Effects of Artificial Sweat Formulation and Extraction Temperature on Estimation of the Dermal Bioaccessibility of Potentially Toxic Elements in a Contaminated Soil from an E-Waste Recycling Site
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Test Substrate
2.2. Determination of pH and Organic Matter (OM)
2.3. Determination of Pseudototal Element Concentration
2.4. Assessment of Soil Contamination
2.5. Dermal Bioaccessibility Tests
- EN 1811:2011, the British Standards Institution artificial sweat formulation for the determination of Ni in body pierced parts [34] (referred to in tables and figures as BSI)
- NIHS 96-10, a quality standard used by the Federation of the Swiss Watch Industry to assess the gold alloy coverings of watch cases and accessories [35] (NIHS)
- A formulation used by Altkofer et al. [36] in their study of the release of nitrosamines from condoms (ALT)
- A formulation used by Ariza et al. [37] in their study of the corrosion resistance of colored ZrNxOy films that have potential applications as decorative coatings on, e.g., eyeglasses (ARI)
- A formulation used by Cheng et al. [38] in their study of the partitioning of volatile organic compounds into sweat (CHE).
2.6. Elemental Analysis and Quality Control
2.7. Statistical Analysis
3. Results and Discussions
3.1. Quality Control
3.2. Characterization of Bulk Composite Soil Sample
| As | Cd | Cr | Cu | Fe | Mn | Ni | Pb | Zn | |
|---|---|---|---|---|---|---|---|---|---|
| Found | 10.1 ± 1.8 | 78.3 ± 5.4 | 88.6 ± 12.0 | 13,700 ± 843 | 73,500 ± 5260 | 1120 ± 92 | 355 ± 26 | 3340 ± 132 | 11,100 ± 618 |
| World soil [45] | 0.1 | 1.1 | 42 | 14 | - | 418 | 18 | 25 | 62 |
| Background † | 5.2 | 0.87 | <37 | 17 | 18,000 | 330 | 13 | 16 | 48 |
| Dutch target values [46] | 29 | 0.8 | 100 | 36 | - | - | 35 | 85 | 140 |
| Dutch intervention values [46] | 55 | 12 | 380 | 190 | - | - | 210 | 530 | 720 |
| Isimekhai et al. [43] | - | 26.4 ± 2.6 | 35.4 ± 3.6 | 3280 ± 277 | - | 115 ± 9 | 40.8 ±4.9 | 2420 ± 289 | 2200 ± 180 |
| Anselm et al. [44] | 5.48 ± 0.38 | 34.9 ± 1.4 | 106 ± 4 | 13,300 ± 195 | 88,000 ± 2700 | 974 ± 38 | 240 ± 15 | 1690 ± 56 | 6600 ± 243 |
| Liu et al. [47] (Zhejiang, China) | nr | 0.2–6.2 | 56–172 | 30–1860 | nr | nr | 21–305 | 18–2060 | 15–2710 |
| Cao et al. [48] (Accra, Ghana) | 4.3–26.2 | <LOD-28.5 | nd | 100–20,400 | nr | nr | nr | 51–12,500 | nr |
| Chakraborty et al. [49] (Indian cites) | <LOD-111 | <LOD-11 | 2–386 | 15–4900 | nr | nr | 4–872 | 2–706 | nr |
