Initial Accretion in Hamelin Pool Microbialites: The Role of Entophysalis in Precipitation of Microbial Micrite
Abstract
:1. Introduction
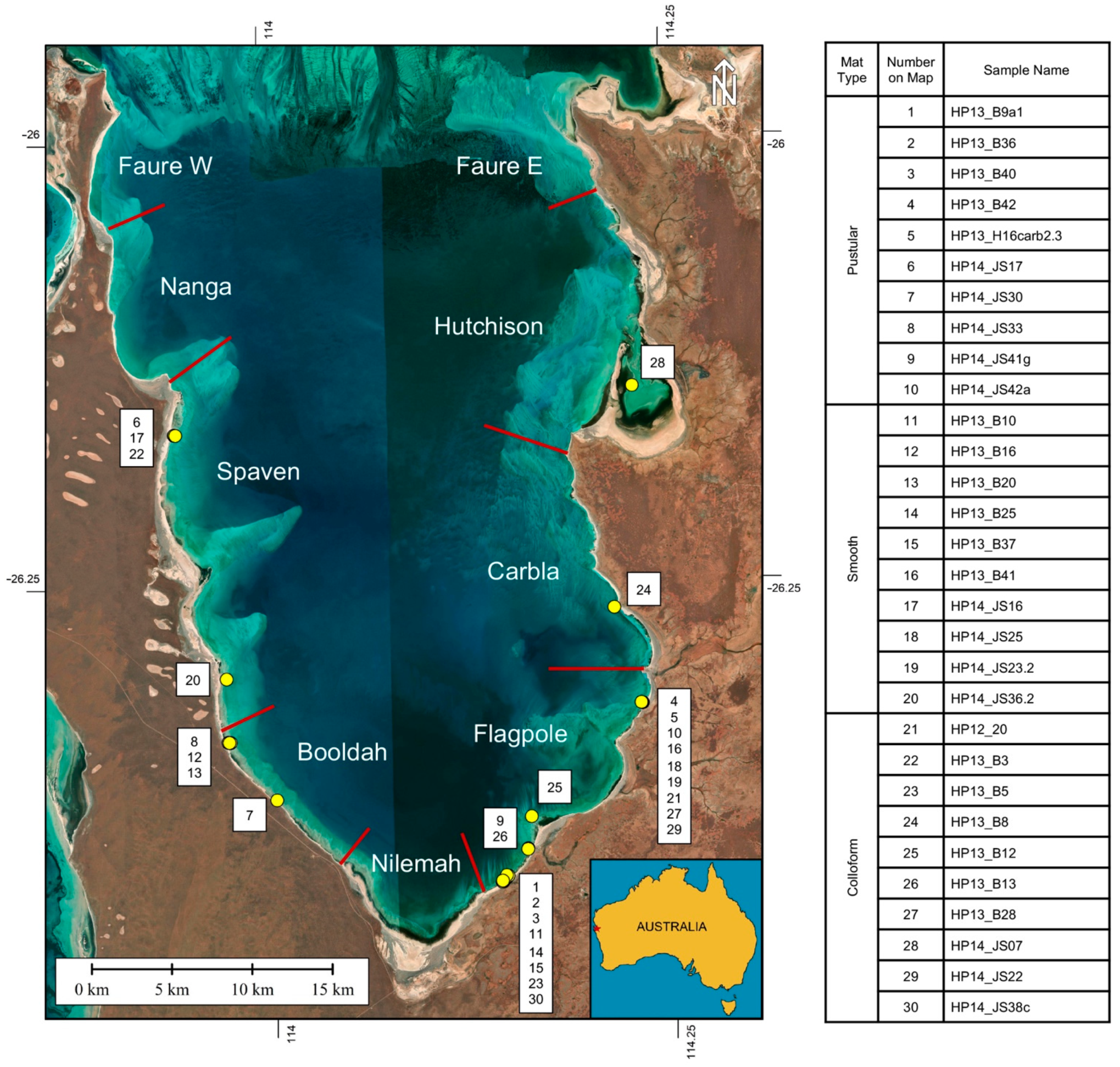
2. Background: Microbialite-Forming Microbial Mats in Hamelin Pool
3. Materials and Methods
3.1. Terminology
3.2. Sample Collection
3.3. Thin Section Preparation
3.4. Petrographic Analysis
3.5. Light Microscopy
3.6. SEM Analysis
4. Results
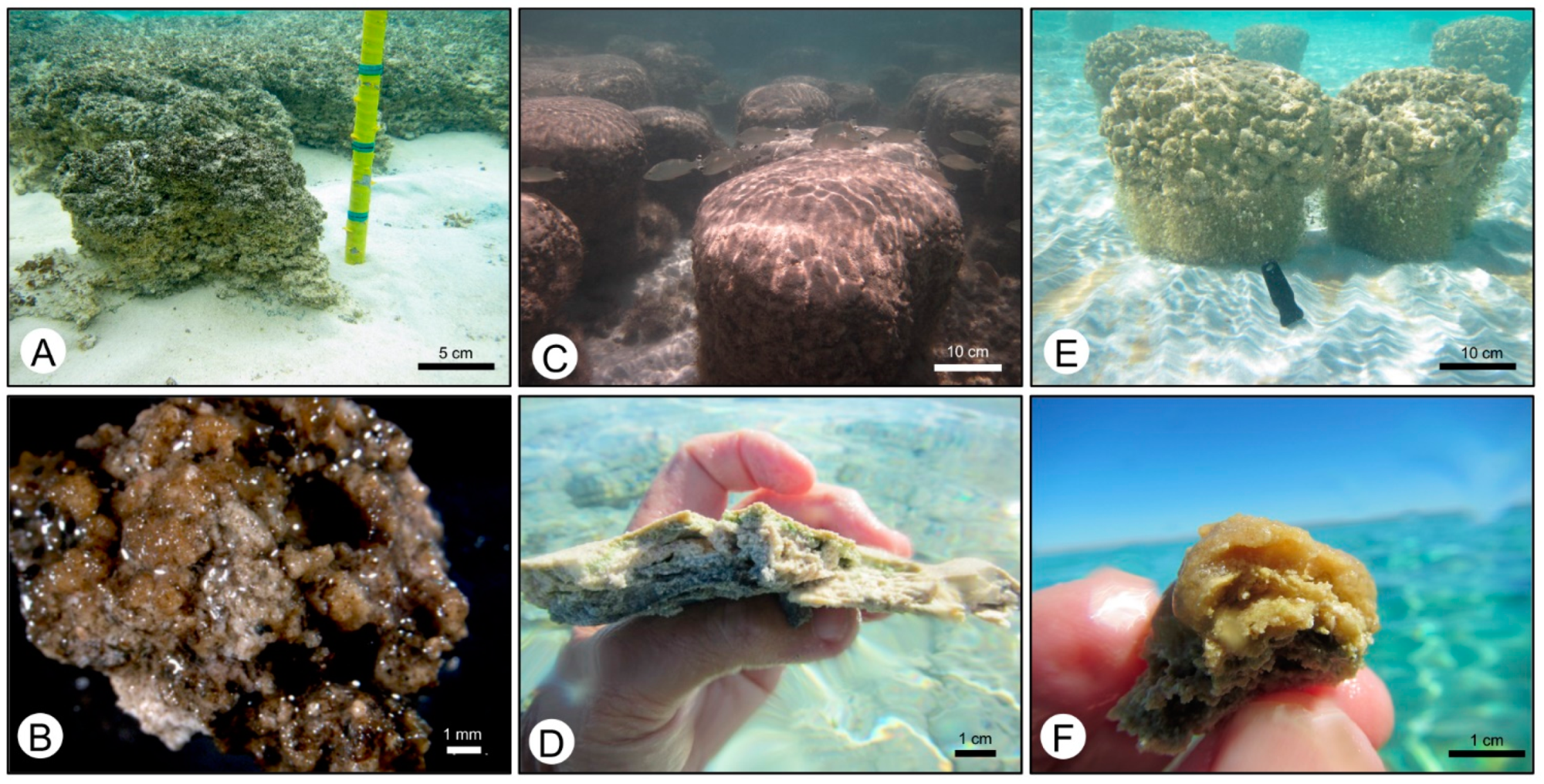
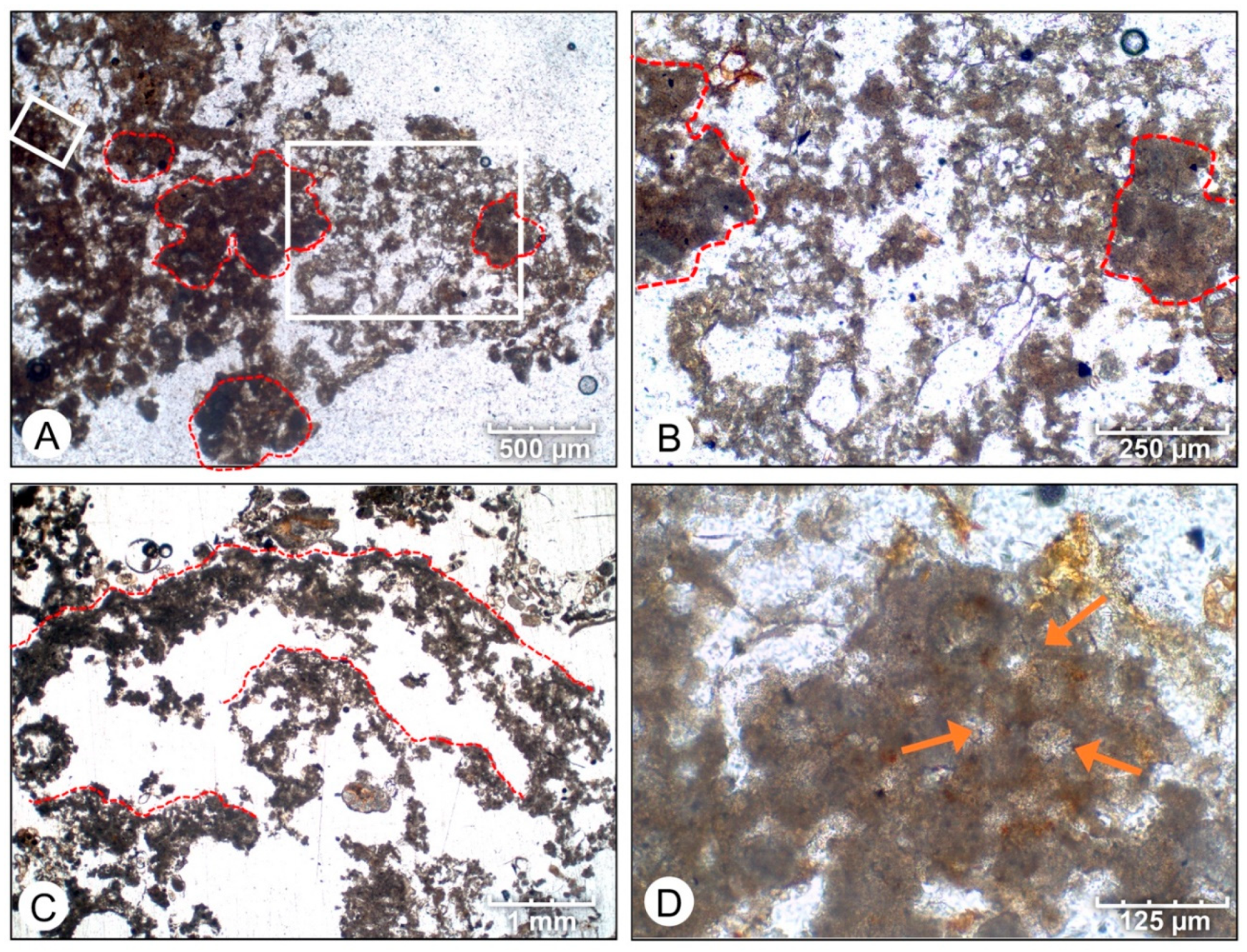
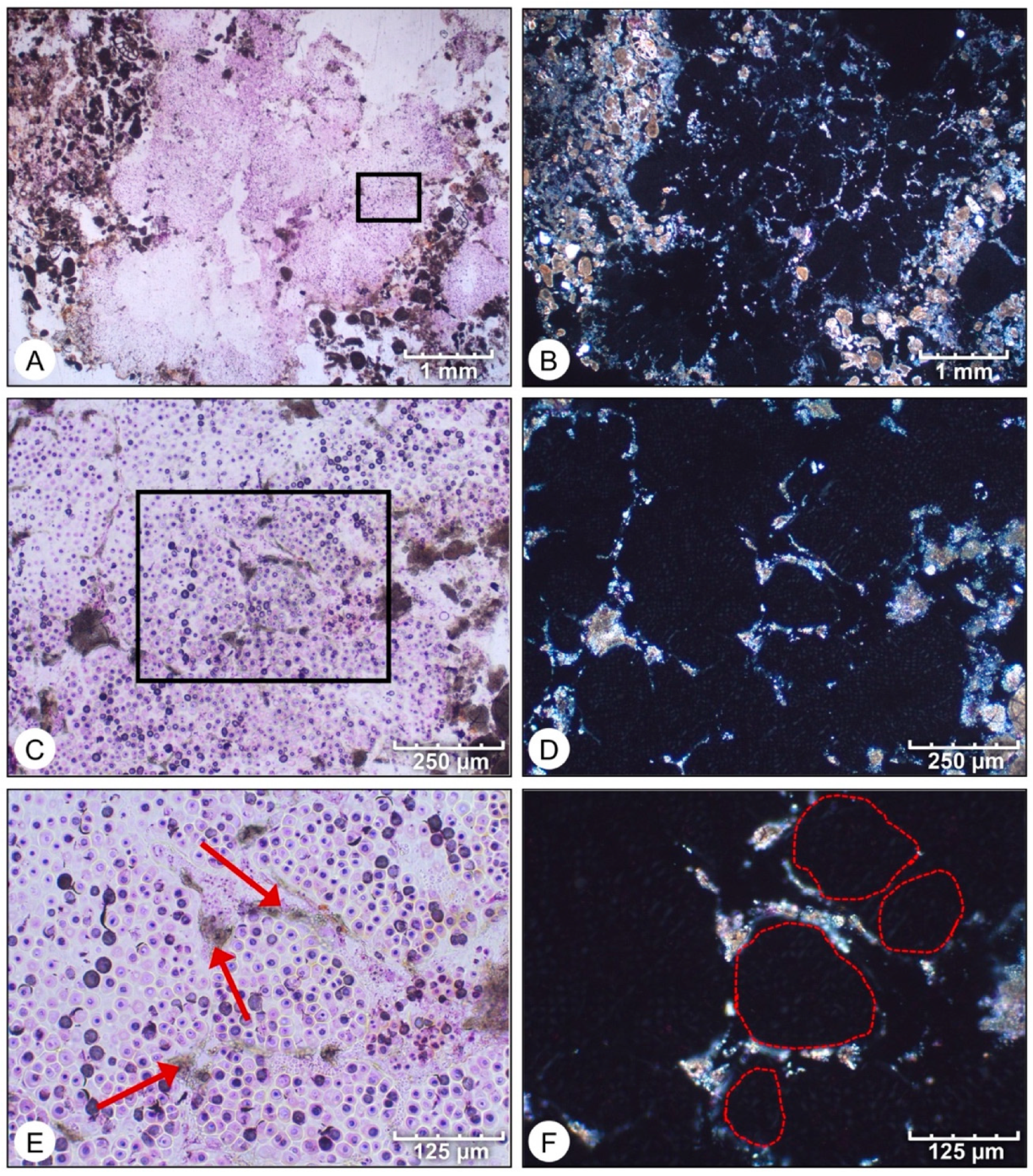
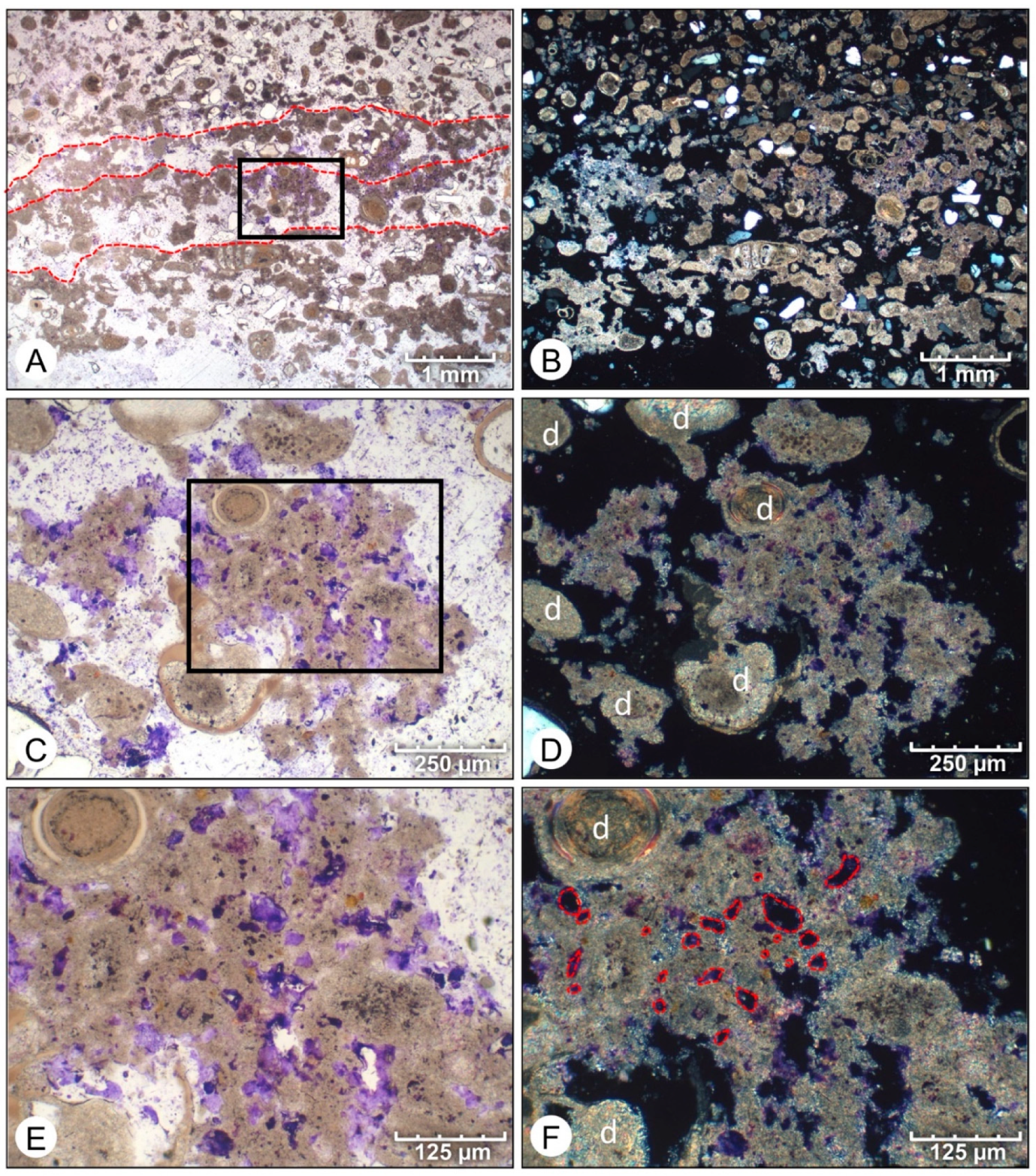
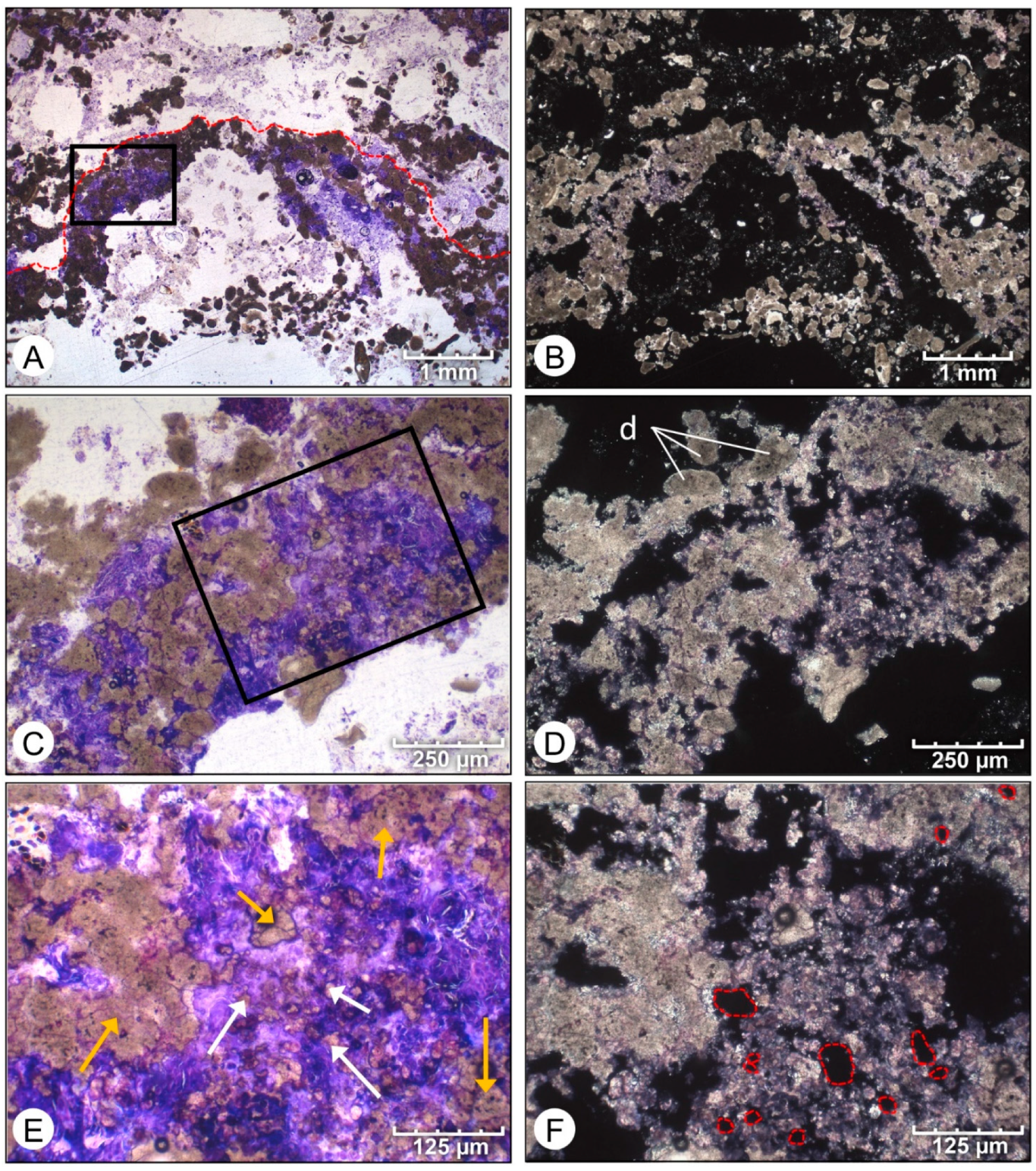
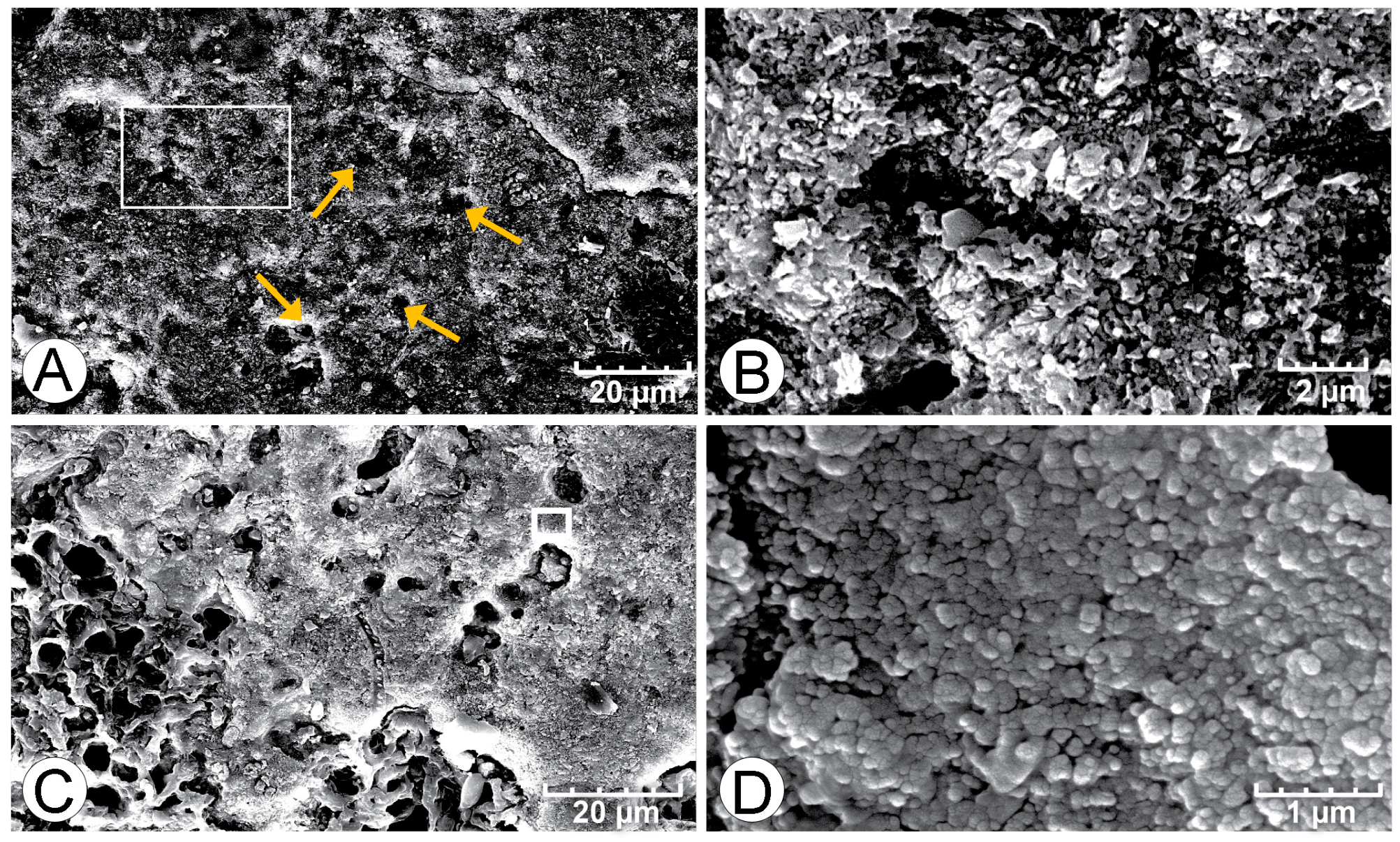
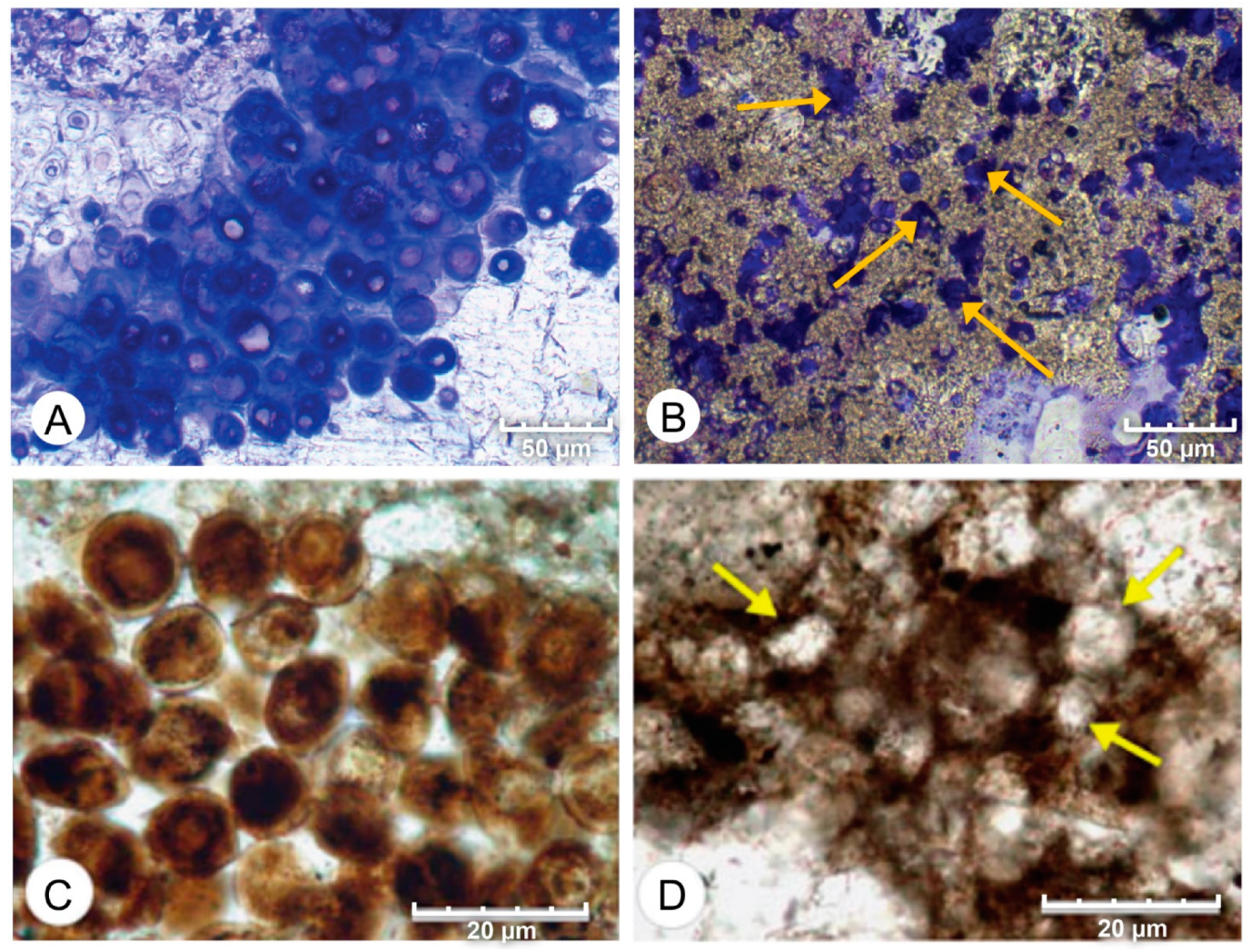
4.1. Petrographic Analysis
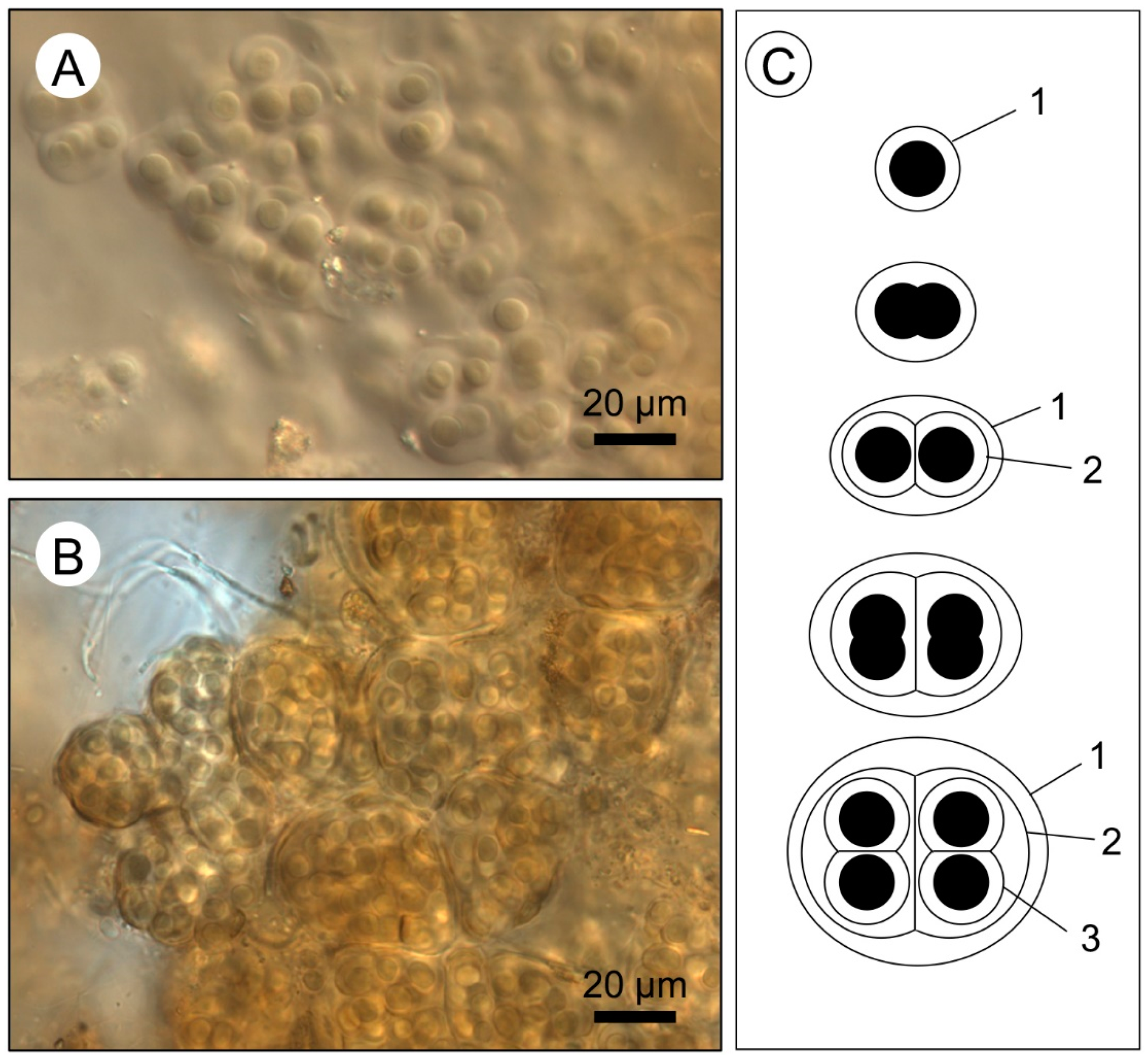
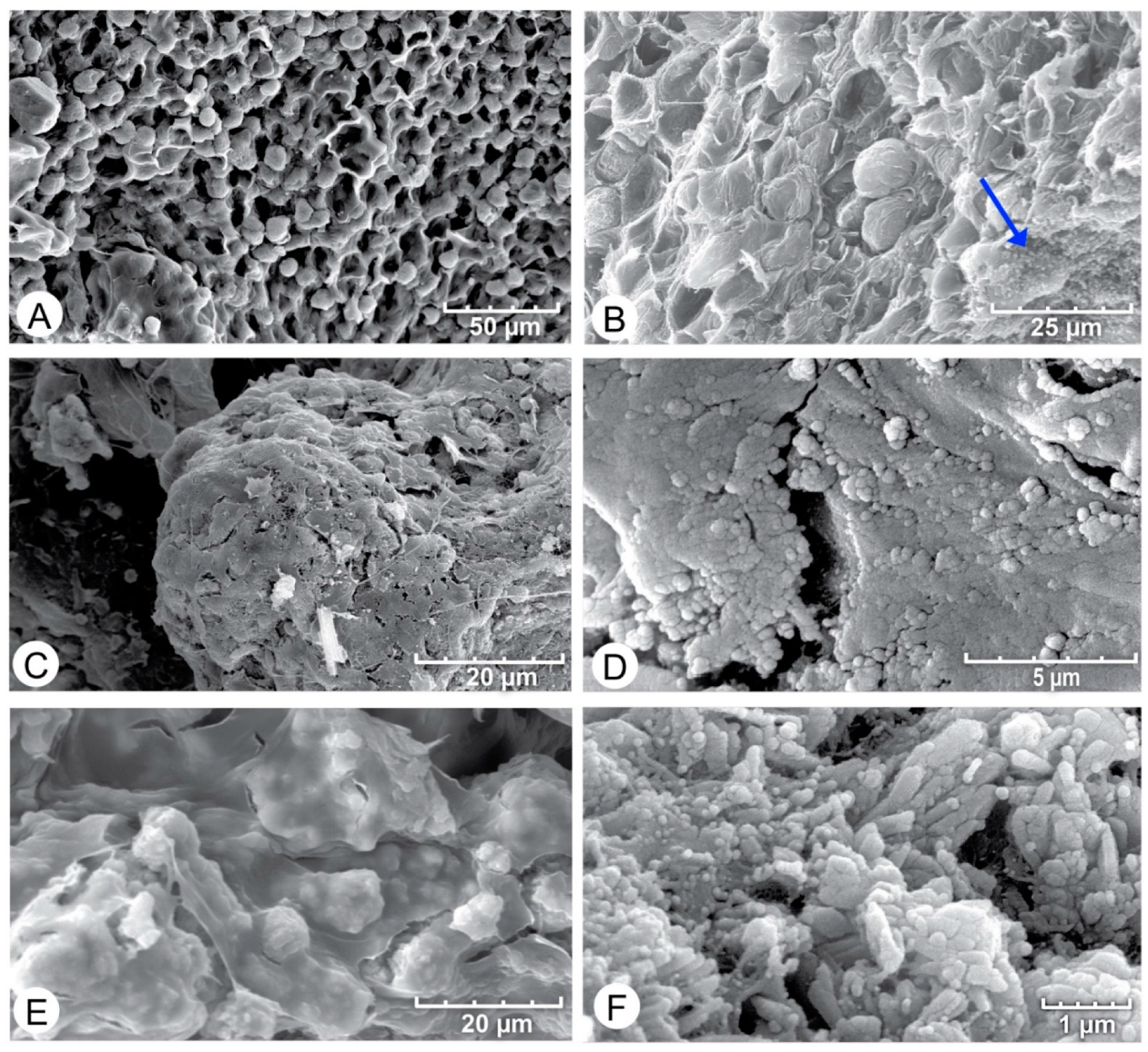
4.2. SEM Analysis
5. Discussion
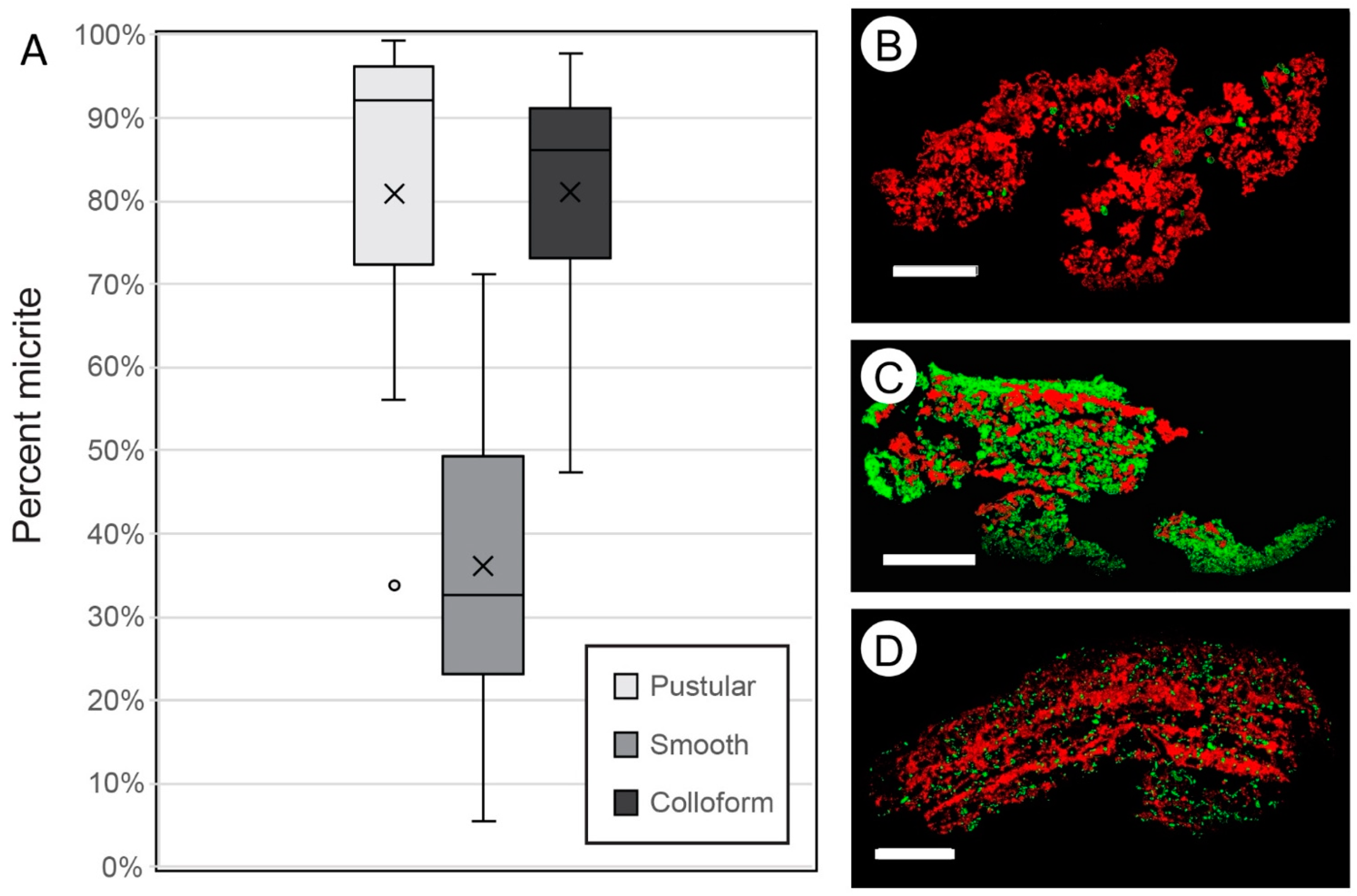
5.1. What Are the Amounts and Distribution of Micrite?
5.2. What Is the Origin of Micrite?
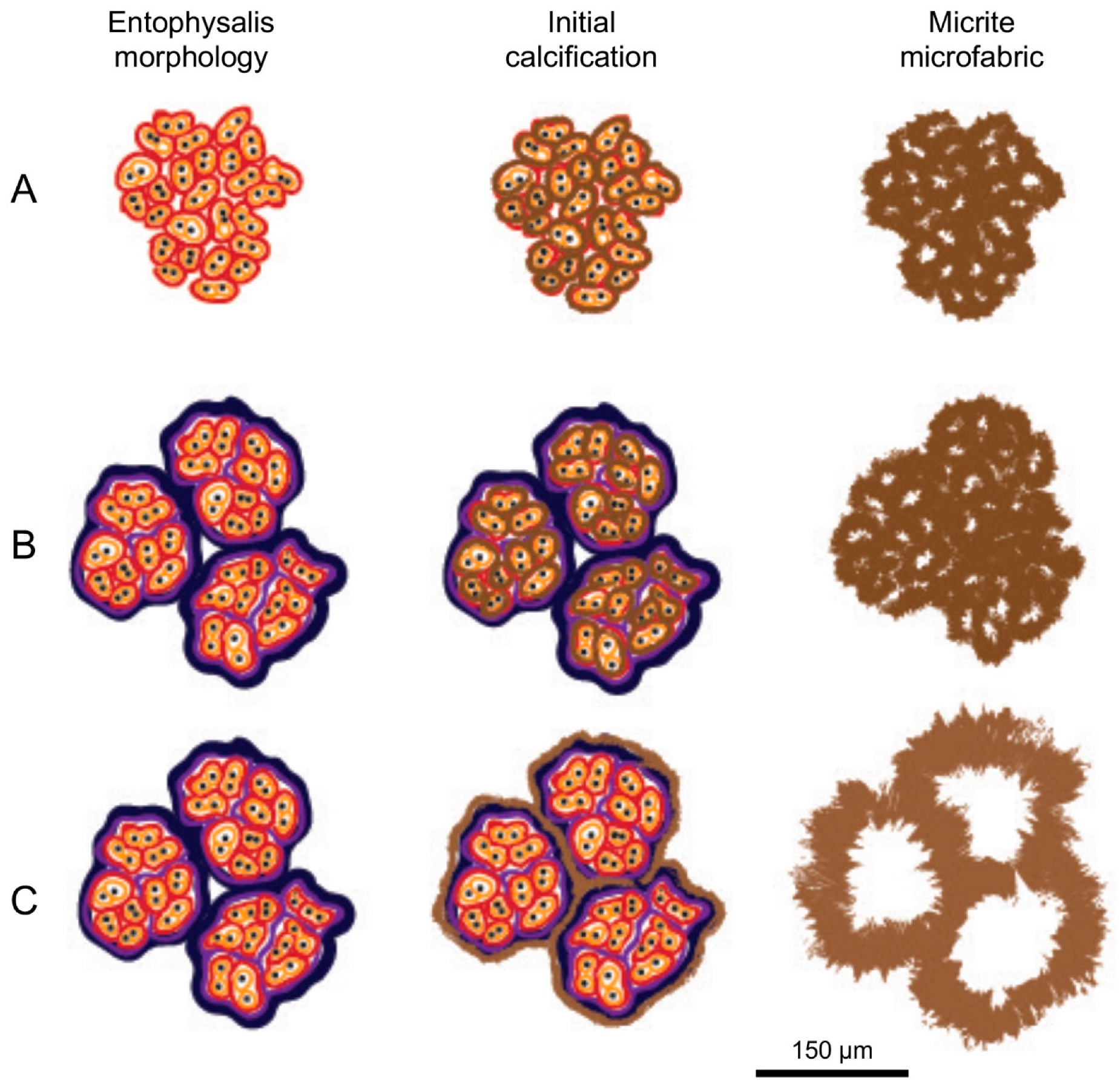
5.3. Do Microbes Influence the Micritic Textures?
6. Implications
6.1. Mat Community and Initial Accretion
6.2. Significance to the Geologic Record and Beyond
7. Conclusions
- In situ precipitation of micrite is an important accretion mechanism in Hamelin Pool microbialites, averaging 36% to 81% of accretion in surface mats.
- Wet thin sections of surface mats from microbialites reveal intimate relationships between Entophysalis cyanobacteria and micrite across all mat types (pustular, smooth, and colloform). This is of particular interest as Entophysalis is a living analog of Eoentophysalis, a coccoid cyanobacteria commonly found throughout early and middle Proterozoic stromatolite assemblages.
- Initial micrite distribution within each mat type reflects the size and distribution of Entophysalis colonies within the surface mats. Random clots of micrite within pustular mats reflect the occurrence of large colonies of Entophysalis distributed randomly throughout the mat. Micrite in smooth and colloform mats is aligned along horizons that are enriched in smaller Entophysalis colonies and forms laminae.
- Micrite formed by the calcification of Entophysalis cell envelopes in Hamelin microbialites has a characteristic honeycomb appearance resulting from the entombment of cells or colonies, with the size of honeycombs hypothesized to reflect the maturity of the Entophysalis community. Crystal shapes range from nanobulbous to tabular or rod-shaped, with an aragonite mineralogy.
