Fluid-Rock Interactions in a Paleo-Geothermal Reservoir (Noble Hills Granite, California, USA). Part 1: Granite Pervasive Alteration Processes away from Fracture Zones
Abstract
:1. Introduction
2. Geological Setting
2.1. Death Valley
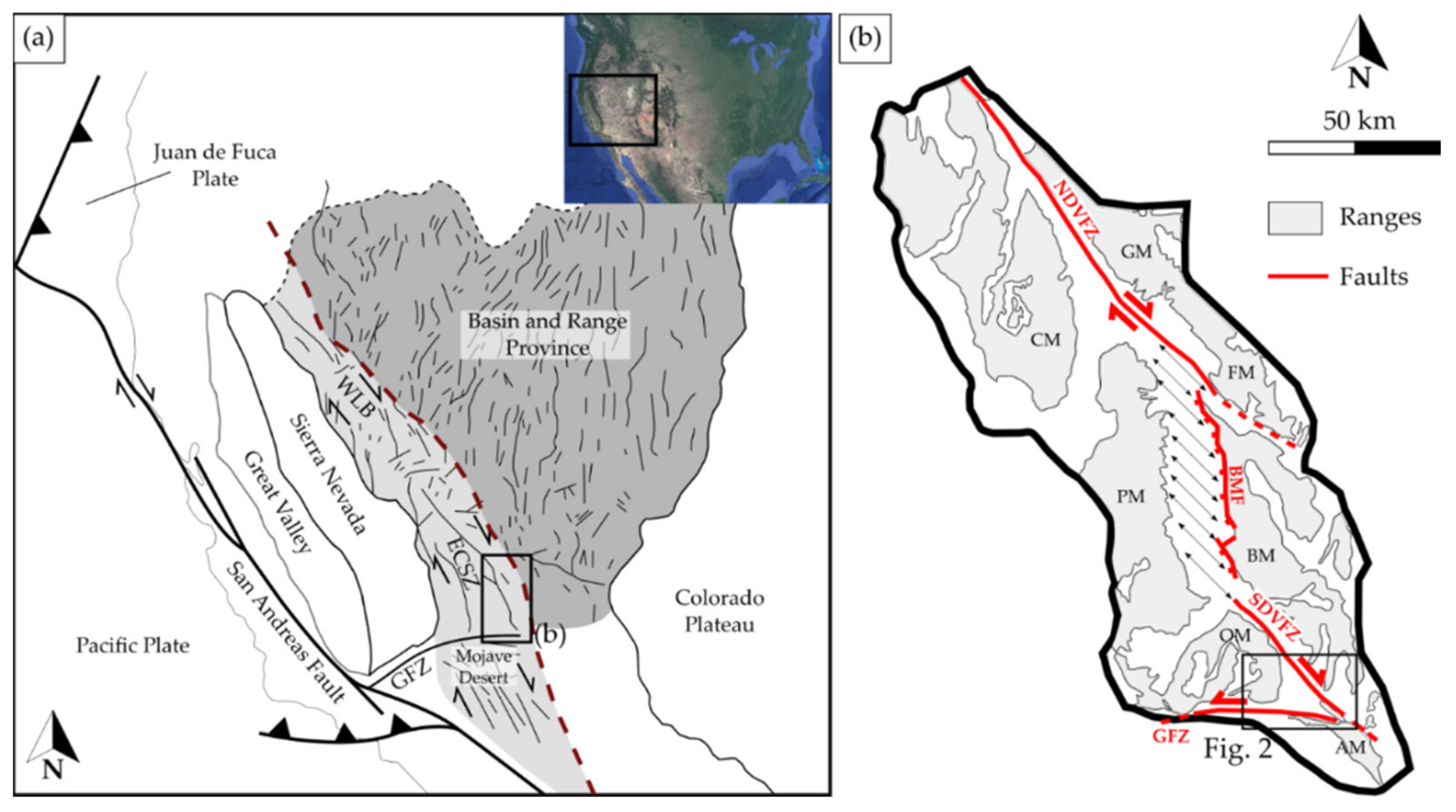
2.2. Noble Hills
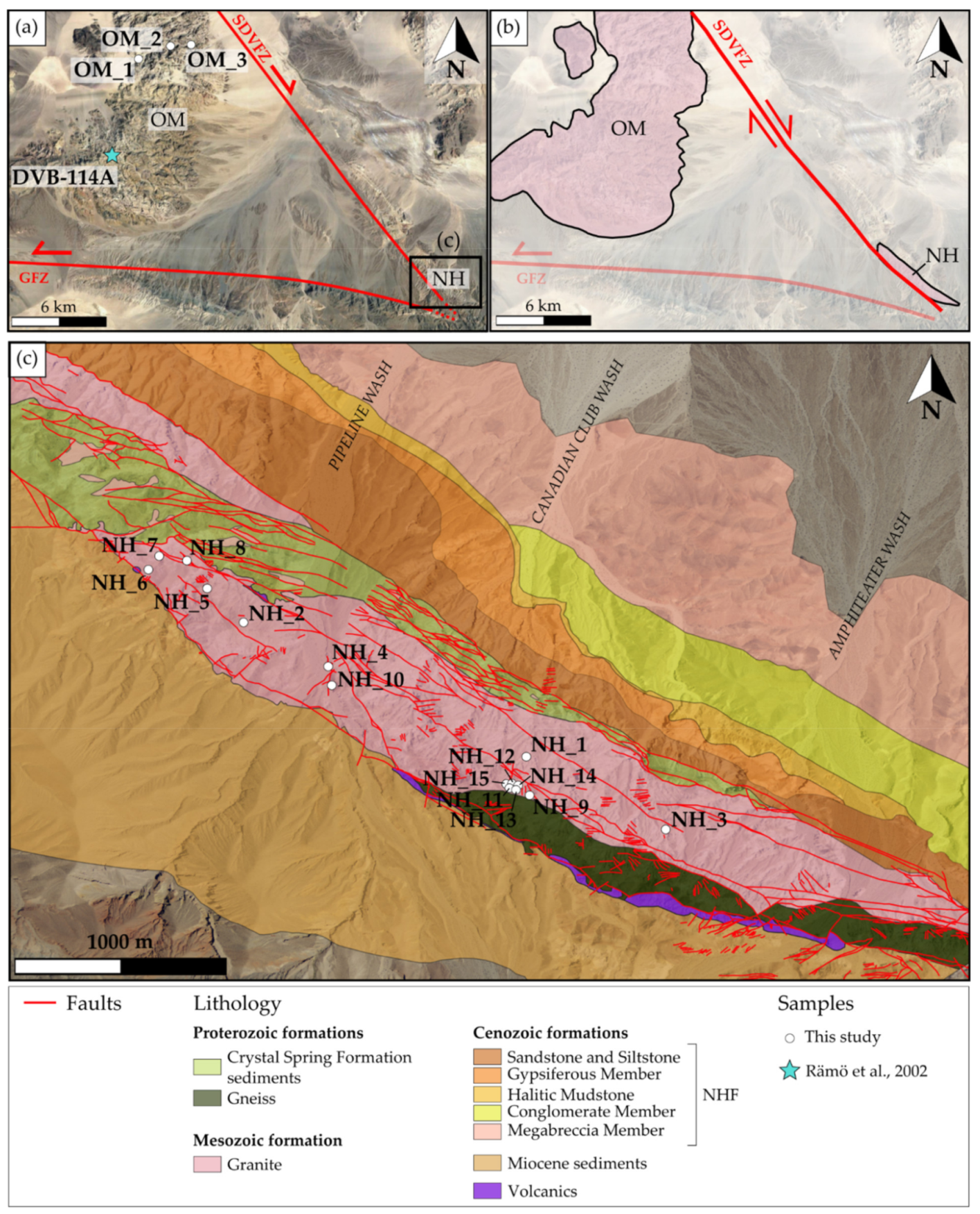
3. Material and Methods
3.1. Material and Sampling Strategy
3.2. Methods
3.2.1. Microscopic Observations
3.2.2. Fractures Density
- Fd0 < 1687 fracs/m—no to very low microfracturing
- Fd1 = 1687 fracs/m—microfracturing of order less than the grain size
- Fd2 = 2694 fracs/m, with a multiplicator factor of 1.6—microfracturing of grain size order with interconnections
- Fd3 = 3549 fracs/m, with a multiplicator factor of 1.3—abundant microfracturing
- Fd4 ≥ 5140 fracs/m ([12] this issue), with a multiplicator factor of 1.4—very abundant microfracturing
- Samples selected for this study have so a fracture density lower or equal to Fd2.