3.3. Comparison of Artificial Sweat Solutions
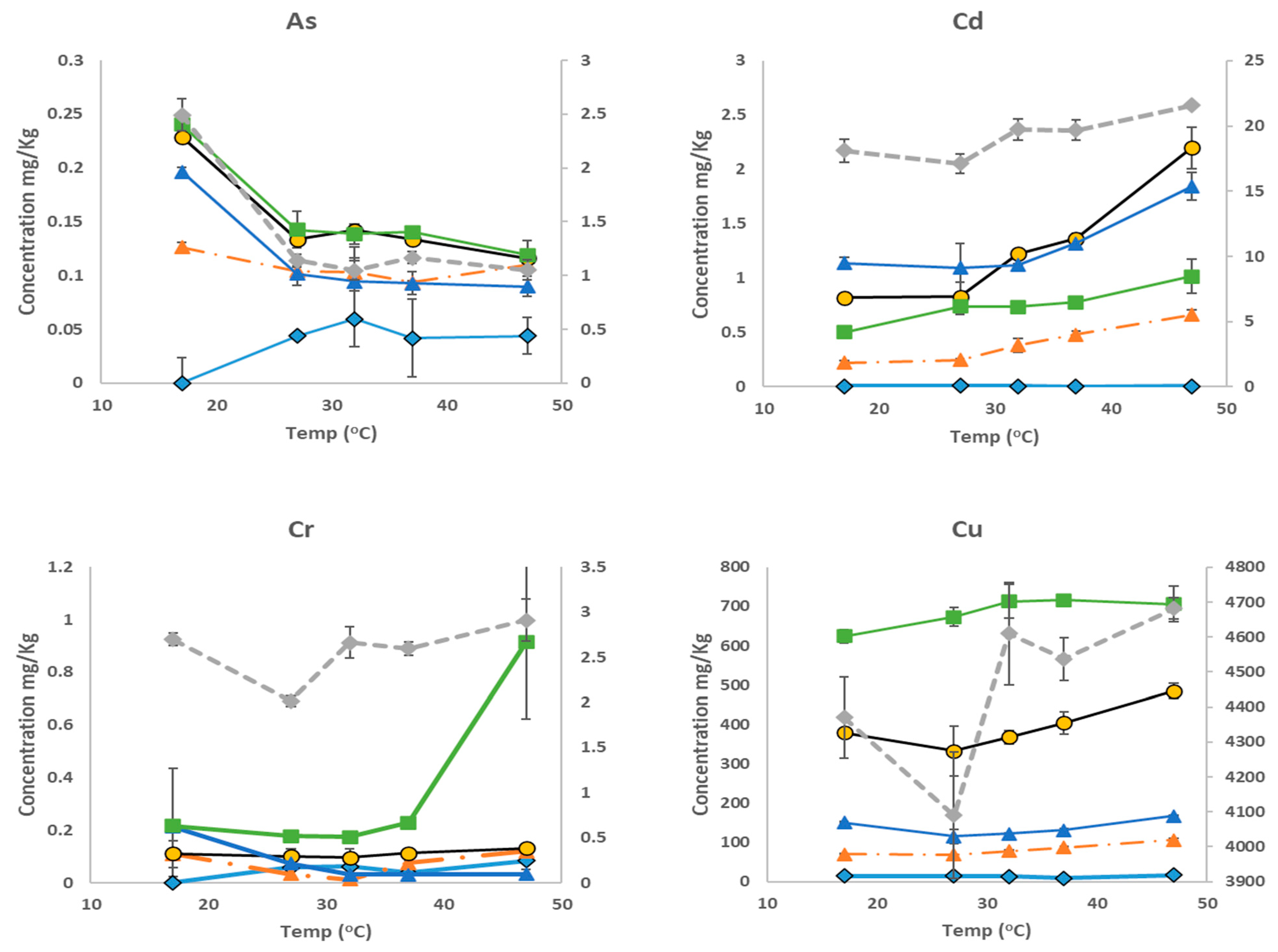
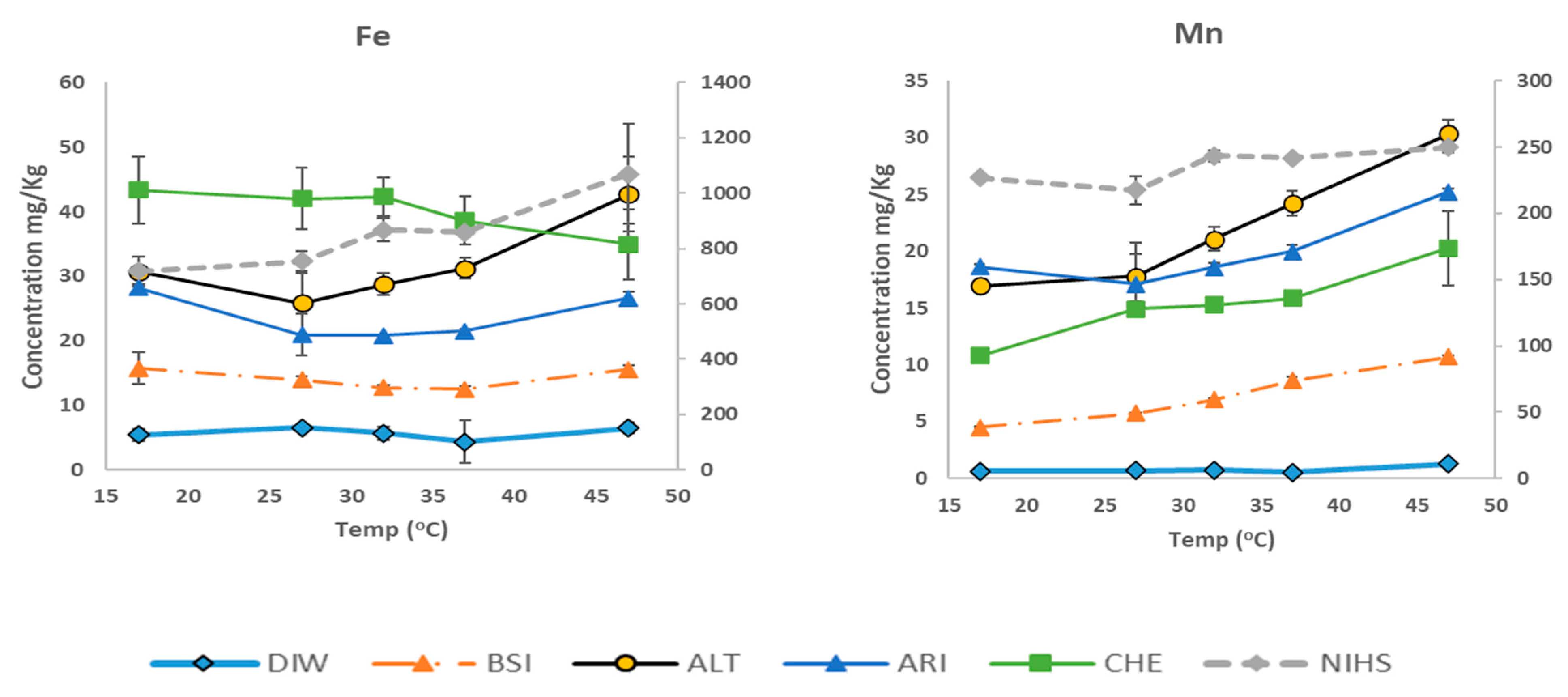
3.4. Variation in Bioaccessible Analyte Concentrations with Extraction Temperatures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chatterjee, A.; Abraham, J. Efficient management of e-wastes. Int. J. Environ. Sci. Technol. 2017, 14, 211–222. [Google Scholar] [CrossRef]
- Tansel, B. From electronic consumer products to e-wastes: Global outlook, waste quantities, recycling challenges. Environ. Int. 2017, 98, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Vaish, B.; Sharma, B.; Singh, P.; Singh, R.P. E-waste and their implications on the Environmental and Human Health. In E-Waste Recycling and Management: Present Scenarios and Environmental Issues, 1st ed.; Khan, A., Inamuddin, A., Asiri, A.M., Eds.; Springer Nature: Basingstoke, UK, 2020; Volume 3, pp. 219–232. [Google Scholar] [CrossRef]
- Perkins, D.N.; Drisse, M.-N.B.; Nxele, T.; Sly, P.D. E-Waste: A Global Hazard. Ann. Glob. Health 2014, 1, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Grant, K.; Goldizen, F.C.; Sly, P.D.; Brune, M.-N.; Neira, M.; Van den Berg, M.; Norman, R.E. Health consequences of exposure to e-waste: A systematic review. Lancet Glob. Health 2013, 1, e350–e361. [Google Scholar] [CrossRef] [Green Version]
- Ackah, M. Informal E-waste recycling in developing countries: Review of metal(loid)s pollution, environmental impacts and transport pathways. Environ. Sci. Pollut. Res. 2017, 24, 24092–24101. [Google Scholar] [CrossRef]
- Bin Hameed, H.; Ali, Y.; Petrillo, A. Environmental risk assessment of E-waste in developing countries by using the modified-SIRA method. Sci. Total Environ. 2020, 733, 138525. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Li, J. A review on human health consequences of metals exposure to e-waste in China. Environ. Pollut. 2015, 196, 450–461. [Google Scholar] [CrossRef]
- Awasthi, A.K.; Zeng, X.; Li, J. Environmental pollution of electronic waste recycling in India: A critical review. Environ. Pollut. 2016, 211, 259–270. [Google Scholar] [CrossRef]
- Orisakwe, O.E.; Frazzoli, C.; Ilo, C.E.; Oritsemuelebi, B. Public Health Burden of E-waste in Africa. J. Health Pollut. 2019, 9, 190610. [Google Scholar] [CrossRef] [Green Version]
