Asbestos Bodies in Human Lung: Localization of Iron and Carbon in the Coating
Abstract
1. Introduction
2. Materials and Methods
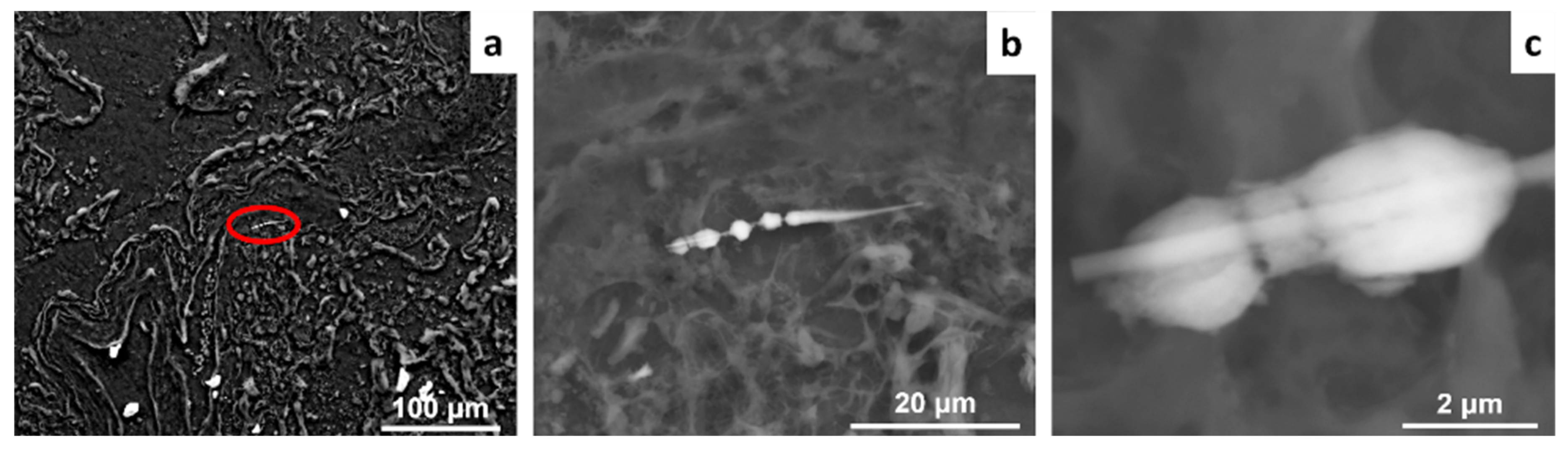
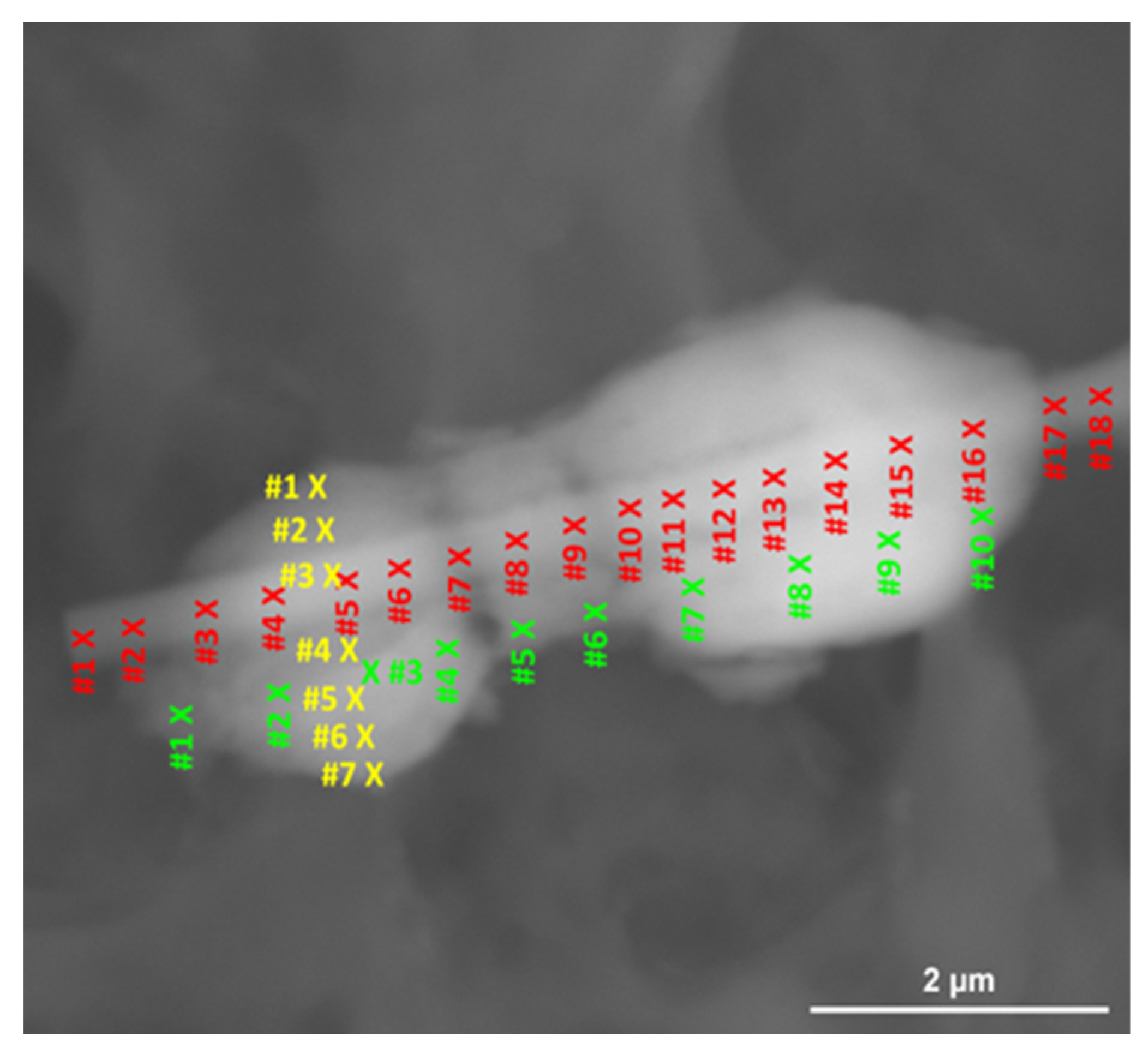
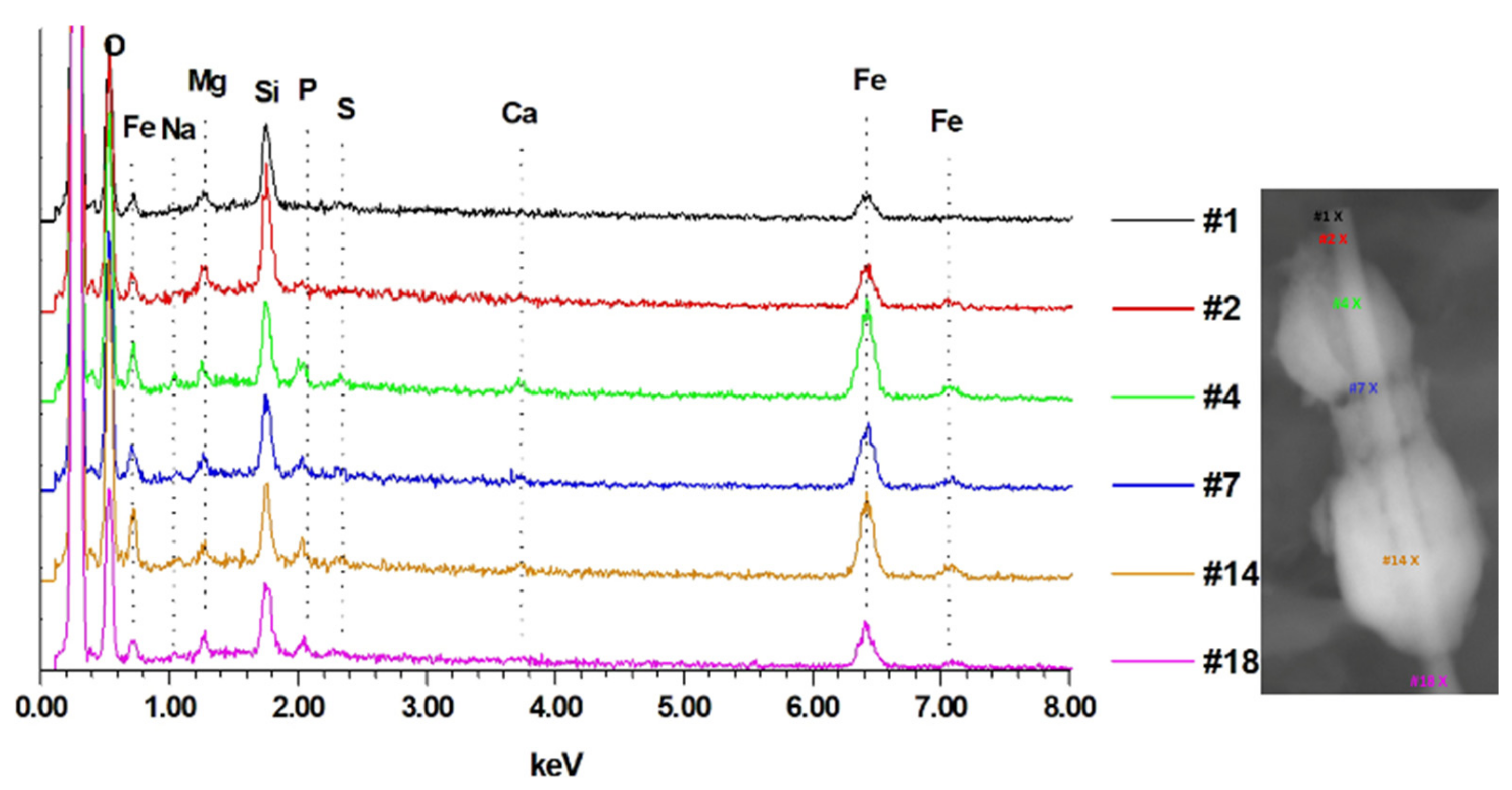
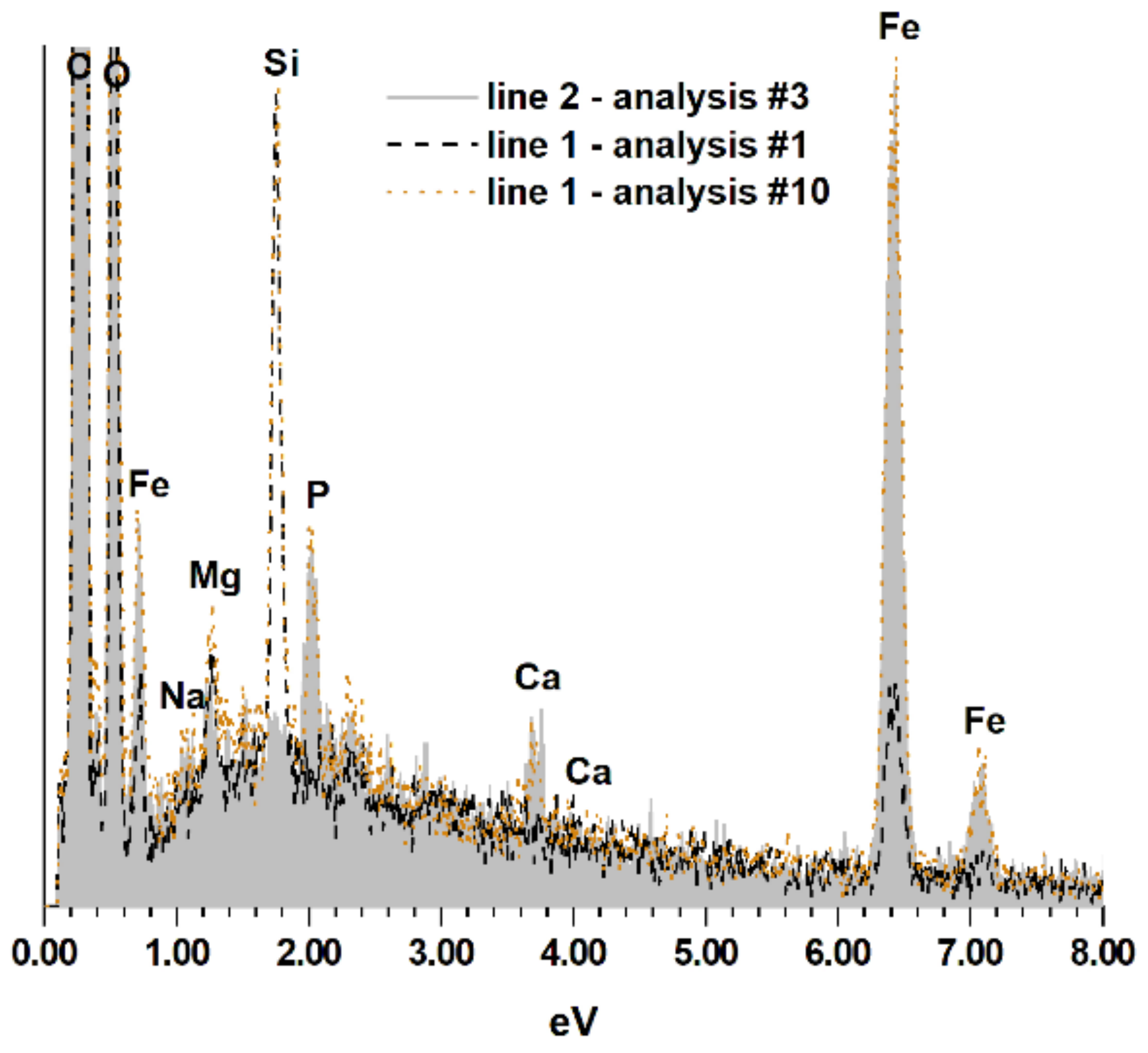
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Agency for Research on Cancer (IARC). Asbestos (chrysotile, amosite, crocidolite, tremolite, actinolite, and anthophyllite). In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2012; Volume 100C, pp. 219–309. ISBN 978-92-832-1320-8. [Google Scholar]
- Italian Government. Legislative Decree No. 277 of 15 August 1991, Implementing EU Directives No. 80/1107/EEC, No. 82/605/EEC, No. 83/477/EEC, No. 86/188/EEC, and No. 88/642/EEC, on the Protection of Workers from the Risks Related to Exposure to Chemical, Physical and Biological Agents at Work. In Gazzetta Ufficiale Supplemento Ordinario No. 200; Italian Government: Rome, Italy, 1991. [Google Scholar]
- Hawthorne, F.C.; Oberti, R.; Harlow, G.E.; Maresch, W.V.; Martin, R.F.; Schumacher, J.C.; Welch, M.D. Nomenclature of the amphibole supergroup. Am. Mineral. 2012, 97, 2031–2048. [Google Scholar] [CrossRef]
- Leake, B.E.; Woolley, A.R.; Arps, C.E.S.; Birch, W.D.; Gilbert, M.C.; Grice, J.D.; Hawthorne, F.C.; Kato, A.; Kisch, H.J.; Krivovichev, V.G.; et al. Nomenclature of amphiboles: Report of the Subcommittee on amphiboles of the International Mineralogical Association, Commission on New Minerals and Mineral Names. Can. Mineral. 1997, 35, 219–246. [Google Scholar]
- Whittaker, E.J.W.; Zussman, J. The characterization of serpentine minerals by X-ray diffraction. Mineral. Mag. 1956, 233, 107–126. [Google Scholar] [CrossRef]
- Bartrip, P.W.J. History of asbestos related disease. Postgrad. Med. J. 2004, 80, 72–76. [Google Scholar] [CrossRef]
- Donaldson, K.; Seaton, A. A short history of the toxicology of inhaled particles. Part. Fibre Toxicol. 2012, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Caraballo-Arias, Y.; Roccuzzo, F.; Graziosi, F.; Danilevskaia, N.; Rota, S.; Zunarelli, C.; Caffaro, P.; Boffetta, P.; Bonetti, M.; Violante, F.S. Quantitative assessment of asbestos fibers in abdominal organs: A scoping review. Med. Lav. 2023, 114, e2023048. [Google Scholar]
