Abstract
This study focused on identifying Th-, Nb-Ta-, Zr-, and REE-bearing minerals with a multivariate statistical approach in alkaline syenite to evaluate their radiological risks, at Nikeiba, Egypt. Through microchemical analyses, by utilizing electron probe microanalysis, horite, microlite, monazite, zircon, columbite, and fergusonite were shown to bear uranium and thorium. These minerals have played an important role in higher radioactive zones in the studied alkaline syenite. REE-minerals comprising bastnäsite, monazite, and fluorite and apatite are well recorded. The total rare earth elements (TREE2O3) reveal higher concentrations in bastnäsite than monazite, with averages 74.87 and 63.8 wt%. Ce is considered the most predominant LREE in the analyzed bastnäsite and monazite. The mean values of radionuclide activity concentrations of 238U, 232Th, and 40K are 108 ± 20 Bq/kg, 107 ± 9 Bq/kg, and 1255 ± 166 Bq/kg, respectively. Radiological assessments revealed a radium equivalent activity of 357 Bq/kg, below global limits, but an air-absorbed dose rate (166 nGy/h) and annual effective doses (0.81 mSv/y indoors, 0.20 mSv/y outdoors) exceeding safe thresholds. Additionally, the excess lifetime cancer risk (ELCR) was calculated at 0.00071, surpassing the acceptable limit of 0.00029, making these rocks unsafe for construction use. Statistical analyses further underscored the relationships between radionuclide concentrations and associated risks, highlighting the necessity for continuous monitoring and mitigation.
1. Introduction
Natural radioactivity is an inherent property of certain rocks and minerals that contain trace amounts of radioactive elements such as uranium (238U), thorium (232Th), and potassium-40 (40K). These elements, which have been present since the formation of the Earth, emit radiation in the form of alpha, beta, and gamma particles. This radioactivity varies widely across different types of rocks, influenced by their mineral composition and geological history. Understanding the natural radioactivity in rocks is important for several fields, including environmental monitoring, geochronology, and radiological safety [1,2,3].
The study of natural radioactivity in rocks is critical for assessing radiation exposure to human populations, particularly in regions where high levels of radioactive elements are present. Prolonged exposure to ionizing radiation from naturally occurring radioactive materials (NORMs) can pose health risks, including an increased likelihood of developing cancer. Thus, the distribution and concentration of these radioactive elements in different rock types play a crucial role in determining the radiological safety of residential and industrial areas, especially in construction and mining [4,5,6,7].
In addition to health implications, natural radioactivity serves as a powerful tool in geochronology, the study of the age of rocks and geological events. Radioactive isotopes like uranium-238 and potassium-40 decay over time at known rates, allowing scientists to date the formation of rocks and the timing of geological processes. The presence of radioactive minerals in specific rock types also provides insights into the thermal and tectonic history of the Earth, contributing to our understanding of plate tectonics, mountain building, and continental drift [8,9].
Rare metal mineralization is widely distributed in granites, especially in the Arabian–Nubian Shield (ANS), and has great attention from most economic geologists. These granites are good resources for Zr, Nb, Ta, Sn, Au, B, Be, W, Li, Mo, Th, U, and REEs mineralization and possess higher radiological hazards in many regions such as Ras Abda, Muweilha, Um Naggate, Nuweibi, Abu Dabbab, Um Safi, Um Ara-Um Shilman, and El Sela [10,11,12,13,14,15,16,17,18,19]. Nikeiba granites are considered one of the most predominant rare metals, occurring in the South Eastern Desert (SED), Egypt. It could be selected as a case study of the Arabian–Nubian Shield (ANS) (Figure 1a,b). Geochemically, Nikeiba granites and quartz syenite have been enriched in large-ion lithophile elements such as Rb, Ba, and Sr as well as high field-strength elements like Y, Nb, and Zr. These granitic rocks reveal metaluminous characteristics, calc-alkaline affinity, A-type granites, and emplacement in within-plate environs under extensional regime. The area investigated is of great interest to many researchers [20,21].
Alkaline syenite is a type of igneous rock that could be identified as having higher concentrations of the total alkalis, especially Na and K, than those found in feldspars, the excess manifesting as sodic pyroxenes such as aegirine, sodic amphiboles like riebeckite and arfvedsonite, and feldspathoids with other alkali phases [22,23,24]. Such rock types are distinguished by being deficient in SiO2 and Al2O3 than Na2O and K2O, and will contain acmite and/or nepheline in their norms. The alkaline syenite is distinguished by enrichments of valuable rare metals such as Zr, Nb, Ta, Li, Be, Th, and REEs as well as volatile such as F and Cl [25,26,27]. The objective of the present study is to provide micro-chemical analyses of the investigated rare metals mineralization in the alkaline syenite at Nikeiba. Furthermore, this work strives to measure the natural radionuclides’ concentration and their related environmental hazards from the alkaline syenite rocks. The radioactive risk is determined through many radiological hazard indices.
2. Geologic Setting
The exposed rock types in the investigated Nikeiba are metavolcanics, syenogranite, alkali feldspar granite, quartz syenite, and their related pegmatite bodies. These rocks were invaded by microgranite dikes and quartz veins (Figure 1c). Metavolcanics are found as thick sequences of lava flows of basic to acidic types. They have interbanded with their pyroclastics. They are composed of metarhyolite, metadacite, meta-andesite, and metabasalt (Figure 2a). Syenogranite occurs as medium- to coarse-grained and varies from pink to reddish brown colors. It reveals cavernous weathering, and is highly fractured, jointed, and exfoliated. It is composed essentially of K-feldspar, quartz, plagioclase, and biotite. Alkali feldspar granite is distinguished by its low- to moderate-relief, and being medium- to coarse-grained, grayish white color, strongly weathered, fractured, and highly exfoliated. It consists of quartz, K-feldspar, plagioclase, and biotite. Quartz syenite is characterized by higher peaks (Figure 2b), being massive, medium- to coarse-grained, and pale pink to grayish green color. It shows a boulder appearance (Figure 2c,d), and is strongly weathered, highly fractured, jointed, and exfoliated. It consists of quartz, K-feldspars, plagioclase, amphibole, and biotite. It intrudes into syenogranite with intrusive contacts (Figure 2b). Pegmatite pockets are irregular bodies mostly predominant in the syenogranite and alkali feldspar granite. They are very coarse-grained, red to buff in color, and composed of quartz, K-feldspar, plagioclase, and mica. Microgranite dikes dissected all granites in the prospected area. They have E–W, NNW, NW, and NE structural trends with nearly vertical dipping. Quartz veins are predominant and widely distributed in the different basement rocks of the investigated area. They range in thickness between 0.5 and 20 m, and have E–W, NE, and NW structural trends with nearly steep dipping.
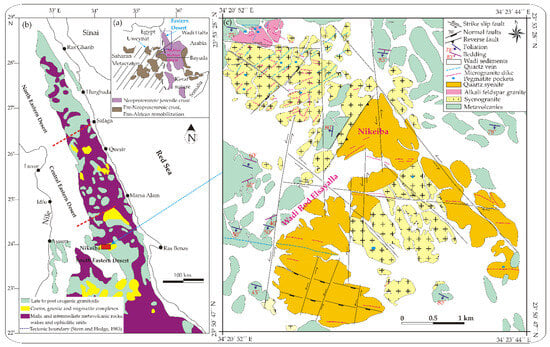
Figure 1.
