Palaeoecological Implications of Lower-Middle Triassic Stromatolites and Microbe-Metazoan Build-Ups in the Germanic Basin: Insights into the Aftermath of the Permian–Triassic Crisis
Abstract
1. Introduction
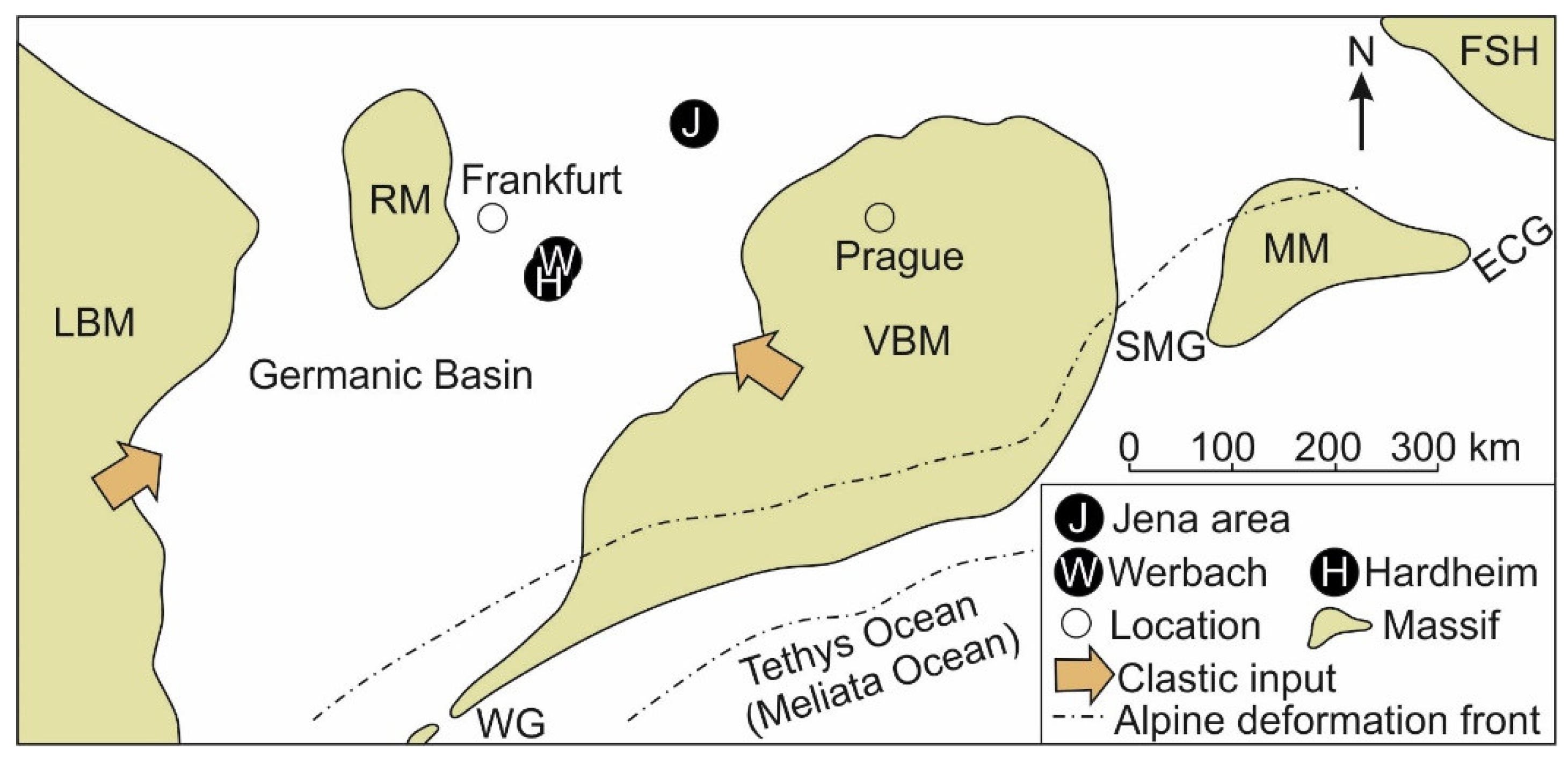
2. Geological Setting
3. Materials and Methods
3.1. Fieldwork and Petrography
3.2. Analytical Imaging Techniques
3.3. Stable Isotope Analyses (δ13Ccarb, δ18Ocarb)
4. Results
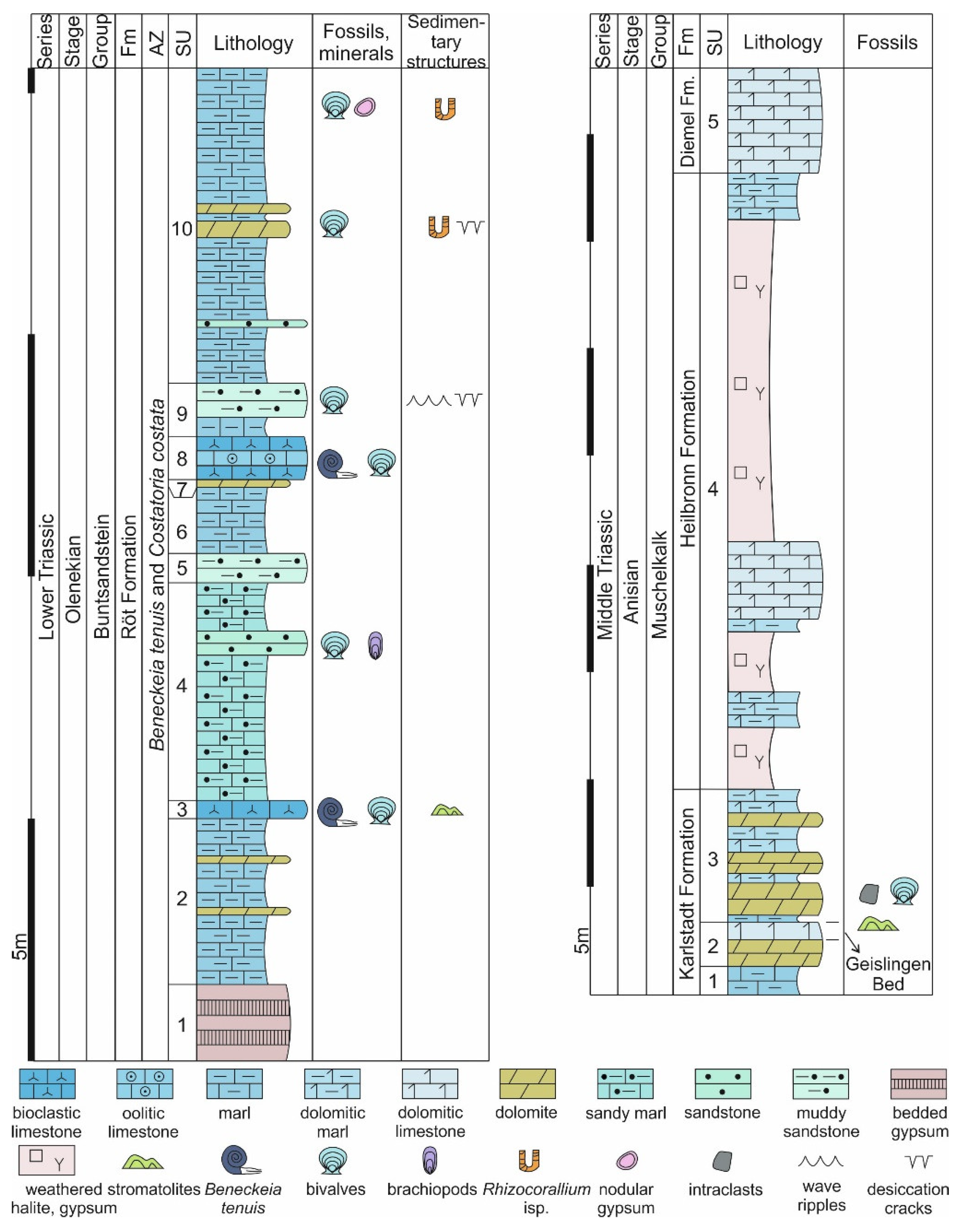
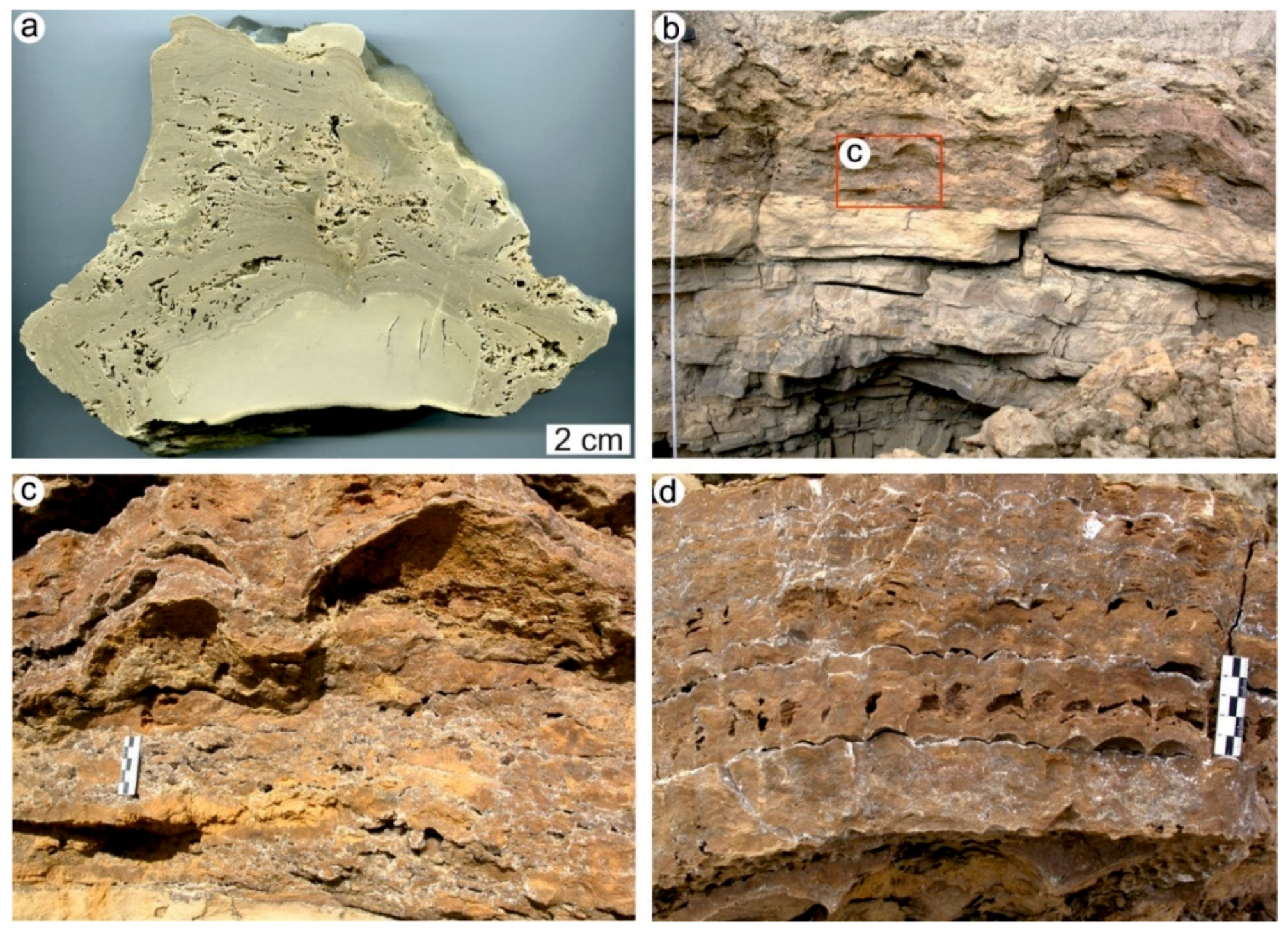
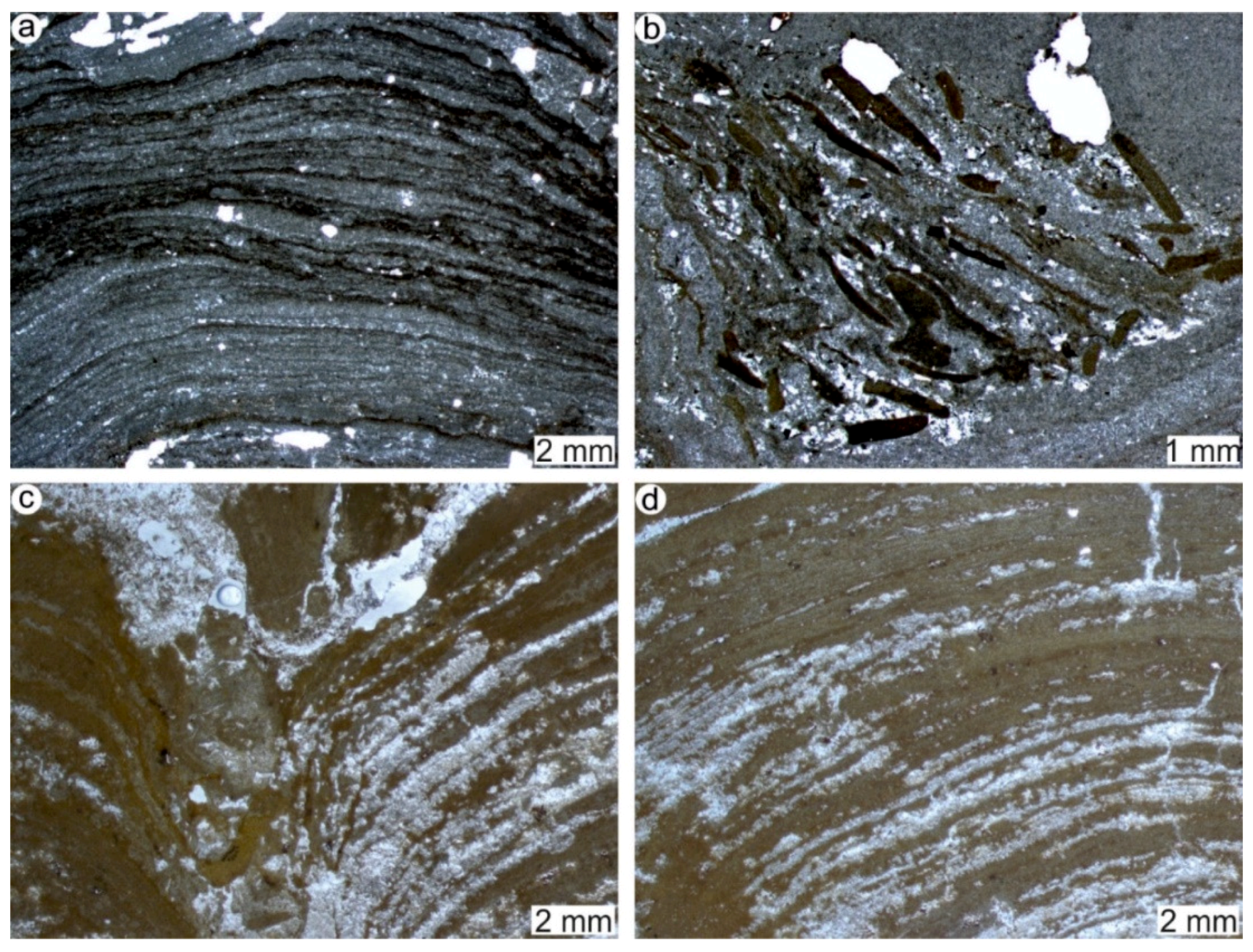
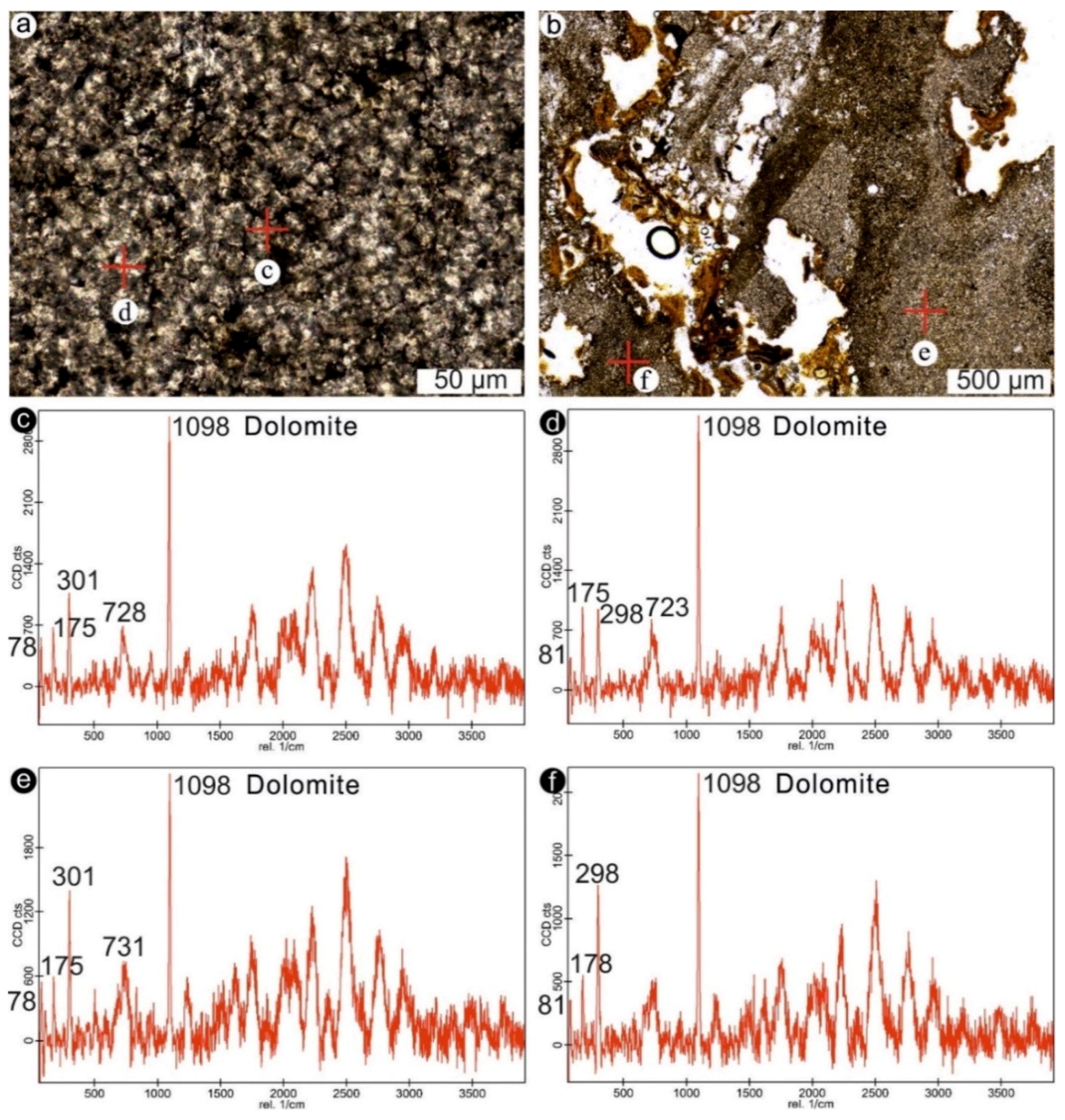
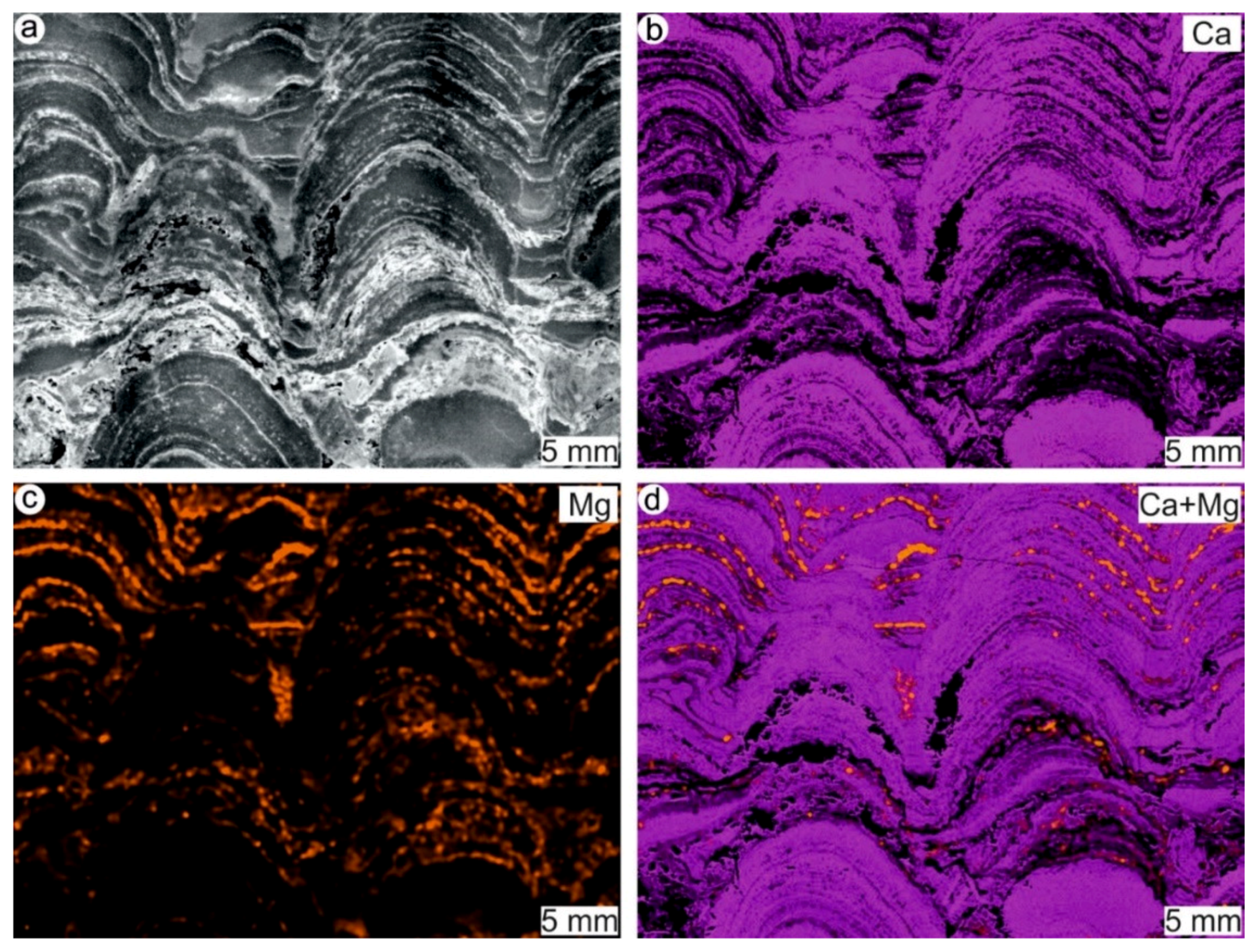
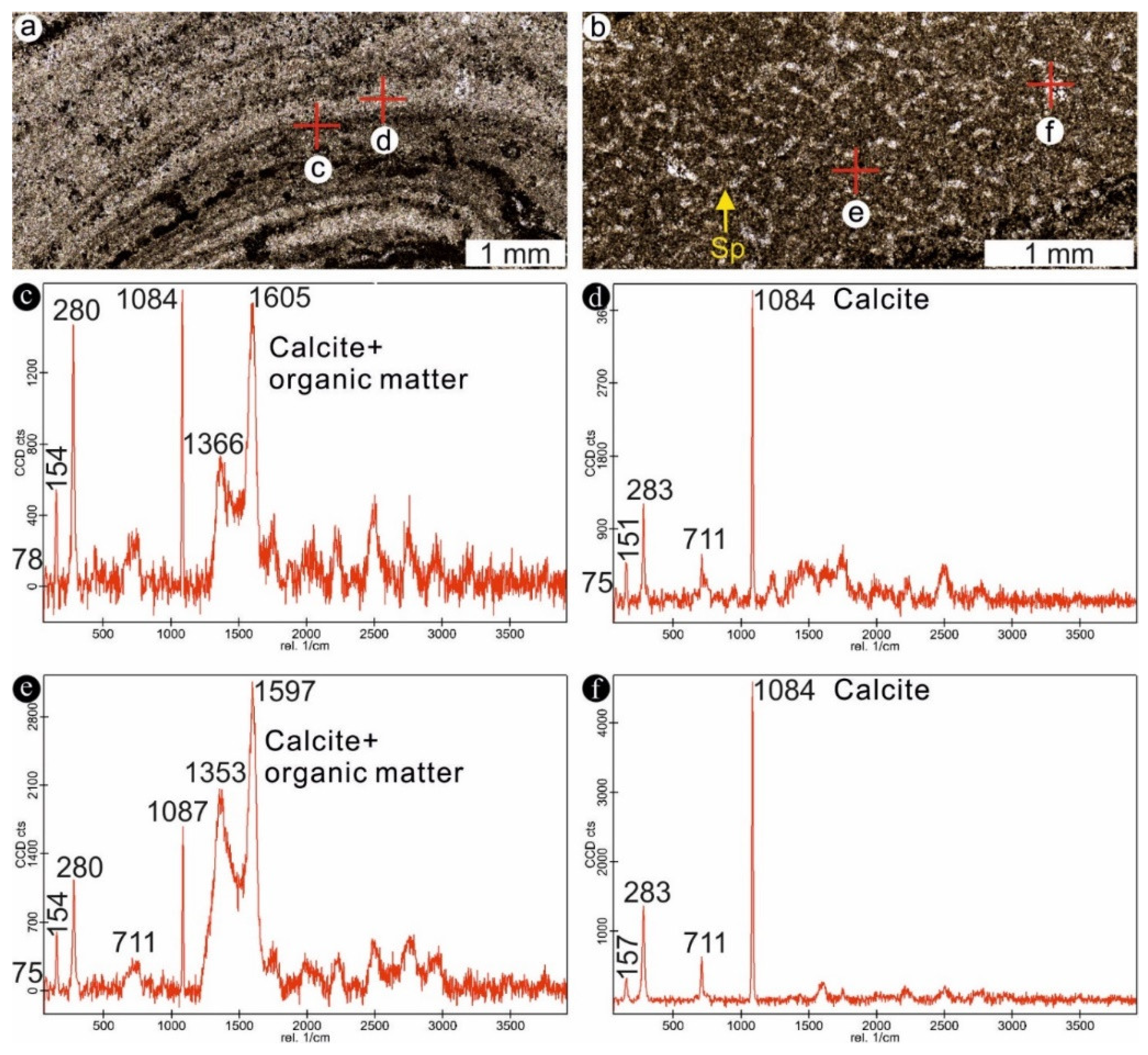
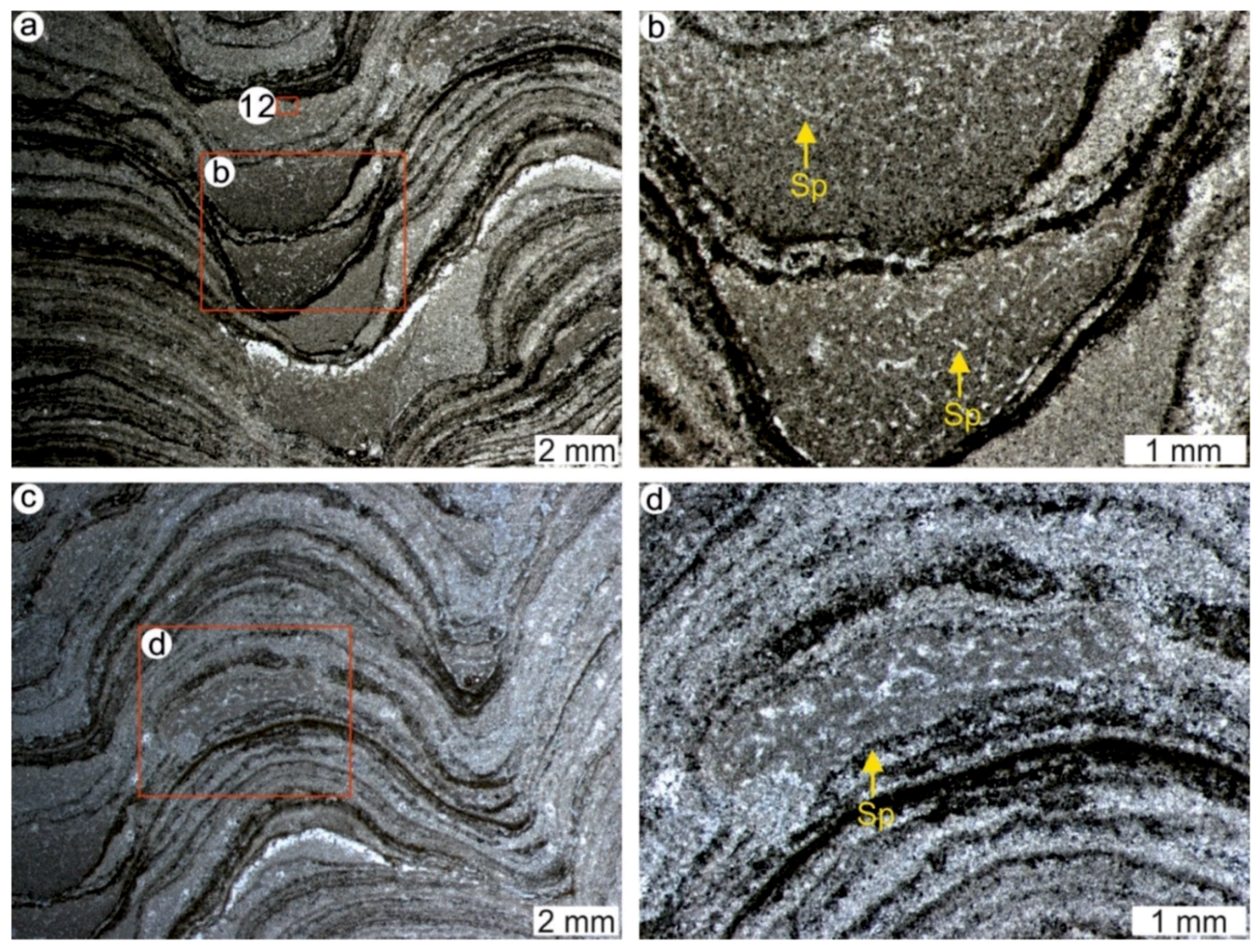
4.1. Stromatolites from the Jena Area (Upper Buntsandstein, Olenekian, Lower Triassic)
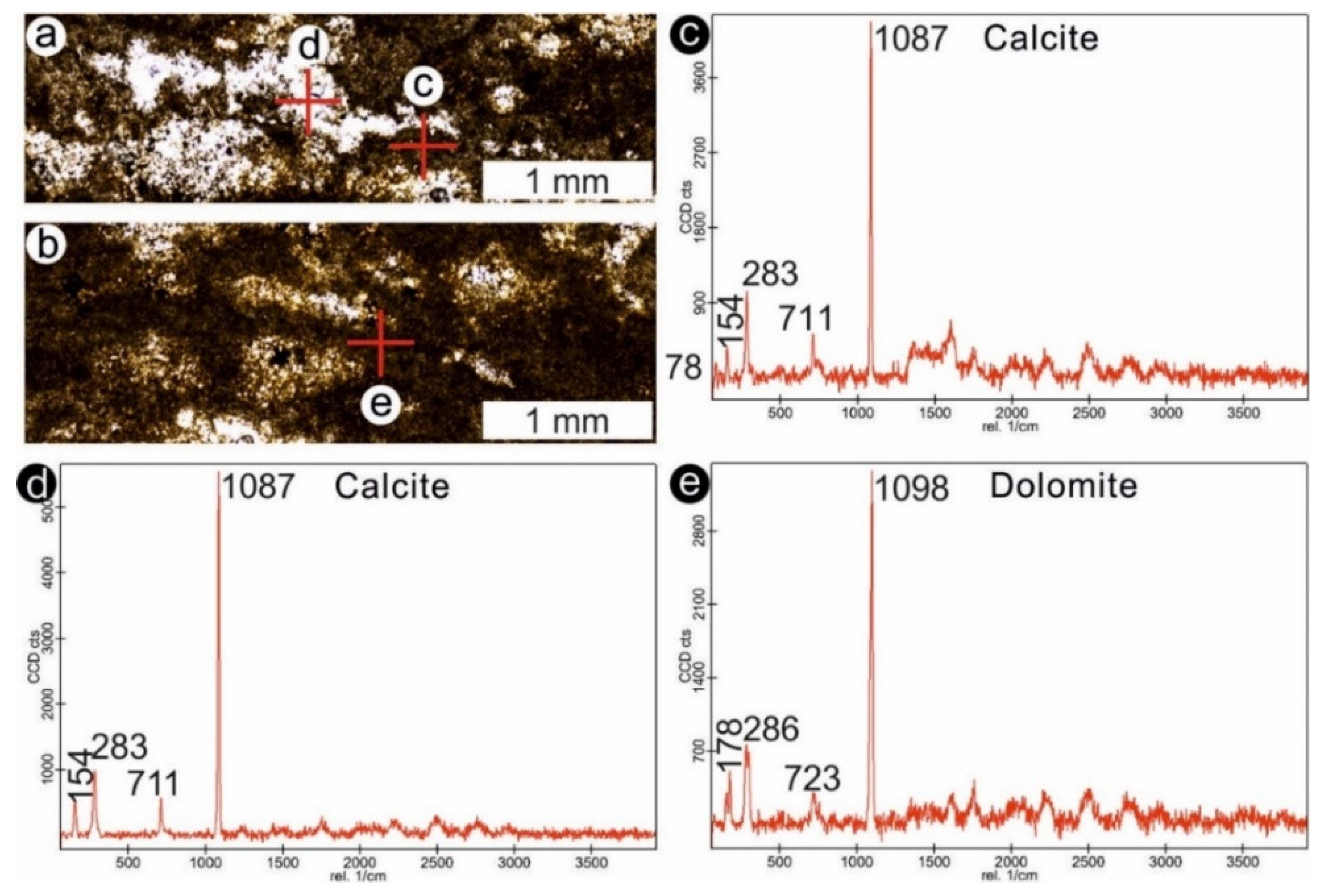
4.2. Stromatolites from Werbach (Middle Muschelkalk, Anisian, Middle Triassic)
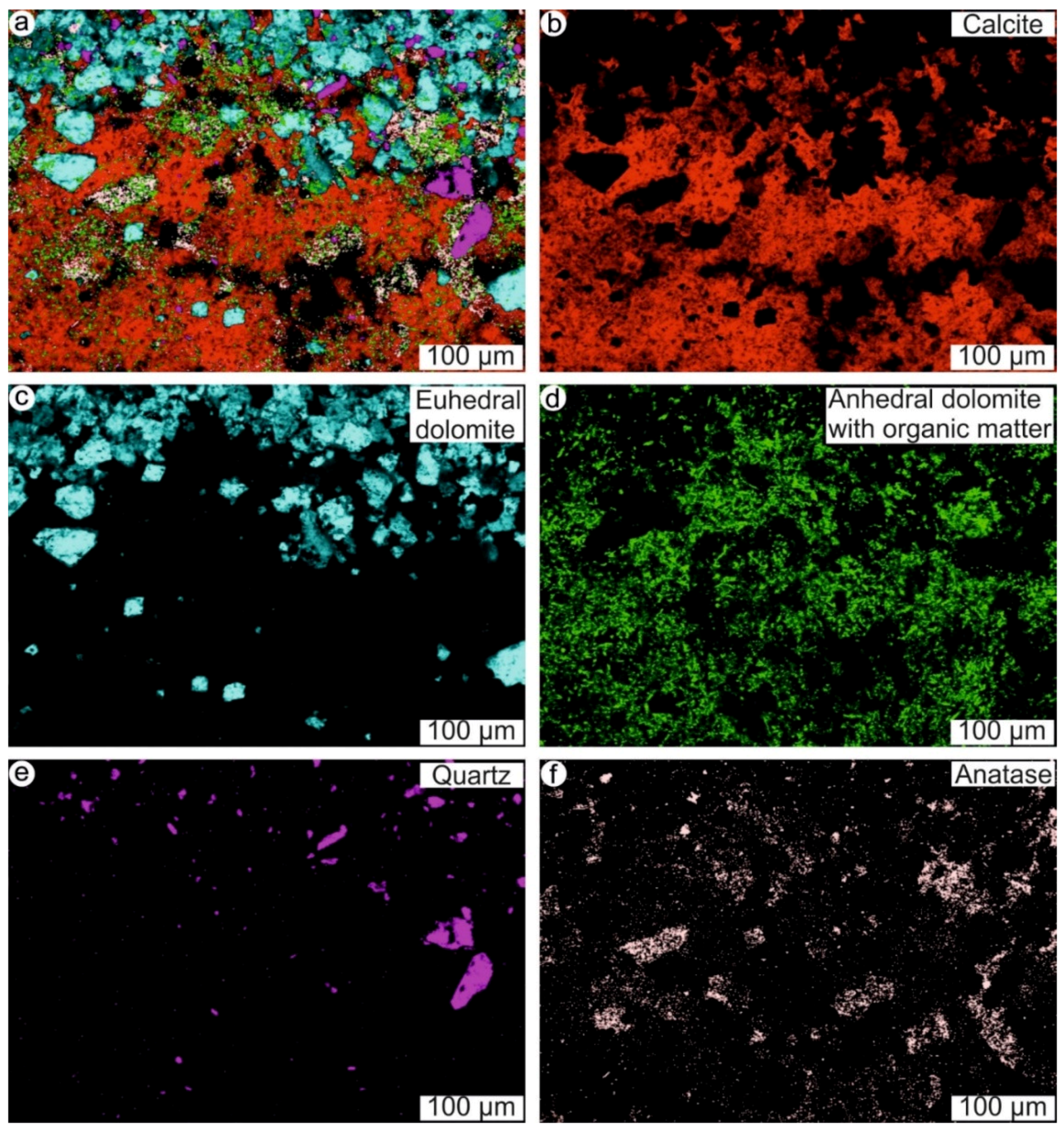
4.3. Microbe-Metazoan Build-Ups from Hardheim (Middle Muschelkalk, Anisian, Middle Triassic)
4.4. Carbon and Oxygen Stable Isotopes (δ13Ccarb, δ18Ocarb)
5. Discussion
5.1. Sedimentary Environments
5.2. Stromatolites vs. Microbe-Metazoan Build-Ups
5.3. Palaeoecological Implications of the Microbialites
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burne, R.V.; Moore, L.S. Microbialites: Organosedimentrary deposits of benthic microbial communities. Palaios 1987, 2, 241–254. [Google Scholar] [CrossRef]
- Arp, G.; Reimer, A.; Reitner, J. Photosynthesis-induced biofilm calcification and calcium concentrations in Phanerozoic oceans. Science 2001, 292, 1701–1704. [Google Scholar] [CrossRef] [PubMed]
- Riding, R.; Liang, L. Geobiology of microbial carbonates: Metazoan and seawater saturation state influences on secular trends during the Phanerozoic. Palaeogeogr. Palaeoclim. Palaeoecol. 2005, 219, 101–115. [Google Scholar] [CrossRef]
- Riding, R. Microbial carbonate abundance compared with fluctuations in metazoan diversity over geological time. Sediment. Geol. 2006, 185, 229–238. [Google Scholar] [CrossRef]
- Walter, M.R.; Buick, R.; Dunlop, J.S.R. Stromatolites 3400–3500 Myr old from the North Pole area, Western Australia. Nature 1980, 284, 443–445. [Google Scholar] [CrossRef]
- Riding, R. The nature of stromatolites: 3500 million years of history and a century of research. In Advances in Stromatolite Geobiology; Reitner, J., Quéric, N.V., Arp, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 29–74. [Google Scholar]
- Duda, J.-P.; Van Kranendonk, M.J.; Thiel, V.; Ionescu, D.; Strauss, H.; Schäfer, N.; Reitner, J. A Rare glimpse of Paleo-archean life: Geobiology of an exceptionally preserved microbial mat facies from the 3.4 Ga Strelley Pool Formation, Western Australia. PLoS ONE 2016, 11, e0147629. [Google Scholar] [CrossRef]
- Pratt, B.R. Stromatolite decline—A reconsideration. Geology 1982, 10, 512–515. [Google Scholar] [CrossRef]
