Mineralogy and Mineral Chemistry of the REE-Rich Black Sands in Beaches of the Kavala District, Northern Greece
Abstract
1. Introduction
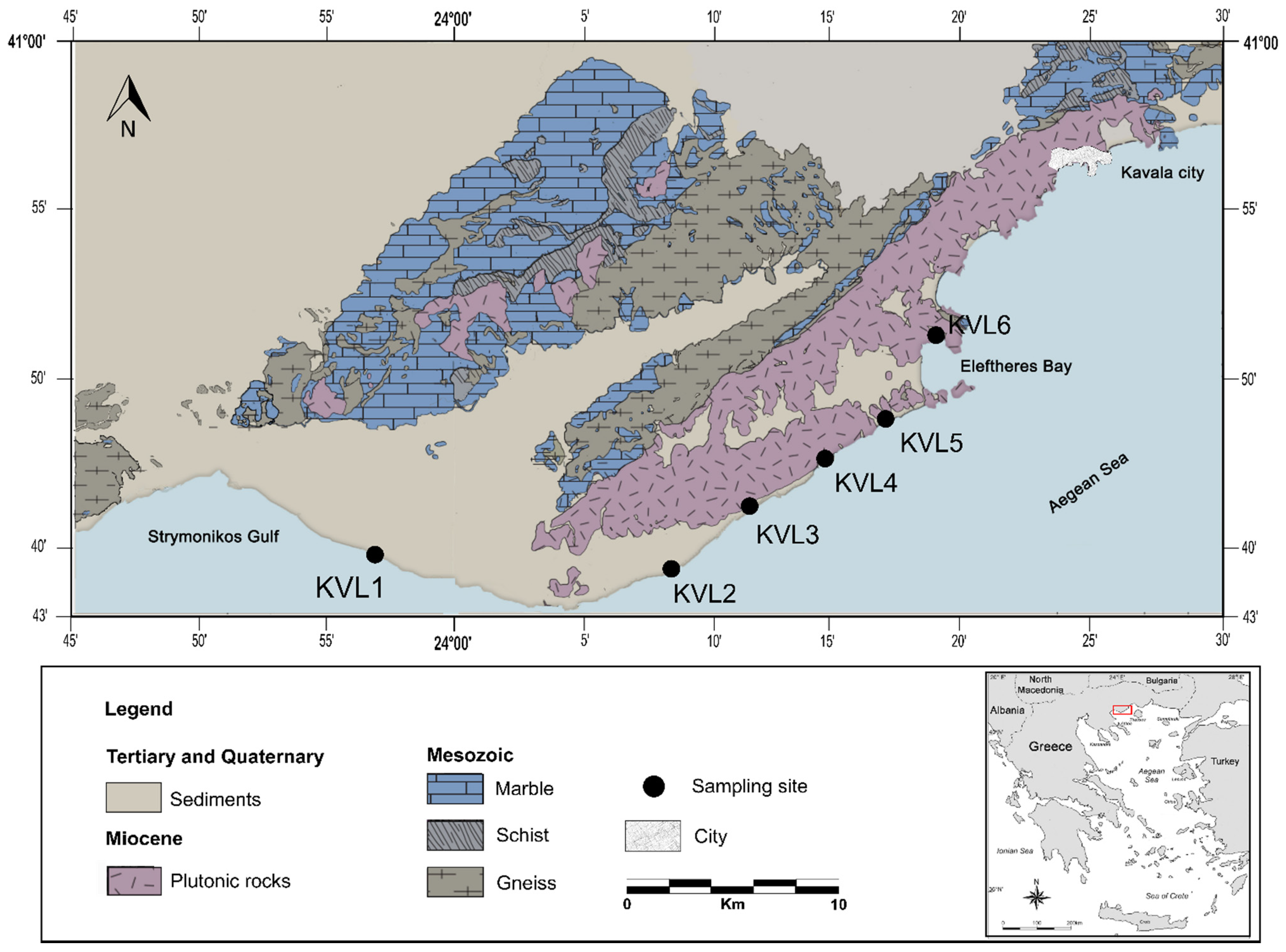
2. Geological Setting
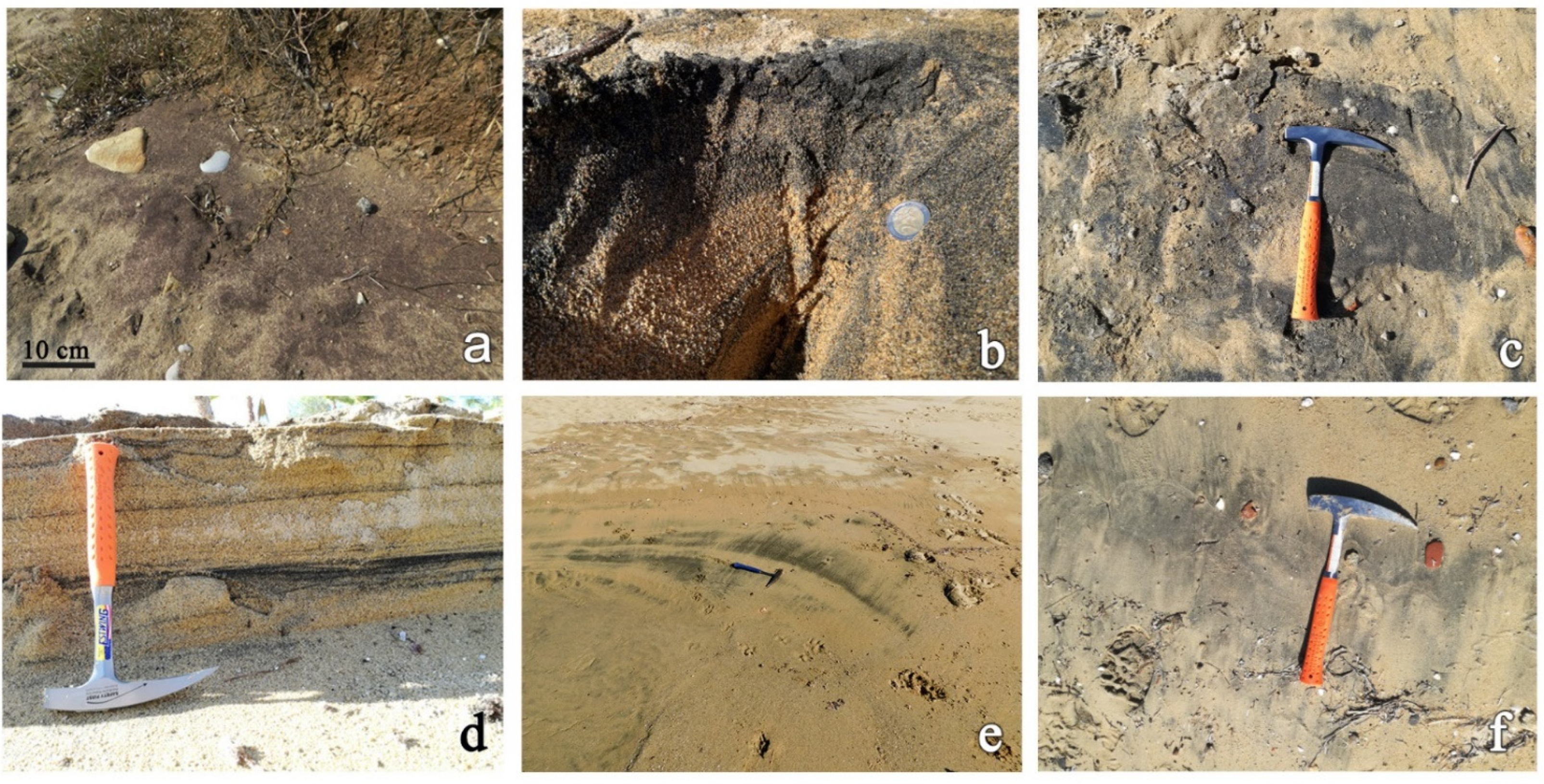
3. Materials and Methods
4. Results
4.1. Mineralogical Composition of the REE-Bearing Minerals
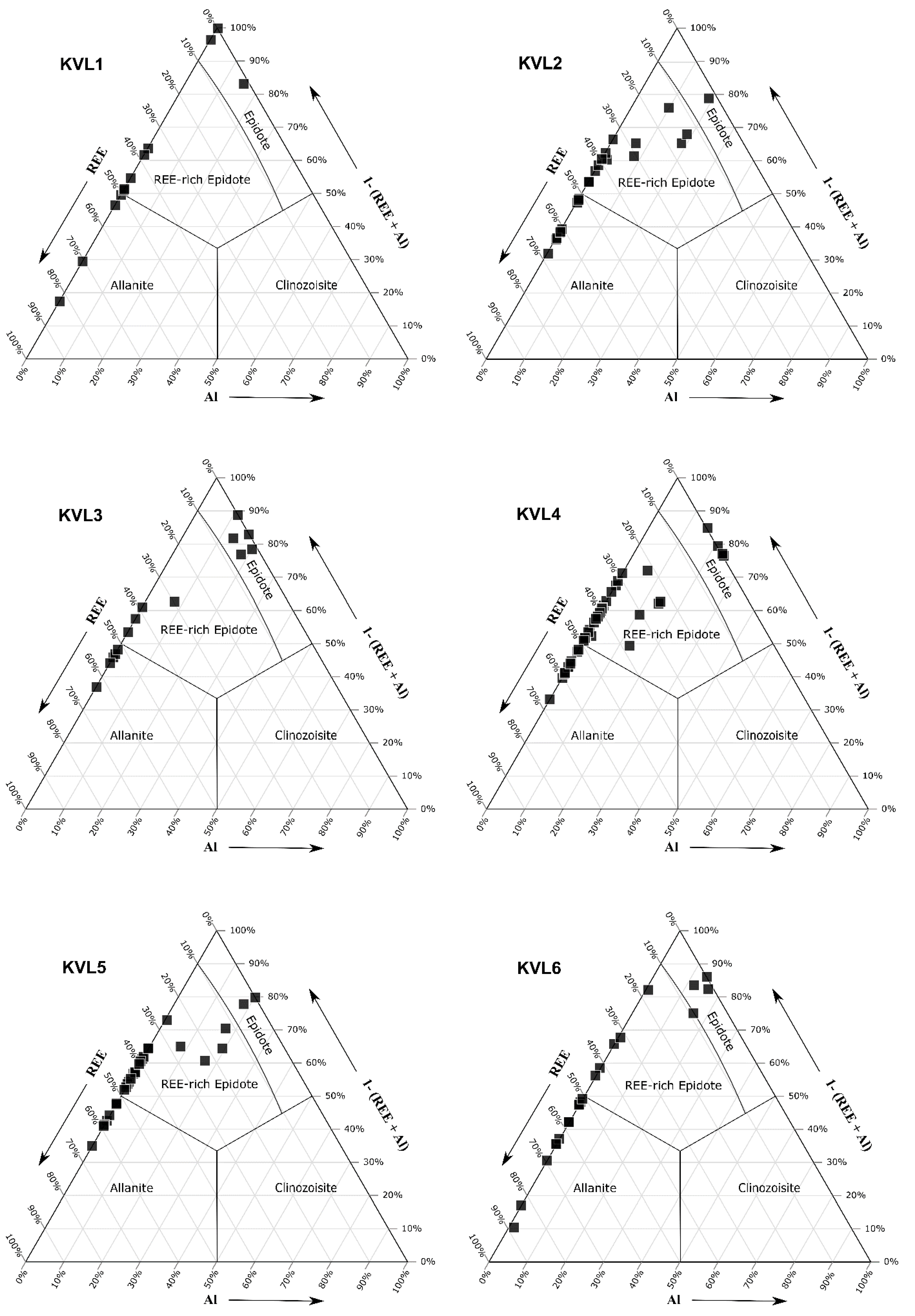
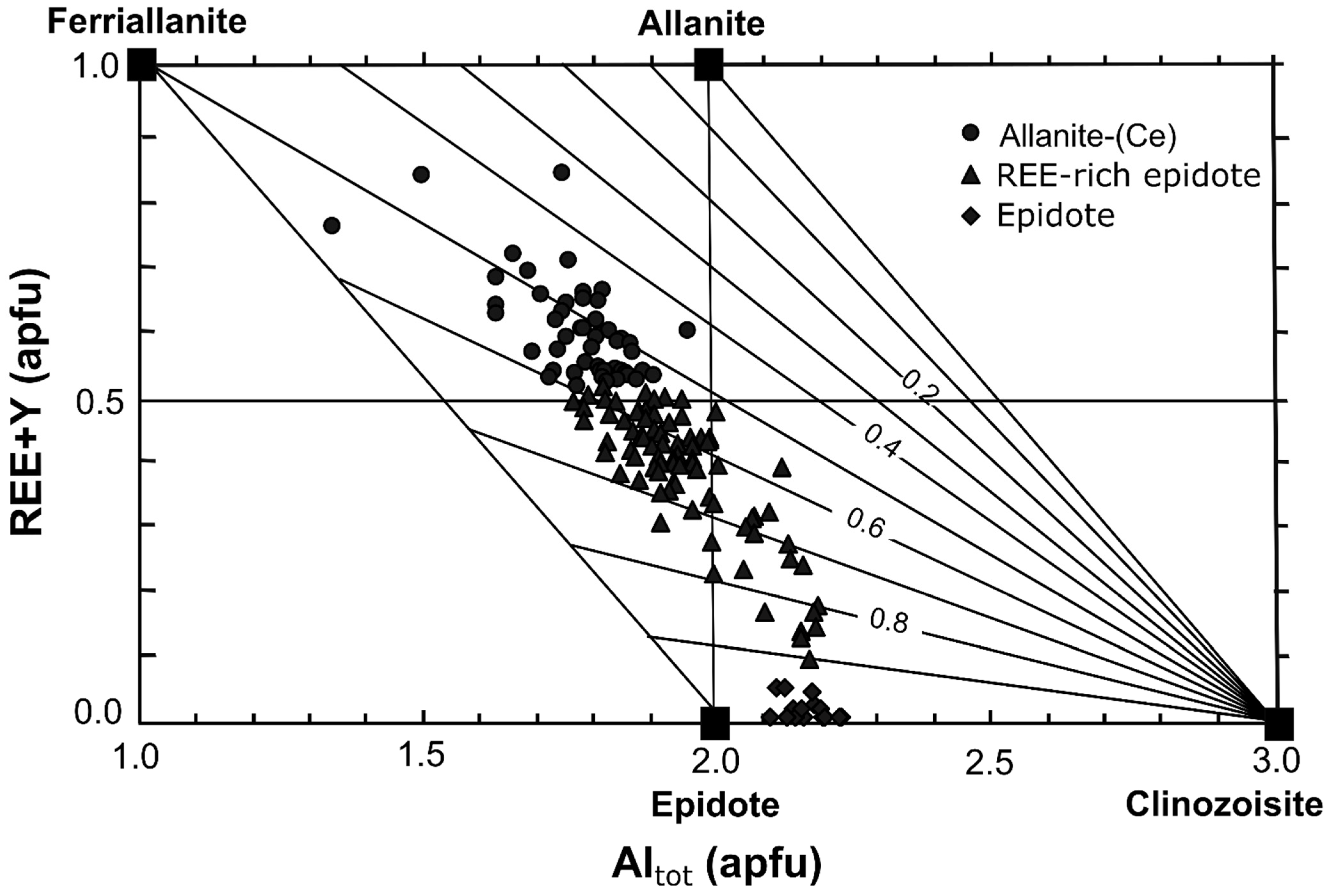
4.2. Chemical Composition of the REE-Bearing Minerals
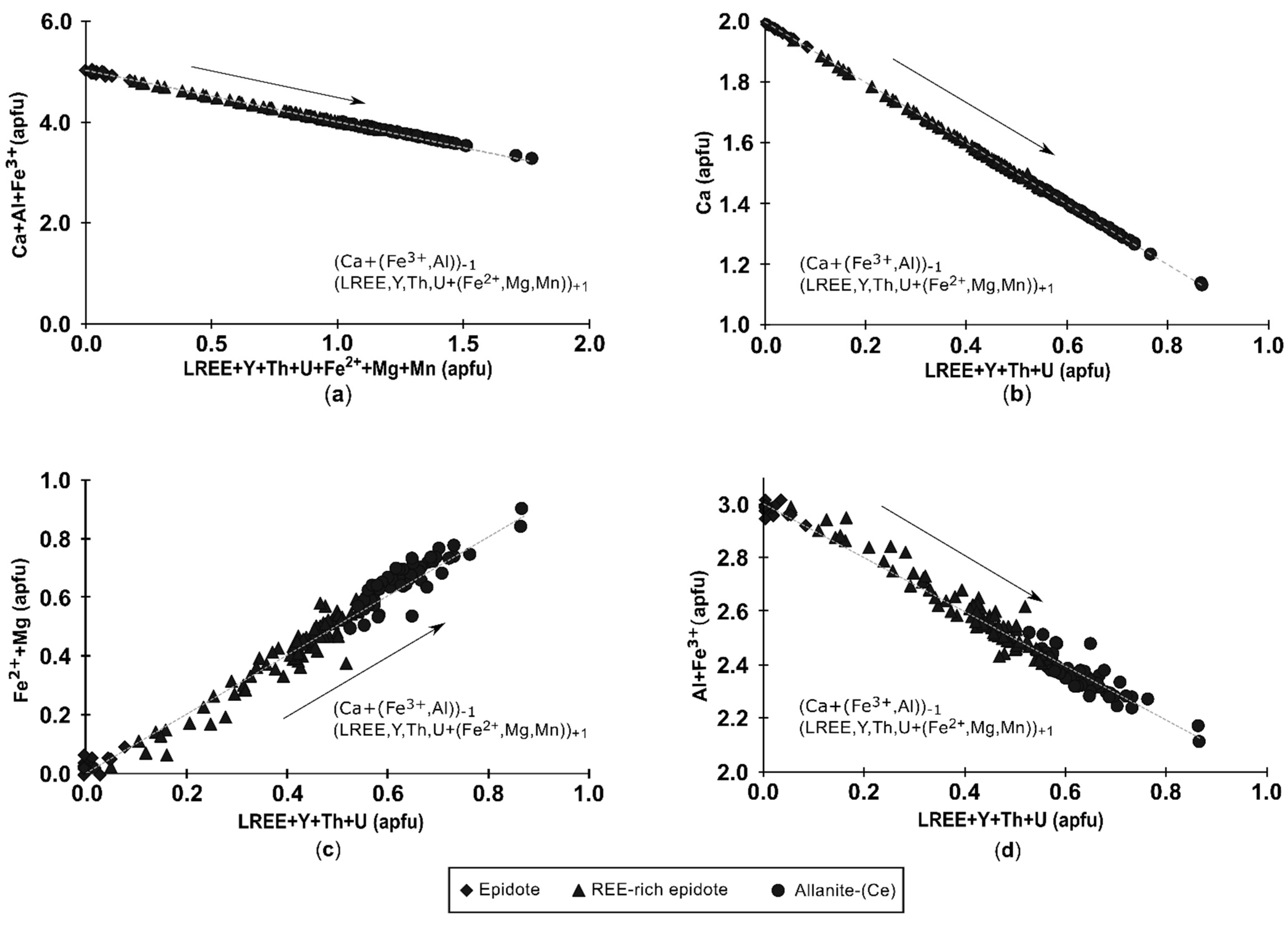
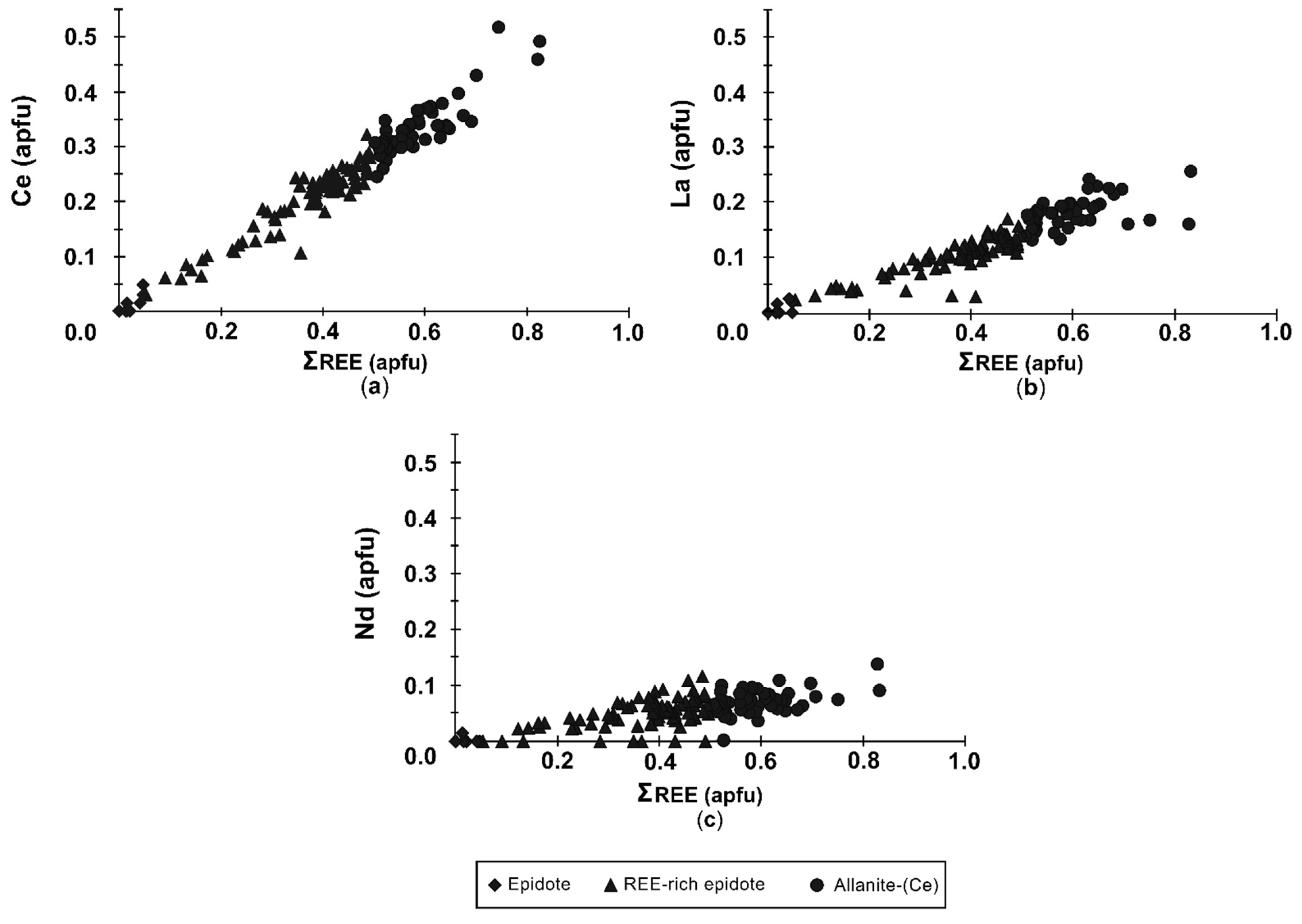
4.2.1. Composition of Allanite
4.2.2. Composition of Epidote
4.2.3. Composition of Monazite and Thorite
4.2.4. Composition of Zircon and Titanite
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watari, T.; McLellan, B.C.; Giurco, D.; Dominish, E.; Yamasue, E.; Nansai, K. Total material requirement for the global energy transition to 2050: A focus on transport and electricity. Resour. Conserv. Recycl. 2019, 148, 91–103. [Google Scholar] [CrossRef]
- European Commission. Critical Raw Materials Resilience: Charting a Path towards Greater Security and Sustainability; European Commission: Brussels, Belgium, 2020; p. 4. [Google Scholar]
- Emsley, J. Nature’s Building Blocks: An AZ Guide to the Elements, 2nd ed.; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Van Gosen, B.S.; Verplanck, P.L.; Seal, R.R., II; Long, K.R.; Gambogi, J. Rare-Earth Elements, Chap. 0. In Critical Mineral Resources of the United States: Economic and Environmental Geology and Prospects for Future Supply, No. 1802-O; U.S. Geological Survey Professional Paper 1802; Schulz, K.J., De Young, J.H., Seal, R.R., Bradley, D.C., Eds.; U.S. Geological Survey: Reston, VA, USA, 2018; pp. 1–31. [Google Scholar] [CrossRef]
- Dushyantha, N.; Batapola, N.; Ilankoon, I.M.S.K.; Rohitha, S.; Premasiri, R.; Abeysinghe, B.; Ratnayake, N.; Dissanayake, K. The story of rare earth elements (REEs): Occurrences, global distribution, genesis, geology, mineralogy and global production. Ore Geol. Rev. 2020, 122, 103521. [Google Scholar] [CrossRef]
- Goodenough, K.M.; Schilling, J.; Jonsson, E.; Kalvig, P.; Charles, N.; Tuduri, J.; Deady, E.A.; Sadeghi, M.; Schiellerup, H.; Müller, A.; et al. Europe’s Rare Earth Element Resource Potential: An Overview of REE Metallogenetic Provinces and Their Geodynamic Setting. Ore Geol. Rev. 2016, 72, 838–856. [Google Scholar] [CrossRef]
- Barakos, G.; Gutzmer, J.; Mischo, H. Strategic evaluations and mining process optimization towards a strong global REE supply chain. J. Sustain. Min. 2016, 15, 26–35. [Google Scholar] [CrossRef]
- Verplanck, P.L.; Hitzman, M.W. Rare Earth and Critical Elements in Ore Deposits. Rev. Econ. Geol. 2016, 18, 1–3. [Google Scholar] [CrossRef]
- Long, K.R.; van Gosen, B.S.; Foley, N.K.; Cordier, D. The Principal Rare Earth Elements Deposits of the United States: A Summary of Domestic Deposits and a Global Perspective. In Non-Renewable Resource Issues: Geoscientific and Societal Challenges; Sinding-Larsen, R., Wellmer, F.W., Eds.; Springer: Berlin, Germany, 2012; pp. 131–156. [Google Scholar] [CrossRef]
