1. Introduction
Carbonate rocks are primarily made up of particles (biological and chemical) with more than 50% carbonate minerals embedded either in clasts or in cement [
1]. Calcareous organisms commonly form carbonate rocks through the accumulation of their bioclasts; thus, these rocks are primarily deposited in areas that are favorable for biological activities, such as shallow marine shelf environments [
2,
3]. Carbonates have been used as raw materials in industrial, construction, and environmental applications for decades [
4,
5,
6].
Carbonate rocks are heterogeneous in nature, both physically and chemically. The main mineral—calcium carbonate (CaCO
3)—is often found in association with other detrital minerals such as clays and quartz [
7]. Depending on their origin, depositional environment conditions, and composition, these rocks are deposited as limestones, travertine, marble, chalk, tufa, and coquina [
8,
9]. Carbonate rocks have different crystalline polymorphs, of which the anhydrous calcite (rhombic), aragonite (needle-like), and vaterite (spherical) varieties are the most common [
10]. Amongst these, calcite is the most stable form of carbonate under ambient conditions [
2]. The magnesium/calcium (Mg/Ca) content results in the precipitation of either dolomite (high MgCO
3 content) or low-Mg calcite (<5% MgCO
3 content) [
11].
In industry, lime, cement, building stone, and crushed stone production relies primarily on carbonate rocks. These rocks and their derived products are commonly used as fluxes, aggregates, fillers, soil conditioners, and in many other industrial applications [
6,
12]. However, specific physical or chemical characteristics are required to properly implement these stones in the context of certain industrial applications. These include compositions with CaCO
3 > 93%, SiO
2 < 3%, MgO < 1.2% (and higher for certain products), Fe
2O
3 < 1.5 (and lower for certain products), and/or S, P
2O
5, and alkaline salt contents as low as possible [
13,
14].
In Jordan, the use of natural stones for construction dates back to the Neolithic (ca. 10,000–4500 years BC) [
15]. Several ancient uses of natural stones as a building material can be clearly observed in the city of Petra, which was carved into sandstone during the Nabatenean times (first century BC); the Roman City of Jerash (ca. 6000 years ago); and the Byzantine and Islamic heritage found across Jordan [
16,
17]. Due to its abundance in the geological record, Jordanian limestone is one of the most-used types of stone for building during these periods and even up to the present time.
The carbonate rocks in Jordan include both the Ajlun and Belqa Groups [
18,
19]. These primarily carbonate rocks were deposited under various hydrodynamic and environmental conditions due to sea level fluctuations and tectonic activity [
19,
20]. These deposits are widespread in Jordan and record the environmental variations in the Tethys Sea area over time [
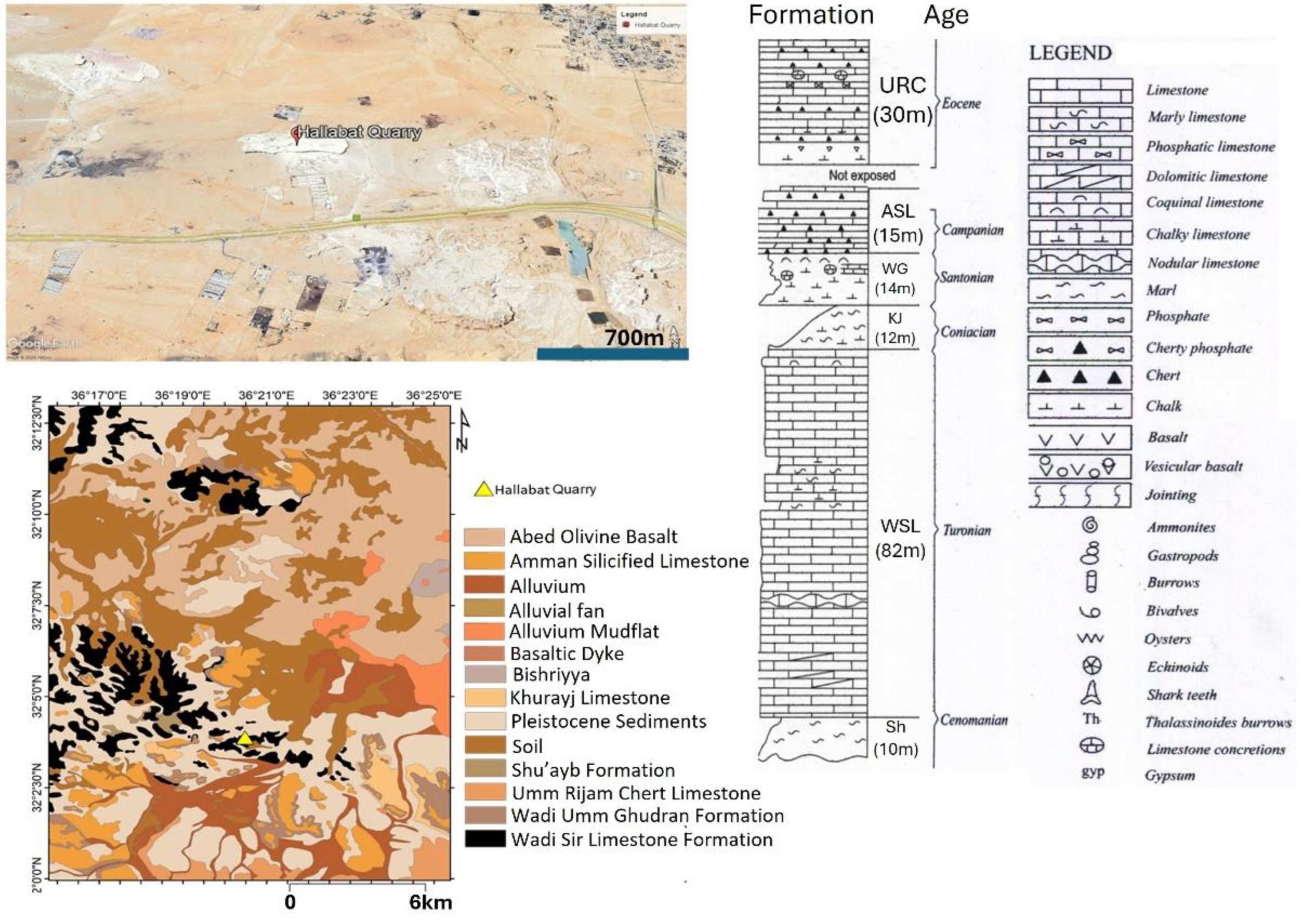
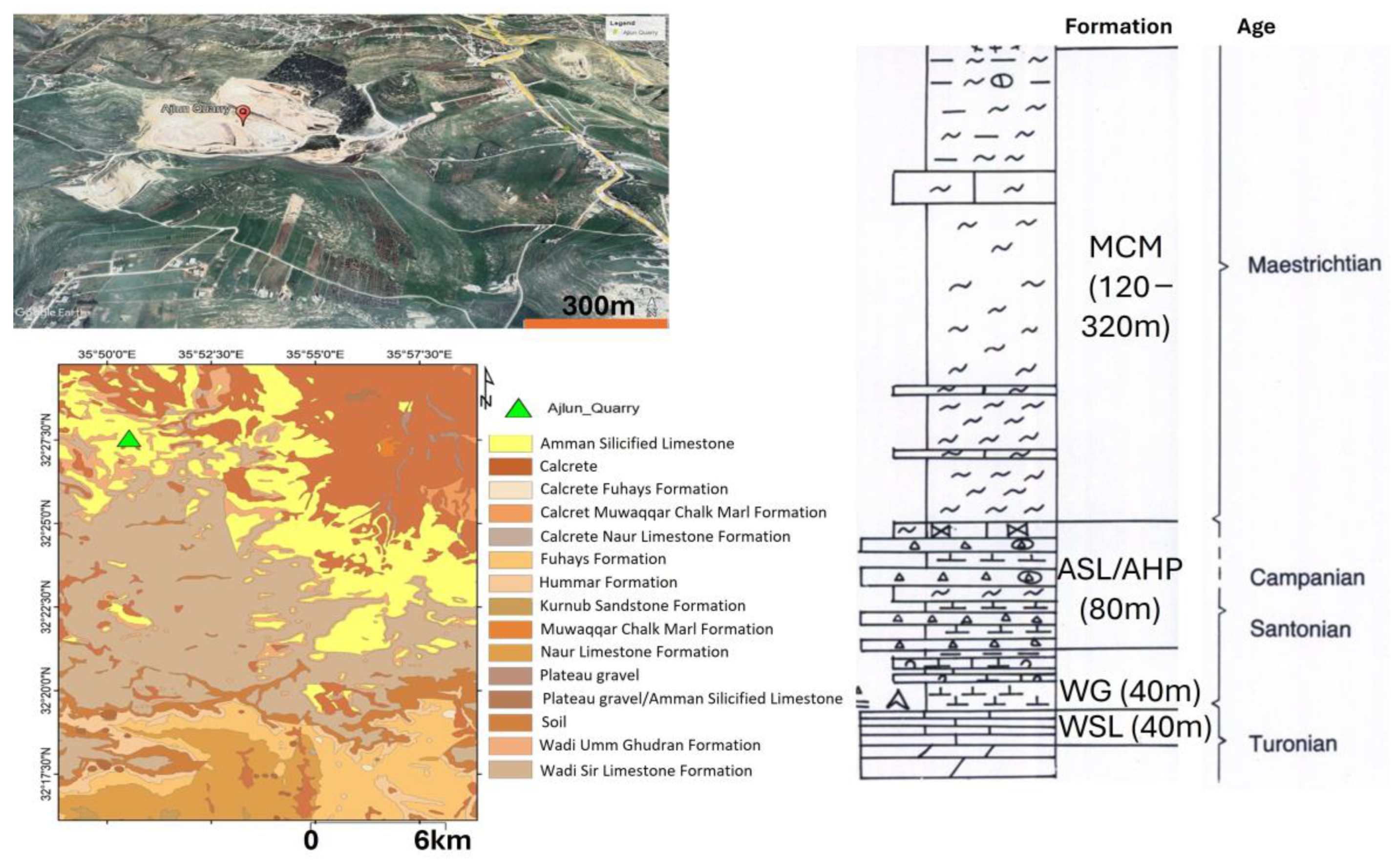
21]. Upper Cretaceous Limestone Formations—namely, the Turonian Wadi As Sir Limestone (WSL) Formation (Ajlun Group) and the Santonian–Campanian Amman Silicified Limestone (ASL) Formation (Belqa Group)—are the main sources of Jordanian limestone.
