Arsenic Mineral in Volcanic Tuff, a Source of Arsenic Anomaly in Groundwater: City of Chihuahua, Mexico
Abstract
1. Introduction
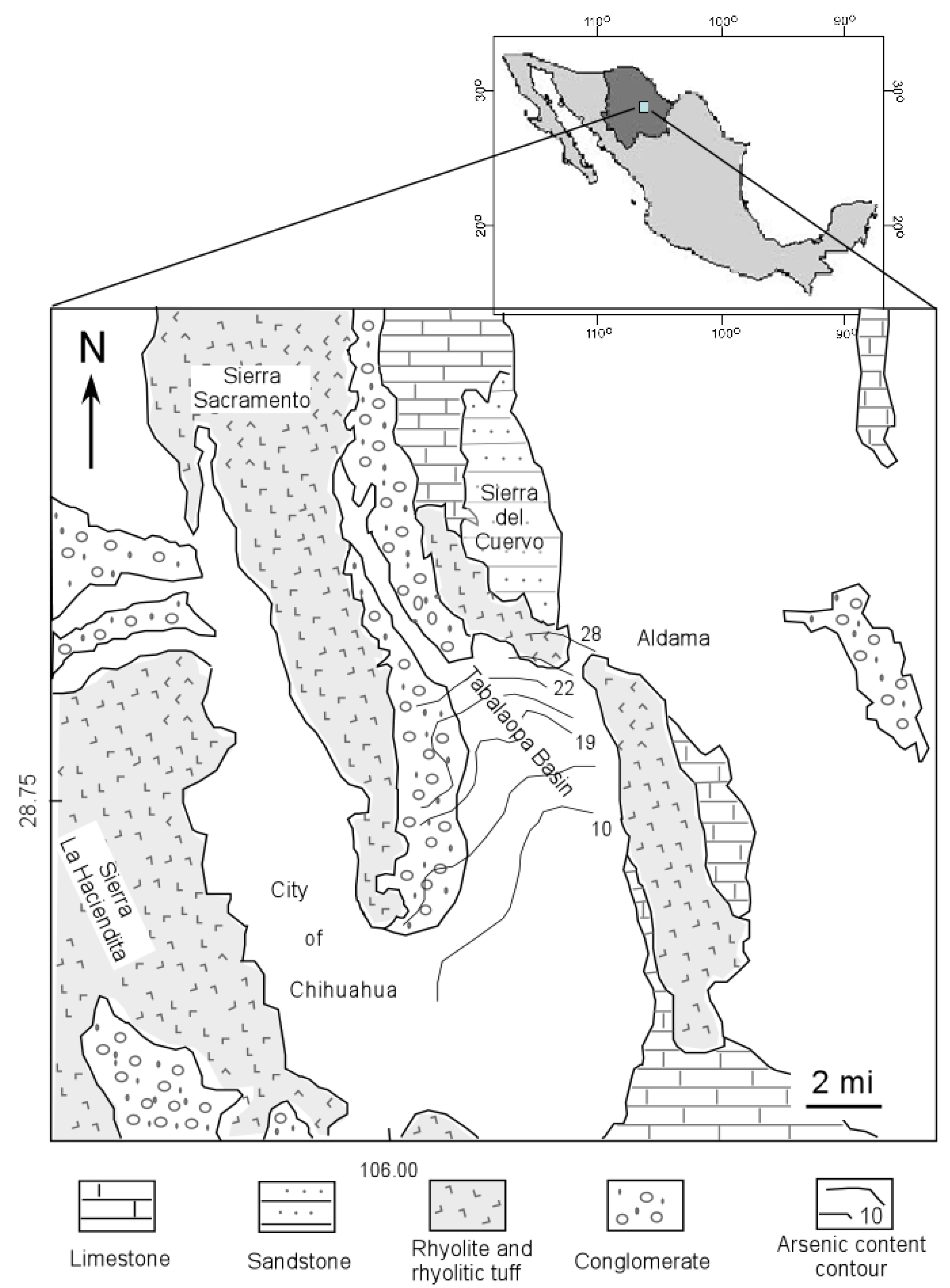
2. Regional Geology
3. Methods
4. Results
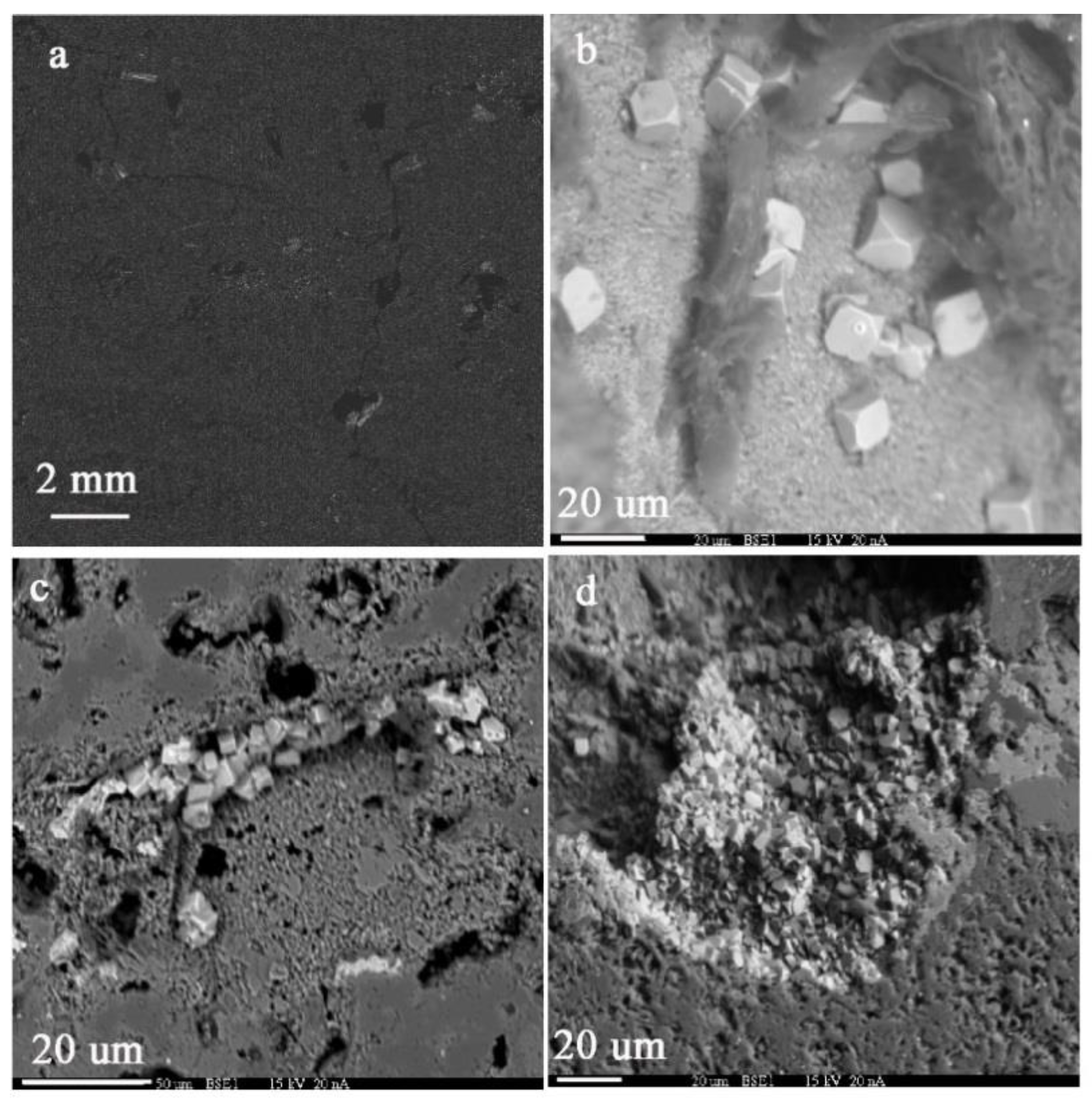
4.1. Petrographic Characteristics
4.2. Volcanic Shard and Mineral Chemistry
4.2.1. Volcanic Shard
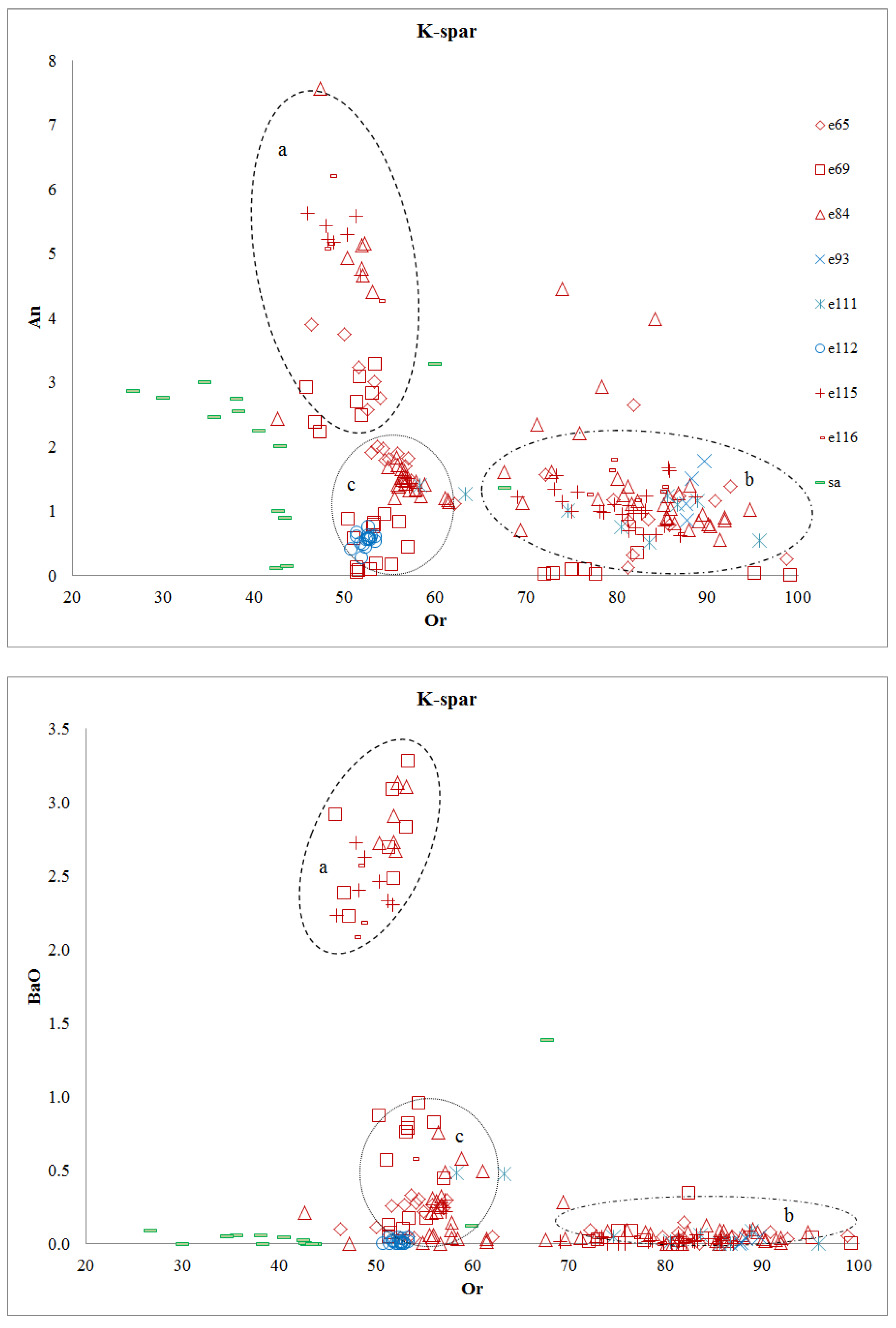
4.2.2. Feldspar
4.2.3. Biotite
4.2.4. Fe Oxides
4.2.5. Other Major Minerals
4.2.6. As Minerals
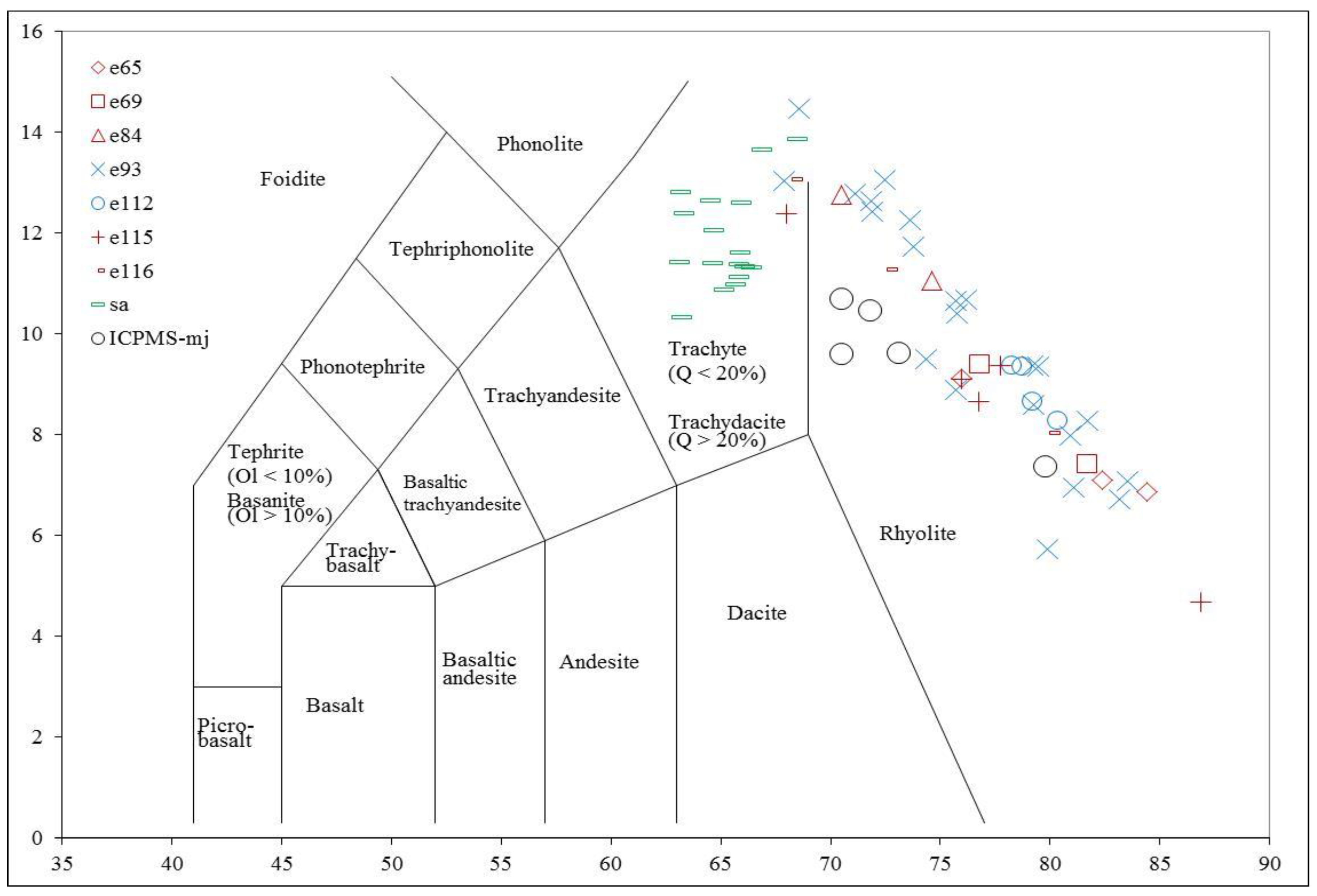
4.3. Whole-Rock Major and Trace Elements
5. Discussion
5.1. Arsenic Containing Minerals
5.2. Whole-Rock Geochemistry
5.3. Possible REE Source
5.4. As in Groundwater
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Welch, A.H.; Westjohn, D.B.; Helsel, D.R.; Wanty, R.B. Arsenic in groundwater of the United States: Occurrence and geochemistry. Groundwater 2000, 38, 589–604. [Google Scholar] [CrossRef]
- González-Horta, C.; Ballinas-Casarrubias, L.; Sánchez-Ramírez, B.; Ishida, M.C.; Barrera-Hernández, A.; Gutiérrez-Torres, D.; Zacarias, O.L.; Saunders, R.J.; Drobna, Z.; Mendez, M.A.; et al. A Concurrent Exposure to Arsenic and Fluoride from Drinking Water in Chihuahua, Mexico. Int. J. Environ. Res. Public Health 2015, 12, 4587–4601. [Google Scholar] [CrossRef]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Fuoco, I.; De Rosa, R.; Barca, D.; Figoli, A.; Gabriele, B.; Apollaro, C. Arsenic polluted waters: Application of geochemical modelling as a tool to understand the release and fate of the pollutant in crystalline aquifers. J. Environ. Manag. 2022, 301, 113796. [Google Scholar] [CrossRef]
- Rodrıgueza, R.; Ramos, J.A.; Armienta, A. Groundwater arsenic variations: The role of local geology and rainfall. Appl. Geochem. 2004, 19, 245–250. [Google Scholar] [CrossRef]
- Abdullah, M.I.; Shiyu, Z.; Mosgren, K. Arsenic and selenium species in the oxic and anoxic waters of the Oslofjord, Norway. Mar. Pollut. Bull. 1995, 31, 116–126. [Google Scholar] [CrossRef]
- Rodriguez-Pineda, J.A. A Geophysical, Geochemical and Remote Sensing Investigation of the Water Resources at the City of Chihuahua, Mexico. Ph.D. Thesis, Department of Geology, University of Texas, El Paso, TX, USA, 2000. [Google Scholar]
- Mahlknecht, J.; Horst, A.; Hernandez-Limon, G.; Aravena, R. Groundwater geochemistry of the Chihuahua City region in the Rio Conchos Basin (northern Mexico) and implications for water resources management. Hydrol. Processes 2008, 22, 4736–4751. [Google Scholar] [CrossRef]
- Comité de la Carta Geológica de México; Universidad Nacional Autónoma de México; Instituto de Geología. Carta Geológica de la República Mexicana, Escala 1:2000000; Universidad Nacional Autónoma de México and Instituto de Geología: Mexico City, Mexico, 1962. [Google Scholar]
- Handschy, J.W.; Dyer, R. Polyphase deformation in Sierra del Cuervo, Chihuahua, Mexico: Evidence for Ancestral Rocky Mountain tectonics in the Ouachita foreland of northern Mexico. Geol. Soc. Am. Bull. 1987, 99, 618–632. [Google Scholar] [CrossRef]
