Agglutinated Foraminiferal Acmes and Their Role in the Biostratigraphy of the Campanian–Eocene Outer Carpathians
Abstract
1. Introduction
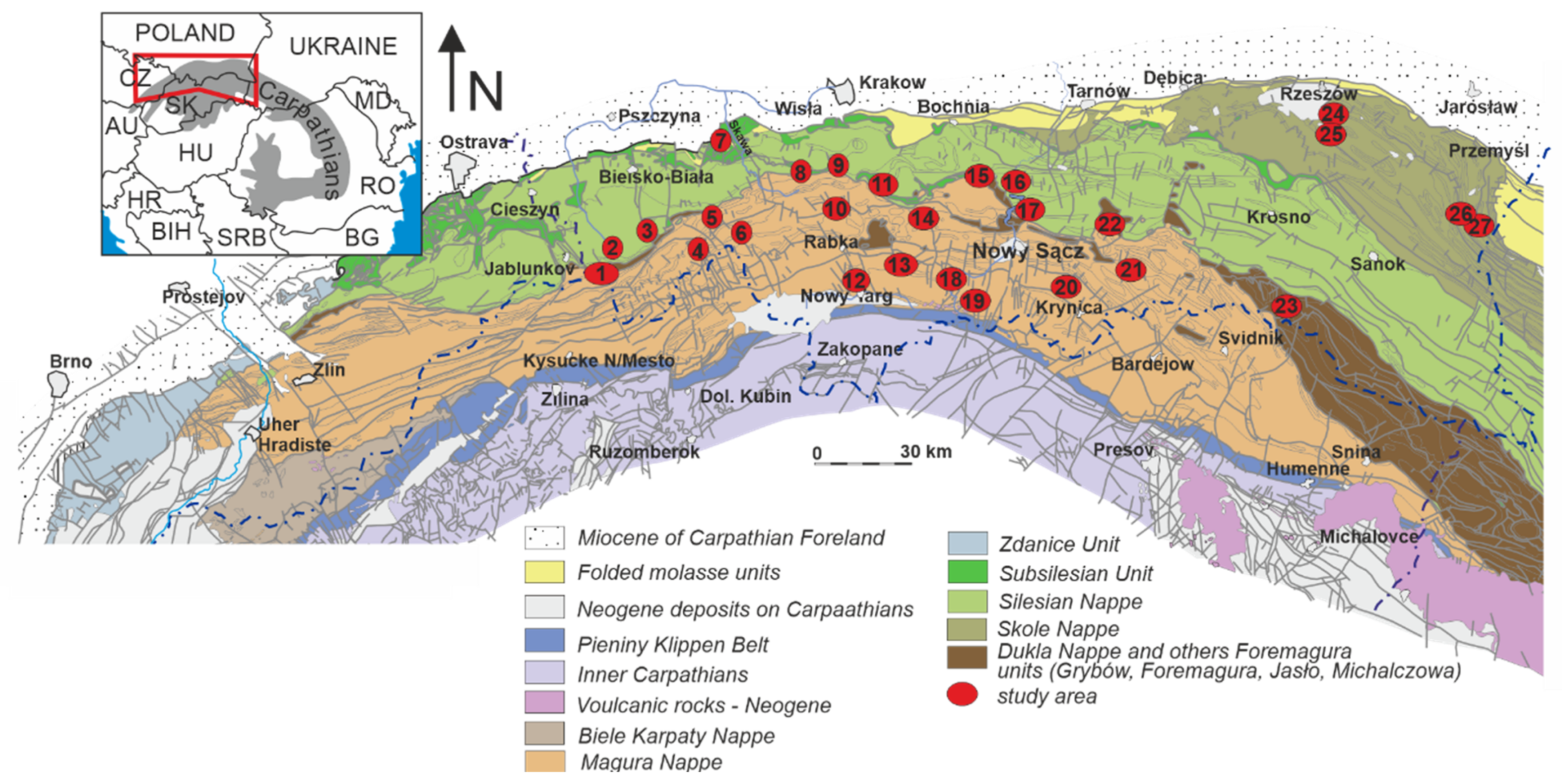
2. Study Area
3. Materials and Methods
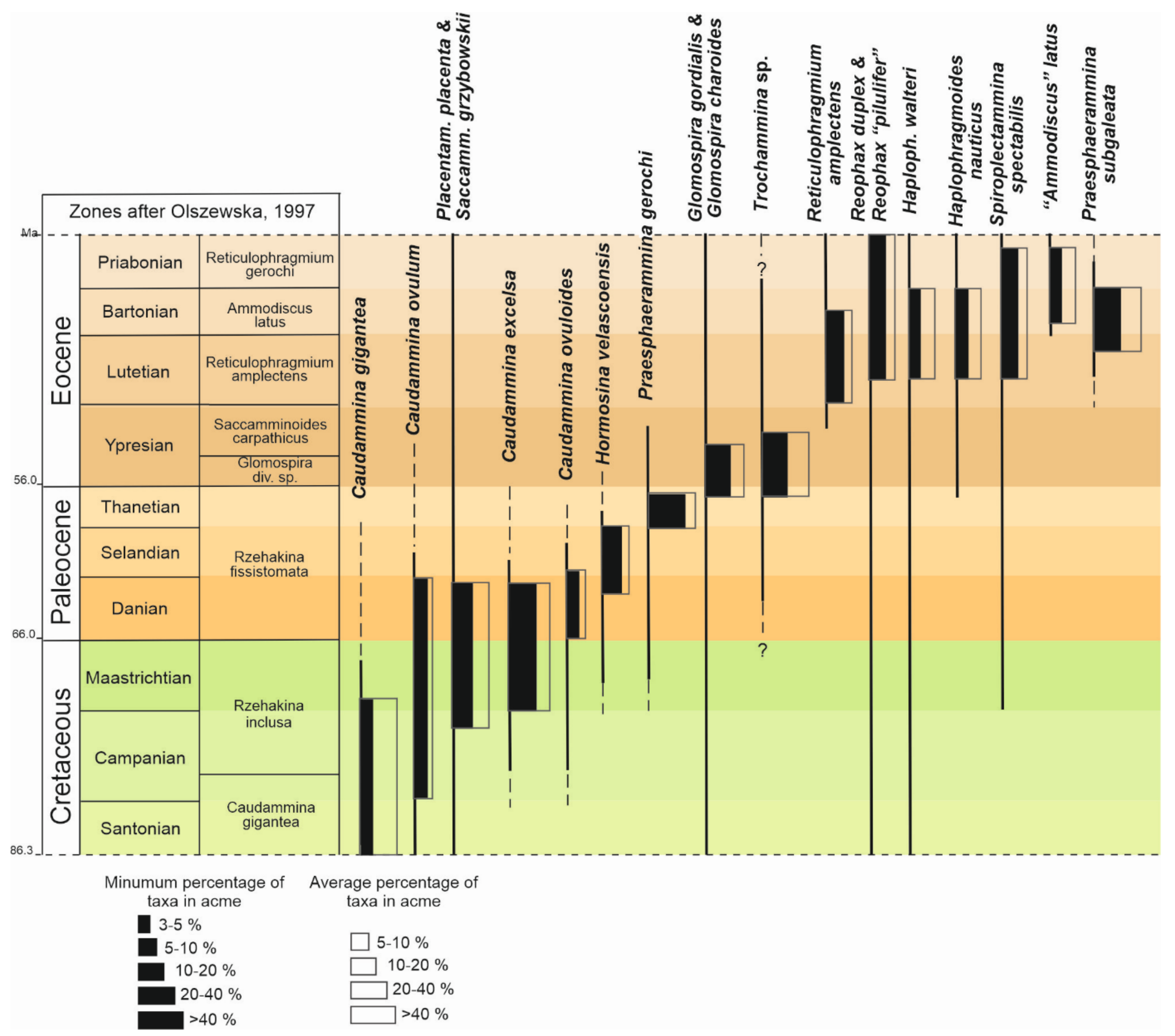
4. Results
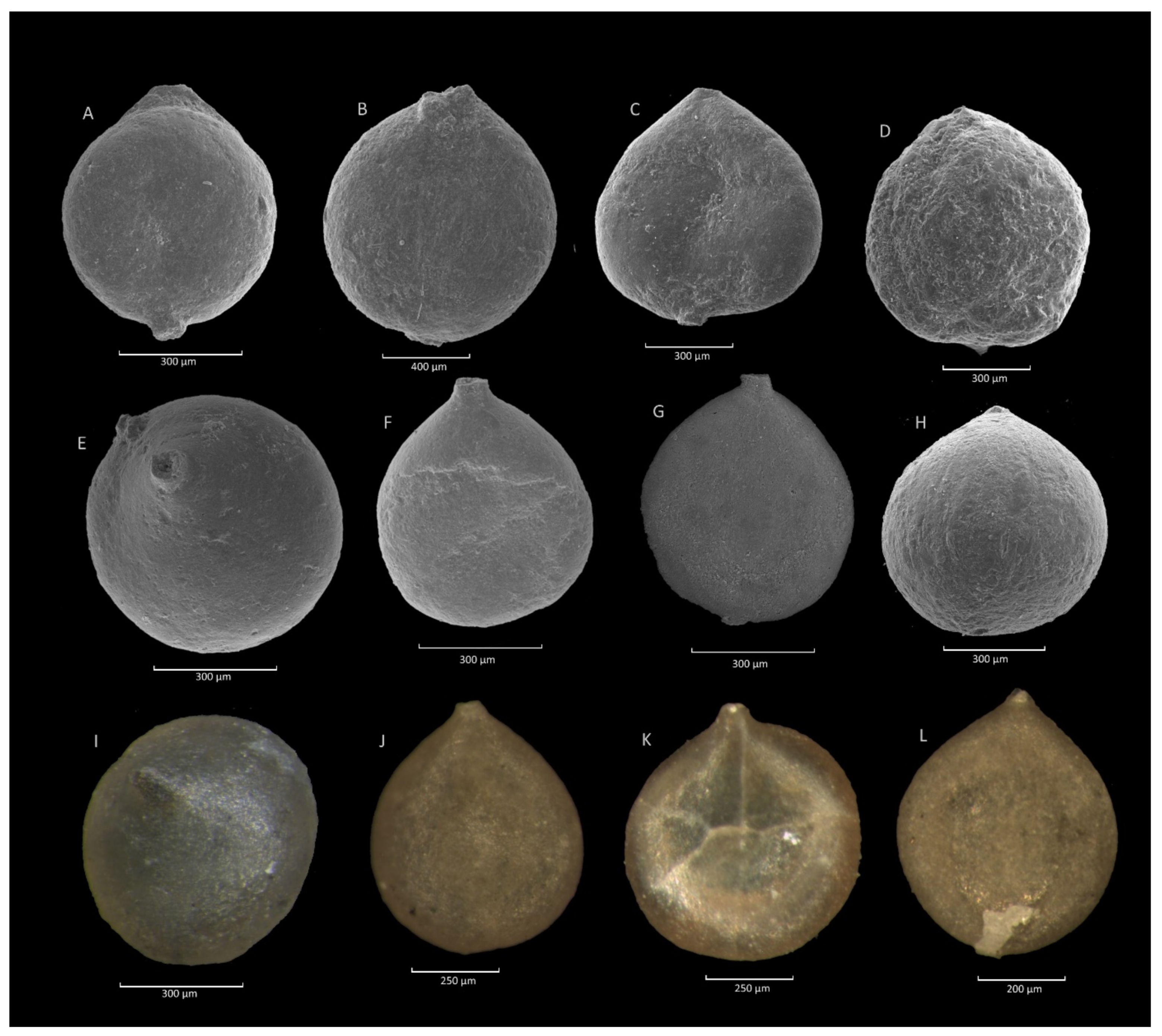
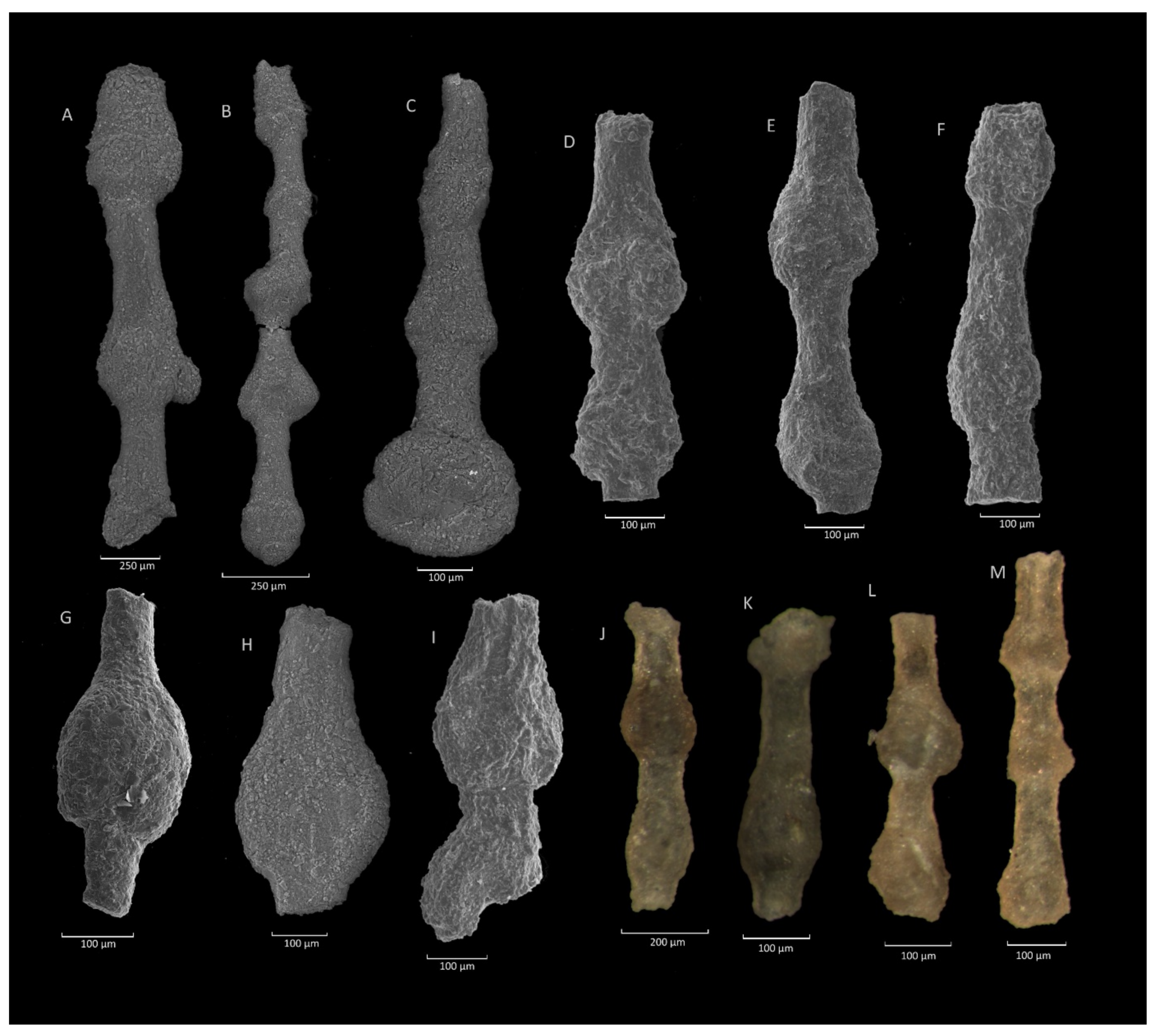
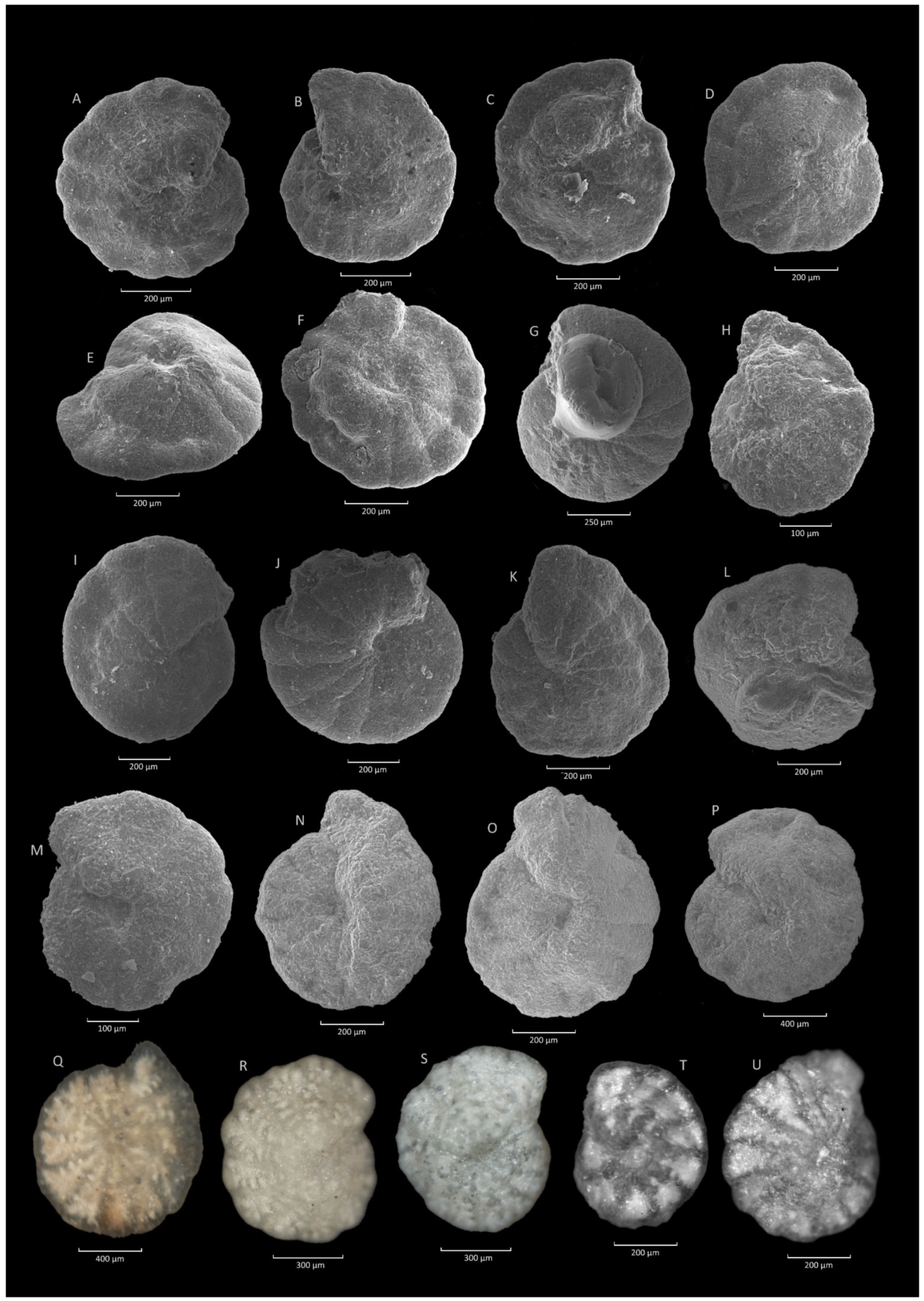
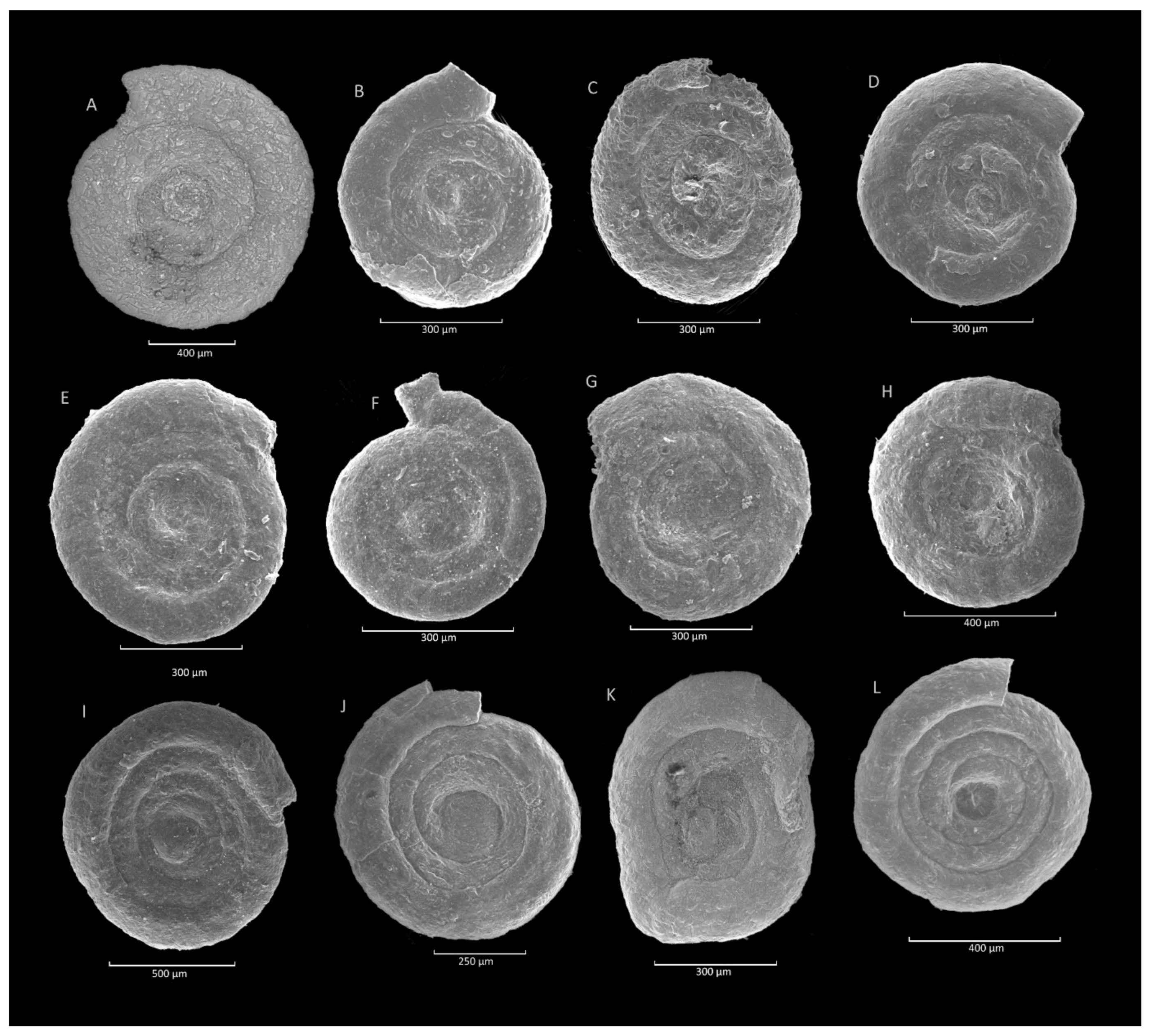
4.1. Caudammina gigantea (Geroch) Acme
4.1.1. Definition
4.1.2. Occurrence and Age Assignment
4.1.3. Remarks
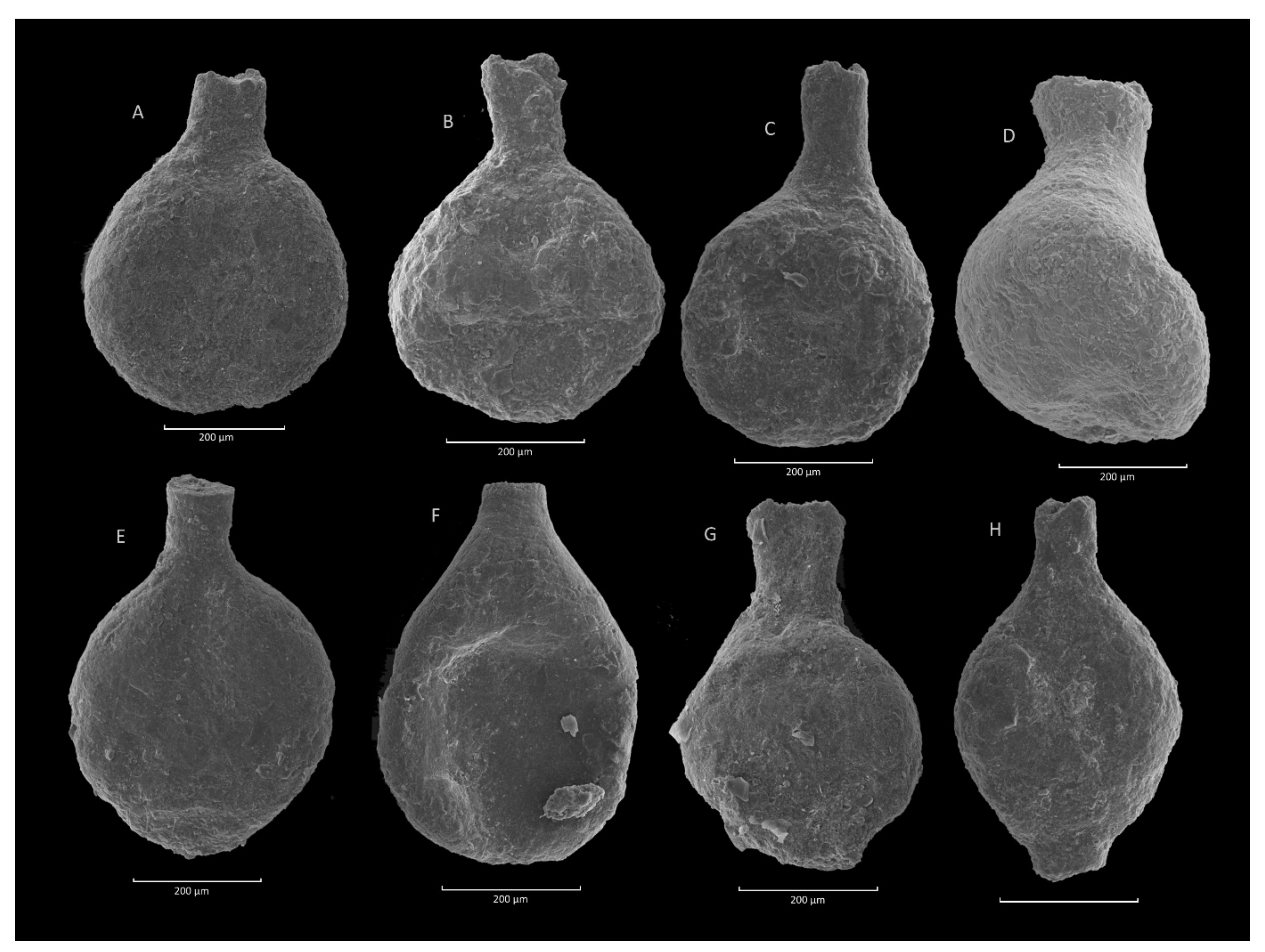
4.2. Caudammina ovulum (Grzybowski) Acme
4.2.1. Definition
4.2.2. Occurrence and Age Assignment
4.2.3. Remarks
4.3. Placentammina placenta (Grzybowski)-Saccammina grzybowskii (Schubert) Acme
4.3.1. Definition
4.3.2. Occurrence and Age Assignment
4.3.3. Remarks
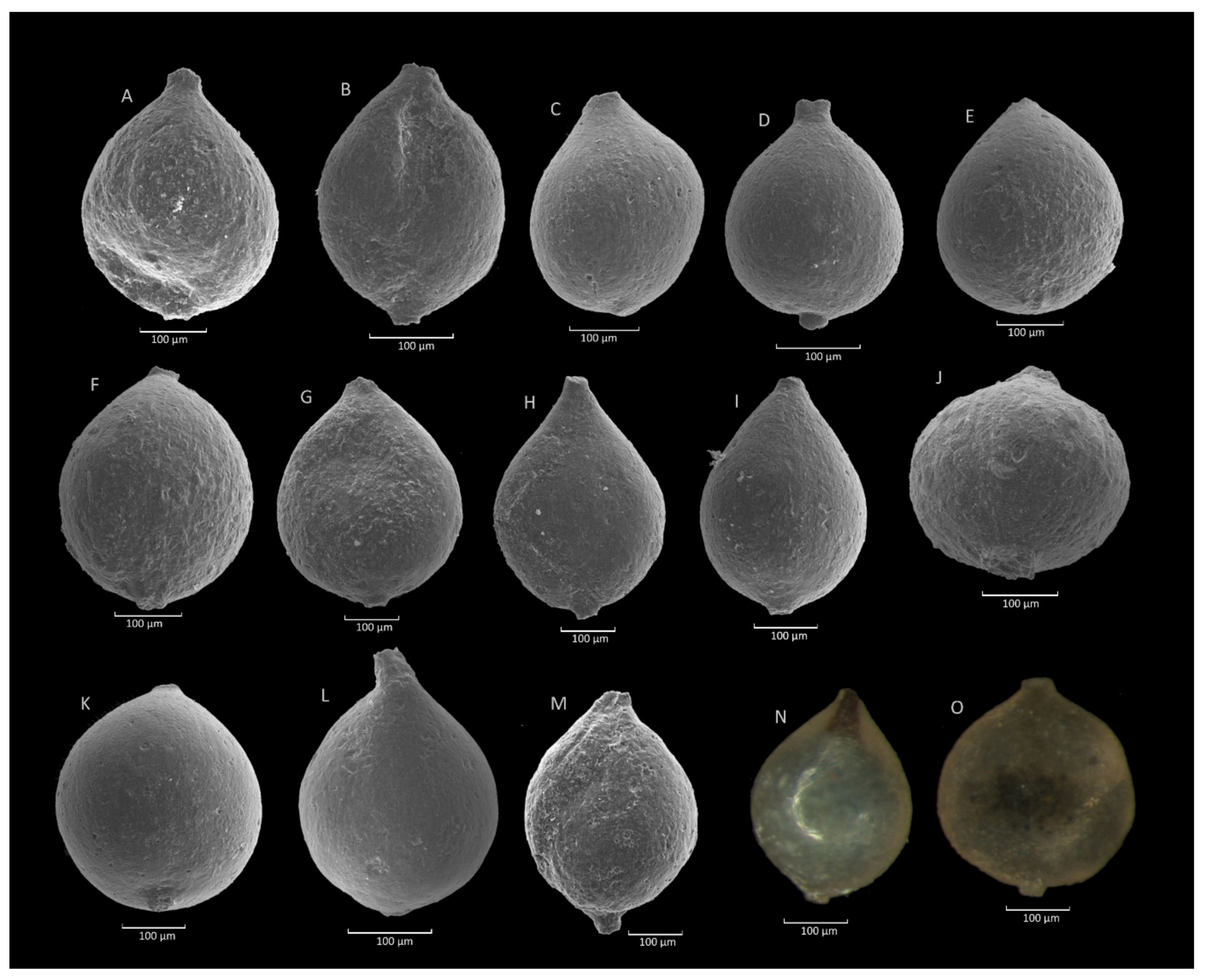
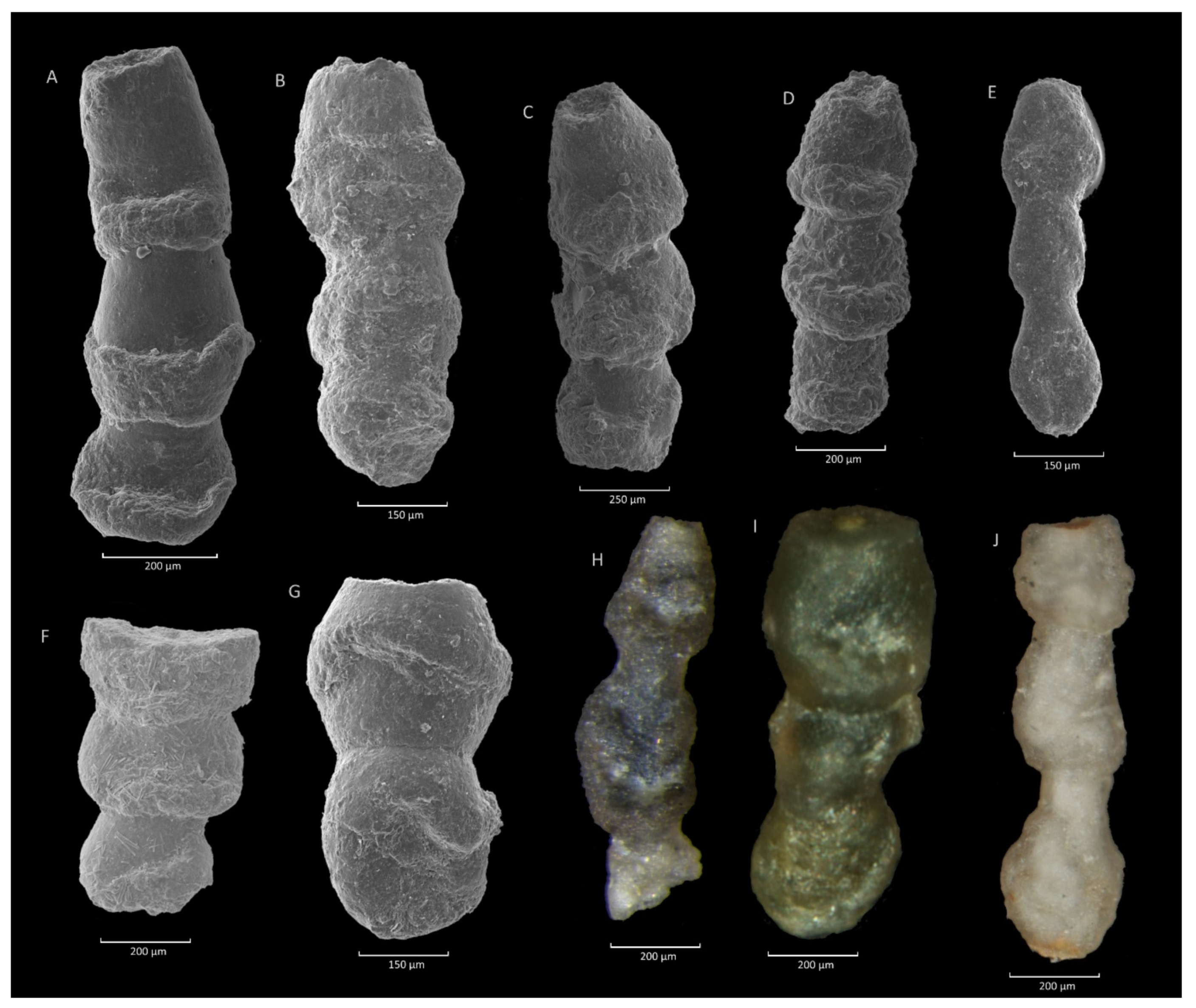
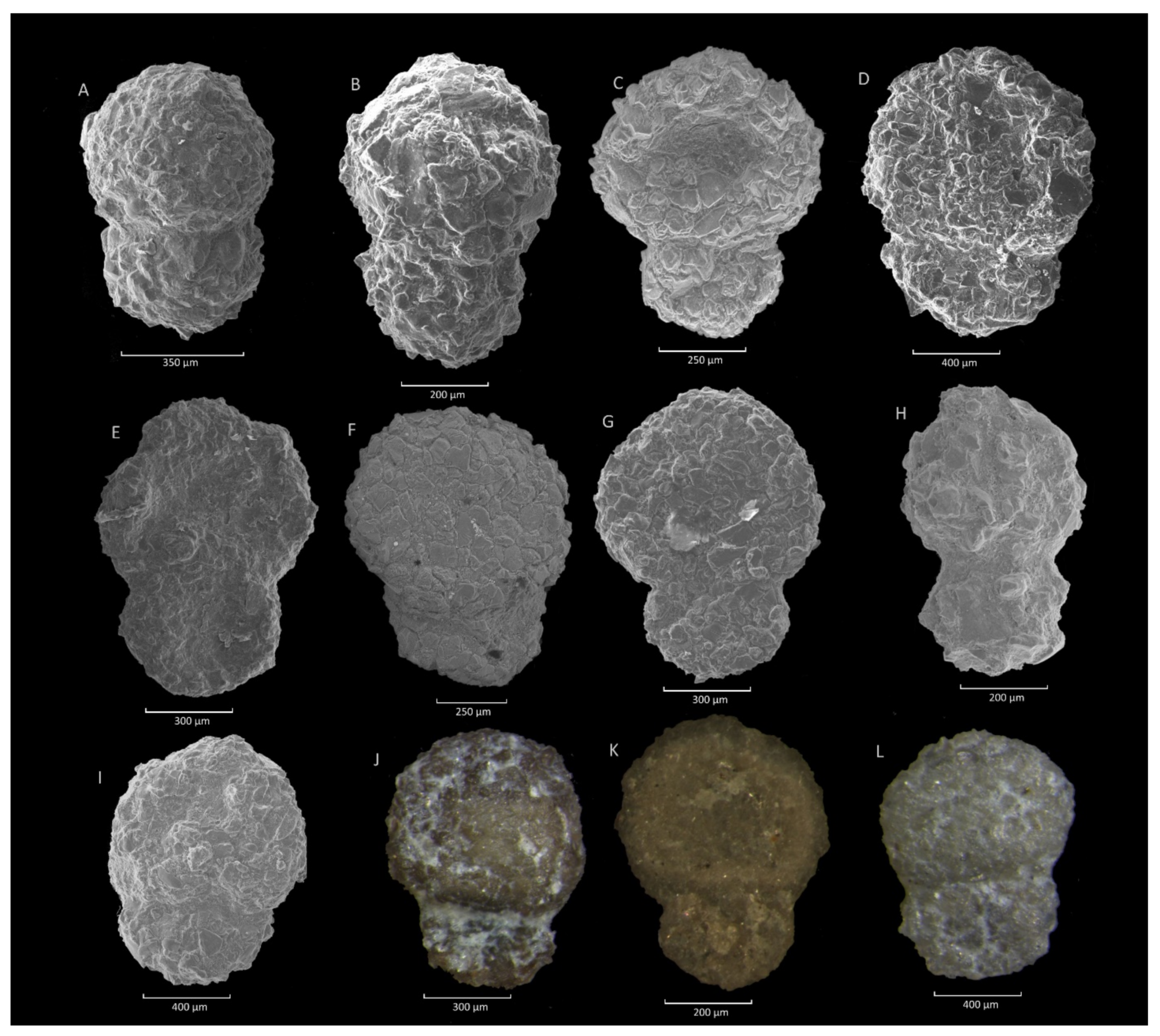
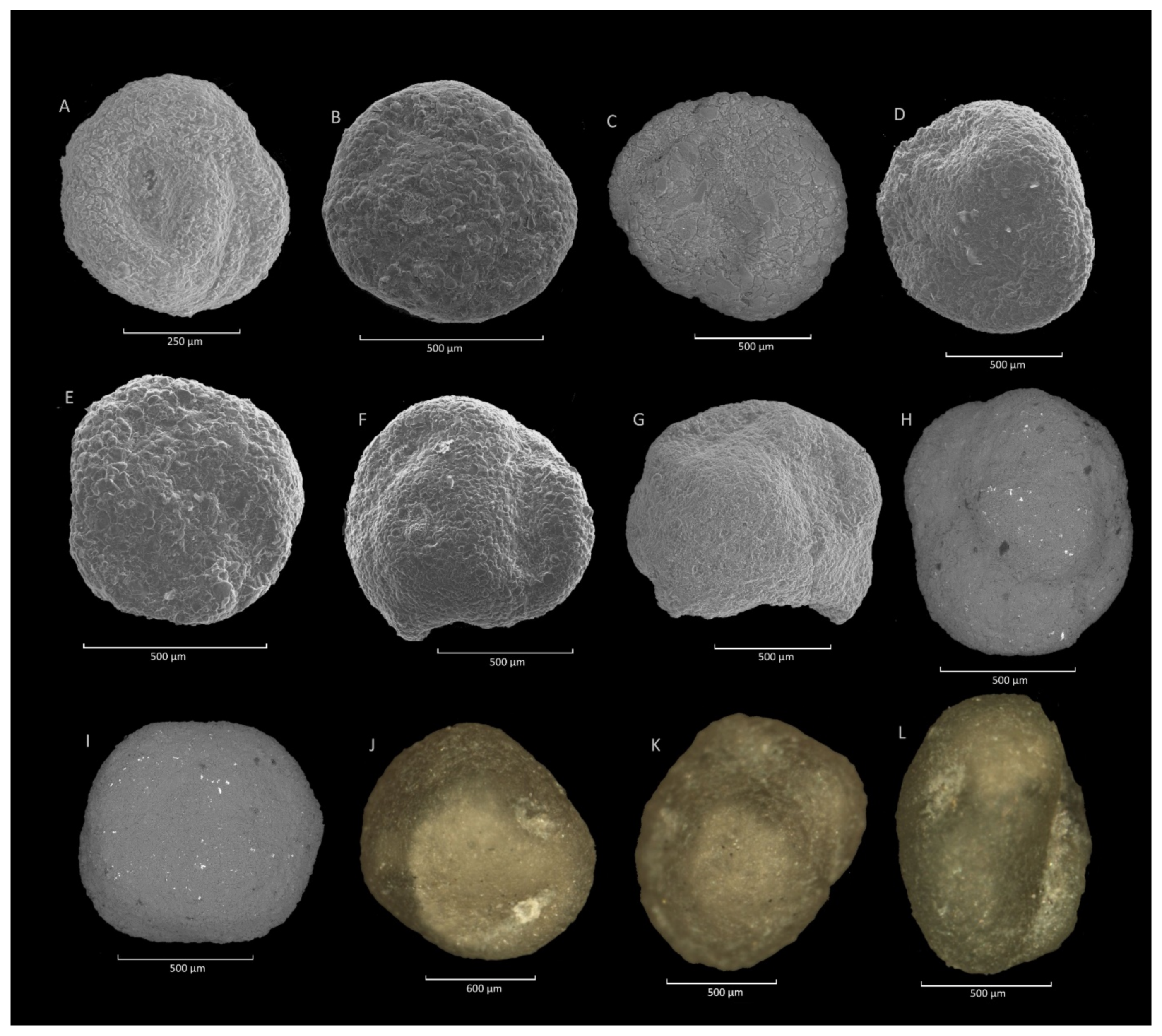
4.4. Caudammina excelsa (Dylążanka) Acme
4.4.1. Definition
4.4.2. Occurrence and Age Assignment
4.4.3. Remarks