3.2.3. SEM-EDS
3.2.4. X-ray Diffraction (XRD)
Experimental Conditions
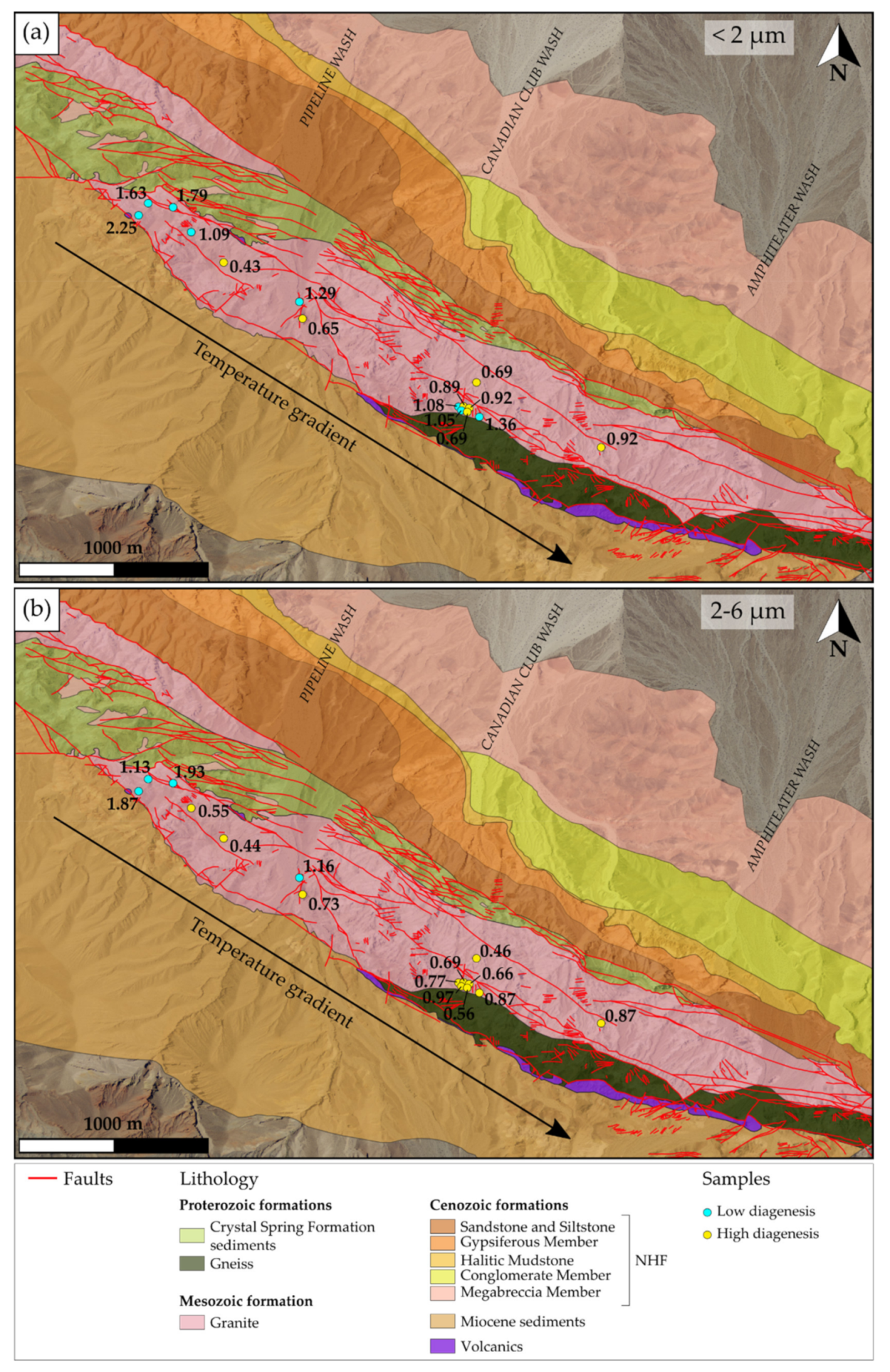
Determination of Illite Crystallinity and Kübler Index
3.2.5. ICP-MS—ICP-AES
3.2.6. Manocalcimetry
3.2.7. Ethanol Saturation Porosimetry
4. Results
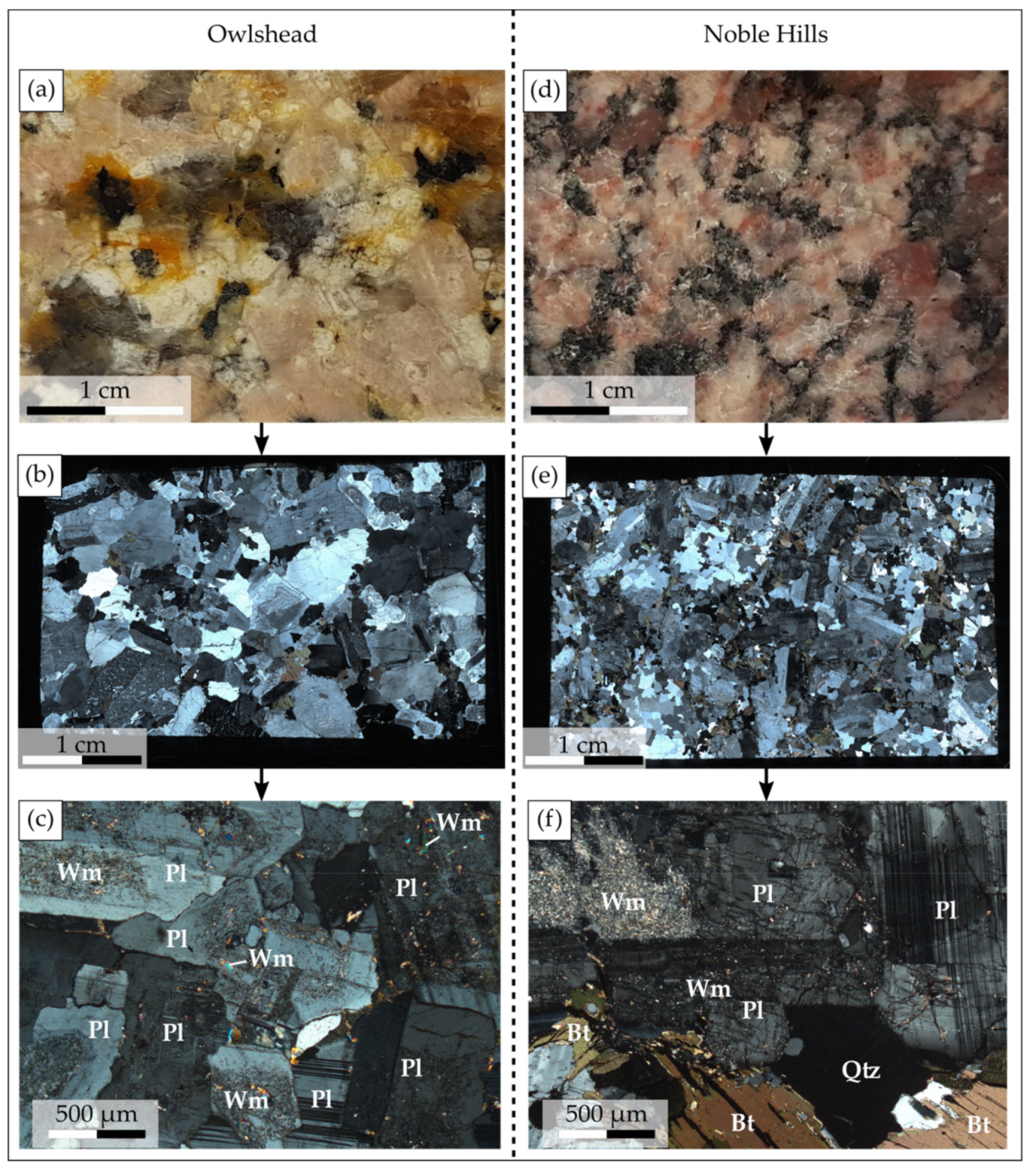
4.1. Petrographic Description
4.1.1. The Owlshead Granite
4.1.2. The Noble Hills Granite
- Kaolinite is present as fan shape (Figure 5e,f) of 25 µm to 40 µm in diameter. Under SEM (Figure 5f), well crystallized kaolinite presents a porous structure which can contribute to the porosity of the rock. It is only present in the NH granite (Table 3) indicating that the NH granite has undergone a different alteration from that of the OM granite.
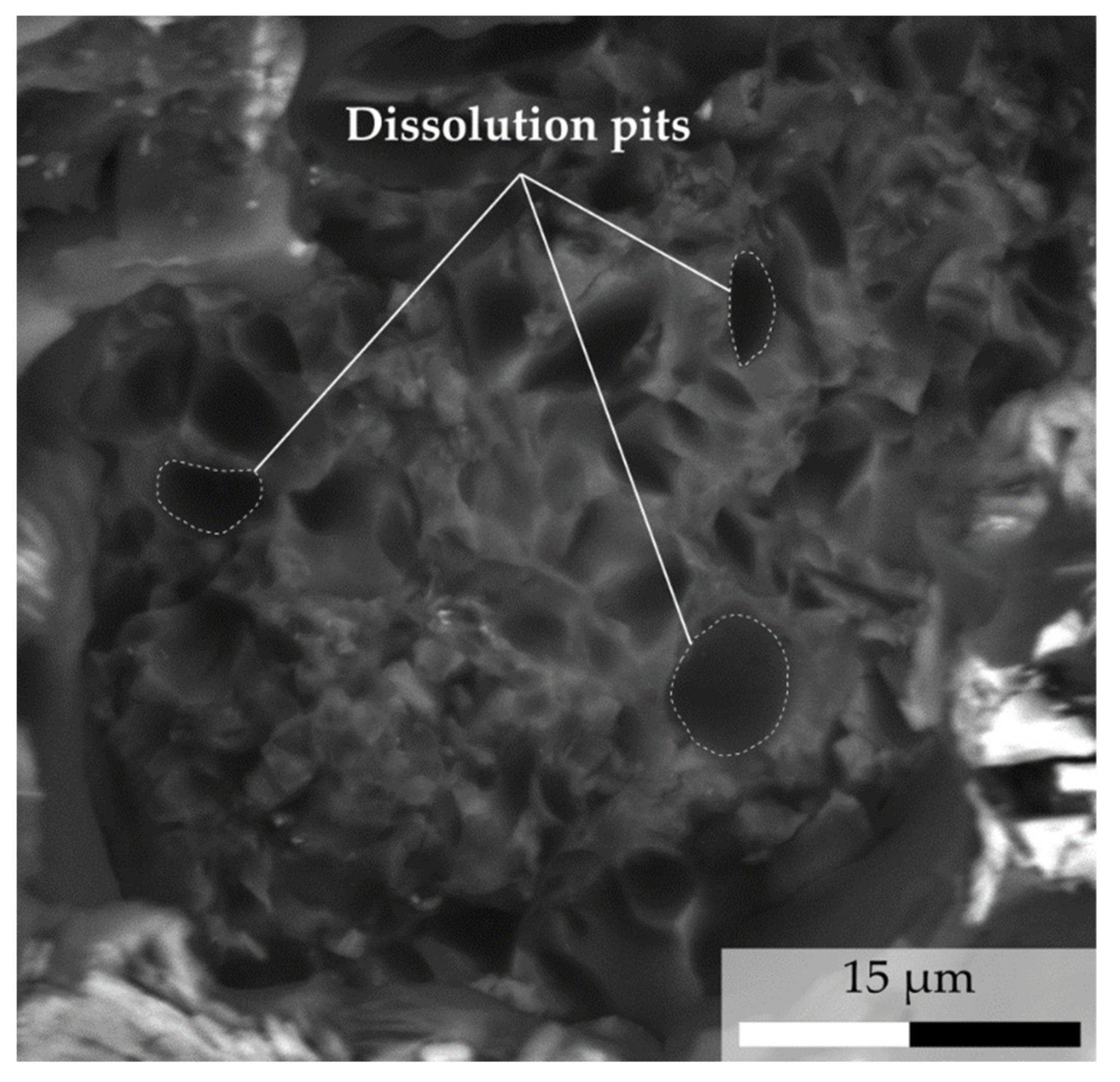
- Calcite, which occurs as small spots, is mainly associated with kaolinite (Figure 5d,e). It crystallizes in the porosity created by plagioclase dissolution.