(a) Geologic map of the ANS; (b) Geologic map presenting the Neoproterozoic basement complex in the SED of Egypt [28]; (c) Detailed geologic map of Nikeiba, SED of Egypt [6].

Figure 2.
(a) Associations of metabasaltic-metarhyolitic (MBS-MRH) metavolcanics. (b) Higher peaks of quartz syenite (QZ SY) intrude into the syenogranite (SYGR). (c,d) Boulder appearances of quartz syenite, Nikeiba, SED of Egypt.
3. Petrography of Alkaline Syenite
The alkaline syenite is medium- to coarse-grained, grayish-green to dark gray in color, and shows higher relief than the surrounding rocks. Microscopically, it is mainly composed of K-feldspars, quartz, biotite, arfvedsonite, riebeckite, and plagioclase (Figure 3a–f). The accessories are zircon, apatite, iron oxides, and opaques, while chlorite, sericite, kaolinite, and carbonates are alteration products. K-feldspar occurs as euhedral to subhedral crystals, prismatic to sub-prismatic in shape, and represented by orthoclase perthite. Orthoclase perthite shows simple twinning and is well presented as string, patchy, and flame types. It encloses fine crystals of biotite, quartz, zircon, and opaques. Quartz occurs as subhedral to anhedral, medium- to coarse-grained, and it poikilitically encloses zoned zircon crystals (Figure 3f). Biotite occurs as large flakes, pleochroic, and partially to completely alters to chlorite. Biotite is sometimes corroded with quartz and plagioclase crystals with the presence of iron oxide minerals. It poikilitically encloses zoned allanite and zircon crystals (Figure 3e). Riebeckite occurs as subhedral prismatic crystals, dark blue to lighter-blue in color, and is highly pleochroic and corroded along their peripheries by K-feldspar and quartz (Figure 3d,e). It encloses opaques, zircon, and quartz. Arfvedsonite presents as euhedral to subhedral prismatic crystals, grayish green to pale green in color, having two sets of cleavage with simple twinning (Figure 3a,f). It is highly altered to chlorite, and encloses opaques. Plagioclase occurs as subhedral crystals; most crystals are enclosed or interlocked with perthite. It shows lamellar, and percline twining. Fine crystals of plagioclase have occurred along the peripheries between arfvedsonite and antiperthite crystals (Figure 3f). Zircon occurs as tiny prismatic crystals with zonation, and is always enclosed in quartz, biotite, feldspars, and riebeckite (Figure 3b,c,f). Allanite presents as burned to brown prismatic crystals and shows faint pleochorism. Titanite occurs as rhombic, euhedral to subhedral crystals enclosed within biotite, riebeckite, and perthite.

Figure 3.
Photomicrographs in the investigated alkaline syenite at Nikeiba, SED of Egypt. (a) Pale green arfvedsonite associated with perthite and antiperthite; (b) Zoned zircon crystal adjacent to opaques; (c) Zircon crystals coated with iron oxides enclosed in quartz; (d) Dark bluish color of corroded riebeckite crystal; (e) Association of corroded burned biotite, pale bluish riebeckite, and quartz crystals; (f) Zircon crystals occurred along peripheries of arvedsonite crystal. Abbreviations: Pth, perthite; Plg, plagioclase; Qz, quartz; Arv, arfvedsonite; Rbk, riebeckite; Bt, Biotite; Zrn, zircon.
4. Materials and Methods
4.1. Analytical Methods
Fourteen polished sections have been completed for the microchemical analyses in the investigated rare metals mineralization from the alkaline syenite rocks. This work has been completed by utilizing a scanning electron microscope that has been fitted with the Pegasus 4000 (EDAX, USA). Microchemical analyses have been performed by utilizing electron probe microanalysis (EPMA) equipped with a CAMECA SX 100 (Wisconsin, USA). The diameter of the electron beam that has been adopted on the surface of the analyzed spot is 1–2 μm. This could be followed by the standard conditions including a 15 kV acceleration potential, 15 nA beam current, and a counting time of 10 s for the peak and 5 s for the background. The microprobe instrument is equipped with an automated wavelength dispersive spectrometer (WDS) and energy dispersive spectrometer (EDS). Natural standards comprise olivine for Mg (Kα), orthoclase for Si (Kα), albite for Al (Kα), wollastonite for Ca (Kα), monazite for Yb (Lα), and hematite for Fe (Kα). The synthetic standard compounds have been used, including UO2, ThO2, ZrO2, PbCrO4, CePO4, NdPO4, SmPO4, GdTiGe, DyRu2Ge2, and YPO4, which are the corresponding standards for U (Mβ), Th (Mα), Zr (Lα), Pb (Mα), Ce (Lα), Nd (Lα), Sm (Lα), Gd (Lα), Dy (Lα), and Y (Lα), respectively. The standard of fluorite is used for F (Kα).
4.2. Sample Collection and Preparation for Radiometric Analysis
A total of 50 alkaline syenite samples, with a mean weight of approximately 250 g each, were collected and dispatched to the Nuclear and Radiological Regulatory Authority in Cairo, Egypt, for subsequent analysis. Upon arrival, the samples were subjected to a drying process. This process was conducted at a temperature of 100 °C for a duration of 72 h. Subsequent to the drying process, each sample was divided into four equal parts, weighed, and transferred into 200 mL polyethylene Marinelli beakers. These sealed beakers were then set aside for approximately 28 days to allow for secular equilibrium before radiometric measurements commenced.
4.3. Gamma Spectrometry Using HPGe Detector
The measurement of granitic samples using high-purity germanium (HPGe) spectrometry commences with the calibration of the HPGe detector. This step is crucial to ensure the accuracy and precision of the spectrometric analysis. Calibration entails the utilization of standard reference materials, which possess known radionuclide concentrations, to establish a baseline for the detector’s response. These standards are certified materials, including RGU-1 for 238U, RGTh-1 for 232Th, and RGK-1 for 40K, which are employed in quantities that are commensurate with those used for construction materials [29,30,31]. Regular calibration checks are performed over time to ensure the accuracy of the detector. The subsequent step involves the identification of the characteristic gamma-ray peaks of the radionuclides of interest, including 238U, 232Th, and 40K. The net peak area, which is defined as the number of counts detected for a specific energy peak, is determined by subtraction: the net peak area is calculated by subtracting the background counts from the total counts under the peak. The activity concentration (A) of a radionuclide in the sample is subsequently calculated using the following formula [32,33]:
where C is the net count rate (counts per second), ε is the detector efficiency for the specific gamma-ray energy, P is the emission probability of the gamma-ray, t is the counting time (seconds), and m is the mass of the sample (kg). This calculation provides the activity concentration in units of Becquerel per kilogram (Bq/kg), allowing for the comparison of radionuclide levels across different samples. Identifying key radionuclides in granitic samples through HPGe spectrometry involves the detection of specific gamma-ray peaks associated with their decay products. Potassium-40 (40K) is directly detected at 1460.7 keV. Uranium-238 (238U) is identified through its decay products, including lead-214 (352 and 295 keV), protactinium-234 (1001 keV), and bismuth-214 (609, 1120, and 1765 keV). Thorium-232 (232Th) is detected via its decay products, with actinium-228 observed at 911 and 338.4 keV and thallium-208 at 583 and 2614 keV. These gamma-ray peaks are crucial for the indirect measurement of 238U and 232Th, assuming secular equilibrium is maintained between parent and progeny nuclides in the samples [34,35]. The Minimum Detectable Activity (MDA) for a radionuclide is a subjective measurement that is influenced by several factors. These include the background radiation (B, in counts per second), the detection efficiency (ε) of the HPGe detector, the probability of emission gamma rays (P), and the counting time (t, in seconds). The formula for calculating the MDA is as follows:
For samples recorded up to 20,000 s, the Minimum Detectable Activities (MDAs) are as follows: 2 Bq/kg for 238U, 4 Bq/kg for 232Th, and 12 Bq/kg for 40K [36]. Moreover, the uncertainties in the measurements are expressed in terms of the standard deviation (±2σ), where σ is calculated as σ = , with Ns and Ts representing the sample counts and measurement time, and Nb and Tb representing the background counts and measurement time, respectively [30].
