Abstract
The establishment of quality requirements of clay-based products, for medicinal, wellness, and aesthetic purposes, is mainly sustained by the good interactions between the clay-based formulation and the skin. The release of ionizable elements and their availability to percutaneous absorption should be, ideally, physiologically effective during passive percutaneous absorption. Clay-based products are promoted in the European market as therapeutic clays or aesthetics, which is labeling that combines characteristics of medicinal products along with cosmetics. Different countries regulate these products under different legal frameworks. This study focuses on the mineralogical, chemical, and technological characterization of some clay-based products available on the market, designed for topical use, framed in the peloids concept, and claimed as natural products. The main goals are to contribute to the establishment of clay-based products quality criteria as reliable scientific information, aiming for the compliance of intended use, the information for the potential health hazards and toxicological effects of clay-based products, and the distinction in what concerns therapeutic compliance and aesthetic or wellbeing product certification. There were 13 clayed products for cosmetic purposes, available online and in commercial stores, together with three thermal peloids, that were studied. Mineralogical composition of the 16 studied samples reveals a polymineralic association with the presence of variable quantities of quartz, calcite, and feldspars, whereas clay minerals are not predominant and characterized by the presence of clay-based fraction content, composed mainly by illite, smectite, and kaolinite in variable amounts and with several mineral associations. The clay-based products contain median values of 17 ppm As, 315 ppm Ba, 79 ppm Cr, 11 ppm Co, 29 ppm Pb, 26 ppm Ni, and 62 ppm Zn. One sample presented 4.1 ppm of Cd. The studied samples have safety concerns about specific limits of As, Ba, Cd, Cr, Co, Pb, Ni, and Zn which are above the regulated avoidable limits. Samples’ pH is out of range of skin’s natural pH as well.
1. Introduction
Clays for medicinal, wellness, and aesthetic purposes were ancestrally used by mankind topically (peloids or muds) or by ingestion [1].
Peloids, resulting from the mixing of clay and mineral–medicinal water, have been used in many thermal centers, more recently in designated spas, as a distinguished therapeutic modality, pelotherapy. All over the world, we can find different peloids that can be highlighted by their peculiar chemical and mineralogical composition, physical proprieties, and also by the biological–metabolic activity of micro-organisms, e.g., Poça da Dona Beija (Portugal—S. Miguel Island, Azores), Porto Santo (Portugal—Madeira Island), Dax peloid (France), Copahue spa (Argentina), Arnedillo, Caldas de Boí, and El Raposo spas (Spain), Abano–Montegrotto spas (Padua, Italy), among others.
The majority of these peloids are naturally maturated with the autochthonous water and clays on the outskirts of thermal centers, or they are prepared artificially (paramud), sometimes enhanced with cosmetic ingredients performers—botanicals, algae, or diatoms—as well as colored additives or flavors. Some of these peloids are sold for cosmetic purposes and personal healthcare usage, a parallel business to the gist of pelotherapy, and are now gaining some relevance in the wellness field and in Health Tourism.
The heated muds, when in contact with the skin, promote several reactions responsible for the therapeutic effect of the clay essential elements [2]. Several clay-based products (peloids) have a traditional therapeutic history, being easily available in nature for people use [3,4,5,6] or are available on the market as 100% natural products [7]. Many of these products have no pharmaceutical or cosmetic control, and they are not free of possible side effects on human health [8,9].
The pharmaceutical and cosmetic industry uses clays in their formulations that are subject to prior control before being used [10,11]. Clays are used in pharmaceuticals, as excipients or as active substances, due to their high retention capacity, colloidal and expansive properties, usefulness for the modulation of drug release in the organism [12], chemical inertia, and low or non-toxicity to the patient [13].
Pelotherapy’s studies emphasized the suitable properties of peloids when applied to the skin, such as water retention capacity, consistency, adhesiveness, heat capacity, cooling rate, cation exchange capacity, handling, and pleasant sensation [14,15,16]. The focus on the thermotherapeutic effect of the peloid as having an important healing action, by the heat released when in contact with the skin, is still a transversal property for the characterization of peloids for therapeutic purposes [17]. The importance of not neglecting the mobility of some chemical elements with toxicity potentiality after their absorption by the skin [9,13,18] is now considered with strong scientific relevance, which is framed by the legal conformity associated with consumer safety and health users. Contemporaneous studies found evidence of the need for quality criteria establishment and certification of clay-based products intended to be used topically, namely thermal muds, which have therapeutic action and are applied directly onto the skin on thermal centers [15,16,19], as well as the proposal of some methodologies for clays’ decontamination before their incorporation into cosmetics, to achieve the limits required for cosmetic safety [20].
The requirements’ definitions for good interaction between the clay-based formulation and the skin are sustained on the increased release of ionizable elements and their disposal to percutaneous absorption. The results should, ideally, be physiologically effective during passive percutaneous absorption [18]. The solubility, molecular mass, depth of penetration, and toxicology of the clay components while a topic vehicle need to be considered in the percutaneous absorption. The cation exchange capacity (CEC) and the other formulation characteristics may define the percutaneous depth efficacy that ions may reach, as well as the desirable absorption by the skin. The success of a complete percutaneous action can be observed by the pain relief, the anti-inflammatory action, the range improvement of the upper limb movements, the antibacterial action, the healing action [21], and others therein. These clay-based products are mainly used in rehabilitation programs at thermal centers and spas, being associated with musculoskeletal and tendon injuries, rheumatic pathologies, dermatological infirmities, or for aesthetic purposes and skincare. The biological and physiological mechanisms of how mud applications alleviate symptoms of several pathologies, in the dermatological and rheumatological field, are still not completely understood [22,23,24,25,26,27].
Considering the current European regulatory and legal framework on cosmetic products, namely the EC No. 1223/2009, it is observed that it is the nearest compliance guideline for the quality criteria establishment of peloids or clay-based products. The Directive 76/768/EC, which was adopted in 1976, was replaced by Regulation (EC) No 1223/2009 from 2013 onwards due to the many amendments made to it and the new amendments that were required. According to the actual European Regulation on cosmetic products, a cosmetic product is defined as “any substance or mixture intended to be placed in contact with the external parts of the human body with the main, or exclusive aim, of cleansing or perfuming it, changing their appearance or smell, as well as protecting and maintaining it”. We can find a similar definition by the Federal Food, Drug, and Cosmetic Act, 2021 [28]; however, the labeling requirements of the same product, regulated as cosmetic in Europe, could be regulated as a drug in the United States. The term sunscreen or sun protection meets the definition of a cosmetic product by the European regulation, whereas the FDA considers it as a drug because it is expected to be a product for skin protection from the harmful effects of the sun. In Europe, the legislation on medicinal products is not fully harmonized and may be classified under national regulations. The safety evaluation of cosmetics, in what concerns the chemical content, is regulated by the provisions of EC No. 1223/2009, the EU REACH Regulation (EC No. 1907/2006), Commission Regulation on claims in cosmetics products (EU No. 655/2013), and with national laws where cosmetics products will be on market. However, some products are defined as borderline products when it is unclear whether a product is a cosmetic within the definition in the cosmetic regulation or whether it falls under other sectorial legislation [29]. Nevertheless, from a technological standpoint, there is no regulation or criteria established either for clay-based cosmetics or for peloid formulations.
