1. Introduction
In the context of persistently declining production of oil from conventional reservoirs, studies of oil shale are of high priority. They are often confined to predominantly carbonate (siliceous-carbonate) deposits where hydrocarbons occur in situ, with no evidence of migration into overlying layers.
A possible source for maintaining production at the present-day level is involvement into development of hard-to recover oil from low-permeability siliceous-carbonate deposits situated in areas, which do not require additional expenses for constructing new logistical infrastructure. The Domanik Formation is the most famous carbonate-rich unconventional reservoir in Russia located in the Volga-Ural and Timan-Pechora petroleum basins in the central and northern parts of the East European Craton (the Russian Platform) [
1,
2]. Domanik Formation is represented by organic-rich carbonate shales. These rocks are considered both as source rocks and unconventional reservoirs due to extremely low porosity and permeability with high amount of organic matter. In term of lithology, the rocks are limestone with different genesis such as biogenic and clastic [
2]. In accordance with the concepts of the sediments-and-migration theory, these deposits are considered as “excellent” and “rich” petroleum-source rocks [
3]. Such hydrocarbon-saturated deposits are characterized of low porosity and permeability values [
4], lack of structural and stratigraphic control of oil-bearing intervals, that makes it impossible to identify them by logging data. The main aim of the oil companies is to find affordable options for recovering hydrocarbons from such organic-rich shales, and to this it is essential to clearly understand the controlling factors behind the organic-mineral matrix.
Carbonate rocks with a high content of fossilized organic matter (OM) have complex and non-uniform structure, rocks of various structure and composition alternate in them. Phase composition of organic matter is also non-uniform, high content of hydrocarbons may be encountered both in free state and within the rock matrix [
5,
6].
Association of organic matter with minerals is often discussed in literature as biomineralization, sorption, and intercalation processes during sedimentation and diagenesis [
7,
8,
9]. Evidence of such processes in Domanik Formation is for the first time described in this paper.
This paper discusses evaluation of influence of the mineral matrix on the presence of different components of organic matter in a rock–kerogen, free and bound hydrocarbons in the non-uniform section of Upper Devonian Domanik Formation. Understanding the character of kerogen distribution and fluid saturation is necessary for evaluating the possibility of involving in development of hard-to-recover oil from low-permeability siliceous-carbonate rocks.
3. Results
3.1. Lithological Characteristics
The studied Upper Devonian deposits are represented by bioclastic limestones, in some cases with an admixture of argillaceous and siliceous material (chalcedony replacing shells of tentaculites and mollusks), of fine-crystalline varieties. Limestones are recrystallized, secondary grains of dolomite encountered, and pyrite was practically absent.
By results of XRD optical microscopy it was established that the prevalent component in the mineral composition is calcite—from 50 to 87%, amount of dolomite is up to 7%. Content of silica varies within the range of 1–8%, that of clay minerals—4–17%. The clay minerals detected include: hydromica (1–10%), mixed layer clays (1–4%), chlorite (1–4%), kaolinite (0–1%). The rocks contain a significant amount of amorphous OM, which non-uniformly impregnates the rock, it also is encountered in the form of inclusions, veinlets, coating of calcite crystals, fills interior spaces between walls of shells.