- Castor, S.B.; Hedrick, J.B. Rare earth elements. Ind. Miner. Rocks 2006, 769–792. [Google Scholar] [CrossRef]
- Jordens, A.; Cheng, Y.P.; Waters, K.E. A Review of the Beneficiation of Rare Earth Element Bearing Minerals. Miner. Eng. 2013, 41, 97–114. [Google Scholar] [CrossRef]
- Papadakis, A. The black sands of Loutra Eleftheron near Kavala, Greece. Sci. Ann. Fac. Phys. Math. Univ. Thessalon. 1975, 15, 331–390. [Google Scholar]
- Perissoratis, C.; Moorby, S.A.; Angelopoulos, I.; Cronan, D.S.; Papavasiliou, C.; Konispoliatis, N.; Sakellariadou, F.; Mitropoulos, D. Mineral Concentrations in the Recent Sediments Off Eastern Macedonia, Northern Greece: Geological and Geochemical Considerations. In Mineral Deposits within the European Community; Springer: Berlin/Heidelberg, Germany, 1988. [Google Scholar] [CrossRef]
- Pergamalis, F.; Karageorgiou, D.E.; Koukoulis, A.; Katsikis, I. Mineralogical and chemical composition of sand ore deposits in the seashore zone N. Peramos-L. Eleftheron (N. Greece). Bull. Geol. Soc. Greece 2001, 34, 845–850. [Google Scholar] [CrossRef]
- Pergamalis, F.; Karageorgiou, D.E.; Koukoulis, A. The location of Ti, REE, Th, U, Au deposits in the seafront zones of Nea Peramos-Loutra Eleftheron area, Kavala (N. Greece) using radiation. Bull. Geol. Soc. Greece 2001, 34, 1023–1029. [Google Scholar] [CrossRef]
- Aggelatou, V.; Papamanoli, S.; Stouraiti, C.; Papavasileiou, K. REE distributions in the black sands of Kavala coastal zone, northern Greece: Mineralogical and geochemical characterization of beneficiation products. In Proceedings of the 1st International Electronic Conference on Mineral Science, virtual, 16–31 July 2018; Volume 1. [Google Scholar] [CrossRef]
- Papadopoulos, A.; Koroneos, A.; Christofides, G.; Stoulos, S. Natural Radioactivity Distribution and Gamma Radiation Exposure of Beach Sands Close to Kavala Pluton, Greece. Open Geosci. 2015, 7, 407–422. [Google Scholar] [CrossRef]
- Papadopoulos, A.; Koroneos, A.; Christofides, G.; Papadopoulou, L. Geochemistry of Beach Sands from Kavala, Northern Greece. Ital. J. Geosci. 2016, 135, 526–539. [Google Scholar] [CrossRef]
- Papadopoulos, A.; Tzifas, I.T.; Tsikos, H. The Potential for REE and Associated Critical Metals in Coastal Sand (Placer) Deposits of Greece: A Review. Minerals 2019, 9, 469. [Google Scholar] [CrossRef]
- Tzifas, I.T.; Papadopoulos, A.; Misaelides, P.; Godelitsas, A.; Göttlicher, J.; Tsikos, H.; Gamaletsos, P.N.; Luvizotto, G.; Karydas, A.G.; Petrelli, M.; et al. New Insights into Mineralogy and Geochemistry of Allanite-Bearing Mediterranean Coastal Sands from Northern Greece. Chem. Der. Erde. 2019, 79, 247–267. [Google Scholar] [CrossRef]