- Kershaw, S.; Zhang, T.; Lan, G. A microbialite crust at the Permian–Triassic boundary in South China, and its palaeoenvironmental significance. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1999, 146, 1–18. [Google Scholar] [CrossRef]
- Kershaw, S.; Crasquin, S.; Li, Y.; Collin, P.-Y.; Forel, M.-B.; Mu, X.; Baud, A.; Wang, Y.; Xie, S.; Maurer, F.; et al. Microbialites and global environmental change across the Permian-Triassic boundary: A synthesis. Geobiology 2012, 10, 25–47. [Google Scholar] [CrossRef]
- Kershaw, S.; Tang, H.; Li, Y.; Guo, L. Oxygenation in carbonate microbialites and associated facies after the end-Permian mass extinction: Problems and potential solutions. J. Palaeogeogr. 2018, 7, 32–47. [Google Scholar] [CrossRef]
- Lehrmann, D.J. Early Triassic calcimicrobial mounds and biostromes of the Nanpanjiang basin, south China. Geology 1999, 27, 359–362. [Google Scholar] [CrossRef]
- Woods, A.D. Assessing Early Triassic paleoceanographic conditions via unusual sedimentary fabrics and features. Earth Sci. Rev. 2014, 137, 6–18. [Google Scholar] [CrossRef]
- Wu, Y.S.; Yu, G.L.; Li, R.H.; Song, L.R.; Jiang, H.X.; Riding, R.; Liu, L.J.; Liu, N.Y.; Zhao, R. Cyanobacterial fossils from 252 Ma old microbialites and their environmental significance. Sci. Rep. 2014, 4, 3820. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Chen, Z.-Q.; Fang, Y.; Pei, Y.; Yang, H.; Ogg, J. A Permian-Triassic boundary microbialite deposit from the eastern Yangtze Platform (Jiangxi Province, South China): Geobiologic features, ecosystem composition and redox conditions. Palaeogeogr. Palaeoclim. Palaeoecol. 2017, 486, 58–73. [Google Scholar] [CrossRef]
- Heindel, K.; Foster, W.J.; Richoz, S.; Birgel, D.; Roden, V.J.; Baud, A.; Brandner, R.; Krystyn, L.; Mohtat, T.; Koşun, E.; et al. The formation of microbial-metazoan bioherms and biostromes following the latest Permian mass extinction. Gondwana Res. 2018, 61, 187–202. [Google Scholar] [CrossRef]
- Chen, Z.-Q.; Tu, C.; Pei, Y.; Ogg, J.; Fang, Y.; Wu, S.; Feng, X.; Huang, Y.; Guo, Z.; Yang, H. Biosedimentological features of major microbe-metazoan transitions (MMTs) from Precambrian to Cenozoic. Earth Sci. Rev. 2019, 189, 21–50. [Google Scholar] [CrossRef]
- Pei, Y.; Chen, Z.-Q.; Fang, Y.; Kershaw, S.; Wu, S.; Luo, M. Volcanism, redox conditions, and microbialite growth linked with the end-Permian mass extinction: Evidence from the Xiajiacao section (western Hubei Province), South China. Palaeogeogr. Palaeoclim. Palaeoecol. 2019, 519, 194–208. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Li, Y.; Wang, G.; Yang, H.-Q. Different accretion and diagenetic patterns within the fabrics of the Permian–Triassic boundary microbialites on the Leye isolated carbonate platform, South China Block. J. Palaeogeogr. 2021, 10, 1–12. [Google Scholar] [CrossRef]