Viseras et al. (2022) debated the European cosmetic regulation, supported by the rules of the intended use and by the different categories and typologies, according to the European cosmetic directive 76/768/ECC list in annex I and the European association of cosmetics fabricants. The use of clays and derivates as ingredients were used in numerous formulations for commercial cosmetics products with both technological and cosmetological functions [28].
Some clay-based products (zeolites and bentonite-montmorillonite) are presented in the market as natural medicines for ingestion, with a detoxification action and a protective effect on the mucous membrane of the gastrointestinal tract, and they are certified as medical devices.
The European legal framework governing medical devices, Regulation (EU) 2017/745, defines a medical device as “any instrument, apparatus, appliance, software, implant, reagent, material or other article intended by the manufacturer to be used, alone or in combination, for human beings for one or more specific medical purposes”. This medical device regulation is much closer to that of the FDA prerequisites for the quality conformity assessment. European Regulation and FDA requires the determination of a product as a medical device to be settled on the intended use and indications for use. After that, it should be verified if it meets the medical device definition. The definition of a medical device by the FDA consists as “an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including a component part or accessory which is: (i) recognized in the official National Formulary, or the United States Pharmacopoeia, or any supplement to them; (ii) intended for use in the diagnosis of disease or other conditions, or (iii) in the cure, mitigation, treatment, or prevention of disease, in man or other animals, or (iv) intended to affect the structure or any function of the body of man or other animals, and which does not achieve its primary intended purposes through chemical action within or on the body of man or other animals and which is not dependent upon being metabolized for the achievement of any of its primary intended purposes.”
This study aims at the mineralogical, chemical, and technological characterization of some clay-based products available on the market, designed for topical use, framed in peloids concept, and claimed as natural products. The main goals are to yield comparative data that can contribute to the establishment of specific quality parameters and clarify the effect of the differentiated usage of peloids.
2. Materials and Methods
2.1. Materials Description
There were 13 clayed products for cosmetic purposes, available online and in commercial stores, together with three thermal peloids (C5, C10, and C16), that were studied. There were eight samples obtained in a semi-solid formulation (paste), and the remaining samples were acquired in the form of powder. Despite sample C7, which is for ingestion, all samples are indicated for dermal application, generally for face masks, cataplasm, or whole-body use (Table 1 and Table 2). The sample C7 is sold as a medical device. The samples C1, C2, C3, C4, C6, and C8 were formulated with mineral thermal water.

Table 1.
Label composition information of the studied samples.

Table 2.
Label indications for use of the studied samples.
The paste samples are specially designed as peloids with preparations ‘ready-to-use’. The labeled indications/information commonly included healing, cleanse, detox, absorbent, refreshing, calming, decongesting, and energizing actions. However, we can find that there is no harmonized labeled information for the usage and dosage indication. The expected adverse events are centralized in skin reactions, and the safety alerts are based on eye and mouth avoidance.
2.2. Methods
2.2.1. Mineralogy and Granulometry
The samples were frozen, then freeze-dried, and gently disaggregated before testing. Grain size distribution bellow 100 µm particle size was assessed using an X-ray grain size analyzer (Sedigraph III Plus).
Mineralogical analysis of both fine and clay fractions was carried out by X-ray diffraction, using a Philips/Panalytical X’Pert-Pro MPD, Kα Cu (λ = 1,5405 Å) radiation, with 0.02° 2θ s−1 steps in goniometer speed by the random-oriented powders (total sample) and oriented aggregates (<2 µm). The oriented aggregates were treated with glycerol and heat treatment at 500 °C. The identification and semi-quantification of the different mineral phases were based on measured peak areas of the basal reflections, considering the full width at half maximum, and then, weighted by empirically estimated factors or reflection powers [36,37].
2.2.2. Physicochemical Properties
The chemical composition of major and minor elements was assessed by X-Ray fluorescence using a Panalytical AX-IOS PW4400/40 X-ray fluorescence spectrometer, and it provided the data for major chemical elements: SiO2, Al2O3, Fe2O3, TiO2, MnO, CaO, MgO, K2O, Na2O, and P2O5, as well as loss on ignition (LOI) and minor chemical elements: As, Ba, Ce, Co, Cr, Cu, Ga, Mo, Ni, Pb, Rb, Sc, Sn, Th, U, V, Zn, and Zr.
The cation exchange capacity (CEC) was determined using ammonium acetate (CH3COOHNH4) as the saturation solution. This method involves the saturation of 10 g of dried sample with 200 mL of ammonium acetate for 24 h. The exchangeable cations (Na+, K+, Mg2+, and Ca2+) were carried out by ICP Mass Spectrometry (Agilent Technologies 7700Series) after collection of 100 mL of the filtered solution, under vacuum extraction, using Macherey–Nagel MN640d filter paper. The excess of ammonium acetate was rinsed with ethanol until completely cleaned by testing with Nessler reagent. Before conducting CEC analyses, 200 mL deionized water and 2 g of oxide magnesium (MgO) were added to the sample and distillate. Additionally, 100 mL of distilled solution was collected into a volumetric flask with 50 mL of boric acid solution 4% (H3BO3) and a bromocresol indicator (0.1%). CEC determination was concluded with 0.1 N chloride acid (HCl) titration.
The pH was determined by using a HANNA HI 9126 pH meter, previously calibrated with standards (Titisol standard solutions) at pH 4 and pH 7, with an accuracy of ±0.05.
The specific surface area (SSA) was estimated by BET analysis using Micromeritics Instrument Corporation—Gemini II 2370 equipment, and specific heat was found by differential scanning calorimetry (DSC) using a Dynamic Scanning Calorimeter DSC-50 Shimadzu.
Expandability was determined following the Portuguese LNEC Standard E200-1967. It was weighted with 100 g of dried sample and packed into a cylinder supported on a porous plate. The measurement was periodically made using a deflectometer, after hydration of the porous base, until constant value. The difference between initial and final measurement corresponds to the expandability/swelling result [38].
The liquid and plastic limit (LL and PL, respectively), as well as the plasticity index (PI), have been determined following an adaptation of the Portuguese standard NP143-1969 and calculated from Atterberg Limits [38,39].
It was weighted with 100 g of dried clay-based sample and was moistened with demineralized water to obtain a semisolid formulation. For the LL determination, the standard Casagrande cup was half filled with the formulation. Paste was grooved and two rotations per second of the apparatus were inflicted until the narrow cut closed. Number of rotations and weight were taken, and the moisture was dried. There were four measurements taken at decreasing water contents. LL is, by definition, the sample moisture content when the groove is closed by 25 blows. This amount was determined by the number of blows log versus water content graph projection. The PL is based on the determination of moisture content of a 3 mm diameter and 10 cm length cylinder made with the sample when it starts to fracture. The water content is measured by the weight cylinder difference after dried-up. The difference between LL and PL is called the PI.
