Modern River-Sand Geochemical Mapping in the Manufahi Municipality and Its Surroundings, Timor-Leste: Implications for Provenance
Abstract
1. Introduction
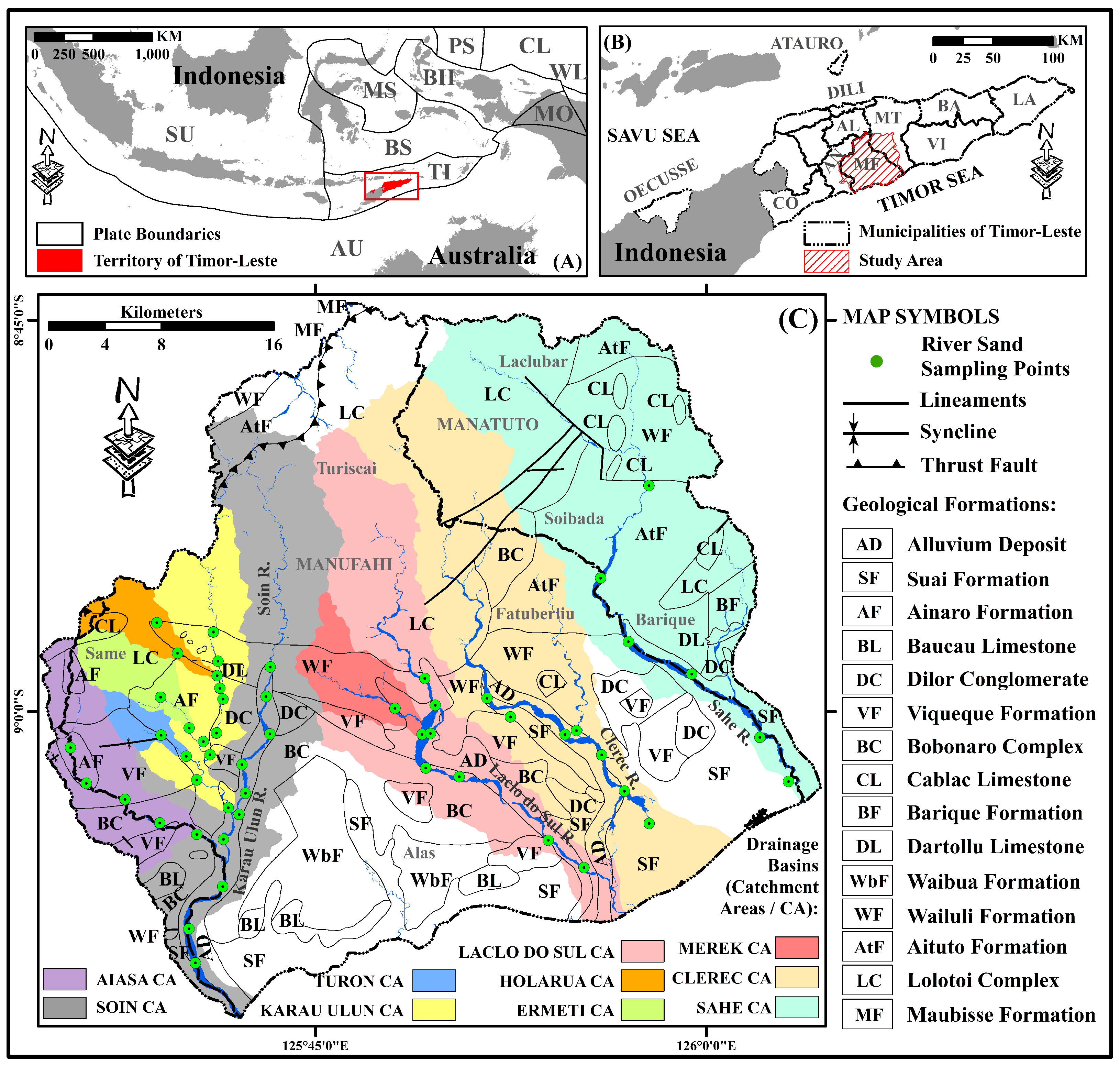
2. Geographical and Geological Setting of Timor-Leste and the Study Area
- (a)
- Pleistocene–Holocene Suai FormationIt is mostly composed of rudites and arenites, with minor amounts of mud and marls. The particles of the formation were mostly sourced from the Viqueque Formation, Dilor Conglomerate, and Lolotoi Complex. This formation belongs to the Synorogenic Megasequence.
- (b)
- Pleistocene–Holocene Ainaro Formation (Ainaro Gravels)This formation is part of the Synorogenic Megasequence and is primarily composed of matrix-supported conglomerates that are believed to be sediments from an ancient river terrace. Occasionally, calcite lateritic cements fill these sediments, and the irregular surfaces of the river terrace sediments are frequently covered by ferruginous horizons.
- (c)
- Lower Pleistocene–Holocene Baucau Limestone (Baucau Formation)It is predominantly composed of coral reef limestones along with a minor proportion of calcarenites, calcirudites, and conglomerates (submature graywackes). This formation is expected to provide evidence of the uplift of the island. This formation belongs to the Synorogenic Megasequence.
- (d)
- Pliocene Dilor Conglomerate (Dilor Formation)This formation is part of the Synorogenic Megasequence and consists of conglomerates and sandstones with a significant contribution of detritus from the Lolotoi Complex, particularly quartzite.
- (e)
- Upper Miocene–Lower Pliocene Viqueque Formation (Synorogenic Viqueque Megasequence)Lithologically, this formation is separated into lower (described as “more clayey and silty”) and upper (characterized as “more silty and sandy”) sections. Large amounts of silty marls, marly siltstones, silty claystones, siltstones, and sandstones, along with minor proportions of calcilutites and biocalcarenites are present in the upper section. The lower section is mostly composed of marls, clayey marls, silty marls, claystones, silty claystones, calcilutites, and tuffs, with minor amounts of basal conglomerates and mottled marls. These rocks are mostly formed by foraminifera fossils and skeletal radiolarians as well as rock fragments and mineral particles, which are associated with carbonate, metamorphic, volcanic, and other sedimentary rocks found in the Lolotoi Complex, Maubisse Formation, Aitutu Formation, Wailuli Formation, and Bobonaro Complex. This formation is included in the Synorogenic Megasequence.
- (f)
- Middle Miocene Bobonaro Complex (Bobonaro Formation or Bobonaro Scaly-Clay or Bobonaro Mélange or Synorogenic Mélange)This unit is part of the Synorogenic Mélange and is primarily composed of exotic blocks within a scaly clay matrix. The matrix lithology is similar to that of mudstone of the Wailuli Formation. Exotic blocks of Permian to Cretaceous ages are common and widely distributed, although absent in several areas.
- (g)
- Oligocene–Miocene Cablac Limestone (Cablac Formation)This formation is largely composed of oolitic and peloidal limestones and pelagic carbonates with small amounts of intraformational conglomerates, calcilutites, calcarenites, agglomerates, and tuffaceous rocks. The most common clasts of conglomerates are volcanic rocks, calcilutites containing foraminifera, radiolarian chert, biomicarenites, and detrital minerals, such as quartz and magnetite. Several rocks have been affected by alteration processes such as dolomitization and a few have undergone partial silicification, desilicification, and dedolomitization. This formation belongs to the Banda Terrane unit.
- (h)
- Oligocene Barique FormationThis formation belongs to the Banda Terrane unit and is largely composed of mafic to acidic lavas and tuffs, with minor amounts of serpentinites, volcanic conglomerates, and sandstones. Pillow lava is also observed. Significant alterations are observed in most volcanic rocks. Volcanic rocks are considered to have formed at mid-oceanic ridges and volcanic arcs.
- (i)
- Middle–Upper Eocene Dartollu Limestone (Dartolu Formation)This limestone is part of the Banda Terrane unit and its primary constituents are algal and alveolina biomicarenites with minor proportions of calcilutites, siliceous shales, and siltstones. Dolomitization or silicification was not observed.
- (j)
- Lower–Upper Cretaceous Waibua FormationThis formation is part of the Australian-Margin Megasequence and is largely composed of radiolarites, radiolarian cherts, marls, and shales, with several percentages of calcilutites, marls, and calcarenites. Radiolaria and pelagic foraminifera are important components of these rocks and most limestones are completely or partially silicified. Radiolarian shales, marls, and radiolarites often occur in association with Mn nodules and ferromanganiferous rocks. The formation processes of radiolarites and cherts are closely associated with Mn-rich strata.
- (k)
- Late Triassic–Middle Jurassic Wailuli FormationThis formation predominantly consists of gray shales and blue-gray marls with minor amounts of sandstones, mudstones, quartz-arenites, coarse polymictic conglomerates, calcarenites, and calcilutites. Most shales are composed of fine micaceous minerals and microcrystalline carbonates. In some areas, small amounts of pyrite are present in the shales, whereas salt pseudomorphs and gypsum are present in gypsiferous shales, calcilutites, calcarenites, and quartz-arenites. Quartz-arenites contain considerable amounts of mica, and radiolarian and foraminiferal tests are the primary constituents of the calcilutites. This formation is a part of the Gondwana Megasequence.
- (l)
- Middle–Upper Triassic Aitutu FormationThis formation consists mostly of calcilutites, shales, and calcareous shales with minor amounts of marls, calcarenites, lumachelles, quartz-arenites, radiolarites, bituminous rocks, and chert. Radiolarian fossils are significant components of limestones but are partly or almost entirely filled with sparry calcite. Several limestones have been affected by alteration processes such as silicification, dedolomitization, and pyritization. This formation belongs to the Gondwana Megasequence.
- (m)
- Triassic–Late Cretaceous Lolotoi Complex (Lolotoi Formation or Lolotoi Metamorphic Complex)Belonging to the Banda Terrane units, this complex is composed of regionally metamorphosed sedimentary and volcanic rocks, as well as basic and ultrabasic volcanic rocks. These include greenschists, graphitic phyllites, quartz mica schists, amphibolite gneisses and schists, garnet-bearing pelitic gneisses and schists, metagabbros, granulites, garnet mica schists, mafic and felsic igneous, pelitic schists, metabasite schists, carbonate-rich greenschists, peridotites, blueschists, serpentinites, and pyroxenites. The metamorphic rocks of the Lolotoi Complex mostly originate from sedimentary rocks and some metavolcanic rocks have undergone considerable alteration. The forearc region is thought to have been the location of the deposition of sedimentary rocks, and the rock components are thought to have originated from intermediate to mafic continental and oceanic arcs. Volcanic rocks were formed at volcanic arcs and mid-oceanic ridges.
- (n)
- Permian–Triassic Maubisse Formation (Maubisse Limestone)This formation belongs to the Gondwana Megasequence and is mainly composed of fossiliferous limestones and volcanic rocks, as well as other types of sedimentary rocks. These include well-bedded dense biocalcarenites, massive reef limestones, pink crinoidal limestones, calcirudites, sandstones, calcareous shales, micaceous siltstones, tuffs, volcanic conglomerates, basalts, marbles, and metamorphosed basic volcanics. The basalts are pillowed and amygdaloidal and have an alkaline chemical composition. Alteration processes have affected most rocks of the Maubisse Formation, and limestones have been partially affected by alteration processes such as silicification, dolomitization, and chloritization.