- Our observations redefine our understanding of microbialites accretion in Hamelin Pool stromatolites, showing that primary microbial micrite is important in the accretion of these living structures and that coccoid mats are capable of producing laminated structures.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burne, R.V.; Moore, L.S. Microbialites: Organosedimentary Deposits of Benthic Microbial Communities. Palaios 1987, 2, 241–254. [Google Scholar] [CrossRef]
- Awramik, S.M.; Grey, K. Stromatolites: Biogenicity, Biosignatures, and Bioconfusion; SPIE: Bellingham, WA, USA, 2005; Volume 5906, 59060p. [Google Scholar] [CrossRef]
- WWalter, M.R. Stromatolites: The Main Geological Source of Information on the Evolution of the Early Benthos. In Early Life on Earth; Nobel Symposium No. 84; Bengtson, S., Ed.; Columbia University Press: New York, NY, USA, 1994; pp. 270–286. [Google Scholar]
- Grey, K.; Awramik, S.M. Handbook for the Study and Description of Microbialites; Geological Survey of Western Australia: Perth, Australia, 2020; ISBN 9781741688610. [Google Scholar]
- Riding, R. Microbial Carbonates: The Geological Record of Calcified Bacterial-Algal Mats and Biofilms. Sedimentology 2000, 47, 179–214. [Google Scholar] [CrossRef]
- Reid, R.P.; James, N.P.; Macintyre, I.G.; Dupraz, C.P.; Burne, R.V. Shark Bay Stromatolites: Microfabrics and Reinterpretation of Origins. Facies 2003, 49, 299–324. [Google Scholar] [CrossRef]
- Thompson, J.B.; Ferris, F.G. Cyanobacterial Precipitation of Gypsum, Calcite, and Magnesite from Natural Alkaline Lake Water. Geology 1990, 18, 995–998. [Google Scholar] [CrossRef]
- Stolz, J.F.; Feinstein, T.N.; Salsi, J.; Visscher, P.T.; Reid, R.P. TEM Analysis of Microbial Mediated Sedimentation and Lithification in Modern Stromatolites. Am. Mineral. 2001, 86, 826–833. [Google Scholar] [CrossRef]
- Dupraz, C.; Reid, R.P.; Braissant, O.; Decho, A.W.; Norman, R.S.; Visscher, P.T. Processes of Carbonate Precipitation in Modern Microbial Mats. Earth-Science Rev. 2009, 96, 141–162. [Google Scholar] [CrossRef]
- Mann, S. Biomineralization: Principles and Concepts in Bioinorganic Materials Chemistry; Oxford chemistry masters; Oxford University Press: Oxford, UK, 2001; ISBN 9780198508823. [Google Scholar]