Radium equivalent activity (Raeq) is a standard metric used to evaluate the potential health risks associated with radiation exposure. To ensure that the annual effective dose (AED) for the general population remains below 1 mSv, Raeq values must be less than 370 Bq/kg (Equation (3)). The contribution of gamma radiation from radionuclides such as 238U, 232Th, and 40K in the environment is assessed by determining the absorbed dose rate at a height of 1 m (Equation (4)). The annual effective dose, both in the indoor and outdoor environment, reflects the total radiation exposure over the course of a year and is calculated to determine radioactivity levels (Equations (5) and (6)). Furthermore, the excess lifetime cancer risk is utilized to estimate the probability of developing fatal cancer as a result of prolonged exposure to gamma radiation (see Equation (7)) [37,38].
Raeq (Bq/kg) = AU + 1.43 ATh + 0.077 AK
Dair (nGy·h−1) = 0.430 AU + 0.666 ATh + 0.042 AK
AEDout (mSv/y) = Dair (nGy/h) × 0.2× 8760 (h/y) × 0.7 (Sv/Gy) × 10−6 (mSv/nGy)
AEDin (mSv/y) = Dair (nGy/h) × 0.8× 8760 (h/y) × 0.7 (Sv/Gy) × 10−6 (mSv/nGy)
ELCR = AED × DL × RF
5. Results and Discussion
5.1. Rare Metals Mineralization
5.1.1. Radioactive Minerals
Thorite is a radioactive mineral found in the alkaline syenite. It presents as bright subhedral to anhedral, fine-grained, and is mostly enclosed in quartz, K-feldspar, and plagioclase (Figure 4a–c). From EPMA data, thorite is composed mainly of ThO2, which ranges from 55.91 to 61.6 wt%, and SiO2 ranges from 19.22 to 20.9 wt%. Y2O3 is well recorded in the analyzed thorite crystals and reached up to 7.71 wt% and could indicate a replacement for Th by Y. P2O5, CaO, Fe2O3, and Al2O3 are recorded in the analyzed thorite with low concentrations (Table 1). Ce2O3 and Nd2O3 are reported in thorite and reached up to 1.11 and 1.01 wt%, respectively. The total is very close to 90 wt% in the analyzed thorite crystals (Table 1). This could be attributed to extensive metamictization and/or hydration processes [39,40,41].
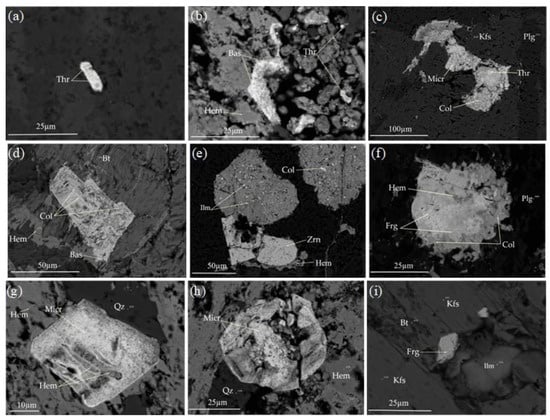
Figure 4.
Back-scattered images of Th-, Nb-, Ta-bearing mineralization from alkaline syenite at Nikeiba, SED, Egypt. (a) Fine-grained thorite; (b) Association of thorite and bastnäsite, surrounded by hematite; (c) Columbite encloses microinclusion of thorite, and associated with microlite; (d) Zoned columbite crystals associated with bastnäsite along their peripheries; (e) Large crystals of ilmenite enclose microinclusions of columbite, associated with zircon; (f) Highly corroded columbite crystal encloses fergusonite (light gray color) and hematite (dark gray color); (g) Rhombic crystal of microlite encloses hematite (gray color); (h) Large crystal of microlite is highly fractured related to deformation; (i) fine-grained fergusonite associated with ilmenite. Abbreviations: Thr, thorite; Bas, bastnäsite; Col, columbite; Micr, microlite; Zrn, zircon; Frg, fergusonite; Kfs, K-feldspar; Plg, plagioclase; Qz, quartz; Bt, biotite; Ilm, ilmenite; Hem, hematite.

Table 1.
Representative EPMA (oxides, Wt%) of thorite from alkaline syenite at Nikeiba, SED of Egypt.
5.1.2. Nb–Ta-Rich Minerals
Alkaline syenite is enriched in Nb–Ta-rich rare metals mineralization. They include columbite, microlite, and fergusonite.
Columbite occurs as massive, euhedral to anhedral crystals, elongated crystals, which are highly corroded and fine-grained (Figure 4c–f). It occurs as fine microinclusions in ilmenite, and is always enclosed in K-feldspar, quartz, plagioclase, and biotite crystals. It is always associated with thorite, bastnäsite, microlite, zircon, ilmenite, hematite, and rutile. Some columbite crystals enclose fergusonite and hematite (Figure 4f). The analyzed EPMA data reveal that columbite is composed of Nb2O5, which ranges from 66.49 to 71.57 wt%, Fe2O3 from 21.08 to 26.32 wt%, Ta2O5 from 2.55 to 3.69 wt%, and TiO2 from 1.33 to 2.84 wt%. Y2O3 and MnO are recorded as minor constituents and reached up 3.62 and 1.08 wt%, respectively (Table 2). EPMA show that Fe has a higher concentration than Mn (Table 2), and indicates its typical composition of ferro-columbite according to [42]. The ratio of Ta/(Ta + Nb) ranges from 0.02 and 0.03, whereas the ratio of Mn/(Mn + Fe) ranges from 0.02 to 0.05, indicating a marked enrichment of Fe compared to Mn in ferro-columbite crystals (Table 2). The concentrations of radioactive elements uranium and thorium reveal that UO2 and ThO2 reached up 0.98 and 1.5 wt% in the analyzed ferro-columbite crystals.

Table 2.
Representative EPMA (oxides, Wt%) of columbite from alkaline syenite at Nikeiba, SED of Egypt.
Microlite presents as fine- to coarse-grained, reaching to 50 μm in size, is anhedral to subhedral, and has rhombic crystals, while others are corroded and highly deformed (Figure 4c,g,h). It could be associated with ferro-columbite and thorite. Some crystals poikilitically enclose fine-grained hematite and opaques (gray to black grains). It is well-developed in quartz, plagioclase, and K-feldspar. EPMA shows that microlite is composed of Ta2O5, which ranges from 40.46 to 43.21 wt%, and Nb2O5 from 29.33 to 32.86 wt% and FeO, CaO, and TiO2 reached up to 5.29, 4.84, and 1.13 wt%, respectively. Light rare earth elements LRE2O3 (La2O3–Nd2O3) are well recorded and range from 8.47 to 12.68 wt% (Table 3). Microlite is enriched in radioactive elements, especially uranium in which UO2 varies between 4.47 and 8.61 wt% (Table 3).