3. Sampling and Analytical Methods
3.1. Sampling Method
3.2. Analytical Method
3.3. Statistical Analysis
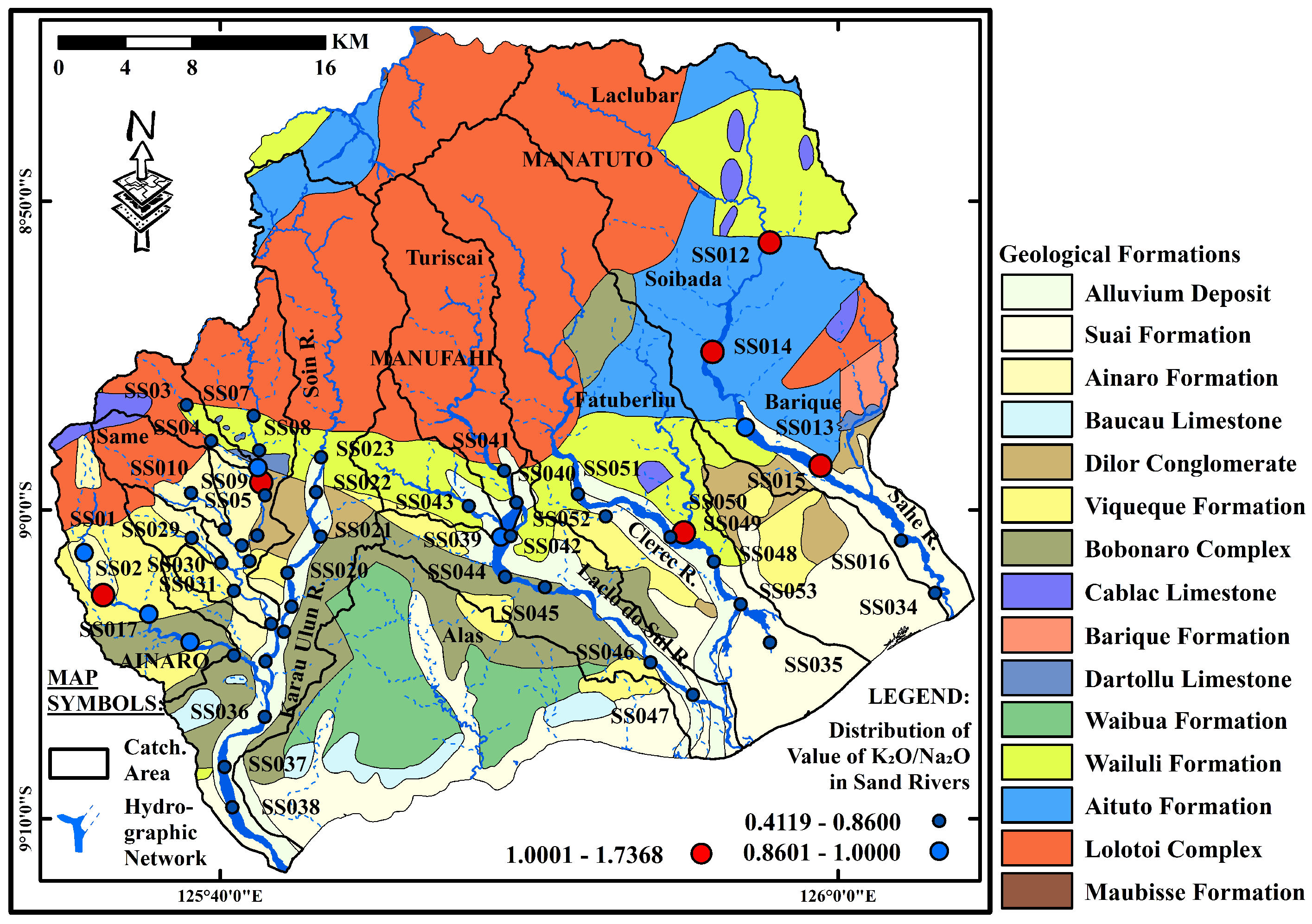
4. Analytical Results
4.1. Geochemical Features of River Sand Samples
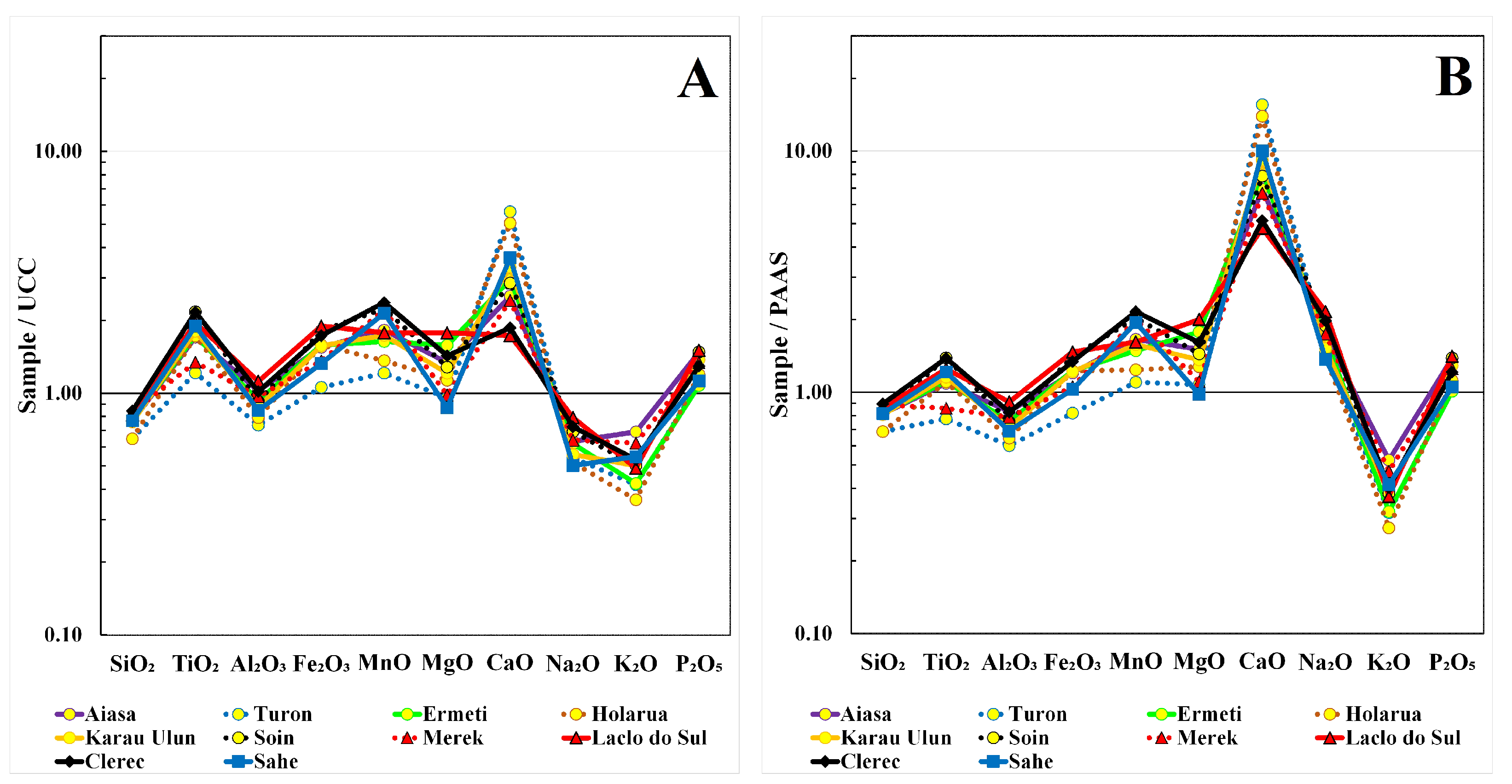
4.2. Comparison with UCC and PAAS
5. Discussion
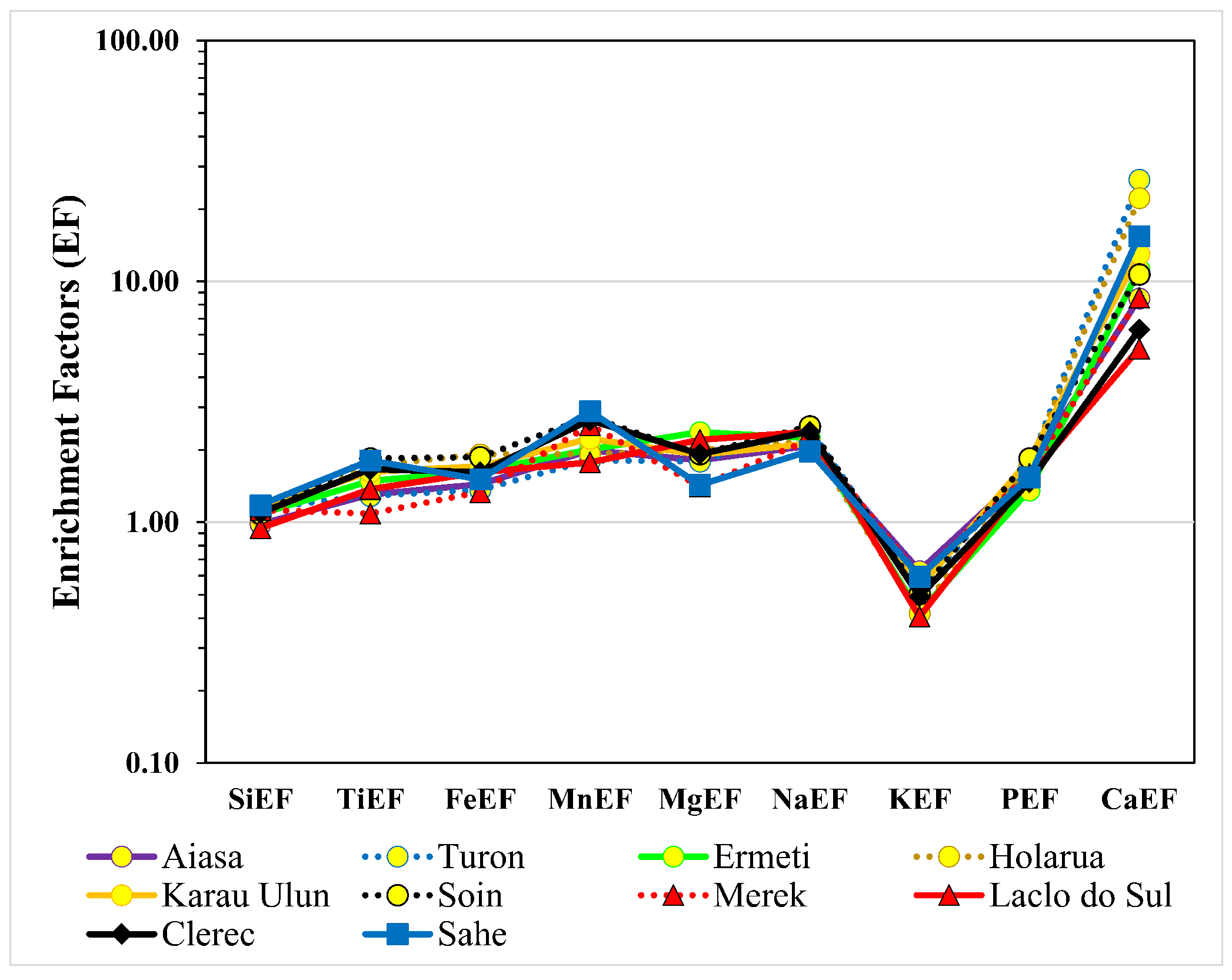
5.1. The Enrichment of the Elements
5.2. Geochemical Characteristics of the Drainage Basins
- (1)
- Aiasa River catchmentThe Aiasa River catchment is covered by the Lolotoi and Bobonaro Complexes, as well as the Viqueque and Ainaro Formations (Figure 1C and Figure 3). This catchment area showed the highest concentrations of K2O and P2O5, with average values of 1.94 wt% (ranging from 1.64 to 2.15 wt%) and 0.22 wt% (ranging from 0.20 to 0.26 wt%), respectively (Table 1). The highest values were registered in the area near the upstream (as shown in Figure 3). The concentrations of Al2O3, K2O, TiO2, Fe2O3, MgO, and P2O5 tended to decrease toward downstream, and on the contrary, CaO and Na2O seemed to increase downstream. However, the high content value of Na2O was recorded near the upstream area. The SiO2 and MnO concentration values showed an irregular distribution pattern from upstream to downstream. The presence of CaO and Na2O might be attributed to the presence of the calcite and halite minerals associated with carbonate components. Near the upstream area, Na2O could be partly attributed to the existence of silicate and aluminosilicate minerals such as plagioclases, clays, and micas. Meanwhile, TiO2, Fe2O3, MgO, K2O, and P2O5 concentrations were mostly concentrated in clay minerals such as illite and others. In the upstream area, the composition of river sand is mostly controlled by clay contents and lithic fragments associated with clastic sedimentary rocks. On the other hand, carbonate components related to the carbonate sedimentary rocks make major contributions in the downstream region.
- (2)
- Turon River catchmentThe highest content of CaO and the lowest concentrations of SiO2, TiO2, Al2O3, Fe2O3, MnO, K2O, and P2O5 were reported in this river catchment (Table 1), which is mostly covered by the Viqueque and Ainaro Formations (Figure 1C and Figure 3). The average value of the highest concentration was measured to be 20.22 wt% (ranging from 16.19 to 24.25 wt%), and the lowest contents were 43.15 wt% (ranging from 39.00 to 47.30 wt%), 0.78 wt% (ranging from 0.67 to 0.88 wt%), 11.35 wt% (ranging from 10.40 to 12.30 wt%), 5.33 wt% (ranging from 4.75 to 5.91 wt%), 0.12 wt% (ranging from 0.09 to 0.15 wt%), 1.17 wt% (ranging from 0.99 to 1.36 wt%), and 0.17 wt% (ranging from 0.15 to 0.18 wt%), respectively (Table 1). The highest and lowest values were recorded in the midstream area (Figure 3). The abundance of CaO might be sourced from carbonate components, which contributed much more than others, while other major elements could be associated with clay and silicate minerals.
- (3)
- Ermeti River catchmentThe Ermeti River catchment is mostly covered by the Ainaro Formation (Figure 1C and Figure 3). This catchment area showed high content of SiO2 and low CaO, P2O5, and MnO concentrations, with average values of 51.36 wt% (ranging from 47.70 to 55.01 wt%), 10.61 wt% (ranging from 6.99 to 14.22 wt%), 0.16 wt% (ranging from 0.15 to 0.18 wt%), and 0.16 wt% (ranging from 0.14 to 0.18 wt%) (Table 1). SiO2, Al2O3, Fe2O3, MgO, Na2O, and K2O contents appeared to increase in the upstream, while the concentrations of TiO2, MnO, CaO, and P2O5 tended to decrease toward the downstream (Figure 3). TiO2, MnO, CaO, and P2O5 could be attributed to the contribution of carbonate components along with manganese and apatite minerals, while clay and silicate minerals could be responsible for SiO2, Al2O3, Fe2O3, MgO, Na2O, and K2O.
- (4)
- Holarua River catchmentThe Holarua River catchment is covered by the Lolotoi Complex, Wailuli Formation, and Ainaro Formation (Figure 1C and Figure 3). This drainage basin also recorded the highest concentration of CaO and the lowest contents of SiO2, Al2O3, MnO, Na2O, and K2O, with the average values reported at 18.11 wt% (ranging from 13.43 to 23.21 wt%), 43.15 wt% (ranging from 36.84 to 47.92 wt%), 12.20 wt% (range 11.00–13.80 wt%), 0.14 wt% (range 0.11–0.16 wt%), 1.69 wt% (range 1.26–2.17 wt%), and 1.01 wt% (range 0.68–1.28 wt%), respectively (Table 1). TiO2, MnO, Na2O, and P2O5 contents showed an increasing trend toward upstream, and SiO2, K2O, and Al2O3 concentrations appeared to increase toward downstream, but their high content values were observed close to the upstream area. Although the concentrations of CaO, Fe2O3, and MgO seemed to decrease toward downstream, their low measured values were found close to the upstream area (Figure 3). SiO2, K2O, and Al2O3 could be derived from the destruction of feldspar and/or muscovite minerals. TiO2, MnO, Na2O, and P2O5 were mostly accumulated in clay minerals, and these elements can be partly attributed to the presence of silicate and accessory minerals. The abundance of CaO, Fe2O3, and MgO could be sourced from calcite, dolomite, and pyrite minerals associated with carbonate components. The upstream and downstream areas are mostly influenced by clay and mica contents, as well as lithic fragments affiliated with clastic sedimentary rocks, which contribute to the composition of river sands. However, there were contributions from silicate and accessory minerals associated with metamorphic rocks in the upstream area. The midstream region is characterized by significant contributions from carbonate components, particularly those associated with carbonate sedimentary rocks.
- (5)
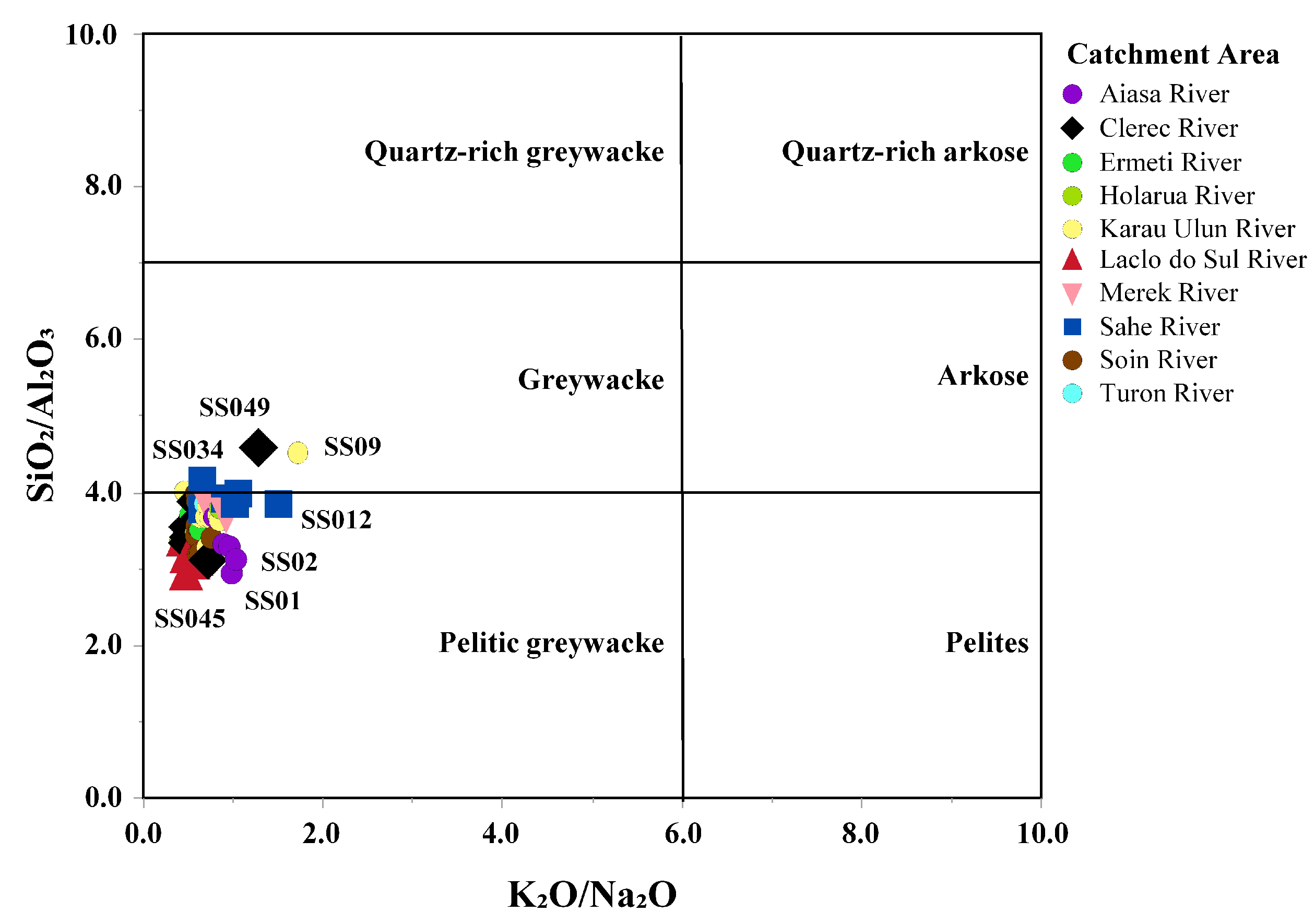
- Karau Ulun River catchmentThis river catchment is mostly covered by the Lolotoi Complex, Wailuli Formation, Dartollu Limestone, Viqueque Formation, Dilor Conglomerate, and Ainaro Formation (Figure 1C and Figure 3). The lowest concentration of Na2O was registered in this catchment area, with an average value of 1.82 wt% (range 1.06–2.47 wt%) (Table 1). The concentration values of CaO almost showed a decreasing trend toward downstream, and Al2O3 contents also nearly appeared to be increasing in the upstream direction. Near the upstream region, mostly high values of SiO2, TiO2, Fe2O3, MnO, MgO, Na2O, K2O, and P2O5 concentrations were recorded. A high content of CaO and low concentrations of TiO2, Al2O3, K2O, P2O5, Fe2O3, MnO, and Na2O were reported in the downstream region. However, low Al2O3 content and high concentration values of SiO2, K2O, MnO, P2O5, and MgO were recorded in the sampling location SS09 (Figure 3). The significant contribution of clay, mica, silicate, and accessory minerals, as well as lithic fragments associated with clastic sedimentary and metamorphic rocks in the upstream region. In the downstream area, carbonate components associated with carbonate sedimentary rocks have important contributions. On the other hand, sample location SS09 is characterized by notable inputs from the silicate and accessory minerals, such as quartz, feldspar, muscovite, garnet, apatite, and chlorite, which are associated with metamorphic rocks.