- Suosaari, E.P.; Reid, R.P.; Playford, P.E.; Foster, J.S.; Stolz, J.F.; Casaburi, G.; Hagan, P.D.; Chirayath, V.; Macintyre, I.G.; Planavsky, N.J.; et al. New Multi-Scale Perspectives on the Stromatolites of Shark Bay, Western Australia. Sci. Rep. 2016, 6, 20557. [Google Scholar] [CrossRef]
- Suosaari, E.P.; Pamela Reid, R.; Oehlert, A.M.; Playford, P.E.; Steffensen, C.K.; Andres, M.S.; Suosaari, G.V.; Milano, G.R.; Eberli, G.P. Stromatolite Provinces of Hamelin Pool: Physiographic Controls on Stromatolites and Associated Lithofacies. J. Sediment. Res. 2019, 89, 207–226. [Google Scholar] [CrossRef]
- Ginsburg, R.N. Controversies about Stromatolites: Vices and Virtues. In Symposium Controversies in Modern Geology; Academic Press: London, UK, 1991; pp. 25–36. [Google Scholar]
- Kaźmierczak, J.; Coleman, M.L.; Gruszczyński, M.; Kempe, S. Cyanobacterial Key to the Genesis of Micritic and Peloidal Limestones in Ancient Seas. Acta Palaeontol. Pol. 1996, 41, 319–338. [Google Scholar]
- Awramik, S.M.; Riding, R. Role of Algal Eukaryotes in Subtidal Columnar Stromatolite Formation. Proc. Natl. Acad. Sci. USA 1988, 85, 1327–1329. [Google Scholar] [CrossRef] [Green Version]
- Browne, K.M. Modern Marine Stromatolitic Structures: The Sediment Dilemma BT—STROMATOLITES: Interaction of Microbes with Sediments; Tewari, V., Seckbach, J., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 291–312. ISBN 978-94-007-0397-1. [Google Scholar]
- Jahnert, R.J.; Collins, L.B. Characteristics, Distribution and Morphogenesis of Subtidal Microbial Systems in Shark Bay, Australia. Mar. Geol. 2012, 303–306, 115–136. [Google Scholar] [CrossRef]
- Hagan, P.D. Internal Fabrics and Microbial Precipitation in the Stromatolites of Hamelin Pool, Western Australia. Ph.D. Thesis, University of Miami, Miami, FL, USA, 2015. [Google Scholar]
- Goh, F.; Allen, M.A.; Leuko, S.; Kawaguchi, T.; Decho, A.W.; Burns, B.P.; Neilan, B.A. Determining the Specific Microbial Populations and Their Spatial Distribution within the Stromatolite Ecosystem of Shark Bay. ISME J. 2009, 3, 383–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffman, P. Shallow and Deepwater Stromatolites in Lower Proterozoic Platform–to–Basin Facies Change, Great Slave Lake, Canada. AAPG Bull. 1974, 58, 856–867. [Google Scholar] [CrossRef]
- Kazmierczak, J.; Kempe, S. Genuine Modern Analogues of Precambrian Stromatolites from Caldera Lakes of Niuafo’ou Island, Tonga. Naturwissenschaften 2006, 93, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Burns, B.P.; Anitori, R.; Butterworth, P.; Henneberger, R.; Goh, F.; Allen, M.A.; Ibañez-Peral, R.; Bergquist, P.L.; Walter, M.R.; Neilan, B.A. Modern Analogues and the Early History of Microbial Life. Precambrian Res. 2009, 173, 10–18. [Google Scholar] [CrossRef]