- Porzio, A.; Feola, A.; Parisi, G.; Lauro, A.; Campobasso, C.P. Colorectal cancer: 35 cases in asbestos-exposed workers. Healthcare 2023, 11, 3077. [Google Scholar] [CrossRef]
- Brandi, G.; Straif, K.; Mandrioli, D.; Curti, S.; Mattioli, S.; Tavolari, S. Exposure to asbestos and increased intrahepatic cholangiocarcinoma risk: Growing evidences of a putative causal link. Ann. Glob. Health 2022, 88, 41. [Google Scholar] [CrossRef]
- Grosso, F.; Croce, A.; Libener, R.; Mariani, N.; Pastormerlo, M.; Maconi, A.; Rinaudo, C. Asbestos fiber identification in liver from cholangiocarcinoma patients living in an asbestos polluted area: A preliminary study. Tumori J. 2019, 105, 404–410. [Google Scholar] [CrossRef]
- Gamble, J.F. Asbestos and colon cancer: A weight-of-the-evidence review. Environ. Health Perspect. 1994, 102, 1038–1050. [Google Scholar] [CrossRef]
- Ehrlich, A.; Rohl, A.N.; Holstein, E.C. Asbestos bodies in carcinoma of colon in an insulation worker with asbestosis. JAMA 1985, 254, 2932–2933. [Google Scholar] [CrossRef]
- Ehrlich, A.; Gordon, R.E.; Dikman, S.H. Carcinoma of the colon in asbestos-exposed workers: Analysis of asbestos content in colon tissue. Am. J. Ind. Med. 1991, 19, 629–636. [Google Scholar] [CrossRef]
- Kobayashi, H.; Ming, Z.W.; Watanabe, H.; Ohnishi, Y. A quantitative study on the distribution of asbestos bodies in extrapulmonary organs. Acta Pathol. Jpn. 1987, 37, 375–383. [Google Scholar] [CrossRef]
- Barrett, J.C.; Lamb, P.W.; Wiseman, R.W. Multiple mechanisms for the carcinogenic effects of asbestos and other mineral fibers. Environ. Health Perspect. 1989, 81, 81–89. [Google Scholar] [CrossRef]
- Wachowski, L.; Domka, L. Sources and effects of asbestos and other mineral fibres present in ambient air. Pol. J. Environ. Stud. 2000, 9, 443–454. [Google Scholar]
- Andolfi, L.; Trevisan, E.; Zweyer, M.; Prato, S.; Troian, B.; Vita, F.; Borelli, V.; Soranzo, M.R.; Melato, M.; Zabucchi, G. The crocidolite fiber interaction with human mesothelial cells as investigated by combining electron microscopy, atomic force and scanning near-field optical microscopy. J. Microsc. 2013, 249, 173–183. [Google Scholar] [CrossRef]
- Aust, A.E.; Cook, P.M.; Dodson, R.F. Morphological and chemical mechanisms of elongated mineral particle toxicities. J. Toxicol. Environ. Health Part B 2011, 14, 40–75. [Google Scholar] [CrossRef]