- (6)
- Soin River catchmentThis catchment area is covered by the Lolotoi Complex, Wailuli Formation, Bobonaro Complex, Viqueque Formation, Dilor Conglomerate, and Ainaro Formation (Figure 1C and Figure 3). The highest P2O5 content was recorded in this drainage basin, with the average value reported at 0.22 wt% (ranging from 0.20 to 0.27 wt%) (Table 1). As shown in Table 2A, SiO2 showed a positive correlation with Al2O3, MgO, Na2O, and K2O, as opposed to Al2O3, which was negatively associated with MnO and CaO and positively correlated with all other major elements. CaO had a strong positive association with MnO and a negative relationship with all other elements. Positive relationships were observed between TiO2, Fe2O3, MgO, Na2O, K2O, and P2O5. High amounts of TiO2, Al2O3, MgO, Na2O, K2O, P2O5, Fe2O3, and SiO2, along with low concentrations of CaO and MnO, were mostly recorded near the upstream areas (Figure 3). The midstream regions had low SiO2 content and high concentrations of CaO, MnO, and Fe2O3. In addition, the downstream regions had high SiO2, CaO, and MnO concentrations, and low content values of P2O5, MgO, TiO2, Fe2O3, and Al2O3. The upstream areas could be influenced by clay and mica content as well as quartz, plagioclase, amphibole, chlorite, hematite, ilmenite, rutile, garnet, sphene, and apatite minerals, which are associated with clastic sedimentary and metamorphic rocks. Carbonate components and Mn minerals affiliated with carbonate sedimentary rocks, along with the clay content associated with clastic sedimentary rocks (e.g., shales and mudstones), made major contributions to the midstream regions. The downstream regions were mostly controlled by silicate minerals, such as quartz associated with clastic sedimentary rocks; carbonate components and manganese minerals affiliated with carbonate sedimentary rocks; along with clay content related to clastic sedimentary rocks.
- (7)
- Laclo do Sul River catchmentThe Laclo do Sul River catchment is covered by the Lolotoi Complex, Wailuli Formation, and Bobonaro Complex, as well as the Viqueque, Ainaro, and Suai Formations (Figure 1C and Figure 3). The lowest content of CaO and highest values of Al2O3, MgO, Fe2O3, Na2O, and P2O5 were registered near the upstream and downstream areas (Figure 3). The average values of the lowest and highest concentrations were measured to be 6.21 wt% (ranging from 4.93 to 7.94 wt%), 17.23 wt% (ranging from 16.40 to 17.90 wt%), 4.41 wt% (ranging from 4.30 to 4.70 wt%), 9.55 wt% (ranging from 8.57 to 11.73 wt%), 2.59 wt% (ranging from 2.28 to 2.74 wt%), and 0.23 wt% (ranging from 0.20 to 0.25 wt%), respectively (Table 1). As shown in Table 2B, SiO2 was positively correlated with Na2O, K2O, P2O5, and Al2O3. Al2O3 was positively associated with SiO2, TiO2, Fe2O3, Na2O, K2O, and P2O5. Moderate-to-very strong correlations were observed between TiO2, Fe2O3, MnO, MgO, and CaO. The Al2O3 concentration appeared to increase in the upstream direction. High SiO2, Al2O3, K2O, P2O5, Na2O, TiO2, Fe2O3, and MgO contents and low CaO and MnO concentrations were recorded near the upstream areas. Downstream and midstream regions, specifically sample location SS045, had high concentrations of Fe2O3, MgO, TiO2, and MnO and low Na2O and K2O contents. In addition, high values of SiO2, Na2O, and CaO, along with low concentrations of TiO2, Fe2O3, and Al2O3, were also observed in the downstream area (Figure 3). The destruction of clay and mica, along with quartz, plagioclase, amphibole, chlorite, hematite, ilmenite, rutile, garnet, sphene, and apatite minerals associated with clastic sedimentary and metamorphic rocks, contributed significantly to the composition of the river sands near the upstream regions. Near the downstream and continuing to the midstream regions, quartz and clay contents affiliated with clastic sedimentary rocks (e.g., shales and mudstones) made major contributions; however, in the sampling location SS045, there was significant inputs from mafic and heavy minerals related to the presence of igneous and metamorphic rocks that associated with the Bobonaro Complex, as exotic blocks that were incorporated into the clastic sedimentary rocks (such as shales and mudstones) [9,13,76]. Silicate minerals and clay related to clastic sedimentary rocks contributed significantly to the downstream area.
- (8)
- Merek River catchmentThe Merek River catchment is mostly covered by the Wailuli Formation and Lolotoi Complex (Figure 1C and Figure 3). The highest and lowest concentrations of SiO2 and TiO2 were also recorded in this river catchment, with the average values reported at 55.76 wt% (ranging from 53.33 to 58.19 wt%) and 0.86 wt% (ranging from 0.78 to 0.94 wt%), respectively (Table 1). CaO and K2O appeared to increase toward downstream, while the other major element contents showed an decreasing trend toward downstream (Figure 3). Silicate (such as quartz, plagioclase, amphibole, and pyroxene), and clay minerals might significantly contribute to the geochemical composition of the river sand samples from this river catchment. The remaining contributions could be attributed to the presence of carbonate components.
- (9)
- Clerec River catchmentThe Clerec River catchment was registered as having the highest concentrations of SiO2, TiO2, Fe2O3, MnO, Na2O, and MgO (Table 1). This catchment area is mostly covered by the Lolotoi and Bobonaro Complexes, as well as the Aitutu, Wailuli, Viqueque, and Suai Formations (Figure 1C and Figure 3). As demonstrated by the Pearson correlation of the Clerec River catchment (Table 2C), SiO2 showed a positive association with CaO and K2O, Al2O3 had a positive correlation with TiO2, Fe2O3, Na2O, P2O5, and MgO, and CaO showed a positive correlation with SiO2, MnO, and K2O. There was a moderate-to-very strong positive relationship between TiO2, Fe2O3, MgO, Na2O, and P2O5, indicating that the destruction of clay, mica, amphibole, pyroxene, biotite, ilmenite, hematite, sphene, rutile, garnet, and apatite, along with calcium carbonate, manganese, and alteration minerals, contributed significantly to the composition of the river sand from this river catchment. The positive correlations between SiO2, CaO, and K2O indicated that they may have been derived from the same source. The elevated K2O level may be related to the presence of secondary K-bearing minerals. The CaO content tended to increase downstream; however, the highest measured concentration in this river catchment was recorded at the sample site SS049. In the midstream regions, high contents of Al2O3, Na2O, and P2O5 and the lowest concentrations of CaO and MnO were recorded. The reported distribution of the elemental concentrations near the downstream regions showed high measured values of TiO2, Al2O3, Fe2O3, MgO, MnO, and P2O5. In contrast, elevated values of SiO2, K2O, and MnO were recorded at sample site SS049. The downstream regions also appeared to have high concentrations of SiO2, Na2O, and Al2O3 and low TiO2 and CaO contents. These findings suggest that the midstream areas were largely influenced by quartz, clay, and mica contents as well as lithic fragments, which are associated with clastic sedimentary rocks. Near the downstream regions, clay, muscovite, quartz, plagioclase, chlorite, sphene, garnet and apatite minerals affiliated with clastic sedimentary rocks and their altered rocks owing to metamorphic processes made major contributions. However, sample location SS049 was characterized by notable inputs from quartz, muscovite, garnet, calcium carbonate, and manganese minerals, which were associated with clastic sedimentary interbedded with carbonate sedimentary rocks (such as micaceous shales and sandstones, marls and calcilutites) of the Wailuli Formation and their altered rocks due to metamorphic processes. In addition, there were major contributions from silicate and clay contents related to clastic sedimentary rocks in the downstream areas.
- (10)
- Sahe River catchmentThe Sahe River catchment is covered by the Lolotoi Complex, Aitutu Formation, Wailuli Formation, Dartollu Limestone, Cablac Limestone, Viqueque Formation, Dilor Conglomerate, and Suai Formation (Figure 1C and Figure 3). The highest concentrations of CaO and K2O, as well as the lowest values of Al2O3, SiO2, MgO, Na2O, and P2O5, were also registered in this river catchment (Table 1). CaO had a positive correlation with TiO2 and MnO, and SiO2 showed a very strong association with Al2O3, and they had a moderate-to-strong positive relationship with MgO, Na2O, and K2O (Table 2D). Positive associations were observed between TiO2, Fe2O3, MnO, MgO, Na2O, and P2O5. The presence of TiO2 in carbonate sedimentary rocks associated with Mn minerals is not common. TiO2 could be associated with Mn minerals in carbonate sedimentary rocks, suggesting the presence of secondary Ti-bearing minerals owing to certain alteration processes. In this river catchment, the highest concentrations of SiO2 and K2O appeared near the upstream areas, along with the lowest concentrations of TiO2, Fe2O3, Na2O, MnO, and CaO. Although high measured concentrations of SiO2, K2O, and MnO were reported in the midstream regions, sample location SS015 also appeared to have high measured contents of CaO and MnO and low concentrations of SiO2, Al2O3, K2O, and Na2O. The downstream regions were also reported to have high SiO2, Na2O, K2O, MgO, and MnO contents. This indicate that the composition of river sand sample near the upstream area was mostly controlled by the destruction of quartz, clay, and mica minerals, which were affiliated with clastic sedimentary rocks; however, silicate and accessory minerals (such as quartz, muscovite and garnet minerals), which are associated with their altered rocks due to metamorphic processes, contributed to the sample location SS013. Sample sites SS014 and SS015 had major contributions from calcium-carbonate minerals associated with carbonate sedimentary rocks of the Aitutu Formation and their altered rocks due to silicification or certain alteration processes [13,72,114]. There were notable inputs from quartz and secondary K-bearing minerals affiliated with altered carbonate sedimentary rocks intercalated with clastic sedimentary (e.g., shales) strata due to silicification processes in the sample location SS014, and considerable contributions to SS015 came from calcium carbonate, manganese, and Ti-bearing minerals, which were also associated with altered carbonate sedimentary rocks intercalated with clastic sedimentary (e.g., shales) strata due to certain alteration processes. The downstream regions were characterized by contributions from quartz, clay, carbonate, mica, amphibole, chlorite, ilmenite, garnet, and other minerals related to clastic sedimentary rocks.