The abrasiveness index (AI) was determined using an Einheler AT-1000 apparatus following Neubold et al.’s (1982) procedure indications [40]. For this purpose, 50 g of dry sample was mixed with 500 mL of distilled water until complete homogenization. The mixture was put in contact with a clean and dry standard bronze wire, previously weighted, onto the Einheler apparatus, for stirring, during 174,000 revolutions (≈ 96 min). For the samples that break up the net, the procedure is repeated for 87,000 revolutions or 43,500 revolutions (≈ 30 min). At the end of the Einheler test, the wire is cleaned, dried, and weighted [40]. The AI is the result of the bronze wire net weight difference before and after abrasion (wear) by area value.
The relative density of the samples was determined using a pycnometer for solids, with precise measurements of weight and volume. Demineralized water was used for the working liquid.
To estimate the cooling kinetics properties of the samples, 20 g of dried sample was heated at 60 °C for 6 h. Every 30 s, a measurement of temperature was made using a dual thermometer, Lutron TM-906A, starting the measurements at 50 °C, until the sample reached 30 °C.
3. Results
3.1. Mineralogy and Granulometry
The mineralogical associations of studied samples are presented in Table 3 and Table 4, considering XRD data after validation by chemical one. Quartz is always present on samples, whereas carbonates, feldspars, and iron minerals are more variable. Low quantities of dolomite are present in some samples in association with calcite; sample C7 shows Mg calcite. Samples C7 and C8 also present a low quantity of opal, which is naturally associated with the volcanic ground.

Table 3.
Mineralogical composition (wt %) of the studied samples.

Table 4.
Clay fraction (wt %) of the studied samples.
Samples are quite rich in clay minerals, except for samples C4 (7%) and C6, C7, C9, and C10 that reveal less than 35% of the clay minerals content. Samples C2, C11, C13, and C16 are almost pure (more than 80% of clay minerals). Kaolinite content is significant in sample C13 (96%). Most of the samples reveal kaolinite and illite as the main clay minerals, but occasionally, smectite is also present, except for sample C2, which reveals the highest content (100%) of smectite.
For samples C1, C2, C8, C10, C11, C13, C15, and C16, the treatment with glycerol causes the shift of smectite reflection to values around 18.5–18.9 Å, and for samples C3 C5, C6, C10, C11, and C14, they are around 15.0–17.6 Å, which indicates the presence of swelling smectites. The basal spacing exhibit by smectite, in referred samples without treatment, showed reflections around 12–15 Å, suggesting that, in these samples, smectite interlayer space was mainly occupied by divalent cations.
Grain-size data (Figure 1) indicates that samples have a high content of <2 µm fraction (>42%). Paste mud samples revealed a content of clay fraction (<2 µm) between 45% and 92% of the total fraction, whereas powder samples revealed a content between 42% and 99%. The average diameter for paste samples ranges between 0.141 µm and 3.17 µm, and for powder samples, it ranges between 0.103 and 3.29 µm.
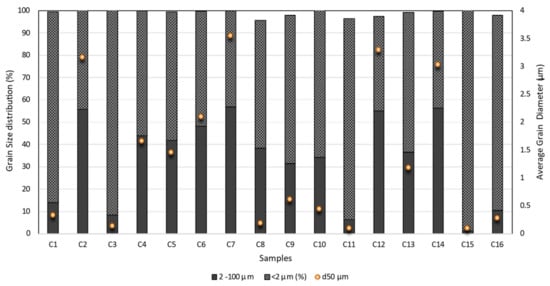
Figure 1.
Grain size distribution and average diameter (d50) of the clay-based samples.
3.2. Physicochemical Properties
3.2.1. Chemical Composition
Geochemical data of the studied samples is provided in Table 5. There are significant variations on SiO2, Al2O3, CaO, and MgO content in the samples. C16 sample shows a very low LOI, considering its phyllosilicate content; this sample was additived by the thermal spa having relevant amounts of paraffin (being defined by them as a parafang), which affects the mineralogical composition assessment. On the contrary, for sample C4, the peculiar high value of LOI is related to calcite and dolomite. MgO content in C1, C2, and C3 samples is very high; the richer, C2, has 85% of phyllosilicates (all smectite), but the other two do not actually show identified (by XRD) mineral phases as being Mg-rich, pointing to the occurrence of amorphous ones (Mg oxides/hydroxides). Sample C8, which has a phyllosilicates content dominated by smectite, shows some disagreements between mineralogical and chemical composition, which is most probably due to amorphous phases, such as on C2, but in this case, sulphates (not oxides) consider the amount of SO3 (8.19%).

Table 5.
Major-element content (wt %) of the studied samples.
The principal component analysis (PCA) was used to characterize samples in what concerns the major chemical elements and LOI composition. The data matrix included the 16 samples studied and 12 variables for chemical composition. The explained variance percentage and the cumulative variance of axes are shown in Table 6 and Table 7. The four axes retention was based on eigenvalues >1 criterion, and selected variables were with values > |0.5| [41]. The first four axes explained 84.61% of the total inertia. The first two axes had eigenvalues of 4.59 and 2.71, respectively, and explain 38.24% and 22.56%, correspondently, of the total inertia. Axis 1 explained Al2O3, Fe2O3, K2O, SO3, MnO, TiO2, and P2O5 in opposition to SiO2, MgO, and LOI; axis 2 explained SiO2 in opposition to CaO, P2O5, and LOI; axis 3 explained MgO and Na2O in opposition to Al2O3; axis 4 explained variables already explained by the other axes.

Table 6.
‘Factor (F) loading’ of the commercial samples and chemical composition, extracted by PCA.

Table 7.
Studied samples’ Principal Analysis Components.
The variable projection on PCA’s first factorial plane established groups of samples according to their chemical affinity (Figure 2).
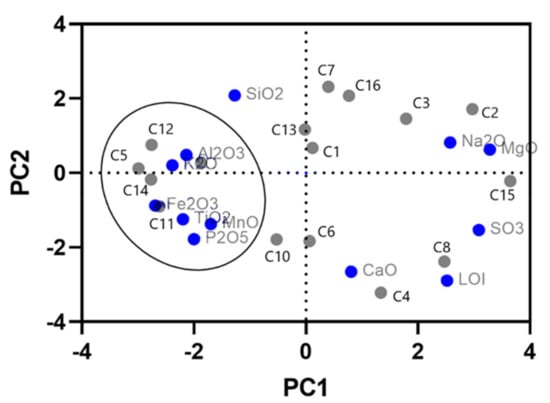
Figure 2.
Studied samples’ Principal Analysis Components projection (PC1/PC2).
Samples C1, C2, C3, C7, C13, and C16 are positively correlated with Na2O–MgO association in opposition to samples C11 and C14 that are positively correlated with CaO–SO3 association.
In axis 2, samples may be distinguished by Al2O3 higher content (C5, C9, and C12) and with more Fe2O3–TiO2–P2O5–MnO content (C11 and C14).