- Foster, W.J.; Heindel, K.; Richoz, S.; Gliwa, J.; Lehrmann, D.J.; Baud, A.; Kolar-Jurkovšek, T.; Aljinović, D.; Jurkovšek, B.; Korn, D.; et al. Suppressed competitive exclusion enabled the proliferation of Permian/Triassic boundary microbialites. Depos. Rec. 2020, 6, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Kalkowsky, E. Oolith und Stromatolith im norddeutschen Buntsandstein. Z. Dtsch. Geol. Ges. 1908, 60, 68–125. [Google Scholar]
- Aitken, J.D. Middle Cambrian to Middle Ordovician cyclic sedimentation, southern Rocky Mountains of Alberta. Bull. Can. Pet. Geol. 1966, 14, 405–441. [Google Scholar]
- Riding, R. (Ed.) Classification of microbial carbonates. In Calcareous Algae and Stromatolites; Springer: Berlin/Heidelberg, Germany, 1991; pp. 21–51. [Google Scholar]
- Braga, J.; Martín, J.; Riding, R. Controls on Microbial Dome Fabric Development along a Carbonate-Siliciclastic Shelf-Basin Transect, Miocene, SE Spain. Palaios 1995, 10, 347. [Google Scholar] [CrossRef]
- Walter, M.R. Stromatolites and the biostratigraphy of the Australian Precambrian and Cambrian. Spec. Pap. Palaeontol. 1972, 11, 1–256. [Google Scholar]
- Dravis, J.J. Hardened Subtidal Stromatolites, Bahamas. Science 1983, 219, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Arp, G.; Thiel, V.; Reimer, A.; Michaelis, W.; Reitner, J. Biofilm exopolymers control microbialite formation at thermal springs discharging into the alkaline Pyramid Lake, Nevada, USA. Sediment. Geol. 1999, 126, 159–176. [Google Scholar] [CrossRef]
- Bosak, T.; Liang, B.; Wu, T.-D.; Templer, S.P.; Evans, A.; Vali, H.; Guerquin-Kern, J.-L.; Klepac-Ceraj, V.; Sim, M.S.; Mui, J. Cyanobacterial diversity and activity in modern conical microbialites. Geobiology 2012, 10, 384–401. [Google Scholar] [CrossRef] [PubMed]
- Playford, P.; Cockbain, A.; Druce, E.; Wray, J. Chapter 10.4: Devonian Stromatolites from the Canning Basin, Western Australia. Dev. Sedimentol. 1976, 20, 543–563. [Google Scholar]
- Böhm, F.; Brachert, T.C. Deep-water stromatolites and, Frutexites Maslov from the early and Middle Jurassic of S-Germany and Austria. Facies 1993, 28, 145–168. [Google Scholar] [CrossRef]
- Heim, C.; Simon, K.; Ionescu, D.; Reimer, A.; De Beer, D.; Quéric, N.-V.; Reitner, J.; Thiel, V. Assessing the utility of trace and rare earth elements as biosignatures in microbial iron oxyhydroxides. Front. Earth Sci. 2015, 3, 1–15. [Google Scholar] [CrossRef][Green Version]
- Heim, C.; Quéric, N.-V.; Ionescu, D.; Schäfer, N.; Reitner, J. Frutexites-like structures formed by iron oxidizing biofilms in the continental subsurface (Äspö Hard Rock Laboratory, Sweden). PLoS ONE 2017, 12, e0177542. [Google Scholar] [CrossRef] [PubMed]
- Mißbach, H.; Duda, J.-P.; van den Kerkhof, A.M.; Lüders, V.; Pack, A.; Reitner, J.; Thiel, V. Ingredients for microbial life preserved in 3.5 billion-year-old fluid inclusions. Nat. Commun. 2021, 12, 1101. [Google Scholar] [CrossRef]