- Adamo, P.; Agrelli, D.; Zampella, M. Chemical speciation to assess bioavailability, bioaccessibility and geochemical forms of potentially toxic metals (PTMs) in polluted soils. In Environmental Geochemistry: Site Characterization, Data Analysis and Case Histories, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 153–194. [Google Scholar] [CrossRef]
- USA EPA. Standard Operating Procedure for an In Vitro Bioaccessibility Assay for Lead in Soil; Environmental Protection Agency: Washington, DC, USA, 2012; pp. 1–16. [Google Scholar]
- Ruby, M.V.; Davis, A.; Schoof, R.; Eberle, S.; Sellstone, C.M. Estimation of Lead and Arsenic Bioavailability Using a Physiologically Based Extraction Test. Environ. Sci. Technol. 1996, 30, 422–430. [Google Scholar] [CrossRef]
- Wragg, J.; Cave, M.; Basta, N.; Brandon, E.; Casteel, S.; Denys, S.; Gron, C.; Oomen, A.; Reimer, K.; Tack, K.; et al. An inter-laboratory trial of the unified BARGE bioaccessibility method for arsenic, cadmium and lead in soil. Sci. Total Environ. 2011, 409, 4016–4030. [Google Scholar] [CrossRef] [Green Version]
- Drysdale, M.; Bjorklund, K.L.; Jamieson, H.E.; Weinstein, P.; Cook, A.; Watkins, R.T. Evaluating the respiratory bioaccessibility of nickel in soil through the use of a simulated lung fluid. Environ. Geochem. Health 2012, 34, 279–288. [Google Scholar] [CrossRef]
- Wiseman, C.L.S.; Zereini, F. Characterizing metal(loid) solubility in airborne PM10, PM2.5 and PM1 in Frankfurt, Germany using simulated lung fluids. Atmos. Environ. 2014, 89, 282–289. [Google Scholar] [CrossRef]
- Boisa, N.; Elom, N.; Dean, J.R.; Deary, M.E.; Bird, G.; Entwistle, J.A. Development and application of an inhalation bioaccessibility method (IBM) for lead in the PM10 size fraction of soil. Environ. Int. 2014, 70, 132–142. [Google Scholar] [CrossRef] [Green Version]
- Alpofead, J.A.H.; Davidson, C.M.; Littlejohn, D. A novel two-step sequential bioaccessibility test for potentially toxic elements in inhaled particulate matter transported into the gastrointestinal tract by mucociliary clearance. Anal. Bioanal. Chem. 2017, 409, 3165–3174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefaniak, A.B.; Harvey, C.J. Dissolution of materials in artificial skin surface film liquids. Toxicol. Vitr. 2006, 20, 1265–1283. [Google Scholar] [CrossRef]