Table 3.
Representative EPMA (oxides, Wt%) of microlite from alkaline syenite at Nikeiba, SED of Egypt.
Fergusonite occurs as fine-grained (Figure 4f,i), subhedral to euhedral, tabular forms, which are highly corroded and well deformed. It is poikilitically enclosed in columbite crystals as fine-grained (white to pale gray grains). It is characterized by its submetallic or vitreous appearance. EPMA shows that fergusonite is composed of Nb2O5 and Y2O3 with averages of 48 wt% and 28.45 wt%, respectively. The total ∑TREE2O3 (Ce2O3–Yb2O3) of fergusonite from quartz syenite contains considerably higher concentrations, with an average of 15.91 wt%. Substantial amounts of TiO2, CaO, Fe2O3, SiO2, as well as ThO2 and UO2, are well reported (Table 4). It has a structural formula of A3+B5+O4, where the A-site is dominated by REE and Y, and the B-site is occupied by Nb, Ta, and Ti. Other cations are recorded in the A-site.

Table 4.
Representative EPMA (oxides, Wt%) of fergusonite from alkaline syenite at Nikeiba, SED of Egypt.
5.1.3. Zircon
Zircon occurs as euhedral to subhedral, prismatic crystals, and fine- to medium-independent crystals, and reaches up to 50 μm (Figure 4e and Figure 5a–c). Some crystals are highly cracked, and enclose opaques (Figure 5a–c), and hematite occurs along the rims of zircon crystals (Figure 4e). It is almost observed in K-feldspar, plagioclase, and quartz, and is almost associated with bastnäsite, columbite, thorite, and ilmenite from the investigated alkaline syenite. The analyzed EPMA data indicate that zircon is almost composed of ZrO2, which ranges from 61.27 to 63.21 wt%, SiO2 from 30.2 to 32.37 wt%, and HfO2 from 0.91 to 3.54 wt%. Other elements including CaO, Fe2O3, and Y2O3 are well presented as minor constituents with averages value 2.13 and 1.11 wt% (Table 5). The radioactive elements including thorium and uranium are reported with averages of 0.62 and 0.78 wt% for ThO2 and UO2, respectively.

Figure 5.
Back-scattered images of Zr-, REEs-bearing mineralization from alkaline syenite at Nikeiba, SED, Egypt. (a) Quartz encloses zircon and rutile; (b,c) Fine-grained bastnäsite associated with zircon crystals; (d) Bastnäsite encloses microinclusions of rutile (gray color); (e) Quartz crystal encloses bastnäsite and ilmenite; (f) Fine-grained bastnäsite and fluorite surrounded by hematite; (g,h) Anhedral to subhedral monazite, enclosed in K-feldspar; (i) Fine-grained monazite surrounded by hematite. Abbreviations: Zrn, zircon; Bas, bastnäsite; Mnz, monazite; Fl, fluorite; Ap, apatite; Kfs, K-feldspar; Plg, plagioclase; Qz, quartz; Bt, biotite; Ilm, ilmenite; Hem, hematite; Rt, rutile.

Table 5.
Representative EPMA (oxides, Wt%) of zircon from alkaline syenite at Nikeiba, SED of Egypt.
5.1.4. REE-Minerals
From the investigated alkaline syenite, two groups of REE-minerals have been recognized: (i) fluorocarbonates and fluoride-REEs (bastnäsite and fluorite); (ii) phosphatic-REEs (monazite and apatite), (Figure 4 and Figure 5 and Tables S1 and S2).
- i.
- Fluorocarbonates and fluoride-REEs
Bastnäsite is the most predominant fluorocarbonate-LREE mineral recorded in the investigated alkaline syenite. It occurs as anhedral, tabular crystals, fine- to medium-grained, and reaches up to 50 µm (Figure 4b,d and Figure 5b–f). The EPMA data show that Ce2O3 has a higher concentration in the analyzed bastnäsite crystals, which ranges from 37.13 to 38.56 wt% with an average of 37.92 wt%, followed by La2O3, then Nd2O3, Pr2O3, and Sm2O3, with averages 20.43, 11.64, 3.10, and 1.79 wt%, respectively. The radioactive element thorium is reported in the analyzed bastnäsite crystals, and ThO2 ranges from 0.58 to 2.21 wt%, with an average of 1.03 wt%. Y2O3, CaO, and Fe2O3 are recorded as minor constituents with averages of 1.18, 1.1, and 1.5 wt%, respectively. Consequently, Ce is considered the most predominant LREE (0.49 apfu) (Table S1), and specifies bastnäsite-(Ce).
Fluorite (CaF2) occurs as anhedral crystals and is mostly predominant in association with bastnäsite, monazite, and zircon crystals (Figure 5f). It is enclosed in quartz, plagioclase, and K-feldspar crystals. EPMA data reveal that fluorite is composed of F 48.11 wt% and Ca 45.76 wt%. LREEs including La, Ce, Nd, as well as Y, are well presented as minor constituents reaching 1.39, 2.78, 1.46, and 2.21wt%, respectively. Its composition shows similarity to those fluorites recorded in the El Sela granite [43].
- ii.
- Phosphatic-REEs
Monazite presents as subhedral to anhedral crystals, fine-grained, and predominant in K-feldspar, plagioclase, and quartz (Figure 5g–i). It is accompanied with apatite, zircon, thorite, and columbite. The EPMA data reveal that monazite is composed of P2O5, which ranges from 28.92 wt%, with especially enriched LREE concentrations ranging from 62.2 to 65.32 wt% (Table S2). Ce2O3 shows a higher concentration other than LREEs in analyzed monazite crystals and ranges from 27.15 to 31.65 wt%. It is followed by Nd₂O₃, La₂O₃, Pr₂O₃, and Sm₂O₃, with average contents of 13.74, 12.08, 3.07, and 2.83 wt%, respectively. Gd2O3 is the only recorded HREEs in the analyzed monazite crystals with an average of 2.27 wt%. Radioactive elements, including thorium and uranium, are present, with ThO₂ and UO₂ reaching 2.72 wt% and 0.52 wt%, respectively. SiO2 is present as a minor component, with an average of 1.21 wt%.
It is very clear to mention herein from the calculated chemical formula that monazite has a predominance of Ce (0.41 apfu) over other REEs, designating it monazite-Ce (Table S2). The geochemical analyses of monazite crystals show a marked similarity in its composition with lower concentrations of thorium. This could be attributed to the geochemical substitutions that have been carried out according to the following reactions:
as recorded by [12,44].
Th4+ + Si4+ ↔ REE3+ + P5+
Th4+ + Ca2+ ↔ 2REE3+
Apatite occurs as anhedral to subhedral, massive, fine-grained, and enclosed in K-feldspar, quartz, and plagioclase (Figure 5g). Apatite is predominately associated with monazite and zircon. The analyzed EPMA data reveal that CaO ranges from 53.21 to 54.61 wt%, and P2O5 ranges from 40.15 to 41.27 wt%. Apatite bears minor concentrations of LREE, especially La2O3, Ce2O3, and Nd2O3, and reached up to 1.05, 2.56, and 0.81 wt%, respectively. Fluorine ranges from 4.62 to 5.81 wt%, designating it fluorapatite.