- Stouraiti, C.; Angelatou, V.; Petushok, S.; Soukis, K.; Eliopoulos, D. Effect of Mineralogy on the Beneficiation of REE from Heavy Mineral Sands: The Case of Nea Peramos, Kavala, Northern Greece. Minerals 2020, 10, 387. [Google Scholar] [CrossRef]
- Kromberg, P.; Schenk, P.F. Nikisiani- Loutra Elevtheron Sheets. In Geological Map of Greece, Scale 1:50.000; Institute of Geology and Mineral Exploration (IGME): Athens, Greece, 1974. [Google Scholar]
- Xidas, S. Rodholivos Sheet. In Geological Map of Greece, Scale 1:50,000; Institute of Geology and Mineral Exploration (IGME): Athens, Greece, 1984. [Google Scholar]
- Kromberg, P. Kavala Sheet. In Geological Map of Greece, Scale 1:50,000; Institute of Geology and Mineral Exploration (IGME): Athens, Greece, 1973. [Google Scholar]
- Brun, J.P.; Sokoutis, D. Kinematics of the Southern Rhodope Core Complex (North Greece). Int. J. Earth Sci. 2007, 96, 1079–1099. [Google Scholar] [CrossRef]
- Kounov, A.; Wüthrich, E.; Seward, D.; Burg, J.P.; Stockli, D. Low-Temperature Constraints on the Cenozoic Thermal Evolution of the Southern Rhodope Core Complex (Northern Greece). Int. J. Earth Sci. 2015, 104, 1337–1352. [Google Scholar] [CrossRef]
- Dinter, D.A.; Macfarlane, A.; Hames, W.; Isachsen, C.; Bowring, S.; Royden, L. U-Pb and 40Ar/39Ar Geochronology of the Symvolon Granodiorite: Implications for the Thermal and Structural Evolution of the Rhodope Metamorphic Core Complex, Northeastern Greece. Tectonics 1995, 14, 886–908. [Google Scholar] [CrossRef]
- Dinter, D.A. Late Cenozoic Extension of the Alpine Collisional Orogen, Northeastern Greece: Origin of the North Aegean Basin. Bull. Geol. Soc. Am. 1998, 110, 1208–1230. [Google Scholar] [CrossRef]
- Neiva, A.M.R.; Christofides, G.; Eleftheriadis, G.; Soldatos, T. Geochemistry of Granitic Rocks and Their Minerals from the Kavala Pluton, Northern Greece. Chem. Der Erde 1996, 56, 117–142. [Google Scholar]
- Christofides, G.; Neiva, A.M.R.; Soldatos, T.; Eleftheriadis, G. Petrology of the Kavala plutonite (Eastern Macedonia, Greece). Proc. XV Congr. CBGA 1995, 489–494. [Google Scholar]
- Xydous, S.; Magganas, A.; Pomonis, P.; Kokkinakis, A. Tourmalinite veins and breccias from the Symvolon-Kavala pluton, northern Greece: Petrogenetic preliminary results. Bull. Geol. Soc. Greece 2016, 50, 2079–2087. [Google Scholar] [CrossRef][Green Version]
- Dinter, D.A.; Royden, L. Late Cenozoic Extension in Northeastern Greece: Strymon Valley Detachment System and Rhodope Metamorphic Core Complex. Geology 1993, 21, 45–48. [Google Scholar] [CrossRef]
- Kokkinakis, A. Geologie und Petrographie des Kavala-Gebietes und des Symvolongebirges in Griechisch-Ostmakedonien. Z. Dtsch. Geol. Ges. 1980, 131, 903–925. [Google Scholar]
- Kyriakopoulos, K.; Pezzino, A.; Del Moro, A. Rb-Sr geochronological, petrological and structural study of the Kavala plutonic complex (N. Greece). Bull. Geol. Soc. Greece 1989, 23, 545–560. [Google Scholar]
- Doebelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef]