- Midander, K.; Julander, A.; Kettelarij, J.; Lidén, C. Testing in artificial sweat–Is less more? Comparison of metal release in two different artificial sweat solutions. Regul. Toxicol. Pharmacol. 2016, 81, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Spalt, E.W.; Kissel, J.C.; Shirai, J.H.; Bunge, A.L. Dermal absorption of environmental contaminants from soil and sediment: A critical review. J. Expo. Sci. Environ. Epidemiol. 2009, 19, 119–148. [Google Scholar] [CrossRef]
- Leal, L.T.C.; Guney, M.; Zagury, G.J. In vitro dermal bioaccessibility of selected metals in contaminated soil and mine tailings and human health risk characterization. Chemosphere 2018, 197, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Khelifi, F.; Caporale, A.G.; Hamed, Y.; Adamo, P. Bioaccessibility of potentially toxic metals in soil, sediments and tailings from a north Africa phosphate-mining area: Insight into human health risk assessment. J. Environ. Manag. 2021, 279, 111634. [Google Scholar] [CrossRef] [PubMed]
- Villegas, C.A.M.; Guney, M.; Zagury, G.J. Comparison of five artificial skin surface film liquids for assessing dermal bioaccessibility of metals in certified reference soils. Sci. Total Environ. 2019, 692, 595–601. [Google Scholar] [CrossRef]
- Choate, L.M.; Ranville, J.F.; Bunge, A.L.; Macalady, D.L. Dermally adhered soil: 1. Amount and particle-size distribution. Integr. Environ. Assess. Manag. 2006, 2, 375–384. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual (Part E, Supplemental Guidance for Dermal Risk Assessment); EPA/540/R/99/005. OSWER 9285. 7-02EP PB99–963312; USEPA: Washington, DC, USA, 2004; E-2. [Google Scholar]
- Midander, K.; Pan, J.; Wallinder, I.O.; Heim, K.; Leygraf, C. Nickel release from nickel particles in artificial sweat. Contact Dermat. 2007, 56, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Torii, M.; Nakayama, H.; Sasaki, T. Thermoregulation of exercising men in the morning rise and evening fall phases of internal temperature. Br. J. Sports Med. 1995, 29, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Budd, G.M. Skin temperature, thermal comfort, sweating, clothing and activity of men sledging in Antarctica. J. Physiol. 1966, 186, 201–215. [Google Scholar] [CrossRef] [Green Version]
- Flouris, A.D.; Cheung, S.S. Thermometry and calorimetry assessment of sweat response during exercise in the heat. Eur. J. Appl. Physiol. 2010, 108, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Ohajinwa, C.M.; van Bodegom, P.M.; Vijver, M.G.; Peijnenburg, W.J. Impact of informal electronic waste recycling on metal concentrations in soils and dusts. Environ. Res. 2018, 164, 385–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- British Standard International. Soil Quality. Determination of pH. BS ISO 10390:2005; British Standard International: London, UK, 2005; pp. 1–7. [Google Scholar]