5.1.5. Radionuclides Concentrations and Hazards
The concentrations of radionuclides 238U, 232Th, and 40K in alkaline syenite were determined, and the statistical analysis is summarized in Table 6. Each element’s concentration was based on 50 samples (n = 50), and the results offer insights into the variability, distribution patterns, and potential geological influences on these radionuclides. The mean concentration of 238U in the alkaline syenite was 108 ± 20 Bq/kg, reflecting moderate variability. The concentration ranged from 73 to 170 Bq/kg, indicating a relatively broad distribution. The positive skewness value of 0.55 suggests a moderate skew towards higher concentrations, while the kurtosis of 0.30 indicates a distribution slightly flatter than normal. The coefficient of variation (CV) was 0.19%, indicating that the concentration values are not significantly dispersed relative to the mean. These findings imply that 238U concentrations in syenite samples demonstrate variability but tend to cluster around the mean. Conversely, thorium (232Th) concentrations exhibited relative consistency, with a mean of 107 ± 9 Bq/kg. The concentration range (89 to 121 Bq/kg) exhibited a narrower variation compared to that of 238 U, suggesting a reduced dispersion in the data. The skewness value of 0.14 suggests that the distribution is nearly symmetrical, while the negative kurtosis value of −1.16 points to a platykurtic distribution, indicating lighter tails compared to a normal distribution. The low coefficient of variation (CV) of 0.09% further corroborates the stability and low relative variability of 232Th in the samples. These findings suggest that 232Th is more uniformly distributed in the alkaline syenite samples. The mean concentration of 40K was found to be significantly higher than that of the other radionuclides, with a mean value of 1255 ± 166 Bq/kg. The concentration exhibited a considerable range, from 783 to 1628 Bq/kg, reflecting the natural abundance of potassium in rocks. The skewness value of −0.16 indicates a slight negative skewness, suggesting a distribution that is marginally skewed towards lower concentrations. The kurtosis value of 0.76 indicates a leptokurtic distribution, with a more pronounced peak than that of a normal distribution. The CV was 0.13%, indicating moderate variability relative to the mean. The mean concentration values of 238U, 232Th, and 40K in alkaline syenite samples were found to be higher than the worldwide average of 33, 45, and 412 Bq/kg, respectively [3,45]. These statistical patterns likely reflect the mineralogical and geochemical processes responsible for the distribution of radionuclides in the alkaline syenite. The elevated concentration of potassium relative to uranium and thorium is anticipated, given potassium’s role as a predominant constituent in numerous rock-forming minerals, including orthoclase and microcline, along with potassium-rich micas such as biotite and muscovite. In contrast, uranium and thorium are typically present in trace amounts. The relatively moderate variability in 238U and 232Th suggests that these radionuclides are uniformly distributed in the syenite rock matrix, with some localized enrichment in uranium-bearing minerals. In alkaline syenite, uranium is predominantly present in the microlite phase, while thorium manifests in the thorite and monazite phases. As demonstrated in the relevant literature, uranium is predominantly present in the microlite form within alkaline syenite. Conversely, thorium manifests in the thorite and monazite forms.

Table 6.
Descriptive statistics of studied alkaline syenite samples in the studied area.
As indicated by the results of the Kolmogorov–Smirnov test (Table 7), the concentrations of 238U, 232Th, and 40K in alkaline syenite samples appear to be normally distributed. This finding is consistent with the descriptive statistics, which revealed that the skewness and kurtosis values were within the acceptable range, suggesting that the data did not significantly deviate from normality. For 238U, the KS statistic of 0.08 and p-value of 1.00 indicate a strong fit to normality, consistent with the descriptive statistics showing moderate skewness and kurtosis. A similar observation can be made with 232Th and 40K, which exhibited KS statistics of 0.12 and 0.09, respectively, with p-values of 0.49 and 0.85. These results, too, support the hypothesis of a normal distribution, despite minor deviations in skewness and kurtosis. These findings serve to validate the underlying assumption of normality.

Table 7.
Results of Kolmogorov–Smirnov (KS) normality test.
Table S3 discloses the radiological hazard parameters in the alkaline syenite samples and presents critical insights into the levels of natural radioactivity within the study area. The mean radium equivalent activity Raeq of 357 Bq/kg is slightly below the worldwide average of 370 Bq/kg [46]. This finding indicates that, on average, the alkaline syenite rock in this region does not demonstrate excessively high levels of radioactivity compared to global standards. Nevertheless, the proximity to the mean value underscores the necessity for continuous monitoring, as variations in geological formations can result in localized increases in radioactivity that may not be reflected in mean measurements. Consequently, continuous assessments are imperative to elucidate the potential environmental and health impacts associated with natural radiation exposure. Notwithstanding the mean Raeq being within a safe range, the air-absorbed dose rate (Dair) of 166 nGy/h is considerably higher than the global average of 59 nGy/h [47,48]. This elevated dose rate indicates that individuals residing in or proximate to the alkaline syenite rock may experience heightened exposure to radiation relative to generally accepted safety limits. Such elevated levels of Dair can contribute to an increased risk of radiation-related health concerns, underscoring the necessity of community awareness initiatives and regulatory measures to mitigate exposure. Furthermore, the annual effective doses calculated for both outdoor (AEDout) at 0.20 mSv/y and indoor (AEDin) at 0.81 mSv/y indicate that outdoor and indoor exposure exceeds the global average of 0.07 and 0.41 mSv/y, respectively. Indoor environments are frequently where individuals spend the majority of their time, thereby increasing their cumulative exposure to radiation. Furthermore, the excess lifetime cancer risk (ELCR) average of 0.00071 indicates a relatively higher risk associated with prolonged exposure to these radionuclides compared to the permissible limit of 0.00029. This is of particular concern, as indoor environments are often where people spend the majority of their time [49,50]. While this risk is relatively low in absolute terms, it is crucial to consider the long-term exposure implications for populations residing in areas with heightened radioactivity. The observed maximum values for Raeq (437 Bq/kg), Dair (202 nGy/h), and AEDout (0.25 mSv/y) suggest that certain locations may pose significant radiological hazards, necessitating targeted investigations and protective measures. The findings underscore the necessity of regular monitoring and risk assessment to safeguard public health and ensure that exposure to radiation from natural sources remains within acceptable limits.
As illustrated in Table S4, the Pearson correlation analysis discloses substantial relationships between radionuclide concentrations and radiological parameters in alkaline syenite samples. The analysis demonstrates a modest positive correlation of 0.15 between 238U and 232Th, suggesting a marginal tendency for their concentrations to increase in tandem. Conversely, the correlation between 238U and 40K is −0.01, indicating the absence of a substantial relationship between these two radionuclides. Furthermore, 232Th and 40K exhibit a modest positive correlation of 0.16, suggesting a negligible interdependence. 238U demonstrates a strong positive correlation with radium equivalent activity Raeq at 0.75 and air-absorbed dose rate Dair at 0.73, indicating its substantial contribution to overall radioactivity and radiation exposure. A similar correlation was observed between 232Th and Raeq (0.61) and Dair (0.58), suggesting its influence on radiation levels. In addition, 40K demonstrates moderate correlations with Raeq (0.49) and Dair (0.55), suggesting its influence on radiological exposure, though to a lesser extent than that observed for 238U and 232Th. The analysis also highlights strong correlations among radiological parameters themselves, such as Dair, AED, and ELCR, reinforcing the idea that higher radionuclide concentrations contribute to increased radiation exposure and associated cancer risks.