According to ordination diagram, associations between chemical composition and mineralogy signature can be established:
- sample C13’s high concentration of Al2O3 reflects its high kaolinite content;
- the highest values on K2O and Fe2O3 can be associated to mica/illite contents and distinguished C5, C9, C11, C12, and C14 samples;
- samples C4 and C6 reveal higher values of CaO and LOI, which can be associated with their carbonate minerals composition (calcite and dolomite contents);
- samples C2 and C3 are differentiated by Na2O content reflecting the halite (C3) and smectite (C2) contents;
- sample C15 is distinguished by the MgO value related to its dolomite content and due to the nature of the clay, which is a magnesian smectite.
The highest concentrations of potential toxic elements were present on the listed samples: C11 (As-44.7 ppm); C14 (Ba-690 ppm); C12 (Cr-230 ppm); C6 (Ni-83.1 ppm); C13 (Pb-61.3 ppm); C5 (Zn-130 ppm) (Table 8).

Table 8.
Trace element content (ppm) of the studied samples and the acceptable limits for heavy metals in topic products [42,43,44].
3.2.2. Cation Exchange Capacity
Exchangeable cations (EC) and CEC (cation exchange capacity) are variable (Table 9); CEC value is, in some samples, relatively unrelated to the semi-quantitative mineralogical composition, especially with the smectite content. CEC is assessed on fine fraction, and mineralogical composition (Table 3) is related to the whole sample.

Table 9.
Cation exchange capacity and individual main exchangeable cations.
The highest CEC value was achieved for C2 sample (45 meq/100 g), which was richer in phyllosilicates (clay faction 100% smectite), and the lowest was for C4 and C8 samples (1 meq/100 g). The values of CEC higher than 40 meq/100 g were registered on samples C2 and C16 with the main exchangeable cation Na+. The samples with illite predominance present a CEC value between 4–34 meq/100 g.
Except for sample C7, which presents K+ as the main exchangeable cation, and C1, C2, C3, and C16, which present Na+, the remaining samples reveal Ca2+ as the main exchangeable cation (values between 48 mg/L and 1225 mg/L). The samples C1, C7, and C10 have an approximate value for CEC despite the difference of the exchangeable cations.
3.2.3. pH
In Table 10, the studied samples present pH values ranging from 3.96 and 10.30. The C8 sample is volcanic, and its mineralogy justifies the lower pH, while the other samples have an average pH of 8.04 and a standard deviation of 1.05.

Table 10.
pH values of the studied samples.
3.2.4. Specific Surface Area, Expandability, Abrasiveness Index, and Relative Density
The technological analyses’ results for specific surface area, expandability, abrasiveness, and relative density are presented in Table 11.

Table 11.
Sample technological analysis.
The plasticity properties of the samples showed a high range of value variation (Figure 3). The liquid limit (LL) values are variable between 32% (sample C4) and 235% (sample C1). Therefore, according to Bain (1971) [45] classification, samples can be distinguished in high plasticity clays due to the LL results above 50%, except for C4, C14, and C12 samples, which are classified as low plasticity clays because of their LL result under 50%.
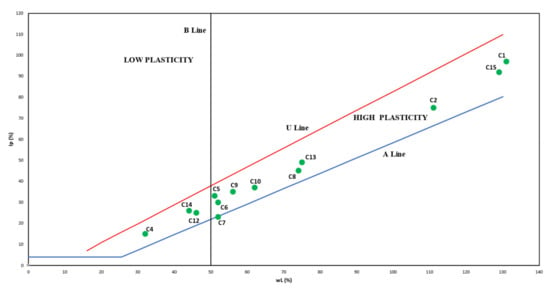
Figure 3.
Projection of plasticity indices and liquid limits in the Casagrande Chart. (U Line, limit above which raw materials do not turn out to be plastic: LL ≥ PL; A Line, frontier between clays with or without organic colloids and B Line, LL = 50%, frontier between low (LL < 50%) and high (LL > 50%) plasticity clays).
The plastic limit (PL) results reveal values between 15% (sample C4) and 186% (sample C1). Classification according to Jenkins [45] assumes that soils are considered with high plasticity when P.L. is above 15%; thus, all samples studied are evaluated as highly plastic. Samples revealed high plasticity according to Atterberg limits because they present high plasticity when PI is above 15%. Sample C4 showed the lowest plasticity index (15%), and C1 showed the highest plasticity index (186%) for a median value of 55% for all samples. It was not possible to test samples C3 and C16 regarding their high LL. Although it was possible to measure samples C1 and C2, samples C11 and C15 also reveal higher LL results and, therefore, better plasticity behavior among the studied samples. These samples should exhibit the higher water retention capacity and, consequently, be the most appropriate to form pastes with good consistency for topic application.
The specific surface area (SSA) of minerals is an important parameter to quantify the clay minerals’ dissolution and adsorptive interactions. The grain size distribution and clay mineralogy have important roles on SSA properties. All samples have a predominant content of <2 µm fraction and different mineralogy, showing values that are sometimes far away from what is expected from the mineralogical composition and literature [46]; studied samples are not pure clays, containing (at least some of them) amorphous components (oxides/hydroxides and/or sulphates) and additives (even organics), disturbing the theoretical relationships between mineralogical composition and technological parameters.
The lowest SSA, <11 m2/g, is found in samples C4, C6, C8, C12, C13, and C14, and the highest values are in samples C1 (81 m2/g) and C11 (70 m2/g). The remaining samples present SSA values between 22 m2/g and 46 m2/g.
The density samples present values around 2ρs and 3ρs. The specific heat enhances the samples C5, C6, C8, and C12 for the lower heat capacity (≤104 j/kg.K).
The abrasiveness index indicates the ability of a sample to cause abrasion and could differentiate the use purpose of a product. Results reveal that abrasiveness varies between 296 (sample C15) and 9 (sample C16) at 174,000 rpm, and the median value is 148 g/m2. Samples C4, C5, C8, C10, C12, C14, and C15 have the AI above 100 g/m2 (Table 12), detaching C5, C6, C12, and C14 as the most abrasive, followed by C4 and C10.

Table 12.
Einlehner abrasivity of the studied samples.
3.2.5. Cooling Kinetics
The cooling rate ranged between 8 min (sample C1 and C3) and 56 min (sample C15, with 100% on clay fraction) (Figure 4). The higher cooling rates were achieved by C10, C11, C15, and C16 (>45 min). Samples C1, C2, and C3 achieve quick cooling. All the other samples, except C7 for oral intake (geophagy), which are intended for aesthetic or thermal mud formulation, cooled from 60 °C to 30 °C within 25–56 min, which is favorable to the established therapeutic procedures.
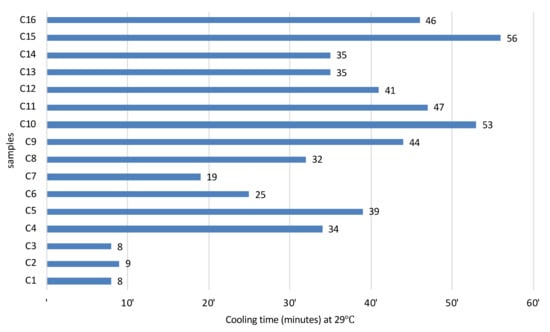
Figure 4.