- Playford, P.E.; Cockbain, A.E.; Berry, P.F.; Roberts, A.P.; Haines, P.W.; Brooke, B.P. Geology of Shark Bay; Geological Survey of Western Australia: Perth, Australia, 2013; ISBN 9781741685039. [Google Scholar]
- Logan, B.W.; Hoffman, P.; Gebelein, C.D. Algal Mats, Cryptalgal Fabrics, and Structures, Hamelin Pool, Western Australia. In Memoir 22: Evolution and Diagenesis of Quaternary Carbonate Sequences, Shark Bay, Western Australia; American Association of Petroleum Geologists (AAPG): Tulsa, OK, USA, 1974; pp. 140–194. [Google Scholar]
- Playford, P.E. Geology of the Shark Bay area, Western Australia; Geological Survey of Western Australia: Perth, Australia, 1990. [Google Scholar]
- BBabilonia, J.; Conesa, A.; Casaburi, G.; Pereira, C.; Louyakis, A.S.; Reid, R.P.; Foster, J.S. Comparative metagenomics provides insight into the ecosystem functioning of the Shark Bay Stromatolites, Western Australia. Front. Microbiol. 2018, 9, 1359. [Google Scholar] [CrossRef] [Green Version]
- Golubic, S.; Hofmann, H.J. Comparison of Holocene and Mid-Precambrian Entophysalidaceae (Cyanophyta) in Stromatolitic Algal Mats: Cell Division and Degradation. J. Paleontol. 1976, 50, 1074–1082. [Google Scholar]
- Golubic, S.; Abed, R.M.M. Entophysalis Mats as Environmental Regulators. In Microbial Mats; Seckbach, J., Oren, A., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 237–251. ISBN 9789048137992. [Google Scholar]
- Playford, P.E.; Cockbain, A.E. Modern Algal Stromatolites at Hamelin Pool, A Hypersaline Barred Basin in Shark Bay, Western Australia. In Stromatolites; Walter, M.R.B.T.-D., Ed.; Elsevier: Amsterdam, The Netherlands, 1976; Volume 20, pp. 389–411. ISBN 0070-4571. [Google Scholar]
- Nye, O.B.; Dean, D.A.; Hinds, R.W. Improved Thin Section Techniques for Fossil and Recent Organisms Published by: SEPM Society for Sedimentary Geology Stable. J. Paleontol. 1972, 46, 271–275. [Google Scholar]
- Stolz, J.F.; Reid, R.P.; Visscher, P.T.; Decho, A.W.; Norman, R.S.; Aspden, R.J.; Bowlin, E.M.; Franks, J.; Foster, J.S.; Paterson, D.M.; et al. The Microbial Communities of the Modern Marine Stromatolites at Highborne Cay, Bahamas; National Museum Of Natural History Smithsonian Institution: Washington, DC, USA, 2009; pp. 1–29. [Google Scholar]
