] [CrossRef]
- Hu, R.G.; Wijbrans, J.R.; Brouwer, F.M.; Bai, X.J.; Qiu, H.N. Constraints on retrograde metamorphism of UHP eclogites in North Qinling, central China, from 40Ar/39Ar dating of amphibole and phengite. Gondwana Res. 2020, 87, 83–106. [Google Scholar] [CrossRef]
- Sagna, I.; Quidelleur, X.; Dioh, E.; Gillot, P.Y.; Diallo, D.P. Proterozoic magmatic events recorded in 40Ar/39Ar data from the northern part of the Kedougou Kenieba Inlier (Eastern Senegal). J. Afr. Earth Sci. 2021, 175, 104109. [Google Scholar] [CrossRef]
- Scibiorski, E.; Jourdan, F.; Mezger, K.; Tohver, E.; Vollstaedt, H. Cryptic excess argon in metamorphic biotite: Anomalously old 40Ar/39Ar plateau dates tested with Rb/Sr thermochronology and Ar diffusion modelling. Geochim. Cosmochim. Acta 2021, 315, 1–23. [Google Scholar] [CrossRef]
- Bai, X.J.; Li, Y.L.; Hu, R.G.; Liu, X.; Tang, B.; Gu, X.P.; Qiu, H.N. High-precision microcline 40Ar/39Ar dating by combined techniques. Chem. Geol. 2024, 655, 122086. [Google Scholar] [CrossRef]
- Nie, F.; Jiang, S.; Liu, Y.; Chen, W.; Zhang, S. 40Ar/39Ar isotopic age dating on k-feldspar separates from eastern Huaniushan granite Gansu province, and its geological significance. Sci. Geol. Sin. 2002, 37, 415–422. [Google Scholar]
- Wang, Y.; Zhou, S. 40Ar/39Ar dating constraints on the high-angle normal faulting along the southern segment of the Tan-Lu fault system: An implication for the onset of eastern china rift-systems. J. Asian Earth Sci. 2009, 34, 51–60. [Google Scholar] [CrossRef]
- Liu, Z.S.; Shi, H.S.; Zhu, J.Z.; Qiu, H.N.; Zhang, Z.L.; Yun, J.B. Detrital K-feldspar 40Ar/39Ar ages: Source constraints of the lower Miocene sandstones in the Pearl River Mouth basin, South China Sea. Acta Geol. Sin. 2012, 86, 383–392. [Google Scholar]
- Zhu, X.; Li, G.; Chen, H.; Ma, D.; Huang, H. Zircon U-Pb, molybdenite Re–Os and K-feldspar 40Ar/39Ar dating of the bolong porphyry Cu–Au deposit, Tibet, China. Resour. Geol. 2015, 65, 122–135. [Google Scholar] [CrossRef]
- Davids, C.; Benowitz, J.A.; Layer, P.W.; Bergh, S.G. Direct 40Ar/39Ar K-feldspar dating of late Permian—Early Triassic brittle faulting in northern Norway. Terra Nova 2018, 30, 263–269. [Google Scholar] [CrossRef]
- Sun, X.L.; Tian, Y.T.; Kuiper, K.F.; Li, C.A.; Zhang, Z.J.; Wijbrans, J.R. No Yangtze river prior to the late Miocene: Evidence from detrital muscovite and K-feldspar 40Ar/39Ar geochronology. Geophys. Res. Lett. 2021, 48, e2020GL089903. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Liu, W.M. Lithostratigraphy of Shandong Province; China University of Geosciences Press: Wuhan, China, 1996; pp. 207–254. (In Chinese) [Google Scholar]
- Liu, M.W.; Zhang, Q.Y.; Song, W.Q. Division of the Cretaceous lithostratigraphy and volcanic sequences of Shandong. J Stratigr. 2003, 27, 247–253. [Google Scholar]
- Qin, H.F.; Hao, W.X.; Deng, C.L.; Zhao, P.; Shen, Z.S.; Han, F.; He, H.Y.; Pan, Y.X.; Zhu, R.X. Sinistral displacement along the Tan–Lu Fault during the Cretaceous induced by Paleo-Pacific subduction: Constraints from new paleomagnetic and U–Pb geochronological data. J. Asian Earth Sci. 2022, 237, 105362. [Google Scholar] [CrossRef]
- Renne, P.R.; Balco, G.; Ludwig, K.R.; Mundil, R.; Min, K. Response to the comment by W.H. Schwarz et al. on “Joint determination of K-40 decay constants and 40Ar*/40K for the Fish Canyon sanidine standard, and improved accuracy for Ar-40/Ar-39 geochronology” by PR Renne et al. (2010). Geochim. Cosmochim. Acta 2011, 75, 5097–5100. [Google Scholar] [CrossRef]
- Wang, F.; Jourdan, F.; Lo, C.H.; Nomade, S.; Guillou, H.; Zhu, R.X.; Yang, L.K.; Shi, W.B.; Feng, H.L.; Wu, L.; et al. YBCs: A new standard for 40Ar/39Ar dating. Chem. Geol. 2014, 388, 87–97. [Google Scholar] [CrossRef]
- McDougall, I.; Harrison, T.M. Geochronology and Thermochronology by the 40Ar/39Ar Method; Oxford University Press: Oxford, NY, USA, 1999; pp. 199–204. [Google Scholar]