5.3. Relationship between River Sand Geochemistry and Provenance Geology
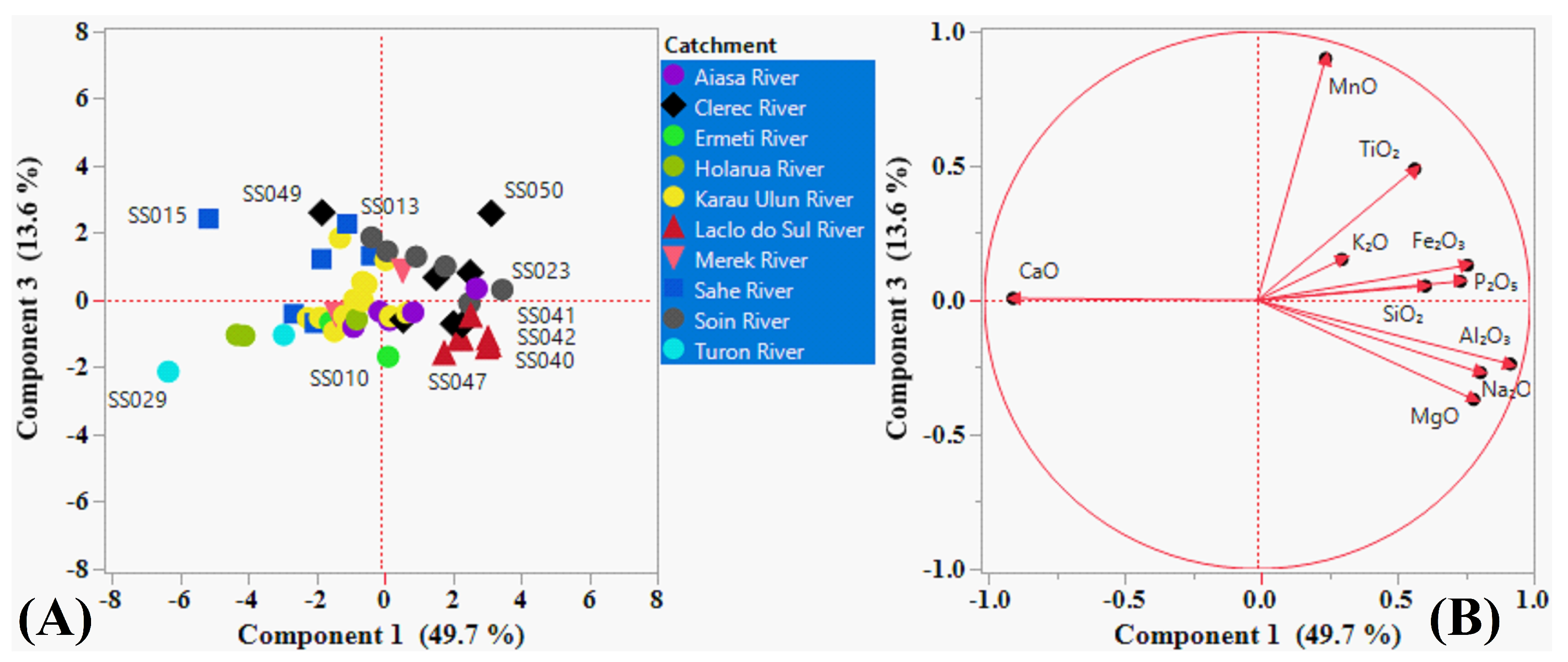
5.4. Principal Component Analysis
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PCA | Principal component analysis |
| PC1 | Principal component 1 |
| PC2 | Principal component 2 |
| PC3 | Principal component 3 |
| ICV | Index of compositional variability |
| XRF | X-ray fluorescence |
| UCC | Upper continental crust |
| PAAS | Post-Archean Australian Shale |
| Mn | Manganese |
| K-bearing minerals | Potassium-bearing minerals |
| Ti-bearing minerals | Titanium-bearing minerals |
| JMP | Jump, statistical analysis software developed by JMP |
| ArcGIS | Aeronautical Reconnaissance Coverage Geographic Information System |
| JICA | Japan International Cooperation Agency |
References
- Audley-Charles, M.G. The Geology of Portuguese Timor. Mem. Geol. Soc. Lond. 1968, 4, 1–75. [Google Scholar]
- Audley-Charles, M.G. Rates of Neogene and Quaternary Tectonic Movements in the Southern Banda Arc Based on Micropalaeontology. J. Geol. Soc. Lond. 1986, 143, 161–175. [Google Scholar] [CrossRef]
- Boger, S.D.; Spelbrink, L.G.; Lee, R.I.; Sandiford, M.; Maas, R.; Woodhead, J.D. Isotopic (U-Pb, Nd) and Geochemical Constraints on the Origins of the Aileu and Gondwana Sequences of Timor. J. Asian Earth Sci. 2017, 134, 330–351. [Google Scholar] [CrossRef]
- Chamalaun, F.H.; Grady, A.E. The Tectonic Development of Timor: A New Model and its Implications for Petroleum Exploration. APEA J. 1978, 18, 102–108. [Google Scholar] [CrossRef]
- Charlton, T.R. The Petroleum Potential of East Timor. J. Aust. Pet. Prod. Explor. Assoc. (APPEA) 2002, 42, 351–369. [Google Scholar] [CrossRef]
- Ely, K.S.; Sandiford, M.; Hawke, M.L.; Phillips, D.; Quigley, M.; dos Reis, J.E. Evolution of Ataúro Island: Temporal constraints on subduction processes beneath the Wetar zone, Banda Arc. J. Asian Earth Sci. 2011, 41, 477–493. [Google Scholar] [CrossRef]
- Grady, A.E.; Berry, R.F. Some Palaeozoic-Mesozoic Stratigraphic-Structural Relationships in East Timor and Their Significance in the Tectonics of Timor. J. Geol. Soc. Aust. 1977, 24, 203–214. [Google Scholar] [CrossRef]
- Haig, D.W. Palaeobathymetric Gradients Across Timor During 5.7–3.3 Ma (Latest Miocene-Pliocene) and Implications for Collision Uplift. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2012, 331–332, 50–59. [Google Scholar] [CrossRef]
- Harris, R.A. The Nature of the Banda Arc—Continent Collision in the Timor Region. In Arc-Continent Collision; Brown, D., Ryan, P.D., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 163–211. [Google Scholar] [CrossRef]
- Price, N.J.; Audley-Charles, M.G. Tectonic Collision Processes After Plate Rupture. Tectonophysics 1987, 140, 121–129. [Google Scholar] [CrossRef]
- Tate, G.W.; McQuarrie, N.; Van Hinsbergen, D.J.J.; Bakker, R.R.; Harris, R.; Jiang, H. Australia Going Down Under: Quantifying Continental Subduction During Arc-Continent Accretion in Timor-Leste. Geosphere 2015, 11, 1860–1883. [Google Scholar] [CrossRef]
- Wittouck, S.F. Exploration of Portuguese Timor; Report of Allied Mining Corporation to Asia Investment. Co. Ltd.; Kolff & Co.: Batavia/Amsterdam, The Netherlands, 1937. [Google Scholar]
- Audley-Charles, M.G. The Geology of Portuguese Timor. Ph.D. Thesis, University of London, London, UK, 1965. [Google Scholar]
- Audley-Charles, M.G. Tectonic Post-Collision Processes in Timor. Geol. Soc. Lond. Spec. Publ. 2011, 355, 241–266. [Google Scholar] [CrossRef]
- Barber, A.J.; Audley-Charles, M.G.; Carter, D.J. Thrust Tectonics in Timor. J. Geol. Soc. Aust. 1977, 24, 51–62. [Google Scholar] [CrossRef]
- Carter, D.J.; Audley-Charles, M.G.; Barber, A.J. Stratigraphical Analysis of Island Arc - Continental Margin Collision in Eastern Indonesia. J. Geol. Soc. Lond. 1976, 132, 179–198. [Google Scholar] [CrossRef]
- Charlton, T.R. The Structural Setting and Tectonic Significance of the Lolotoi, Laclubar and Aileu Metamorphic Massifs, East Timor. J. Asian Earth Sci. 2002, 20, 851–865. [Google Scholar] [CrossRef]
- Charlton, T.R.; Barber, A.J.; Harris, R.A.; Barkham, S.T.; Bird, P.R.; Archbold, N.W.; Morris, N.J.; Nicoll, R.S.; Owen, H.G.; Owens, R.M.; et al. The Permian of Timor: Stratigraphy, Palaeontology and Palaeogeography. J. Asian Earth Sci. 2002, 20, 719–774. [Google Scholar] [CrossRef]
- Kaneko, Y.; Maruyama, S.; Kadarusman, A.; Ota, T.; Ishikawa, M.; Tsujimori, T.; Ishikawa, A.; Okamoto, K. On-Going Orogeny in The Outer-Arc of the Timor-Tanimbar Region, Eastern Indonesia. Gondwana Res. 2007, 11, 218–233. [Google Scholar] [CrossRef]
- Park, S.I.; Kwon, S.; Kim, S.W. Evidence for the Jurassic Arc Volcanism of the Lolotoi complex, Timor: Tectonic Implications. J. Asian Earth Sci. 2014, 95, 254–265. [Google Scholar] [CrossRef]
- Bird, P. An Updated Digital Model of Plate Boundaries. Geochem. Geophys. Geosyst. 2003, 4, 1027–1080. [Google Scholar] [CrossRef]
- Poiata, N.; Koketsu, K.; Miyake, H. Source Processes of the 2009 Irian Jaya, Indonesia, Earthquake Doublet. Earth Planet Space 2010, 62, 475–481. [Google Scholar] [CrossRef][Green Version]
- Pisut, D. Plate Tectonic and Boundaries. 2020. Available online: https://services.arcgis.com/jIL9msH9OI208GCb/arcgis/rest/services/Tectonic_Plates_and_Boundaries/FeatureServer (accessed on 12 November 2022).
- Bachri, S.; Situmorang, R.L. Geological Map of the Dili Quadrangle 2406-2407, East Timor, Scale 1: 250.000; Geological Research and Development Centre: Bandung, Indonesia, 1994. [Google Scholar]
- Partoyo, E.; Hermanto, B.; Bachri, S. Geological Map of the Baucau Quadrangle 2057, East Timor, Scale 1: 250.000; Geological Research and Development Centre: Bandung, Indonesia, 1995. [Google Scholar]
- Franzinelli, E.; Potter, P.E. Petrology, Chemistry, and Texture of Modern River Sands, Amazon River System. J. Geol. 1983, 91, 23–39. [Google Scholar] [CrossRef]
- Garzanti, E.; Resentini, A. Provenance Control on Chemical Indices of Weathering (Taiwan River Sands). Sediment. Geol. 2016, 336, 81–95. [Google Scholar] [CrossRef]
- He, J.; Garzanti, E.; Jiang, T.; Barbarano, M.; Resentini, A.; Liu, E.; Chen, S.; Shi, G.; Wang, H. Mineralogy and Geochemistry of Modern Red River Sediments (North Vietnam): Provenance and Weathering Implications. J. Sediment. Res. 2022, 92, 1169–1185. [Google Scholar] [CrossRef]
- Liang, W.; Hu, X.; Garzanti, E.; Wen, H.; Hou, M. Petrographic Composition and Heavy Minerals in Modern River Sand: A Global Database. Geosci. Data J. 2023, 1–9. [Google Scholar] [CrossRef]
- Potter, P.E. Petrology and Chemistry of Modern Big River Sands. J. Geol. 1978, 86, 423–449. [Google Scholar] [CrossRef]
- Potter, P.E. Modern Sands of South America: Composition, Provenance and Global Significance. Geol Rundsch. 1994, 83, 212–232. [Google Scholar] [CrossRef]
- Fletcher, W.K. Stream Sediment Geochemistry in Today’s Exploration World. 1997. Available online: https://www.911metallurgist.com/blog/wp-content/uploads/2015/10/Stream-Sediment-Geochemistry-in-Todays-Exploration-World.pdf (accessed on 26 May 2024).
- Darnley, A.G. International Geochemical Mapping: A New Global Project. J. Geochem. Explor. 1990, 39, 1–13. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Young, G.M.; McLennan, S.M.; Keays, R.R. Effects of Chemical Weathering and Sorting on the Petrogenesis of Siliciclastic Sediments, with Implications for Provenance Studies. J. Geol. 1996, 104, 525–542. [Google Scholar] [CrossRef]
- Cocker, M.D. Geochemical Mapping in Georgia, USA: A Tool for Environmental Studies, Geologic Mapping and Mineral Exploration. J. Geochem. Explor. 1999, 67, 345–360. [Google Scholar] [CrossRef]
- Guagliardi, I.; Apollaro, C.; Scarciglia, F.; De Rosa, R. Influence of Particle-Size on Geochemical Distribution of Stream Sediments in the Lese River Catchment, Southern Italy. Biotechnol. Agron. Soc. Environ. 2013, 17, 43–55. [Google Scholar]
- Ortiz, E.; Roser, B.P. Major and trace element provenance signatures in stream sediments from the Kando River, San’in district, southwest Japan. Island Arc. 2006, 15, 223–238. [Google Scholar] [CrossRef]
- Formoso, M.L.L. Some Topics on Geochemistry of Weathering: A Review. Ann. Braz. Acad. Sci. 2006, 78, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, E.; Roser, B. Geochemistry of Stream Sediments from the Hino River, SW Japan: Source Rock Signatures, Downstream Compositional Variations, and Influence of Sorting and Weathering. Earth Sci. 2006, 60, 131–146. [Google Scholar]
- Yamamoto, K.; Tanaka, T.; Minami, M.; Mimura, K.; Asahara, Y.; Yoshida, H.; Yogo, S.; Takeuchi, M.; Inayoshi, M. Geochemical Mapping in Aichi Prefecture, Japan: Its Significance as a Useful Dataset for Geological Mapping. Appl. Geochem. 2007, 22, 306–319. [Google Scholar] [CrossRef]
- Oliva, P.; Viers, J.; Dupré, B. Chemical Weathering in Granitic Environments. Chem. Geol. 2003, 202, 225–256. [Google Scholar] [CrossRef]
- Borges, J.B.; Huh, Y.; Moon, S.; Noh, H. Provenance and Weathering Control on River Bed Sediments of the Eastern Tibetan Plateau and the Russian Far East. Chem. Geol. 2008, 254, 52–72. [Google Scholar] [CrossRef]
- Reimann, C.; Melezhik, V. Metallogenic Provinces, Geochemical Provinces and Regional Geology—What Causes Large-Scale Patterns in Low Density Geochemical Maps of the C-Horizon of Podzols in Arctic Europe? Appl. Geochem. 2001, 16, 963–983. [Google Scholar] [CrossRef]
- Tanaka, T.; Kawabe, I.; Hirahara, Y.; Iwamori, H.; Mimura, K.; Sugisaki, R.; Asahara, Y.; Ito, T.; Yarai, H.; Yonezawa, C.; et al. Geochemical Survey of the Sanaga-Yama Area in Aichi Perfecture for Environmental Assessment. J. Earth Planet. Sci. Nagoya Univ. 1994, 41, 1–31. [Google Scholar]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution; Blackwell Scientific Publications: Oxford, UK, 1985. [Google Scholar]
- McLennan, S.M.; Hemming, S.; McDaniel, D.K.; Hanson, G.N. Geochemical Approaches to Sedimentation, Provenance, and Tectonics. Geol. Soc. Am. 1993, 284, 21–40. [Google Scholar] [CrossRef]
- Grunsky, E.C.; Drew, L.J.; Sutphin, D.M. Process recognition in multi-element soil and stream-sediment geochemical data. Appl. Geochem. 2009, 24, 1602–1616. [Google Scholar] [CrossRef]
- Johnsson, M.J. The System Controlling the Composition of Clastic Sediments. Geol. Soc. Am. 1993, 284, 1–21. [Google Scholar]
- Ottesen, R.T.; Theobald, P.K. Stream Sediments in Mineral Exploration. In Handbook of Exploration Geochemistry: Drainage Geochemistry; Hale, M., Plant, J.A., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1994; pp. 147–184. [Google Scholar]
- Bineli, M.T.N.; Onana, V.L.; Noa Tang, S.D.; Bikoy, Y.R.; Ekodeck, G.E. Mineralogy and geochemistry of sands of the lower course of the Sanaga River, Cameroon: Implications for weathering, provenance, and tectonic setting. Acta Geochim. 2021, 40, 348–365. [Google Scholar] [CrossRef]
- Liyouck, P.R.; Ngueutchoua, G.; Armstrong-Altrin, J.S.; Sonfack, A.N.; Kontchipe Ngagoum, Y.S.; Ekoa Bessa, A.Z.; Ambassa Bela, V.; Tsanga, D.A.; Wouatong, A.S.L. Petrography and geochemistry of the Sanaga river sediments, central Cameroon: Constraints on weathering, provenance, and tectonic setting. J. Afr. Earth Sci. 2023, 199, 104840. [Google Scholar] [CrossRef]
- Rahman, M.A.; Das, S.C.; Pownceby, M.I.; Tardio, J.; Alam, M.S.; Zaman, M.N. Geochemistry of Recent Brahmaputra River Sediments: Provenance, Tectonics, Source Area Weathering and Depositional Environment. Minerals 2020, 10, 813. [Google Scholar] [CrossRef]
- Kontchipe, Y.S.N.; Sopie, F.T.; Ngueutchoua, G.; Sonfack, A.N.; Nkouathio, D.G.; Tchatchueng, R.; Nguemo, G.R.K.; Njanko, T. Mineralogy and Geochemistry Study of the Nyong River Sediments, SW Cameroon: Implications for Provenance, Weathering, and Tectonic Setting. Arab. J. Geosci. 2021, 14, 1018. [Google Scholar] [CrossRef]
- Hossain, H.M.Z.; Kawahata, H.; Roser, B.P.; Sampei, Y.; Manaka, T.; Otani, S. Geochemical Characteristics of Modern River Sediments in Myanmar and Thailand: Implications for Provenance and Weathering. Geochemistry 2017, 77, 443–458. [Google Scholar] [CrossRef]
- Pang, H.; Pan, B.; Garzanti, E.; Gao, H.; Zhao, X.; Chen, D. Mineralogy and geochemistry of modern Yellow River sediments: Implications for weathering and provenance. Chem. Geol. 2018, 488, 76–86. [Google Scholar] [CrossRef]
- Singh, P. Geochemistry and provenance of stream sediments of the Ganga River and its major tributaries in the Himalayan region, India. Chem. Geol. 2010, 269, 220–236. [Google Scholar] [CrossRef]
- Chandrajith, R.; Dissanayake, C.B.; Tobschall, H.J. Application of Multi-Element Relationships in Stream Sediments to Mineral Exploration: A Case Study of Walawe Ganga Basin, Sri Lanka. Appl. Geochem. 2001, 16, 339–350. [Google Scholar] [CrossRef]
- Darwish, M.A.G. Stream Sediment Geochemical Patterns Around an Ancient Gold Mine in the Wadi El Quleib Area of the Allaqi Region, South Eastern Desert of Egypt: Implications for Mineral Exploration and Environmental Studies. J. Geochem. Explor. 2017, 175, 156–175. [Google Scholar] [CrossRef]
- Kelley, K.D.; Graham, G.E.; Pfaff, K.; Lowers, H.A.; Koenig, A.E. Indicator Mineral Analyses of Stream-Sediment Samples Using Automated Mineralogy and Mineral Chemistry: Applicability to Exploration in Covered Terranes in Eastern Alaska, USA. Ore Geol. Rev. 2022, 148, 1–22. [Google Scholar] [CrossRef]
- Obeid, M.; Ali, M.; Mohamed, N. Geochemical Exploration on the Stream Sediments of Gabal El Mueilha Area, Central Eastern Desert, Egypt: An Overview on the Rare Metals. Resour. Geol. 2001, 51, 217–227. [Google Scholar] [CrossRef]
- Pan, T.; Zuo, R.; Wang, Z. Geological Mapping via Convolutional Neural Network Based on Remote Sensing and Geochemical Survey Data in Vegetation Coverage Areas. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2023, 16, 3485–3494. [Google Scholar] [CrossRef]
- Audley-Charles, M.G. Geometrical Problems and Implications of Large-Scale Over-Thrusting in the Banda Arc—Australian Margin Collision Zone; The Geological Society of London: London, UK, 1981; pp. 407–416. [Google Scholar]
- Audley-Charles, M.G. Ocean Trench Blocked and Obliterated by Banda Forearc Collision with Australian Proximal Continental Slope. Tectonophysics 2004, 389, 65–79. [Google Scholar] [CrossRef]
- Barber, A.J. Structural Interpretations of the Island of Timor, Eastern Indonesia. In The Geology and Tectonics of Eastern Indonesia; Geological Research and Development Centre: Bandung, Indonesia, 1981; Volume 2, pp. 183–197. [Google Scholar]
- Berry, R.F.; Jenner, G.A. Basalt Geochemistry as a Test of the Tectonic Models of Timor. J. Geol. Soc. Lond. 1982, 139, 593–604. [Google Scholar] [CrossRef]
- Duffy, B.; Quigley, M.; Harris, R.; Ring, U. Arc-Parallel Extrusion of the Timor Sector of the Banda Arc-Continent Collision. Tectonics 2013, 32, 641–660. [Google Scholar] [CrossRef]
- Tate, G.W.; McQuarrie, N.; Tiranda, H.; van Hinsbergen, D.J.J.; Harris, R.; Zachariasse, W.J.; Fellin, M.G.; Reiners, P.W.; Willett, S.D. Reconciling Regional Continuity with Local Variability in Structure, Uplift and Exhumation of the Timor Orogen. Gondwana Res. 2017, 49, 364–386. [Google Scholar] [CrossRef]
- Haig, D.W.; McCartain, E.; Barber, L.; Backhouse, J. Triassic-Lower Jurassic Foraminiferal Indices for Bahaman-Type Carbonate-Bank Limestones, Cablac Mountain, East Timor. J. Foraminifer. Res. 2007, 37, 248–264. [Google Scholar] [CrossRef]
- Haig, D.W.; McCartain, E.W.; Keep, M.; Barber, L. Re-evaluation of the Cablac Limestone at Its Type Area, East Timor: Revision of the Miocene Stratigraphy of Timor. J. Asian Earth Sci. 2008, 33, 366–378. [Google Scholar] [CrossRef]
- Keep, M.; Haig, D.W. Deformation and Exhumation in Timor: Distinct Stages of a Young Orogeny. Tectonophysics 2010, 483, 93–111. [Google Scholar] [CrossRef]
- Audley-Charles, M.G.; Carter, D.J.; Barber, A.J. Stratigraphic Basis for Tectonic Interpretation of the Outer Banda Arc, Eastern Indonesia. 1974. Available online: https://archives.datapages.com/data/ipa/data/003/003001/25_ipa0030025.htm (accessed on 26 May 2024).