- Friedman, G.M. Classification of sediments and sedimentary rocks. In Encyclopedia of Sediments and Sedimentary Rocks; Middleton, G.V., Church, M.J., Coniglio, M., Hardie, L.A., Longstaffe, F.J., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 1978; pp. 127–136. ISBN 978-1-4020-3609-5. [Google Scholar]
- Folk, R.L. Practical Petrographic Classification of Limestones1. Am. Assoc. Pet. Geol. Bull. 1959, 43, 1–38. [Google Scholar] [CrossRef]
- Bontognali, T.R.R.; Vasconcelos, C.; Warthmann, R.J.; Dupraz, C.; Bernasconi, S.M.; McKenzie, J.A. Microbes Produce Nanobacteria-like Structures, Avoiding Cell Entombment. Geology 2008, 36, 663–666. [Google Scholar] [CrossRef]
- Weiner, S.; Levi-Kalisman, Y.; Raz, S.; Addadi, L. Biologically Formed Amorphous Calcium Carbonate. Connect. Tissue Res. 2003, 44, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.M.; Marzec, B.; Nudelman, F. Solid-State Transformation of Amorphous Calcium Carbonate to Aragonite Captured by CryoTEM. Angew. Chemie Int. Ed. 2017, 56, 11740–11743. [Google Scholar] [CrossRef] [PubMed]
- Enyedi, N.T.; Makk, J.; Kótai, L.; Berényi, B.; Klébert, S.; Sebestyén, Z.; Molnár, Z.; Borsodi, A.K.; Leél-Őssy, S.; Demény, A.; et al. Cave Bacteria-Induced Amorphous Calcium Carbonate Formation. Sci. Rep. 2020, 10, 8696. [Google Scholar] [CrossRef] [PubMed]
- Kazmierczak, J.; Kremer, B.; Racki, G. Late Devonian Marine Anoxia Challenged by Benthic Cyanobacterial Mats. Geobiology 2012, 10, 371–383. [Google Scholar] [CrossRef]
- Baumgartner, R.J.; Van Kranendonk, M.J.; Wacey, D.; Fiorentini, M.L.; Saunders, M.; Caruso, S.; Pages, A.; Homann, M.; Guagliardo, P. Nano-Porous Pyrite and Organic Matter in 3.5-Billion-Year-Old Stromatolites Record Primordial Life. Geology 2019, 47, 1039–1043. [Google Scholar] [CrossRef]
- Dupraz, C.; Visscher, P.T. Microbial Lithification in Marine Stromatolites and Hypersaline Mats. Trends Microbiol. 2005, 13, 429–438. [Google Scholar] [CrossRef] [Green Version]
- Braissant, O.; Decho, A.W.; Dupraz, C.; Glunk, C.; Przekop, K.M.; Visscher, P.T. Exopolymeric Substances of Sulfate-Reducing Bacteria: Interactions with Calcium at Alkaline PH and Implication for Formation of Carbonate Minerals. Geobiology 2007, 5, 401–411. [Google Scholar] [CrossRef]
- Gallagher, K.L.; Kading, T.J.; Braissant, O.; Dupraz, C.; Visscher, P.T. Inside the Alkalinity Engine: The Role of Electron Donors in the Organomineralization Potential of Sulfate-Reducing Bacteria. Geobiology 2012, 10, 518–530. [Google Scholar] [CrossRef]