Cooling kinetics of the studied samples, from 60 °C to 30 °C.
The measurement of the dry clay may be an indicator of how it will be the thermal kinetics when prepared for pelotherapy use.
4. Discussion
Personal care products (PCP) that contain substances of natural origin are considered, by the natural and organic cosmetics consumers, safe alternatives to synthetic ingredients. The main orientations in clay-based products and peloids cover, beside the mineralogical and chemical composition characterization of the raw materials for a basic formulation, the interaction mechanism between the product and the skin to aid consumers’ therapeutic or aesthetic expectations, ensuring toxicological safety and low risk to the health.
The toxicological effects of clay-based hazardous components exceeding the recommended level by the international regulations may be difficult to avoid, during the raw material selection and manufacturing process, because of the high variability on toxic minerals and/or trace chemical elements in the geological materials. Considering factors such as how humans are exposed to heavy metals, how often, and in what amounts, surveys should be conducted to evaluate the extent of the toxicity of elements such as arsenic, cadmium, chromium, cobalt, lead, mercury, and nickel.
The safety evaluation of cosmetics is settled out in the European Regulation on cosmetic products (EC No. 1223/2009), as amended, with a list of more than 1300 substances and group substances that cannot be included in the composition of cosmetic products (Annex II). A list substance, which is a cosmetic or PCP, may be contained only under the restrictions and conditions laid down (Annex III).
The EC No.1223/2009 also contains lists of colorings (Annex IV), preservatives (Annex VI), and UV filters (Annex VII) permitted in cosmetic products. Germany reduced, even more, those limits for heavy metals in cosmetics and PCP, according to the typology: cosmetic products in general and toothpaste (Lead 2.0 and 0.5 mg/kg; Arsenic 0.5 and 2.5 mg/kg, respectively; Mercury 0.1 mg/kg; Cadmium 0.1 mg/kg; Antimony 0.5 mg/kg and Nickel’s limit remained unchangeable at 10 mg/kg) [44].
The United States FDA (Food and Drug Administration) also limits the presence of metals in cosmetics, as well as prohibits and restricts ingredients, by conducting regular surveys of cosmetics on the market [47].
It is urgent to clarify the classification of natural mineralogical products or mineralogical PCP ingredients and provide their declared information (CAS number) for the safe use of chemicals and replacement of substances that give cause for concern, with the supervision of ECHA—European Chemicals Agency, and they need to be registered according to REACH regulation. Cosmetic and personal care products’ chemical safety is an important issue with transversal concerns, documented in several studies [48,49,50,51,52,53,54].
As cosmetics and medicinal products are intended to be used topically or orally, technological features must be evaluated and adequate for the intended use. The technological characterization of materials is also imperative on clay-based products, mainly when used directly on the skin, such as peloids, which have no recommendation values or quality criteria established.
Taking into consideration that cosmetics and peloids have the same exposure route, it is reliable to assume that the technologic characterization of clay-based commercial products for aesthetic purposes may contribute to the establishment of normative values and criteria for peloids. However, when a clay-based product or peloid also meets the definition of the medicinal product, it will oblige the required compliance for the intended use.
For this study, the 13 clay-based products were acquired with the assumption that these were well accepted by the user and, therefore, presented pleasant and adequate characteristics for dermatological application. The same assumption is applied for the thermal peloids: they are applied under therapeutic personnel supervision in thermal centers and welcomed by their users.
Samples reveal a polymineralic association with the presence of variable quantities of quartz, calcite, and feldspars, whereas clay minerals are predominant and common to other peloid compositions [16,55,56].
The mineralogical composition of samples reflects the chemical signature, which was evaluated by PCA analysis and could be affecting some technological features exhibited by samples, such as plasticity, abrasivity index, and cation exchange capacity. Nevertheless, some technological property values seem to be inconsistent with mineralogical analysis results; our samples are commercial products, not pure clays, having (at least some of them) amorphous components (oxides/hydroxides and/or sulphates) and additives (even organics), disturbing the theoretical relationships between mineralogical composition and technological parameters.
Some elements, such as Ca, Mg, K, and Na, are considered essential elements for human health [56,57]. Recent studies proved the chemical elements mobilization between thermal muds and artificial sweat—or even human skin—with prominence to the significant supply of Na and Ca [11,18]. Samples studied show Ca2+ as the main exchangeable cation, which can be a useful feature concerning human health. The CEC results suggest that cation exchange may be influenced by the particle median size, as well as clay minerals content. This fact can explain the CEC results, which are relatively low when compared to some reference values (kaolinite: <15–20 meq/100 g; illite and chlorite: 10–40 meq/100 g; smectite: 100–200 meq/100 g) [45,58].
Skin pH plays an important role in its protection mechanism. The skin’s natural pH is slightly acidic, and its values range from 4.1 to 5.8, which is important to prevent the development of bacteria and the maintenance of natural flora. Inadequate skin pH may contribute to various dermatological infirmities [59]. The pH assessment of peloids is an important parameter not only to predict the risk of developing some side effects of the skin but also to stabilize its natural balance. Alkaline clay-based samples are out of the skin’s natural pH, which should be paid attention to as quality and safety control.
Plasticity behavior may be affected by several physico-chemical parameters such as mineralogy, particle size, aggregation/disaggregation situation, and CEC. The studied samples reveal plasticity index values above the reference for kaolinite (26–37) but, as expected, lower than the smectite reference value (101–251) [45]. Nevertheless, all samples were considered highly plastic, which makes them suitable during their manipulation and application.
Abrasiveness and cooling kinetics can characterize the handling, pleasant sensation, and drying time of the products into the skin. Samples with higher AI may be more adequate for the exfoliating purpose and cleansing, which is ideal for establishing skin balance.
The association between abrasivity index (AI) and mineralogical signature of samples can be made. Sample C13, on PCA analysis, reflected kaolinite content and revealed lower AI (13 g/m2) when compared to groups formed by the association with carbonate minerals (samples C4, C6, and C8) or illite and iron minerals (samples C5, C9, C11, C12, and C14).
The highest abrasiveness behavior occurs on samples with higher content on detrital minerals, such as quartz, feldspars, and iron minerals (samples C4, C5, C6, C10, C12, and C14). The clay-based samples, C4, C5, C6, and C10, are semi-solid formulations (paste), with mineral medicinal water as an ingredient, and C12 and C14 are natural clays in form of powder.
The lowest abrasiveness behavior of samples C1, C2, C3, C9, and C16 may be explained by the fact that these samples are the result of a blend of natural origin ingredients and other artificial performer additives, defined by Gomes et al. (2013) as Paramud or Parapeloid [2].
Cooling kinetics characterize the sample’s ability to retain or release heat. This feature is more relevant in what concerns thermal mud application because, in most cases, the intent of use is to sustain the product temperature above corporal temperature for about 15 to 20 min. The measurement of the cooling rate was made on dry samples, which means that an improvement of cooling rate is expected with the moisturizing of materials [13,16,60,61].