The cluster analysis (Figure S1a) reveals distinct groupings among the radionuclides and radiological risk parameters based on their similarity. Notably, 40K forms a discrete group, indicating its unique behavior due to its significantly higher concentration compared to 238U and 232Th. Conversely, 232Th and ELCR exhibit a close proximity, thereby implying that thorium concentrations bear a significant correlation with cancer risk estimates. Radiological dose-related parameters such as AED (indoor and outdoor), Dair, and Raeq are grouped, reflecting their interrelationship in radiation exposure assessment. 238U is grouped with Raeq, emphasizing its contribution to radium equivalent activity and radiation exposure. The principal component analysis (PCA) (Figure S1b) corroborates these findings by demonstrating that two primary factors account for the majority (88.46%) of the variance. The most significant variable along Factor 1 is 40K, followed by 232Th and 238U, indicating the predominance of potassium in terms of radiological influence. The close proximity of AED, Dair, and Raeq in the clustering analysis suggests a strong correlation between them. ELCR, on the other hand, exhibits a more independent behavior from the radionuclides, positioned along Factor 2. The integration of cluster analysis and PCA elucidates that 40K exerts a pronounced influence on radiological variance, while the remaining variables exhibit more pronounced interrelationships in terms of exposure and risk.
The statistical analysis (Figure S1) indicates that 40K is the primary contributor to the observed radiological variance in the studied alkaline syenite samples. In contrast to 238U and 232Th, which are predominantly found in accessory minerals such as thorite, monazite, and zircon, 40K is a significant component of rock-forming minerals like feldspars and micas. Consequently, its concentration is considerably higher and more homogeneously distributed in the rock matrix. Although 40K exhibits a lower radiotoxicity compared to uranium and thorium, it contributes significantly to the external gamma dose due to its relatively high energy gamma emissions (1460 keV). This underscores the need to consider its role in radiological risk assessments of alkaline syenite and similar rock types. While 40K does not pose significant internal exposure risks unless ingested or inhaled in substantial amounts, its contribution to ambient gamma radiation should not be overlooked, particularly in indoor environments where prolonged exposure may increase cumulative dose rates. In light of the statistical findings, future radiological assessments of building materials derived from alkaline syenite should evaluate 40K contributions alongside uranium- and thorium-related hazards to provide a more comprehensive risk assessment.
6. Conclusions
The investigated alkaline syenite at Nikeiba area, South Eastern Desert of Egypt is mostly enriched in rare metal minerals. Thorite is the main Th-silica mineral, while microlite is a Ta-, Nb-rich mineral containing a higher concentration of UO2, reaching up to 8.61 wt%. Bastnäsite, monazite, and microlite are considered as the main sources for LREE contents, whereas fergusonite is a good source for Y and HREE. Monazite reveals low Th contents of 2.72 wt% ThO2, which could be attributed to the effect of hydrothermal alteration. Additionally, ferro-columbite and fergusonite have higher Nb₂O₅ contents, while microlite shows a higher Ta₂O₅ concentration, indicating good mineral diversity of the investigated alkaline syenite. Radiological assessments revealed that the investigated alkaline syenite shows higher 238U, 232Th, and 40K levels, exceeding the global averages, raising health concerns, which could be attributed to the presence of radioactive minerals such as thorite and monazite as well as microlite and zircon. Although the radium equivalent activity (Raeq) is slightly below the safety limit, elevated air-absorbed dose rates and annual effective doses indicate a need for vigilance. The excess lifetime cancer risk (ELCR) highlights the long-term impact of exposure to these radionuclides. Statistical analysis confirmed a normal distribution of radionuclide concentrations, with 238U and 232Th being primary contributors to radiological hazards. These findings call for ongoing monitoring, public awareness, and regulatory actions to mitigate risks, ensuring public safety and minimizing the environmental impact of natural radioactivity.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/geosciences15040138/s1. Table S1. Representative EPMA (oxides, Wt%) of bastnäsite from alkaline syenite at Nikeiba, SED of Egypt.; Table S2. Representative EPMA (oxides, Wt%) of monazite from alkaline syenite at Nikeiba, SED of Egypt.; Table S3. Radium equivalent content and radiological hazard parameters of the alkaline syenite samples in the studied area.; Table S4. Pearson correlation between natural radionuclides and the radiological hazard coefficients of the alkaline syenite samples in the studied area.; Figure S1. (a) Dendrogram of radionuclide and hazard indices and (b) principal component analysis of the radionuclides concentrations and radiological hazards.
Author Contributions
Conceptualization, A.E.A.G. and M.Y.H.; Methodology, A.E.A.G. and M.Y.H.; Software, A.E.A.G. and M.Y.H.; Validation, A.E.A.G., M.Y.H., S.J.A., A.S. and N.M.A.; Formal Analysis, E.G.P., M.M.G. and S.Y.Y.; Investigation, A.E.A.G., M.Y.H. and M.M.G.; Resources, A.E.A.G., M.Y.H., M.M.G., S.J.A., A.S. and N.M.A.; Data Curation, A.E.A.G. and M.Y.H.; Writing—original draft preparation, A.E.A.G. and M.Y.H.; Writing—review and editing, A.E.A.G. and M.Y.H.; Visualization, A.E.A.G. and M.Y.H.; Supervision, E.G.P., A.E.A.G., M.Y.H. and S.Y.Y.; Project administration, A.E.A.G., M.Y.H., S.J.A., A.S. and N.M.A.; Funding acquisition, A.E.A.G., S.J.A., A.S. and N.M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Taif University, Saudi Arabia, Project No. (TU-DSPP-2024-118).