- Håkanson, L. An ecological risk index for aquatic pollution control: A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- British Standard International. Reference Test Method for Release of Nickel from All Post Assemblies Which Are Inserted into Pierced Parts of the Human Body and Articles Intended to Come into Direct and Prolonged Contact with the Skin. BS EN 1811:2011+A1:2015; British Standard International: London, UK, 2011; pp. 1–29. [Google Scholar]
- Wainman, T.; Hazen, R.E.; Lioy, P.J. The extractability of Cr(VI) from contaminated soil in synthetic sweat. J. Expo. Anal. Environ. Epidemiology 1994, 4, 171–181. [Google Scholar]
- Altkofer, W.; Braune, S.; Ellendt, K.; Kettl-Grömminger, M.; Steiner, G. Migration of nitrosamines from rubber products-are balloons and condoms harmful to the human health? Mol. Nutr. Food Res. 2005, 49, 235–238. [Google Scholar] [CrossRef]
- Ariza, E.; Rocha, L.A.; Vaz, F.; Cunha, L.; Ferreira, S.C.; Carvalho, P.; Rebouta, L.; Alves, E.; Goudeau, P.; Rivière, J.P. Corrosion resistance of ZrNxOy thin films obtained by rf reactive magnetron sputtering. Thin Solid Films 2004, 469–470, 274–281. [Google Scholar] [CrossRef] [Green Version]
- Cheng, W.-H.; Chu, F.-S.; Su, T.-I. Effects of liquid VOC concentration and salt content on partitioning equilibrium of hydrophilic VOC at air–sweat interface. Atmos. Environ. 2005, 39, 5509–5516. [Google Scholar] [CrossRef]
- Alpofead, J.A.H.; Davidson, C.M.; Littlejohn, D. Oral bioaccessibility tests to measure potentially toxic elements in inhalable particulate matter collected during routine air quality monitoring. Anal. Methods 2016, 8, 5466–5474. [Google Scholar] [CrossRef] [Green Version]
- Davidson, C.M.; Nordon, A.; Urquhart, G.J.; Ajmone-Marsan, F.; Biasioli, M.; Duarte, A.C.; Diaz-Barrientos, E.; Grcman, H.; Hodnik, A.; Hossack, I.; et al. Quality and comparability of measurement of potentially toxic elements in urban soils by a group of European laboratories. Int. J. Environ. Anal. Chem. 2007, 87, 589–601. [Google Scholar] [CrossRef]
- Allen, T.T. Software overview and methods review: Minitab. In Introduction to Engineering. Statistics and Lean Six Sigma, 3rd ed.; Allen, T., Ed.; Springer: London, UK, 2019; pp. 575–600. [Google Scholar] [CrossRef]