- Reitner, J.; Peckmann, J.; Blumenberg, M.; Michaelis, W.; Reimer, A.; Thiel, V. Concretionary methane-seep carbonates and associated microbial communities in Black Sea sediments. Palaeogeogr. Palaeoclim. Palaeoecol. 2005, 227, 18–30. [Google Scholar] [CrossRef]
- Reitner, J.; Peckmann, J.; Reimer, A.; Schumann, G.; Thiel, V. Methane-derived carbonate build-ups and associated microbial communities at cold seeps on the lower Crimean shelf (Black Sea). Facies 2005, 51, 66–79. [Google Scholar] [CrossRef]
- Arp, G.; Ostertag-Henning, C.; Yücekent, S.; Reitner, J.; Thiel, V. Methane-related microbial gypsum calcitization in stromatolites of a marine evaporative setting (Münder Formation, Upper Jurassic, Hils Syncline, north Germany). Sedimentology 2008, 55, 1227–1251. [Google Scholar] [CrossRef]
- Reitner, J. Architecture of archaeal-dominated microbial mats from cold seeps in the Black Sea (Dnjepr Canyon, Lower Crimean Shelf). In Microbial Mats: Modern and Ancient Microorganisms in Stratified Systems, Cellular Origin, Life in Extreme Habitats and Astrobiology; Seckbach, J., Oren, A., Eds.; Springer: Dordrecht, The Netherlands, 2010; Volume 14, pp. 207–220. [Google Scholar]
- Luo, C.; Reitner, J. First report of fossil “keratose” demosponges in Phanerozoic carbonates: Preservation and 3-D reconstruction. Naturwissenschaften 2014, 101, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Reitner, J. ‘Stromatolites’ built by sponges and microbes—A new type of Phanerozoic bioconstruction. Lethaia 2016, 49, 555–570. [Google Scholar] [CrossRef]
- Pei, Y.; Duda, J.; Schönig, J.; Luo, C.; Reitner, J. Late Anisian microbe-metazoan build-ups in the Germanic Basin: Aftermath of the Permian–Triassic crisis. Lethaia 2021, 54, 823–844. [Google Scholar] [CrossRef]
- Reitner, J.; Hühne, C.; Thiel, V. Porifera-rich mud mounds and microbialites as indicators of environmental changes within the Devonian/Lower Carboniferous critical interval. Terra Nostra 2001, 4, 60–65. [Google Scholar]
- Rodríguez-Martínez, M.; Sánchez, F.; Walliser, E.O.; Reitner, J. An Upper Turonian fine-grained shallow marine stromatolite bed from the Muñecas Formation, Northern Iberian Ranges, Spain. Sediment. Geol. 2012, 263–264, 96–108. [Google Scholar] [CrossRef]
- Szulc, J. Middle Triassic (Muschelkalk) sponge-microbial stromatolites, diplopores and Girvanella-oncoids from the Silesian Cracow Upland. In Proceedings of the 3rd IFAA Regional Symposium and IGCP 380 International Meeting, Kraków, Poland, 14–20 September 1997; pp. 10–15. [Google Scholar]
- Brayard, A.; Vennin, E.; Olivier, N.; Bylund, K.G.; Jenks, J.F.; Stephen, D.A.; Bucher, H.; Hofmann, R.; Goudemand, N.; Escarguel, G. Transient metazoan reefs in the aftermath of the end-Permian mass extinction. Nat. Geosci. 2011, 4, 693–697. [Google Scholar] [CrossRef]
- Marenco, P.J.; Griffin, J.M.; Fraiser, M.L.; Clapham, M.E. Paleoecology and geochemistry of Early Triassic (Spathian) microbial mounds and implications for anoxia following the end-Permian mass extinction. Geology 2012, 40, 715–718. [Google Scholar] [CrossRef]