- Carbone, M.; Ly, B.H.; Dodson, R.F.; Pagano, I.; Morris, P.T.; Dogan, U.A.; Gazdar, A.F.; Pass, H.I.; Yang, H. Malignant mesothelioma: Facts, myths, and hypotheses. J. Cell. Physiol. 2012, 227, 44–58. [Google Scholar] [CrossRef]
- Crawford, D. Electron microscopy applied to studies of the biological significance of defects in crocidolite asbestos. J. Microsc. 1980, 120, 181–192. [Google Scholar] [CrossRef]
- Fubini, B.; Mollo, L. Role of iron in the reactivity of mineral fibers. Toxicol. Lett. 1995, 82–83, 951–960. [Google Scholar] [CrossRef]
- Goodglick, L.A.; Kane, A.B. Cytotoxicity of long and short crocidolite asbestos fibers in vitro and in vivo. Cancer Res. 1990, 50, 5153–5163. [Google Scholar]
- Hearne, G.R.; Kolk, B.; Pollak, H.; van Wyk, J.A.; Gulumian, M. Bulk and surface modifications in detoxified crocidolite. J. Inorg. Biochem. 1993, 50, 145–156. [Google Scholar] [CrossRef]
- Martra, G.; Chiardola, E.; Coluccia, S.; Marchese, L.; Tomatis, M.; Fubini, B. Reactive sites at the surface of crocidolite asbestos. Langmuir 1999, 15, 5742–5752. [Google Scholar] [CrossRef]
- Mossman, B.; Light, W.; Wei, E. Asbestos: Mechanisms of toxicity and carcinogenicity in the respiratory tract. Annu. Rev. Pharmacol. 1983, 23, 595–615. [Google Scholar] [CrossRef] [PubMed]
- Pacella, A.; Fantauzzi, M.; Turci, F.; Cremisini, C.; Montereali, M.R.; Nardi, E.; Atzei, D.; Rossi, A.; Andreozzi, G.B. Dissolution reaction and surface iron speciation of UICC crocidolite in buffered solution at pH 7.4: A combined ICP-OES, XPS and TEM investigation. Geochim. Cosmochim. Acta 2014, 127, 221–232. [Google Scholar] [CrossRef]
- Rihn, B.; Coulais, C.; Kauffer, E.; Bottin, M.C.; Martin, P.; Yvon, F.; Vigneron, J.C.; Binet, S.; Monhoven, N.; Steiblen, G.; et al. Inhaled crocidolite mutagenicity in lung DNA. Environ. Health Perspect. 2000, 108, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.C.; Berry, G.; Timbrell, V. Mesotheliomata in rats after inoculation with asbestos and other materials. Br. J. Cancer 1973, 28, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.C.; Griffiths, D.M.; Hill, R.J. The effect of fiber size on the in vivo activity of UICC crocidolite. Br. J. Cancer 1984, 49, 453–458. [Google Scholar] [CrossRef]