- Charlton, T.R.; Barber, A.J.; McGowan, A.J.; Nicoll, R.S.; Roniewicz, E.; Cook, S.E.; Barkham, S.T.; Bird, P.R. The Triassic of Timor: Lithostratigraphy, Chronostratigraphy and Palaeogeography. J. Asian Earth Sci. 2009, 36, 341–363. [Google Scholar] [CrossRef]
- Duffy, B.; Kalansky, J.; Bassett, K.; Harris, R.; Quigley, M.; van Hinsbergen, D.J.J.; Strachan, L.J.; Rosenthal, Y. Mélange Versus Forearc Contributions to Sedimentation and Uplift, During Rapid Denudation of a Young Banda Forearc-Continent Collisional Belt. J. Asian Earth Sci. 2017, 138, 186–210. [Google Scholar] [CrossRef]
- Haig, D.W.; McCartain, E.; Mory, A.J.; Borges, G.; Davydov, V.I.; Dixon, M.; Ernst, A.; Groflin, S.; Håkansson, E.; Keep, M.; et al. Postglacial Early Permian (late Sakmarian-early Artinskian) shallow-marine carbonate deposition along a 2000km transect from Timor to west Australia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 409, 180–204. [Google Scholar] [CrossRef]
- Haig, D.W.; McCartain, E. Triassic Organic-Cemented Siliceous Agglutinated Foraminifera from Timor Leste: Conservative Development in Shallow-Marine Environments. J. Foraminifer. Res. 2010, 40, 366–392. [Google Scholar] [CrossRef]
- Harris, R.A.; Sawyer, R.K.; Audley-Charles, M.G. Collisional Melange Development: Geologic Associations of Active Melange-Forming Processes with Exhumed Melange Facies in the Western Banda Orogen, Indonesia. Tectonics 1998, 17, 458–479. [Google Scholar] [CrossRef]
- Kenyon, C.S. Stratigraphy and Sedimentology of the Late Miocene to Quaternary Deposits of Timor. Ph.D. Thesis, University of London, London, UK, May 1974. [Google Scholar]
- Lisboa, J.V.V.; Silva, T.P.; De Oliveira, D.P.S.; Carvalho, J.F. Mineralogical and Geochemistry Characteristics of the Bobonaro Melange of Western East Timor: Provenance Implications. Comun. GeolóGicas 2020, 106, 35–49. [Google Scholar]
- Standley, C.E.; Harris, R. Tectonic Evolution of Forearc Nappes of the Active Banda Arc-Continent Collision: Origin, Age, Metamorphic History and Structure of the Lolotoi Complex, East Timor. Tectonophysics 2009, 479, 66–94. [Google Scholar] [CrossRef]
- Darnley, A.G.; Bjorklund, A.; Bolviken, B.; Gustavsson, N.; Koval, P.V.; Plant, J.A.; Steenfelt, A.; Tauchid, M.; Xuejing, X.; Garrett, R.G.; et al. A Global Geochemical Reference Network & Field Methods for Regional Surveys. In A Global Geochemical Database for Environmental and Resource Management: Recommendations for International Geochemical Mapping; United Nations Educational, Scientific and Cultural Organization (UNESCO) Publishing House: Paris, France, 1995; pp. 37–53. [Google Scholar]
- Hale, M.; Plant, J.A. Introduction: The Foundation of Modern Drainage Geochemistry. In Handbook of Exploration Geochemistry: Drainage Geochemistry; Govett, G.J.S., Ed.; Elsevier Science B.V.: Amsterdam, The Netherlands, 1994; pp. 3–9. [Google Scholar]
- Ohta, A.; Imai, N.; Terashima, S.; Tachibana, Y. Application of Multi-Element Statistical Analysis for Regional Geochemical Mapping in Central Japan. Appl. Geochem. 2005, 20, 1017–1037. [Google Scholar] [CrossRef]
- Tanaka, T.; Kawabe, I.; Yamamoto, K.; Iwamori, H.; Hirahara, Y.; Mimura, K.; Asahara, Y.; Ito, T.; Yonezawa, C.; Dragusanu, C.; et al. Distributions of Elements in Stream Sediments in and around Seto City, Aichi Prefecture: An Attempt to a Geoenvironmental Assessment by Geochemical Mapping. Geochemistry 1995, 29, 113–125. [Google Scholar]
- Yamamoto, K.; Morishita, T. Preparation of Standard Composites for the Trace Elements Analysis by X-ray Fluorescence. Geol. Soc. Jpn. 1997, 103, 1037–1045. [Google Scholar] [CrossRef]
- Rudnick, R.L.; Gao, S. Composition of the Continental Crust. In Treatise on Geochemistry: The Crust; Rudnick, R.L., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2003; Volume 3, pp. 1–64. [Google Scholar]
- Algeo, T.J.; Tribovillard, N. Environmental Analysis of Paleoceanographic Systems Based on Molybdenum-Uranium Covariation. Chem. Geol. 2009, 268, 211–225. [Google Scholar] [CrossRef]
- Tribovillard, N.; Algeo, T.J.; Baudin, F.; Riboulleau, A. Analysis of Marine Environmental Conditions Based on Molybdenum-Uranium Covariation-Applications to Mesozoic Paleoceanography. Chem. Geol. 2012, 224–325, 46–58. [Google Scholar] [CrossRef]
- He, T.; Lu, S.; Li, W.; Sun, D.; Pan, W.; Zhang, B.; Tan, Z.; Ying, J. Paleoweathering, Hydrothermal Activity and Organic Matter Enrichment During the Formation of Earliest Cambrian Black Strata in the Northwest Tarim Basin, China. J. Pet. Sci. Eng. 2020, 189, 106987. [Google Scholar] [CrossRef]
- Liu, D.; Lin, B.; Kandasamy, S.; Wang, H.; Liu, Q.; Zou, W.; Zhu, A.; Zou, J.; Lou, J.Y.; Shi, X. Geochemical Appraisal of Chemical Weathering and Metal Contamination in Coastal Surface Sediments, Off Northwest Hainan Island, the Gulf of Tonkin. Front. Mar. Sci. 2019, 6, 363. [Google Scholar] [CrossRef]
- Biswas, P.K.; Alam, M.S.; Hasan, A.S.M.M.; Ahmed, S.S.; Zaman, M.N. Geochemical signatures of recent bar deposits in the Tista river, Bangladesh: Implications to provenance, paleoweathering and tectonics. J. Nepal Geol. Soc. 2020, 60, 1–20. [Google Scholar] [CrossRef]
- He, M.; Zheng, H.; Clift, P.D.; Tada, R.; Wu, W.; Luo, C. Geochemistry of fine-grained sediments in the Yangtze River and the implications for provenance and chemical weathering in East Asia. Prog. Earth Planet. Sci. 2015, 2, 1–20. [Google Scholar] [CrossRef]
- Hossain, H.M.Z. Major, trace, and REE geochemistry of the Meghna River sediments, Bangladesh: Constraints on weathering and provenance. Geol. J. 2020, 55, 3321–3343. [Google Scholar] [CrossRef]
- Kimeli, A.; Ocholla, O.; Okello, J.; Koedam, N.; Westphal, H.; Kairo, J. Geochemical and petrographic characteristics of sediments along the transboundary (Kenya-Tanzania) Umba River as indicators of provenance and weathering. Open Geosci. 2021, 13, 1064–1083. [Google Scholar] [CrossRef]
- Sonfack, A.N.; Ngueutchoua, G.; Kontchipe, Y.S.N.; Sopie, F.T.; Nkouathio, D.G.; Wouatong, A.S.L.; Tchatchueng, R.; Kenfack Nguemo, G.R.; Njanko, T. Mineralogical and Geochemical Signatures of Surface Stream Sediments from Dibamba River Basin, SW Cameroon: Implications for Provenance, Weathering, and Tectonic Setting. J. Afr. Earth Sci. 2021, 181, 1–26. [Google Scholar] [CrossRef]
- Cox, R.; Lowe, D.R.; Cullers, R.L. The Influence of Sediment Recycling and Basement Composition on Evolution of Mudrock Chemistry in the Southwestern United States. Geochim. Cosmochim. Acta 1995, 59, 2919–2940. [Google Scholar] [CrossRef]
- Zuffa, G.G. Unravelling Hinterland and Offshore Palaeogeography from Deep-water Arenites. In Marine Clastic Seimentology: Models and Case Studies (A Volume in Memory of C. Tarquin Teale); Leggett, J.K., Zuffa, G.G., Eds.; Graham and Trotman: London, UK, 1987; pp. 39–61. [Google Scholar]
- Pearson, K. On Lines and Planes of Closest Fit to Systems of Points in Space. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1901, 2, 559–572. [Google Scholar] [CrossRef]
- Demšar, U.; Harris, P.; Brunsdon, C.; Fotheringham, A.S.; McLoone, S. Principal Component Analysis on Spatial Data: An Overview. Ann. Assoc. Am. Geogr. 2013, 103, 106–128. [Google Scholar] [CrossRef]
- Reimann, C.; Filzmoser, P.; Garrett, R.G.; Dutter, R. Principal Component Analysis (PCA) and Factor Analysis (FA). In Statistical Data Analysis Explained: Applied Environmental Statistics with R; John Wiley & Sons, Ltd.: West Sussex, UK, 2008; pp. 211–232. [Google Scholar]
- Armstrong-Altrin, J.S.; Botello, A.V.; Villanueva, S.F.; Soto, L.A. Geochemistry of Surface Sediments from the Northwestern Gulf of Mexico: Implications for Provenance and Heavy Metal Contamination. Geol. Q. 2019, 63, 522–538. [Google Scholar] [CrossRef]
- Kassi, A.M.; Grigsby, J.D.; Khan, A.S.; Kasi, A.K. Sandstone Petrology and Geochemistry of the Oligocene-Early Miocene Panjgur Formation, Makran Accretionary Wedge, Southwest Pakistan: Implications for Provenance, Weathering and Tectonic Setting. J. Asian Earth Sci. 2015, 105, 192–207. [Google Scholar] [CrossRef]
- Turekian, K.K.; Wedepohl, K.H. Distribution of the Elements in Some Major Units of the Earth’s Crust. Bull. Geol. Soc. Am. 1961, 72, 175–192. [Google Scholar] [CrossRef]
- Greber, N.D.; Dauphas, N. The Chemistry of Fine-Grained Terrigenous Sediments Reveals a Chemically Evolved Paleoarchean Emerged Crust. Geochim. Cosmochim. Acta 2019, 255, 247–264. [Google Scholar] [CrossRef]
- Hayashi, K.-I.; Fujisawa, H.; Holland, H.D.; Ohmoto, H. Geochemistry of 1.9 Ga Sedimentary Rocks from Northeastern Labrador, Canada. Geochim. Cosmochim. Acta 1997, 61, 4115–4137. [Google Scholar] [CrossRef]