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors extend their appreciation to Taif University, Saudi Arabia, for supporting this work through project number (TU-DSSP-2024-118).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pavlidou, S.; Koroneos, A.; Papastefanou, C.; Christofides, G.; Stoulos, S.; Vavelides, M. Natural Radioactivity of Granites Used as Building Materials. J. Environ. Radioact. 2006, 89, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Trevisi, R.; Leonardi, F.; Risica, S.; Nuccetelli, C. Updated Database on Natural Radioactivity in Building Materials in Europe. J. Environ. Radioact. 2018, 187, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Taalab, S.A.; Ismail, A.M.; El Maadawy, W.M.; Abdelrahman, K.; Khandaker, M.U.; Sakr, A.K.; Hanfi, M.Y. Natural Radioactivity, Mineralogy and Hazard Assessment of Syenogranites (Ornamental Stones) Using a Statistical Approach. Nucl. Eng. Technol. 2024, 56, 4141–4148. [Google Scholar] [CrossRef]
- Rétif, J.; Zalouk-Vergnoux, A.; Briant, N.; Poirier, L. From Geochemistry to Ecotoxicology of Rare Earth Elements in Aquatic Environments: Diversity and Uses of Normalization Reference Materials and Anomaly Calculation Methods. Sci. Total Environ. 2023, 856, 158890. [Google Scholar] [CrossRef]
- Malain, D.; Regan, P.H.; Bradley, D.A.; Matthews, M.; Santawamaitre, T.; Al-Sulaiti, H.A. Measurements of NORM in Beach Sand Samples along the Andaman Coast of Thailand after the 2004 Tsunami. Nucl. Instrum. Methods Phys. Res. A 2010, 619, 441–445. [Google Scholar] [CrossRef]
- Abdel Gawad, A.E.; Eliwa, H.; Ali, K.G.; Alsafi, K.; Murata, M.; Salah, M.S.; Hanfi, M.Y. Cancer Risk Assessment and Geochemical Features of Granitoids at Nikeiba, Southeastern Desert, Egypt. Minerals 2022, 12, 621. [Google Scholar] [CrossRef]
- Taskin, H.; Karavus, M.; Ay, P.; Topuzoglu, A.; Hidiroglu, S.; Karahan, G. Radionuclide Concentrations in Soil and Lifetime Cancer Risk Due to Gamma Radioactivity in Kirklareli, Turkey. J. Environ. Radioact. 2009, 100, 49–53. [Google Scholar] [CrossRef]
- Abbasi, A. Radiation Risk Assessment of Coastal Biota from a Quasi-Fukushima Hypothetical Accident in the Mediterranean Sea. Mar. Pollut. Bull. 2023, 194, 115363. [Google Scholar] [CrossRef]
- Asaduzzaman, K.; Mannan, F.; Khandaker, M.U. Assessment of Natural Radioactivity Levels and Potential Radiological Risks of Common Building Materials Used in Bangladeshi Dwellings. PLoS ONE 2015, 10, e0140667. [Google Scholar] [CrossRef]
- Zoheir, B.; Lehmann, B.; Emam, A.; Radwan, A.; Zhang, R.; Bain, W.M.; Steele-MacInnis, M.; Nolte, N. Extreme Fractionation and Magmatic–Hydrothermal Transition in the Formation of the Abu Dabbab Rare-Metal Granite, Eastern Desert, Egypt. Lithos 2020, 352–353, 105329. [Google Scholar] [CrossRef]
- Abdalla, H.M.; Helba, H.A.; Mohamed, F.H. Chemistry of Columbite-Tantalite Minerals in Rare Metal Granitoids, Eastern Desert, Egypt. Miner. Mag. 1998, 62, 821–836. [Google Scholar] [CrossRef]
- Abdel Gawad, A.E. Mineral Chemistry (U, Th, Zr, REE) in Accessory Minerals from Wadi Rod Elsayalla Granitoids, South Eastern Desert, Egypt. Arab. J. Geosci. 2021, 14, 1996. [Google Scholar] [CrossRef]
- Hanfi, M.Y.; Abdel Gawad, A.E.; Abu-Donia, A.M.; Abu Khoziem, H.A.; Mira, H.I.; Khandaker, M.U.; Alqahtani, M.S.; Elshoukrofy, A.S.M. Risk Assessment and Rare Metals Mineralization Associated with Alteration Aspects of Rhyolite Flow Tuffs, Egypt. Radiat. Phys. Chem. 2024, 215, 111379. [Google Scholar] [CrossRef]
- Ghoneim, M.M.; Abdel Gawad, A.E.; El-Dokouny, H.A.; Dawoud, M.; Panova, E.G.; El-Lithy, M.A.; Mahmoud, A.S. Petrogenesis and Geodynamic Evolution of A-Type Granite Bearing Rare Metals Mineralization in Egypt: Insights from Geochemistry and Mineral Chemistry. Minerals 2024, 14, 583. [Google Scholar] [CrossRef]
- Abdelfadil, K.M.; Mahdy, N.M.; Ondrejka, M.; Putiš, M. Mineral Chemistry and Monazite Chemical Th–U–Total Pb Dating of the Wadi Muweilha Muscovite Pegmatite, Central Eastern Desert of Egypt: Constraints on Its Origin and Geodynamic Evolution Relative to the Arabian Nubian Shield. Int. J. Earth Sci. 2022, 111, 823–860. [Google Scholar] [CrossRef]
- Surour, A.A. Sn-W-Ta-Mo-U-REE Mineralizations Associated with Alkali Granite Magmatism in Egyptian Nubian Shield. In The Geology of the Egyptian Nubian Shield; Hamimi, Z., Arai, S., Fowler, A.-R., El-Bialy, M.Z., Eds.; Springer International Publishing: Cham, Switzerland, 2021; ISBN 978-3-030-49770-5. [Google Scholar]
- Azer, M.K.; Abdelfadil, K.M.; Ramadan, A.A. Geochemistry and Petrogenesis of Late Ediacaran Rare-Metal Albite Granite of the Nubian Shield: Case Study of Nuweibi Intrusion, Eastern Desert, Egypt. J. Geol. 2019, 127, 665–689. [Google Scholar] [CrossRef]
- Abdelfadil, K.M.; Gharib, M.E.; Uher, P.; Putiš, M. Petrogenesis of Post-Orogenic Pan-African Rare-Element Granitic Pegmatites in the Western Arabian-Nubian Shield, Aswan Area, Southern Egypt. J. Asian Earth Sci. 2022, 224, 105003. [Google Scholar]
- Sami, M.; El Monsef, M.A.; Abart, R.; Toksoy-Koksal, F.; Abdelfadil, K.M. Unraveling the genesis of highly fractionated rare metal granites in the Nubian shield via the rare-earth elements tetrad effect, Sr–Nd isotope systematics, and mineral chemistry. ACS Earth Space Chem. 2022, 6, 2368–2384. [Google Scholar] [CrossRef]
- Abdel Gawad, A.E.; Ali, K.; Eliwa, H.; Sayyed, M.I.; Khandaker, M.U.; Bradley, D.A.; Osman, H.; Elesawy, B.H.; Hanfi, M.Y. Radiological Investigation on Sediments: A Case Study of Wadi Rod Elsayalla the Southeastern Desert of Egypt. Appl. Sci. 2021, 11, 11884. [Google Scholar] [CrossRef]
- Hanfi, M.Y.; Abdel Gawad, A.E.; Eliwa, H.; Ali, K.; Taki, M.M.; Sayyed, M.I.; Khandaker, M.U.; Bradley, D.A. Assessment of Radioactivity in Granitoids at Nikeiba, Southeastern Desert, Egypt; Radionuclides Concentrations and Radiological Hazard Parameters. Radiat. Phys. Chem. 2022, 200, 110113. [Google Scholar] [CrossRef]
- Gibson, S. LE MAITRE, R.W. (Ed.) 2002. Igneous Rocks. A Classification and Glossary of Terms. Recommendations of the International Union of Geological Sciences Subcommission on the Systematics of Igneous Rocks, 2nd Ed. Xvi + 236 Pp. Cambridge, New York, Melbourne: Cambridge University Press. Geol. Mag. 2003, 140, 367. [Google Scholar] [CrossRef]