The WSL Formation outcrops in several sites across Jordan and forms the uppermost part of the Ajlun Group. This succession primarily consists of bedded massive limestone, hard dolomitic limestone with thin layers of marls and chert nodules, mainly in the middle and upper parts of the formation. The fossil assemblages include bivalves and gastropods [
22]. The depositional environments vary from lagoonal conditions to open-shelf conditions and shallow marine environments. The thickness varies from ca. 125 to 150 m [
18,
23,
24]. The ASL Formation is also found in various localities across Jordan. This formation comprises silicified limestones that alternate with chert, phosphatic chert, and limestone. The fossil assemblages in this formation include foraminifera, ammonites, gastropods, and bivalves [
21,
25]. The formation in northern Jordan was deposited in a marine shelf environment.
These limestones have a suitable composition for industrial uses and have been used as building stones for decades [
14,
26], as well as in various industrial applications such as paint and concrete production. Nonetheless, based on observations reported by several researchers (e.g., [
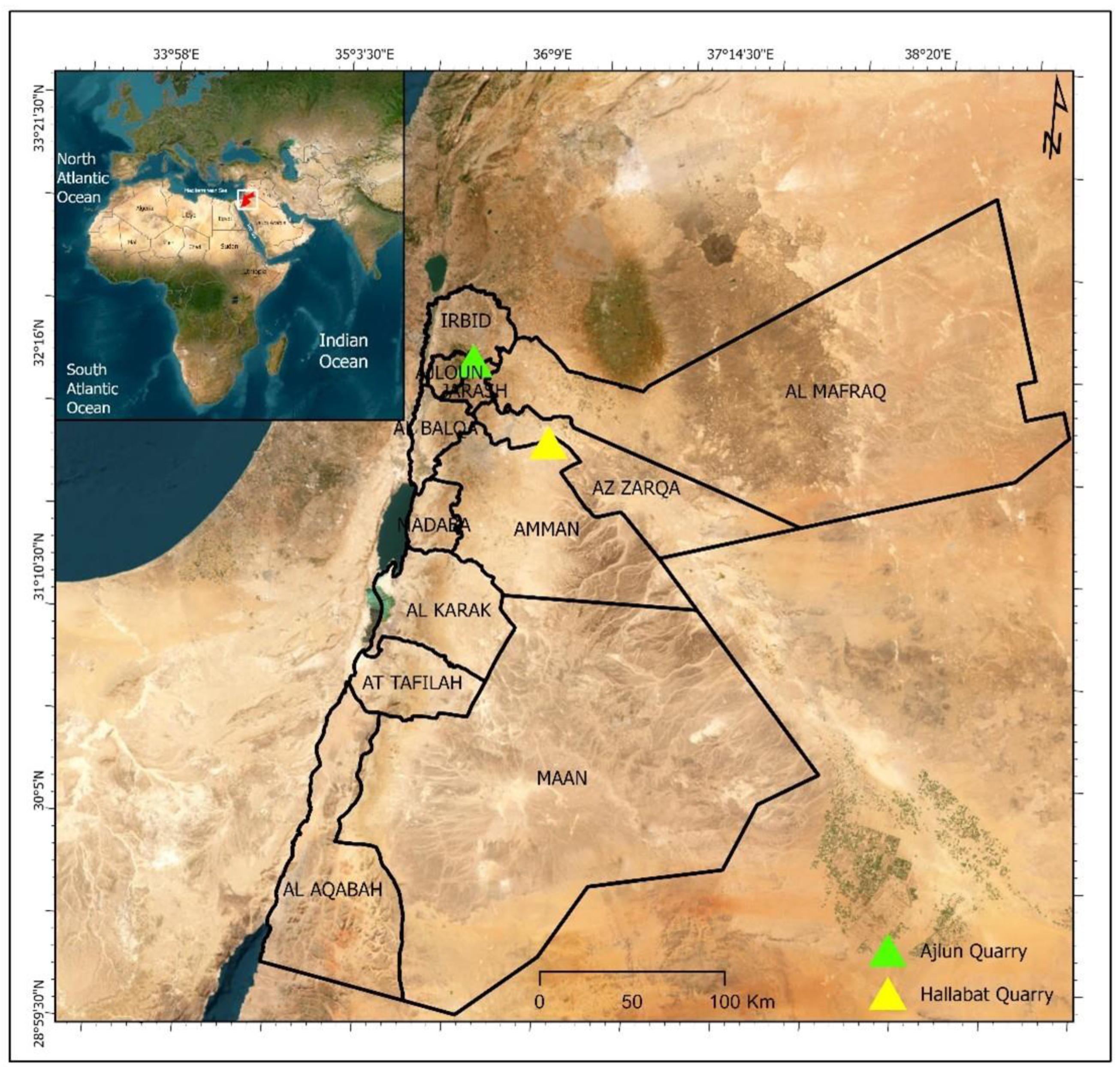
26]), buildings built using such stones have shown deterioration over time due to weathering; therefore, a more precise examination of the composition of these limestones is required. This study, focusing on two main localities in northern Jordan (Al Hallabat and Ajlun; see
Figure 1), provides new insights into the physical and chemical composition of the WSL and ASL carbonate rocks. The two study sites are currently used for limestone quarrying.