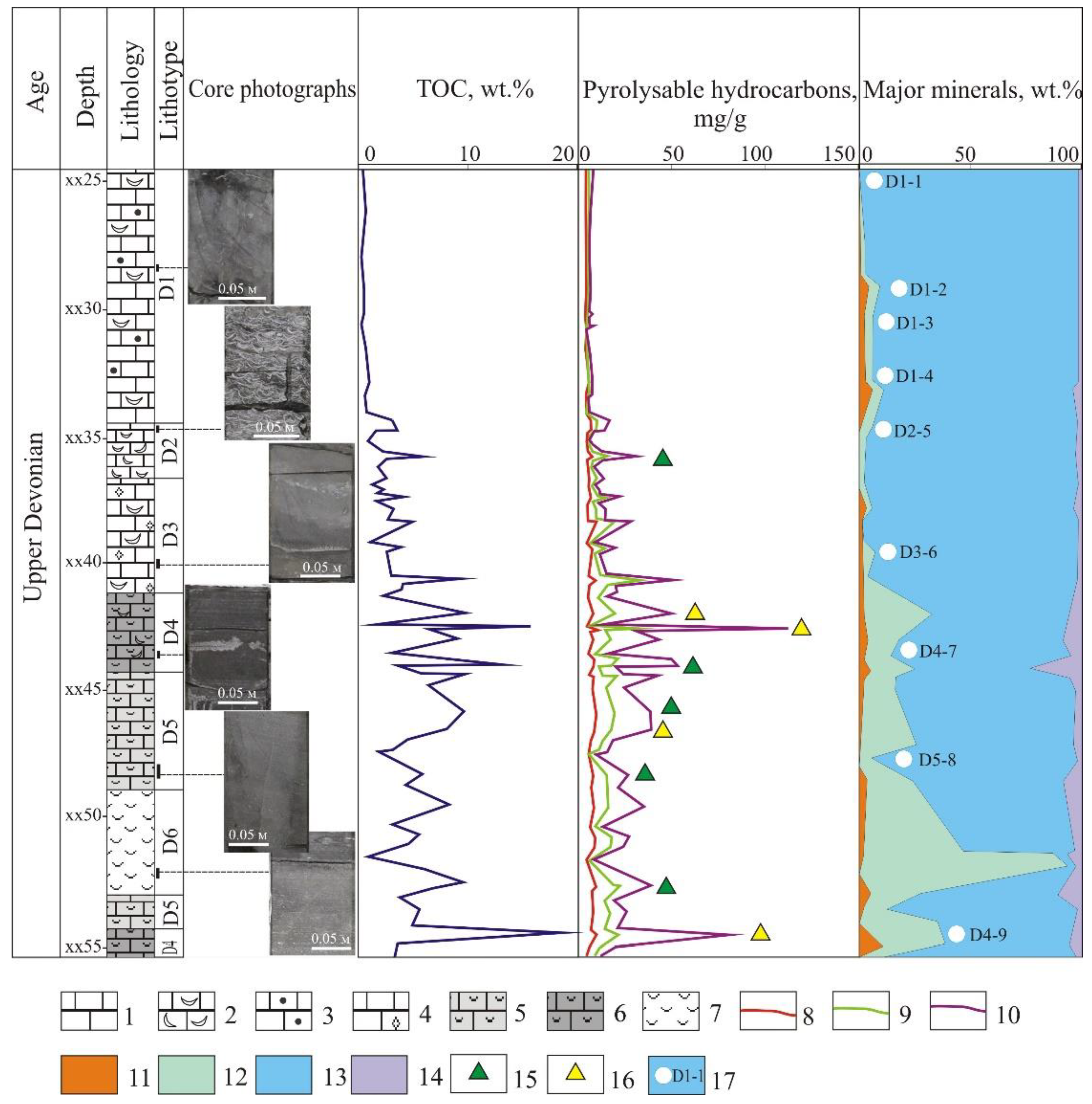
The main rock lithotypes identified by results of petrographic analysis are as follows: micro-clotted limestone with bioclasts (D1); bioclastic limestone with debris (D2); fine-close-crystalline limestone with bioclasts (D3); bioclastic (tentaculitic) organic-rich limestone (D4); limestone, micro-fine-crystalline with bioclasts, siliceous, organic-rich (D5); organic-rich tentaculitic siliceous rocks (D6) (
Figure 2 and
Figure 3).
Lithotype D1. Micro-clotted limestones with bioclasts. The rock is predominantly composed of clots of pelitomorphic calcite. Rhomboidal crystals of dolomite up to 0.15 mm in size are presented. Bioclasts (5–10%) are represented by shells of tentaculites of various sections (up to 0.7 mm in size); fragments of mollusks, ostracode shells up to 2 mm in size; individual fragments of trilobite shells, gastropods, and spicules of sponges up to 0.1 mm in size. Detected are individual quartz fragments of 0.01–0.03 mm size. OM in rock reaches 1–5% in the form of dispersed inclusions. Recrystallization of calcite is non-uniform, often over bioclasts, in individual cases in nests (up to 1–1.5 mm). Dolomitization–hypidiomorphic dolomite crystals up to 0.15 mm in size, quantity about 1–3%. Fracturing individual, fading, tortuous, intersecting open fissures, with aperture up to 0.01 mm. Pyritization in the form of finely dispersed particles, less frequently inclusions (0.01–0.5 mm).