- McDowell, F.W.; McIntosh, W.C. Timing of intense magmatic episodes in the northern and central Sierra Madre Occidental, western Mexico. Geosphere 2012, 8, 1505–1526. [Google Scholar] [CrossRef]
- Flawn, P.T.; Goldstein, A., Jr.; King, P.B.; Weaver, C.E. The Ouachita System; Bureau of Economic Geology, University of Texas Publication: Austin, TX, USA, 1961. [Google Scholar]
- Stege, B.R. Stratigraphy and Significance of the Carbonates of the Peña Blanci Uranium District, Chihuahua, Mexico. Master’s Thesis, University of Texas, El Paso, TX, USA, 1979; 81p. [Google Scholar]
- Gries, J.C. Problems of delineation of the Rio Grande Rift into the Chihuahua tectonic belt of northern Mexico. In Rio Grande Rift: Tectonics and Magmatism; Riecker, R.E., Ed.; AGU: Washington, DC, USA, 1979; Volume 14, pp. 107–113. [Google Scholar]
- Hennings, P.H. Structural transect of the southern Chihuahua Fold Belt between Ojinaga and A’dama, Chihuahua, Mexico. Tectonics 1994, 13, 1445–1460. [Google Scholar] [CrossRef]
- Humphreys, E.D. Post-Laramide removal of the Farallon slab, western United States. Geology 1995, 23, 987–990. [Google Scholar] [CrossRef]
- Ferrari, L.; López-Martinez, M.; Rosas-Elguera, J. Ignimbrite flare-up and deformation in the southern Sierra Madre Occidental, western Mexico—Implications for the late subduction history of the Farallon plate. Tectonics 2002, 21, 17-1–17-24. [Google Scholar] [CrossRef]
- Murray, B.P.; Busby, C.J.; Ferrari, L.; Solari, L.A. Synvolcanic crustal extension during the mid-Cenozoic ignimbrite flare-up in the northern Sierra Madre Occidental, Mexico: Evidence from the Guazapares Mining District region, western Chihuahua. Geosphere 2013, 9, 1201–1235. [Google Scholar] [CrossRef]
- McDowell, F.W. Geologic Transect across the Northern Sierra Madre Occidental Volcanic Field, Chihuahua and Sonora, Mexico. Geol. Soc. Am. 2007, 6, 70p. [Google Scholar]
- Campbell, A.R. Volcanic Rocks of the La Perla Area, Chihuahua, Mexico. Master’s Thesis, University of Texas, Austin, TX, USA, 1977; 110p. [Google Scholar]
- Keller, P.C.; Bockover, N.T.; McDowell, F.W. Tertiary volcanic history of the Sierra del Gallego area, Chihuahua, Mexico. Geol. Soc. Am. Bull. 1982, 93, 303–314. [Google Scholar] [CrossRef]
- Cameron, M.; Bagby, W.C.; Cameron, K.L. Petrogenesis of voluminous mid-tertiary ignimbrites of the Sierra Madre Occidental. Contrib. Mineral. Petrol. 1980, 74, 271–284. [Google Scholar] [CrossRef]
- Cather, S.M.; Dunbar, N.W.; McDowell, F.W.; McIntosh, W.C.; Scholle, P.A. Climate forcing by iron fertilization from repeated ignimbrite eruptions: The icehouse-silicic large igneous province (SLIP) hypothesis. Geosphere 2009, 5, 315–324. [Google Scholar] [CrossRef]
- Bryan, S.E.; Ferrari, L.; Reiners, P.W.; Allen, C.M.; Petrone, C.M.; Ramos-Rosique, A.; Campbell, I.H. New Insights into Crustal Contributions to Large-volume Rhyolite Generation in the Mid-Tertiary Sierra Madre Occidental Province, Mexico, Revealed by U-Pb Geochronology. J. Petrol. 2008, 49, 47–77. [Google Scholar] [CrossRef]