- Werner, A.J.; Hochella, M.F., Jr.; Guthrie, G.D.; Hardy, J.A.; Aust, A.E.; Rimstidt, J.D. Asbestiform riebeckite (crocidolite) dissolution in presence of Fe chelators: Implications for mineral-induced disease. Am. Mineral. 1995, 80, 1093–1103. [Google Scholar] [CrossRef]
- Croce, A.; Musa, M.; Allegrina, M.; Trivero, P.; Rinaudo, C. Environmental scanning electron microscopy technique to identify asbestos phases inside ferruginous bodies. Microsc. Microanal. 2013, 19, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Musa, M.; Croce, A.; Allegrina, M.; Rinaudo, C.; Belluso, E.; Bellis, D.; Toffalorio, F.; Veronesi, G. The use of Raman spectroscopy to identify inorganic phases in iatrogenic pathological lesions of patients with malignant pleural mesothelioma. Vib. Spectrosc. 2012, 61, 66–71. [Google Scholar] [CrossRef]
- Croce, A.; Arrais, A.; Rinaudo, C. Raman micro-spectroscopy identifies carbonaceous particles lying on the surface of crocidolite, amosite, and chrysotile fibers. Minerals 2018, 8, 249. [Google Scholar] [CrossRef]
- Rinaudo, C.; Croce, A. Micro-Raman spectroscopy, a powerful technique allowing sure identification and complete characterization of asbestiform minerals. Appl. Sci. 2019, 9, 3092. [Google Scholar] [CrossRef]
- Avramescu, M.L.; Potiszil, C.; Kunihiro, T.; Okabe, K.; Nakamura, E. An investigation of the internal morphology of asbestos ferruginous bodies: Constraining their role in the onset of malignant mesothelioma. Part. Fibre Toxicol. 2023, 20, 19. [Google Scholar] [CrossRef] [PubMed]
- Bardelli, F.; Giacobbe, C.; Ballirano, P.; Borelli, V.; Di Benedetto, F.; Montegrassi, G.; Bellis, D.; Pacella, A. Closing the knowledge gap on the composition of the asbestos bodies. Environ. Geochem. Health 2023, 45, 5039–5051. [Google Scholar] [CrossRef] [PubMed]
- Vigliaturo, R.; Jannik, M.; Dražić, G.; Podobnik, M.; Tušek Žnidarič, M.; Della Ventura, G.; Redhammer, G.J.; Žnidarič, N.; Caserman, S.; Gieré, R. Nanoscale transformations of amphiboles within human alveolar epithelial cells. Sci. Rep. 2022, 12, 1782. [Google Scholar] [CrossRef]