4.1.3. Clay Minerals Identification and Kübler INDEX
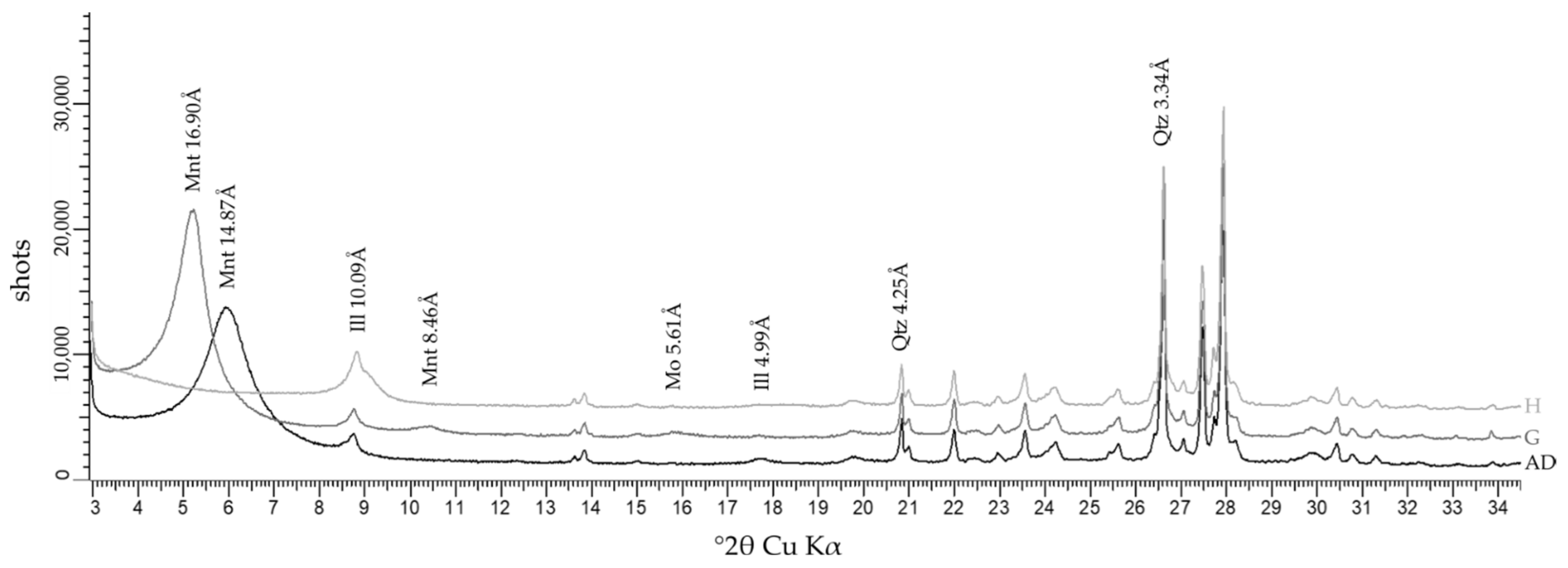
Owlshead Mountains
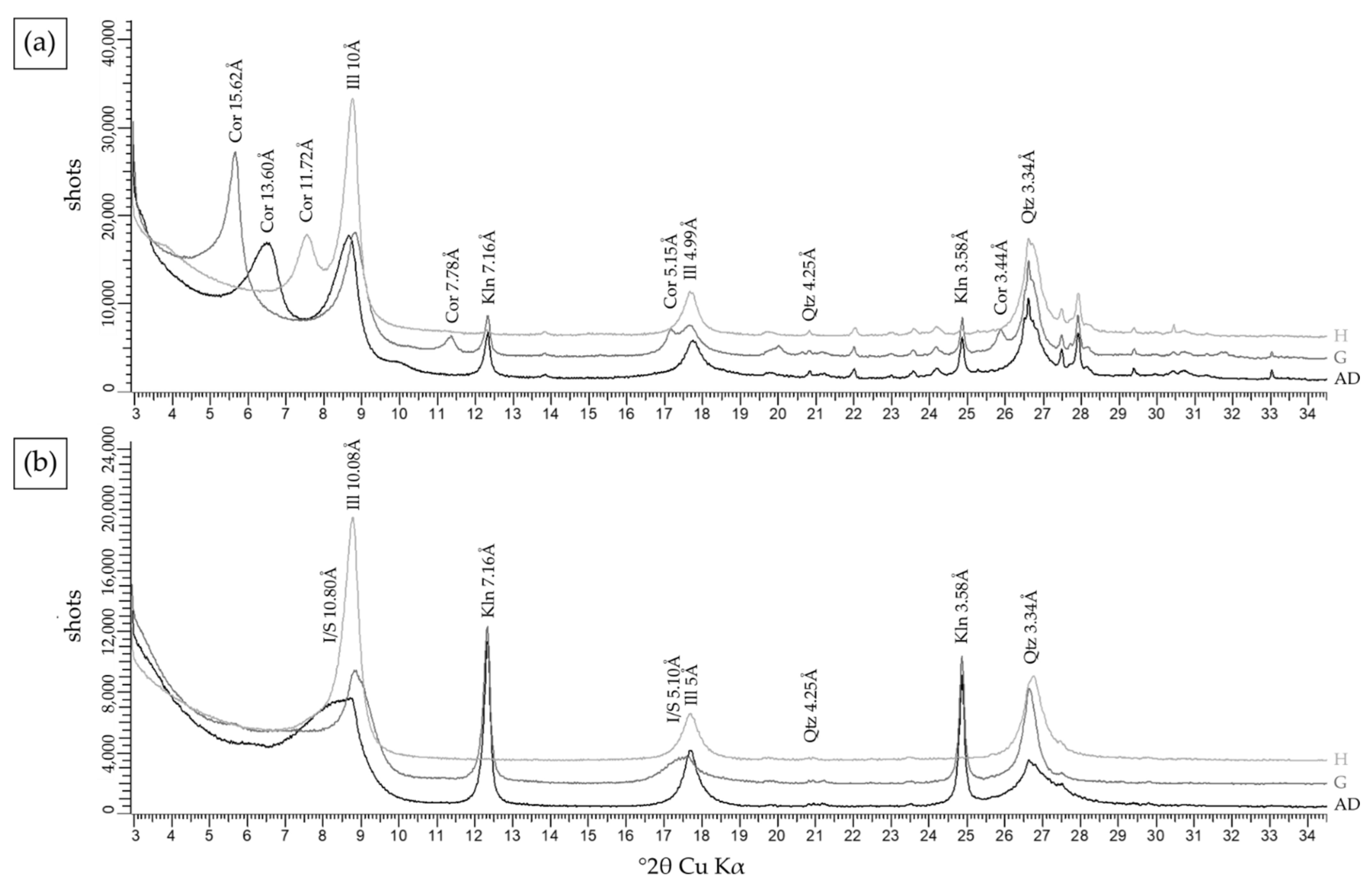
Noble Hills
- Corrensite, kaolinite and illite are identified in the first pattern, where corrensite is well known as the trioctahedral variety of regular 50:50 mixed-layer chlorite/smectite [69] (Figure 9a). It is characterized by (1) the peak at 13.60 Å in air-dried conditions, shifting to 15.62 Å after glycol solvation and collapses to 11.72 Å after heating, and (2) new peaks at 7.78 Å, 5.15 Å and 3.44 Å appear after glycol solvation and disappear after heating. The corrensite found in the NH granite is considered as a low charge corrensite after [51].
- Illite/smectite (I/S) mixed-layer, kaolinite and illite (Figure 9b) are identified in the second pattern, where I/S is illite-rich (R3), with more than 90% of illite and R representing the