- Onder, M.; Yigit, E. Assessment of respirable dust exposures in an opencast coal mine. Environ. Monit. Assess. 2008, 152, 393–401. [Google Scholar] [CrossRef]
- Isimekhai, K.A.; Garelick, H.; Watt, J.; Purchase, D. Heavy metals distribution and risk assessment in soil from an informal E-waste recycling site in Lagos State, Nigeria. Environ. Sci. Pollut. Res. 2017, 24, 17206–17219. [Google Scholar] [CrossRef] [PubMed]
- Anselm, O.H.; Cavoura, O.; Davidson, C.M.; Oluseyi, T.O.; Oyeyiola, A.O.; Togias, K. Mobility, spatial variation and human health risk assessment of mercury in soil from an informal e-waste recycling site, Lagos, Nigeria. Environ. Monit. Assess. 2021, 193, 416. [Google Scholar] [CrossRef] [PubMed]
- Alloway, B. Trace metals and metalloids in soils and their bioavailability. In Heavy Metals in Soils; Springer: London, UK, 2013; pp. 11–50. [Google Scholar]
- Dutch Ministry of Housing, Spatial Planning and Environment. Circular on Target and Intervention Values for Soil Remediation; Netherlands Government Gazette: The Hague, The Netherlands, 2000; pp. 1–51. [Google Scholar]
- Liu, X.; Gu, S.; Yang, S.; Deng, J.; Xu, J. Heavy metals in soil-vegetable system around E-waste site and the health risk assessment. Sci. Total Environ. 2021, 779, 146438. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Fujimori, T.; Juhasz, A.; Takaoka, M.; Oshita, K. Bioaccessibility and human health risk assessment of metal(loid)s in soil from an e-waste open burning site in Agbogbloshie, Accra, Ghana. Chemosphere 2020, 240, 124909. [Google Scholar] [CrossRef]
- Chakraborty, P.; Sampath, S.; Mukhopadhyay, M.; Selvaraj, S.; Bharat, G.K.; Nizzetto, L. Baseline investigation on plasticizers, bisphenol A, polycyclic aromatic hydrocarbons and heavy metals in the surface soil of the informal electronic waste recycling workshops and nearby open dumpsites in Indian metropolitan cities. Environ. Pollut. 2019, 248, 1036–1045. [Google Scholar] [CrossRef]
- Robinson, B.H. E-waste: An assessment of global production and environmental impacts. Sci. Total Environ. 2009, 408, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Buchman, M.F. Screening Quick Reference Tables (SQuiRTs). 2008. Available online: https://repository.library.noaa.gov/view/noaa/9327 (accessed on 4 September 2021).