4.5. Caudammina ovuloides (Grzybowski) Acme
4.5.1. Definition
4.5.2. Occurrence and Age Assegment
4.5.3. Remarks
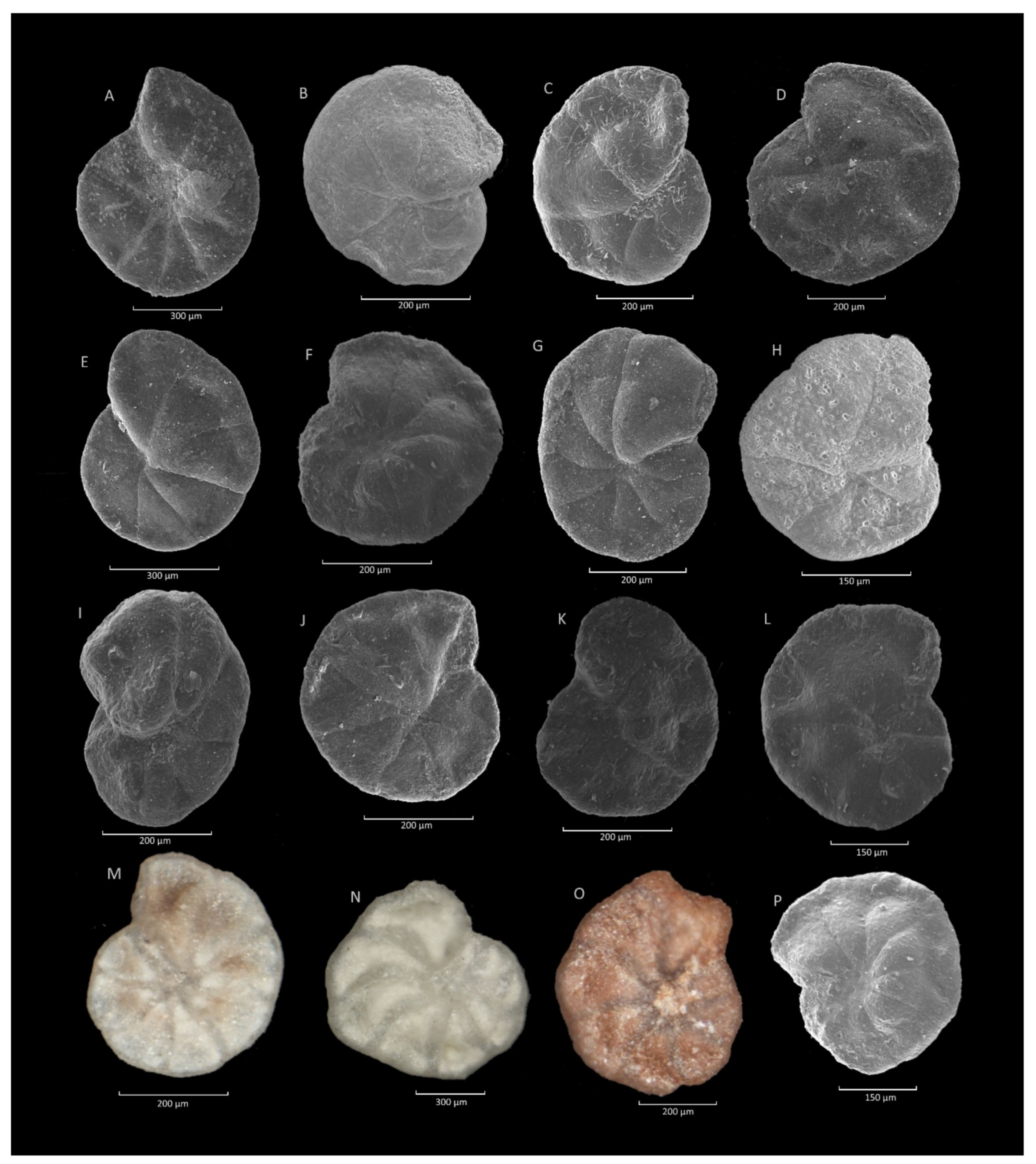
4.6. Hormosina velascoensis (Cushman) Acme
4.6.1. Definition
4.6.2. Occurrence and Age Assignment
4.6.3. Remarks
4.7. Praesphaerammina gerochi (Hanzlíková) Acme
4.7.1. Definition
4.7.2. Occurrence and Age Assignment
4.7.3. Remarks
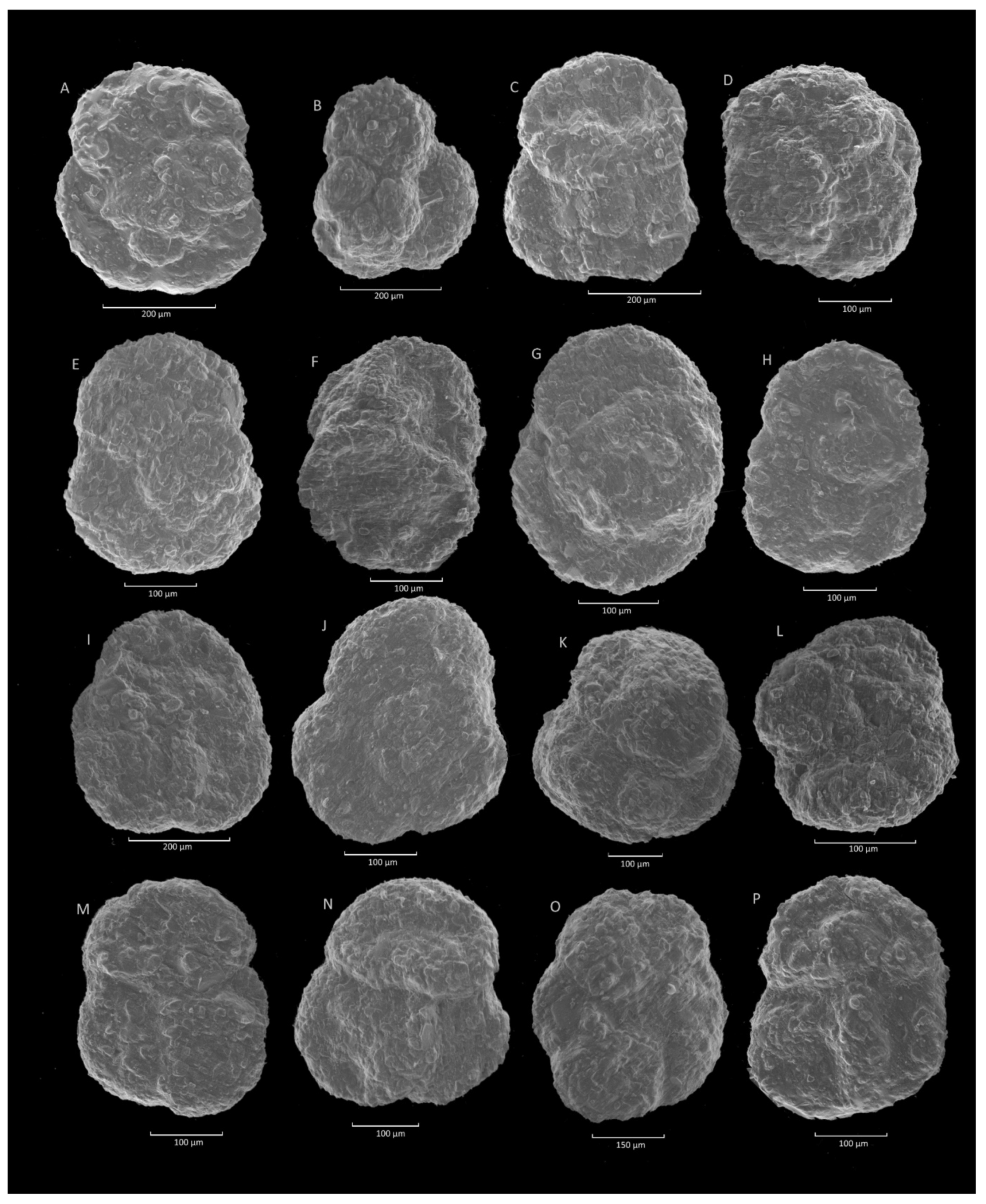
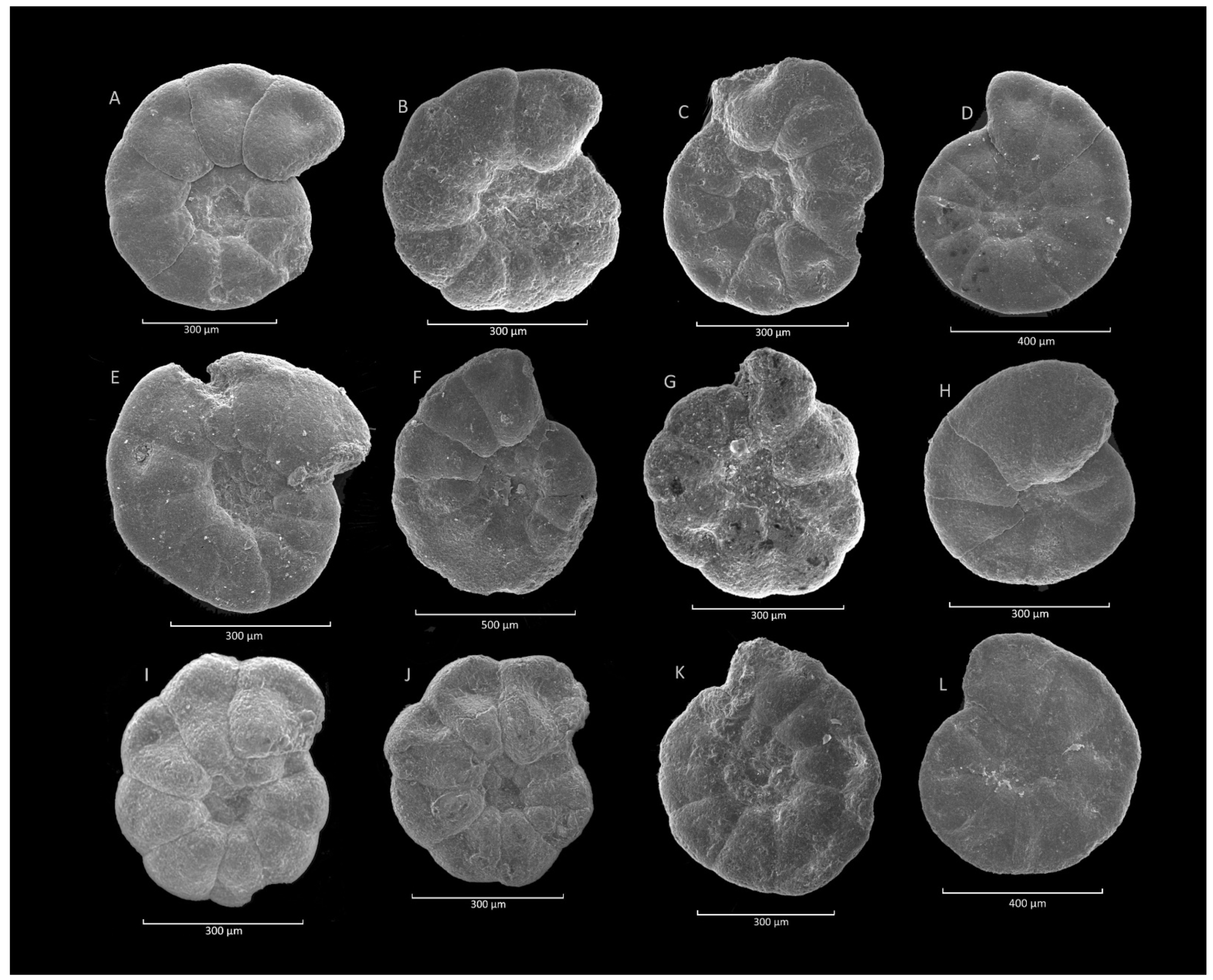
4.8. Glomospira charoides (Jones et Parker)-Glomospira gordialis (Jones et Parker) Acme
4.8.1. Definition
4.8.2. Occurrence and Age Assignment
4.8.3. Remarks
4.9. Trochammina spp. Acme
4.9.1. Definition
4.9.2. Occurrence and Age Assignment
4.9.3. Remarks
4.10. Reticulophragmium amplectens (Grzybowski) Acme
4.10.1. Definition
4.10.2. Occurrence and Age Assignment
4.10.3. Remarks
4.11. Reophax duplex (Grzybowski)-Reophax “pilulifer” Brady Acme
4.11.1. Definition
4.11.2. Occurrence and Age Assignment
4.11.3. Remarks
4.12. Haplophragmoides walteri (Grzybowski)-Haplophragmoides nauticus Kender, Kaminski et Jones Acme
4.12.1. Definition
4.12.2. Occurrence and Age Assignment