Reichweite parameter [70]. I/S is characterized by a large peak at 10.08 Å in air-dried, becoming narrower when it collapses to 9.93 Å after glycol solvation and by a peak at 5 Å swelling after glycol solvation.
4.2. Geochemical Analyses
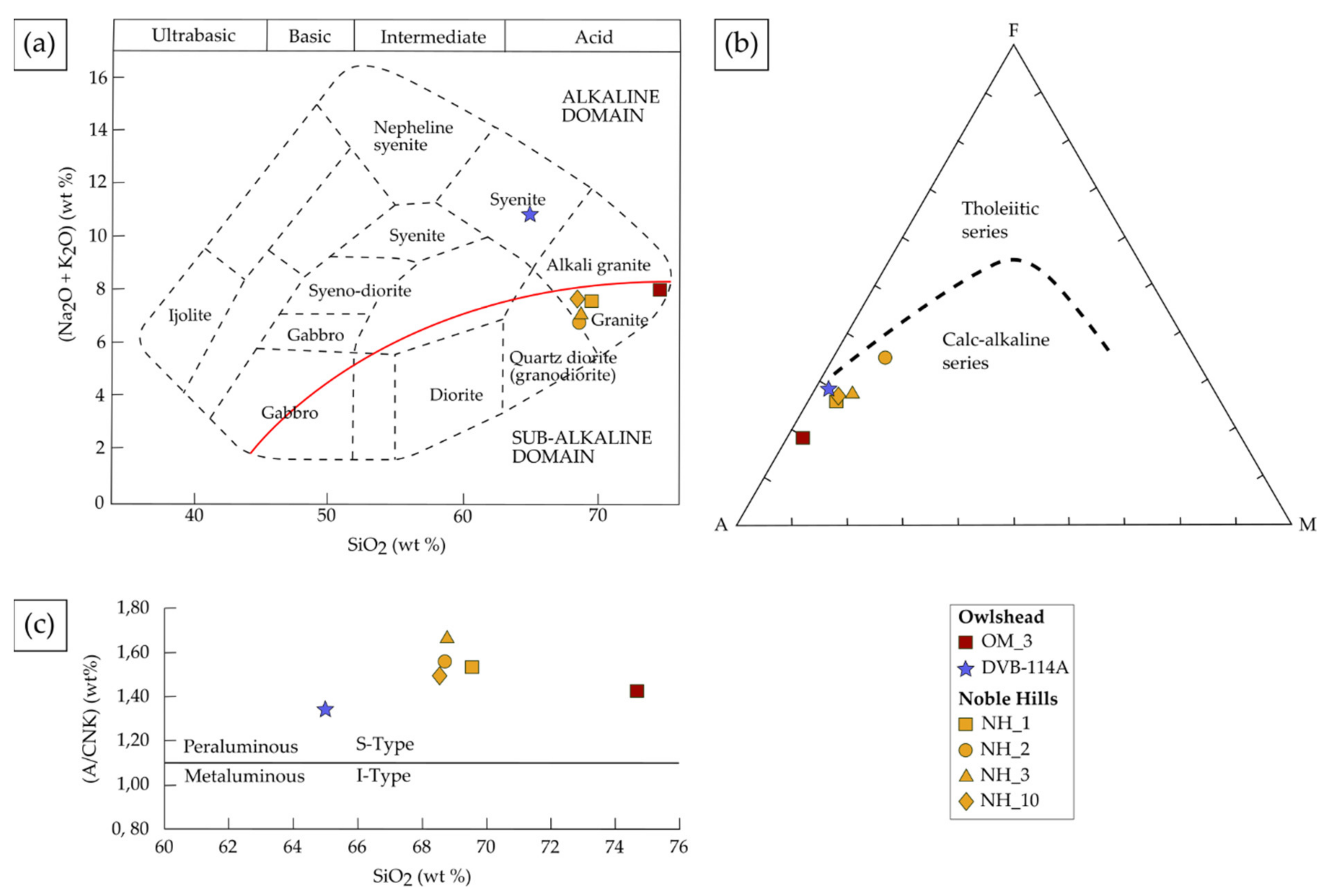
4.2.1. Major Element Bulk Rock Chemistry

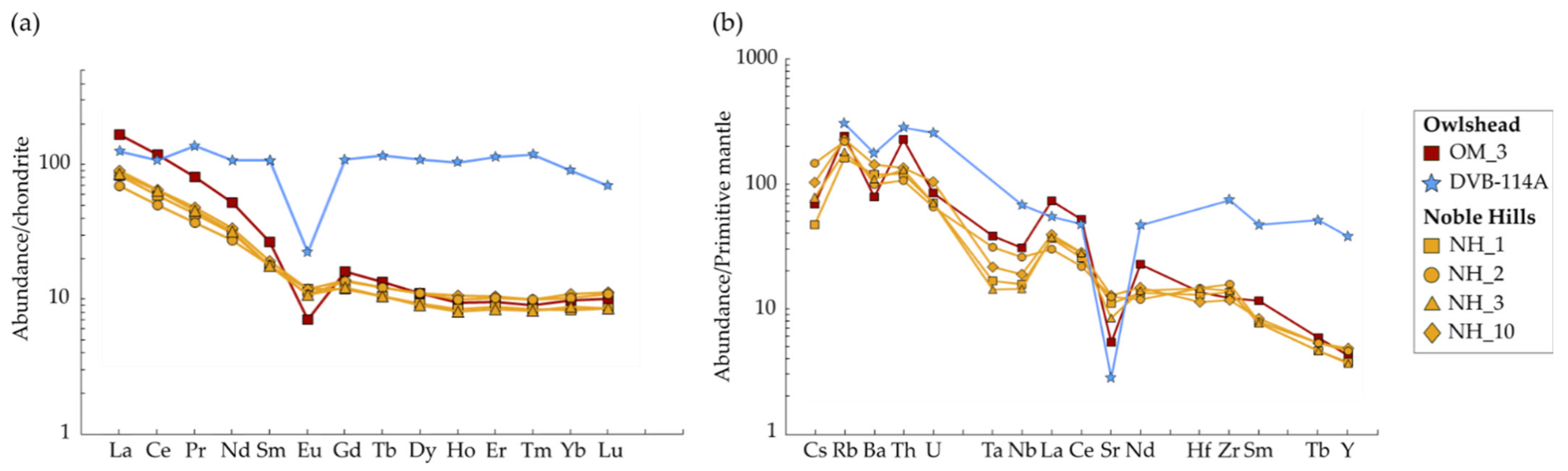
4.2.2. Trace Element and REE Bulk Chemistry
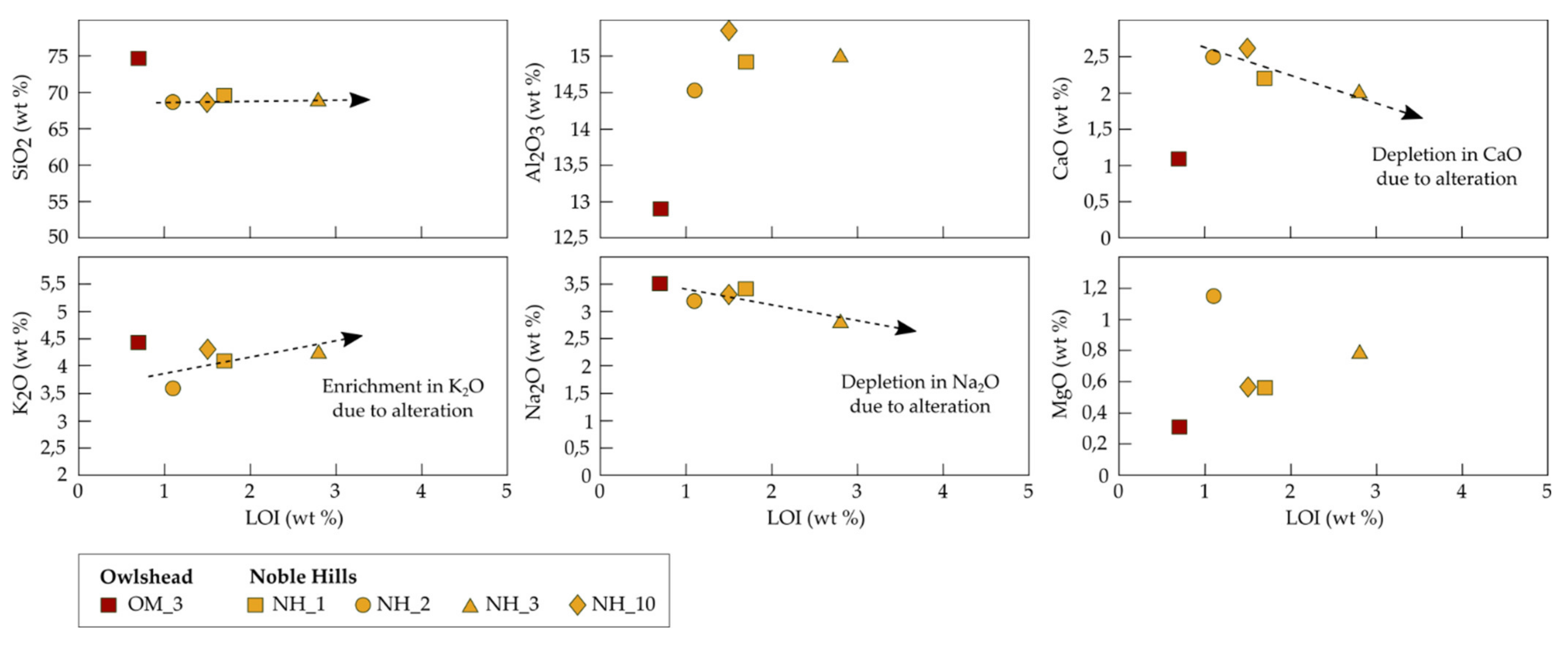
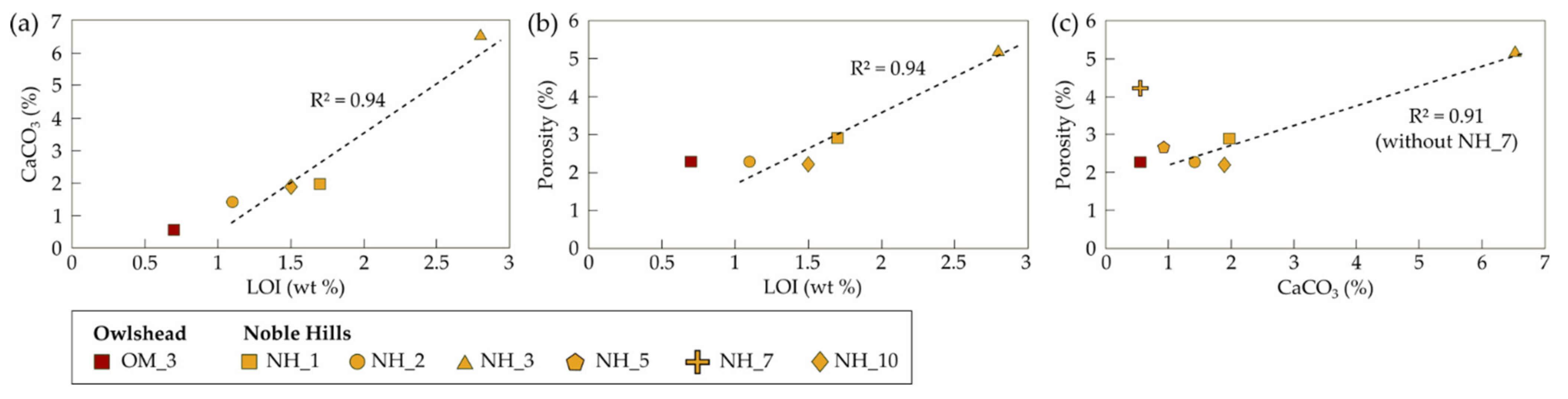
4.3. Calcimetry and Porosimetry
5. Discussion
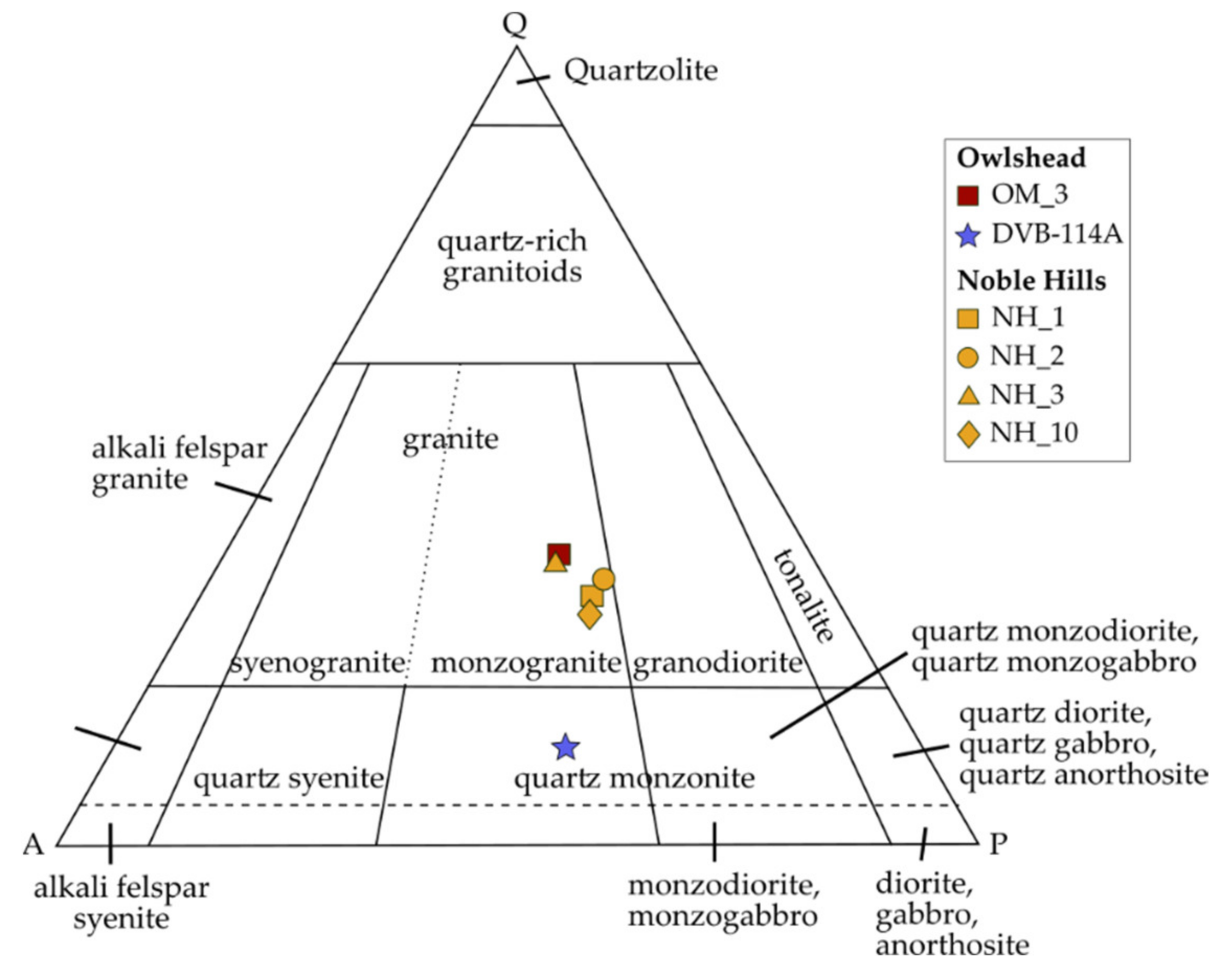
5.1. Petrogenesis of the OM and NH Granites
5.2. Thermal Evolution of the NH Granite
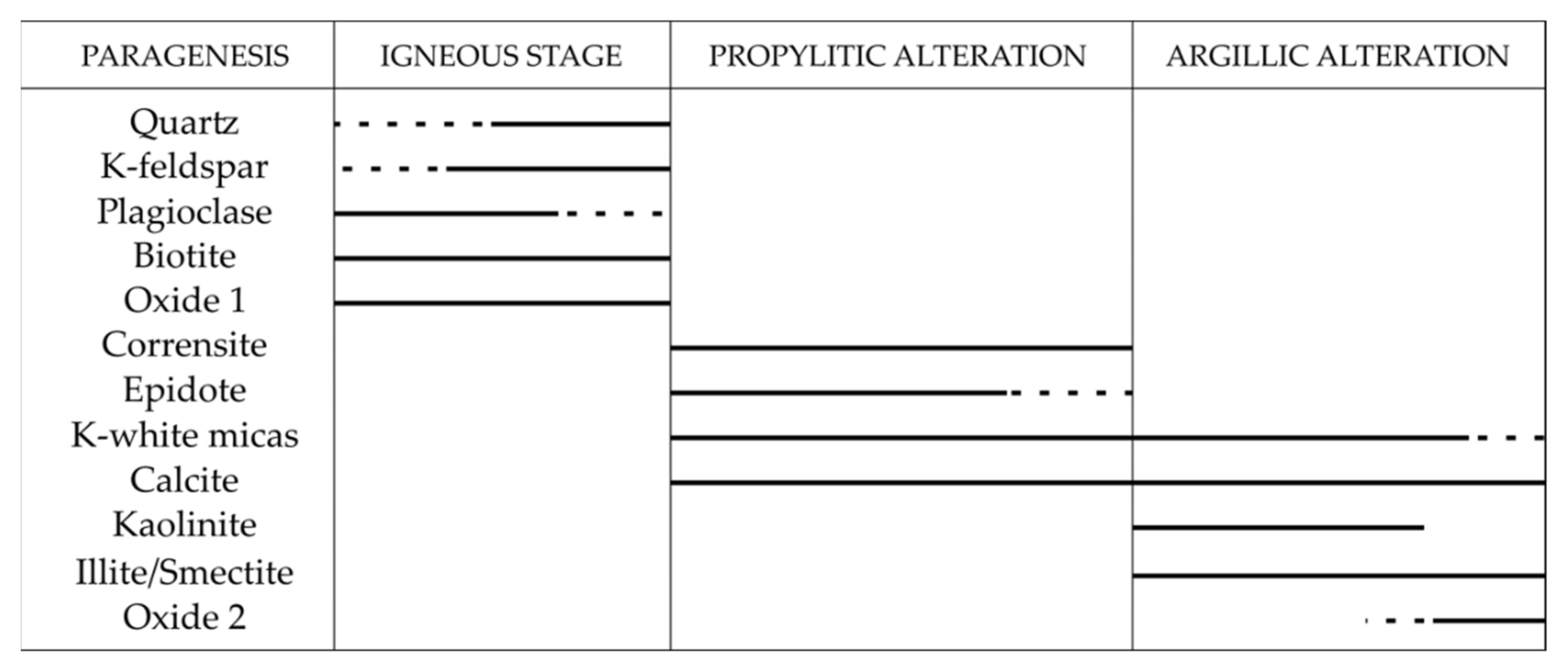
5.3. Alteration Parageneses
5.3.1. Propylitic Alteration
- The calcite as in the OM granite occurs as infills of the microcracks without interacting with the surrounding rock, as well as at grain boundaries.