- Dollase, W.A. Refinement of the Crystal Structures of Epidote, Allanite and Hancockite. Am. Mineral. 1971, 56, 447–464. [Google Scholar]
- Deer, W.A.; Howie, R.A.; Zussman, J. Rock-forming minerals. Disilicates and Ring Silicates, 2nd ed.; Longman: Harlow, UK, 1997; 629p. [Google Scholar]
- Gieré, R.; Sorensen, S.S. Allanite and Other: REE-Rich Epidote-Group Minerals. Rev. Mineral. Geochem. 2004, 56, 431–493. [Google Scholar] [CrossRef]
- Armbruster, T.; Bonazzi, P.; Akasaka, M.; Bermanec, V.; Chopin, C.; Gieré, R.; Heuss-Assbichler, S.; Liebscher, A.; Menchetti, S.; Pan, Y.; et al. Recommended Nomenclature of Epidote-Group Minerals. Eur. J. Mineral. 2006, 18, 551–567. [Google Scholar] [CrossRef]
- Thomson, T. Experiments on allanite, a new mineral from Greenland. Trans. R. Soc. Edinburg 1810, 8, 371–386. [Google Scholar]
- Franz, G.; Liebscher, A. Physical and Chemical Properties of the Epidote Minerals—An Introduction. Rev. Mineral. Geochem. 2004, 56, 1–82. [Google Scholar] [CrossRef]
- Graser, G.; Markl, G. Ca-Rich Ilvaite-Epidote-Hydrogarnet Endoskarns: A Record of Late-Magmatic Fluid Influx into the Persodic Ilímaussaq Complex, South Greenland. J. Petrol. 2008, 49, 239–265. [Google Scholar] [CrossRef]
- Ercit, T.S. The Mess That Is “Allanite”. Can. Mineral. 2002, 40, 1411–1419. [Google Scholar] [CrossRef]
- Kartashov, P.M.; Ferraris, G.; Ivaldi, G.; Sokolova, E.; McCammon, C.A. Ferriallanite-(Ce), CaCeFe3+AlFe2+(SiO4(Si2O7)O(OH), a new member of the Epidote Group: Description, X-Ray and Mössbauer Study. Can. Mineral. 2003, 41, 829–830. [Google Scholar] [CrossRef]
- Petrik, I.; Broska, I.; Lipka, J.; Siman, P. Granitoid Allanite-(Ce): Substitution Relations, Redox Conditions and REE Distributions (on an Example of I-Type Granitoids, Western Carpathians, Slovakia). Geol. Carpathica 1995, 46, 79–94. [Google Scholar]
- Mills, S.J.; Hatert, F.; Nickel, E.H.; Ferraris, G. The Standardisation of Mineral Group Hierarchies: Application to Recent Nomenclature Proposals. Eur. J. Mineral. 2009, 21, 1073–1080. [Google Scholar] [CrossRef]
- Poitrasson, F. In Situ Investigations of Allanite Hydrothermal Alteration: Examples from Calc-Alkaline and Anorogenic Granites of Corsica (Southeast France). Contrib. Mineral. Petrol. 2002, 142, 485–500. [Google Scholar] [CrossRef]
- Chen, W.T.; Zhou, M.F. Ages and Compositions of Primary and Secondary Allanite from the Lala Fe-Cu Deposit, SW China: Implications for Multiple Episodes of Hydrothermal Events. Contrib. Mineral. Petrol. 2014, 168, 1–20. [Google Scholar] [CrossRef]
- Sawka, W.N.; Chappell, B.W.; Norrish, K. Light-Rare-Earth-Element Zoning in Sphene and Allanite during Granitoid Fractionation. Geology 1984, 12, 131–134. [Google Scholar] [CrossRef]



| Sample | Location |
|---|---|
| KVL1 | Kariani |
| KVL2 | Pirgos |
| KVL3 | Mirtofito |
| KVL4 | Eleochori |
| KVL5 | Agia Marina |
| KVL6 | Nea Peramos |
| Sample | MF % | NMF % |
|---|---|---|