- Vennin, E.; Olivier, N.; Brayard, A.; Bour, I.; Thomazo, C.; Escarguel, G.; Fara, E.; Bylund, K.G.; Jenks, J.F.; Stephen, D.A.; et al. Microbial deposits in the aftermath of the end-Permian mass extinction: A diverging case from the Mineral Mountains (Utah, USA). Sedimentology 2015, 62, 753–792. [Google Scholar] [CrossRef]
- Luo, C. “Keratose” Sponge Fossils and Microbialites: A Geobiological Contribution to the Understanding of Metazoan Origin. Ph.D. Thesis, Georg-August-Universität Göttingen, Göttingen, Germany, 2015. [Google Scholar]
- Friesenbichler, E.; Richoz, S.; Baud, A.; Krystyn, L.; Sahakyan, L.; Vardanyan, S.; Peckmann, J.; Reitner, J.; Heindel, K. Sponge-microbial build-ups from the lowermost Triassic Chanakhchi section in southern Armenia: Microfacies and stable carbon isotopes. Palaeogeogr. Palaeoclim. Palaeoecol. 2018, 490, 653–672. [Google Scholar] [CrossRef]
- Baud, A.; Richoz, S.; Brandner, R.; Krystyn, L.; Heindel, K.; Mohtat, T.; Mohtat-Aghai, P.; Horacek, M. Sponge Takeover from End-Permian Mass Extinction to Early Induan Time: Records in Central Iran Microbial Buildups. Front. Earth Sci. 2021, 9, 586210. [Google Scholar] [CrossRef]
- Lee, J.-H.; Riding, R. The ‘classic stromatolite’ Cryptozoön is a keratose sponge-microbial consortium. Geobiology 2021, 19, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.; Hong, J.; Lee, J.-H. Keratose sponge–microbial consortia in stromatolite-like columns and thrombolite-like mounds of the Lower Ordovician (Tremadocian) Mungok Formation, Yeongwol, Korea. Palaeogeogr. Palaeoclim. Palaeoecol. 2021, 572, 110409. [Google Scholar] [CrossRef]
- Turner, E.C. Possible poriferan body fossils in early Neoproterozoic microbial reefs. Nature 2021, 596, 87–91. [Google Scholar] [CrossRef]
- Paul, J.; Peryt, T.M.; Burne, R.V. Kalkowsky’s stromatolites and oolites (Lower Buntsandstein, Northern Germany). In Advances in Stromatolite Geobiology; Reitner, J., Quéric, N.-V., Arp, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 13–28. [Google Scholar]
- Naumann, E. Erläuterungen zur Geologischen Karte von Preußen und Benachbarten Deutschen Ländern: Blatt Jena, Gradabteilung 71, Blatt 2, 65 S; Preußische Geologische Landesanstalt: Berlin, Germany, 1928. [Google Scholar]
- Genser, C. Zur Stratigraphie und Chemie des Mittleren Muschelkalks in Franken: Geologisch und Paläontologische Abhandlungen, Neue Folge 17; Verlag Gustav Fischer: Jena, Germany, 1930; p. 111. [Google Scholar]
- Schwarz, H.-U. Zur Sedimentologie und Fazies des Unteren Muschelkalkes in Südwestdeutschland und angrenzenden Gebieten. Ph.D. Thesis, Eberhard-Karls-Universität Tübingen, Tübingen, Germany, 1970, unpublished. [Google Scholar]
- Vossmerbäumer, H. Organosedimentäre Gefüge im oberen Wellenkalk (mu3, Trias) Frankens. Neues Jahrb. Geol. Paläontol. Mon. 1971, 437–448. [Google Scholar]