- Mixed-layer clay minerals are the intermediate products of reactions involving end-member clays [87]. Corrensite, a chlorite/trioctahedral smectite mixed-layer phyllosicilate is considered as a stable mineral and also as an indicator of propylitic alteration [86,88,89]. It replaces partially biotite and occurs between 160–250 °C in geothermal fields [6,84,90].
5.3.2. Argillic Alteration
- Illitic minerals are well known to be indicators of fluid circulation as well as paleo-circulation systems [93]. [19,94] show that illite crystallization episodes can occur, for example, in a temperature range of 120 to 160 °C, corresponding to the argillic alteration facies. The illitization process mainly develops in plagioclase and biotite. It is a form of alteration product found extensively in granitoids, and felsic rocks, whereas K-feldspar remains relatively unaltered [8,67,85].
- According to [84], the presence of kaolinite in alteration paragenesis indicates a fluid temperature lower than 200–150 °C. Kaolinite is stable under more acidic conditions than illite, with pH values ranging from about 4.5 to 6. It also represents a more advanced product of hydrolysis reaction due to a high H+ activity in hydrothermal fluids.
- Plagioclase, oligoclase in composition, presents patches of calcite. Those patches are interpreted as a product of Ca release due to plagioclase alteration.
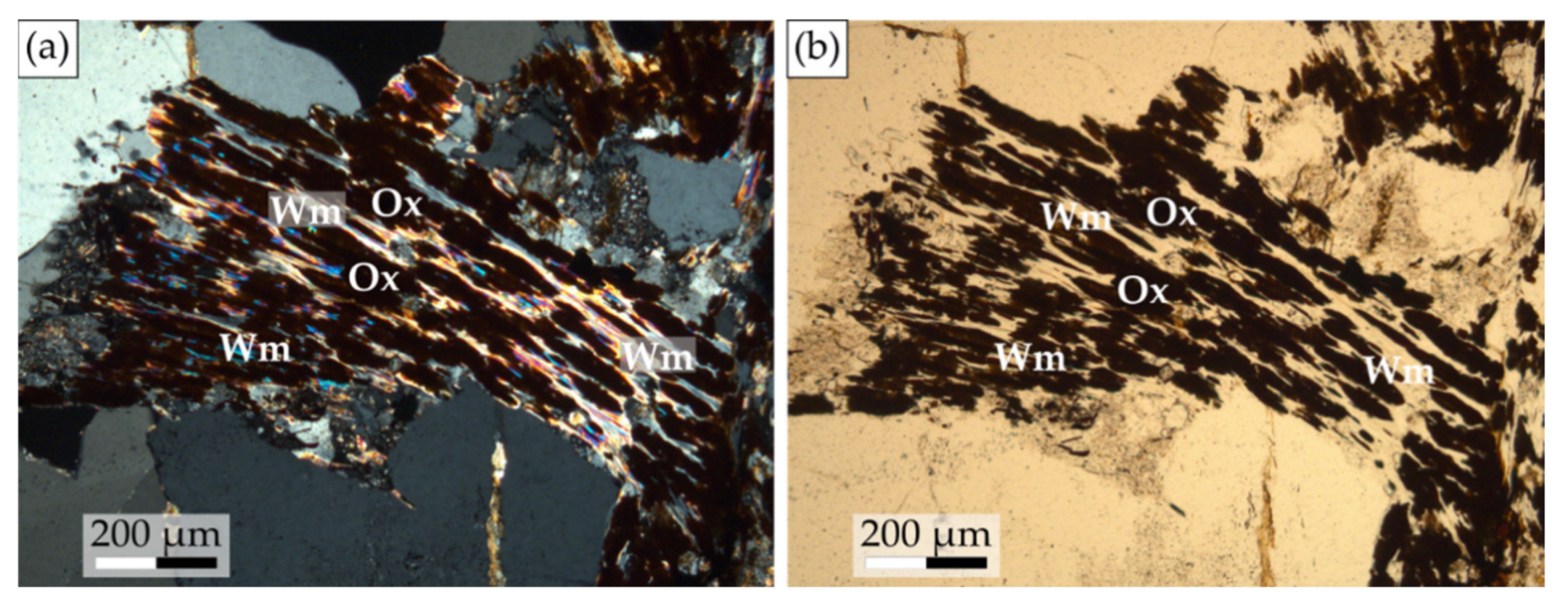
- Oxides can be present along the cleavages of the altered biotite. They are interpreted as the result of Mg and Fe release during biotite alteration.
5.3.3. Evidences of Weathering
5.3.4. Alteration Stage Occurrences
5.4. Effects of Alteration on Petrogaphic and Petrophysical Behaviour
5.5. The NH: A Paleo-Geothermal Reservoir?
6. Conclusions
- A pervasive propylitic alteration. This alteration is present in the OM granite (the freshest one considered as the protolith) and in the NH granite and characterized by the presence of corrensite and/or epidote.
- A local argillic alteration. This alteration was identified only locally in the NH granite by the occurrence of clay minerals such as kaolinite, illite/smectite mixed-layers and illite, all of which crystallize at a lower temperature than the propylitic alteration. Kaolinite and illite might reflect a different amount of leaching or different pH, meaning that several fluids have circulated.
- Weathering identified in the OM granite by the presence of montmorillonite, thus formed at surface temperature.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, C.F.; Reed, M.J.; Anderson, A.F. Updating the classification of geothermal resources. In Proceedings of the Thirty Sixth Workshop on Geothermal Reservoir Engineering Standford University, Standford, CA, USA, 31 January–2 February 2011. [Google Scholar]
- Moeck, I.S. Catalog of geothermal play types based on geologic controls. Renew. Sustain. Energy Rev. 2014, 37, 867–882. [Google Scholar] [CrossRef] [Green Version]
- Olasolo, P.; Juárez, M.C.; Morales, M.P.; D’Amico, S.; Liarte, I.A. Enhanced Geothermal Systems (EGS): A Review. Renew. Sustain. Energy Rev. 2016, 56, 133–144. [Google Scholar] [CrossRef]
- Trullenque, G.; Genter, A.; Leiss, B.; Wagner, B.; Bouchet, R.; Leoutre, E.; Malnar, B.; Bär, K.; Rajšl, I. Upscaling of EGS in different geological conditions: A European perspective. In Proceedings of the 43rd Workshop on Geothermal Reservoir Engineering Standford University, Standford, CA, USA, 12–14 February 2018; p. 10. [Google Scholar]
- Faulds, J.E.; Hinz, N.H.; Dering, G.M.; Siler, D.L. The hybrid model—The most accommodating structural setting for geothermal power generation in the Great Basin, Western USA. Geotherm. Resour. Counc. Trans. 2013, 37, 3–10. [Google Scholar]
- Nishimoto, S.; Yoshida, H. Hydrothermal alteration of deep fractured granite: Effects of dissolution and precipitation. Lithos 2010, 115, 153–162. [Google Scholar] [CrossRef]
- Dezayes, C.; Lerouge, C. Reconstructing paleofluid circulation at the hercynian basement/mesozoic sedimentary cover interface in the Upper Rhine Graben. Geofluids 2019, 2019, 1–30. [Google Scholar] [CrossRef]
- Plumper, O.; Putnis, A. The complex hydrothermal history of granitic rocks: Multiple feldspar replacement reactions under subsolidus conditions. J. Petrol. 2009, 50, 967–987. [Google Scholar] [CrossRef]
- Inoue, A. Formation of clay minerals in hydrothermal environments. In Origin and Mineralogy of Clays; Velde, B., Ed.; Springer: Berlin/Heidelberg, Germany, 1995; pp. 268–329. ISBN 978-3-642-08195-8. [Google Scholar]
- Burchfiel, B.C.; Stewart, J.H. “Pull-apart” origin of the central segment of Death Valley, California. GSA Bull. 1966, 77, 439–442. [Google Scholar] [CrossRef]
- Pavlis, T.L.; Trullenque, G. Evidence for 40–41 km of dextral slip on the Southern Death Valley fault: Implications for the Eastern California shear zone and extensional tectonics. Geology 2021, 49, 767–772. [Google Scholar] [CrossRef]
- Chabani, A.; Trullenque, G.; Ledésert, B.A.; Klee, J. Multiscale Characterization of fracture patterns: A case study of the Noble Hills Range (Death Valley, CA, USA), application to geothermal reservoirs. Geosciences 2021, 11, 280. [Google Scholar] [CrossRef]
- Kisch, H.J. Correlation between indicators of very low-grade metamorphism. In Low Temperature Metamorphism; Frey, M., Ed.; Chapman & Hall: London, UK, 1987; pp. 227–300. [Google Scholar]
- Árkai, P.; Sassi, F.; Desmons, J. Very low- to low-grade metamorphic rocks. In Metamorphic Rocks A Classification and Glossary Terms; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Frey, M. Very low-grade metamorphism of clastic sedimentary rocks. In Low Temperature Metamorphism; Chapman & Hall: London, UK, 1987; pp. 9–58. [Google Scholar]
- Árkai, P.; Maehlmann, R.; Suchy, V.; Balogh, K.; Sykorová, I.; Frey, M. Possible Effects of Tectonic Shear Strain on Phyllosilicates: A Case Study from the Kandersteg Area, Helvetic Domain, Central Alps, Switzerland. TMPM Tschermaks Mineral. Petrogr. Mitt. 2002, 82, 273–290. [Google Scholar]
- Mullis, J.; Mählmann, R.F.; Wolf, M. Fluid Inclusion Microthermometry to Calibrate Vitrinite Reflectance (between 50 and 270 °C), Illite Kübler-Index Data and the Diagenesis/Anchizone Boundary in the External Part of the Central Alps. Appl. Clay Sci. 2017, 143, 307–319. [Google Scholar] [CrossRef]
- Ferreiro Mählmann, R.; Bozkaya, Ö.; Potel, S.; le Bayon, R.; Šegvić, B.; Nieto, F. The Pioneer Work of Bernard Kübler and Martin Frey in Very Low-Grade Metamorphic Terranes: Paleo-Geothermal Potential of Variation in Kübler-Index/Organic Matter Reflectance Correlations. A Review. Swiss. J. Geosci. 2012, 105, 121–152. [Google Scholar] [CrossRef]
- Ledésert, B.; Berger, G.; Meunier, A.; Genter, A.; Bouchet, A. Diagenetic-Type Reactions Related to Hydrothermal Alteration in the Soultz-Sous-Forets Granite, France. Eur. J. Mineral. 1999, 11, 731–741. [Google Scholar] [CrossRef]
- Wernicke, B.; Axen, G.J.; Snow, J.K. Basin and Range Extensional Tectonics at the Latitude of Las Vegas, Nevada. GSA Bull. 1988, 100, 1738–1757. [Google Scholar] [CrossRef]