| KVL1 | 59.89 | 40.11 |
| KVL2 | 29.10 | 70.90 |
| KVL3 | 26.64 | 73.36 |
| KVL4 | 34.76 | 65.24 |
| KVL5 | 27.32 | 72.68 |
| KVL6 | 22.60 | 77.40 |
| KVL1 | KVL2 | KVL3 | KVL4 | KVL5 | KVL6 | |
|---|---|---|---|---|---|---|
| Allanite-(Ce) | 3 | 3 | 3 | 5 | 2 | 3 |
| Amphibole | 4 | 26 | 31 | 30 | 27 | 23 |
| Chlorite | n.d. | n.d. | 5 | n.d. | n.d. | 6 |
| Epidote | 6 | 20 | 32 | 21 | 38 | 28 |
| Garnet | 68 | 3 | traces | traces | n.d. | traces |
| Goethite | n.d. | 9 | n.d. | n.d. | n.d. | n.d. |
| Hematite | 4 | 1 | 14 | traces | n.d. | 1 |
| Ilmenite | 7 | n.d. | n.d. | 2 | n.d. | n.d. |
| K-feldspar | n.d. | 7 | 5 | 15 | 8 | 8 |
| Magnetite | 2 | n.d. | traces | n.d. | traces | n.d. |
| Mica | 6 | 5 | n.d. | n.d. | n.d. | 6 |
| Monazite-(Ce), -(La) | traces | n.d. | n.d. | n.d. | 2 | 2 |
| Plagioclase | n.d. | 16 | n.d. | 11 | 11 | 8 |
| Quartz | n.d. | 9 | 8 | 12 | 10 | 11 |
| Rutile | n.d. | traces | 1 | 2 | 2 | n.d. |
| Thorite | traces | n.d. | n.d. | n.d. | traces | n.d. |
| Titanite | traces | traces | n.d. | 2 | traces | 3 |
| Zircon | traces | 1 | 1 | traces | traces | 1 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 |
| KVL1 1.3 | KVL2 5.2 | KVL2 5.4 | KVL3 1.2 | KVL3 1.3 | KVL4 3.1 | KVL6 2.3 | KVL6 3.1 | KVL6 3.3 | KVL6 3.4 | |
|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | 30.43 | 31.63 | 32.11 | 31.73 | 32.74 | 31.87 | 30.01 | 32.21 | 31.40 | 32.48 |
| Al2O3 | 13.04 | 15.19 | 16.75 | 16.14 | 17.28 | 14.72 | 15.14 | 16.75 | 15.88 | 16.78 |
| FeO* | 15.57 | 14.22 | 13.71 | 13.61 | 14.08 | 13.52 | 14.02 | 14.09 | 13.82 | 11.97 |
| MgO | 1.88 | 1.29 | 1.31 | 0.99 | 0.81 | 1.86 | 1.27 | 1.13 | 1.03 | 1.17 |
| MnO | bdl | 0.68 | bdl | 0.45 | 0.51 | 0.92 | bdl | bdl | 1.19 | 1.03 |
| CaO | 10.82 | 12.56 | 14.32 | 13.72 | 15.22 | 12.82 | 10.85 | 14.47 | 13.27 | 14.44 |
| La2O3 | 4.43 | 6.16 | 3.84 | 5.71 | 5.56 | 6.50 | 7.12 | 5.05 | 6.99 | 5.15 |
| Ce2O3 | 12.72 | 10.23 | 8.65 | 8.85 | 8.86 | 11.44 | 13.59 | 8.07 | 9.73 | 7.15 |
| Nd2O3 | 3.91 | 1.83 | 2.66 | 1.14 | 1.50 | 1.63 | 2.52 | 1.29 | 1.67 | 1.72 |
| Eu2O3 | 2.35 | bdl | 0.34 | bdl | 0.13 | bdl | bdl | 1.42 | bdl | bdl |
| Y2O3 | bdl | 1.10 | bdl | bdl | bdl | bdl | bdl | bdl | bdl | 0.75 |
| UO3 | bdl | bdl | bdl | bdl | bdl | 1.00 | bdl | bdl | bdl | bdl |
| ThO2 | 2.00 | 2.57 | 3.19 | 3.81 | bdl | 1.00 | 1.70 | 2.30 | 1.89 | 3.02 |
| Total | 97.15 | 97.46 | 96.89 | 96.14 | 96.69 | 97.29 | 96.22 | 96.77 | 96.87 | 95.67 |
| Chemical Formulae Calculated on the Basis of 12.5 O | ||||||||||
| Si | 2.963 | 2.969 | 2.953 | 2.976 | 2.952 | 2.984 | 2.928 | 2.955 | 2.934 | 2.991 |
| Al | 0.037 | 0.031 | 0.047 | 0.024 | 0.048 | 0.016 | 0.072 | 0.045 | 0.066 | 0.009 |
| T | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 |
| Fe3 | 0.541 | 0.350 | 0.235 | 0.242 | 0.209 | 0.391 | 0.328 | 0.235 | 0.319 | 0.189 |
| Al | 0.459 | 0.650 | 0.768 | 0.760 | 0.789 | 0.609 | 0.669 | 0.766 | 0.683 | 0.813 |