- Hagdorn, H.; Simon, T. Rinnenbildung und Emersion in den Basisschichten des Mittleren Muschelkalks von Eberstadt (Nordbaden). Neues Jahrb. Geol. Paläontol. 1993, 189, 119–145. [Google Scholar]
- Krause, T.; Weller, H. Aufbau und palökologische Bedeutung der Stromatolithe im Übergang vom Mittleren zum Oberen Muschelkalk der Thüringer Mulde. Beitr. Geol. Thüring. 2000, 7, 147–193. [Google Scholar]
- Bachmann, G.H.; Gwinner, M.P. Algen-Stromatolithen von der Grenze Unterer/Mittlerer Keuper (Obere Trias) bei Schwäbisch Hall (Nordwürttemberg, Deutschland). Neues Jahrbuch für Geologie und Paläontologie, Monatshefte; Geologisch-Paläontologisches Institut der Technischen Hochschule Stuttgart: Stuttgart, Germany, 1971; pp. 594–604. [Google Scholar]
- Bachmann, G.H. A lamellibranch-stromatolite bioherm in the Lower Keuper (Ladinian, Middle Triassic), South Germany. Facies 2002, 46, 83–88. [Google Scholar] [CrossRef]
- Scotese, C.; Mc Kerrow, W.S. Revised World maps and introduction. Geol. Soc. Lond. Mem. 1990, 12, 1–21. [Google Scholar] [CrossRef]
- Ziegler, P.A. Geological Atlas of Western and Central Europe; Geological Society Publishing House: Bath, UK, 1990; pp. 1–239. [Google Scholar]
- Stampfli, G.M. Tethyan oceans. In Tectonics and Magmatism in Turkey and the Surrounding Area; Bozkurt, E., Winchester, J.A., Piper, J.D.A., Eds.; Special Publications 173; Geological Society: London, UK, 2000; pp. 1–23. [Google Scholar]
- Hagdorn, H. Paläobiogeographie des Mitteleuropäischen Beckens in der Frühen und Mittleren Trias und Faunenimmigration ins Muschelkalkmeer. Schr. Dtsch. Ges. Geowiss. 2020, 91, 111–123. [Google Scholar]
- Simon, T.; Hagdorn, H.; Dittrich, D.; Röhling, S.; Voigt, T. Lithostratigraphie der Mittlerer-Muschelkalk-Subgruppe. Schr. Dtsch. Ges. Geowiss. 2020, 91, 441–466. [Google Scholar]
- Bishop, J.W.; Osleger, D.A.; Montanez, I.; Sumner, D. Meteoric diagenesis and fluid-rock interaction in the Middle Permian Capitan backreef: Yates Formation, Slaughter Canyon, New Mexico. AAPG Bull. 2014, 98, 1495–1519. [Google Scholar] [CrossRef][Green Version]
- Craig, H. Isotopic Variations in Meteoric Waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef] [PubMed]
- Hauck, T.E.; Corlett, H.J.; Grobe, M.; Walton, E.L.; Sansjofre, P. Meteoric diagenesis and dedolomite fabrics in precursor primary dolomicrite in a mixed carbonate-evaporite system. Sedimentology 2018, 65, 1827–1858. [Google Scholar] [CrossRef]
- Awramik, S.; Margulis, L.; Barghoorn, E. Chapter 4.4 Evolutionary Processes in the Formation of Stromatolites. Dev. Sedimentol. 1976, 20, 149–162. [Google Scholar]
- Reitner, J. Microbial mats. In Encyclopedia of Geobiology; Reitner, J., Thiel, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 606–608. [Google Scholar]
- Suarez-Gonzalez, P.; Reitner, J. Ooids forming in situ within microbial mats (Kiritimati atoll, central Pacific). PalZ 2021, 95, 809–821. [Google Scholar] [CrossRef]