- Croce, A.; Allegrina, M.; Rinaudo, C.; Gaudino, G.; Yang, H.; Carbone, M. Numerous iron-rich particles lie on the surface of erionite fibers from Rome (Oregon, USA) and Karlik (Cappadocia, Turkey). Microsc. Microanal. 2015, 21, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Bowes, D.R.; Farrow, C.M. Major and trace element compositions of the UICC standard asbestos samples. Am. J. Ind. Med. 1997, 32, 592–594. [Google Scholar] [CrossRef]
- Burns, R.G.; Prentice, F.J. Distribution of iron cations in the crocidolite structure. Am. Mineral. 1968, 53, 770–776. [Google Scholar]
- Galumian, M.; Pollak, H. Effect of microwave radiation on surface charge, surface sites and ionic state of iron, and the activity of crocidolite asbestos fibres. Hyperfine Interact. 1998, 111, 291–298. [Google Scholar] [CrossRef]
- Graham, A.; Higinbotham, J.; Doug, A.; Donaldson, K.; Beswick, P.H. Chemical differences between long and short amosite asbestos: Differences in oxidation state and coordination sites of iron, detected by infrared spectroscopy. Occup. Environ. Med. 1999, 56, 606–611. [Google Scholar] [CrossRef]
- Gunter, M.E.; Sanchez, M.S.; Williams, T.J. Characterization of chrysotile samples for the presence of amphiboles: The Carey Canadian deposit, Southeastern Quebec, Canada. Can. Mineral. 2007, 45, 263–280. [Google Scholar] [CrossRef]
- Hilborn, J.J.; Thomas, R.S.; Lao, R.C. The organic content of the international reference samples of asbestos. Sci. Total Environ. 1974, 3, 129–140. [Google Scholar] [CrossRef]
- Harington, J.S. Chemical studies of asbestos. Ann. N. Y. Acad. Sci. 1965, 132, 31–47. [Google Scholar] [CrossRef]
- Steel, E.B.; Small, J.A. Accuracy of transmission electron microscopy for the analysis of asbestos in ambient environments. Anal. Chem. 1985, 57, 209–213. [Google Scholar] [CrossRef]
- Platek, S.F.; Riley, R.D.; Simon, S.D. The classification of asbestos fibers by scanning electron microscopy and computer-digitizing tablet. Ann. Occup. Hyg. 1992, 36, 155–171. [Google Scholar] [PubMed]
- Rinaudo, C.; Croce, A.; Musa, M.; Fornero, E.; Allegrina, M.; Trivero, P.; Bellis, D.; Sferch, D.; Toffalorio, F.; Veronesi, G.; et al. Study of inorganic particles, fibers, and asbestos bodies by variable pressure scanning electron microscopy with annexed energy dispersive spectroscopy and micro-Raman spectroscopy in thin sections of lung and pleural plaque. Appl. Spectrosc. 2010, 64, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Prashanth, M.; Verma, O. Medical geology: An interdisciplinary approach intended to unfold the issues of natural environment on public health. J. Geosci. Res. 2022, 7, 139–144. [Google Scholar] [CrossRef]