- Hagan, G.M.; Logan, B.W. Development of Carbonate Banks and Hypersaline Basins, Shark Bay, Western Australia; American Association of Petroleum Geologists (AAPG): Tulsa, OK, USA, 1974. [Google Scholar]
- Jahnert, R.J.; Collins, L.B. Controls on Microbial Activity and Tidal Flat Evolution in Shark Bay, Western Australia. Sedimentology 2013, 60, 1071–1099. [Google Scholar] [CrossRef]
- Balci, N.; Demirel, C.; Akcer Ön, S.; Gültekin, A.H.; Kurt, M.A. Evaluating Abiotic and Microbial Factors on Carbonate Precipitation in Lake Acigöl, a Hypersaline Lake in Southwestern Turkey. Quat. Int. 2018, 486, 116–128. [Google Scholar] [CrossRef]
- Golubic, S. Stromatolites, Fossil and Recent: A Case History. In Biomineralization and Biological Metal Accumulation; Westbroek, P., de Jong, E.W., Eds.; D. Reidel Publishing Company: Dordrecht, The Netherlands, 1983; pp. 313–326. [Google Scholar]
- Golubic, S. Microbial Mats and Modern Stromatolites in Shark Bay, Western Australia. In Planet Ecology; Van Nostrand Reinhold Co.: New York, NY, USA, 1985; pp. 3–16. [Google Scholar]
- Jahnert, R.J.; Collins, L.B. Significance of Subtidal Microbial Deposits in Shark Bay, Australia. Mar. Geol. 2011, 286, 106–111. [Google Scholar] [CrossRef]
- Riding, R. Calcareous Algae and Stromatolites. In Calcareous Algae and Stromatolites; Riding, R., Ed.; Springer-Verlag: Berlin, Germany, 1991; pp. 21–51. ISBN 9783642523359. [Google Scholar]
- Horodyski, R.J.; Vonder Haar, S.P. Recent Calcareous Stromatolites from Laguna Mormona (Baja California) Mexico. J. Sediment. Petrol. 1975, 45, 894–906. [Google Scholar] [CrossRef]
- Allen, C.C.; Albert, F.G.; Chafetz, H.S.; Combie, J.; Graham, C.R.; Kieft, T.L.; Kivett, S.J.; McKay, D.S.; Steele, A.; Taunton, A.E.; et al. Microscopic Physical Biomarkers in Carbonate Hot Springs: Implications in the Search for Life on Mars. Icarus 2000, 147, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Kazmierczak, J.; Altermann, W.; Kremer, B.; Kempe, S.; Eriksson, P.G. Mass Occurrence of Benthic Coccoid Cyanobacteria and Their Role in the Production of Neoarchean Carbonates of South Africa. Precambrian Res. 2009, 173, 79–92. [Google Scholar] [CrossRef] [Green Version]
- Melim, L.A.; Northup, D.E.; Boston, P.J.; Spilde, M.N. Preservation of Fossil Microbes and Biofilm in Cave Pool Carbonates and Comparison to Other Microbial Carbonate Environments. Palaios 2016, 31, 177–189. [Google Scholar] [CrossRef]