4.12.3. Remarks
4.13. Spiroplectammina spectabilis (Grzybowski) Acme
4.13.1. Definition
4.13.2. Occurrence and Age Assignment
4.13.3. Remarks
4.14. “Ammodiscus” latus Grzybowski Acme
4.14.1. Definition
4.14.2. Occurrence and Age Assignment
4.14.3. Remarks
4.15. Praesphaerammina subgaleata (Vašíček) Acme
4.15.1. Definition
4.15.2. Occurrence and Age Assignment
4.15.3. Remarks
5. Discussion
6. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaminski, M.A.; Gradstein, F.M. Atlas of Paleogene Cosmopolitan Deep-Water Foraminifera; Grzybowski Foundation, Grzybowski Foundation Special Publication: Kraków, Poland, 2005; Volume 10, pp. 1–547. [Google Scholar]
- Geroch, S.; Nowak, W. Proposal of zonation for the Late Tithonian–Late Eocene, based upon arenaceous Foraminifera from the Outer Carpathians, Poland. In Proceedings of the BENTHOS ‘83: 2nd International Symposium on Benthic Foraminifera, Pau, France, 11–15 April 1983; Oertli, H.J., Ed.; ESO REP and TOTAL CFP: Pau and Bordeoux, Elf-Aquitane, France, 1984; pp. 225–239. [Google Scholar]