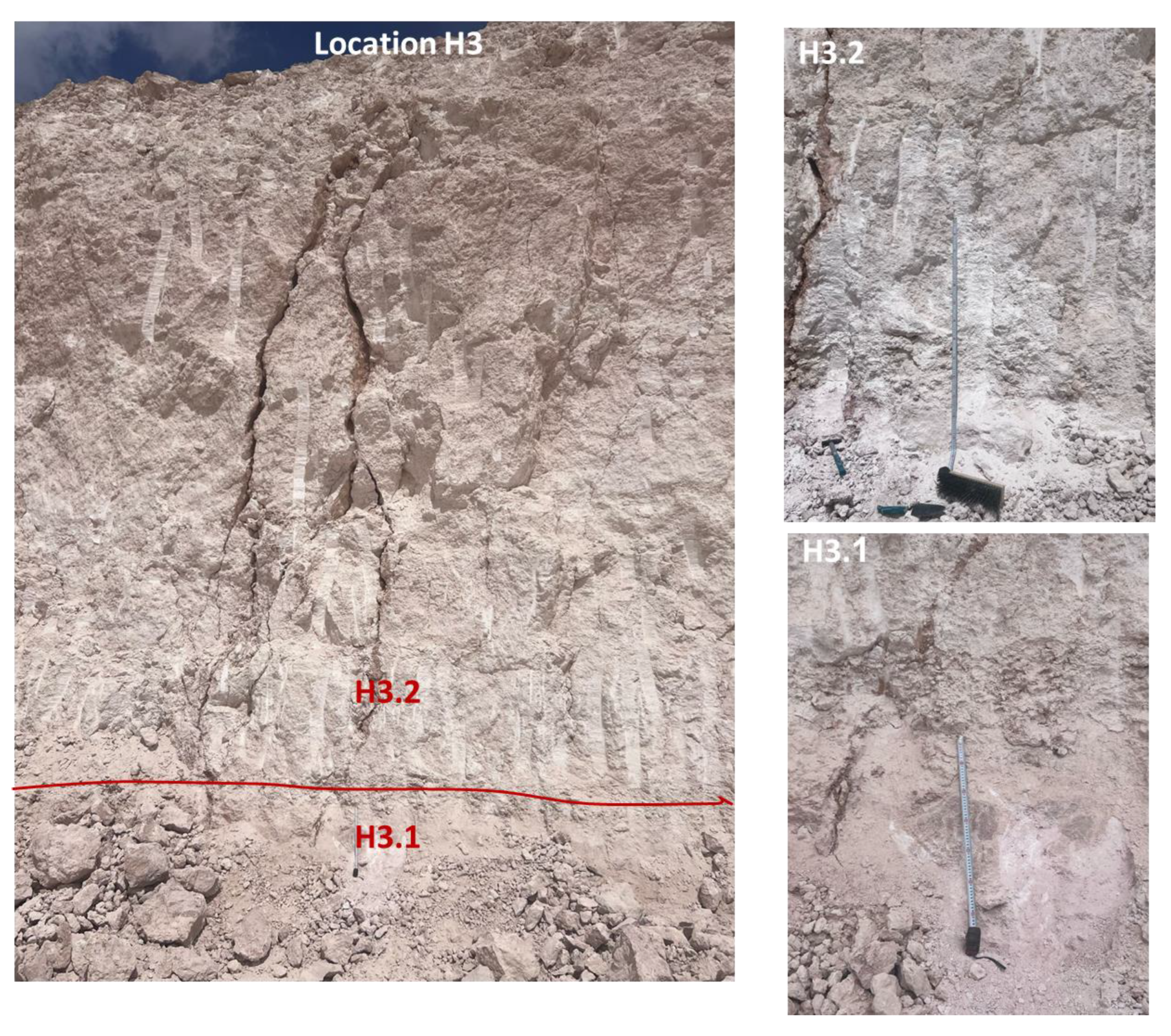
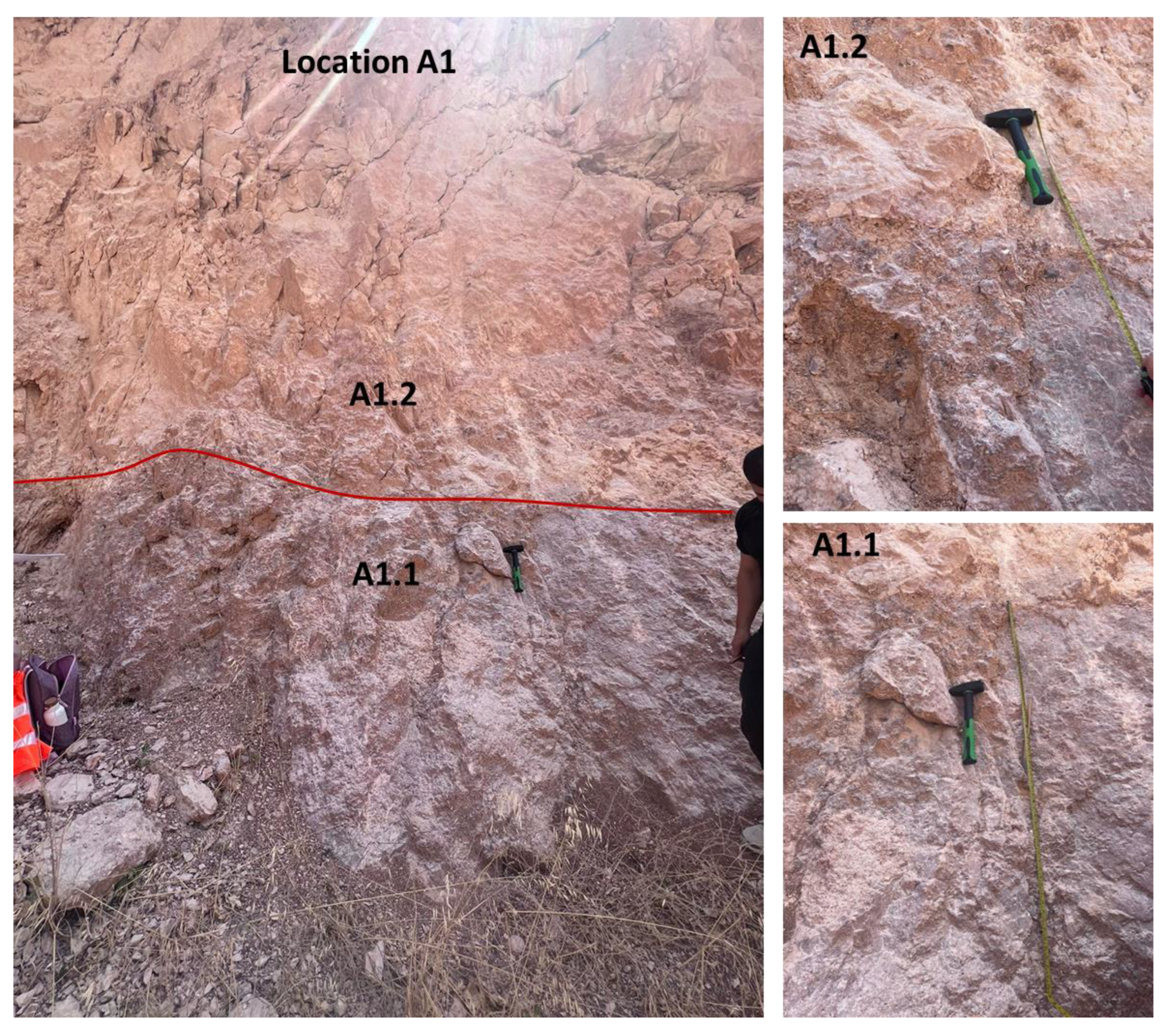
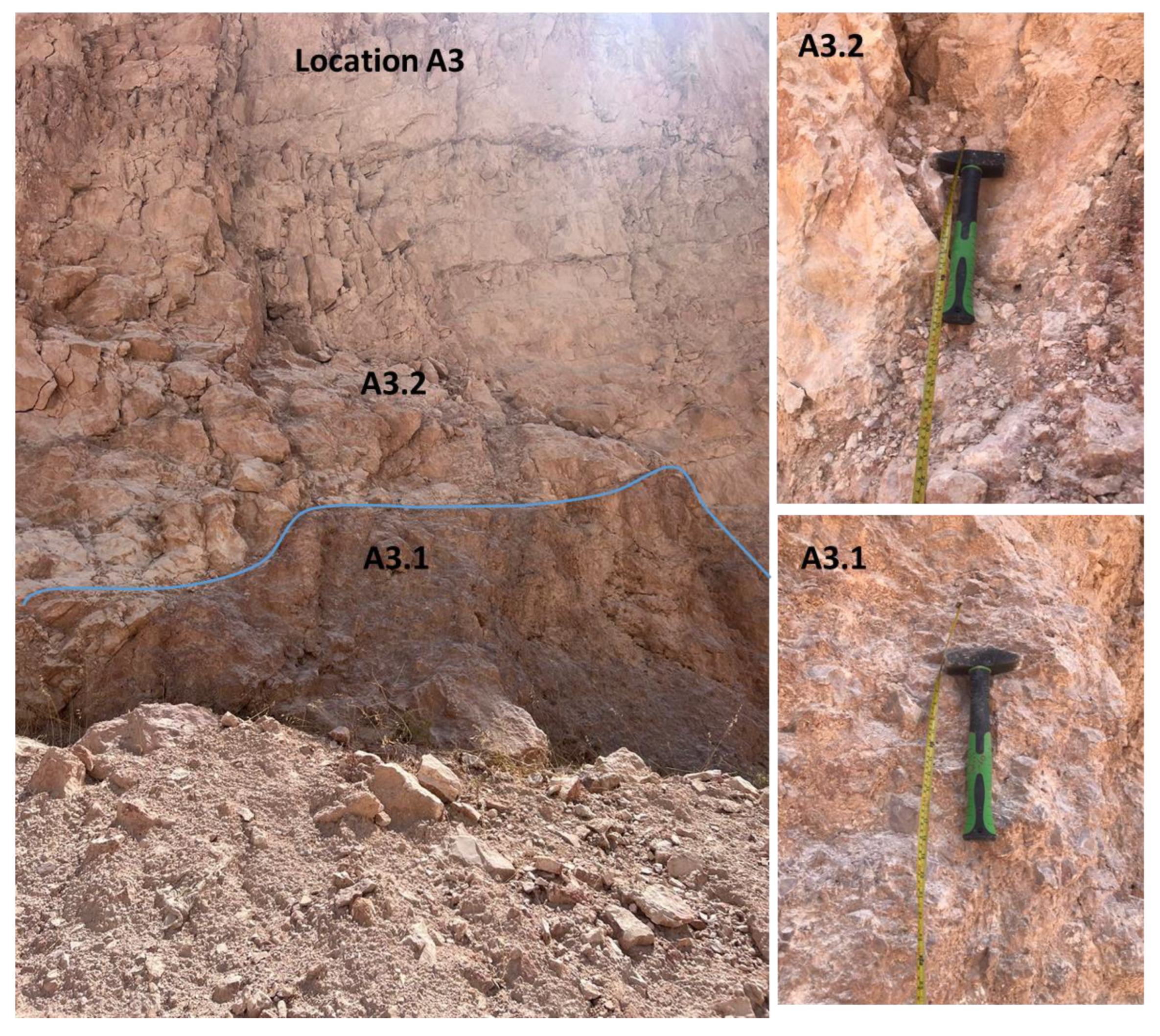
Limestones from two main localities—namely, Hallabat and Ajlun—were investigated. A total of 12 limestone samples were collected from two quarries in the two study sites and analyzed for their mineralogical (XRD), petrographic (microscopic), and elemental (XRF) compositions. The samples were collected from four distinctive beds (two beds in each quarry), representative of the WSL and ASL Formations. This paper provides an overview of the study sites, followed by the methods employed. Then, the results are discussed and put into a wider context for comparison between the two studied quarries and the regional extent. Finally, the composition and potential industrial uses of the carbonates are explored and defined.