- Croce, A.; Re, G.; Bisio, C.; Gatti, G.; Coluccia, S.; Marchese, L. On the correlation between Raman spectra and structural properties of activated carbons derived by hyper-crosslinked polymers. Res. Chem. Intermed. 2021, 47, 419–431. [Google Scholar] [CrossRef]
- Chasteen, N.D.; Harrison, P.M. Mineralization in ferritin: An efficient means of iron storage. J. Struct. Biol. 1999, 126, 182–194. [Google Scholar] [CrossRef]
- Harrison, P.M.; Fischbach, F.A.; Hoy, T.G.; Haggis, G.H. Ferric oxyhydroxide core of ferritin. Nature 1967, 216, 1188–1190. [Google Scholar] [CrossRef] [PubMed]
- St. Pierre, T.G.; Kim, K.S.; Webb, J.; Mann, S.; Dickson, D.P.E. Biomineralization of iron: Mossbauer spectroscopy and electron microscopy of ferritin cores from the chiton Acanthopleura hirtosa and the limpet Patella laticostata. Inorg. Chem. 1990, 29, 1870–1874. [Google Scholar] [CrossRef]
- Wade, V.J.; Treffry, A.; Laulhere, J.P.; Bauminger, E.R.; Cleton, M.I.; Mann, S.; Briat, J.F.; Harrison, P.M. Structure and composition of ferritin cores from pea seed (Pisum sativum). Biophys. Biochim. Acta 1993, 1161, 91–96. [Google Scholar] [CrossRef]
- Mian, S.A.; Colley, H.E.; Thornhill, M.H.; Rehman, I.U. Development of a dewaxing protocol for tissue-engineered models of the oral mucosa used for Raman spectroscopic analysis. Appl. Spectrosc. Rev. 2014, 49, 614–617. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Carbon black, titanium dioxide, and talc. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2010; Volume 93, pp. 43–192. ISBN 978-92-832-1293-5. [Google Scholar]
- Zhang, J.; Li, X.; Cheng, W.; Li, Y.; Shi, T.; Jiang, Y.; Wang, T.; Wang, H.; Ren, D.; Zhang, R.; et al. Chronic carbon black nanoparticle exposure increases lung cancer risk by affecting the cell cycle via circulatory inflammation. Environ. Pollut. 2022, 305, 119293. [Google Scholar] [CrossRef] [PubMed]