- Lee, J.Y.; Marti, K.; Severinghaus, J.P.; Kawamura, K.; Yoo, H.-S.; Lee, J.B.; Kim, J.S. A redetermination of the isotopic abundances of atmospheric Ar. Geochim. Cosmochim. Acta 2006, 70, 4507–4512. [Google Scholar] [CrossRef]
- Koppers, A.A.P. ArArCALC-software for 40Ar/39Ar age calculations. Comput. Geosci. 2002, 28, 605–619. [Google Scholar] [CrossRef]
- Li, X.H.; Liu, Y.; Li, Q.L.; Guo, C.H.; Chamberlain, K.R. Precise determination of Phanerozoic zircon Pb/Pb age by multi-collector SIMS without external standardization. Geochem. Geophys. Geosyst. 2009, 10, Q04010. [Google Scholar]
- Sláma, J.; Košler, J.; Condon, D.J.; Crowley, J.L.; Gerdes, A.; Hanchar, J.M.; Horstwood, M.S.A.; Morris, G.A.; Nasdala, L.; Norberg, N.; et al. Plešovice zircon—A new natural reference material for U–Pb and Hf isotopic microanalysis. Chem. Geol. 2008, 249, 1–35. [Google Scholar] [CrossRef]
- Li, Q.L.; Li, X.H.; Liu, Y.; Tang, G.Q.; Yang, J.H.; Zhu, W.G. Precise U-Pb and Pb-Pb dating of Phanerozoic baddeleyite by SIMS with oxygen flooding technique. J. Anal. At. Spectrom. 2010, 25, 1107–1113. [Google Scholar] [CrossRef]
- Stacey, J.S.; Kramers, J.D. Approximation of terrestrial lead isotope evolution by a two-stage model. Earth Planet. Sc. Lett. 1975, 26, 207–221. [Google Scholar] [CrossRef]
- Li, X.H.; Tang, G.Q.; Gong, B.; Yang, Y.H.; Hou, K.J.; Hu, Z.C.; Li, Q.L.; Liu, Y.; Li, W.X. Qinghu zircon: A working reference for microbeam analysis of U-Pb age and Hf and O isotopes. Chin. Sci. Bull. 2013, 58, 4647–4654. [Google Scholar] [CrossRef]
- Tang, X.; Li, J.H. Transmission Electron Microscopy: New Advances and Applications for Earth and Planetary Sciences. J. China Univ. Geosci. 2021, 46, 1374–1415. [Google Scholar]
- Ma, H.W. Comparison of k-feldspar ordering by x-ray powder diffraction and infrared spectroscopy and its relations to Al occupancy and equilibrium temperature. Acta Minera. Sin. 1988, 8, 143–150. [Google Scholar]
- Harris, M.; Salje, E.; Guttler, B.; Carpenter, M. Structural states of natural potassium-feldspar—An infrared spectroscopic study. Phys. Chem. Miner. 1989, 16, 649–658. [Google Scholar] [CrossRef]
- Hecker, C.; Meijde, M.V.D.; Meer, F.D.V.D. Thermal infrared spectroscopy on feldspars—Successes, limitations and their implications for remote sensing. Earth Sci. Rev. 2010, 103, 60–70. [Google Scholar] [CrossRef]
- Theodosoglou, E.; Koroneos, A.; Soldatos, T.; Zorba, T.; Paraskevopoulos, K. Comparative Fourier transform infrared and X-ray powder diffraction analysis of naturally occurred K-feldspars. Bull. Geol. Soc. Greece 2010, 43, 2752–2761. [Google Scholar] [CrossRef]
- Li, S.P.; Xu, H.; Gao, D.; Chen, J.K. Structure and forming temperature of k-feldspar and their metallogenic significance in the Jinchang gold deposit, Heilongjiang. Bull. Mineral. Petrol. Geochemistry 2012, 31, 31–37. [Google Scholar]
- Shoji, T.; Sugi, M. Alkali feldspars: Ordering degree of Al/Si distribution and difference of enthalpies between the low and high forms. J. Mineral. Soc. Japan 1976, 12, 415–427. [Google Scholar]
- Badejoko, T.A. Triclinicity of k-feldspars and trace-element content of Mesozoic granites of Central Nigeria. Chem. Geol. 1986, 54, 43–51. [Google Scholar] [CrossRef]
- Chen, W.M.; Sheng, J.F.; Qian, H.D. Degrees of ordering and origin of K-feldspar phenocrysts in a mineralized porphyry of the Yulong porphyry copper deposit, Tibet. Acta Petrol. Sin. 2006, 22, 1017–1022. [Google Scholar]
- Yang, F.; Wang, Y.; Fan, Z.Y.; Yu, F.J.; Chao, N.F.; Zhang, G.Y.; Liu, H. The order degree and origin of k-feldspar phenocrysts in the mineralized porphyry of the Weilasituo polymetallic deposit in Inner Mongolia. Geol. Resour. 2017, 26, 124–128. [Google Scholar]