Lithotype D2. Bioclastic limestone. The rock is mainly composed of big-size bioclasts that are oriented in layers and represented by big-size mollusk shells (up to 10 mm long, 0.2–1 mm thick), individual ostracode shells (up to 0.3 mm); smaller fragments are also present (60–65%). Bioclasts are cemented with fine-grained calcite mass. Within this mass, encountered are individual dolomite crystals, idiomorphic to xenomorphic (up to 0.1 mm). The mass is pigmented with OM, which uniformly impregnates the rock and is present in the form of fine-dispersed inclusions and veinlets. Recrystallization is insignificant, mainly over organic remains.
Lithotype D3. Fine-crystalline limestone with bioclasts, with spotted structure due to non-uniform recrystallization and saturation with OM. The rock is composed of recrystallized fine-crystalline calcite crystals of irregular shapes (0.05–0.12 mm, in individual cases up to 0.4 mm). In most cases, crystals are coated with a film of organic matter. In the rock fragments and relicts of mollusk shells 0.1–0.6 mm in size (10–15%) are present. Organic matter is located in intercrystalline voids, in a form of films over crystal faces, veinlets, fills individual fissures (over 5%). Recrystallization is actively developed and is distributed non-uniformly, newly formed calcite crystals up to 0.3 mm, mainly 0.05–0.12 mm. Leaching is detected over individual valves of shells. Dolomitization—insignificant, individual dolomite crystals up to 0.05 mm in size. Pyritization—individual fine-dispersed particles of pyrite up to 0.02 mm in size. Detected are individual fading, sometimes branching fissures, aperture up to 0.02 mm.
Lithotype D4. Bioclastic (tentaculitic) limestones, siliceous-carbonic, with lenticular-laminar fabric due to concentration of bioclasts into lenses. Bioclasts are represented dominantly by shells of tentaculuites of various sections (up to 1 mm in size), mollusk shells and fragments of mollusks, ostracode shells, (0.1–2 mm). The lithotype is characteristic of the presence of shells with walls encrusted with or partly built of organic matter. Silicification over shells is also detected. Its amount often alternates from layer to layer from 10–20% to 25–30%, sometimes is concentrated and forms tentaculite interbeds of various thicknesses (up to 1–2 mm). Bioclasts are “embedded” into the siliceous-carbonate organic mass. Carbonate and organic components dominate, siliceous matter is present in a lesser amount (10–15%). Organic matter is present in the form of finely dispersed detached inclusions, veinlets, films, fills intergranular voids, and sometimes partly builds shell walls. Amount of OM is about 15–20%.
Lithotype D5. Micro-fine-crystalline limestone with siliceous-carbonic bioclasts. Present in the rock are rare bioclasts and relicts thereof (3%), represented by tentaculite shells of various sections (up to 1.2 mm), in addition, shells and shell valves of bivalve mollusks (0.3–0.65 mm) are encountered. Organic matter is distributed uniformly over the rock (5%), and is present in the form of thin veinlets between crystals, it also fills interctystalline interstices.
Lithotype D6. In this case, rock is composed of siliceous matter of fine-micro-crystalline structure and is uniformly impregnated with organic matter. Bioclasts are represented mainly by tentaculite shells (various sections: longitudinal 0.35–0.9 mm, infrequently up to 2.2 mm; transverse sections up to 0.08–0.42 mm), mollusk shells (about 0.25 mm). Bioclasts are built of siliceous matter and chalcedony, less frequently of calcite. Amount of bioclasts is 15–20%. Shell walls are partly built of calcite. Organic matter is uniformly distributed over the rock and saturates the organogenic detritus. Amount of OM is about 20%. Pyrite is encountered in the form of micro-nodules, often confined to OM, size up to 0.01–0.03 mm, amount less than 1%.