- Lequy, E.; Siemiatycki, J.; de Hoogh, K.; Vienneau, D.; Dupuy, J.F.; Garès, V.; Hertel, O.; Christensen, J.H.; Goldberg, M.; Zins, M.; et al. Contribution of long-term exposure to outdoor black carbon to the carcinogenicity of air pollution: Evidence regarding risk of cancer in the Gazel Cohort. Environ. Health Perspect. 2021, 129, 37005. [Google Scholar] [CrossRef]
- Grahame, T.J.; Klemm, R.; Schlesinger, S.B. Public health and components of particulate matter: The changing assessment of black carbon. J. Air Waste Manag. 2014, 64, 620–660. [Google Scholar] [CrossRef]

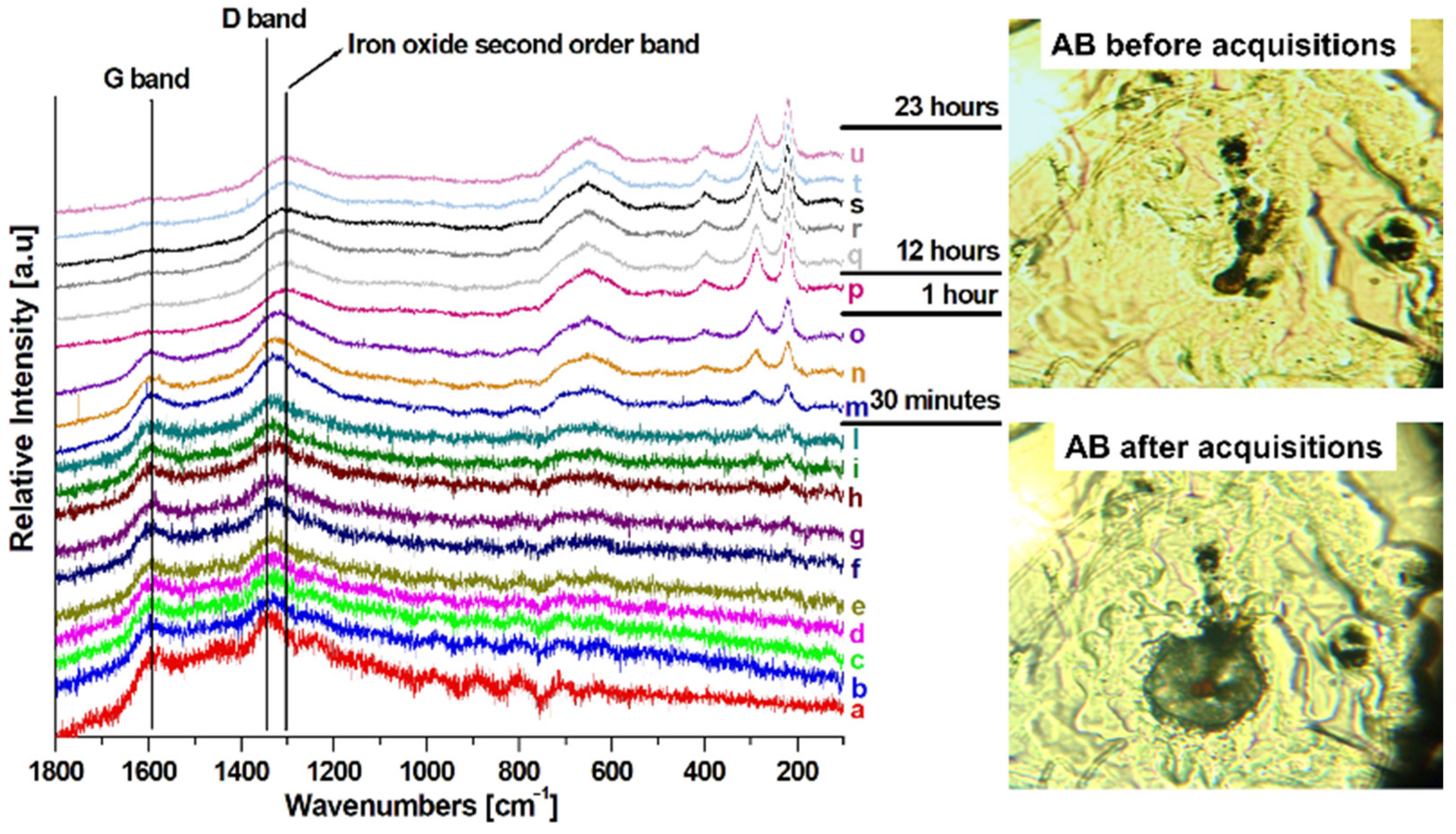
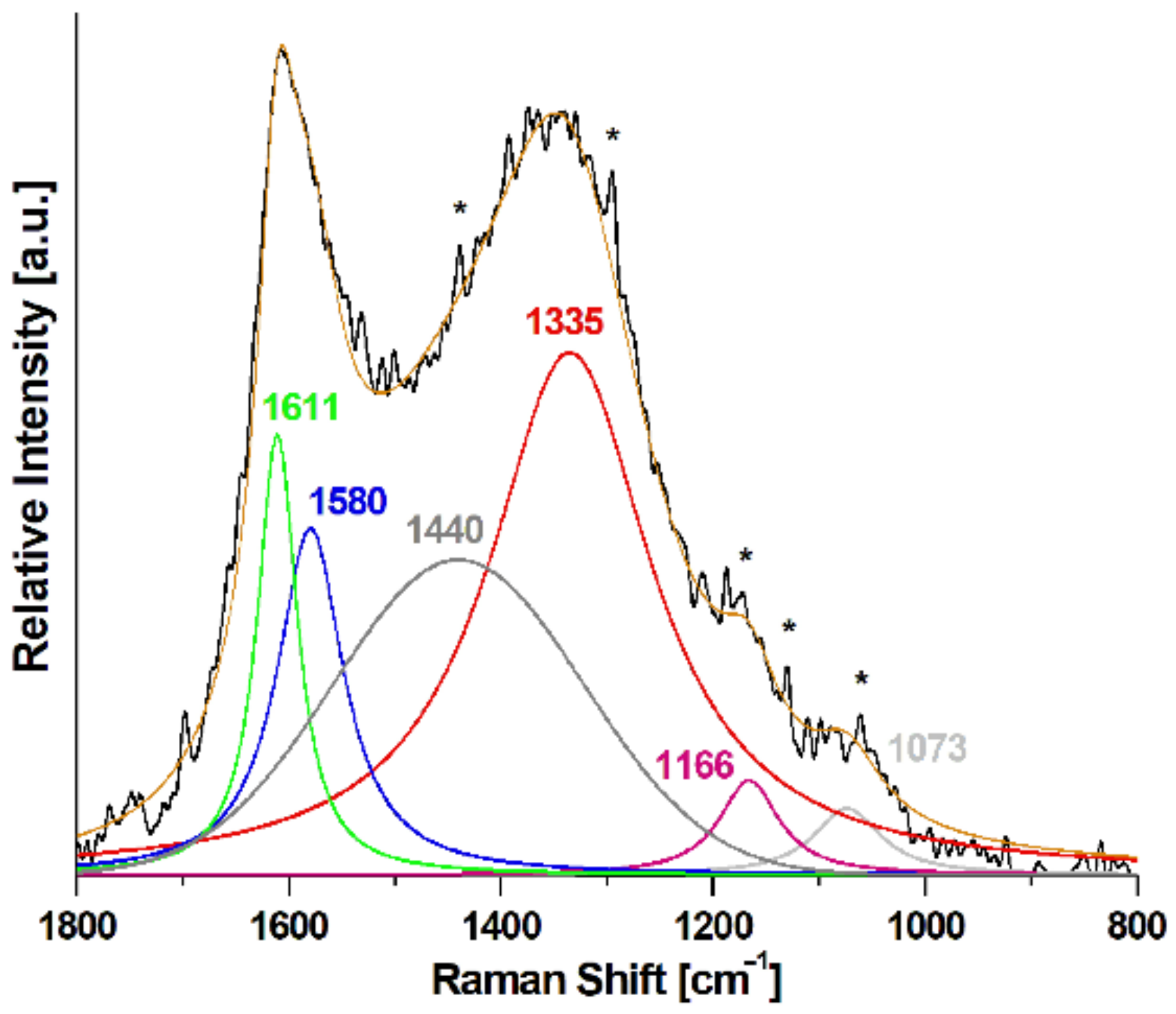
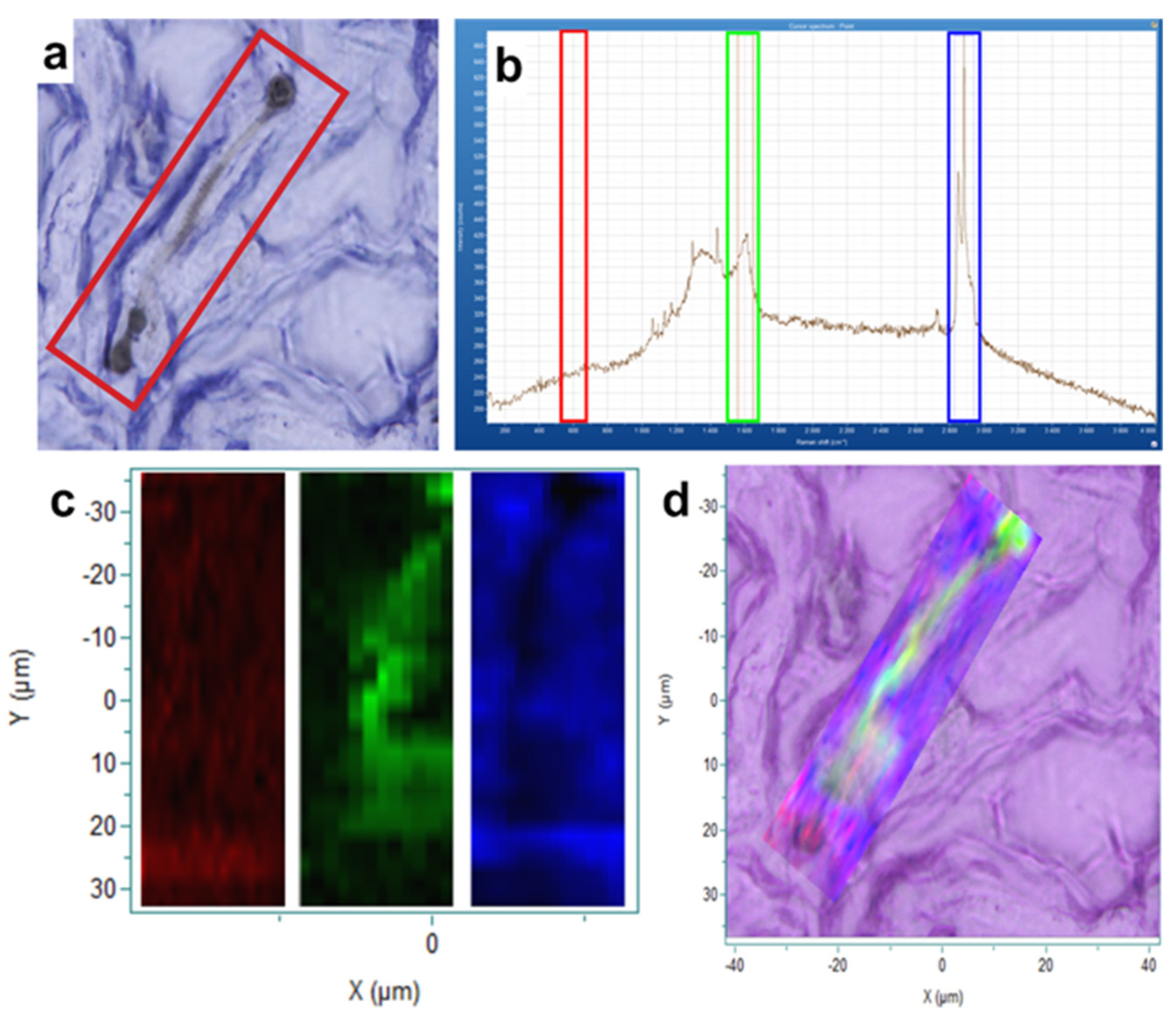
| Atoms % | P/Fe Ratio | |||||||
|---|---|---|---|---|---|---|---|---|
| Si | Mg | Fe | Na | Ca | S | P | ||
| Line 1 | ||||||||
| #1 | 0.31 | 0.10 | 0.21 | 0.02 | 0.01 | 0.01 | 0.00 | 0.00 |
| #2 | 0.34 | 0.12 | 0.25 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| #3 | 0.31 | 0.15 | 0.44 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| #4 | 0.27 | 0.09 | 0.65 | 0.06 | 0.05 | 0.03 | 0.07 | 0.11 |
| #5 | 0.27 | 0.12 | 0.59 | 0.06 | 0.03 | 0.02 | 0.07 | 0.10 |
| #6 | 0.29 | 0.12 | 0.47 | 0.06 | 0.03 | 0.03 | 0.06 | 0.13 |
| #7 | 0.29 | 0.12 | 0.50 | 0.06 | 0.03 | 0.02 | 0.07 | 0.10 |
| #8 | 0.29 | 0.12 | 0.50 | 0.06 | 0.03 | 0.03 | 0.06 | 0.12 |
| #9 | 0.27 | 0.09 | 0.47 | 0.07 | 0.04 | 0.04 | 0.06 | 0.13 |
| #10 | 0.28 | 0.13 | 0.53 | 0.09 | 0.04 | 0.04 | 0.08 | 0.15 |
| #11 | 0.26 | 0.10 | 0.63 | 0.06 | 0.04 | 0.03 | 0.09 | 0.14 |
| #12 | 0.25 | 0.11 | 0.71 | 0.03 | 0.04 | 0.04 | 0.04 | 0.13 |
| #13 | 0.22 | 0.10 | 0.70 | 0.04 | 0.04 | 0.03 | 0.08 | 0.11 |
| #14 | 0.20 | 0.12 | 0.74 | 0.08 | 0.05 | 0.03 | 0.11 | 0.15 |
| #15 | 0.20 | 0.10 | 0.66 | 0.07 | 0.03 | 0.02 | 0.09 | 0.14 |
| #16 | 0.21 | 0.08 | 0.48 | 0.07 | 0.03 | 0.02 | 0.04 | 0.08 |
| #17 | 0.26 | 0.11 | 0.25 | 0.04 | 0.02 | 0.01 | 0.03 | 0.12 |
| #18 | 0.25 | 0.11 | 0.29 | 0.06 | 0.02 | 0.02 | 0.04 | 0.14 |
| Line 2 | ||||||||
| #1 | 0.04 | 0.05 | 0.31 | 0.03 | 0.05 | 0.01 | 0.05 | 0.16 |
| #2 | 0.04 | 0.06 | 0.55 | 0.05 | 0.04 | 0.04 | 0.10 | 0.18 |
| #3 | 0.02 | 0.08 | 0.78 | 0.07 | 0.06 | 0.03 | 0.10 | 0.13 |
| #4 | 0.04 | 0.06 | 0.59 | 0.07 | 0.05 | 0.05 | 0.10 | 0.17 |
| #5 | 0.06 | 0.05 | 0.21 | 0.07 | 0.04 | 0.04 | 0.04 | 0.19 |
| #6 | 0.05 | 0.04 | 0.24 | 0.02 | 0.02 | 0.03 | 0.05 | 0.21 |