- Olszewska, B. Foraminiferal biostratigraphy of the Polish Outer Carpathians: A record of basin geohistory. Ann. Soc. Geol. Pol. 1997, 67, 325–337. [Google Scholar]
- Golonka, J.; Gahagan, L.; Krobicki, M.; Marko, F.; Oszczypko, N.; Ślączka, A. Plate tectonic evolution and paleogeography of the Circum-Carpathian region. In The Carpathians and Their Foreland: Geology and Hydrocarbon Resources; Golonka, J., Picha, F., Eds.; American Association of Petroleum Geologists: Tulsa, OK, USA, 2006; Volume 84, pp. 11–46. [Google Scholar]
- Golonka, J.; Waśkowska, A.; Ślączka, A. The Western Outer Carpathians: Origin and evolution. Zeitsch. Deut. Gesell. Geowiss. 2019, 170, 229–254. [Google Scholar] [CrossRef]
- Golonka, J.; Gawęda, A.; Waśkowska, A. Carpathians. In Reference Module in Earth Systems and Environmental Sciences, Encyclopedia of Geology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 372–381. [Google Scholar] [CrossRef]
- Oszczypko, N.; Uchman, A.; Malata, E. Palaeotectonic Evolution of the Outer Carpathian and Pieniny Klippen Belt Basins; Instytut Nauk Geologicznych UJ: Kraków, Poland, 2006; pp. 1–199. [Google Scholar]