- Suarez-Gonzalez, P.; Benito, M.; Quijada, I.; Mas, R.; Campos-Soto, S. ‘Trapping and binding’: A review of the factors controlling the development of fossil agglutinated microbialites and their distribution in space and time. Earth Sci. Rev. 2019, 194, 182–215. [Google Scholar] [CrossRef]
- Olivier, N.; Brayard, A.; Vennin, E.; Escarguel, G.; Fara, E.; Bylund, K.G.; Jenks, J.F.; Caravaca, G.; Stephen, D.A. Evo-lution of depositional settings in the Torrey area during the Smithian (Early Triassic, Utah, USA) and their significance for the biotic recovery. Geol. J. 2016, 51, 600–626. [Google Scholar] [CrossRef]
- Olivier, N.; Fara, E.; Vennin, E.; Bylund, K.G.; Jenks, J.F.; Escarguel, G.; Stephen, D.A.; Goudemand, N.; Snyder, D.; Thomazo, C.; et al. Late Smithian microbial deposits and their lateral marine fossiliferous limestones (Early Triassic, Hurricane Cliffs, Utah, USA). Facies 2018, 64, 13. [Google Scholar] [CrossRef]
- Adachi, N.; Asada, Y.; Ezaki, Y.; Liu, J. Stromatolites near the Permian–Triassic boundary in Chongyang, Hubei Province, South China: A geobiological window into palaeo-oceanic fluctuations following the end-Permian extinction. Palaeogeogr. Palaeoclim. Palaeoecol. 2017, 475, 55–69. [Google Scholar] [CrossRef]
- Murray, J.W.; Wright, C.A. The Carboniferous Limestone of Chipping Sodbury and Wick, Gloucestershire. Geol. J. 1971, 7, 255–270. [Google Scholar] [CrossRef]
- Reitner, J. Modern cryptic microbialite/metazoan facies from Lizard Island (Great Barrier Reef, Australia) formation and concepts. Facies 1993, 29, 3–39. [Google Scholar] [CrossRef]
- Reitner, J.; Gautret, P.; Marin, F.; Neuweiler, F. Automicrite in a modern marine microbialite: Formation model via organic matrices (Lizard Island, Great Barrier Reef, Australia). Bull. Inst. Océanogr. 1995, 4, 237–263. [Google Scholar]
- Preto, N.; Breda, A.; Corso, J.D.; Spötl, C.; Zorzi, F.; Frisia, S. Primary dolomite in the Late Triassic Travenanzes Formation, Dolomites, Northern Italy: Facies control and possible bacterial influence. Sedimentology 2015, 62, 697–716. [Google Scholar] [CrossRef]
- Warthmann, R.; Van Lith, Y.; Vasconcelos, C.; Mc Kenzie, J.A.; Karpoff, A.M. Bacterially induced dolomite precipitation in anoxic culture experiments. Geology 2000, 28, 1091–1094. [Google Scholar] [CrossRef]
- Liu, D.; Fan, Q.; Papineau, D.; Yu, N.; Chu, Y.; Wang, H.; Qiu, X.; Wang, X. Precipitation of protodolomite facilitated by sulfate-reducing bacteria: The role of capsule extracellular polymeric substances. Chem. Geol. 2020, 533, 119415. [Google Scholar] [CrossRef]
- Reitner, J.; Schumann-Kindel, G. Pyrite in mineralized sponge tissue—Product of sulfate reducing sponge related bacteria? Facies 1997, 36, 272–284. [Google Scholar]
- Schumann-Kindel, G.; Bergbauer, M.; Manz, W.; Szewzyk, U.; Reitner, J. Aerobic and anaerobic microorganisms in modern sponges: A possible relationship to fossilization-processes. Facies 1997, 36, 268–272. [Google Scholar]