- Awramik, S.M. Ancient Stromatolites and Microbial Mats. In Microbial Mats: Stromatolites; Cohen, Y., Castenholz, R.W., Halvorson, H.O., Eds.; Alan, R. Liss Inc.: New York, NY, USA, 1984; pp. 1–21. [Google Scholar]
- Kennard, J.; James, N.P. Thrombolites and Stromatolites: Two Distinct Types of Microbial Structures. Palaios 1986, 1, 492–503. [Google Scholar] [CrossRef]
- Sumner, D.Y. Microbial vs Environmental Influences on the Morphology of Late Archean Fenestrate Microbialites. In Microbial Sediments; Riding, R.E., Awramik, S.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 307–314. ISBN 978-3-662-04036-2. [Google Scholar]
- Suarez-Gonzalez, P.; Benito, M.I.I.; Quijada, I.E.E.; Mas, R.; Campos-Soto, S. ‘Trapping and Binding’: A Review of the Factors Controlling the Development of Fossil Agglutinated Microbialites and Their Distribution in Space and Time. Earth-Sci. Rev. 2019, 194, 182–215. [Google Scholar] [CrossRef] [Green Version]
- Sugitani, K.; Mimura, K.; Nagaoka, T.; Lepot, K.; Takeuchi, M. Microfossil Assemblage from the 3400Ma Strelley Pool Formation in the Pilbara Craton, Western Australia: Results Form a New Locality. Precambrian Res. 2013, 226, 59–74. [Google Scholar] [CrossRef]
- Gong, Y. Sedimentary Fabrics for Thrombolite Bioherm in Cambrian Zhangxia Formation in the Western Part of Shandong Province. IOP Conf. Ser. Earth Environ. Sci. 2021, 781, 052006. [Google Scholar] [CrossRef]
- Bahniuk, A.M.; Anjos, S.; França, A.B.; Matsuda, N.; Eiler, J.; Mckenzie, J.A.; Vasconcelos, C. Development of Microbial Carbonates in the Lower Cretaceous Codó Formation (North-East Brazil): Implications for Interpretation of Microbialite Facies Associations and Palaeoenvironmental Conditions. Sedimentology 2015, 62, 155–181. [Google Scholar] [CrossRef]
- Warren, L.V.; Varejão, F.G.; Quaglio, F.; Simões, M.G.; Fürsich, F.T.; Poiré, D.G.; Catto, B.; Assine, M.L. Stromatolites from the Aptian Crato Formation, a Hypersaline Lake System in the Araripe Basin, Northeastern Brazil. Facies 2017, 63, 3. [Google Scholar] [CrossRef] [Green Version]
- Backus, D.H.; Johnson, M.E. Stromatolitic Mats from an Uplifted Pleistocene Lagoon at Punta Chivato on the Gulf of California (Mexico). Palaios 2014, 29, 460–466. [Google Scholar] [CrossRef]