- Forte, G.; Petrucci, F.; Bocca, B. Metal allergens of growing significance: Epidemiology, immunotoxicology, strategies for testing and prevention. Inflamm. Allergy-Drug Targets 2008, 7, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Cipullo, S.; Prpich, G.; Campo, P.; Coulon, F. Assessing bioavailability of complex chemical mixtures in contaminated soils: Progress made and research needs. Sci. Total Environ. 2018, 615, 708–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ojeh, V.N.; Balogun, A.A.; Okhimamhe, A.A. Urban-Rural Temperature Differences in Lagos. Climate 2016, 4, 29. [Google Scholar] [CrossRef]
- Murthy, S.N.; Sen, A.; Zhao, Y.-L.; Hui, S.W. Temperature Influences the Postelectroporation Permeability State of the Skin. J. Pharm. Sci. 2004, 93, 908–915. [Google Scholar] [CrossRef] [PubMed]
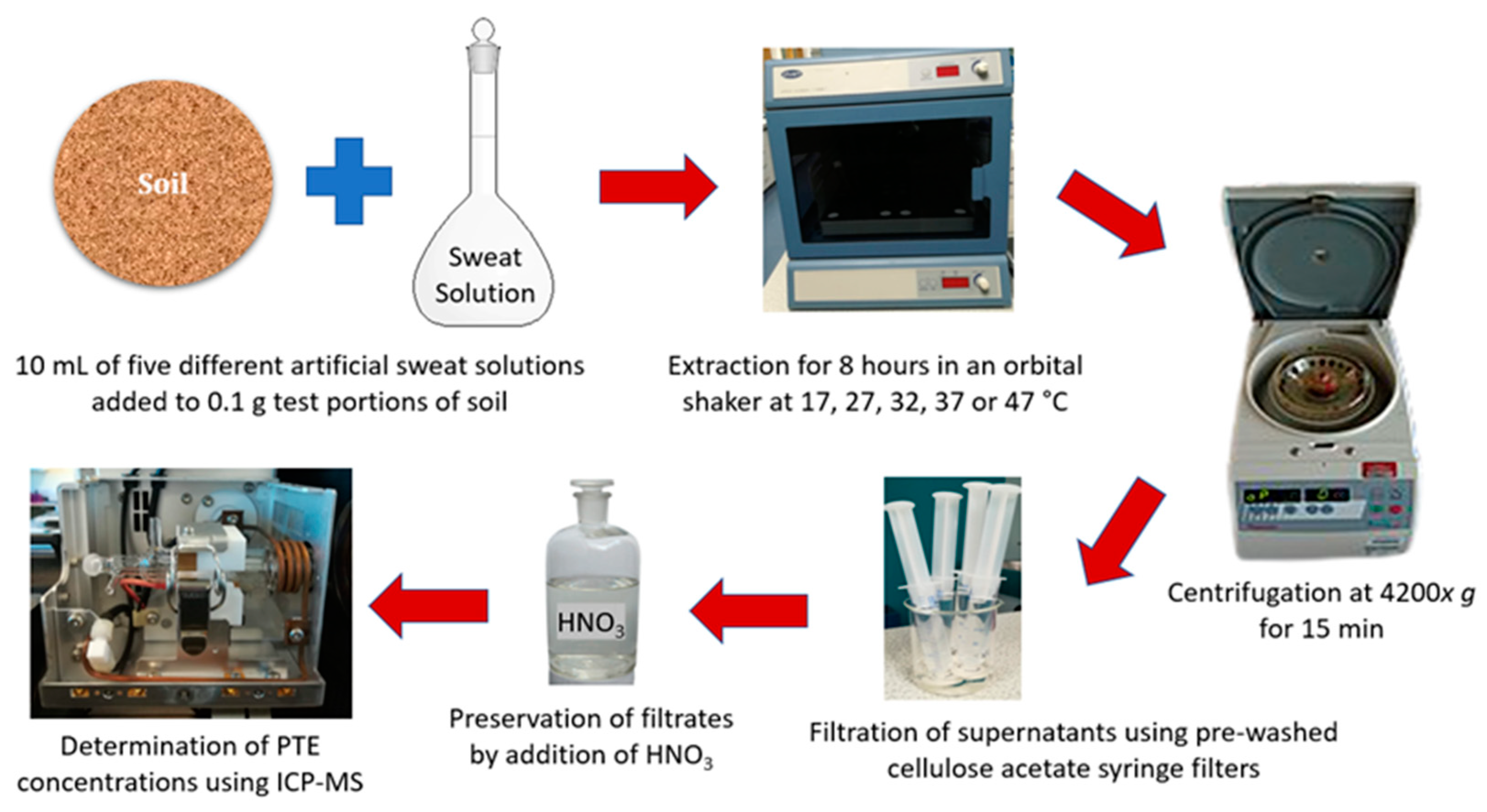

| BSI [34] | NIHS [35] | ALT [36] | ARI [37] | CHE [38] | |
|---|---|---|---|---|---|
| Acetic acid | - | 0.25 | - | - | - |
| Ammonia | - | - | - | - | 0.0343 |
| Ammonium Chloride | - | 1.75 | 0.04 | - | - |
| Lactic acid | 0.1 | 1.5 | 0.3 | 0.1 mL | - |
| Potassium chloride | - | - | 0.03 | 0.12 | - |
| Sodium chloride | 0.5 | 2.0 | 0.45 | 0.75 | 0.468 |
| Sodium lactate | - | - | - | - | 0.6 |
| Sodium sulfate | - | - | 0.03 | - | - |
| Urea | 0.1 | 0.5 | 0.02 | 0.1 | 0.0516 |
| pH | 6.5 | 4.7 | (5.3)* | 4.5 | 6 |
| As | Cd | Cr | Cu | Fe | Mn | Ni | Pb | Zn | |
|---|---|---|---|---|---|---|---|---|---|
| Found | 17.2 ± 1.5 | 0.524 ± 0.060 | 41.2 ± 4.4 | 92.3 ± 9.9 | 29,500 ± 2800 | 407 ± 31 | 43.5 ± 4.7 | 380 ± 26 | 180 ± 19 |
| Indicative [40] | 17.7 ± 4 | 0.646 ± 0.184 | 43.2 ± 3 | 111 ± 5 | 30,600 ± 1200 | 442 ± 18 | 48.8 ± 7 | 389 ± 25 | 177 ± 11 |
| Recovery (%) | 97.2 | 81.1 | 95.4 | 83.2 | 96.4 | 92.1 | 89.1 | 97.7 | 102 |
| As | Cd | Cr | Cu | Fe | Mn | Ni | Pb | Zn | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Concentration (mg/kg) | DIW | 0.0419 ± 0.0361 | 0.000924 ± 0.001401 | 0.0379 ± 0.0298 | 8.77 ± 6.52 | 4.37 ± 3.35 | 0.545 ± 0.413 | 0.0749 ± 0.0568 | 0.0580 ± 0.0372 | 0.0478 ± 1.3222 |
| BSI | 0.0939 ± 0.0016 | 0.479 ± 0.036 | 0.0763 ± 0.0108 | 87.4 ± 1.8 | 12.5 ± 0.4 | 8.61 ± 0.30 | 0.711 ± 0.0223 | 0.155 ± 0.019 | 9.98 ± 6.21 | |
| NIHS | 1.17 ± 0.06 | 19.7 ± 0.8 | 2.59 ± 0.08 | 4540 ± 62 | 859 ± 7 | 242 ± 0 | 42.1 ± 0.5 | 579 ± 3 | 3190 ± 19 | |
| ALT | 0.134 ± 0.013 | 1.36 ± 0.05 | 0.114 ± 0.011 | 405 ± 28 | 31.2 ± 1.6 | 24.2 ± 1.1 | 2.74 ± 0.11 | 0.343 ± 0.162 | 157 ± 18 | |