The concentration of trace elements (As, Ba, Cd, Ce, Co, Cr, Cu, Ga, Mo, Ni, Pb, Rb, Sc, Sn, Th, U, V, Zn, and Zr) in each sample was studied to evaluate the health risk through exposure to different elements. The presence of hazardous elements must be previously determined to evaluate the risk of dermal exposure to toxic elements. The clay-based products contain median values of 17 ppm As, 315 ppm Ba, 79 ppm Cr, 11 ppm Co, 29 ppm Pb, 26 ppm Ni, and 62 ppm Zn. Only sample C11 presented 4.1 ppm of Cd. It was not detected with As, Co, Cr, Mo, Ni, and V at sample C7 for ingestion intake. Arsenic is mostly found in a free state; it is formed as sulfur, oxygen, and iron compounds. Therefore, it must be free to cause a skin problem.
Table 13 presents the technological properties of the samples studied and their framework with their qualitative requirements for skin and clay-based product interaction previously debated. It also introduced the relevance in comparative terms with a clay-based product, for ingestion intake, certified as a medical device.

Table 13.
Studied samples’ qualitative requirements establishment by technological, thermal, and rheological properties.
5. Conclusions
Different countries regulate these clay-based products under different frameworks. The absence of premarket approval, mainly for clay-based products that are intended for a transdermal therapeutic purpose, such as treating or preventing infirmities, makes it difficult for consumers to determine the safety and effective clinical compliance. The importance of labeling uniformity and product safety information, ensuring the use of warning statements, is also an issue of quality compliance that is missing.
The mineralogical composition of the 16 studied samples reveals a polymineralic association with the presence of variable quantities of quartz, calcite, and feldspars, whereas clay minerals are not predominant and characterized by the presence of a clay-based fraction content, composed mainly by illite, smectite, and kaolinite in variable amounts and several mineral associations. The mineralogical composition of the studied samples is not always in accordance with the label information, which is the case for C1 and C3 samples.
The studied samples have safety concerns about specific limits of As, Ba, Cd, Cr, Co, Pb, Ni, and Zn, which are above the regulated avoidable limits. Additionally, the pH of the samples is out of range of the skin’s natural pH.
It was important to compare the results of a clay-based product for ingestion intake, which were certified and safe monitored (pharmacovigilance). The postmarketing surveillance databases of these clay-based medical devices, which monitor the adverse reactions and product effectiveness, can be a way to previously refine the safety of a topical clay-based product or peloid.
Author Contributions
Conceptualization, C.M.B. and F.R.; methodology, C.M.B. and F.R.; resources, C.M.B. and F.R.; investigation, C.M.B. and F.R.; writing—original draft preparation, C.M.B.; writing—review and editing, C.M.B. and F.R.; supervision, F.R.; project administration, C.M.B.; funding acquisition, C.M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FCT—Fundação para a Ciência e a Tecnologica and Exatronic, Lda., grant number SFRH/BDE/11062/2015 and also supported by GeoBioTec Research Center (UIDB/04035/2020), funded by FCT, FEDER funds through the Operational Program Competitiveness Factors—COMPETE.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to Balneario El Raposo, Termas de Monchique and Régie Municipale des Boues de Dax for providing samples for this study, of great clinical relevance in the treatment and prevention of musculoskeletal disorders.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gomes, C.D.S.F. Healing and edible clays: A review of basic concepts, benefits and risks. Environ. Geochem. Health 2018, 40, 1739–1765. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.; Carretero, M.I.; Pozo, M.; Maraver, F.; Cantista, P.; Armijo, F.; Legido, J.L.; Teixeira, F.; Rautureau, M.; Delgado, R. Peloids and pelotherapy: Historical evolution, classification and glossary. Appl. Clay Sci. 2013, 75–76, 28–38. [Google Scholar] [CrossRef]
- Calderan, A.; Carraro, A.; Honisch, C.; Lalli, A.; Ruzza, P.; Tateo, F. Euganean therapeutic mud (NE Italy): Chlorophyll a variations over two years and relationships with mineralogy and geochemistry. Appl. Clay Sci. 2020, 185, 105361. [Google Scholar] [CrossRef]
- Komar, D.; Dolenec, T.; Dolenec, M.; Vrhovnik, P.; Lojen, S.; Belak, Z.L.; Kniewald, G.; Šmuc, R. Physico-chemical and geochemical characterization of Makirina Bay peloid mud and its evaluation for potential use in balneotherapy (N Dalmatia, Republic of Croatia). Indian J. Tradit. Knowl. 2015, 1, 5–12. [Google Scholar]
- Da Silva, P.S.C.; Torrecilha, J.K.; Gouvea, P.F.D.M.; Máduar, M.F.; de Oliveira, S.M.B.; Scapin, M.A. Chemical and radiological characterization of Peruíbe Black Mud. Appl. Clay Sci. 2015, 118, 221–230. [Google Scholar] [CrossRef]
- Baschini, M.; Pettinari, G.; Vallés, J.; Aguzzi, C.; Cerezo, P.; Galindo, A.L.; Setti, M.; Viseras, C. Suitability of natural sulphur-rich muds from Copahue (Argentina) for use as semisolid health care products. Appl. Clay Sci. 2010, 49, 205–212. [Google Scholar] [CrossRef]
- Khiari, I.; Mefteh, S.; Sánchez-Espejo, R.; Cerezo, P.; Aguzzi, C.; López-Galindo, A.; Jamoussi, F.; Iborra, C.V. Study of traditional Tunisian medina clays used in therapeutic and cosmetic mud-packs. Appl. Clay Sci. 2014, 101, 141–148. [Google Scholar] [CrossRef]
- Khiari, I.; Sánchez-Espejo, R.; García-Villén, F.; Cerezo, P.; Aguzzi, C.; López-Galindo, A.; Jamoussi, F.; Viseras, C. Rheology and cation release of tunisian medina mud-packs intended for topical applications. Appl. Clay Sci. 2019, 171, 110–117. [Google Scholar] [CrossRef]