- Wright, L. Late Cenozoic Fault Patterns and Stress Fields in the Great Basin and Westward Displacement of the Sierra Nevada Block. Geology 1976, 4, 489–494. [Google Scholar] [CrossRef]
- Wernicke, B.; Spencer, J.E.; Burchfiel, B.C.; Guth, P.L. Magnitude of Crustal Extension in the Southern Great Basin. Geology 1982, 10, 499–502. [Google Scholar] [CrossRef]
- Stewart, J.H. Extensional Tectonics in the Death Valley Area, California: Transport of the Panamint Range Structural Block 80 Km Northwestward. Geology 1983, 11, 153–157. [Google Scholar] [CrossRef]
- Calzia, J.P.; Rämö, O.T. Late Cenozoic Crustal Extension and Magmatism, Southern Death Valley Region, California. GSA Field Guides 2000, 2, 135–164. [Google Scholar] [CrossRef]
- Norton, I. Two-Stage Formation of Death Valley. Geosphere 2011, 7, 171–182. [Google Scholar] [CrossRef]
- Luckow, H.; Pavlis, T.; Serpa, L.; Guest, B.; Wagner, D.; Snee, L.; Hensley, T.; Korjenkov, A. Late Cenozoic Sedimentation and Volcanism during Transtensional Deformation in Wingate Wash and the Owlshead Mountains, Death Valley. Earth Sci. Rev. 2005, 73, 177–219. [Google Scholar] [CrossRef]
- Hill, M.L.; Troxel, B.W. Tectonics of Death Valley Region, California. GSA Bull. 1966, 77, 435–438. [Google Scholar] [CrossRef]
- Butler, P.R.; Troxel, B.W.; Verosub, K.L. Late Cenozoic History and Styles of Deformation along the Southern Death Valley Fault Zone, California. GSA Bull. 1988, 100, 402–410. [Google Scholar] [CrossRef]
- Brady, R.H., III. Cenozoic Geology of the Northern Avawatz Mountains in Relation to the Intersection of the Garlock and Death Valley Fault Zones, San Bernardino County, California. Ph.D. Thesis, University of California, Berkeley, CA, USA, 1986. [Google Scholar]
- Brady, R.H.; Clayton, J.; Troxel, B.W.; Verosub, K.L.; Cregan, A.; Abrams, M. Thematic Mapper and Field Investigations at the Intersection of the Death Valley and Garlock Fault Zones, California. Remote Sens. Environ. 1989, 28, 207–217. [Google Scholar] [CrossRef]
- Lifton, Z.M.; Newman, A.V.; Frankel, K.L.; Johnson, C.W.; Dixon, T.H. Insights into Distributed Plate Rates across the Walker Lane from GPS Geodesy. Geophys. Res. Lett. 2013, 40, 4620–4624. [Google Scholar] [CrossRef] [Green Version]
- Nagorsen-Rinke, S.; Lee, J.; Calvert, A. Pliocene Sinistral Slip across the Adobe Hills, Eastern California-Western Nevada: Kinematics of Fault Slip Transfer across the Mina Deflection. Geosphere 2013, 9, 37–53. [Google Scholar] [CrossRef]
- Miller, M.B.; Wright, L.A. Geology of Death Valley National Park, 3rd ed.; Kendall Hunt Publishing Company: Dubuque, IA, USA, 2015. [Google Scholar]
- Rämö, T.O.; Calzia, J.P.; Kosunen, P.J. Geochemistry of Mesozoic Plutons, Southern Death Valley Region, California: Insights into the Origin of Cordilleran Interior Magmatism. Contrib. Mineral. Petrol. 2002, 143, 416–437. [Google Scholar] [CrossRef]
- Troxel, B.W.; Butler, P.R. Rate of Cenozoic Slip on Normal Faults, South-Central Death Valley, California; Department of Geology, University of California: Berkeley, CA, USA, 1979. [Google Scholar]
- Butler, P.R. Geology: Structural History and Fluvial Geomorphology of the Southern Death Valley Fault Zone, Inyo and San Bernardino Counties, California. Ph.D. Thesis, University of California, Davis, CA, USA, 1984. [Google Scholar]
- Brady, R.H.; Troxel, B.W. Stratigraphy and Tectonics of the Northern Avawatz Mountains at the Intersection of the Garlock and Death Valley Fault Zones, San Bernardino County, California. In Quaternary Tectonics of Southern Death Valley, California—Field Trip Guide: Shoshone, California, Friends of the Pleistocene, Pacific Cell; USGS: Shoshone, CA, USA, 1986; pp. 1–12. [Google Scholar]
- Niles, J.H. Post-Middle Pliocene Tectonic Development of the Noble Hills, Southern Death Valley, California. Ph.D. Thesis, San Francisco State University, San Francisco, CA, USA, 2016. [Google Scholar]
- Mahon, R.C.; Dehler, C.M.; Link, P.K.; Karlstrom, K.E.; Gehrels, G.E. Detrital Zircon Provenance and Paleogeography of the Pahrump Group and Overlying Strata, Death Valley, California. Precambrian Res. 2014, 251, 102–117. [Google Scholar] [CrossRef]
- DeCelles, P.G. Late Jurassic to Eocene Evolution of the Cordilleran Thrust Belt and Foreland Basin System, Western USA. Am. J. Sci. 2004, 304, 105–168. [Google Scholar] [CrossRef]
- Stamm, J.F. Geology at the Intersection of the Death Valley and Garlock Fault Zones, Southern Death Valley, California. Ph.D. Thesis, Pennsylvania State University, State College, PA, USA, 1981. [Google Scholar]
- Klee, J.; Trullenque, G.; Ledésert, B.; Potel, S.; Hébert, R.; Chabani, A.; Genter, A. Petrographic analyzes of fractured granites used as an analogue of the soultz-sous-forêts geothermal reservoir: Noble Hills, CA, USA. In Proceedings of the Extended Abstract, Reykjavik, Iceland, 26 May 2021. [Google Scholar]
- Troxel, B.W. Right-Lateral Offset of ca. 28 Km along a Strand of the Southern Death Valley Fault Zone. Calif. Geol. Soc. Am. Abstr. Programs 1994, 26, 99. [Google Scholar]
- Castaing, C.; Rabu, D. Apports de La Géologie à La Recherche et à l’Exploitation de Pierres de Taille (Roches Ornementales et de Construction). Available online: https://hal.archives-ouvertes.fr/hal-01860154 (accessed on 12 July 2021).
- Kretz, R. Symbols for Rock-Forming Minerals. Am. Mineral. 1983, 68, 277–279. [Google Scholar]
- Bisdom, K.; Gauthier, B.D.M.; Bertotti, G.; Hardebol, N.J. Calibrating Discrete Fracture-Network Models with a Carbonate Three-Dimensional Outcrop Fracture Network: Implications for Naturally Fractured Reservoir Modeling. AAPG Bull. 2014, 98, 1351–1376. [Google Scholar] [CrossRef]
- Gillespie, P.A.; Howard, C.B.; Walsh, J.J.; Watterson, J. Measurement and Characterisation of Spatial Distributions of Fractures. Tectonophysics 1993, 226, 113–141. [Google Scholar] [CrossRef]
- Schmidt, D.; Schmidt, S.T.; Mullis, J.; Ferreiro Mählmann, R.; Frey, M. Very Low Grade Metamorphism of the Taveyanne Formation of Western Switzerland. Contrib. Mineral. Petrol. 1997, 129, 385–403. [Google Scholar] [CrossRef]
- Kisch, H.J. Illite Crystallinity: Recommendations on Sample Preparation, X-ray Diffraction Settings, and Interlaboratory Samples. J. Metamorph. Geol. 1991, 9, 665–670. [Google Scholar] [CrossRef]
- Ferreiro Mählmann, R.; Frey, M. Standardisation, Calibration and Correlation of the Kübler-Index and the Vitrinite/Bituminite Reflectance: An Inter-Laboratory and Field Related Study. Swiss J. Geosci. 2012, 105, 153–170. [Google Scholar] [CrossRef]
- Moore, D.M.; Reynolds, R.C. X-ray diffraction and the identification and analysis of clay minerals. In X-ray Diffraction and the Identification and Analysis of Clay Minerals; Oxford University Press: Oxford, UK, 1989. [Google Scholar]
- Starkey, H.C.; Blackmon, P.D.; Hauff, P.L. The Routine Mineralogical Analysis of Clay-Bearing Samples; United States Government Publishing Office: Washington, DC, USA, 1984.