- Caudwell, C.; Lang, J.; Pascal, A. Lamination of Swampy-Rivulets Rivularia Haematites Stromatolites in a Temperate Climate. Sediment. Geol. 2001, 143, 125–147. [Google Scholar] [CrossRef]
- Lundberg, J.; McFarlane, D.A. Subaerial Freshwater Phosphatic Stromatolites in Deer Cave, Sarawak—A Unique Geobiological Cave Formation. Geomorphology 2011, 128, 57–72. [Google Scholar] [CrossRef]
- Shirokova, L.S.; Mavromatis, V.; Bundeleva, I.A.; Pokrovsky, O.S.; Bénézeth, P.; Gérard, E.; Pearce, C.R.; Oelkers, E.H. Using Mg Isotopes to Trace Cyanobacterially Mediated Magnesium Carbonate Precipitation in Alkaline Lakes. Aquat. Geochemistry 2013, 19, 1–24. [Google Scholar] [CrossRef]
- Sanz-Montero, M.E.; Cabestrero, Ó.; Sánchez-Román, M. Microbial Mg-Rich Carbonates in an Extreme Alkaline Lake (Las Eras, Central Spain). Front. Microbiol. 2019, 10, 148. [Google Scholar] [CrossRef] [Green Version]
- Balci, N.; Gunes, Y.; Kaiser, J.; On, S.A.; Eris, K.; Garczynski, B.; Horgan, B.H.N. Biotic and Abiotic Imprints on Mg-Rich Stromatolites: Lessons from Lake Salda, SW Turkey. Geomicrobiol. J. 2020, 37, 401–425. [Google Scholar] [CrossRef]
- Popall, R.M.; Bolhuis, H.; Muyzer, G.; Sánchez-Román, M. Stromatolites as Biosignatures of Atmospheric Oxygenation: Carbonate Biomineralization and UV-C Resilience in a Geitlerinema sp.—Dominated Culture. Front. Microbiol. 2020, 11, 948. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitek, B.E.; Suosaari, E.P.; Stolz, J.F.; Oehlert, A.M.; Reid, R.P. Initial Accretion in Hamelin Pool Microbialites: The Role of Entophysalis in Precipitation of Microbial Micrite. Geosciences 2022, 12, 304. https://doi.org/10.3390/geosciences12080304
Vitek BE, Suosaari EP, Stolz JF, Oehlert AM, Reid RP. Initial Accretion in Hamelin Pool Microbialites: The Role of Entophysalis in Precipitation of Microbial Micrite. Geosciences. 2022; 12(8):304. https://doi.org/10.3390/geosciences12080304
Chicago/Turabian StyleVitek, Brooke E., Erica P. Suosaari, John F. Stolz, Amanda M. Oehlert, and R. Pamela Reid. 2022. "Initial Accretion in Hamelin Pool Microbialites: The Role of Entophysalis in Precipitation of Microbial Micrite" Geosciences 12, no. 8: 304. https://doi.org/10.3390/geosciences12080304
APA StyleVitek, B. E., Suosaari, E. P., Stolz, J. F., Oehlert, A. M., & Reid, R. P. (2022). Initial Accretion in Hamelin Pool Microbialites: The Role of Entophysalis in Precipitation of Microbial Micrite. Geosciences, 12(8), 304. https://doi.org/10.3390/geosciences12080304