- Walenta, K.; Dunn, P.J. Arsenogoyazit, ein neues Mineral der Crandallitgruppe aus dem Schwarzwald. Schweiz. Mineral. Und Petrogr. Mitt. 1984, 64, 11–19. (In German) [Google Scholar]
- McDowell, F.W.; Clabaugh, S.E. Ignimbrites of the Sierra Madre Occidental and their relation to the tectonic history of western Mexico. In Ash-Flow Tuffs; Chapin, C.E., Elston, W.E., Eds.; Geological Society of America: Boulder, CO, USA, 1979; Volume 180, pp. 113–124. [Google Scholar]
- Ferrari, L.; Valencia-Moreno, M.; Bryan, S. Magmatism and tectonics of the Sierra Madre Occidental and its relation with the evolution of the western margin of North America. In Geology of Mexico: Celebrating the Centenary of the Geological Society of Mexico; Alaniz-Àlvarez, S.A., Nieto Samaniego, A.F., Eds.; Geological Society of America: Boulder, CO, USA, 2007; Volume 422, pp. 1–39. [Google Scholar]
- Hildreth, W. Gradients in silicic magma chambers: Implications for lithospheric magmatism. J. Geophys. Res. 1981, 86, 10153–10192. [Google Scholar] [CrossRef]
- Wark, D.A. Oligocene ash flow volcanism, northern Sierra Madre Occidental: Role of mafic and intermediate-composition magmas in rhyolite genesis. J. Geophys. Res. 1991, 96, 13389–13411. [Google Scholar] [CrossRef]
- Hanson, R.B.; Glazner, A.F. Thermal requirements for extensional emplacement of granitoids. Geology 1995, 23, 213–216. [Google Scholar] [CrossRef]
- Costa, A.; Gottsmann, J.; Melnik, O.; Sparks, R.S.J. A stress-controlled mechanism for the intensity of very large magnitude explosive eruptions. Earth Planet. Sci. Lett. 2011, 310, 161–166. [Google Scholar] [CrossRef]
- Henry, C.D.; Price, J.G.; James, E.W. Mid-Cenozoic stress evolution and magmatism in the southern Cordillera, Texas and Mexico: Transition from continental arc to intraplate extension. J. Geophys. Res. 1991, 96, 13545–13560. [Google Scholar] [CrossRef]
- McDowell, F.; Keizer, R.P. Timing of mid-Tertiary volcanism in the Sierra Madre Occidental between Durango city and Mazatlán, Mexico. Geol. Soc. Am. Bull. 1977, 88, 1479–1487. [Google Scholar] [CrossRef]
- McDowell, F.W.; Mauger, R.L. K-Ar and U-Pb zircon chronology of Late Cretaceous and Tertiary magmatism in central Chihuahua State, Mexico. Geol. Soc. Am. Bull. 1994, 106, 118–132. [Google Scholar] [CrossRef]
- McDowell, F.W.; Roldan-Quintana, J.; Connelly, J. Duration of Late Cretaceous-early Tertiary magmatism in east-central Sonora, Mexico. Geol. Soc. Am. Bull. 2001, 113, 521–531. [Google Scholar] [CrossRef]
- Bryan, S.E. Silicic Large Igneous Provinces. Episodes 2007, 30, 20–31. [Google Scholar] [CrossRef]
- Swanson, E.R.; Kempter, K.A.; McDowell, F.W.; McIntosh, W.C. Major ignimbrites and volcanic centers of the Copper Canyon area; a view into the core of Mexico’s Sierra Madre Occidental. Geosphere 2006, 2, 125–141. [Google Scholar] [CrossRef]
- Verma, S.; Carrasco-Núñez, G. Reappraisal of the Geology and Geochemistry of Volcán Zamorano, Central Mexico: Implications for Discriminating the Sierra Madre Occidental and Mexican Volcanic Belt Provinces. Int. Geol. Rev. 2003, 45, 724–752. [Google Scholar] [CrossRef]