- Warr, L.N.; Rice, A.H.N. Interlaboratory Standardization and Calibration of Day Mineral Crystallinity and Crystallite Size Data. J. Metamorph. Geol. 1994, 12, 141–152. [Google Scholar] [CrossRef]
- Kübler, B. La Cristallinite de l’illite et Les Zones Tout a Fait Superieures Du Metamorphisme. In Etages Tectoniques; van Bemmelen, R.W., Chapman, C.A., Watznauer, A., Eds.; La Baconniere: Boudry, Switzerland, 1981; pp. 105–121. [Google Scholar]
- Warr, L.N.; Mählmann, R.F. Recommendations for Kübler Index Standardization. Clay Minerals 2015, 50, 283–286. [Google Scholar] [CrossRef]
- Winkler, H.G.F. Anatexis, Formation of Migmatites, and Origin of Granitic Magmas. In Petrogenesis of Metamorphic Rocks; Winkler, H.G.F., Ed.; Springer Study Edition; Springer: New York, NY, USA, 1979; pp. 283–339. ISBN 978-1-4757-4215-2. [Google Scholar]
- Tilley, C.E. A Preliminary Survey of Metamorphic Zones in the Southern Highlands of Scotland. Q. J. Geol. Soc. 1925, 81, 100–112. [Google Scholar] [CrossRef]
- Barrow, G. On an Intrusion of Muscovite-Biotite Gneiss in the South-Eastern Highlands of Scotland, and Its Accompanying Metamorphism. Q. J. Geol. Soc. 1893, 49, 330–358. [Google Scholar] [CrossRef]
- Merriman, R.J.; Frey, M. Patterns of very low-grade metamorphism in metapelitic rocks. In Low-Grade Metamorphism; Frey, M., Robinson, D., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 1998; pp. 61–107. ISBN 978-1-4443-1334-5. [Google Scholar]
- Abad, I. Physical meaning and applications of the Illite Kübler index: Measuring reaction progress in low-grade metamorphism. In Diagenesis and Low-Temperature Metamorphism, Theory, Methods and Regional Aspects; Sociedad Española de Mineralogía: Jaén, Spain, 2007; pp. 53–64. [Google Scholar]
- Ledésert, B.; Hébert, R.L.; Grall, C.; Genter, A.; Dezayes, C.; Bartier, D.; Gérard, A. Calcimetry as a Useful Tool for a Better Knowledge of Flow Pathways in the Soultz-Sous-Forêts Enhanced Geothermal System. J. Volcanol. Geotherm. Res. 2009, 181, 106–114. [Google Scholar] [CrossRef]
- Dullien, F.A.L. Porous Media: Fluid Transport and Pore Structure; Academic Press: San Diego, CA, USA, 1979; ISBN 978-0-323-13933-5. [Google Scholar]
- Navelot, V.; Géraud, Y.; Favier, A.; Diraison, M.; Corsini, M.; Lardeaux, J.-M.; Verati, C.; Mercier de Lépinay, J.; Legendre, L.; Beauchamps, G. Petrophysical Properties of Volcanic Rocks and Impacts of Hydrothermal Alteration in the Guadeloupe Archipelago (West Indies). J. Volcanol. Geotherm. Res. 2018, 360, 1–21. [Google Scholar] [CrossRef]
- Gates, W.P.; Nefiodovas, A.; Peter, P. Permeability of an Organo-Modified Bentonite to Ethanol-Water Solutions. Clays Clay Miner. 2004, 52, 192–203. [Google Scholar] [CrossRef]
- Bard, J.P. Microtextures des Roches Magmatiques et Métamorphiques; Masson: Paris, France, 1980. [Google Scholar]
- Goldich, S.S. A Study in Rock-Weathering. J. Geol. 1938, 46, 17–58. [Google Scholar] [CrossRef]
- Que, M.; Allen, A.R. Sericitization of Plagioclase in the Rosses Granite Complex, Co. Donegal, Ireland. Mineral. Mag. 1996, 60, 927–936. [Google Scholar] [CrossRef]
- Streckeisen, A.; Le Maître, R.W. A Chemical Approximation to Modal QAPF Classification of the Igneous Rocks. Neues Jahrb. Mineral. Abh. 1979, 136, 169–206. [Google Scholar]
- Beaufort, D.; Baronnet, A.; Lanson, B.; Meunier, A. Corrensite; a Single Phase or a Mixed-Layer Phyllosilicate in Saponite-to-Chlorite Conversion Series? A Case Study of Sancerre-Couy Deep Drill Hole (France). Am. Mineral. 1997, 82, 109–124. [Google Scholar] [CrossRef]
- Jagodzinski, H. Eindimensionale Fehlordnung in Kristallen und ihr Einfluss auf die Röntgeninterferenzen. I. Berechnung des Fehlordnungsgrades aus den Röntgenintensitäten. Acta Cryst. 1949, 2, 201–207. [Google Scholar] [CrossRef]
- Cox, K.G. The Interpretation of Igneous Rocks; Springer Science & Business Media: London, UK, 1979; ISBN 978-94-017-3373-1. [Google Scholar]
- Wilson, M. Review of Igneous Petrogenesis: A Global Tectonic Approach. Terra Nova 1989, 1, 218–222. [Google Scholar] [CrossRef]
- Jensen, L.S. A New Plot for Classifying Subalkalic Volcanic Rocks. Ont. Div. Mines Misc. Pap. 1976, 66, 1–22. [Google Scholar]
- White, A.J.R.; Chappell, B.W. Granitoid types and their distribution in the Lachlan Fold Belt, southeastern Australia. In Geological Society of America Memoirs; Geological Society of America: Boulder, CO, USA, 1983; Volume 159, pp. 21–34. ISBN 978-0-8137-1159-1. [Google Scholar]
- Le Maître, R.W.; Bateman, P.; Dudek, A.; Keller, J.; Lameyre Le Bas, M.J.; Sabine, P.A.; Schmid, R.; Sorensen, H.; Streckeisen, A.; Woolley, A.R.; et al. A Classification of Igneous Rocks and Glossary of Terms; Blackwell: Oxford, UK, 1989. [Google Scholar]
- Rollinson, H.R. Using Geochemical Data: Evaluation, Presentation, Interpretation; Routledge: Abingdon, UK, 1993; ISBN 978-1-317-89819-1. [Google Scholar]
- Boynton, W.V. Cosmochemistry of the rare earth elements: Meteorite studies. In Developments in Geochemistry; Elsevier: Amsterdam, The Netherlands, 1984; Volume 2, pp. 63–114. ISBN 978-0-444-42148-7. [Google Scholar]
- McDonough, W.; Sun, S.-S.; Ringwood, A.; Jagoutz, E.; Hofmann, A. Potassium, Rubidium, and Cesium in the Earth and Moon and the Evolution of the Mantle of the Earth. Geochim. Cosmochim. Acta 1992, 56, 1001–1012. [Google Scholar] [CrossRef]
- Sun, S.; McDonough, W.F. Chemical and Isotopic Systematics of Oceanic Basalts: Implications for Mantle Composition and Processes. Geol. Soc. Lond. Spec. Publ. 1989, 42, 313–345. [Google Scholar] [CrossRef]
- Koljonen, T.; Rosenberg, R.J. Rare Earth Elements in Granitic Rocks. Lithos 1974, 7, 249–261. [Google Scholar] [CrossRef]
- Alderton, D.H.M.; Pearce, J.A.; Potts, P.J. Rare Earth Element Mobility during Granite Alteration: Evidence from Southwest England. Earth Planet. Sci. Lett. 1980, 49, 149–165. [Google Scholar] [CrossRef]
- Li, X.-C.; Fan, H.-R.; Santosh, M.; Hu, F.-F.; Yang, K.-F.; Lan, T.-G. Hydrothermal Alteration Associated with Mesozoic Granite-Hosted Gold Mineralization at the Sanshandao Deposit, Jiaodong Gold Province, China. Ore Geol. Rev. 2013, 53, 403–421. [Google Scholar] [CrossRef]
- Warr, L.N.; Cox, S.C. Correlating Illite (Kübler) and Chlorite (Árkai) “Crystallinity” Indices with Metamorphic Mineral Zones of the South Island, New Zealand. Appl. Clay Sci. 2016, 134, 164–174. [Google Scholar] [CrossRef]
- Fulignati, P. Clay Minerals in Hydrothermal Systems. Minerals 2020, 10, 919. [Google Scholar] [CrossRef]
- Creasey, S.C. Hydrothermal alteration. In Geology of the Porphyry Copper Deposits Southwestern North America; Titley & Hicks: Tucson, AZ, USA, 1966; pp. 51–74. [Google Scholar]
- Traineau, H.; Genter, A.; Cautru, J.P.; Fabriol, H.; Chevremont, P. Petrography of the Granite Massif from Drill Cutting Analysis and Well Log Interpretation in the Geothermal HDR Borehole GPK1 (Soultz, Alsace, France). Geotherm. Sci. Technol. 1991, 3, 1–29. [Google Scholar]
- Środoń, J. Nature of Mixed-Layer Clays and Mechanisms of Their Formation and Alteration. Annu. Rev. Earth Planet. Sci. 1999, 27, 19–53. [Google Scholar] [CrossRef]
- Genter, A. Géothermie Roches Chaudes Sèches: Le Granite de Soultz-Sous-Forêts (Bas-Rhin, France): Fracturation Naturelle, Altérations Hydrothermales et Interaction Eau-Roche. Ph.D. Thesis, Université d’Orléans, Orléans, France, 1989. [Google Scholar]
- Burnham, C.W. Facies and Types of Hydrothermal Alteration. Econ. Geol. 1962, 57, 768–784. [Google Scholar] [CrossRef]
- Velde, B. Clays and Clay Minerals in Natural and Synthetic Systems; Development in Sedimentology; Elsevier: Amsterdam, The Netherlands, 1977; ISBN 978-0-08-086933-9. [Google Scholar]
- Steiner, A. Clay Minerals in Hydrothermally Altered Rocks at Wairakei, New Zealand. Clays Clay Miner. 1968, 16, 193–213. [Google Scholar] [CrossRef]
- Ledésert, B.; Hebert, R.; Genter, A.; Bartier, D.; Clauer, N.; Grall, C. Fractures, Hydrothermal Alterations and Permeability in the Soultz Enhanced Geothermal System. Comptes Rendus Geosci. 2010, 342, 607–615. [Google Scholar] [CrossRef]
- Vidal, J.; Patrier, P.; Genter, A.; Beaufort, D.; Dezayes, C.; Glaas, C.; Lerouge, C.; Sanjuan, B. Clay Minerals Related to the Circulation of Geothermal Fluids in Boreholes at Rittershoffen (Alsace, France). J. Volcanol. Geotherm. Res. 2018, 349, 192–204. [Google Scholar] [CrossRef]
- Ledésert, B.A.; Hébert, R.L. How Can Deep Geothermal Projects Provide Information on the Temperature Distribution in the Upper Rhine Graben? The Example of the Soultz-Sous-Forêts-Enhanced Geothermal System. Geosciences 2020, 10, 459. [Google Scholar] [CrossRef]
- Sardini, P.; Ledésert, B.; Touchard, G. Quantification of microscopic porous networks by image analysis and measurements of permeability in the Soultz-Sous-Forêts Granite (Alsace, France). In Fluid Flow and Transport in Rocks: Mechanisms and Effects; Jamtveit, B., Yardley, B.W.D., Eds.; Springer: Dordrecht, The Netherlands, 1997; pp. 171–189. ISBN 978-94-009-1533-6. [Google Scholar]