The section of Upper Devonian deposits under investigation is predominantly represented by carbonate and mixed siliceous-carbonate varieties. The mineral matrix is characterized by both crystalline structure (processes of recrystallization, individual crystals of dolomite, etc.) and by bioclastic structure (numerous fragments of shells). A considerable role in the rock composition is played by fossilized organic matter, which may be presented in various forms: it may both fill intercystalline space and build layers and interlayers. In a number of cases joint occurrence of OM and mineral component is encountered, and the mineral component is more often represented by calcite whose dimensions are at the verge of resolution of the optical microscopy.
3.2. Geochemical Characteristics
The variability of the Rock-Eval pyrolysis data along the stratigraphic columns illustrates the non-uniform OM distribution. Total organic carbon content (TOC) varies within a wide range from decimal fractions up to 18 wt.% (see
Figure 3). The lower part of the section of the Domanik Formation (lithotypes D4, D5 and D6) is enriched in OM (average TOC value is 7.27 with variations from 0.98 to 17.79 wt.%). The highest TOC values are fixed within the lithotype D4 (8.5 wt.% on the average). Individual high TOC values (above 10 wt.%) are also encountered in the lithotype D5. OM content in the lithotype D6 is 4.5 wt.% on average. In the upper interval of the studied section in the lithotypes D1, D2, and D3, TOC amount is low, it gradually increases down the section from decimal fractions to 4 wt.%, being 2 wt.% on the average. Notwithstanding the high percentage of TOC values over 10 wt.%, non-uniform alternation of high and low values emphasizes non-uniformity of the studied carbonate section. By TOC amount, the lithotypes D2 and D3 contain domanikoid, lithotype D4—domanikite, D5 and D6—dominantly domankite concentrations of organic matter.
Amount of pyrolyzable hydrocarbons (generation potential (S0 + S1 + S2)) in the studied section reaches 112.53 mg HC/g rock and varies in proportion to amount of organic carbon in the rock (that confirming syngenetic nature of hydrocarbons in organic-rich rocks). A low S1 and high S2 in combination with a medium to high TOC are also arguments for a syngenetic or indigenous nature of the HC. The deposits under study contain a wide spectrum of source rocks—from poor to excellent (
Figure 4); in that, the rocks of the lithotype D1 only fall within “poor” ones. All other lithotypes are characterized by dominantly high oil-generating potential. Rocks of the lithotype D4 have excellent petroleum potential.
Degree of catagenetic transformation of OM is evaluated as “mature” due to average Tmax_ex = 443 °C and production index PI 0.15–0.35, it corresponds to the middle of the main oil-generation zone (oil window) (the MC
2 stage at the catagenesis scale of Vassoyevich [
21]), i.e., OM is at the peak of oil generation.
The distribution of light and heavy thermodesorbable hydrocarbons is extremely non-uniform. The experiments with extraction by different solvents set up by the authors did show that pores, fissures, and films on recrystallized carbonates of the studied rocks contain a big amount of resin-asphaltene components, which dissolve only in case of direct access through pore network. This amount depends on the type of rock and on secondary alterations thereof; heavy components are able to block free hydrocarbons, therefore high-melting heteroatomic hydrocarbon compounds and sorbed parautochthonous bitumoids are detected in the rocks, which increase the pyrolytic parameters S2 and decrease S0 + S1 [
15]. Thermodestruction of kerogen and thermal evaporation of asphaltenes take place at a practically same temperature as pyrolysis, and these components can only be separated by extraction in organic solvents. According to the comparison of the results before and after extraction in organic solvent, depth distribution of pyrolyzable hydrocarbons is constructed (see
Figure 3).
Values of the parameter S2ex vary within a wide range from 0.14 to 63.45 mg HC/g rock, being 9.6 mg HC/g rock on average. After extraction, this parameter changes significantly, 1.5 to 2.5 times, thus confirming a high contribution of high-boiling resin-asphaltene components in S2, which are absolutely impossible to be considered as “products of kerogene cracking”.
For some samples with high content of TOC and of pyrolyzable HC components, uncharacteristic liberation of CO
2 during the oxidation stage of the pyrolysis Rock-Eval was detected. For these samples (shown with triangles of different colors in the diagram, see
Figure 3), non-distinct separation of the peaks S4 (organic carbon) and S5 (mineral carbon) was detected.