- Orozco-Esquivel, M.T.; Nieto-Samaniego, A.F.; Alaniz-Álvarez, S. Origin of rhyolitic lavas in the Mesa central, Mexico, by crustal melting related to extension. J. Volca. Geoth. Res. 2002, 118, 37–56. [Google Scholar] [CrossRef]
- Huppert, H.E.; Sparks, R.S.J. The generation of granitic magmas by intrusion of basalt into continental crust. J. Petrol. 1988, 29, 599–642. [Google Scholar] [CrossRef]
- Harry, D.L.; Leeman, W.P. Partial melting of melt metasomatized subcontinental mantle and the magma source potential of the lower lithosphere. J. Geophys. Res. 1995, 100, 10255–10269. [Google Scholar] [CrossRef]
- Mauger, R.L. The mid-Eocene Majalca Canyon caldera, Chihuahua, Mexico. In Geology and Mineral Resources of Northern Sierra Madre Occidental, Mexico: Guidebook for the 1992 Field Conference; Clark, K.F., Roldán- Quintana, J., Schmitt, R.H., Eds.; El Paso Geological Society: El Paso, TX, USA, 1992; pp. 127–132. [Google Scholar]
- Centeno-García, E.; Guerrero-Suastegui, M.; Talavera-Mendoza, O. The Guerrero Composite Terrane of western Mexico: Collision and subsequent rifting in a supra-subduction zone. In Formation and Applications of the Sedimentary Record in Arc Collision Zones; Draut, A., Clift, P.D., Scholl, D.W., Eds.; Geological Society of America: Boulder, CO, USA, 2008; Volume 436, pp. 279–308. [Google Scholar]
- Draut, A.E.; Clift, P.D.; Scholl, D.W.; Ryan, P.D.; Waltham, D.; Hall, R.; Smyth, H.R.; Ebinger, C.J.; Dorobek, S.L.; Hoffmann, G.; et al. Formation and Applications of the Sedimentary Record in Arc Collision Zones; Geological Society of America: Boulder, CO, USA, 2008; 435p. [Google Scholar]
- Coney, P.J.; Campa, M.F. Lithotectonic Terrane Map of México (West of the 91st Meridian); Reston, Va.: Denver, CO, USA, 1987. [Google Scholar]
- Stewart, J.H.; Roldán-Quintana, J. Upper Triassic Barranca Group: Nonmarine and shallow-marine rift-basin deposits of northwestern Mexico. In Studies of Sonoran Geology; Pérez-Segura, E., Jacques Ayala, C., Eds.; Geological Society of America: Boulder, CO, USA, 1991; Volume 254, pp. 19–36. [Google Scholar]
- Gao, J.; Green, T.H. Barium partitioning between alkali feldspar and silicate liquid at high temperature and pressure. Contrib. Miner. Pet. 1989, 102, 328–335. [Google Scholar] [CrossRef]
- Meyers, P.A.; Pratt, L.M.; Nagy, B. Introduction to geochemistry of metalliferous black shales. Chem. Geol. 1992, 99, 7–11. [Google Scholar] [CrossRef][Green Version]
- Boyle, R.W.; Jonasson, I.R. The geochemistry of arsenic and its use as an indicator element in geochemical prospecting. J. Geochem. Explor. 1973, 2, 251–296. [Google Scholar] [CrossRef]
- Quinby-Hunt, M.S.; Wide, P.; Berry, W.B.N. Element geochemistry of low calcic black shales—statistical comparison with other shales. U.S. Geol. Surv. Circ. 1989, 1037, 8–15. [Google Scholar]
- Ketris, M.P.; Yudovitch, Y.E. Estimations of Clarkes for Carbonaceous bioliths: World averages for trace element contents in black shales and coals. Int. J. Coal Geol. 2009, 78, 135–148. [Google Scholar] [CrossRef]
- Reimann, C.; de Caritat, P. Chemical Elements in the Environment: Fact Sheets for the Geochemist and Environmental Scientist; Springer: Berlin, Germany, 1998; 398p. [Google Scholar]