- Hoffmann, F.; Larsen, O.; Thiel, V.; Rapp, H.T.; Pape, T.; Michaelis, W.; Reitner, J. An Anaerobic World in Sponges. Geomicrobiol. J. 2005, 22, 1–10. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, W.; Feng, G.; Zhang, F.; Anbuchezhian, R.; Li, Z.; Jiang, Q. Phylogenetic diversity of sulphate-reducing Desulfovibrio associated with three South China Sea sponges. Lett. Appl. Microbiol. 2015, 60, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Benton, M.J.; Twitchett, R.J. How to kill (almost) all life: The end-Permian extinction event. Trends Ecol. Evol. 2003, 18, 358–365. [Google Scholar] [CrossRef]
- Wignall, P.B. The End-Permian mass extinction—How bad did it get? Geobiology 2007, 5, 303–309. [Google Scholar] [CrossRef]
- Chen, Z.-Q.; Benton, M. The timing and pattern of biotic recovery following the end-Permian mass extinction. Nat. Geosci. 2012, 5, 375–383. [Google Scholar] [CrossRef]
- Payne, J.L.; Clapham, M.E. End-Permian Mass Extinction in the Oceans: An Ancient Analog for the Twenty-First Century? Annu. Rev. Earth Planet. Sci. 2012, 40, 89–111. [Google Scholar] [CrossRef]



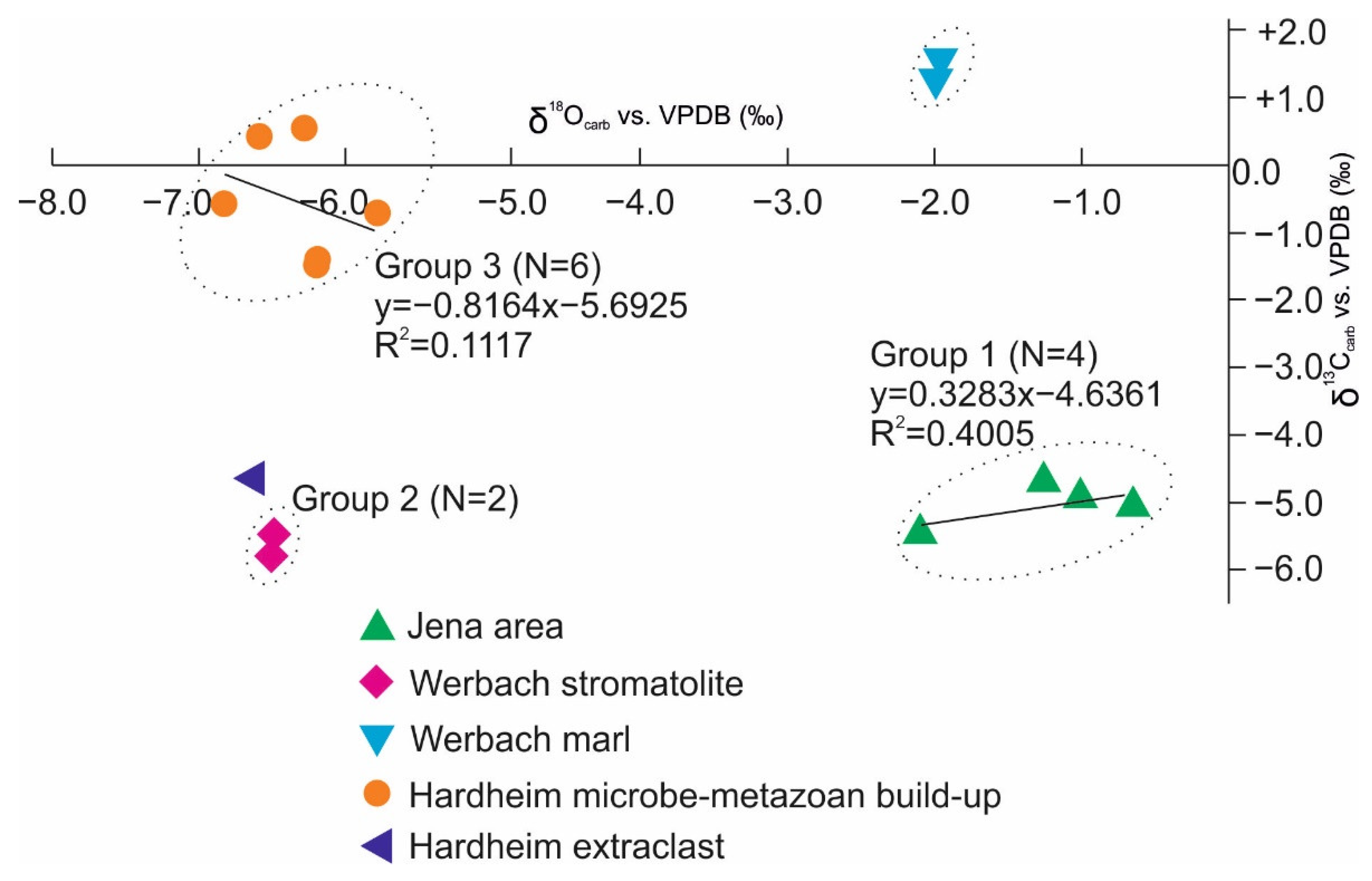
| Sample Name | Sample Number | δ13Ccarb vs. VPDB (‰) | δ18Ocarb vs. VPDB (‰) |
|---|---|---|---|
| Jena area stromatolite | 4 | −4.7 | −1.3 |
| −4.9 | −1.0 | ||
| −5.5 | −2.1 | ||
| −5.1 | −0.6 | ||
| Werbach stromatolite | 2 | −5.8 | −6.5 |
| −5.5 | −6.5 | ||
| Werbach marl | 2 | 1.3 | −2.0 |
| 1.5 | −1.9 | ||
| Hardheim microbe-metazoan build-up | 6 | −0.6 | −6.8 |
| −0.7 | −5.8 | ||
| −1.4 | −6.2 | ||
| −1.5 | −6.2 | ||
| 0.4 | −6.6 | ||
| 0.6 | −6.3 | ||
| Hardheim extraclast | 1 | −4.7 | −6.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, Y.; Hagdorn, H.; Voigt, T.; Duda, J.-P.; Reitner, J. Palaeoecological Implications of Lower-Middle Triassic Stromatolites and Microbe-Metazoan Build-Ups in the Germanic Basin: Insights into the Aftermath of the Permian–Triassic Crisis. Geosciences 2022, 12, 133. https://doi.org/10.3390/geosciences12030133
Pei Y, Hagdorn H, Voigt T, Duda J-P, Reitner J. Palaeoecological Implications of Lower-Middle Triassic Stromatolites and Microbe-Metazoan Build-Ups in the Germanic Basin: Insights into the Aftermath of the Permian–Triassic Crisis. Geosciences. 2022; 12(3):133. https://doi.org/10.3390/geosciences12030133
Chicago/Turabian StylePei, Yu, Hans Hagdorn, Thomas Voigt, Jan-Peter Duda, and Joachim Reitner. 2022. "Palaeoecological Implications of Lower-Middle Triassic Stromatolites and Microbe-Metazoan Build-Ups in the Germanic Basin: Insights into the Aftermath of the Permian–Triassic Crisis" Geosciences 12, no. 3: 133. https://doi.org/10.3390/geosciences12030133
APA StylePei, Y., Hagdorn, H., Voigt, T., Duda, J.-P., & Reitner, J. (2022). Palaeoecological Implications of Lower-Middle Triassic Stromatolites and Microbe-Metazoan Build-Ups in the Germanic Basin: Insights into the Aftermath of the Permian–Triassic Crisis. Geosciences, 12(3), 133. https://doi.org/10.3390/geosciences12030133