| M1 | 1.000 | 1.000 | 1.003 | 1.002 | 0.998 | 1.000 | 0.997 | 1.001 | 1.002 | 1.002 |
| Al | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| M2 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| Mn2 | 0.000 | 0.054 | 0.000 | 0.036 | 0.039 | 0.073 | 0.000 | 0.000 | 0.094 | 0.081 |
| Fe2 | 0.615 | 0.531 | 0.436 | 0.514 | 0.339 | 0.339 | 0.645 | 0.426 | 0.410 | 0.388 |
| Mg | 0.273 | 0.180 | 0.180 | 0.138 | 0.108 | 0.259 | 0.184 | 0.154 | 0.144 | 0.160 |
| Fe3 | 0.112 | 0.235 | 0.384 | 0.312 | 0.514 | 0.329 | 0.171 | 0.420 | 0.352 | 0.371 |
| M3 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| M | 3.000 | 3.000 | 3.003 | 3.002 | 2.998 | 3.000 | 2.997 | 3.001 | 3.002 | 3.002 |
| Ca | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| A1 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| Ca | 0.128 | 0.263 | 0.411 | 0.379 | 0.471 | 0.287 | 0.134 | 0.422 | 0.329 | 0.425 |
| REE | 0.826 | 0.625 | 0.519 | 0.540 | 0.530 | 0.670 | 0.830 | 0.528 | 0.630 | 0.473 |
| Y | 0.000 | 0.055 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.037 |
| U | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.021 | 0.000 | 0.000 | 0.000 | 0.000 |
| Th | 0.044 | 0.055 | 0.067 | 0.081 | 0.000 | 0.021 | 0.038 | 0.048 | 0.040 | 0.063 |
| A2 | 0.998 | 0.998 | 0.997 | 1.000 | 1.001 | 0.999 | 1.002 | 0.998 | 0.999 | 0.998 |
| A | 1.998 | 1.998 | 1.997 | 2.000 | 2.001 | 1.999 | 2.002 | 1.998 | 1.999 | 1.998 |
| Σcation charge | 24.985 | 24.990 | 24.993 | 24.996 | 24.992 | 24.981 | 25.000 | 24.998 | 25.000 | 25.000 |
| Feox | 0.515 | 0.524 | 0.587 | 0.519 | 0.681 | 0.680 | 0.436 | 0.606 | 0.621 | 0.591 |
| REE-Rich Epidote | Epidote | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| KVL1 3.3 | KVL4 2.2 | KVL4 2.5 | KVL5 2.3 | KVL5 7.1 | KVL5 5.8 | KVL6 5.1 | KVL4 3.5 | KVL5 14.3 | |
| SiO2 | 33.14 | 33.44 | 34.10 | 32.14 | 32.93 | 32.27 | 33.03 | 37.20 | 36.77 |
| Al2O3 | 17.34 | 19.05 | 21.11 | 17.16 | 18.03 | 16.28 | 18.17 | 23.77 | 23.82 |
| FeO* | 13.15 | 13.19 | 12.37 | 13.40 | 12.81 | 13.63 | 13.27 | 11.48 | 12.94 |
| MgO | 0.93 | bdl | bdl | 1.08 | 0.63 | 1.13 | 0.59 | bdl | bdl |
| MnO | 0.66 | bdl | 0.53 | 0.64 | 2.42 | 0.93 | 0.55 | 0.34 | 0.98 |
| CaO | 15.83 | 15.56 | 18.67 | 14.47 | 16.67 | 14.78 | 15.84 | 23.32 | 23.51 |
| La2O3 | 3.70 | 5.17 | 2.52 | 4.81 | 0.92 | 4.00 | 2.83 | bdl | bdl |
| Ce2O3 | 7.31 | 7.05 | 3.96 | 9.70 | 3.25 | 6.85 | 6.89 | bdl | bdl |
| Nd2O3 | bdl | 1.97 | 1.22 | 1.24 | 2.43 | 3.53 | 1.84 | bdl | bdl |
| Eu2O3 | bdl | bdl | bdl | bdl | bdl | bdl | 1.24 | bdl | bdl |
| Y2O3 | bdl | bdl | bdl | bdl | 3.11 | bdl | bdl | bdl | bdl |
| UO3 | bdl | bdl | 0.87 | 0.76 | bdl | bdl | bdl | bdl | bdl |
| ThO2 | 5.08 | 1.75 | 1.01 | 2.03 | 3.28 | 3.18 | 2.96 | 0.23 | bdl |
| Total | 97.15 | 97.19 | 96.36 | 97.42 | 96.48 | 96.58 | 97.20 | 96.33 | 98.02 |
| Chemical Formulae Calculated on the Basis of 12.5 O | |||||||||
| Si | 2.990 | 2.999 | 2.932 | 2.934 | 2.909 | 2.963 | 2.963 | 2.972 | 2.924 |