- Chappell, B.W. A chemical database for the New England batholith. In Proceedings of the New England Orogen Conference, University of New England, Armidale, NSW, Australia; 2010; pp. 119–123. [Google Scholar]
- Albrecht, A.; Goldstein, S.L. Effects of basement composition and age on silicic magmas across an accreted terrane-Precambrian crust boundary, Sierra Madre Occidental, Mexico. J. S. Am. Earth Sci. 2000, 13, 255–273. [Google Scholar] [CrossRef]
- Hildreth, W.; Moorbath, S. Crustal contributions to arc magmatism in the Andes of Central Chile. Contrib. Miner. Pet. 1988, 98, 455–489. [Google Scholar] [CrossRef]
- Rapp, R.P.; Watson, E.B. Dehydration melting of metabasalt at 8-32 kbar: Implications for continental growth and crust-mantle recycling. J. Petrol. 1995, 36, 891–931. [Google Scholar] [CrossRef]
- Hervig, R.L.; Dunbar, N.W. Cause of chemical zoning in the Bishop (California) and Bandelier (New Mexico) magma chambers. Earth Planet. Sci. Lett. 1992, 111, 97–108. [Google Scholar] [CrossRef]
- Reyes-Gómez, V.M.; Alarcón-Herrera, M.T.; Gutiérrez, M.; López, D.N. Fluoride and Arsenic in an Alluvial Aquifer System in Chihuahua, Mexico: Contaminant Levels, Potential Sources, and Co-occurrence. Water Air Soil Pollut. 2013, 224, 1433. [Google Scholar] [CrossRef]
- Morán-Ramírez, J.; Ledesma-Ruiz, R.; Mahlknecht, J.; Ramos-Leal, J.A. Rockewater interactions and pollution processes in the volcanic aquifer system of Guadalajara, Mexico, using inverse geochemical modeling. Appl. Geochem. 2016, 68, 79–94. [Google Scholar] [CrossRef]
- Apollaro, C.; Fuoco, I.; Bloise, L.; Calabrese, E.; Marini, L.; Vespasiano, G.; Muto, F. Geochemical Modeling of Water-Rock Interaction Processes in the Pollino National Park. Geofluids 2021, 2021, 6655711. [Google Scholar] [CrossRef]
- Apollaro, C.; Di Curzio, D.; Fuoco, I.; Buccianti, A.; Dinelli, E.; Vespasiano, G.; Castrignanò, A.; Rusi, S.; Barca, D.; Figoli, A.; et al. A multivariate non-parametric approach for estimating probability of exceeding the local natural background level of arsenic in the aquifers of Calabria region (Southern Italy). Sci. Total Environ. 2022, 806, 150345. [Google Scholar] [CrossRef] [PubMed]


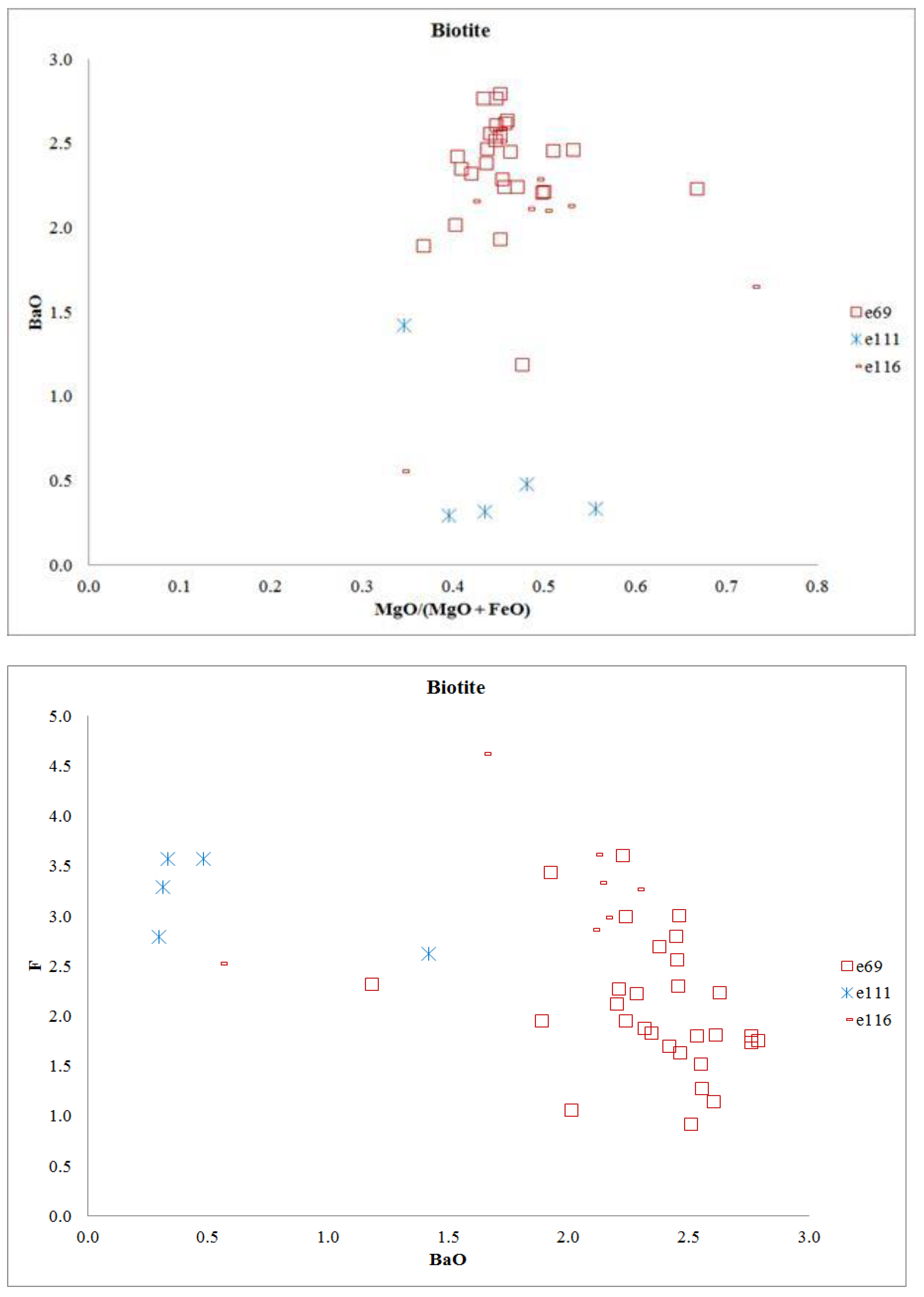

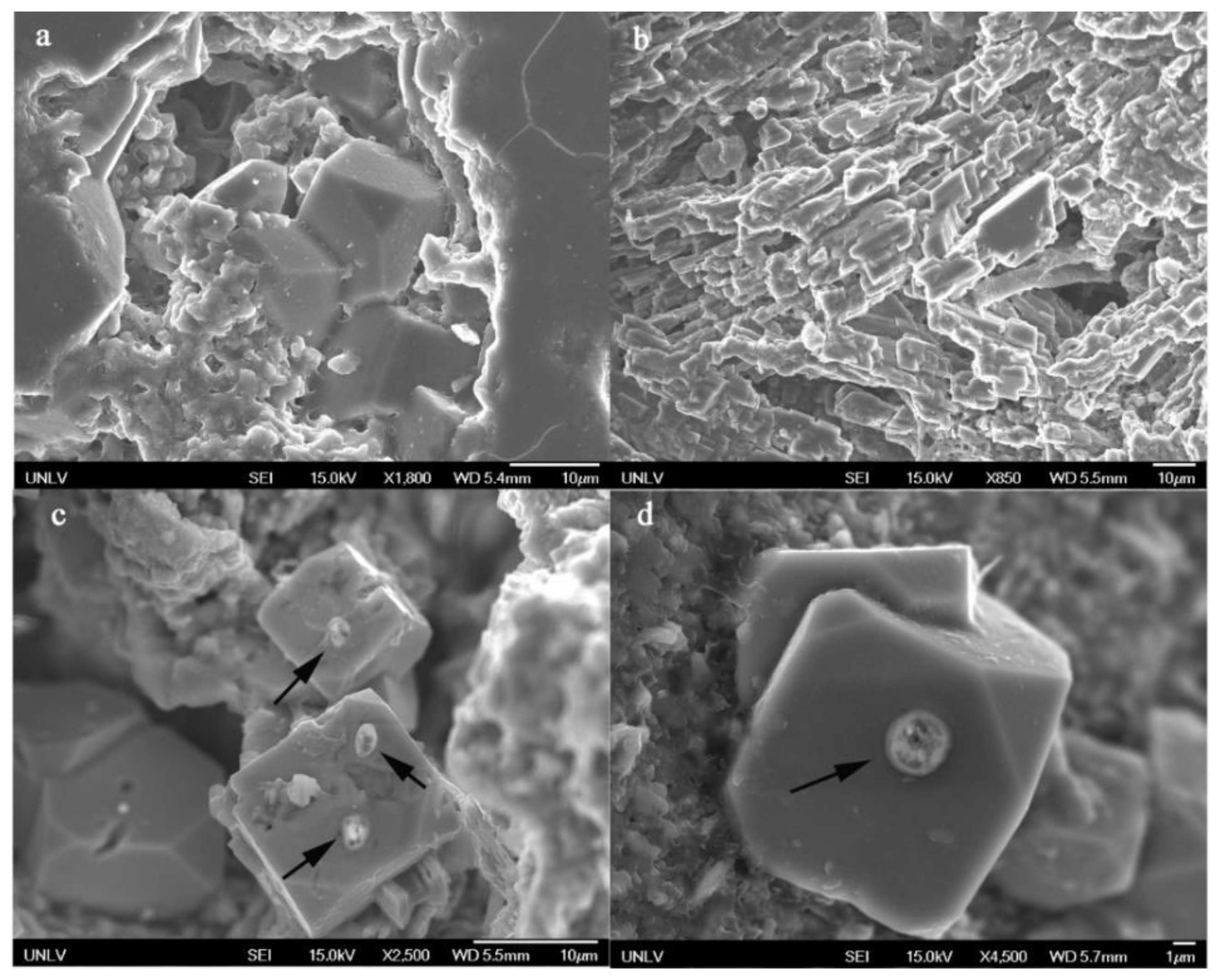

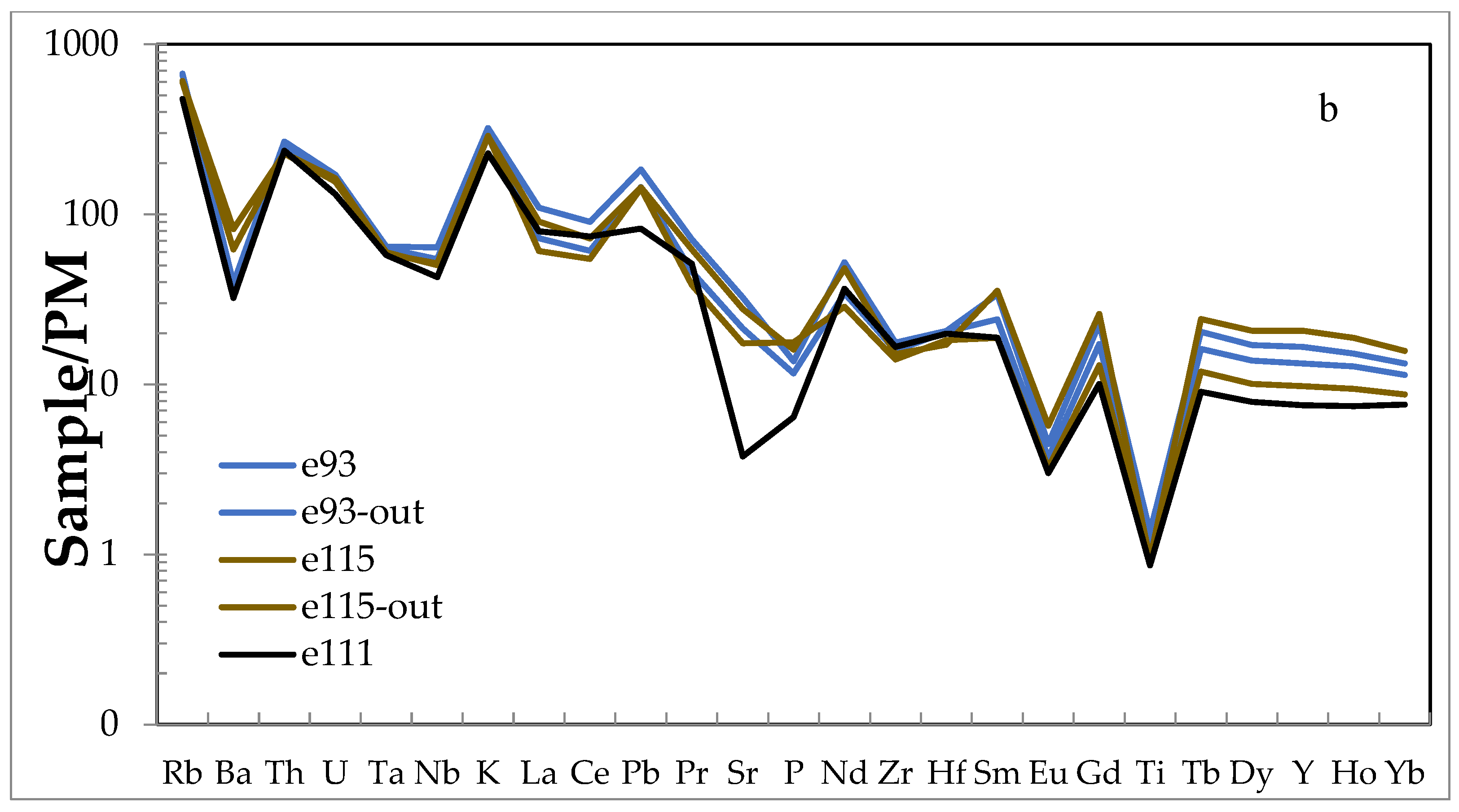
| Sample | e65 | e69 | e84 | e93 | e112 | e115 | e116 | sa3 |
|---|---|---|---|---|---|---|---|---|
| SiO2 | 82.38 | 76.74 | 70.47 | 76.18 | 78.20 | 77.75 | 72.57 | 64.66 |
| TiO2 | bdl | 0.10 | 0.07 | 0.15 | 0.15 | bdl | 0.02 | 0.19 |
| Al2O3 | 8.61 | 10.90 | 15.75 | 12.64 | 10.97 | 10.95 | 14.21 | 18.77 |
| FeO | 0.14 | 0.28 | 0.35 | 0.38 | 0.53 | 0.15 | 0.33 | 0.83 |
| MnO | bdl | bdl | bdl | bdl | 0.06 | bdl | 0.02 | 0.03 |
| MgO | 0.04 | 0.14 | 0.04 | bdl | bdl | 0.01 | 0.03 | 0.27 |
| CaO | 0.10 | 0.55 | 0.23 | 0.09 | 0.03 | 0.20 | 0.28 | 0.75 |
| Na2O | 0.73 | 1.37 | 1.75 | 1.20 | 1.71 | 1.24 | 1.45 | 3.90 |
| K2O | 6.35 | 8.05 | 11.01 | 9.47 | 7.68 | 8.14 | 9.82 | 8.14 |