- Cai, G.; Guo, F.; Liu, X.; Sui, S.; Li, C.; Zhao, L. Geochemistry of Neogene Sedimentary Rocks from the Jiyang Basin, North China Block: The Roles of Grain Size and Clay Minerals. Geochem. J. 2008, 42, 381–402. [Google Scholar] [CrossRef]
- Nesbitt, W.H.; Markovics, G.; Price, R.C. Chemical Processes Affecting Alkalis and Alkaline Earths During Continental Weathering. Geochim. Cosmochim. Acta 1980, 44, 1659–1666. [Google Scholar] [CrossRef]
- Kronberg, B.I.; Nesbitt, H.W.; Fyfe, W.S. Mobilities of Alkalis, Alkaline Earths and Halogens During Weathering. Chem. Geol. 1987, 60, 41–49. [Google Scholar] [CrossRef]
- Price, J.R.; Velbel, M.A. Chemical Weathering Indices Applied to Weathering Profiles Developed on Heterogeneous Felsic Metamorphic Parent Rocks. Chem. Geol. 2003, 202, 397–416. [Google Scholar] [CrossRef]
- Garzanti, E.; Padoan, M.; Peruta, L.; Setti, M.; Najman, Y.; Villa, I.M. Weathering Geochemistry and Sr-Nd Fingerprints of Equatorial Upper Nile and Congo Muds. Geochem. Geophys. Geosyst. 2013, 14, 292–316. [Google Scholar] [CrossRef]
- Lim, D.; Choi, J.Y.; Shin, H.H.; Rho, K.C.; Jung, H.S. Multielement Geochemistry of Offshore Sediments in the Southeastern Yellow Sea and Implications for Sediment Origin and Dispersal. Quat. Int. 2013, 298, 196–206. [Google Scholar] [CrossRef]
- Von Eynatten, H.; Tolosana-Delgado, R.; Karius, V.; Bachmann, K.; Caracciolo, L. Sediment Generation in Humid Mediterranean Setting: Grain-Size and Source-Rock Cntrol on Sediment Geochemistry and Mineralogy (Sila Massif, Calabria). Sediment. Geol. 2016, 336, 68–80. [Google Scholar] [CrossRef]
- Armstrong-Altrin, J.S. Detrital zircon U–Pb geochronology and geochemistry of the Riachuelos and Palma Sola beach sediments, Veracruz State, Gulf of Mexico: A new insight on palaeoenvironment. J. Palaeogeogr. 2020, 9, 1–27. [Google Scholar] [CrossRef]
- Armstrong-Altrin, J.S.; Lee, Y.I.; Verma, S.P.; Ramasamy, S. Geochemistry of Sandstones from the Upper Miocene Kudankulam Formation, Southern Inida: Implications for Provenance, Weathering, and Tectonic Setting. J. Sediment. Res. 2004, 74, 285–297. [Google Scholar] [CrossRef]
- Barkham, S.T. The Structure and Stratigraphy of the Permo-Triassic Carbonate Formations of West Timor, Indonesia. Ph.D. Thesis, University of London, London, UK, 1993. [Google Scholar]
- Herron, M.M. Geochemical Classification of Terrigenous Sands and Shales from Core or Log Data. Sediment. Petrol. 1988, 58, 820–829. [Google Scholar] [CrossRef]
- Pettijohn, F.J.; Potter, P.E.; Siever, R. Sand and Sandstone; Springer: New York, NY, USA, 1972; pp. 1–618. [Google Scholar] [CrossRef]
- Roser, B.P.; Cooper, R.A.; Nathan, S.; Tulloch, A.J. Reconnaissance Sandstone Geochemistry, Provenance, and Tectonic Setting of the Lower Paleozoic Terranes of the West Coast and Nelson, New Zealand. N. Z. J. Geol. Geophys. 1996, 39, 1–16. [Google Scholar] [CrossRef]
- Wimmenauer, W. Das pravariskische Kristallin im Schwarzwald. Fortschr. Mineral. 1984, 62, 69–86. [Google Scholar]
- Armstrong-Altrin, J.S.; Nagarajan, R.; Madhavaraju, J.; Rosalez-Hoz, L.; Lee, Y.I.; Balaram, V.; Cruz-Martínez, A.; Avila-Ramírez, G. Geochemistry of the Jurassic and Upper Cretaceous Shales from the Molango Region, Hidalgo, Eastern Mexico: Implications for Source-Area Weathering, Provenance, and Tectonic Setting. Comptes Rendus - Geosci. 2013, 345, 185–202. [Google Scholar] [CrossRef]
- Vicente, V.A.S.; Pratas, J.A.M.S.; Santos, F.C.M.; Silva, M.M.V.G.; Favas, P.J.C.; Conde, L.E.N. Geochemical Anomalies from a Survey of Stream Sediments in the Maquelab Area (Oecusse, Timor-Leste) and Their Bearing on the Identification of Mafic - Ultramafic Chromite Rich Complex. Appl. Geochem. 2021, 126, 104868. [Google Scholar] [CrossRef]




| Catchment Area | Sample Code | Weight % (wt%) | ||||||
| SiO2 | TiO2 | Al2O3 | Fe2O3 | MnO | MgO | CaO | ||
| Aiasa River | SS01 | 51.65 | 1.32 | 17.60 | 9.32 | 0.21 | 3.67 | 5.58 |
| SS02 | 50.78 | 1.13 | 16.30 | 7.98 | 0.18 | 3.38 | 8.84 | |
| SS017 | 50.61 | 1.05 | 15.40 | 7.88 | 0.17 | 3.26 | 9.48 | |
| SS018 | 50.74 | 1.03 | 15.30 | 7.33 | 0.19 | 3.15 | 10.47 | |
| SS032 | 51.74 | 0.92 | 14.10 | 6.53 | 0.17 | 3.12 | 10.83 | |
| Avg. | 51.10 | 1.09 | 15.74 | 7.81 | 0.18 | 3.32 | 9.04 | |
| Std. | 0.54 | 0.15 | 1.30 | 1.02 | 0.02 | 0.22 | 2.09 | |
| Turon River | SS029 | 39.00 | 0.67 | 10.40 | 4.75 | 0.09 | 2.11 | 24.25 |
| SS030 | 47.30 | 0.88 | 12.30 | 5.91 | 0.15 | 2.63 | 16.19 | |
| Avg. | 43.15 | 0.78 | 11.35 | 5.33 | 0.12 | 2.37 | 20.22 | |
| Std. | 5.87 | 0.15 | 1.34 | 0.82 | 0.04 | 0.37 | 5.70 | |
| Ermeti River | SS010 | 55.01 | 1.08 | 15.70 | 8.40 | 0.14 | 3.99 | 6.99 |
| SS011 | 47.70 | 1.14 | 12.90 | 7.54 | 0.18 | 3.84 | 14.22 | |
| Avg. | 51.36 | 1.11 | 14.30 | 7.97 | 0.16 | 3.92 | 10.61 | |
| Std. | 5.17 | 0.04 | 1.98 | 0.61 | 0.03 | 0.11 | 5.11 | |
| Holarua River | SS03 | 47.92 | 1.20 | 13.80 | 8.19 | 0.16 | 3.03 | 13.43 |
| SS04 | 36.84 | 1.11 | 11.00 | 8.22 | 0.14 | 3.12 | 23.21 | |
| SS06 | 44.69 | 1.00 | 11.80 | 7.49 | 0.11 | 2.27 | 17.70 | |
| Avg. | 43.15 | 1.10 | 12.20 | 7.97 | 0.14 | 2.81 | 18.11 | |
| Std. | 5.70 | 0.10 | 1.44 | 0.41 | 0.03 | 0.47 | 4.90 | |
| Karau Ulun River | SS05 | 51.79 | 1.25 | 13.60 | 8.82 | 0.17 | 3.01 | 10.71 |
| SS07 | 50.77 | 1.25 | 15.50 | 8.76 | 0.18 | 3.43 | 9.86 | |
| SS08 | 53.59 | 1.16 | 14.60 | 8.94 | 0.17 | 3.15 | 9.56 | |
| SS09 | 55.50 | 1.13 | 12.30 | 6.61 | 0.24 | 2.47 | 10.00 | |
| SS024 | 45.52 | 1.08 | 12.40 | 7.30 | 0.17 | 3.09 | 15.99 | |
| SS026 | 47.29 | 1.11 | 12.90 | 7.80 | 0.16 | 3.11 | 13.83 | |
| SS027 | 49.50 | 1.21 | 13.40 | 8.45 | 0.17 | 3.08 | 12.32 | |
| SS028 | 49.00 | 1.15 | 13.50 | 8.17 | 0.15 | 2.98 | 12.47 | |
| SS031 | 52.88 | 1.05 | 13.20 | 6.41 | 0.16 | 2.66 | 12.60 | |
| Avg. | 50.65 | 1.15 | 13.49 | 7.92 | 0.17 | 3.00 | 11.93 | |
| Std. | 3.16 | 0.07 | 1.02 | 0.96 | 0.02 | 0.28 | 2.12 | |
| Soin River | SS019 | 48.53 | 1.52 | 13.80 | 10.79 | 0.23 | 3.14 | 10.84 |
| SS020 | 52.72 | 1.48 | 15.40 | 9.36 | 0.24 | 3.29 | 8.43 | |
| SS021 | 44.86 | 1.37 | 13.20 | 10.07 | 0.26 | 3.02 | 13.31 | |
| SS022 | 52.47 | 1.60 | 16.40 | 9.94 | 0.17 | 3.56 | 6.98 | |
| SS023 | 52.82 | 1.71 | 16.60 | 11.54 | 0.18 | 3.61 | 6.04 | |
| SS025 | 47.69 | 1.48 | 13.40 | 9.13 | 0.25 | 3.22 | 13.01 | |
| SS033 | 52.87 | 1.20 | 13.30 | 7.55 | 0.22 | 2.98 | 11.31 | |
| SS036 | 52.30 | 1.18 | 13.20 | 7.52 | 0.23 | 2.97 | 11.10 | |
| SS037 | 52.35 | 1.37 | 13.50 | 8.77 | 0.24 | 3.10 | 10.28 | |
| SS038 | 54.53 | 1.00 | 13.60 | 6.69 | 0.21 | 2.88 | 11.05 | |
| Avg. | 51.11 | 1.39 | 14.24 | 9.13 | 0.22 | 3.18 | 10.24 | |
| Std. | 3.03 | 0.21 | 1.35 | 1.54 | 0.03 | 0.25 | 2.39 | |
| Laclo do Sul River | SS040 | 55.81 | 1.20 | 17.90 | 9.28 | 0.17 | 4.32 | 4.93 |
| SS041 | 54.09 | 1.25 | 17.60 | 9.50 | 0.18 | 4.41 | 5.15 | |
| SS042 | 54.66 | 1.25 | 17.60 | 9.29 | 0.16 | 4.33 | 5.18 | |
| SS044 | 54.23 | 1.23 | 17.20 | 9.21 | 0.18 | 4.40 | 6.59 | |
| SS045 | 50.13 | 1.50 | 17.10 | 11.73 | 0.19 | 4.70 | 7.94 | |
| SS046 | 54.53 | 1.19 | 16.80 | 9.26 | 0.18 | 4.30 | 6.85 | |
| SS047 | 55.33 | 1.14 | 16.40 | 8.57 | 0.17 | 4.39 | 6.86 | |
| Avg. | 54.11 | 1.25 | 17.23 | 9.55 | 0.18 | 4.41 | 6.21 | |
| Std. | 1.86 | 0.12 | 0.52 | 1.00 | 0.01 | 0.14 | 1.14 | |
| Catchment Area | Sample Code | Weight % (wt%) | Ratios | ICV | ||||
| Na2O | K2O | P2O5 | SiO2/ Al2O3 | K2O/ Na2O | Na2O/ TiO2 | |||
| Aiasa River | SS01 | 2.17 | 2.15 | 0.26 | 2.93 | 0.99 | 1.64 | 1.18 |
| SS02 | 2.00 | 2.08 | 0.23 | 3.12 | 1.04 | 1.78 | 1.14 | |
| SS017 | 2.04 | 1.97 | 0.21 | 3.29 | 0.97 | 1.95 | 1.18 | |
| SS018 | 2.06 | 1.86 | 0.21 | 3.32 | 0.90 | 1.99 | 1.14 | |
| SS032 | 2.06 | 1.64 | 0.20 | 3.67 | 0.80 | 2.24 | 1.16 | |
| Avg. | 2.07 | 1.94 | 0.22 | 3.26 | 0.94 | 1.92 | 1.16 | |
| Std. | 0.06 | 0.20 | 0.02 | 0.27 | 0.09 | 0.23 | 0.02 | |
| Turon River | SS029 | 1.57 | 0.99 | 0.15 | 3.75 | 0.63 | 2.34 | 1.12 |
| SS030 | 1.96 | 1.36 | 0.18 | 3.85 | 0.69 | 2.22 | 1.19 | |
| Avg. | 1.77 | 1.17 | 0.17 | 3.80 | 0.66 | 2.28 | 1.15 | |
| Std. | 0.28 | 0.27 | 0.02 | 0.07 | 0.05 | 0.08 | 0.05 | |
| Ermeti River | SS010 | 2.09 | 1.34 | 0.15 | 3.50 | 0.64 | 1.93 | 1.21 |
| SS011 | 1.95 | 1.03 | 0.18 | 3.70 | 0.53 | 1.72 | 1.35 | |
| Avg. | 2.02 | 1.19 | 0.16 | 3.60 | 0.58 | 1.82 | 1.28 | |
| Std. | 0.10 | 0.22 | 0.02 | 0.14 | 0.08 | 0.15 | 0.10 | |
| Holarua River | SS03 | 2.17 | 1.28 | 0.21 | 3.47 | 0.59 | 1.81 | 1.30 |
| SS04 | 1.64 | 0.68 | 0.16 | 3.35 | 0.41 | 1.48 | 1.49 | |
| SS06 | 1.26 | 1.09 | 0.16 | 3.79 | 0.86 | 1.26 | 1.22 | |
| Avg. | 1.69 | 1.01 | 0.18 | 3.54 | 0.62 | 1.51 | 1.34 | |
| Std. | 0.46 | 0.31 | 0.03 | 0.23 | 0.23 | 0.28 | 0.14 | |
| Karau Ulun River | SS05 | 1.81 | 1.33 | 0.20 | 3.81 | 0.73 | 1.45 | 1.32 |
| SS07 | 2.11 | 1.53 | 0.22 | 3.28 | 0.72 | 1.68 | 1.24 | |
| SS08 | 2.06 | 1.45 | 0.21 | 3.67 | 0.70 | 1.77 | 1.29 | |
| SS09 | 1.06 | 1.84 | 0.23 | 4.51 | 1.74 | 0.94 | 1.16 | |