- Landoll, J.D.; Foland, K.A.; Henderson, C.M.B. Nd Isotopes Demonstrate the Role of Contamination in the Formation of Coexisting Quartz and Nepheline Syenites at the Abu Khruq Complex, Egypt. Contrib. Mineral. Petrol. 1994, 117, 305–329. [Google Scholar] [CrossRef]
- Abdel Gawad, A.E.; Hammam, H.F.; Abd El Rahman, R.M.; Hanfi, M.Y. Environmental Impact Assessment of Granites Bearing Rare Metals Mineralization Utilizing Airborne Gamma-Ray Spectrometric Data, Egypt. J. Radioanal. Nucl. Chem. 2025, 334, 817–834. [Google Scholar] [CrossRef]
- Mogahed, M.M. Petrogenesis of Cogenetic Silica-Oversaturated and -Undersaturated Syenites of Abu Khruq Ring Complex, South Eastern Desert, Egypt. J. Afr. Earth Sci. 2016, 124, 44–62. [Google Scholar] [CrossRef]
- Sørrensen, H. Agpaitic Nepheline Syenites: A Potential Source of Rare Elements. Appl. Geochem. 1992, 7, 417–427. [Google Scholar] [CrossRef]
- Emad, B.M. Alkaline Igneous Rocks, a Potential Source of Rare Metals and Radioactive Minerals: Case Study at Amreit Area, South Eastern Desert, Egypt. Acta Geochim. 2025, 44, 189–214. [Google Scholar] [CrossRef]
- Liégeois, J.-P.; Stern, R.J. Sr–Nd Isotopes and Geochemistry of Granite-Gneiss Complexes from the Meatiq and Hafafit Domes, Eastern Desert, Egypt: No Evidence for Pre-Neoproterozoic Crust. J. Afr. Earth Sci. 2010, 57, 31–40. [Google Scholar] [CrossRef]
- Iqbal, M.; Tufail, M.; Mirza, S.M. Measurement of Natural Radioactivity in Marble Found in Pakistan Using a NaI(Tl) Gamma-Ray Spectrometer. J. Environ. Radioact. 2000, 51, 255–265. [Google Scholar] [CrossRef]
- Abedin, M.J.; Karim, M.R.; Hossain, S.; Deb, N.; Kamal, M.; Miah, M.H.A.; Khandaker, M.U. Spatial Distribution of Radionuclides in Agricultural Soil in the Vicinity of a Coal-Fired Brick Kiln. Arab. J. Geosci. 2019, 12, 236. [Google Scholar] [CrossRef]
- IAEA. Measurement of Radionuclides in Food and the Environment; Technical Reports; IAEA: Vienna, Austria, 1983. [Google Scholar]
- Khandaker, M.U. Radiometric Analysis of Comstruction Materials Using HPGe Gamma-Ray Spectrometry. Radiat. Prot. Dosim. 2012, 152, 33–37. [Google Scholar] [CrossRef]
- El-Gamal, H.; Negm, H.; Hasabelnaby, M. Detection Efficiency of NaI(Tl) Detector Based on the Fabricated Calibration of HPGe Detector. J. Radiat. Res. Appl. Sci. 2019, 12, 360–366. [Google Scholar] [CrossRef]
- Paiva, J.D.S.; Farias, E.E.G.; Franca, E.J. De Assessment of the Equilibrium of Th-228 and Ra-228 by Gamma-Ray Spectrometry in Mangrove Soils. In Proceedings of the INAC 2015: International Nuclear Atlantic Conference Brazilian Nuclear Program State Policy for a Sustainable World, Sao Paulo, Brazil, 4–9 October 2015. [Google Scholar]
- Shahrokhi, A.; Kovacs, T. Radiological Survey on Radon Entry Path in an Underground Mine and Implementation of an Optimized Mitigation System. Environ. Sci. Eur. 2021, 33, 66. [Google Scholar] [CrossRef]
- Papadopoulos, A.; Christofides, G.; Koroneos, A.; Papadopoulou, L.; Papastefanou, C.; Stoulos, S. Natural Radioactivity and Radiation Index of the Major Plutonic Bodies in Greece. J. Environ. Radioact. 2013, 124, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Shuaibu, H.K.; Khandaker, M.U.; Alrefae, T.; Bradley, D.A. Assessment of Natural Radioactivity and Gamma-Ray Dose in Monazite Rich Black Sand Beach of Penang Island, Malaysia. Mar. Pollut. Bull. 2017, 119, 423–428. [Google Scholar] [CrossRef]
- Hasan, M.; Hossain Chaity, A.; Haydar, A.; Ali, I.; Uddin Khandaker, M. Elevated Concentrations of Terrestrial Radionuclides in Sand: An Essential Raw Material Used in Bangladeshi Dwellings. Indoor Built Environ. 2021, 30, 1051–1061. [Google Scholar] [CrossRef]
- Abd El-Naby, H.H. High and Low Temperature Alteration of Uranium and Thorium Minerals, Um Ara Granites, South Eastern Desert, Egypt. Ore Geol. Rev. 2009, 35, 436–446. [Google Scholar] [CrossRef]
- Gaafar, I.; Cuney, M.; Abdel Gawad, A.A. Mineral Chemistry of Two-Mica Granite Rare Metals: Impact of Geophysics on the Distribution of Uranium Mineralization at El Sela shear zone, Egypt. Open J. Geol. 2014, 4, 137–160. [Google Scholar]
- Abdel Gawad, A.E. Mineral Chemistry Aspects of Radioactive Mineralization Associated with Zr-, Nb-, and REE-Bearing Minerals from Felsic Dikes at Abu Hawis, North Eastern Desert, Egypt. Arab. J. Geosci. 2022, 15, 791. [Google Scholar] [CrossRef]
- Černý, P.; Ercit, T.S. Some Recent Advances in the Mineralogy and Geochemistry of Nb and Ta in Rare-Element Granitic Pegmatites. Bull. Minéral. 1985, 108, 499–532. [Google Scholar] [CrossRef]
- Abdel Gawad, A.E.; Ibrahim, E.M. Activity Ratios as a Tool for Studying Uranium Mobility at El Sela Shear Zone, Southeastern Desert, Egypt. J. Radioanal. Nucl. Chem. 2016, 308, 129–142. [Google Scholar] [CrossRef]
- Abd El Ghaffar, N.I. Enrichment of Rare Earth and Radioactive Elements Concentration in Accessory Phases from Alkaline Granite, South Sinai- Egypt. J. Afr. Earth Sci. 2018, 147, 393–401. [Google Scholar] [CrossRef]
- UNSCEAR. Sources and Effects of Ionizing Radiation—Exposures of The Public and Workers From Various Sources of Radiation—UNSCEAR 2008 Report; UNSCEAR: New York, NY, USA, 2010. [Google Scholar]
- Abbasi, A.; Mirekhtiary, S.F. Risk Assessment Due to Various Terrestrial Radionuclides Concentrations Scenarios. Int. J. Radiat. Biol. 2019, 95, 179–185. [Google Scholar] [CrossRef] [PubMed]
- UNSCEAR. Sources and Effects of Ionizing Radiation. In UNSCEAR 2000 Report to the General Assembly; UNSCEAR: New York, NY, USA, 2000; pp. 1–10. [Google Scholar]
- El Dabe, M.M.; Ismail, A.M.; Metwaly, M.; Taalab, S.A.; Hanfi, M.Y.; Ene, A. Hazards of Radioactive Mineralization Associated with Pegmatites Used as Decorative and Building Material. Materials 2022, 15, 1224. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.A.; Tariq, S.; Kamal, U.; Manzoor, S.; Calligaris, C.; Waheed, A. Evaluation of Excessive Lifetime Cancer Risk Due to Natural Radioactivity in the Rivers Sediments of Northern Pakistan. J. Radiat. Res. Appl. Sci. 2014, 7, 438–447. [Google Scholar] [CrossRef]
- Abdullahi, S.; Ismail, A.F.; Samat, S. Determination of Indoor Doses and Excess Lifetime Cancer Risks Caused by Building Materials Containing Natural Radionuclides in Malaysia. Nucl. Eng. Technol. 2019, 51, 325–336. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).