| Al | 0.010 | 0.001 | 0.068 | 0.066 | 0.091 | 0.037 | 0.037 | 0.028 | 0.076 |
| T | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 |
| Fe3 | 0.168 | 0.000 | 0.000 | 0.219 | 0.209 | 0.274 | 0.117 | 0.207 | 0.207 |
| Al | 0.834 | 1.003 | 1.001 | 0.780 | 0.787 | 0.725 | 0.884 | 0.793 | 0.793 |
| M1 | 1.002 | 1.003 | 1.001 | 0.999 | 0.996 | 0.999 | 1.001 | 1.000 | 1.000 |
| Al | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| M2 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| Mn2 | 0.051 | 0.000 | 0.039 | 0.049 | 0.181 | 0.072 | 0.042 | 0.023 | 0.066 |
| Fe2 | 0.398 | 0.546 | 0.155 | 0.328 | 0.143 | 0.358 | 0.382 | 0.000 | 0.000 |
| Mg | 0.125 | 0.000 | 0.000 | 0.147 | 0.082 | 0.155 | 0.079 | 0.000 | 0.000 |
| Fe3 | 0.426 | 0.444 | 0.735 | 0.476 | 0.594 | 0.415 | 0.497 | 0.560 | 0.568 |
| Al | 0.000 | 0.010 | 0.071 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| M3 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.001 | 0.998 |
| M | 3.002 | 3.003 | 3.001 | 2.999 | 2.996 | 2.999 | 3.001 | 3.001 | 2.998 |
| Ca | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| A1 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| Ca | 0.530 | 0.496 | 0.720 | 0.416 | 0.578 | 0.454 | .523 | 0.996 | 1.003 |
| REE | 0.364 | 0.466 | 0.243 | 0.527 | 0.212 | 0.482 | 0.417 | 0.000 | 0.000 |
| Y | 0.000 | 0.000 | 0.000 | 0.000 | 0.146 | 0.000 | 0.000 | 0.000 | 0.000 |
| U | 0.000 | 0.000 | 0.017 | 0.015 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Th | 0.104 | 0.036 | 0.020 | 0.042 | 0.066 | 0.066 | 0.006 | 0.004 | 0.000 |
| A2 | 0.998 | 0.998 | 1.000 | 0.999 | 1.002 | 1.0002 | 1.000 | 1.000 | 1.003 |
| A | 1.998 | 1.998 | 2.000 | 1.999 | 2.002 | 2.002 | 2.000 | 2.000 | 2.003 |
| Σcharge cation | 24.990 | 24.996 | 24.990 | 24.981 | 24.985 | 24.993 | 25.000 | 24.960 | 24.858 |
| Feox | 0.599 | 0.448 | 0.826 | 0.679 | 0.849 | 0.658 | 0.616 | 1.000 | 1.000 |
| KVL1.1 4 | KVL1.1 5 | KVL5.7 5 | KVL1.4 1 | |
|---|---|---|---|---|
| Monazite-(Ce) | Monazite-(La) | Monazite–Cheralite | Thorite | |
| P2O5 | 31.25 | 32.26 | 25.94 | bdl |
| SiO2 | bdl | bdl | bdl | 19.87 |
| CaO | 6.07 | 5.82 | 1.81 | 0.29 |
| La2O3 | 13.08 | 20.64 | 8.27 | 1.34 |
| Ce2O3 | 21.69 | 19.49 | 7.98 | 2.48 |
| Nd2O3 | 5.92 | 15.08 | 5.98 | bdl |
| ThO2 | 21.45 | 6.33 | 49.61 | 75.95 |
| Total | 99.45 | 99.62 | 99.58 | 99.94 |
| Chemical Formulae Calculated on the Basis of 4 O | ||||
| P | 1.011 | 1.015 | 0.959 | 0.000 |
| Si | 0.000 | 0.000 | 0.000 | 1.036 |
| Ca | 0.248 | 0.232 | 0.085 | 0.016 |
| La | 0.184 | 0.283 | 0.133 | 0.026 |
| Ce | 0.303 | 0.265 | 0.128 | 0.047 |
| Nd | 0.081 | 0.200 | 0.093 | 0.000 |
| Th | 0.186 | 0.054 | 0.493 | 0.901 |
| Total | 2.014 | 2.049 | 1.891 | 2.026 |
| KVL3 3.5 | KVL3 6.7 | KVL4 1.6 | KVL4 6.6 | KVL6 2.5 | |
|---|---|---|---|---|---|
| SiO2 | 32.34 | 31.85 | 32.26 | 32.07 | 32.09 |
| ZrO2 | 67.12 | 65.33 | 65.66 | 64.63 | 64.06 |