- Bian, Q.J.; Zhang, H.K. Studies on typomorphism of K-feldspar from Wulashan gold deposit and its relationship with gold mineralization. Gold Geology 1997, 3, 8–15. [Google Scholar]
- Spence, J.C.H. High-Resolution Electron Microscopy, 3rd ed.; Oxford University Press: Oxford, NY, USA, 2003. [Google Scholar]
- Williams, D.B.; Carter, C.B. Transmission Electron Microscopy, 3rd ed.; Springer Science Press: New York, NY, USA, 2009. [Google Scholar]
- Benzerara, K.; Menguy, N.; Obst, M.; Stolarski, J.; Mazur, M.; Tylisczak, T.; Brown, G.; Meibom, A. Study of the crystallographic architecture of corals at the nanoscale by scanning transmission x-ray microscopy and transmission electron microscopy. Ultramicroscopy 2011, 111, 1268–1275. [Google Scholar] [CrossRef]
- Lovera, O.M.; Richter, F.M.; Harrison, T.M. Diffusion domains determined by 39Ar released during step heating. J. Geophys. Res. Solid Earth 1991, 96, 2057–2069. [Google Scholar] [CrossRef]
- Inger, S.; Ramsbotham, W.; Cliff, R.A.; Rex, D.C. Metamorphic evolution of the Sesia-Lanzo zone, western Alps: Time constraints from multi-system geochronology. Contrib. Mineral. Petr. 1996, 126, 152–168. [Google Scholar] [CrossRef]
- Popov, D.V.; Spikings, R.A.; Scaillet, S.; O’Sullivan, G.; Chew, D.; Badenszki, E.; Daly, J.S.; Razakamanana, T.; Davies, J.H.F.L. Diffusion and fluid interaction in itrongay pegmatite (madagascar): Evidence from in situ 40Ar/39Ar dating of gem-quality alkali feldspar and U-Pb dating of protogenetic apatite inclusions. Chem. Geol. 2020, 556, 119841. [Google Scholar] [CrossRef]
- Li, H.; Yonezu, K.; Watanabe, K.; Tindell, T. Fluid origin and migration of the Huangshaping W–Mo polymetallic deposit, South China: Geochemistry and 40Ar/39Ar geochronology of hydrothermal k-feldspars. Ore Geol. Rev. 2017, 86, 117–129. [Google Scholar] [CrossRef]
- Betsi, T.B.; Kelepile, T.; Shindo, K.; Mapeo, R.B.; Camacho, A. Ar/Ar geochronology of hydrothermal K-feldspar from the Mowana Cu mine and implications for geotectonothermal evolution and Cu mineralisation in the Archean matsitama greenstone belt, Zimbabwe craton, Northeastern Botswana. J. Afr. Earth Sci. 2025, 223, 105521. [Google Scholar] [CrossRef]
- Popov, D.V.; Brovchenko, V.D.; Nekrylov, N.A.; Plechov, P.Y.; Spikings, R.A.; Tyutyunnik, O.A.; Krigman, L.V.; Anosova, M.O.; Kostitsyn, Y.A.; Soloviev, A.V. Removing a mask of alteration: Geochemistry and age of the Karadag volcanic sequence in SE Crimea. Lithos 2019, 324–325, 371–384. [Google Scholar] [CrossRef]
- Popov, D.V.; Spikings, R.A.; Paul, A.N.; Kutzschbach, M.; Ulianov, A.; O’Sullivan, G.; Badenszki, E.; Daly, J.S.; Ovtcharova, M.; Chiaradia, M.; et al. Excess 40Ar in alkali feldspar and 206, 207Pb in apatite caused by fluid-induced recrystallisation in a semi-closed environment in proterozoic (meta) granites of the Mt Isa Inlier, NE Australia. Geosciences 2024, 14, 358. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Yang, L.; Shi, W.; Wu, L.; Wang, F. Challenges in Interpreting 40Ar/39Ar Age Spectra: Clues from Hydrothermally Altered Alkali Feldspars. Geosciences 2025, 15, 188. https://doi.org/10.3390/geosciences15050188
Wang Y, Yang L, Shi W, Wu L, Wang F. Challenges in Interpreting 40Ar/39Ar Age Spectra: Clues from Hydrothermally Altered Alkali Feldspars. Geosciences. 2025; 15(5):188. https://doi.org/10.3390/geosciences15050188
Chicago/Turabian StyleWang, Yinzhi, Liekun Yang, Wenbei Shi, Lin Wu, and Fei Wang. 2025. "Challenges in Interpreting 40Ar/39Ar Age Spectra: Clues from Hydrothermally Altered Alkali Feldspars" Geosciences 15, no. 5: 188. https://doi.org/10.3390/geosciences15050188
APA StyleWang, Y., Yang, L., Shi, W., Wu, L., & Wang, F. (2025). Challenges in Interpreting 40Ar/39Ar Age Spectra: Clues from Hydrothermally Altered Alkali Feldspars. Geosciences, 15(5), 188. https://doi.org/10.3390/geosciences15050188