4. Results and Discussion
4.1. Elemental Composition
The six samples collected from the Hallabat area were analyzed for their elemental composition (
Table 1). The results indicated variability between the middle and upper parts of the WSL carbonates in the area. Samples H1.1, 2.1, and 3.1 represent the middle part, while samples H1.2, 2.2, and 3.2 represent the upper part. Generally, higher silica content was observed in the upper part (mean 2.023 wt.%) compared with the middle part (mean 0.9 wt.%). This was also associated with higher percentages of detrital elements such as Ti and Al. This suggests that the two parts were probably deposited under different hydrodynamic conditions, with the detrital contribution being higher during the deposition of the upper part of the formation. Furthermore, the CaO content was higher for the middle part (mean 55 wt.%).
The correlation analysis (
Table 2) indicated several statistically significant correlations. The silica content co-varied with detrital elements such as Ti and Al, and positive correlations (
p < 0.05) were observed between SiO
2 and Mg, Na, K, and P, while significant negative correlations were observed between CaO and all elements except for MnO. It has been previously reported in [
20] that the lower part of the WSL Formation in the Al-Tayyar area—located in close proximity to the Hallabat area—may have been deposited under low-energy hydrodynamic conditions based on its microfacies and fossil content, in agreement with lower detrital input and higher levels of in-basin deposition. In addition, the upper parts of the WSL Formation in the same area were reported to have been deposited during a period of high-energy hydrodynamics with current activity [
20], in agreement with higher detrital input and basin disturbance.
Six samples collected from Ajlun were also analyzed for their elemental composition (
Table 3). The results show that, in contrast with the Hallabat WSL carbonate rocks, the lower part of the Ajlun ASL carbonates was more influenced by detrital input compared with the upper part. Higher silica content was recorded in the lower part (mean 2.023 wt.%) compared with the upper part (mean 0.3 wt.%). In addition, higher percentages of Ti, Al, and Fe were present in the lower part, suggesting that the two parts were probably deposited under variable hydrodynamic conditions, with the detrital contribution being higher during the deposition of the lower part of the formation. Furthermore, the upper part’s CaO content was relatively higher (mean 56 wt.%).
The correlation analysis (
Table 4) indicated that the silica content was positively correlated with detrital elements such as Ti and Al (
p < 0.05). Unlike the Hallabat samples, the SiO
2 content only showed a positive correlation (
p < 0.05) with K and negative correlations with Mn, Ca, and Na, likely suggesting that these elements are more related to in-basin depositional processes than basin in-wash. The negative correlations between these elements and other detrital indicators, such as Ti, Al, and Fe, support this observation. On the other hand, the statistically significant negative correlations between CaO with all elements suggest that it may have a specific depositional behavior compared with the others. This agrees with previous research reporting on the environmental deposition of the ASL Formation in northern Jordan; for example, in [
21], it was reported that, based on microfossil and geochemical analyses, the upper parts of the ASL Formation show less submarine weathering and detrital indicators compared to the lower parts, where higher submarine and continental weathering is recorded.
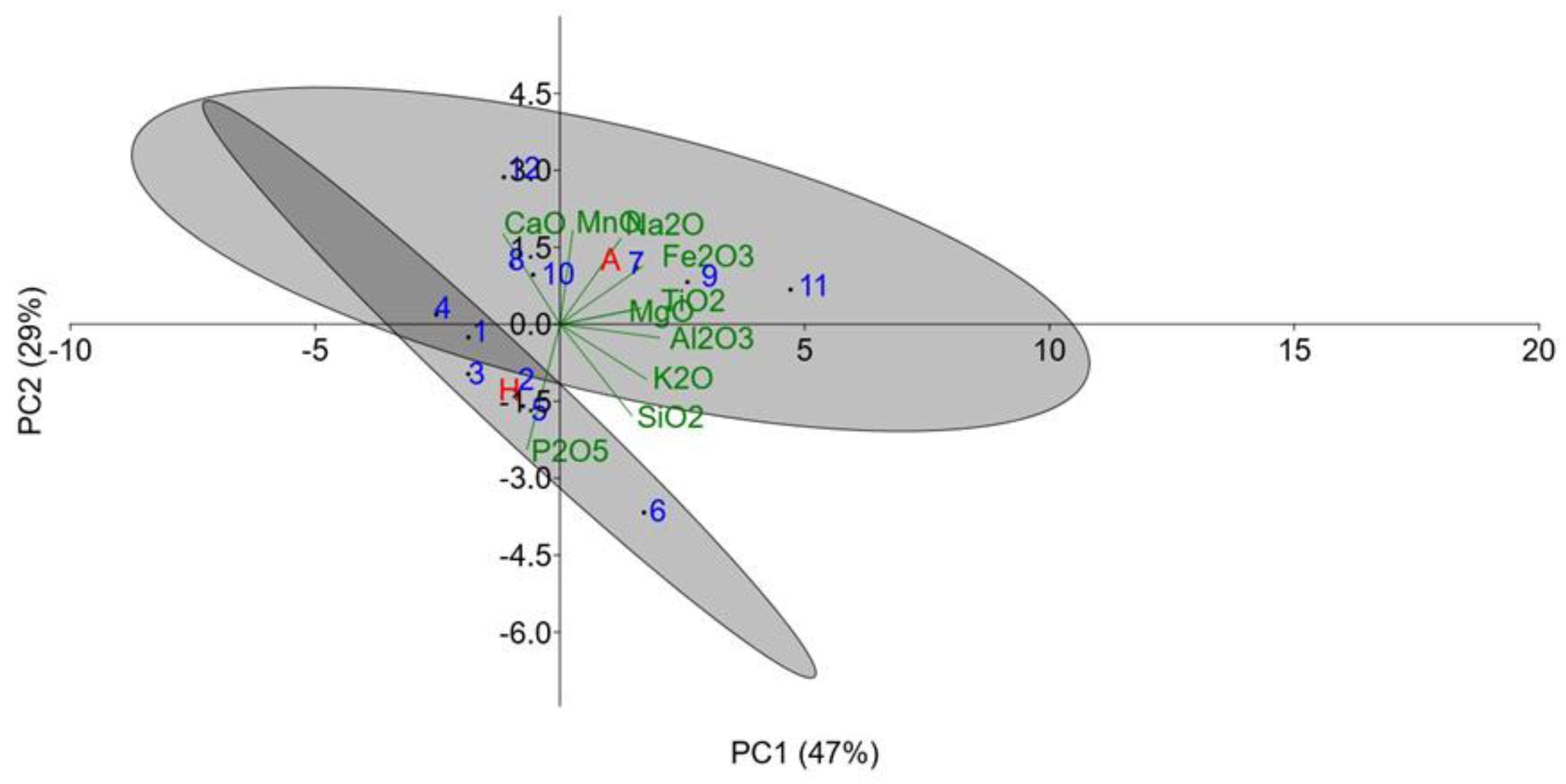
These differences in the elemental composition between the two study sites were further investigated using PCA analyses. The PCA plot (
Figure 5) clearly shows the distinction between samples from the two study sites, namely, the Hallabat samples (1–6) and the Ajlun samples (7–12). The two eigenvectors (PC1 and PC2) explain 76% of the variance within the dataset. The data form two primary directional trends: a horizontal spread along PC1 (−10 to +20) and a vertical spread along PC2 (−6 to +4.5), resulting in two main clusters (as indicated by the grey ellipses). PC1 (47%) represents mixing between carbonate minerals (CaO and MgO) and siliciclastic elements (SiO
2, TiO
2, Al
2O
3), indicating variable depositional environments and suggests an environmental gradient from deep marine (−20) to terrestrial influence (+20). On the other hand, PC2 (29%) reflects diagenetic and authigenic processes.