| ThO2 | bdl | bdl | bdl | 1.93 | 1.42 |
| HfO2 | bdl | bdl | 1.73 | bdl | 1.19 |
| UO3 | bdl | 2.24 | bdl | 1.09 | 1.00 |
| Total | 99.46 | 99.42 | 99.64 | 99.71 | 99.76 |
| Chemical Formulae Calculated on the Basis of 16 O | |||||
| Si | 3.976 | 3.969 | 3.984 | 3.992 | 3.998 |
| Zr | 4.024 | 3.969 | 3.955 | 3.923 | 3.891 |
| Th | 0.000 | 0.000 | 0.000 | 0.055 | 0.040 |
| Hf | 0.000 | 0.000 | 0.061 | 0.000 | 0.042 |
| U | 0.000 | 0.062 | 0.000 | 0.030 | 0.028 |
| KVL2 1.6 | KVL2 7.1 | KVL5 2 9.1 | KVL5 10.1 | KVL5 1.5 | KVL5 4.6 | |
|---|---|---|---|---|---|---|
| SiO2 | 30.83 | 30.55 | 31.22 | 30.39 | 30.79 | 30.84 |
| TiO2 | 38.09 | 39.17 | 36.98 | 37.47 | 38.10 | 38.64 |
| Al2O3 | 1.59 | 0.92 | 2.40 | 1.84 | 1.78 | 1.42 |
| FeO | 1.61 | 1.63 | bdl | 4.50 | 1.66 | 1.47 |
| MnO | bdl | 0.52 | 0.27 | bdl | bdl | bdl |
| MgO | bdl | bdl | bdl | bdl | bdl | 0.29 |
| CaO | 26.28 | 25.87 | 28.82 | 24.14 | 26.42 | 26.62 |
| V2O5 | 0.62 | bdl | bdl | bdl | 0.57 | bdl |
| Nb2O5 | 0.66 | 0.87 | bdl | 1.03 | bdl | 0.55 |
| Total | 99.68 | 99.52 | 99.69 | 99.37 | 99.88 | 99.82 |
| Chemical Formulae Calculated on the Basis of 4 O | ||||||
| Si | 4.000 | 4.000 | 4.000 | 4.000 | 4.000 | 4.000 |
| Ti | 3.716 | 3.856 | 3.563 | 3.708 | 3.722 | 3.768 |
| Al | 0.243 | 0.142 | 0.362 | 0.285 | 0.273 | 0.216 |
| Y | 3.959 | 3.998 | 3.925 | 3.994 | 3.995 | 3.985 |
| Fe | 0.174 | 0.178 | 0.000 | 0.496 | 0.180 | 0.159 |
| Mn | 0.000 | 0.058 | 0.030 | 0.000 | 0.000 | 0.000 |
| Mg | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.056 |
| Ca | 3.654 | 3.629 | 3.956 | 3.404 | 3.677 | 3.698 |
| V | 0.065 | 0.000 | 0.000 | 0.108 | 0.060 | 0.000 |
| Nb | 0.106 | 0.140 | 0.000 | 0.000 | 0.090 | 0.088 |
| X | 3.999 | 4.004 | 3.986 | 4.008 | 4.007 | 4.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peristeridou, E.; Melfos, V.; Papadopoulou, L.; Kantiranis, N.; Voudouris, P. Mineralogy and Mineral Chemistry of the REE-Rich Black Sands in Beaches of the Kavala District, Northern Greece. Geosciences 2022, 12, 277. https://doi.org/10.3390/geosciences12070277
Peristeridou E, Melfos V, Papadopoulou L, Kantiranis N, Voudouris P. Mineralogy and Mineral Chemistry of the REE-Rich Black Sands in Beaches of the Kavala District, Northern Greece. Geosciences. 2022; 12(7):277. https://doi.org/10.3390/geosciences12070277
Chicago/Turabian StylePeristeridou, Eftychia, Vasilios Melfos, Lambrini Papadopoulou, Nikolaos Kantiranis, and Panagiotis Voudouris. 2022. "Mineralogy and Mineral Chemistry of the REE-Rich Black Sands in Beaches of the Kavala District, Northern Greece" Geosciences 12, no. 7: 277. https://doi.org/10.3390/geosciences12070277
APA StylePeristeridou, E., Melfos, V., Papadopoulou, L., Kantiranis, N., & Voudouris, P. (2022). Mineralogy and Mineral Chemistry of the REE-Rich Black Sands in Beaches of the Kavala District, Northern Greece. Geosciences, 12(7), 277. https://doi.org/10.3390/geosciences12070277