- Książkiewicz, M. Tectonics of the Carpathians. In Geology of Poland. Vol. IV. Tectonics; Pożaryski, W., Ed.; Wydawnictwa Geologiczne: Warszawa, Poland, 1977; pp. 476–604. [Google Scholar]
- Lexa, J.; Bezák, V.; Elečko, M.; Mello, J.; Polák, M.; Potfaj, M.; Vozár, J. Geological Map of Western Carpathians and Adjacent Areas 1: 500,000; Geological Survey of Slovak Republic: Bratislava, Slovakia, 2000. [Google Scholar]
- Bubik, M. Remarks on the quantitative analysis of deep-sea agglutinated foraminiferal taphocoenoses with special attention to tubular astrorhizids. Micropaleontology 2019, 65, 63–74. [Google Scholar]
- Gradstein, F.M.; Ogg, J.G.; Schmitz, M.D.; Ogg, G.M. Geologic Time Scale 2020; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–1357. [Google Scholar]
- Geroch, S.; Jednorowska, A.; Książkiewicz, M.; Liszkowa, J. Stratigraphy based upon microfauna in the Western Polish Carpathians. Biul. Inst. Geol. 1967, 211, 185–282. [Google Scholar]
- Malinowska, L. Budowa Geologiczna Polski, T.3, Atlas Skamieniałości Przewodnich i Charakterystycznych, cz. 2c, Mezozoik, Kreda; Wydawnictwa Geologiczne: Warszawa, Poland, 1984; pp. 1–579.