| P2O5 | 0.01 | 0.03 | 0.02 | bdl | bdl | bdl | 0.04 | 0.10 |
| F | bdl | 0.09 | 0.14 | bdl | bdl | 0.03 | bdl | 0.03 |
| Cl | 0.02 | 0.02 | 0.02 | 0.01 | 0.02 | bdl | ||
| SO3 | 0.04 | 1.01 | 0.04 | 0.03 | 0.07 | 0.01 | ||
| BaO | bdl | 0.04 | 0.01 | 0.03 | 0.02 | bdl | 0.04 | 0.08 |
| SrO | 0.09 | bdl | bdl | bdl | 0.03 | bdl | 0.06 | |
| Total | 98.63 | 99.35 | 99.84 | 100.23 | 99.36 | 98.57 | 98.81 | 97.92 |
| TA | 7.09 | 9.41 | 12.76 | 10.67 | 9.39 | 9.38 | 11.27 | 12.03 |
| ASI | 1.05 | 0.92 | 1.04 | 1.02 | 0.98 | 0.98 | 1.06 | 1.15 |
| AI | 0.94 | 1.01 | 0.94 | 0.97 | 1.01 | 0.99 | 0.92 | 0.81 |
| e65 | e69 | e84 | e93 | e111 | |||||||
| ano | matrix | ano | sa | matrix | ano | sa | matrix | sa | sa | ||
| SiO2 | 64.91 | 65.23 | 63.67 | 65.79 | 64.90 | 61.97 | 64.46 | 66.62 | 63.15 | 63.85 | |
| TiO2 | 0.14 | 0.02 | 0.03 | bdl | 0.06 | 0.03 | bdl | 0.06 | 0.01 | bdl | |
| Al2O3 | 20.22 | 18.98 | 21.06 | 18.55 | 20.14 | 19.78 | 18.24 | 18.56 | 18.84 | 18.79 | |
| FeO | 0.23 | 0.12 | 0.28 | 0.24 | 0.31 | 0.26 | 0.28 | 0.09 | 0.20 | 0.20 | |
| MnO | bdl | 0.03 | bdl | bdl | bdl | bdl | 0.06 | bdl | 0.01 | bdl | |
| MgO | 0.01 | bdl | 0.02 | 0.02 | 0.01 | 0.01 | 0.02 | 0.03 | 0.03 | bdl | |
| CaO | 0.65 | 0.15 | 0.92 | 0.21 | 0.76 | 0.93 | 0.19 | 0.33 | 0.30 | 0.23 | |
| Na2O | 5.30 | 1.50 | 4.86 | 1.86 | 5.01 | 4.95 | 1.12 | 4.86 | 1.20 | 1.17 | |
| K2O | 8.68 | 13.80 | 8.33 | 13.22 | 8.87 | 7.97 | 14.94 | 9.31 | 14.81 | 14.99 | |
| P2O5 | bdl | 0.01 | bdl | 0.55 | bdl | bdl | bdl | 0.01 | 0.01 | 0.12 | |
| BaO | 0.26 | bdl | 2.48 | 0.34 | 0.76 | 2.72 | 0.08 | 0.29 | 0.03 | 0.09 | |
| SrO | 0.06 | 0.08 | 0.08 | bdl | 0.10 | 0.13 | bdl | bdl | bdl | bdl | |
| Total | 100.46 | 99.95 | 101.75 | 101.15 | 101.00 | 98.77 | 99.48 | 100.26 | 98.67 | 99.70 | |
| An wt% | 3.23 | 0.78 | 4.79 | 1.10 | 3.82 | 4.93 | 0.94 | 1.65 | 1.50 | 1.16 | |
| Ab wt% | 45.16 | 13.38 | 43.33 | 16.56 | 43.01 | 44.74 | 9.57 | 42.06 | 10.20 | 9.92 | |
| Or wt% | 51.62 | 85.85 | 51.88 | 82.35 | 53.17 | 50.33 | 89.49 | 56.29 | 88.29 | 88.92 | |
| e111 | e112 | e115 | e116 | sa3 | |||||||
| matrix | ano | ano | sa | matrix | ano | sa | ano | pl | |||
| SiO2 | 65.52 | 66.78 | 62.31 | 65.31 | 63.94 | 63.42 | 65.27 | 66.51 | 59.26 | ||
| TiO2 | 0.07 | 0.05 | bdl | 0.13 | bdl | 0.13 | 0.22 | 0.12 | 0.00 | ||
| Al2O3 | 19.65 | 19.19 | 20.79 | 17.87 | 18.88 | 20.92 | 18.63 | 19.82 | 25.15 | ||
| FeO | 0.09 | 0.24 | 0.23 | 0.14 | 0.16 | 0.20 | 0.13 | ||||
| MnO | bdl | bdl | 0.01 | 0.01 | 0.04 | 0.03 | bdl | 0.24 | 0.27 | ||
| MgO | 0.01 | 0.01 | bdl | 0.02 | bdl | 0.01 | bdl | 0.02 | 0.05 | ||
| CaO | 0.25 | 0.09 | 1.04 | 0.12 | 0.24 | 0.96 | 0.13 | bdl | bdl | ||
| Na2O | 4.15 | 5.58 | 4.72 | 1.40 | 1.16 | 5.21 | 1.48 | 0.51 | 6.70 | ||
| K2O | 10.61 | 8.86 | 8.04 | 14.10 | 14.58 | 7.59 | 14.56 | 6.92 | 6.93 | ||
| P2O5 | 0.05 | bdl | bdl | 0.01 | 0.20 | bdl | 0.01 | 6.43 | 0.93 | ||
| BaO | 0.48 | 0.04 | 2.33 | 0.00 | 0.08 | 2.08 | 0.02 | 0.03 | 0.02 | ||
| SrO | 0.11 | bdl | 0.17 | 0.01 | 0.11 | bdl | bdl | bdl | bdl | ||
| Total | 101.02 | 100.84 | 99.83 | 99.28 | 99.46 | 100.59 | 100.45 | 100.62 | 99.60 | ||