Hallabat samples (1–6) present low PC1 values (mean = −5.6; −8 to −3) compared to the Ajlun samples’ (7–12) PC1 values (mean = +4.8; +2 to +8), suggesting carbonate-dominated compositions and siliciclastic influence, respectively. For the diagenetic trends, the PC2 values for Hallabat (mean = −1.2, −6 to +1) and Ajlun (mean = 0.5, −1 to +2) suggest a wider range of diagenetic alteration compared to a more consistent diagenetic signature, respectively. The centroid distance between the two groups (10.63) indicates differences in depositional history, confirming that the two groups originate from two different geological formations, namely, the WSL and ASL Formations. The Hallabat samples show variable CaO and MgO contents and siliciclastic content with variable Fe2O3 content. In terms of depositional history, the Hallabat samples suggest more carbonate facies with variable detrital input, while the Ajlun samples represent transitional facies with terrigenous input.
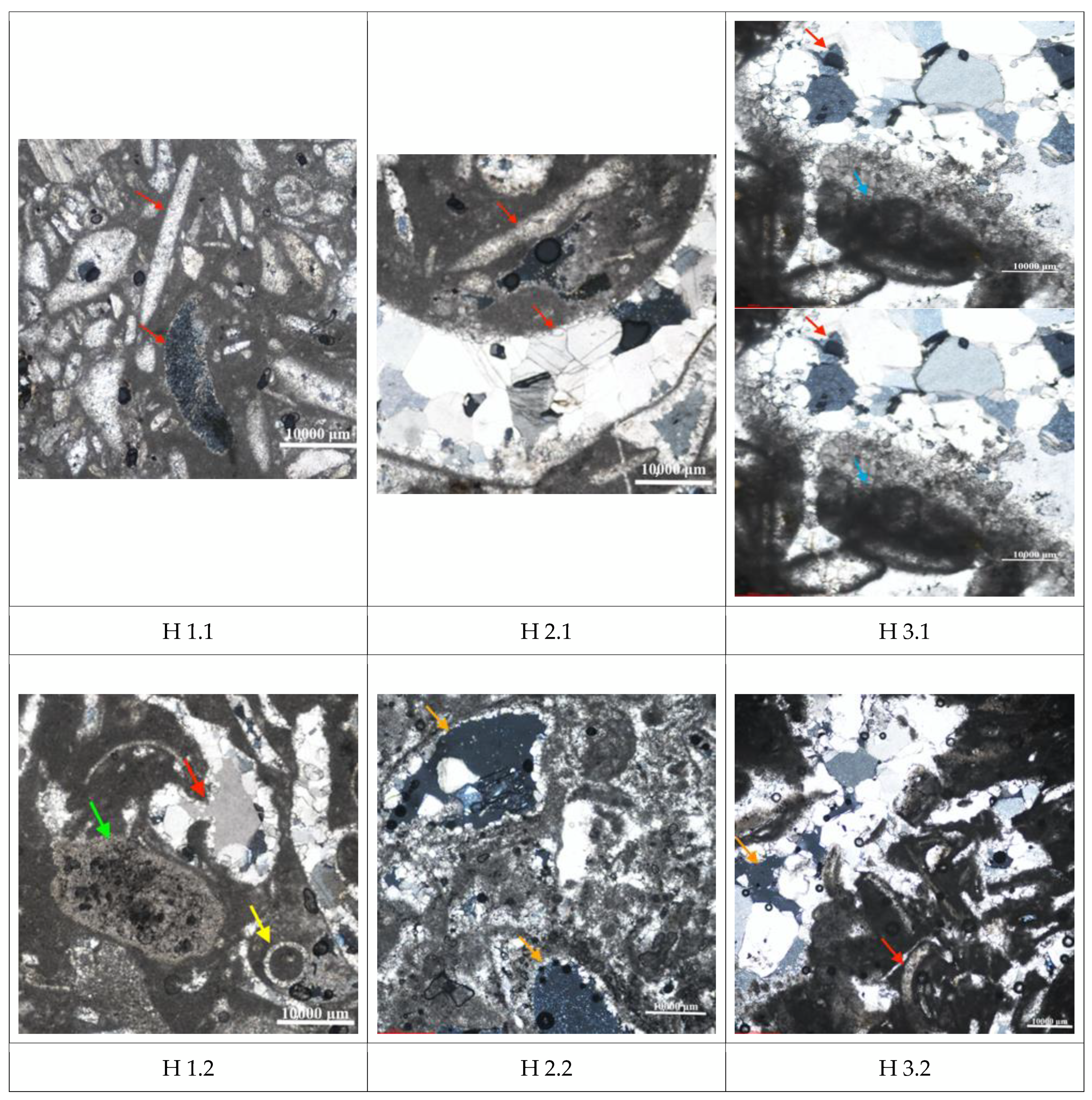
4.2. Petrography
The petrographic analysis (
Figure 6 and
Figure 7) indicated that the main component of the studied carbonates is microcrystalline calcite (micrite). Referring to widely known classifications of carbonates [
30,
31], the Hallabat limestone is composed of >10% mud-supported allochems (grains), where the allochem types are bioclasts (>25%) and intraclasts (<25%). Therefore, according to [
30], the rock class is Biomicrite. These findings are similar to previous studies reporting the composition of the WSL Formation to include various fossil assemblages, micrite, and lithoclasts [
20,
22].
The Ajlun samples contain small lithoclasts of ancient carbonate grains—pellets that are composed of fine-grained carbonates without nuclei—in agreement with the PCA results, where PC 3 (11%) positively correlates with CaO and detrital indicators such as TiO
2, likely suggesting that minor amounts of carbonates were supplied through basin in-wash processes. The cracks developed by dissolution are filled with coarse-grained recrystallized silica. The Ajlun limestone is composed of <1% allochems with spray patches. According to Folk (1959) [
30], the rock class is Dismicrite. These findings are in agreement with previous research into the composition of the WSL Formation [
20,
22].