- Waśkowska-Oliwa, A. The Paleocene assemblages of agglutinated foraminifera from deep-water basin sediments of the Carpathians (Subsilesian Unit, Poland)—Biostratigraphical remarks. In Proceedings of the Seventh International Workshop on Agglutinated Foraminifera; Hart, M., Kaminski, M.A., Smart, C., Eds.; Grzybowski Foundation, Special Publication: Kraków, Poland, 2008; Volume 13, pp. 227–265. [Google Scholar]
- Waśkowska, A.; Golonka, J.; Starzec, K.; Cieszkowski, M. Campanian–Paleocene Jaworzynka Formation in its type area (Magura Nappe, Outer Carpathians). Acta Geol. Pol. 2021, 71, 345–370. [Google Scholar] [CrossRef]
- Bieda, F.; Jednorowska, A.; Książkiewicz, M. Stratigraphy of the Magura Series around Babia Góra. Biul. Inst. Geol. 1967, 211, 293–325. [Google Scholar]
- Bleicher, J. Mikrofauna serii magurskiej okolic Grybowa. Kwart. Geol. 1958, 2, 285–399. [Google Scholar]
- Geroch, S.; Gradzinski, R. Stratygrafia serii podślaskiej żywieckiego okna tektonicznego. Rocz. Pol. Tow. Geol. 1955, 24, 1–62. [Google Scholar]
- Jednorowska, A. Zespoły małych otwornic w warstwach jednostki magurskiej rejonu Babiej Góry i ich znaczenie stratygraficzne. In Przewodnik XXXIX Zjazdu Polskiego Towarzystwa Geologicznego—Babia Góra; Wydawnictwa Geologiczne: Warszawa, Poland, 1966; pp. 71–90. [Google Scholar]
- Lemańska, A. Comparison of deep-water agglutinated foraminiferafrom the hemipelagic variegated shales (Lower Turonian–Lower Santonian) and the turbiditic Godula beds (Upper Santonian–Campanian) in the Lanckorona-Wadowice area (Silesian Unit, Outer Carpathians, Poland). Stud. Geol. Pol. 2005, 124, 259–272. [Google Scholar]
- Malata, E. The stratigraphy of the Magura Nappe in the Western part of the Beskid Wysoki Mts., Poland based on microfauna. Biul. Inst. Geol. 1981, 331, 103–116. [Google Scholar]
- Malata, E. Albian–Early Miocene foraminiferal assemblages of the Magura Nappe (Polish Outer Carpathians). Geol. Carpath. 2002, 53, 77–79. [Google Scholar]
- Malata, E.; Malata, T.; Oszczypko, N. Litho- and biostratigraphy of the Magura Nappe in the eastern part of the Beskid Wyspowy Range (Polish Western Carpathians). Ann. Soc. Geol. Pol. 1996, 66, 269–284. [Google Scholar]
- Oszczypko, N.; Dudziak, J.; Malata, E. Stratygrafia osadów płaszczowiny magurskiej (kreda–paleogen) w Beskidzie Sądeckim, Karpaty Zewnętrzne. Stud. Geol. Polon. 1990, 97, 109–181. [Google Scholar]
- Oszczypko, N.; Malata, E.; Bąk, K.; Kędzierski, M.; Oszczypko-Clowes, M. Lithostratigraphy and biostratigraphy of the Upper Albian-Lower/Middle Eocene flysch deposits in the Bystrica and Rača subunits of the Magura Nappe (Beskid Wyspowy and Gorce Ranges; Poland). Ann. Soc. Geol. Pol. 2005, 75, 27–69. [Google Scholar]
- Sikora, W.; Żytko, K.; Sikora, W.; Żytko, K. Budowa Beskidu Wysokiego na południe od Żywca. Biul. Inst. Geol. 1960, 141, 61–204. [Google Scholar]
- Stráník, Z.; Bubík, M.; Švábenická, L. Oráztockých vrstvách račanské jednotky na listu 25–314 Otrokovice. Geol. Výzk. Morav. Slez. 2016, 7, 74–76. [Google Scholar]
- Geroch, S. Microfaunal assemblages from the Cretaceous and Paleogene Silesian unit in the Beskid Slaski Mountains. Biul. Inst. Geol. 1960, 153, 1–138. [Google Scholar]
- Kępińska, B. Subdivision of the Upper Cretaceous and Paleocene in central part of the Brzanka-Liwocz fold (Silesian Unit). Geo. Quart. 1986, 30, 63–76. [Google Scholar]
- Olszewska, B. Stratigraphy of the middle part of the Dukla Unit in the Polish Carpathians based on foraminifera (Upper Cretaceous–Paleogene). Biul. Inst. Geol. 1980, 326, 59–97. [Google Scholar]
- Waśkowska, A.; Joniec, A.; Kotlarczyk, J.; Siwek, P. The Late Cretaceous fucoid marl of the Ropianka Formation in the Kąkolówka structure (Skole Nappe, Outer Carpathians, Poland)—lithology and foraminiferal biostratigraphy. Ann. Soc. Geol. Pol. 2019, 89, 259–284. [Google Scholar] [CrossRef]
- Ślączka, A. Geologia jednostki dukielskiej. Pr. Inst. Geol. 1971, 63, 1–97. [Google Scholar]
- Bąk, K. Biostratigraphy of deep-water agglutinated Foraminifera in Scaglia Rossa-type deposits of the Pieniny Klippen Belt, Carpathians, Poland. In Proceedings of the Fifth International Workshop on Agglutinated Foraminifera, Plymouth, UK, 6–16 September 1997; Hart, M., Kaminski, M.A., Smart, C.W., Eds.; Grzybowski Foundation, Special Publication: Kraków, Poland, 2000; Volume 7, pp. 15–41. [Google Scholar]
- Morgiel, J.; Olszewska, B. Biostratigraphy of the Polish External Carpathians Based on agglutinated foraminifera. Micropaleontology 1981, 27, 1–30. [Google Scholar] [CrossRef]
- Kuhnt, W.; Moullade, M.; Kaminski, M.A. Upper Cretaceous, K/T boundary, and Paleocene agglutinated foraminifers from Hole 959D (Côte d’Ivoire-Ghana transform margin). In Proceedings of the Ocean Drilling Program: Scientific Results; Ocean Drilling Program: Austin, TX, USA, 1998; Volume 159, pp. 389–411. [Google Scholar]
- Kuhnt, W.; Geroch, S.; Kaminski, M.A.; Moullade, M.; Neagu, T. Upper Cretaceous Abyssal Claystones in the North Atlantic and the Western Tethys—urrent Status of Biostratigraphical Correlation using Agglutinated Foraminifers. Cret. Res. 1992, 13, 467–478. [Google Scholar] [CrossRef]
- Moullade, M.; Kuhnt, W.; Thurow, J. Agglutinated benthic foraminifers from Upper Cretaceous variegated clays of the North Atlantic Ocean (DSDP Leg 93 and ODP Leg 103). In Proceedings of the Ocean Drilling Program: Scientific Results; Ocean Drilling Program: Austin, TX, USA, 1988; Volume 103, pp. 349–377. [Google Scholar]
- Setoyama, E.; Kaminski, M.A.; Tyszka, J. Late Cretaceous agglutinated foraminifera and implications for the biostratigraphy and palaeobiogeography of the southwestern Barents Sea. In Proceedings of the Eighth International Workshop on Agglutinated Foraminifera, Cluj-Napoca, Romania, 7–13 September 2008; Bubík, M., Kaminski, M.A., Eds.; Grzybowski Foundation Special Publication: Kraków, Poland, 2011; Volume 16, pp. 251–309. [Google Scholar]
- Kaminski, M.A.; Cetean, C.G.; Balc, R.; Coccioni, R. Upper Cretaceous Deep-Water Agglutinated Foraminifera from the Contessa Highway Section, Umbria-Marche Basin, Italy: Taxonomy and Biostratigraphy. In Proceedings of the Eighth International Workshop on Agglutinated Foraminifera, Cluj-Napoca, Romania, 7–13 September 2008; Kaminski, M.A., Filipescu, S., Eds.; Grzybowski Foundation, Special Publication: Kraków, Poland, 2011; Volume 16, pp. 71–106. [Google Scholar]
- Jurkiewicz, H. Otwornice paleogenu podmenilitowego polskich Karpat Środkowych. Biul. Inst. Geol. 1967, 210, 5–103. [Google Scholar]
- Jurkiewicz, H. Poziomy otwornicowe paleogenu wschodniej części jednostki śląskiej. Ann. Soc. Geol. Pol. 1959, 29, 235–263. [Google Scholar]
- Książkiewicz, M. Stratygrafia serii magurskiej w Beskidzie Średnim. Startigraphy of the Magura Series North of the Babia Góra, Western Carpathians. Biul. Inst. Geol. 1958, 135, 43–82. [Google Scholar]
- Sikora, W. Budowa geologiczna płaszczowiny magurskiej między Szymbarkiem Ruskim a Nawojową. Biul. Inst. Geol. 1970, 235, 5–121. [Google Scholar]
- Waśkowska, A.; Golonka, J.; Machowski, G.; Pstrucha, E. Potential source rocks in the Ropianka Formation of the Magura Nappe (Outer Carpathians, Poland)—geochemical and foraminiferal case study. Geol. Geoph. Environ. 2018, 44, 49–68. [Google Scholar] [CrossRef]
- Hnylko, S.; Hnylko, O. Foraminiferal stratigraphy and palaeobathymetry of Paleocene-lowermost Oligocene deposits (Vezhany and Monastyrets nappes, Ukrainian Carpathians). Geol. Quart. 2016, 60, 77–105. [Google Scholar] [CrossRef]
- Ţabără, D.; Slimani, H.; Mare, S.; Chira, C.M. Integrated biostratigraphy and palaeoenvironmental interpretation of the Upper Cretaceous to Paleocene succession in the northern Moldavidian Domain (Eastern Carpathians, Romania). Cret. Res. 2017, 77, 102–123. [Google Scholar] [CrossRef]
- Hanzlíková, E. Paleogene stratigraphy and foraminifera of the Outer Flysch Belt. Miscell. Micropal. Knihov. Zemn. Plynu Nafty 1983, 4, 43–70. [Google Scholar]
- Beckmann, J.P.; Bolli, H.M.; Kleboth, P.; Proto-Decima, F. Micropaleontology and biostratigraphy of the Campanian to Paleocene of the Monte Giglio, Bergamo Province, Italy. Mem. Sc. Geol. 1982, 35, 91–172. [Google Scholar]
- Cieszkowski, M.; Golonka, J.; Waśkowska-Oliwa, A.; Chodyń, R. Type Locality of the Mutne Sandstone Member of the Jaworzynka Formation, Western Outer Carpathians, Poland. Ann. Soc. Geol. Pol. 2007, 77, 269–290. [Google Scholar]
- Jurkiewicz, H. Otwornice z łupków czarnorzeckich wschodniej części jednostki śląskiej. Ann. Soc. Geol. Pol. 1960, 30, 333–343. [Google Scholar]
- Teťák, F.; Cieszkowski, M.; Golonka, J.; Waśkowska, A.; Szczęch, M. The Ropianka Formation of the Bystrica Zone (Magura Nappe, Outer Carpathians: Proposal for a new reference section in northwestern Orava. Ann. Soc. Geol. Pol. 2017, 87, 259–274. [Google Scholar] [CrossRef][Green Version]