- Glaas, C.; Patrier, P.; Vidal, J.; Beaufort, D.; Genter, A. Clay Mineralogy: A Signature of Granitic Geothermal Reservoirs of the Central Upper Rhine Graben. Minerals 2021, 11, 479. [Google Scholar] [CrossRef]
- Meunier, A.; Velde, B.D.; Dudoignon, P.; Beaufort, D. Identification of Weathering and Hydrothermal Alteration in Acidic Rocks: Petrography and Mineralogy of Clay Minerals. Sci. Géologiques Bull. Mémoires 1983, 72, 93–99. [Google Scholar]
- Tardy, Y.; Paquet, H.; Millot, G. Trois modes de genèse des montmorillonites dans les altérations et les sols. Bull. Groupe Français Argiles 1970, 22, 69–77. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, C.; Li, C.; Li, S.; Santosh, M.; Wang, M.; Fan, J. Breakup of the Northern Margin of Gondwana through Lithospheric Delamination: Evidence from the Tibetan Plateau. GSA Bull. 2018, 131. [Google Scholar] [CrossRef]
- Chambefort, I.; Moritz, R.; von Quadt, A. Petrology, Geochemistry and U-Pb Geochronology of Magmatic Rocks from the High-Sulfidation Epithermal Au–Cu Chelopech Deposit, Srednogorie Zone, Bulgaria. Miner. Depos. 2007, 42, 665–690. [Google Scholar] [CrossRef] [Green Version]
- Garrels, R.M.; MacKenzie, F.T. Origin of the Chemical Compositions of Some Springs and Lakes. In Equilibrium Concepts in Natural Water Systems; Advances in Chemistry; American Chemical Society: Washington, DC, USA, 1967; Volume 67, pp. 222–242. ISBN 978-0-8412-0068-5. [Google Scholar]
- Rosener, M.; Géraud, Y. Using Physical Properties to Understand the Porosity Network Geometry Evolution in Gradually Altered Granites in Damage Zones. Geol. Soc. Lond. Spec. Publ. 2007, 284, 175–184. [Google Scholar] [CrossRef]
- Cassiaux, M.; Proust, D.; Siitari-Kauppi, M.; Sardini, P.; Leutsch, Y. Clay Minerals Formed during Propylitic Alteration of a Granite and Their Influence on Primary Porosity: A Multi-Scale Approach. Clays Clay Miner. 2006, 54, 541–554. [Google Scholar] [CrossRef]
- White, A.F.; Schulz, M.S.; Lowenstern, J.B.; Vivit, D.V.; Bullen, T.D. The Ubiquitous Nature of Accessory Calcite in Granitoid Rocks: Implications for Weathering, Solute Evolution, and Petrogenesis. Geochim. Cosmochim. Acta 2005, 69, 1455–1471. [Google Scholar] [CrossRef]



| Metamorphic Zone | KI (∆°2θ) | Temperature (°C) |
|---|---|---|
| Low Diagenesis | >1 | ~100 |
| High Diagenesis | 0.42–1 | ~200 |
| Low Anchizone | 0.30–0.42 | |
| High Anchizone | 0.25–0.30 | ~300 |
| Epizone | <0.25 |
| Sample ID | Primary Minerals | Major Secondary Phases Within | Other Secondary Minerals | Microfracturing | |||||
|---|---|---|---|---|---|---|---|---|---|
| Pl | Bt | Kfs | |||||||
| Wm | Kln | Cal | Wm | Ox | Wm | ||||
| OM_1_2_3 | Qtz, Pl, Mc, Or, Bt, Ox | √ | Ep, Ox, Wm, Cal | Fd1 | |||||
| NH_1 | Qtz, Pl, Mc, Or, Bt, Ox | √ | √ | √ | √ | Cal, Dol, Wm | Fd1 | ||
| NH_2 | Qtz, Pl, Or, Bt, Ox | √ | √ | √ | Dol, Wm | Fd1 | |||
| NH_3 | Qtz, Pl, Or, Bt, Ox | √ | √ | √ | √ | √ | Cal, Dol, Wm | Fd1-2 | |
| NH_4 | Qtz, Pl, Or, Bt, Ox | √ | √ | √ | √ | √ | Cal, Hem | Fd0-1 | |
| NH_5 | Qtz, Pl, Or, Bt, Ox | √ | √ | √ | Hem, Wm | Fd0-1 | |||
| NH_6 | Qtz, Pl, Or, Bt, Ox | √ | √ | √ | √ | √ | Cal, Hem, Wm | Fd1-2 | |
| NH_7 | Qtz, Pl, Or, Bt, Ox | √ | √ | √ | √ | Cal, Ox, Wm | Fd1 | ||
| NH_8 | Qtz, Pl, Or, Bt, Ox | √ | √ | √ | √ | √ | Cal, Ox, Wm | Fd1-2 | |
| NH_9 | Qtz, Pl, Or, Bt, Ox | √ | √ | Cal, Ox, Wm | Fd0-1 | ||||
| NH_10 | Qtz, Pl, Or, Bt, Ox | √ | √ | √ | √ | √ | Cal, Hem, Wm, Ep | Fd2 | |
| NH_11 | Qtz, Pl, Or, Bt, Ox | √ | √ | √ | √ | √ | Cal, Hem, Wm | Fd1-2 | |
| NH_12 | Qtz, Pl, Mc, Or, Bt, Ox | √ | √ | √ | √ | √ | Cal, Hem, Gp, Wm | Fd0-1 | |
| NH_13 | Qtz, Pl, Mc, Or, Bt, Ox | √ | √ | √ | √ | √ | Cal, Hem, Gp, Wm | Fd0-1 | |
| NH_14 | Qtz, Pl, Mc, Or, Bt, Ox | √ | √ | √ | √ | √ | Cal, Hem, Gp, Wm | Fd0-1 | |
| NH_15 | Qtz, Pl, Or, Bt, Ox | √ | √ | √ | √ | Cal, Mag, Wm | Fd1-2 | ||
| Sample ID | <2 µm | 2–6 µm | <2 µm (AD) | 2–6 µm (AD) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ill | Kln | Cor | I/S | Bt | Ill | Kln | Cor | I/S | Mnt | Bt | FWHM | KI | FWHM | KI | |
| OM_2 | no material | - | + | 0.18 | 0.22 | ||||||||||
| NH_1 | + | − | ++ | − | + | ++ | 0.48 | 0.69 | 0.34 | 0.46 | |||||
| NH_2 | −− | − | ++ | −− | − | ++ | 0.31 | 0.43 | 0.32 | 0.44 | |||||
| NH_3 | + | + | ++ | + | + | ++ | 0.63 | 0.92 | 0.59 | 0.87 | |||||
| NH_4 | ++ | + | − | ++ | + | − | 0.81 | 1.29 | 0.73 | 1.16 | |||||
| NH_5 | + | − | − | − | − | − | + | 0.69 | 1.09 | 0.37 | 0.55 | ||||
| NH_6 | + | ++ | − | + | ++ | − | 1.37 | 2.25 | 1.15 | 1.87 | |||||
| NH_7 | + | + | − | + | + | − | + | 1.01 | 1.63 | 0.71 | 1.13 | ||||
| NH_8 | + | + | + | + | ++ | + | 1.10 | 1.79 | 1.18 | 1.93 | |||||
| NH_9 | + | + | ++ | + | + | ++ | 0.85 | 1.36 | 0.56 | 0.87 | |||||
| NH_10 | + | − | ++ | + | − | ++ | 0.46 | 0.65 | 0.51 | 0.73 | |||||
| NH_11 | ++ | − | + | ++ | + | + | 0.67 | 1.05 | 0.62 | 0.97 | |||||
| NH_12 | ++ | + | − | ++ | + | − | 0.57 | 0.89 | 0.46 | 0.69 | |||||
| NH_13 | + | − | + | + | − | + | 0.46 | 0.69 | 0.38 | 0.56 | |||||
| NH_14 | + | −− | − | + | + | −− | − | + | 0.59 | 0.92 | 0.44 | 0.66 | |||
| NH_15 | + | − | + | + | − | − | 0.69 | 1.08 | 0.50 | 0.77 | |||||
| Sample ID | OM_3 | NH_10 | NH_1 | NH_2 | NH_3 |
|---|---|---|---|---|---|
| Oxides (weight %) | |||||
| SiO2 | 74.65 | 68.54 | 69.56 | 68.69 | 68.78 |
| Al2O3 | 12.90 | 15.36 | 14.93 | 14.53 | 14.99 |
| Fe2O3 | 1.83 | 3.02 | 2.83 | 4.29 | 2.95 |
| MgO | 0.31 | 0.57 | 0.56 | 1.15 | 0.78 |
| CaO | 1.09 | 2.62 | 2.20 | 2.50 | 1.99 |
| Na2O | 3.51 | 3.31 | 3.42 | 3.19 | 2.78 |
| K2O | 4.43 | 4.31 | 4.10 | 3.60 | 4.22 |
| TiO2 | 0.20 | 0.29 | 0.26 | 0.46 | 0.28 |
| P2O5 | 0.07 | 0.14 | 0.13 | 0.20 | 0.14 |
| MnO | 0.08 | 0.09 | 0.08 | 0.12 | 0.08 |
| Sample ID | OM_3 | NH_10 | NH_1 | NH_2 | NH_3 |
|---|---|---|---|---|---|
| Loss on ignition (LOI) (wt.%) | 0.7 | 1.5 | 1.7 | 1.1 | 2.8 |
| Alteration degree (%) | 2 | 10 | 9 | 4 | 18 |
| Plagioclase alteration (%) | 5 | 20 | 20 | 10 | 40 |
| Biotite alteration (%) | 0 | 15 | 10 | 0 | 20 |
| Sample ID | OM_3 | NH_10 | NH_1 | NH_2 | NH_3 |
|---|---|---|---|---|---|
| Trace elements (ppm) | |||||
| Be | 2 | 2 | 2 | 3 | 2 |
| Co | 1.5 | 4.2 | 2.9 | 6.4 | 3.5 |
| Cs | 1.6 | 2.4 | 1.1 | 3.4 | 1.8 |
| Ga | 15.6 | 16.7 | 16.0 | 17.2 | 14.1 |
| Hf | 4.3 | 3.6 | 4.1 | 4.6 | 4.6 |
| Nb | 22.3 | 13.8 | 11.4 | 18.9 | 10.5 |
| Rb | 154.7 | 144.9 | 103.0 | 139.9 | 115.7 |
| Sn | 2 | 1 | <1 | 2 | <1 |
| Sr | 116.2 | 275.2 | 237.5 | 268.2 | 182.7 |
| Ta | 1.6 | 0.9 | 0.7 | 1.3 | 0.6 |
| Th | 19.3 | 11.4 | 10.2 | 9.0 | 11.0 |
| U | 1.8 | 2.2 | 1.5 | 1.4 | 1.5 |
| V | 16 | 33 | 22 | 40 | 25 |
| W | <0.5 | <0.5 | 0.7 | <0.5 | <0.5 |
| Zr | 139.8 | 134.2 | 156.6 | 180.6 | 159.0 |
| Y | 19.7 | 22.5 | 17.5 | 21.4 | 17.0 |
| Ba | 554 | 1009 | 845 | 704 | 772 |
| Ni | <20 | <20 | <20 | <20 | <20 |
| Sc | 4 | 5 | 4 | 8 | 4 |
| Cr2O3 | <0.002 | <0.002 | <0.002 | <0.002 | <0.002 |
| Be | 2 | 2 | 2 | 3 | 2 |
| Rare Earth Elements (ppm) | |||||
| La | 52.1 | 28.3 | 26.2 | 21.7 | 26.8 |
| Ce | 97.0 | 53.0 | 48.1 | 40.8 | 52.2 |
| Pr | 9.97 | 5.93 | 5.31 | 4.59 | 5.62 |
| Nd | 31.7 | 20.6 | 18.8 | 16.6 | 19.5 |
| Sm | 5.29 | 3.82 | 3.51 | 3.56 | 3.48 |
| Eu | 0.53 | 0.89 | 0.89 | 0.81 | 0.81 |
| Gd | 4.24 | 3.66 | 3.14 | 3.60 | 3.22 |
| Tb | 0.65 | 0.59 | 0.51 | 0.59 | 0.51 |
| Dy | 3.66 | 3.63 | 3.05 | 3.65 | 2.96 |
| Ho | 0.69 | 0.78 | 0.62 | 0.73 | 0.59 |
| Er | 2.05 | 2.25 | 1.87 | 2.18 | 1.81 |
| Tm | 0.30 | 0.33 | 0.28 | 0.33 | 0.27 |
| Yb | 2.08 | 2.32 | 1.77 | 2.16 | 1.86 |
| Lu | 0.33 | 0.37 | 0.28 | 0.36 | 0.28 |
| TOT/C | 0.02 | 0.14 | 0.21 | 0.04 | 0.26 |
| TOT/S | <0.02 | <0.02 | <0.02 | <0.02 | <0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klee, J.; Potel, S.; Ledésert, B.A.; Hébert, R.L.; Chabani, A.; Barrier, P.; Trullenque, G. Fluid-Rock Interactions in a Paleo-Geothermal Reservoir (Noble Hills Granite, California, USA). Part 1: Granite Pervasive Alteration Processes away from Fracture Zones. Geosciences 2021, 11, 325. https://doi.org/10.3390/geosciences11080325
Klee J, Potel S, Ledésert BA, Hébert RL, Chabani A, Barrier P, Trullenque G. Fluid-Rock Interactions in a Paleo-Geothermal Reservoir (Noble Hills Granite, California, USA). Part 1: Granite Pervasive Alteration Processes away from Fracture Zones. Geosciences. 2021; 11(8):325. https://doi.org/10.3390/geosciences11080325
Chicago/Turabian StyleKlee, Johanne, Sébastien Potel, Béatrice A. Ledésert, Ronan L. Hébert, Arezki Chabani, Pascal Barrier, and Ghislain Trullenque. 2021. "Fluid-Rock Interactions in a Paleo-Geothermal Reservoir (Noble Hills Granite, California, USA). Part 1: Granite Pervasive Alteration Processes away from Fracture Zones" Geosciences 11, no. 8: 325. https://doi.org/10.3390/geosciences11080325
APA StyleKlee, J., Potel, S., Ledésert, B. A., Hébert, R. L., Chabani, A., Barrier, P., & Trullenque, G. (2021). Fluid-Rock Interactions in a Paleo-Geothermal Reservoir (Noble Hills Granite, California, USA). Part 1: Granite Pervasive Alteration Processes away from Fracture Zones. Geosciences, 11(8), 325. https://doi.org/10.3390/geosciences11080325