| SS024 | 1.80 | 1.16 | 0.20 | 3.67 | 0.65 | 1.67 | 1.31 | |
| SS026 | 1.72 | 1.29 | 0.19 | 3.67 | 0.75 | 1.55 | 1.30 | |
| SS027 | 1.69 | 1.42 | 0.22 | 3.69 | 0.84 | 1.40 | 1.31 | |
| SS028 | 1.70 | 1.45 | 0.21 | 3.63 | 0.86 | 1.48 | 1.27 | |
| SS031 | 2.47 | 1.17 | 0.18 | 4.01 | 0.47 | 2.35 | 1.22 | |
| Avg. | 1.82 | 1.40 | 0.21 | 3.77 | 0.83 | 1.59 | 1.27 | |
| Std. | 0.38 | 0.21 | 0.02 | 0.34 | 0.36 | 0.37 | 0.05 | |
| Soin River | SS019 | 2.22 | 1.39 | 0.23 | 3.52 | 0.63 | 1.46 | 1.54 |
| SS020 | 2.54 | 1.51 | 0.23 | 3.42 | 0.59 | 1.72 | 1.34 | |
| SS021 | 1.81 | 1.39 | 0.22 | 3.40 | 0.77 | 1.32 | 1.48 | |
| SS022 | 2.57 | 1.64 | 0.24 | 3.20 | 0.64 | 1.61 | 1.33 | |
| SS023 | 2.59 | 1.60 | 0.27 | 3.18 | 0.62 | 1.52 | 1.42 | |
| SS025 | 2.13 | 1.27 | 0.23 | 3.56 | 0.60 | 1.44 | 1.45 | |
| SS033 | 2.24 | 1.32 | 0.20 | 3.98 | 0.59 | 1.87 | 1.32 | |
| SS036 | 2.17 | 1.30 | 0.20 | 3.96 | 0.60 | 1.84 | 1.31 | |
| SS037 | 2.12 | 1.28 | 0.20 | 3.88 | 0.61 | 1.54 | 1.39 | |
| SS038 | 2.26 | 1.39 | 0.20 | 4.01 | 0.62 | 2.25 | 1.21 | |
| Avg. | 2.27 | 1.41 | 0.22 | 3.61 | 0.62 | 1.66 | 1.38 | |
| Std. | 0.24 | 0.13 | 0.02 | 0.32 | 0.05 | 0.27 | 0.10 | |
| Laclo do Sul River | SS040 | 2.74 | 1.64 | 0.23 | 3.12 | 0.60 | 2.29 | 1.22 |
| SS041 | 2.62 | 1.48 | 0.25 | 3.07 | 0.57 | 2.10 | 1.24 | |
| SS042 | 2.68 | 1.52 | 0.25 | 3.11 | 0.57 | 2.14 | 1.23 | |
| SS044 | 2.54 | 1.24 | 0.22 | 3.15 | 0.49 | 2.07 | 1.23 | |
| SS045 | 2.28 | 1.10 | 0.22 | 2.93 | 0.48 | 1.52 | 1.38 | |
| SS046 | 2.57 | 1.40 | 0.22 | 3.25 | 0.54 | 2.16 | 1.26 | |
| SS047 | 2.72 | 1.21 | 0.20 | 3.37 | 0.45 | 2.40 | 1.26 | |
| Avg. | 2.59 | 1.37 | 0.23 | 3.14 | 0.53 | 2.10 | 1.26 | |
| Std. | 0.16 | 0.19 | 0.02 | 0.14 | 0.06 | 0.28 | 0.05 | |
| Catchment Area | Sample Code | Weight % (wt%) | ||||||
| SiO2 | TiO2 | Al2O3 | Fe2O3 | MnO | MgO | CaO | ||
| Merek River | SS039 | 53.33 | 0.78 | 14.40 | 6.17 | 0.19 | 2.40 | 10.70 |
| SS043 | 58.19 | 0.94 | 15.40 | 7.53 | 0.26 | 2.48 | 6.68 | |
| Avg. | 55.76 | 0.86 | 14.90 | 6.85 | 0.22 | 2.44 | 8.69 | |
| Std. | 3.44 | 0.11 | 0.71 | 0.96 | 0.05 | 0.06 | 2.84 | |
| Clerec River | SS035 | 56.32 | 1.48 | 15.70 | 8.82 | 0.23 | 3.53 | 6.86 |
| SS048 | 52.79 | 1.54 | 17.00 | 9.85 | 0.25 | 4.41 | 6.58 | |
| SS049 | 59.94 | 0.76 | 13.10 | 5.87 | 0.32 | 1.66 | 8.17 | |
| SS050 | 52.31 | 2.17 | 15.70 | 11.94 | 0.29 | 4.05 | 6.99 | |
| SS051 | 56.96 | 1.35 | 16.10 | 8.46 | 0.19 | 3.86 | 5.80 | |
| SS052 | 57.58 | 1.34 | 16.90 | 8.51 | 0.19 | 3.90 | 5.86 | |
| SS053 | 57.71 | 1.06 | 14.90 | 7.55 | 0.20 | 3.37 | 6.57 | |
| Avg. | 56.23 | 1.38 | 15.63 | 8.72 | 0.24 | 3.54 | 6.69 | |
| Std. | 2.75 | 0.44 | 1.33 | 1.88 | 0.05 | 0.90 | 0.80 | |
| Sahe River | SS012 | 56.85 | 0.78 | 14.80 | 6.11 | 0.14 | 2.02 | 8.20 |
| SS013 | 52.80 | 1.49 | 13.50 | 7.51 | 0.26 | 2.23 | 11.23 | |
| SS014 | 52.50 | 0.83 | 13.20 | 5.99 | 0.16 | 2.10 | 12.05 | |
| SS015 | 38.30 | 1.44 | 9.97 | 6.59 | 0.25 | 1.86 | 23.49 | |
| SS016 | 49.74 | 1.32 | 13.20 | 6.81 | 0.24 | 2.41 | 13.76 | |
| SS034 | 57.20 | 1.42 | 13.80 | 7.10 | 0.23 | 2.33 | 9.40 | |
| Avg. | 51.23 | 1.21 | 13.08 | 6.68 | 0.21 | 2.16 | 13.02 | |
| Std. | 6.94 | 0.32 | 1.63 | 0.58 | 0.05 | 0.20 | 5.49 | |
| - | UCC | 66.62 | 0.64 | 15.40 | 5.04 | 0.10 | 2.48 | 3.59 |
| - | PAAS | 62.80 | 1.00 | 18.90 | 6.50 | 0.11 | 2.20 | 1.30 |
| Catchment Area | Sample Code | Weight % (wt%) | Ratios | ICV | ||||
| Na2O | K2O | P2O5 | SiO2/ Al2O3 | K2O/ Na2O | Na2O/ TiO2 | |||
| Merek River | SS039 | 1.92 | 1.78 | 0.19 | 3.70 | 0.93 | 2.47 | 1.04 |
| SS043 | 2.26 | 1.70 | 0.21 | 3.78 | 0.75 | 2.41 | 1.12 | |
| Avg. | 2.09 | 1.74 | 0.20 | 3.74 | 0.84 | 2.44 | 1.08 | |
| Std. | 0.24 | 0.06 | 0.01 | 0.05 | 0.12 | 0.04 | 0.05 | |
| Clerec River | SS035 | 2.45 | 1.41 | 0.19 | 3.59 | 0.57 | 1.66 | 1.28 |
| SS048 | 2.24 | 1.63 | 0.21 | 3.11 | 0.73 | 1.45 | 1.29 | |
| SS049 | 1.46 | 1.88 | 0.17 | 4.58 | 1.29 | 1.93 | 1.01 | |
| SS050 | 2.48 | 1.24 | 0.20 | 3.33 | 0.50 | 1.14 | 1.55 | |
| SS051 | 2.73 | 1.38 | 0.21 | 3.54 | 0.50 | 2.03 | 1.27 | |
| SS052 | 2.79 | 1.42 | 0.20 | 3.41 | 0.51 | 2.08 | 1.22 | |
| SS053 | 2.46 | 1.46 | 0.18 | 3.87 | 0.59 | 2.33 | 1.23 | |
| Avg. | 2.37 | 1.49 | 0.19 | 3.63 | 0.67 | 1.80 | 1.27 | |
| Std. | 0.44 | 0.21 | 0.01 | 0.48 | 0.28 | 0.41 | 0.16 | |
| Sahe River | SS012 | 1.38 | 2.09 | 0.15 | 3.84 | 1.51 | 1.77 | 0.93 |
| SS013 | 1.69 | 1.54 | 0.18 | 3.91 | 0.91 | 1.13 | 1.20 | |
| SS014 | 1.62 | 1.73 | 0.17 | 3.98 | 1.06 | 1.94 | 1.05 | |
| SS015 | 1.13 | 1.16 | 0.16 | 3.84 | 1.03 | 0.79 | 1.35 | |
| SS016 | 1.92 | 1.27 | 0.17 | 3.77 | 0.66 | 1.46 | 1.19 | |
| SS034 | 2.11 | 1.40 | 0.19 | 4.14 | 0.66 | 1.49 | 1.20 | |
| Avg. | 1.64 | 1.53 | 0.17 | 3.91 | 0.97 | 1.43 | 1.15 | |
| Std. | 0.35 | 0.34 | 0.01 | 0.13 | 0.32 | 0.42 | 0.14 | |
| - | UCC | 3.27 | 2.80 | 0.15 | 4.33 | 0.86 | 5.11 | 1.16 |
| - | PAAS | 1.20 | 3.70 | 0.16 | 3.32 | 3.08 | 1.20 | 0.85 |
| (A) | SiO2 | TiO2 | Al2O3 | Fe2O3 | MnO | MgO | CaO | Na2O | K2O | P2O5 | SiO2/ Al2O3 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | 1.00 | ||||||||||
| TiO2 | −0.26 | 1.00 | |||||||||
| Al2O3 | 0.36 | 0.71 | 1.00 | ||||||||
| Fe2O3 | −0.43 | 0.93 | 0.59 | 1.00 | |||||||
| MnO | −0.60 | −0.32 | −0.76 | −0.20 | 1.00 | ||||||
| MgO | 0.10 | 0.90 | 0.92 | 0.75 | −0.63 | 1.00 | |||||
| CaO | −0.60 | −0.57 | −0.94 | −0.43 | 0.81 | −0.81 | 1.00 | ||||
| Na2O | 0.68 | 0.45 | 0.87 | 0.25 | −0.78 | 0.73 | −0.91 | 1.00 | |||
| K2O | 0.27 | 0.60 | 0.94 | 0.56 | −0.74 | 0.80 | −0.85 | 0.77 | 1.00 | ||
| P2O5 | −0.20 | 0.91 | 0.79 | 0.90 | −0.43 | 0.88 | −0.60 | 0.53 | 0.74 | 1.00 | |
| SiO2/ Al2O3 | 0.33 | −0.90 | −0.76 | −0.89 | 0.33 | −0.86 | 0.53 | −0.41 | −0.77 | −0.94 | 1.00 |
| (B) | SiO2 | TiO2 | Al2O3 | Fe2O3 | MnO | MgO | CaO | Na2O | K2O | P2O5 | SiO2/ Al2O3 |
| SiO2 | 1.00 | ||||||||||
| TiO2 | −0.96 | 1.00 | |||||||||
| Al2O3 | 0.11 | 0.14 | 1.00 | ||||||||
| Fe2O3 | −0.95 | 0.99 | 0.12 | 1.00 | |||||||
| MnO | −0.77 | 0.64 | −0.31 | 0.69 | 1.00 | ||||||
| MgO | −0.94 | 0.90 | −0.15 | 0.90 | 0.77 | 1.00 | |||||
| CaO | −0.67 | 0.48 | −0.76 | 0.51 | 0.77 | 0.66 | 1.00 | ||||
| Na2O | 0.96 | −0.89 | 0.18 | −0.90 | −0.86 | −0.87 | −0.75 | 1.00 | |||
| K2O | 0.66 | −0.46 | 0.73 | −0.44 | −0.73 | −0.71 | −0.92 | 0.70 | 1.00 | ||
| P2O5 | 0.15 | 0.04 | 0.87 | −0.02 | −0.45 | −0.29 | −0.80 | 0.23 | 0.74 | 1.00 | |
| SiO2/ Al2O3 | 0.71 | −0.86 | −0.61 | −0.84 | −0.39 | −0.64 | 0.00 | 0.64 | 0.01 | −0.49 | 1.00 |
| (C) | SiO2 | TiO2 | Al2O3 | Fe2O3 | MnO | MgO | CaO | Na2O | K2O | P2O5 | SiO2/ Al2O3 |
| SiO2 | 1.00 | ||||||||||
| TiO2 | −0.90 | 1.00 | |||||||||
| Al2O3 | −0.61 | 0.58 | 1.00 | ||||||||
| Fe2O3 | −0.94 | 0.99 | 0.63 | 1.00 | |||||||
| MnO | −0.11 | 0.05 | −0.58 | 0.02 | 1.00 | ||||||
| MgO | −0.78 | 0.74 | 0.94 | 0.79 | −0.52 | 1.00 | |||||
| CaO | 0.21 | −0.28 | −0.82 | −0.32 | 0.91 | −0.77 | 1.00 | ||||
| Na2O | −0.33 | 0.50 | 0.78 | 0.50 | −0.82 | 0.80 | −0.93 | 1.00 | |||
| K2O | 0.51 | −0.76 | −0.57 | −0.73 | 0.49 | −0.72 | 0.64 | −0.87 | 1.00 | ||
| P2O5 | −0.65 | 0.66 | 0.91 | 0.70 | −0.47 | 0.90 | −0.79 | 0.75 | −0.61 | 1.00 | |
| SiO2/ Al2O3 | 0.82 | −0.78 | −0.95 | −0.83 | 0.41 | −0.99 | 0.70 | −0.73 | 0.66 | −0.90 | 1.00 |
| (D) | SiO2 | TiO2 | Al2O3 | Fe2O3 | MnO | MgO | CaO | Na2O | K2O | P2O5 | SiO2/ Al2O3 |
| SiO2 | 1.00 | ||||||||||
| TiO2 | −0.39 | 1.00 | |||||||||
| Al2O3 | 0.97 | −0.46 | 1.00 | ||||||||
| Fe2O3 | 0.03 | 0.88 | −0.03 | 1.00 | |||||||
| MnO | −0.46 | 0.99 | −0.51 | 0.86 | 1.00 | ||||||
| MgO | 0.53 | 0.30 | 0.52 | 0.52 | 0.29 | 1.00 | |||||
| CaO | −0.99 | 0.41 | −0.99 | −0.02 | 0.47 | −0.53 | 1.00 | ||||
| Na2O | 0.62 | 0.26 | 0.53 | 0.47 | 0.22 | 0.95 | −0.58 | 1.00 | |||
| K2O | 0.67 | −0.83 | 0.73 | −0.51 | −0.86 | −0.19 | −0.70 | −0.15 | 1.00 | ||
| P2O5 | 0.38 | 0.60 | 0.24 | 0.75 | 0.56 | 0.81 | −0.33 | 0.89 | −0.40 | 1.00 | |
| SiO2/ Al2O3 | 0.47 | 0.11 | 0.23 | 0.20 | 0.00 | 0.22 | −0.38 | 0.52 | 0.03 | 0.61 | 1.00 |
| Basins | Sample Code | SiEF | TiEF | FeEF | MnEF | MgEF | NaEF | KEF | PEF | CaEF |
|---|---|---|---|---|---|---|---|---|---|---|
| Aiasa | SS01 | 0.88 | 1.42 | 1.54 | 2.03 | 1.79 | 1.94 | 0.62 | 1.74 | 4.61 |
| SS02 | 0.94 | 1.31 | 1.42 | 1.86 | 1.78 | 1.93 | 0.65 | 1.67 | 7.88 | |
| SS017 | 0.99 | 1.28 | 1.49 | 1.87 | 1.82 | 2.09 | 0.65 | 1.60 | 8.95 | |
| SS018 | 1.00 | 1.28 | 1.39 | 2.10 | 1.77 | 2.12 | 0.62 | 1.61 | 9.95 | |
| SS032 | 1.10 | 1.23 | 1.35 | 2.10 | 1.90 | 2.30 | 0.59 | 1.69 | 11.17 | |
| Avg. | 0.98 | 1.30 | 1.44 | 1.99 | 1.81 | 2.08 | 0.63 | 1.66 | 8.51 | |
| Std | 0.08 | 0.07 | 0.08 | 0.12 | 0.05 | 0.15 | 0.02 | 0.06 | 2.50 | |
| Turon | SS029 | 1.13 | 1.22 | 1.33 | 1.52 | 1.74 | 2.38 | 0.48 | 1.68 | 33.90 |
| SS030 | 1.16 | 1.35 | 1.40 | 2.10 | 1.84 | 2.51 | 0.56 | 1.75 | 19.14 | |