- Bąk, K. Deep-water agglutinated foraminiferal changes across the Cretaceous/Tertiary and Paleocene/Eocene transitions in the deep flysch environment; eastern part of Outer Carpathians (Bieszczady Mts., Poland). In Proceedings of the Six International Workshop on Agglutinated Foraminifera, Prague, Czech Republic, 6–7 September 2001; Bubík, M., Kamiński, M.A., Eds.; Grzybowski Foundation, Special Publication: Kraków, Poland, 2004; Volume 8, pp. 1–56. [Google Scholar]
- Jednorowska, A. Zespoły otwornicowe w zewnętrznych strefach jednostki magurskiej Karpat i ich znaczenie stratygraficzne. Pr. Geol. Pol. Ak. Nauk O/Kraków Kom. Nauk Geol. 1968, 50, 1–89. [Google Scholar]
- Waśkowska, A. Response of Early Eocene deep-water benthic foraminifera to volcanic ash falls in the Polish Outer Carpathians: Palaeocological implications. Palaeogeog. Palaeoclim. Palaeoecol. 2011, 305, 50–64. [Google Scholar] [CrossRef]
- Waśkowska, A. The Early Eocene Saccamminoides carpathicus Assemblage in the Outer Flysch Carpathians. In Proceedings of the Eight International Workshop on Agglutinated Foraminifera, Cluj-Napoca, Romania, 7–13 September 2008; Kaminski, M.A., Filipescu, S., Eds.; Grzybowski Foundation, Special Publication: Kraków, Poland, 2011; Volume 16, pp. 331–341. [Google Scholar]
- Waśkowska, A. The Eocene Hieroglyphic beds of the Silesian Nappe in the Western Polish Carpathians—their development and foraminiferal record. Geol. Quart. 2015, 59, 271–299. [Google Scholar] [CrossRef][Green Version]
- Pokorny, V. Microstratigraphie et biofaciès du flysch Carpatique de la Moravie Méridionale (Tchécoslovaquie). Rev. l’Inst. Fran. Pétr. Ann. Comb. Liq. 1960, 15, 1099–1141. [Google Scholar]
- Hanzlíková, E. Carpathian Upper Cretaceous foraminifera of Moravia (Turonian-Maastrichtian); Vydal Ustredni Ustav Geologicky: Praha, Czechoslovakia, 1970; Volume 39, pp. 5–159.
- Waśkowska, A.; Cieszkowski, M.; Golonka, J.; Kowal-Kasprzyk, J. Paleocene sedimentary record of ridge geodynamics in Outer Carpathian basins (Subsilesian Unit). Geol. Carpath. 2014, 65, 35–54. [Google Scholar] [CrossRef]
- Cieszkowski, M.; Waskowska-Oliwa, A. Development and biostratigraphy of the Paleogene Gorzeń beds, Subsilesian Unit, Outer Carpathians, Poland. Geol. Carpath. 2002, 53, 57–58. [Google Scholar]
- Waśkowska-Oliwa, A.; Leśniak, T. Biostratigraphy and palaeoecology of Early Palaeogene deep water foraminiferal assemblages from the Subsilesian Unit in the Żywiec Tectonic Window (Polish Outer Carpathians). Bull. Pol. Ac. Sc. Earth Sc. 2003, 51, 11–27. [Google Scholar]
- Waśkowska-Oliwa, A. Foraminiferal palaeodepth indicators from the lower Palaeogene deposits of the Subsilesian Unit (Polish Outer Carpathians). Stud. Geol. Pol. 2005, 124, 297–324. [Google Scholar]
- Valchev, B. On the stratigraphical distribution of small benthic foraminifera and the biostratigraphy of the Paleocene of the coastal part of east Stara Planina. Geol. Gfizi. 2004, 47, 43–49. [Google Scholar]
- Golonka, J.; Waśkowska, A. Paleogene of the Magura Nappe adjacent to the Pieniny Klippen Belt between Szczawnica and Krościenko (Outer Carpathians, Poland). Geol. Geophis. Environ. 2014, 40, 359–376. [Google Scholar] [CrossRef]
- Olszewska, B.; Odrzywolska-Bieńkowa, E.; Giel, M.D.; Pożaryska, K.; Szczechura, K. Rząd Foraminiferida Eichwald, 1830. In Budowa geologiczna Polski, Atlas skamieniałości przewodnich i charakterystycznych, Kenozoik, Trzeciorzęd, Paleogen, T 3, cz. 3a; Limanowska, L., Piwocki, M., Eds.; Wydawnictwa Geologiczne: Warszawa, Poland, 1996; pp. 45–215. [Google Scholar]
- Huss, F. Otwornice aglutynujące serii podśląskiej roponaśnej jednostki Węglówki, polskie Karpaty fliszowe. Prace Geol. PAN 1966, 34, 7–71. [Google Scholar]
- Jednorowska, A. Small Foraminifera assemblages in the Paleocene of the Polish Western Carpathians. Stud. Geol. Pol. 1975, 47, 3–103. [Google Scholar]
- Liszkowa, J.; Morgiel, J. Mikrofauna typu frydeckiego w polskich Karpatach Zewnętrznych. Biul. Inst. Geol. 1981, 331, 83–102. [Google Scholar]
- Kaminski, M.A.; Alegret, L.; Santoso, S.H.; Waskowska, A. The Paleocene of IODP Site U1511, Tasman Sea: A lagerstatten deposit for deep-water agglutinated foraminifera. Micropaleontology 2021, 4, 41–364. [Google Scholar] [CrossRef]
- Berggren, W.A.; Kent, D.V.; Swisher, C.C.; Aubry, M.P. A revised Cenozoic geochronology and chronostratigraphy. A revised Cenozoic geochronology and chronostratigraphy. Geochr. Time Scales Glob. Stratig. Corr. 1995, 54, 129–212. [Google Scholar]
- Waśkowska-Oliwa, A. Przegląd geologiczny. Biostratygrafia otwornicowa tufitów okna tektonicznego Wiśniowej. Przeg. Geol. 2003, 51, 261–262. [Google Scholar]
- Olszewska, B.; Smagowicz, M. Porównanie podziałów biostratygraficznych górnej kredy i paleogenu jednostki dukielskiej na podstawie otwornic planktonicznych i nannoplanktonu wapiennego. Przeg. Geol. 1977, 25, 359–363. [Google Scholar]
- Bąk, K.; Bąk, M.; Geroch, S.; Manecki, M. Biostratigraphy and paleoenvironmental analysis of benthic foraminifera and radiolarians in Paleogene variegated shales in the Skole unit, Polish Flysch Carpathians. Ann. Soc. Geol. Pol. 1997, 67, 135–154. [Google Scholar]
- Morgiel, J.; Szymakowska, F. Stratygrafia paleocenu i eocenu jednostki skolskiej. Biul. Inst. Geol. 1978, 310, 39–71. [Google Scholar]
- Arreguín-Rodríguez, G.J.; Alegret, L.; Ortiz, S. Glomospira acme during the Paleocene-Eocene Thermal Maximum: Response to CaCO3 dissolution or to ecological forces? J. Foraminifer. Res. 2013, 43, 40–54. [Google Scholar] [CrossRef]
- Arreguín-Rodríguez, G.J.; Alegret, L.; Sepúlveda, J.; Newman, S.; Summons, R.E. Enhanced terrestrial input supporting the Glomospira acme across the Paleocene-Eocene boundary in Southern Spain. Micropaleontology 2014, 60, 43–51. [Google Scholar]
- Galeotti, S.; Kaminski, M.A.; Coccioni, R.; Speijer, R. High resolution Deep Water Agglutinated foraminiferal record across the Paleocene/Eocene transition in the Contessa Road Section (central Italy). In Proceedings of the Sixth International Workshop on Agglutinated Foraminifera, Prague, Czech Republic, 6–7 September 2001; Bubik, M., Kaminski, M.A., Eds.; Grzybowski Foundation, Special Publication: Kraków, Poland, 2011; Volume 8, pp. 83–103. [Google Scholar]
- Waśkowska, A.; Cieszkowski, M.; Bębenek, S. Trochammina semi-specific assemblages from the Upper Paleocene of the Silesian Nappe, Polish Outer Carpathians. In Proceedings of the Ninth International Workshop on Agglutinated Foraminifera, Zaragoza, Spain, 3–7 September 2012; Kaminski, M.A., Alegret, L., Eds.; Grzybowski Foundation, Special Publication: Kraków, Poland, 2017; Volume 22, pp. 239–249. [Google Scholar]
- Waśkowska, A. Small-sized Trochammina assemblages in deep-water Eocene flysch deposits (Outer Carpathians, Poland) and their palaeoecological implications. J. Micropal. 2015, 34, 1–19. [Google Scholar] [CrossRef]
- Nagy, J.; Jargvoll, D.; Dypvik, H.; Jochmann, M.; Riber, L. Environmental changes during the Paleocene–Eocene Thermal Maximum in Spitsbergen as reflected by benthic foraminifera. Polar Res. 2013, 32. [Google Scholar] [CrossRef]
- Oszczypko, N. Budowa geologiczna północnych stoków Beskidu Sądeckiego między Dunajcem a Popradem (płaszczowina magurska). Ann. Soc. Geol. Pol. 1979, 49, 293–325. [Google Scholar]
- Waśkowska, A. The Eocene Hieroglyphic beds and Green shales in the Rożnów Lake area (Silesian Nappe, Outer Carpathians)—facies development and biostratigraphy. Geol. Geoph. Environ. 2014, 40, 5–26. [Google Scholar] [CrossRef][Green Version]
- Waskowska, A.; Cieszkowski, M. Utwory eoceńskie strefy Siar płaszczowiny magurskiej na terenie rezerwatu krajobrazowego Zamczysko nad Rabą (polskie Karpaty zewnętrzne). Chroń. Przyr. Ojcz. 2015, 71, 96–107. [Google Scholar]
- Waśkowska, A. Stratigraphy of the Hieroglyphic Beds with “Black Eocene” facies in the Silesian Nappe (Outer Flysch Carpathians, Poland). Ann. Soc. Geol. Pol. 2015, 85, 321–343. [Google Scholar] [CrossRef]
- Bindiu, R.; Filipescu, S. Foraminiferal biostratigraphy and paleoenvironments of the middle Eocene deposits from the northern part of the Tarcău Nappe (Eastern Carpathians, Romania). Stud. UBB Geol. 2014, 59, 45–59. [Google Scholar] [CrossRef][Green Version]
- Bindiu, R.; Filipescu, S.; Balc, R.; Cocis, L.; Gligor, D. The middle/late Eocene transition in the Eastern Carpathians (Romania) based on foraminifera and calcareous nannofossil assemblages. Geol. Quart. 2016, 60, 38–55. [Google Scholar]
- Săndulescu, M. Geotectonics of Romania; Editura Tehnică: Bucureşti, Romania, 1984; pp. 1–334. [Google Scholar]
- Săndulescu, M.; Micu, M.; Bratu, E. Stratigraphy of the Eocene Flysch formations of the East Carpathians. In The Eocene from the Transylvanian Basin, Romania. Geological Formations of Transylvania; Petrescu, I., Ghergari, L., Mészáros, N., Nicorici, E., Eds.; Babes-Bolyai University: Cluj-Napoca, Romania, 1987; pp. 159–164. [Google Scholar]