- Tateo, F.; Summa, V. Element mobility in clays for healing use. Appl. Clay Sci. 2007, 36, 64–76. [Google Scholar] [CrossRef]
- Carretero, M.I.; Pozo, M. Clay and non-clay minerals in the pharmaceutical industry: Part I. Excipients and medical applications. Appl. Clay Sci. 2009, 46, 73–80. [Google Scholar] [CrossRef]
- Carretero, M.I.; Pozo, M. Clay and non-clay minerals in the pharmaceutical and cosmetic industries Part II. Active ingredients. Appl. Clay Sci. 2010, 47, 171–181. [Google Scholar] [CrossRef]
- Viseras, C.; Aguzzi, C.; Cerezo, P.; Galindo, A.L. Uses of clay minerals in semisolid health care and therapeutic products. Appl. Clay Sci. 2007, 36, 37–50. [Google Scholar] [CrossRef]
- Carretero, M. Clay minerals and their beneficial effects upon human health. A review. Appl. Clay Sci. 2002, 21, 155–163. [Google Scholar] [CrossRef]
- Armijo, F.; Maraver, F.; Pozo, M.; Carretero, M.I.; Armijo, O.; Fernández-Torán, M.; Fernández-González, M.V.; Corvillo, I. Thermal behaviour of clays and clay-water mixtures for pelotherapy. Appl. Clay Sci. 2016, 126, 50–56. [Google Scholar] [CrossRef]
- Karakaya, M.Ç.; Karakaya, N.; Sarıoğlan, Ş.; Koral, M. Some properties of thermal muds of some spas in Turkey. Appl. Clay Sci. 2010, 48, 531–537. [Google Scholar] [CrossRef]
- Veniale, F.; Bettero, A.; Jobstraibizer, P.; Setti, M. Thermal muds: Perspectives of innovations. Appl. Clay Sci. 2007, 36, 141–147. [Google Scholar] [CrossRef]
- Casás, L.; Pozo, M.; Gómez, C.; Pozo, E.; Bessières, L.; Plantier, F.; Legido, J. Thermal behavior of mixtures of bentonitic clay and saline solutions. Appl. Clay Sci. 2013, 72, 18–25. [Google Scholar] [CrossRef]
- Tateo, F.; Ravaglioli, A.; Andreoli, C.; Bonina, F.; Coiro, V.; Degetto, S.; Giaretta, A.; Orsini, A.M.; Puglia, C.; Summa, V. The in-vitro percutaneous migration of chemical elements from a thermal mud for healing use. Appl. Clay Sci. 2009, 44, 83–94. [Google Scholar] [CrossRef]
- Quintela, A.; Terroso, D.; da Silva, E.F.; Rocha, F. Certification and quality criteria of peloids used for therapeutic purposes. Clay Miner. 2012, 47, 441–451. [Google Scholar] [CrossRef]
- Favero, J.D.S.; Parisotto-Peterle, J.; Weiss-Angeli, V.; Brandalise, R.N.; Gomes, L.B.; Bergmann, C.P.; dos Santos, V. Physical and chemical characterization and method for the decontamination of clays for application in cosmetics. Appl. Clay Sci. 2016, 124–125, 252–259. [Google Scholar] [CrossRef]
- Viseras, C.; Carazo, E.; Borrego-Sánchez, A.; García-Villén, F.; Sánchez-Espejo, R.; Cerezo, P.; Aguzzi, C. Clay Minerals in Skin Drug Delivery. Clays Clay Miner. 2019, 67, 59–71. [Google Scholar] [CrossRef]
- Cozzi, F.; Ciprian, L.; Carrara, M.; Galozzi, P.; Zanatta, E.; Scanu, A.; Sfriso, P.; Punzi, L. Balneotherapy in chronic inflammatory rheumatic diseases—A narrative review. Int. J. Biometeorol. 2018, 62, 2065–2071. [Google Scholar] [CrossRef] [PubMed]
- Morer, C.; Roques, C.-F.; Françon, A.; Forestier, R.; Maraver, F. The role of mineral elements and other chemical compounds used in balneology: Data from double-blind randomized clinical trials. Int. J. Biometeorol. 2017, 61, 2159–2173. [Google Scholar] [CrossRef] [PubMed]
- Forestier, R.; Forestier, F.B.E.; Francon, A. Spa therapy and knee osteoarthritis: A systematic review. Ann. Phys. Rehabil. Med. 2016, 59, 216–226. [Google Scholar] [CrossRef]
- Tenti, S.; Cheleschi, S.; Galeazzi, M.; Fioravanti, A. Spa therapy: Can be a valid option for treating knee osteoarthritis? Int. J. Biometeorol. 2015, 59, 1133–1143. [Google Scholar] [CrossRef]
- Güngen, G.; Ardic, F.; Fιndıkoğlu, G.; Rota, S. The effect of mud pack therapy on serum YKL-40 and hsCRP levels in patients with knee osteoarthritis. Rheumatol. Int. 2012, 32, 1235–1244. [Google Scholar] [CrossRef]
- Bellometti, S.; Cecchettin, M.; Galzigna, L. Mud pack therapy in osteoarthrosis: Changes in serum levels of chondrocyte markers. Clin. Chim. Acta 1997, 268, 101–106. [Google Scholar] [CrossRef]
- Viseras, C.; Sánchez-Espejo, R.; Palumbo, R.; Liccardi, N.; García-Villén, F.; Borrego-Sánchez, A.; Massaro, M.; Riela, S.; López-Galindo, A. Clays in cosmetics and personal-care products. Clays Clay Miner. 2021, 69, 561–575. [Google Scholar] [CrossRef]
- Bastos, C.M.; Rocha, F.; Gomes, N.; Marinho-Reis, P. The Challenge in Combining Pelotherapy and Electrotherapy (Iontophoresis) in One Single Therapeutic Modality. Appl. Sci. 2022, 12, 1509. [Google Scholar] [CrossRef]
- Antonelli, M.; Donelli, D. Mud therapy and skin microbiome: A review. Int. J. Biometeorol. 2018, 62, 2037–2044. [Google Scholar] [CrossRef]
- Knorst-Fouran, A.; Casás, L.; Legido, J.; Coussine, C.; Bessières, D.; Plantier, F.; Lagière, J.; Dubourg, K. Influence of dilution on the thermophysical properties of Dax peloid (TERDAX®). Thermochim. Acta 2012, 539, 34–38. [Google Scholar] [CrossRef]
- Rossi, D.; Jobstraibizer, P.G.; Bosco, C.D.; Bettero, A. A combined chemico-mineralogical and tensiometric approach for evaluation of Euganean Thermal Mud (ETM) quality. J. Adhes. Sci. Technol. 2013, 27, 30–45. [Google Scholar] [CrossRef]
- Poli, A.; Romano, I.; Cordella, P.; Orlando, P.; Nicolaus, B.; Berrini, C.C. Anoxybacillus thermarum sp. nov., a novel thermophilic bacterium isolated from thermal mud in Euganean hot springs, Abano Terme, Italy. Extremophiles 2009, 13, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Ortega, E.; Gálvez, I.; Hinchado, M.D.; Guerrero, J.; Martín-Cordero, L.; Torres-Piles, S. Anti-inflammatory effect as a mechanism of effectiveness underlying the clinical benefits of pelotherapy in osteoarthritis patients: Regulation of the altered inflammatory and stress feedback response. Int. J. Biometeorol. 2017, 61, 1777–1785. [Google Scholar] [CrossRef]