| An wt% | 1.26 | 0.44 | 5.59 | 0.61 | 1.22 | 5.08 | 0.65 | 2.56 | 34.15 | ||
| Ab wt% | 35.47 | 47.19 | 43.11 | 12.37 | 10.11 | 47.06 | 12.59 | 59.07 | 60.24 | ||
| Or wt% | 63.27 | 52.37 | 51.30 | 87.02 | 88.67 | 47.86 | 86.76 | 38.37 | 5.61 |
| e69 | e111 | e116 | e93 | sa3 | ||
|---|---|---|---|---|---|---|
| biotite | biotite | biotite | Greenalite | amphibole | ||
| SiO2 | 35.71 | 30.42 | 30.69 | 42.92 | 36.80 | 50.58 |
| TiO2 | 7.09 | 4.61 | 5.84 | 0.06 | 0.11 | 0.48 |
| Al2O3 | 15.53 | 14.34 | 13.34 | 0.14 | 0.14 | 3.09 |
| Cr2O3 | 0.03 | bdl | ||||
| FeO | 14.24 | 20.15 | 18.31 | 51.18 | 57.36 | 6.68 |
| MnO | 0.92 | 1.41 | 1.27 | 0.15 | 0.24 | 0.15 |
| MgO | 12.30 | 13.16 | 13.44 | 0.02 | 0.01 | 15.76 |
| CaO | 0.05 | 1.86 | 0.28 | 0.07 | 0.05 | 20.54 |
| Na2O | 0.77 | 1.00 | 0.63 | 0.11 | 0.11 | 0.38 |
| K2O | 7.88 | 6.04 | 6.63 | 0.07 | 0.05 | bdl |
| P2O5 | 0.03 | 0.15 | 0.03 | bdl | bdl | 0.01 |
| F | 2.79 | 2.80 | 2.99 | bdl | bdl | 0.04 |
| Cl | 0.05 | 0.11 | 0.01 | 0.01 | bdl | |
| SO3 | 0.12 | 2.10 | 0.02 | 0.05 | 0.05 | |
| BaO | 2.45 | 0.30 | 2.16 | bdl | bdl | 0.04 |
| SrO | 0.03 | bdl | bdl | bdl | ||
| Total | 98.75 | 97.21 | 94.33 | 94.87 | 95.46 | 97.75 |
| Sample | e65 | e65 | e69 | e69 | e84 | e84 | e93 | e93 | e69Y |
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| TiO2 | 0.515 | bdl | 0.105 | 0.017 | 0.06 | bdl | bdl | 0.19 | 0.55 |
| Al2O3 | 32.71 | 35.68 | 32.20 | 32.14 | 31.13 | 35.88 | 35.83 | 31.59 | 0.20 |
| Cr2O3 | 0.03 | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| FeO | 0.87 | 0.50 | 0.72 | 0.88 | 0.42 | 0.38 | 1.50 | 3.73 | 1.13 |
| MnO | 0.30 | 0.19 | 0.11 | 0.06 | 0.05 | 0.12 | 0.20 | 0.08 | bdl |
| MgO | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| CaO | 1.71 | 1.65 | 1.19 | 1.22 | 0.38 | 0.47 | 0.98 | 0.60 | 2.41 |
| Na2O | bdl | 0.05 | 0.09 | 0.05 | 0.02 | bdl | 0.03 | 0.01 | bdl |
| K2O | 1.13 | 0.05 | 0.09 | 0.44 | 0.41 | 0.22 | 0.09 | 0.08 | 0.04 |
| P2O5 | 5.64 | 3.52 | 15.28 | 14.01 | 1.86 | 1.51 | 1.81 | 1.52 | 3.11 |
| F | 0.465 | bdl | 2.03 | 1.811 | bdl | bdl | bdl | bdl | 0.07 |
| Cl | bdl | 0.01 | 0.14 | 0.11 | 0.02 | 0.01 | 0.03 | 0.04 | 0.05 |
| SO3 | 0.18 | 0.15 | 0.06 | 0.08 | 0.08 | 0.16 | 0.09 | 0.08 | bdl |
| As2O5 | 18.79 | 22.40 | 9.23 | 13.33 | 27.91 | 30.91 | 32.76 | 28.32 | 30.82 |
| SrO | 11.15 | 12.34 | 4.63 | 5.93 | 7.94 | 10.80 | 11.96 | 10.27 | bdl |
| BaO | 0.977 | 10.443 | 9.152 | bdl | bdl | ||||
| La2O3 | 2.72 | 6.58 | 2.02 | 1.68 | 3.95 | 3.89 | 2.74 | 1.97 | 0.66 |
| Ce2O3 | 2.11 | 0.99 | 3.23 | 3.68 | 8.38 | 7.43 | 3.81 | 3.26 | 8.51 |
| Pr2O3 | 0.72 | 0.72 | 0.45 | 0.41 | 0.69 | 0.64 | 0.76 | 0.28 | 1.26 |
| Nd2O3 | 1.18 | 1.26 | 1.24 | 0.92 | 1.64 | 1.42 | 1.99 | 1.62 | 7.64 |
| Y2O3 | 0.041 | 0.046 | 0.017 | bdl | 0.001 | 0.04 | 0.02 | 0.009 | 20.05 |
| ZrO2 | 0.368 | 0.014 | 2.915 | 2.921 | bdl | bdl | bdl | bdl | 1.71 |
| Total | 81.408 | 86.132 | 85.298 | 88.051 | 84.933 | 93.87 | 94.58 | 83.621 | 78.14 |
| e93 | e93-ot | e111 | e115 | e115-ot | 2σ Uncert * | |
|---|---|---|---|---|---|---|