4.3. Mineralogy
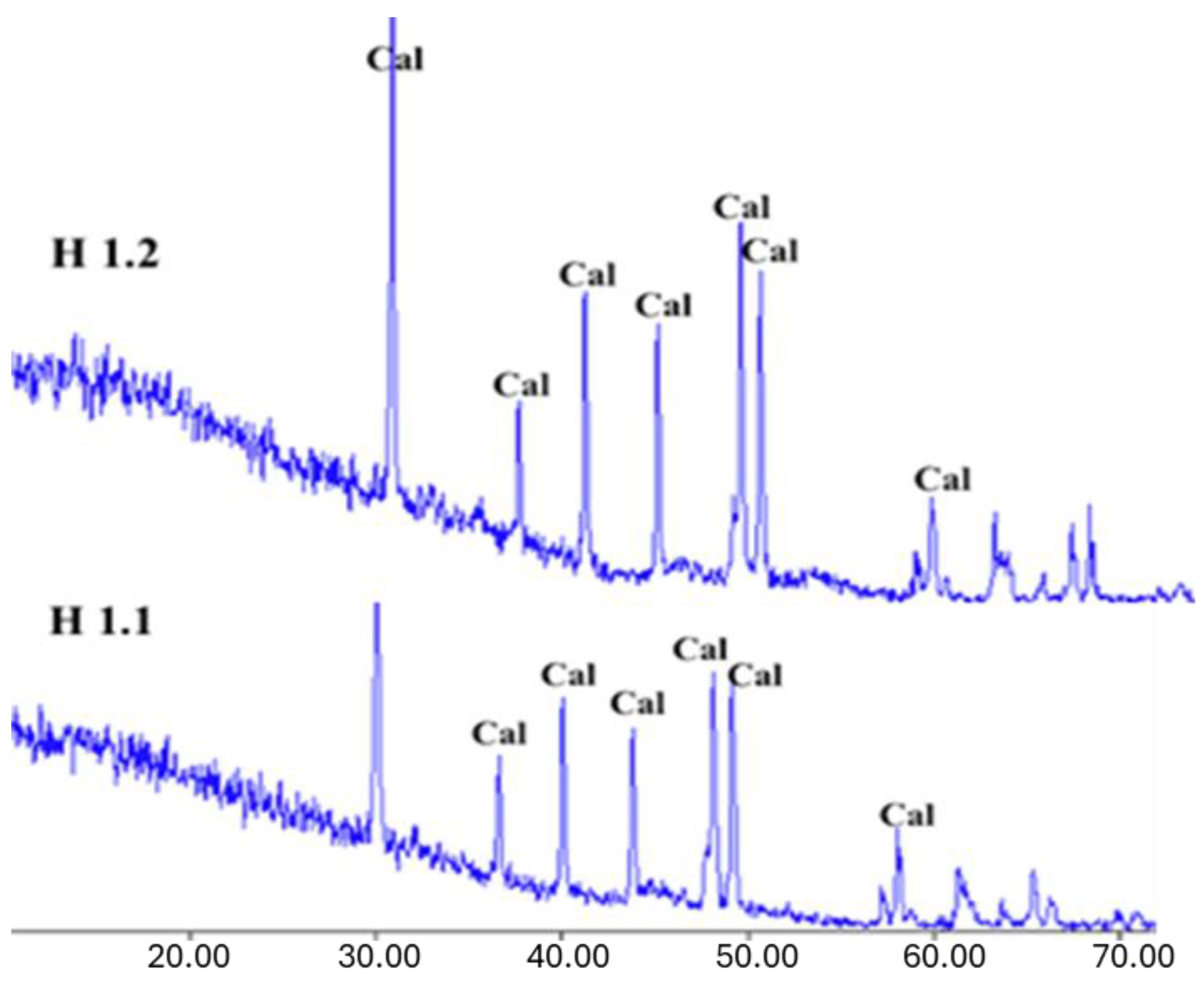
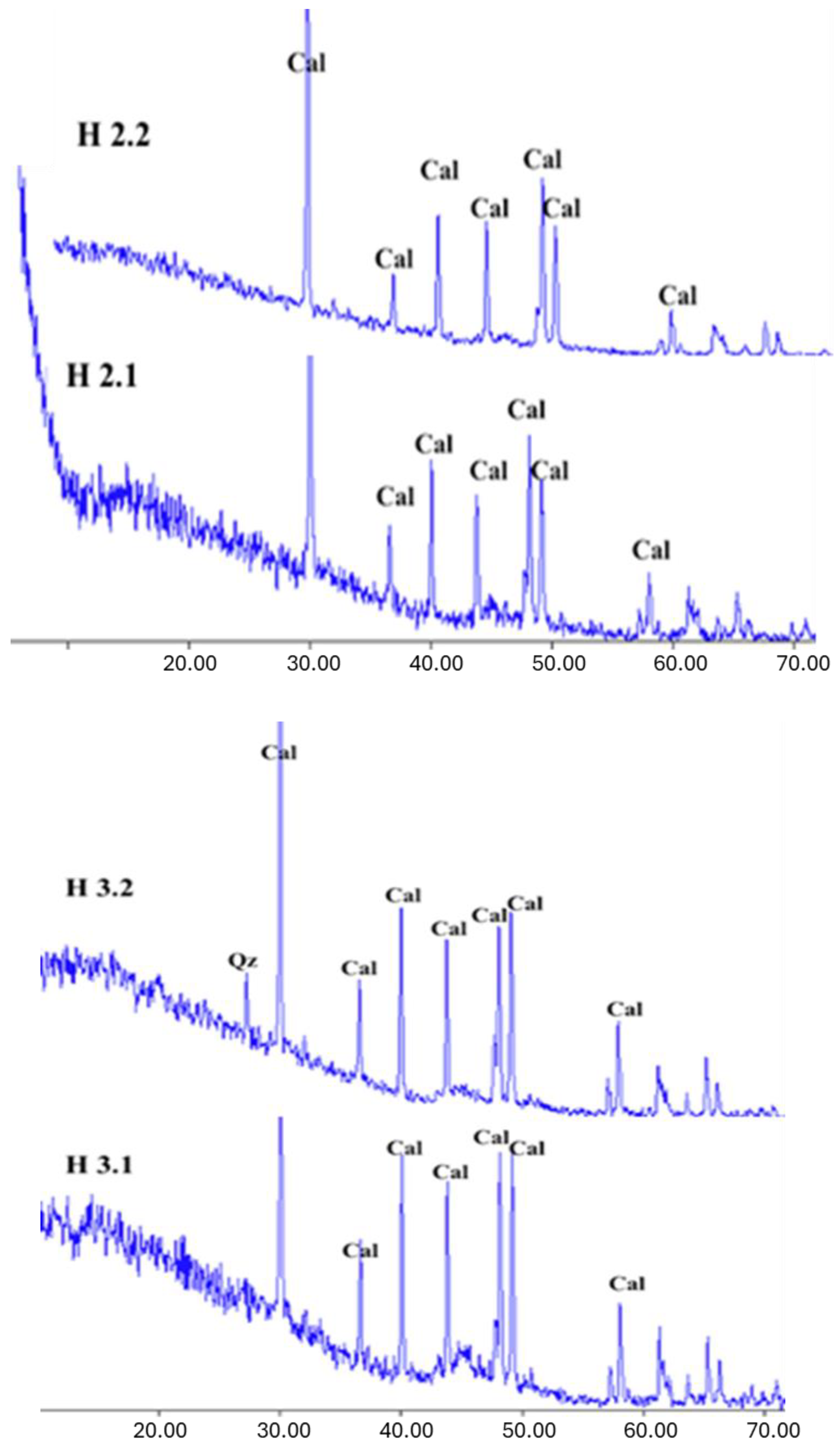
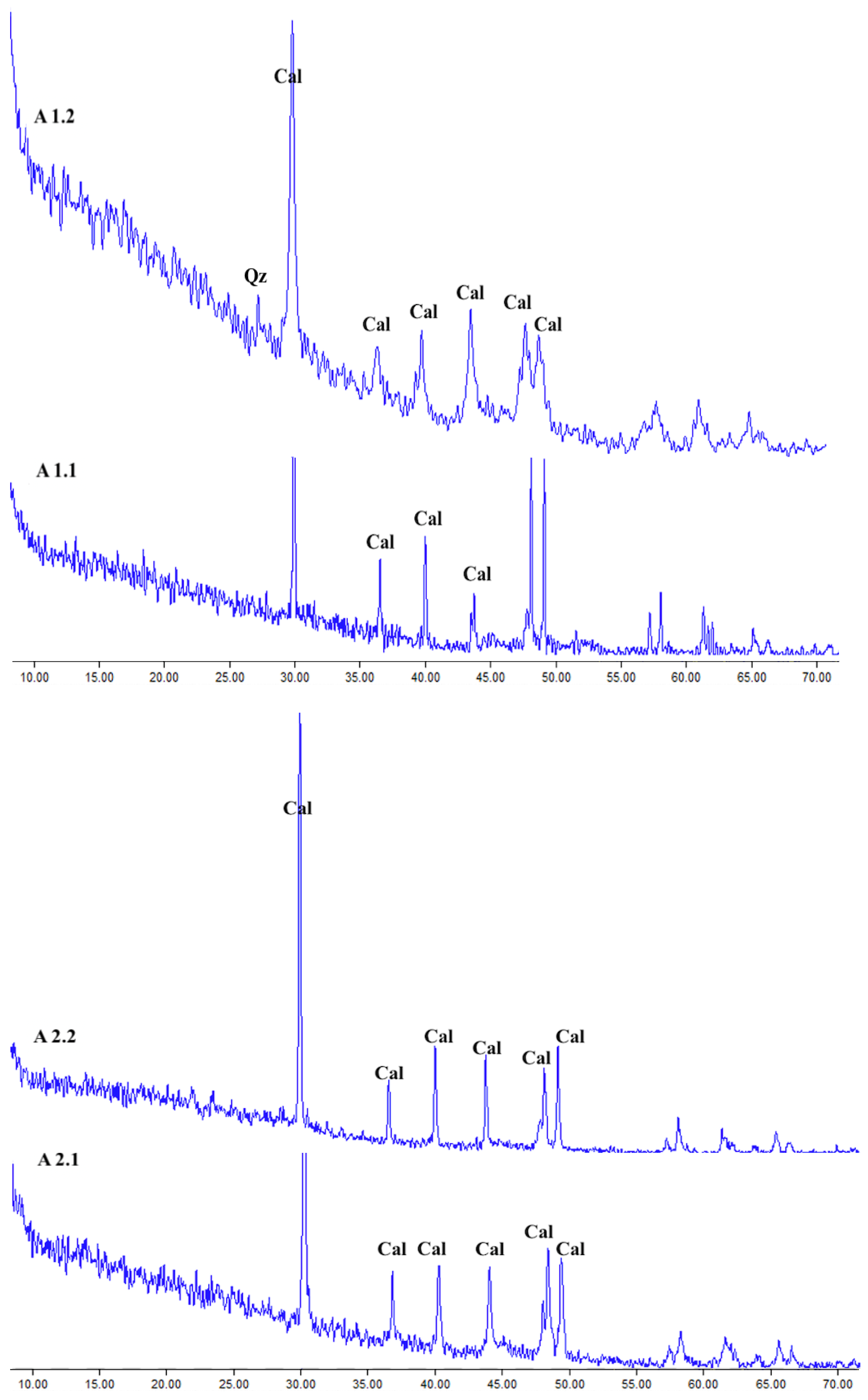
The XRD results for the Halabat and Ajlun samples (
Figure 8 and
Figure 9) revealed that the mineralogical composition is dominated by calcite (CaCO
3), as observed from the diffractograms according to the calcite 2-theta diffraction angle peaks. These observations are in agreement with the elemental composition of these samples, where CaO is dominant with minor amounts of other elements, and with the petrographic results, in which micrite and calcium carbonate were found to dominate. One sample (H3.2) showed a quartz (Qz) peak (26.66°), which was supported by its high content of SiO2 (4.5 wt.%) when compared with the other samples.