| Avg. | 1.14 | 1.29 | 1.36 | 1.81 | 1.79 | 2.44 | 0.52 | 1.71 | 26.52 | |
| Std | 0.02 | 0.10 | 0.05 | 0.40 | 0.07 | 0.09 | 0.06 | 0.05 | 10.44 | |
| Ermeti | SS010 | 1.05 | 1.30 | 1.56 | 1.56 | 2.18 | 2.10 | 0.44 | 1.11 | 6.47 |
| SS011 | 1.11 | 1.66 | 1.70 | 2.45 | 2.56 | 2.38 | 0.41 | 1.61 | 16.03 | |
| Avg. | 1.08 | 1.48 | 1.63 | 2.01 | 2.37 | 2.24 | 0.42 | 1.36 | 11.25 | |
| Std | 0.04 | 0.26 | 0.10 | 0.63 | 0.26 | 0.20 | 0.02 | 0.36 | 6.76 | |
| Holarua | SS03 | 1.05 | 1.64 | 1.73 | 2.02 | 1.89 | 2.48 | 0.47 | 1.81 | 14.15 |
| SS04 | 1.01 | 1.90 | 2.17 | 2.17 | 2.44 | 2.35 | 0.31 | 1.76 | 30.68 | |
| SS06 | 1.14 | 1.61 | 1.85 | 1.57 | 1.65 | 1.68 | 0.47 | 1.63 | 21.81 | |
| Avg. | 1.06 | 1.72 | 1.91 | 1.92 | 1.99 | 2.17 | 0.42 | 1.73 | 22.21 | |
| Std | 0.07 | 0.16 | 0.23 | 0.31 | 0.40 | 0.43 | 0.09 | 0.09 | 8.27 | |
| Karau Ulun | SS05 | 1.15 | 1.74 | 1.88 | 2.17 | 1.90 | 2.10 | 0.50 | 1.72 | 11.45 |
| SS07 | 0.99 | 1.53 | 1.64 | 1.94 | 1.90 | 2.14 | 0.50 | 1.71 | 9.24 | |
| SS08 | 1.10 | 1.50 | 1.78 | 1.94 | 1.85 | 2.22 | 0.51 | 1.68 | 9.52 | |
| SS09 | 1.36 | 1.74 | 1.56 | 3.30 | 1.73 | 1.36 | 0.76 | 2.25 | 11.82 | |
| SS024 | 1.10 | 1.64 | 1.71 | 2.30 | 2.14 | 2.29 | 0.48 | 1.92 | 18.75 | |
| SS026 | 1.10 | 1.63 | 1.76 | 2.13 | 2.07 | 2.10 | 0.51 | 1.71 | 15.59 | |
| SS027 | 1.11 | 1.70 | 1.83 | 2.22 | 1.97 | 1.99 | 0.54 | 1.90 | 13.37 | |
| SS028 | 1.09 | 1.61 | 1.76 | 1.91 | 1.90 | 1.98 | 0.55 | 1.83 | 13.43 | |
| SS031 | 1.21 | 1.51 | 1.41 | 2.12 | 1.73 | 2.95 | 0.45 | 1.61 | 13.88 | |
| Avg. | 1.13 | 1.62 | 1.70 | 2.23 | 1.91 | 2.12 | 0.53 | 1.81 | 13.00 | |
| Std | 0.10 | 0.09 | 0.15 | 0.42 | 0.14 | 0.41 | 0.09 | 0.19 | 2.98 | |
| Soin | SS019 | 1.06 | 2.09 | 2.27 | 2.84 | 1.95 | 2.53 | 0.51 | 1.99 | 11.42 |
| SS020 | 1.03 | 1.81 | 1.77 | 2.68 | 1.84 | 2.60 | 0.50 | 1.78 | 7.96 | |
| SS021 | 1.02 | 1.96 | 2.22 | 3.41 | 1.97 | 2.16 | 0.54 | 2.00 | 14.66 | |
| SS022 | 0.96 | 1.84 | 1.76 | 1.81 | 1.86 | 2.47 | 0.51 | 1.72 | 6.19 | |
| SS023 | 0.96 | 1.94 | 2.02 | 1.86 | 1.87 | 2.46 | 0.49 | 1.89 | 5.29 | |
| SS025 | 1.07 | 2.08 | 1.98 | 3.19 | 2.06 | 2.50 | 0.48 | 2.01 | 14.12 | |
| SS033 | 1.20 | 1.70 | 1.65 | 2.86 | 1.92 | 2.65 | 0.51 | 1.78 | 12.36 | |
| SS036 | 1.19 | 1.69 | 1.66 | 2.93 | 1.93 | 2.59 | 0.50 | 1.79 | 12.23 | |
| SS037 | 1.17 | 1.92 | 1.89 | 3.09 | 1.97 | 2.47 | 0.49 | 1.76 | 11.07 | |
| SS038 | 1.21 | 1.40 | 1.43 | 2.70 | 1.82 | 2.62 | 0.52 | 1.72 | 11.81 | |
| Avg. | 1.09 | 1.84 | 1.86 | 2.74 | 1.92 | 2.51 | 0.51 | 1.84 | 10.71 | |
| Std | 0.10 | 0.21 | 0.26 | 0.52 | 0.07 | 0.14 | 0.02 | 0.12 | 3.19 | |
| Laclo do Sul | SS040 | 0.94 | 1.26 | 1.51 | 1.65 | 2.07 | 2.41 | 0.47 | 1.54 | 4.00 |
| SS041 | 0.92 | 1.34 | 1.57 | 1.74 | 2.15 | 2.34 | 0.43 | 1.65 | 4.25 | |
| SS042 | 0.93 | 1.34 | 1.53 | 1.58 | 2.11 | 2.40 | 0.44 | 1.64 | 4.28 | |
| SS044 | 0.95 | 1.35 | 1.56 | 1.84 | 2.20 | 2.33 | 0.37 | 1.52 | 5.57 | |
| SS045 | 0.88 | 1.66 | 1.99 | 1.95 | 2.36 | 2.10 | 0.33 | 1.49 | 6.75 | |
| SS046 | 0.98 | 1.34 | 1.60 | 1.84 | 2.20 | 2.41 | 0.42 | 1.54 | 5.93 | |
| SS047 | 1.02 | 1.31 | 1.52 | 1.82 | 2.30 | 2.61 | 0.38 | 1.43 | 6.08 | |
| Avg. | 0.95 | 1.37 | 1.61 | 1.77 | 2.20 | 2.37 | 0.41 | 1.54 | 5.27 | |
| Std | 0.04 | 0.13 | 0.17 | 0.13 | 0.10 | 0.15 | 0.05 | 0.08 | 1.08 | |
| Merek | SS039 | 1.11 | 1.02 | 1.25 | 2.21 | 1.43 | 2.10 | 0.63 | 1.58 | 10.80 |
| SS043 | 1.14 | 1.15 | 1.42 | 2.86 | 1.38 | 2.31 | 0.56 | 1.60 | 6.31 | |
| Avg. | 1.13 | 1.09 | 1.33 | 2.53 | 1.41 | 2.21 | 0.60 | 1.59 | 8.56 | |
| Std | 0.02 | 0.09 | 0.12 | 0.46 | 0.03 | 0.15 | 0.05 | 0.01 | 3.18 | |
| Clerec | SS035 | 1.08 | 1.78 | 1.63 | 2.56 | 1.93 | 2.46 | 0.46 | 1.41 | 6.35 |
| SS048 | 0.93 | 1.71 | 1.69 | 2.52 | 2.23 | 2.08 | 0.49 | 1.43 | 5.62 | |
| SS049 | 1.38 | 1.09 | 1.30 | 4.18 | 1.09 | 1.76 | 0.73 | 1.53 | 9.06 | |
| SS050 | 1.00 | 2.61 | 2.21 | 3.14 | 2.22 | 2.49 | 0.40 | 1.51 | 6.47 | |
| SS051 | 1.06 | 1.58 | 1.53 | 1.97 | 2.06 | 2.67 | 0.44 | 1.53 | 5.24 | |
| SS052 | 1.03 | 1.50 | 1.46 | 1.94 | 1.98 | 2.60 | 0.43 | 1.42 | 5.04 | |
| SS053 | 1.17 | 1.34 | 1.47 | 2.25 | 1.94 | 2.60 | 0.50 | 1.44 | 6.41 | |
| Avg. | 1.09 | 1.66 | 1.61 | 2.65 | 1.92 | 2.38 | 0.49 | 1.47 | 6.31 | |
| Std | 0.14 | 0.48 | 0.29 | 0.79 | 0.39 | 0.34 | 0.11 | 0.05 | 1.34 | |
| Sahe | SS012 | 1.16 | 1.00 | 1.20 | 1.64 | 1.17 | 1.47 | 0.72 | 1.21 | 8.05 |
| SS013 | 1.18 | 2.09 | 1.62 | 3.32 | 1.42 | 1.97 | 0.58 | 1.56 | 12.09 | |
| SS014 | 1.20 | 1.19 | 1.32 | 2.11 | 1.37 | 1.93 | 0.67 | 1.48 | 13.27 | |
| SS015 | 1.16 | 2.72 | 1.92 | 4.31 | 1.60 | 1.79 | 0.59 | 1.85 | 34.25 | |
| SS016 | 1.13 | 1.88 | 1.50 | 3.10 | 1.57 | 2.29 | 0.49 | 1.55 | 15.16 | |
| SS034 | 1.25 | 1.94 | 1.50 | 2.89 | 1.45 | 2.41 | 0.52 | 1.60 | 9.90 | |
| Avg. | 1.18 | 1.80 | 1.51 | 2.89 | 1.43 | 1.98 | 0.60 | 1.54 | 15.45 | |
| Std | 0.04 | 0.63 | 0.25 | 0.94 | 0.15 | 0.34 | 0.09 | 0.21 | 9.54 |
| PC1 | PC2 | PC3 | |
|---|---|---|---|
| Eigenvalues | 4.97 | 2.02 | 1.36 |
| Explanation (%) | 49.74 | 20.23 | 13.61 |
| SiO2 | 0.2759 | −0.4652 | 0.0452 |
| TiO2 | 0.2586 | 0.4009 | 0.4187 |
| Al2O3 | 0.4157 | −0.1197 | −0.2033 |
| Fe2O3 | 0.3452 | 0.3725 | 0.1116 |
| MnO | 0.1122 | −0.0577 | 0.7712 |
| MgO | 0.3553 | 0.2546 | −0.3164 |
| CaO | −0.4014 | 0.2846 | 0.0061 |
| Na2O | 0.3666 | 0.0892 | −0.2309 |
| K2O | 0.1390 | −0.5558 | 0.1306 |
| P2O5 | 0.3336 | 0.0618 | 0.0602 |
| PC1 | PC2 | PC3 | PC1 | PC2 | PC3 | ||
|---|---|---|---|---|---|---|---|
| Eigenvalues | 4.97 | 2.02 | 1.36 | Eigenvalues | 4.97 | 2.02 | 1.36 |
| Explanation (%) | 49.74 | 20.23 | 13.61 | Explanation (%) | 49.74 | 20.23 | 13.61 |
| SS01 | 2.8094 | −1.1265 | 0.3508 | SS028 | −1.0743 | 0.2423 | −0.4641 |
| SS02 | 0.9505 | −1.3944 | −0.3555 | SS029 | −6.2286 | 1.1078 | −2.1250 |
| SS03 | −0.7047 | 0.9024 | −0.5657 | SS030 | −2.8438 | −0.1828 | −1.0306 |
| SS04 | −4.2234 | 3.6410 | −1.0284 | SS031 | −1.3621 | −0.1580 | −0.9280 |
| SS05 | −0.5243 | 0.3881 | −0.0213 | SS032 | −0.8008 | −1.1856 | −0.7950 |
| SS06 | −4.0007 | 1.0310 | −1.0535 | SS033 | −0.4160 | −0.0219 | 0.4749 |
| SS07 | 0.7688 | 0.1864 | −0.3698 | SS034 | −0.2811 | −0.8077 | 1.3272 |
| SS08 | 0.2818 | −0.1269 | −0.4798 | SS035 | 1.6275 | −0.0210 | 0.6776 |
| SS09 | −1.1991 | −1.9787 | 1.8580 | SS036 | −0.5419 | 0.0145 | 0.5499 |
| SS010 | 0.2185 | −0.3535 | −1.6808 | SS037 | 0.1410 | 0.5790 | 1.1977 |
| SS011 | −1.4795 | 1.3840 | −0.6523 | SS038 | −0.6504 | −0.8730 | 0.0325 |
| SS012 | −1.9347 | −3.6500 | −0.6833 | SS039 | −1.3181 | −2.2499 | −0.4070 |
| SS013 | −0.9795 | −0.4875 | 2.2749 | SS040 | 3.1957 | −0.4899 | −1.3336 |
| SS014 | −2.5533 | −2.0583 | −0.4066 | SS041 | 3.1437 | 0.1735 | −1.1314 |
| SS015 | −5.0541 | 2.0570 | 2.4259 | SS042 | 3.1120 | 0.0048 | −1.3984 |
| SS016 | −1.7343 | 0.1991 | 1.2251 | SS043 | 0.6433 | −2.2673 | 0.8850 |
| SS017 | 0.2471 | −1.2933 | −0.5948 | SS044 | 2.3801 | 0.5704 | −1.1502 |
| SS018 | −0.0354 | −1.2271 | −0.3313 | SS045 | 2.6404 | 2.3533 | −0.4530 |
| SS019 | 1.0377 | 1.6047 | 1.3011 | SS046 | 2.2837 | 0.2010 | −1.1381 |
| SS020 | 1.8918 | 0.4021 | 1.0118 | SS047 | 1.8602 | 0.2694 | −1.5752 |
| SS021 | −0.2704 | 1.5562 | 1.8742 | SS048 | 2.6183 | 0.4196 | 0.8197 |
| SS022 | 2.5985 | 0.5487 | −0.0845 | SS049 | −1.7195 | −3.8376 | 2.6119 |
| SS023 | 3.5647 | 1.1403 | 0.3147 | SS050 | 3.2405 | 2.6371 | 2.5887 |
| SS024 | −2.1377 | 1.1255 | −0.5264 | SS051 | 2.1386 | −0.1272 | −0.6999 |
| SS025 | 0.1876 | 1.5758 | 1.4588 | SS052 | 2.3937 | −0.3114 | −0.7210 |
| SS026 | −1.7821 | 0.6782 | −0.5172 | SS053 | 0.6559 | −1.1835 | −0.5817 |
| SS027 | −0.7813 | 0.4200 | 0.0228 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilanova, V.; Ohtani, T.; Kojima, S.; Yatabe, K.; Cristovão, N.; Araujo, A. Modern River-Sand Geochemical Mapping in the Manufahi Municipality and Its Surroundings, Timor-Leste: Implications for Provenance. Geosciences 2024, 14, 177. https://doi.org/10.3390/geosciences14070177
Vilanova V, Ohtani T, Kojima S, Yatabe K, Cristovão N, Araujo A. Modern River-Sand Geochemical Mapping in the Manufahi Municipality and Its Surroundings, Timor-Leste: Implications for Provenance. Geosciences. 2024; 14(7):177. https://doi.org/10.3390/geosciences14070177
Chicago/Turabian StyleVilanova, Vital, Tomoyuki Ohtani, Satoru Kojima, Kazuma Yatabe, Nene Cristovão, and Aniceta Araujo. 2024. "Modern River-Sand Geochemical Mapping in the Manufahi Municipality and Its Surroundings, Timor-Leste: Implications for Provenance" Geosciences 14, no. 7: 177. https://doi.org/10.3390/geosciences14070177
APA StyleVilanova, V., Ohtani, T., Kojima, S., Yatabe, K., Cristovão, N., & Araujo, A. (2024). Modern River-Sand Geochemical Mapping in the Manufahi Municipality and Its Surroundings, Timor-Leste: Implications for Provenance. Geosciences, 14(7), 177. https://doi.org/10.3390/geosciences14070177