| ARI | 0.0930 ± 0.0107 | 1.32 ± 0.06 | 0.0321 ± 0.0122 | 132 ± 3 | 21.5 ± 0.4 | 20.0 ± 0.5 | 1.86 ± 0.05 | 0.185 ± 0.157 | 69.3 ± 5.8 | |
| CHE | 0.140 ± 0.001 | 0.779 ± 0.013 | 0.229 ± 0.009 | 717 ± 11 | 38.6 ± 3.8 | 15.8 ± 0.1 | 2.26 ± 0.03 | 0.109 ± 0.010 | 34.2 ± 0.3 | |
| % BA | DIW | 0.415 | 0.00118 | 0.0428 | 0.0640 | 0.00595 | 0.0486 | 0.0211 | 0.00173 | 0.000431 |
| BSI | 0.929 | 0.611 | 0.0860 | 0.638 | 0.0170 | 0.769 | 0.200 | 0.00466 | 0.0900 | |
| NIHS | 11.5 | 25.1 | 2.93 | 33.1 | 1.17 | 21.6 | 11.9 | 17.3 | 28.7 | |
| ALT | 1.32 | 1.74 | 0.128 | 2.95 | 0.0425 | 2.16 | 0.773 | 0.0103 | 1.41 | |
| ARI | 0.920 | 1.69 | 0.0362 | 0.962 | 0.0293 | 1.79 | 0.524 | 0.00553 | 0.625 | |
| CHE | 1.39 | 0.995 | 0.259 | 5.23 | 0.0525 | 1.41 | 0.637 | 0.00327 | 0.309 |
| pH Before Use | Extract pH | |
|---|---|---|
| DIW | 5.00 | 7.96 |
| BSI | 6.48 | 7.67 |
| NIHS | 4.68 | 5.41 |
| ALT | 5.30 | 7.43 |
| ARI | 4.49 | 7.34 |
| CHE | 6.00 | 8.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anselm, O.H.; Davidson, C.M.; Oyeyiola, A.O.; Oluseyi, T.O. Effects of Artificial Sweat Formulation and Extraction Temperature on Estimation of the Dermal Bioaccessibility of Potentially Toxic Elements in a Contaminated Soil from an E-Waste Recycling Site. Geosciences 2022, 12, 31. https://doi.org/10.3390/geosciences12010031
Anselm OH, Davidson CM, Oyeyiola AO, Oluseyi TO. Effects of Artificial Sweat Formulation and Extraction Temperature on Estimation of the Dermal Bioaccessibility of Potentially Toxic Elements in a Contaminated Soil from an E-Waste Recycling Site. Geosciences. 2022; 12(1):31. https://doi.org/10.3390/geosciences12010031
Chicago/Turabian StyleAnselm, Oluwaseun H., Christine M. Davidson, Aderonke O. Oyeyiola, and Temilola O. Oluseyi. 2022. "Effects of Artificial Sweat Formulation and Extraction Temperature on Estimation of the Dermal Bioaccessibility of Potentially Toxic Elements in a Contaminated Soil from an E-Waste Recycling Site" Geosciences 12, no. 1: 31. https://doi.org/10.3390/geosciences12010031
APA StyleAnselm, O. H., Davidson, C. M., Oyeyiola, A. O., & Oluseyi, T. O. (2022). Effects of Artificial Sweat Formulation and Extraction Temperature on Estimation of the Dermal Bioaccessibility of Potentially Toxic Elements in a Contaminated Soil from an E-Waste Recycling Site. Geosciences, 12(1), 31. https://doi.org/10.3390/geosciences12010031