- Kaminski, M.A. The utility of Deep-Water Agglutinated Foraminiferal acmes for correlating Eocene to Oligocene abyssal sediments in the North Atlantic and Western Tethys. Stud. Geol. Pol. 2005, 124, 325–340. [Google Scholar]
- Grzybowski, J. Otwornice czerwonych iłów z Wadowic. Rozpr. Wydz. Mat. Przyr. Ak. Umiej. 1896, 30, 261–308. [Google Scholar]
- Kaminski, M.A.; Silye, L.; Kender, S. Miocene deep-water agglutinated foraminifera from ODP Hole 909c: Implications for the paleoceanography of the Fram Strait Area, Greenland Sea. Micropaleontology 2005, 51, 373–403. [Google Scholar] [CrossRef]
- Cieszkowski, M.; Waśkowska, A.; Kaminski, M. Deep water agglutinated foraminifera in Paleogene hemipelagic sediments of the Magura Basin in the Sucha Beskidzka area—Variegated shales of the Łabowa Shales Formation. In Integrating Microfossil Records from the Oceans and Epicontinental Seas; Bąk, M., Kaminski, M.A., Waśkowska, A., Eds.; Grzybowski Foundation, Special Publication: Kraków, Poland, 2011; Volume 17, pp. 3–63. [Google Scholar]
- Waśkowska, A.; Hnylko, S.; Kaminski, M.A.; Bakayewa, S. Grzybowski’s Lviv Collection of Carpathian Foraminifera; Grzybowski Foundation: Kraków, Poland, 2020; Volume 25, pp. 1–169. [Google Scholar]
- Hnylko, O.; Hnylko, S. Geological environments forming the Eocene black-shale formation of the Silesian Nappe (Ukrainian Carpathians). Geodynamics 2019, 26, 60–75. [Google Scholar] [CrossRef]
- Waśkowska-Oliwa, A. Haplophragmoides nauticus Kender, Kaminski & Jones in the Eocene of the Flysch Carpathians. In Proceedings of the Eighth International Workshop on Agglutinated Foraminifera, Cluj-Napoca, Romania, 7–13 September 2008; Filipescu, S., Kaminski, M.A., Eds.; Grzybowski Foundation, Special Publication: Kraków, Poland, 2008; Volume 14, pp. 63–64. [Google Scholar]
- Kender, S.; Kaminski, M.A.; Jones, R.W. Four new species of deep-water agglutinated foraminifera from the Oligocene-Miocene of the Congo Fan (offshore Angola). Micropaleontology 2007, 52, 465–470. [Google Scholar] [CrossRef]
- Bubik, M. Nove a malo zname aglutinovane foraminifery z paleogenu jizni Moravy. Zpravy Geol. Vyzk. 2009, 82–84. Available online: https://www.semanticscholar.org/paper/Nov%C3%A9-a-m%C3%A1lo-zn%C3%A1m%C3%A9-aglutinovan%C3%A9-foraminifery-z-ji%C5%BEn%C3%AD-Bubi%CC%81k/f5a61358c742c02a0c62d8ccb2ad885c87e8acbb (accessed on 31 August 2021).
- Bubik, M. A tribute to Prof. Anton Rzehak and the first micropaleontological analysis in the Magura Flysch. In Proceedings of the Seventh International Workshop on Agglutinated Foraminifera, Urbino, Italy, 2–8 October 2005; Kaminski, M.A., Cocioni, R., Eds.; Grzybowski Foundation, Special Publication: Kraków, Poland, 2008; Volume 13, pp. 1–12. [Google Scholar]
- Golonka, J.; Waśkowska, A. The Beloveža Formation of the Rača Unit in the Beskid Niski Mts. (Magura Nappe, Polish Flysch Carpathians) and adjacent parts of Slovakia and their equivalents in the western part of the Magura Nappe; remarks on the Beloveža Formation–Hieroglyphic Beds. Geol. Quart. 2012, 56, 821–832. [Google Scholar] [CrossRef][Green Version]
- Waśkowska, A. Bulbobaculites gorlicensis n. sp.—A new agglutinated foraminifera from Eocene of flysch Carpathians. Micropaleontology 2014, 60, 465–473. [Google Scholar]
- Cieszkowski, M.; Leśniak, T.; Środoń, J.; Waśkowska-Oliwa, A. Bentonitized tuffites in the Lower Eocene deposits of the Subsilesian Unit (Wester Outer Carpathians, Poland). Ann. Soc. Geol. Pol. 2006, 76, 197–214. [Google Scholar]
- Morgiel, J. Mikrofauna iłów babickich. Biul. Inst. Geol. 1959, 131, 7–48. [Google Scholar]
- Bindiu-Haitonic, R.; Filipescu, S.; Aroldi, C.; Oltean, G.; Chira, C.M. Eocene Deep-Water Agglutinated Foraminifera from the Eastern Carpathians (Romania): Paleoenvironments and Biostratigraphical Remarks. Micropaleontology 2019, 65, 47–61. [Google Scholar]
- Kuhnt, W.; Kaminski, M.A. The response of benthic foraminifera to the K/T boundary event—a review. Bull. Centres Rech. Explor. Mem. 1996, 16, 433–442. [Google Scholar]
- Nagy, J.; Kaminski, M.A.; Gradstein, F.M.; Johnson, K. Quantitative foraminiferal and palynomorph biostratigraphy of the Paleogene in the southwestern Barents Sea. In Proceedings of the Sixth International Workshop on Agglutinated Foraminifera, Prague, Czech Republic, 6–7 September 2001; Bubík, M., Kaminski, M.A., Eds.; Grzybowski Foundation, Special Publication: Kraków, Poland, 2004; Volume 8, pp. 359–379. [Google Scholar]
- Nagy, J.; Kaminski, M.A.; Johnsen, K.; Mitlehner, A. Benthic foraminiferal and dinoflagellate cyst stratigraphy and paleoenvironments of the Palaeogene Torsk Formation: A reference section for the western Barents Sea. In Micropalaeontology and Paleoceanography of the northern North Atlantic; Hass, C., Kaminski, M.A., Eds.; Grzybowski Foundation, Special Publication: Kraków, Poland, 1997; Volume 5, pp. 15–38. [Google Scholar]
- Waśkowska, A.; Kaminski, M.A. “Ammodiscus” latus Grzybowski, 1898: Its taxonomy, variability, and affinity to the genus Trochamminoides Cushman, 1910. In Proceedings of the Eight International Workshop on Agglutinated Foraminifera; Kaminski, M.A., Filipescu, S., Eds.; Grzybowski Foundation, Special Publication: Kraków, Poland, 2017; Volume 22, pp. 229–238. [Google Scholar]
- Olszewska, B.; Szydło, A. Environmental stress in the northern Tethys during the Paleogene: A review of foraminiferal and geochemical records from the Polish Outer Carpathians. Geol. Quar. 2017, 61, 682–696. [Google Scholar] [CrossRef]
- Olszewska, B. Oniektórych zespołach małych otwornic serii okiennej z Sopotni Małej, Mszany Dolnej, Szczawy i Klęczan. Biul. Inst. Geol. 1981, 21, 141–163. [Google Scholar]
- Jednorowska, A. Some assemblages of planktonic foraminifera from the Eocene of the Magura Series (Polish Flysch Carpathians). Rocz. Pol. Tow. Geol. 1969, 39, 277–294. [Google Scholar]
- Blaicher, J. Poziom wapiennej mikrofauny w górnym eocenie serii magurskiej. Zone with calcareous microfauna in the Upper Eocene of the Magura Series (Flysch Carptahinas). Biul. Inst. Geol. 1961, 166, 5–59. [Google Scholar]
- Golonka, J.; Waśkowska, A. The Beloveža Formation of the Rača Unit, Magura Nappe, in the Beskid Wysoki Mts (Polish Flysch Carpathians) north of Babia Góra Mountain–monospecific assemblages with Praesphaerammina subgaleata (Vašiček). In Integrating Microfossil Records from the Oceans and Epicontinental Seas; Bąk, M., Kaminski, M.A., Waśkowska, A., Eds.; Grzybowski Foundation, Special Publication: Kraków, Poland, 2011; Volume 17, pp. 27–35. [Google Scholar]
- Jednorowska, A.; Węcławik, S. Startygrfia mikropaleontologiczna osadów kredy górnej i paleogenu warstw jednostki magurskiej z rejonu Wysowej (Beskid Niski). Spraw. Pos. PAN Odd. Kraków 1969, 12, 263–266. [Google Scholar]
- Książkiewicz, M. Detailed Geological Map of Poland, 1:50,000, Zawoja Sheet—Explanations; Instytut Geologiczny: Warszawa, Poland, 1971; pp. 1–154.
- Książkiewicz, M. Detailed Geological Map of Poland, 1:50,000, Sucha Beskidzka Sheet—Explanations; Instytut Geologiczny: Warszawa, Poland, 1974; pp. 1–83.
- Vašíček, M. Poznamky k mikrobiostratigrafii magurskeho flyse na Morave. Vest. Stat. Geol. Ust. Ceskosl. Rep. 1947, 22, 235–256. [Google Scholar]
- Węcławik, S. Budowa geologiczna płaszczowiny magurskiej między Uściem Gorlickim a Tyliczem. The geological structure of the Magura Nappe between Uście Gorlickie and Tylicz. Pr. Geol. Kom. Nauk Geol. PAN Odd. Kraków 1969, 59, 1–101. [Google Scholar]
- Săndulescu, M.; Bratu, E. Nouvelles donnees pour la correlation des Formationa Paleogenes des unites allochtonnes de la Zone du Flysch Transcarpathique (Maramures). Dari Seama Instit. Geol. Geof. 1981, 68, 119–135. [Google Scholar]
- Gradstein, F.; Waśkowska, A.; Glinskikh, L. The first 40 million years of planktonic foraminifera. Geoscience 2021, 11, 5. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waśkowska, A. Agglutinated Foraminiferal Acmes and Their Role in the Biostratigraphy of the Campanian–Eocene Outer Carpathians. Geosciences 2021, 11, 367. https://doi.org/10.3390/geosciences11090367
Waśkowska A. Agglutinated Foraminiferal Acmes and Their Role in the Biostratigraphy of the Campanian–Eocene Outer Carpathians. Geosciences. 2021; 11(9):367. https://doi.org/10.3390/geosciences11090367
Chicago/Turabian StyleWaśkowska, Anna. 2021. "Agglutinated Foraminiferal Acmes and Their Role in the Biostratigraphy of the Campanian–Eocene Outer Carpathians" Geosciences 11, no. 9: 367. https://doi.org/10.3390/geosciences11090367
APA StyleWaśkowska, A. (2021). Agglutinated Foraminiferal Acmes and Their Role in the Biostratigraphy of the Campanian–Eocene Outer Carpathians. Geosciences, 11(9), 367. https://doi.org/10.3390/geosciences11090367