4.4. Synthesis
The results obtained for the samples from the two study sites indicate that each of the investigated lithological formations possesses distinct physical and chemical characteristics. The studied samples revealed that the carbonates in these formations were influenced by various environmental, hydrodynamic, and limnological conditions. In turn, these conditions affected the physical and chemical characteristics of the carbonates, rendering them suitable for various industrial uses. The following sections of this paper discuss the current uses of these carbonates and explore additional potential uses based on their specific compositions.
4.5. Limestone Classification
Based on the work of [
32], the Ca/Mg and Mg/Ca ratios are indicators that can be used to identify the type of limestone (
Table 5).
The Ca and Mg contents for the Hallabat and Ajlun samples were calculated using the CaO and MgO percentages, respectively, according to the atomic masses of the different elements (
Table 6). The Ca/Mg ratios for the Ajlun samples showed a range from 114.7 to 347.8, while the Mg/Ca ratios ranged from 0.003 to 0.009. There was a clear distinction between the lower and upper parts, where the lower part samples indicated lower Ca/Mg ratios and higher Mg/Ca ratios. Higher Mg content in the lower part suggests a lower precipitation/evaporation (P/E) ratio during deposition compared with a higher P/E ratio in the upper part. For the Hallabat samples, the results indicated that the lower bed (middle part) of the formation was deposited under a relatively higher E/P ratio compared with the upper part, with Ca/Mg ratios ranging from 188.3 to 466.4 and Mg/Ca ratios ranging from 0.002 to 0.005.
Generally, all samples presented Ca/Mg and Mg/Ca ratios in the range of pure limestone, with no indication of dolomitization. This can likely be attributed to the relatively high P/E ratio during the deposition of the two formations, regardless of slight changes in the environmental conditions during the deposition of the different beds.
In addition to the Ca/Mg ratios, the CaCO
3 content can be used as an indicator of carbonate purity (see, e.g., [
33]). In [
34,
35], a limestone purity classification based on the CaCO
3, CaO, MgO, SiO
2, and Fe
2O
3 contents was proposed, which has been extensively used for the assessment of limestone purity [
36,
37].
The limestone purity of the Hallabat and Ajlun samples was assessed using these values, indicating that the middle part of the WSL Formation in the Hallabat area is of very high purity, while the upper part showed medium purity limestone (
Table 7). This is in agreement with the higher detrital content in the middle part observed in the elemental composition of the beds (
Table 1), which may have affected the purity of these rocks. In Ajlun, the lower bed of the ASL Formation resulted in high purity compared with the very high-purity limestone in the upper bed.
4.6. Potential Industrial Uses for the WSL and ASL Limestones
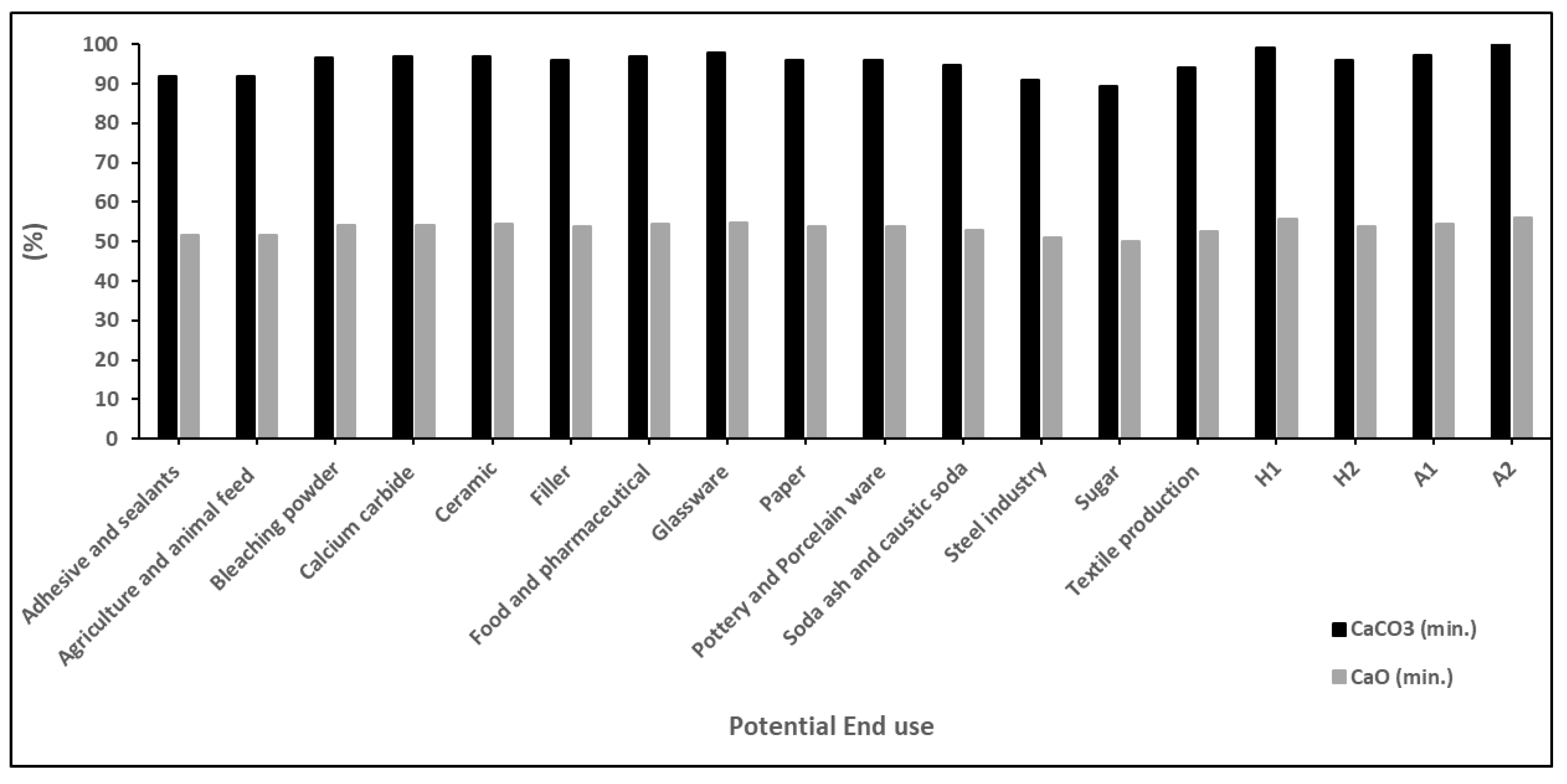
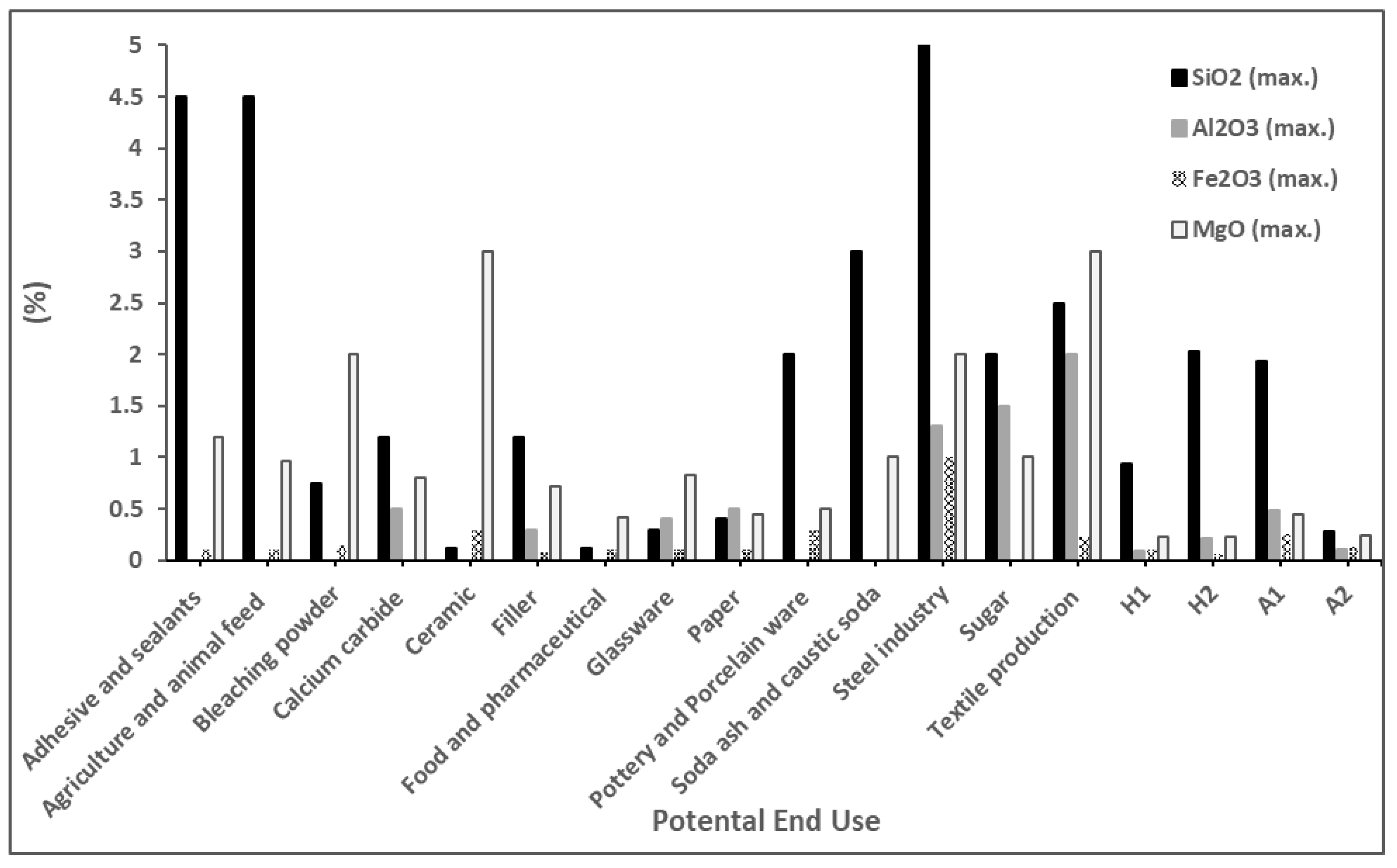
Specific physical and chemical characteristics govern the industrial end uses of limestones [
12,
13,
14]. Several authors have reported on chemical compositions that are suitable for various industries. Starting with the lowest calcium carbonate contents, [
38] reported that limestone with CaCO
3 > 89.3%, CaO > 50%, SiO
2 < 2%, Al
2O
3 < 1.5% and MgO < 1% is suitable for use in the sugar industry. In [
39,
40], it was indicated that limestones with CaCO
3 > 91%, CaO > 51%, SiO
2 < 6%, Al
2O
3 < 1.3%, Fe
2O
3 < 1%, and MgO < 2% are suitable for use in the steel industry. On the other hand, [
40,
41] reported similar contents—namely, CaCO
3 > 92%, CaO > 51.55%, SiO
2 < 4.5%, and Fe
2O
3 < 0.1%—for the use of limestone in the adhesive and sealant industry, with MgO content <1.2% and <0.96% for these industries, respectively. For textile production, suitable carbonates should have a composition characterized by CaCO
3 > 94%, CaO > 52.64%, SiO
2 < 2.5%, Al
2O
3 < 2%, and MgO < 3% [
40], while the soda ash and caustic soda industry requires a limestone composition of CaCO