| #7 | 0.06 | 0.10 | 0.74 | 0.07 | 0.05 | 0.04 | 0.13 | 0.18 |
| #8 | 0.06 | 0.06 | 0.86 | 0.05 | 0.05 | 0.03 | 0.13 | 0.15 |
| #9 | 0.05 | 0.04 | 0.83 | 0.07 | 0.05 | 0.04 | 0.13 | 0.16 |
| #10 | 0.04 | 0.04 | 0.50 | 0.06 | 0.04 | 0.03 | 0.09 | 0.18 |
| Line 3 | ||||||||
| #1 | 0.03 | 0.05 | 0.34 | 0.07 | 0.03 | 0.03 | 0.07 | 0.21 |
| #2 | 0.06 | 0.05 | 0.52 | 0.07 | 0.03 | 0.03 | 0.09 | 0.17 |
| #3 | 0.20 | 0.11 | 0.60 | 0.05 | 0.04 | 0.03 | 0.06 | 0.10 |
| #4 | 0.06 | 0.05 | 0.68 | 0.06 | 0.05 | 0.03 | 0.10 | 0.15 |
| #5 | 0.06 | 0.05 | 0.68 | 0.05 | 0.05 | 0.05 | 0.11 | 0.16 |
| #6 | 0.04 | 0.04 | 0.69 | 0.03 | 0.04 | 0.03 | 0.10 | 0.15 |
| #7 | 0.04 | 0.05 | 0.62 | 0.08 | 0.06 | 0.04 | 0.09 | 0.15 |
| D1 Band Intensity | G Band Intensity | R1 Ratio | |
|---|---|---|---|
| Spectrum #1 | 272.12 | 188.97 | 1.44 |
| Spectrum #2 | 154.78 | 108.24 | 1.43 |
| Spectrum #3 | 150.93 | 101.29 | 1.49 |
| Spectrum #4 | 159.03 | 109.68 | 1.45 |
| Spectrum #5 | 177.60 | 121.64 | 1.46 |
| Spectrum #6 | 169.23 | 116.71 | 1.45 |
| Spectrum #7 | 154.56 | 108.84 | 1.42 |
| Spectrum #8 | 148.96 | 104.90 | 1.42 |
| Spectrum #9 | 143.57 | 99.70 | 1.44 |
| Spectrum #10 | 145.97 | 99.98 | 1.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Croce, A.; Gatti, G.; Calisi, A.; Cagna, L.; Bellis, D.; Bertolotti, M.; Rinaudo, C.; Maconi, A. Asbestos Bodies in Human Lung: Localization of Iron and Carbon in the Coating. Geosciences 2024, 14, 58. https://doi.org/10.3390/geosciences14030058
Croce A, Gatti G, Calisi A, Cagna L, Bellis D, Bertolotti M, Rinaudo C, Maconi A. Asbestos Bodies in Human Lung: Localization of Iron and Carbon in the Coating. Geosciences. 2024; 14(3):58. https://doi.org/10.3390/geosciences14030058
Chicago/Turabian StyleCroce, Alessandro, Giorgio Gatti, Antonio Calisi, Laura Cagna, Donata Bellis, Marinella Bertolotti, Caterina Rinaudo, and Antonio Maconi. 2024. "Asbestos Bodies in Human Lung: Localization of Iron and Carbon in the Coating" Geosciences 14, no. 3: 58. https://doi.org/10.3390/geosciences14030058
APA StyleCroce, A., Gatti, G., Calisi, A., Cagna, L., Bellis, D., Bertolotti, M., Rinaudo, C., & Maconi, A. (2024). Asbestos Bodies in Human Lung: Localization of Iron and Carbon in the Coating. Geosciences, 14(3), 58. https://doi.org/10.3390/geosciences14030058