| SiO2 * | 71.8 | 70.5 | 79.8 | 70.5 | 73.1 | 0.121 |
| TiO2 | 0.22 | 0.19 | 0.17 | 0.27 | 0.20 | 0.084 |
| Al2O3 | 13.80 | 12.78 | 9.89 | 14.13 | 12.99 | 0.101 |
| FeO | 2.01 | 1.56 | 1.44 | 2.28 | 1.87 | 0.079 |
| MnO | 0.09 | 0.07 | 0.05 | 0.10 | 0.10 | 0.134 |
| MgO | 0.20 | 2.74 | 0.03 | 0.30 | 0.48 | 0.086 |
| CaO | 1.20 | 2.16 | 0.32 | 1.40 | 1.24 | 0.081 |
| Na2O | 1.39 | 1.25 | 0.78 | 1.41 | 1.25 | 0.063 |
| K2O | 9.08 | 8.34 | 6.60 | 9.29 | 8.36 | 0.077 |
| P2O5 | 0.23 | 0.32 | 0.13 | 0.27 | 0.35 | 0.106 |
| Total | 100.01 | 99.90 | 99.21 | 99.96 | 99.94 | |
| La | 46.83 | 70.80 | 51.45 | 58.65 | 39.45 | 0.084 |
| Ce | 101.88 | 150.85 | 123.66 | 120.35 | 91.21 | 0.08 |
| Pr | 11.67 | 18.10 | 13.01 | 15.81 | 9.76 | 0.119 |
| Nd | 42.96 | 65.23 | 45.69 | 60.14 | 35.79 | 0.104 |
| Sm | 9.81 | 13.74 | 7.65 | 14.43 | 7.62 | 0.11 |
| Eu | 0.57 | 0.69 | 0.46 | 0.88 | 0.50 | 0.083 |
| Gd | 9.42 | 12.41 | 5.47 | 14.12 | 7.04 | 0.118 |
| Tb | 1.60 | 2.02 | 0.90 | 2.40 | 1.18 | 0.102 |
| Dy | 9.31 | 11.48 | 5.33 | 13.94 | 6.78 | 0.11 |
| Ho | 1.90 | 2.26 | 1.11 | 2.80 | 1.40 | 0.098 |
| Er | 5.26 | 6.32 | 3.24 | 7.58 | 3.93 | 0.135 |
| Tm | 0.80 | 0.96 | 0.53 | 1.16 | 0.61 | 0.173 |
| Yb | 5.01 | 5.85 | 3.36 | 6.95 | 3.85 | 0.136 |
| Lu | 0.71 | 0.84 | 0.50 | 0.97 | 0.56 | 0.076 |
| Cr | 2.48 | 3.23 | 1.54 | 3.11 | 2.93 | 0.113 |
| Ni | 3.88 | 2.08 | 1.61 | 9.74 | 2.92 | 0.103 |
| Sc | 3.85 | 4.77 | 3.04 | 3.78 | 3.64 | 0.087 |
| V | 4.41 | 6.27 | 5.26 | 6.62 | 5.37 | 0.068 |
| Rb | 396.62 | 402.97 | 285.97 | 364.58 | 359.31 | 0.049 |
| Sr | 424.57 | 645.66 | 75.00 | 552.21 | 347.35 | 0.069 |
| Y | 57.08 | 71.52 | 32.42 | 88.93 | 41.95 | 0.108 |
| Zr | 165.89 | 184.81 | 174.47 | 158.16 | 147.38 | 0.113 |
| Nb | 35.88 | 42.01 | 28.12 | 33.03 | 33.85 | 0.178 |
| Ba | 241.78 | 259.17 | 212.63 | 540.37 | 410.01 | 0.078 |
| Hf | 5.77 | 5.83 | 5.62 | 4.84 | 5.16 | 0.138 |
| Ta | 2.33 | 2.39 | 2.13 | 2.21 | 2.16 | 0.156 |
| Pb | 21.68 | 27.57 | 12.35 | 21.63 | 21.33 | 0.131 |
| Th | 19.83 | 21.31 | 18.84 | 18.21 | 18.09 | 0.122 |
| U | 3.15 | 3.48 | 2.67 | 3.31 | 3.16 | 0.103 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, M.; Rodríguez-Pineda, J.A.; Goodell, P. Arsenic Mineral in Volcanic Tuff, a Source of Arsenic Anomaly in Groundwater: City of Chihuahua, Mexico. Geosciences 2022, 12, 69. https://doi.org/10.3390/geosciences12020069
Ren M, Rodríguez-Pineda JA, Goodell P. Arsenic Mineral in Volcanic Tuff, a Source of Arsenic Anomaly in Groundwater: City of Chihuahua, Mexico. Geosciences. 2022; 12(2):69. https://doi.org/10.3390/geosciences12020069
Chicago/Turabian StyleRen, Minghua, José Alfredo Rodríguez-Pineda, and Philip Goodell. 2022. "Arsenic Mineral in Volcanic Tuff, a Source of Arsenic Anomaly in Groundwater: City of Chihuahua, Mexico" Geosciences 12, no. 2: 69. https://doi.org/10.3390/geosciences12020069
APA StyleRen, M., Rodríguez-Pineda, J. A., & Goodell, P. (2022). Arsenic Mineral in Volcanic Tuff, a Source of Arsenic Anomaly in Groundwater: City of Chihuahua, Mexico. Geosciences, 12(2), 69. https://doi.org/10.3390/geosciences12020069