3 > 94.6%, CaO > 53%, SiO
2 < 3%, and MgO < 1% [
40,
42]. Other industries such as those relating to paint and plastic fillers, pottery and porcelain ware, and bleaching powder require CaCO
3 > 96% and CaO > 53.76%, with varying amounts of the other oxides (
Figure 10). Industries such as those relating to calcium carbide, ceramics, and food and pharmaceuticals require a CaCO
3 content > 97%, with different amounts of other oxides (
Figure 10 and
Figure 11). The highest content of CaCO
3 is required for the glassware industry (>98%), along with very low amounts of other oxides (
Figure 10 and
Figure 11) [
39,
42,
43,
44,
45].
Therefore, the average chemical compositions for samples from the middle and upper parts of the WSL Formation in Hallabat and the lower and upper beds of the ASL in Ajlun were calculated in order to evaluate their potential industrial uses (
Figure 10 and
Figure 11). Generally, all investigated limestones were found to be suitable for use in the pottery and porcelain ware, soda ash and caustic soda, steel industry, sugar, and textile production industries. On the other hand, limestone from the Ajlun ASL upper bed was the only one suitable for use in the bleaching powder and calcium carbide industries.
The Hallabat limestones showed several potential industrial uses, varying from one bed to the other. The lower bed (WSL middle part) showed suitability for use in the adhesive and sealants, agriculture and animal feed, calcium carbide, pottery and porcelain ware, soda ash and caustic soda, steel industry, sugar, and textile production industries. Meanwhile, the upper bed (WSL upper part) showed potential for use in the adhesive and sealants, agriculture and animal feed, pottery and porcelain ware, soda ash and caustic soda, steel industry, sugar, and textile production industries.
The ASL Formation samples from Ajlun revealed that the lower bed is suitable for use in pottery and porcelain ware, soda ash and caustic soda, steel industry, sugar, and textile production industries. The upper bed, on the other hand, is appropriate for uses such as bleaching powder, calcium carbide, pottery and porcelain ware, soda and caustic soda, steel industry, sugar, and textile production (
Table 8).
4.7. Investment Opportunities
The Late Cretaceous carbonate rocks evaluated in this study are typical of comparable carbonate formations globally. These formations have similar depositional conditions and economic potential as Late Cretaceous carbonates from the Mediterranean, Middle East, and North Africa; for instance, Iraqi carbonates are primarily used for the cement industry in Kurdistan [
46]. In Greece, carbonate rocks are suitable for the painting industry [
47]. In Yemen, a study [
48] has evaluated the industrial uses of Middle Eocene limestone deposits in Wadi Tanhalin based on geochemical assessment. The authors reported that these limestones are of high purity and suitable for industrial uses such as steel industry, paper, filler, pottery and porcelain ware, bleaching powder, soda ash and caustic soda, calcium carbide, sugar, textile production, adhesives and sealants, and agriculture and animal feed [
48].
The outcomes of this study have implications beyond local applications, providing a framework for appraising similar resources globally.
The production of pure limestone has steadily increased in recent years due to increasing demand by industries for domestic use and exportation. According to [
13], pure limestone in the Hallabat area is exposed on the surface with thicknesses ranging between 1–37 m, and the estimated reserve is about 69 million tons. At present, Jordanian pure limestone in different locations is being mined, produced, and exploited. Local companies are producing pure calcium carbonate at a production capacity of around 450,000 ton/year. Most of the production is used for white cement, carbonates, ground calcium carbonates (GCCs), paints, and magnesia industries, and half of this production is being exported. Thus, there is a promising opportunity for industrial investment in calcium carbonates through the development of paint, plastic, and polymer industries in Jordan and the wider region.