- Moussout, H.; Ahlafi, H.; Aazza, M.; Chfaira, R.; Mounir, C. Interfacial electrochemical properties of natural Moroccan Ghassoul (stevensite) clay in aqueous suspension. Heliyon 2020, 6, e03634. [Google Scholar] [CrossRef]
- Oliveira, A.; Rocha, F.; Rodrigues, A.; Jouanneau, J.; Dias, A.; Weber, O.; Gomes, C. Clay minerals from the sedimentary cover from the Northwest Iberian shelf. Prog. Oceanogr. 2002, 52, 233–247. [Google Scholar] [CrossRef]
- Galhano, C.; Rocha, F.; Gomes, C. Geostatistical analysis of the influence of textural, mineralogical and geochemical parameters on the geotechnical behaviour of the ‘Argilas de Aveiro’ Formation (Portugal). Clay Miner. 1999, 34, 109–116. [Google Scholar] [CrossRef]
- Rebelo, M.; Viseras, C.; López-Galindo, A.; Rocha, F.; da Silva, E.F. Rheological and thermal characterization of peloids made of selected Portuguese geological materials. Appl. Clay Sci. 2011, 52, 219–227. [Google Scholar] [CrossRef]
- Quintela, A.; Costa, C.; Terroso, D.; Rocha, F. Liquid limit determination of clayey material by Casagrande method, fall cone test and EBS parameter. Mater. Technol. 2014, 29 (Suppl. S3), B82–B87. [Google Scholar] [CrossRef]
- Quintela, A.; Costa, C.; Terroso, D.; Rocha, F. Abrasiveness index of dispersions of Portuguese clays using the Einlehner method: Influence of clay parameters. Clay Miner. 2014, 49, 27–34. [Google Scholar] [CrossRef]
- Davis, J.C. Statistics and Data Analysis in Geology; Wiley: New York, NY, USA, 1986; Volume 2, p. 646. ISBN 0-471-83743-1. [Google Scholar]
- Sánchez-Espejo, R.; Aguzzi, C.; Cerezo, P.; Salcedo, I.; López-Galindo, A.; Viseras, C. Folk pharmaceutical formulations in western Mediterranean: Identification and safety of clays used in pelotherapy. J. Ethnopharmacol. 2014, 155, 810–814. [Google Scholar] [CrossRef] [PubMed]
- García-Villén, F.; Sánchez-Espejo, R.; Borrego-Sánchez, A.; Cerezo, P.; Perioli, L.; Viseras, C. Safety of Nanoclay/Spring Water Hydrogels: Assessment and Mobility of Hazardous Elements. Pharmaceutics 2020, 12, 764. [Google Scholar] [CrossRef] [PubMed]
- Bund, B. Technically avoidable heavy metal contents in cosmetic products. J. Consum. Prot. Food Saf. 2017, 12, 51–53. [Google Scholar] [CrossRef]
- Gomes, C. Argilas—Aplicações na Indústria. Aveiro; O Liberal-Empresa de Artes Gráficas, Lda.: Funchal, Portugal, 2002; ISBN 972-8684-12-6. [Google Scholar]
- Karakaya, M.; Karakaya, N.; Aydin, S. The physical and physicochemical properties of some Turkish thermal muds and pure clay minerals and their uses in therapy. Turk. J. Earth Sci. 2017, 26, 395–409. [Google Scholar] [CrossRef]
- Hepp, N.M.; Mindak, W.R.; Gasper, J.W.; Thompson, C.B.; Barrows, J.N. Survey of cosmetics for arsenic, cadmium, chromium, cobalt, lead, mercury, and nickel content. J. Cosmet. Sci. 2014, 65, 125–145. [Google Scholar] [PubMed]
- Aldayel, O.; Hefne, J.; Alharbi, K.N.; Al-Ajyan, T. Heavy Metals Concentration in Facial Cosmetics. Nat. Prod. Chem. Res. 2018, 6, 1. [Google Scholar] [CrossRef]
- Petry, T.; Bury, D.; Fautz, R.; Hauser, M.; Huber, B.; Markowetz, A.; Mishra, S.; Rettinger, K.; Schuh, W.; Teichert, T. Review of data on the dermal penetration of mineral oils and waxes used in cosmetic applications. Toxicol. Lett. 2017, 280, 70–78. [Google Scholar] [CrossRef]
- Iwegbue, C.M.; Bassey, F.I.; Obi, G.; Tesi, G.; Martincigh, B. Concentrations and exposure risks of some metals in facial cosmetics in Nigeria. Toxicol. Rep. 2016, 3, 464–472. [Google Scholar] [CrossRef]
- Ma’Or, Z.; Halicz, L.; Portugal-Cohen, M.; Russo, M.Z.; Robino, F.; Vanhaecke, T.; Rogiers, V. Safety evaluation of traces of nickel and chrome in cosmetics: The case of Dead Sea mud. Regul. Toxicol. Pharmacol. 2015, 73, 797–801. [Google Scholar] [CrossRef]
- Marinovich, M.; Boraso, M.S.; Testai, E.; Galli, C.L. Metals in cosmetics: An a posteriori safety evaluation. Regul. Toxicol. Pharmacol. 2014, 69, 416–424. [Google Scholar] [CrossRef]
- Bocca, B.; Pino, A.; Alimonti, A.; Forte, G. Toxic metals contained in cosmetics: A status report. Regul. Toxicol. Pharmacol. 2014, 68, 447–467. [Google Scholar] [CrossRef] [PubMed]
- Giordani, M.; Mattioli, M.; Cangiotti, M.; Fattori, A.; Ottaviani, M.F.; Betti, M.; Ballirano, P.; Pacella, A.; Di Giuseppe, D.; Scognamiglio, V.; et al. Characterisation of potentially toxic natural fibrous zeolites by means of electron paramagnetic resonance spectroscopy and morphological-mineralogical studies. Chemosphere 2022, 291, 133067. [Google Scholar] [CrossRef] [PubMed]
- Pozo, M.; Carretero, M.I.; Maraver, F.; Pozo, E.; Gómez, I.; Armijo, F.; Rubi, J.A.M. Composition and physico-chemical properties of peloids used in Spanish spas: A comparative study. Appl. Clay Sci. 2013, 83–84, 270–279. [Google Scholar] [CrossRef]
- Summa, V.; Tateo, F. The use of pelitic raw materials in thermal centres: Mineralogy, geochemistry, grain size and leaching tests. Examples from the Lucania area (southern Italy). Appl. Clay Sci. 1998, 12, 403–417. [Google Scholar] [CrossRef]
- De Gomes, C.S.F.; Silva, J.B.P. Minerals and clay minerals in medical geology. Appl. Clay Sci. 2007, 36, 4–21. [Google Scholar] [CrossRef]
- Galindo, A.L.; Viseras, C.; Cerezo, P. Compositional, technical and safety specifications of clays to be used as pharmaceutical and cosmetic products. Appl. Clay Sci. 2007, 36, 51–63. [Google Scholar] [CrossRef]
- Proksch, E. pH in nature, humans and skin. J. Dermatol. 2018, 45, 1044–1052. [Google Scholar] [CrossRef]
- Ferrand, T.; Yvon, J. Thermal properties of clay pastes for pelotherapy. Appl. Clay Sci. 1991, 6, 21–38. [Google Scholar] [CrossRef]
- Veniale, F.; Barberis, E.; Carcangiu, G.; Morandi, N.; Setti, M.; Tamanini, M.; Tessier, D. Formulation of muds for pelotherapy: Effects of “maturation” by different mineral waters. Appl. Clay Sci. 2004, 25, 135–148. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).