There exist cases (green stars) where incomplete separation of peaks takes place (
Figure 5a).
However, in a number of cases it is impossible whatsoever to separate the peaks S4 and S5 (
Figure 5b) (yellow stars in the section,
Figure 3). Superimposed and incompletely separated peaks S4 and S5 are encountered mainly in rocks of lithotypes D4 and D5, although such an example is available in the lithotype D2 as well. In samples after extraction, separation of peaks takes place normally. For the studied section of Upper Devonian deposits, non-uniform distribution of organic matter in neighboring samples at distances of 30–50 was observed. However, we can observe similar non-uniformity at a micro-scale within one and the same sample as well. Analysis was conducted both on standard powders and on small fragments of rock (
Figure 6). There is no distinct correlation between size and shape of a fragment and changes in the parameters S0, S1, and S2. When analyzing powders, higher values of S0 and S2 are observed, while more light oil (S1) is preserved in fragments. Differences are seen in height of S1 peaks, height and width of S2 peaks, and in configuration of S4 and S5 peaks in the oxidation stage. For such paired measurements, difference in determining TOC reaches 30%. For some fragments, the story with non-separated peaks repeats, this is especially characteristic of big-size fragments.
For paired powder-fragment tests over the section and in interlayers enriched in OM, difference in amount of light HC and heavy HC plus HC products of kerogen cracking cannot go unnoticed, and at that sum of pyrolyzable HC is different, which is evidential of heterogeneity of the matrix and of preservation of light hydrocarbons in pores. Sum of organic and mineral carbon also is not constant, by all appearances; the rock is enriched in OM in a “patchy” of “layer-by-layer” manner.
The studied section of Upper Devonian deposits have non-uniform organo-mineral composition of the matrix, manifesting itself in non-uniformities of distribution of oil-producing kerogen and of newly formed hydrocarbons remained in the formation.
The carbonate material facilitates retaining resin-asphaltene high-viscosity components in the deposits; amount thereof may reach 1/3 of the total amount of pyrolyzable hydrocarbons. Heavy hydrocarbons, in their turn, block the light part of oil.
In layers enriched in OM, the phenomenon of “non-separated” S4–S5 peak in the oxidation stage is encountered. Such samples are confined mainly to the lithotypes D4 and D5, which are represented by organic-rich siliceous limestones.
Standard pyrolysis studies are insufficient for characterizing siliceous-carbonate deposits enriched in OM. Conventional studies and classical interpretation of pyrolysis parameters are significantly complicated due to multiscale heterogeneity (even at the micro-level) caused by high-viscosity hydrocarbon components and specific behavior of the organo-mineral matrix.
3.3. Physicochemical Characteristics
In the well under study, cores were drilled out using fiberglass liners, which made it possible to recover the surface isolated full-size cores. In the course of opening these liners in the laboratory, a collection of samples was taken and sealed for subsequent examinations. On a collection of 9 samples over the section of the Domanik Formation, primary water saturation was determined by the evaporation method. For the lithotypes under consideration, primary water saturation varies within the range of 0.03 to 0.43 wt.% (
Table 1). That same table presents percentage proportions of minerals and organic matter in rock samples, recalculated from XRD data with consideration of OM by results of pyrolysis Rock-Eval.
In accordance with the classical concepts, primary water saturation of rocks increases with the increase of the content of hydrophilic (water-wettable) clay minerals and decreases with the increase of the content of hydrophobic (oil-wettable) organic matter. Mineral surface of carbonates is most commonly hydrophobic, while mineral surface of silica SiO
2 is considered to be, as a rule, energetically neutral. However, in the work [
19], the authors detected and described influence of silica content on physicochemical properties (e.g., primary water content, cation exchange capacity, etc.) of the mineral matrix of Bazhenov Formation deposits of Tithonian-Berriasian age (West Siberian Basin, Russia). It was shown [
19] that silica controls to some extent physicochemical heterogeneity of the surface of an organo-mineral system. Therefore, to determine in more detail the role of SiO
2 in the mineral matrix of shale-like carbonate rocks of the Domanic Formation, in this work we considered the dependence of primary water saturation on silica content with consideration of clay minerals content (
Figure 7).
Figure 7 demonstrates that samples with low organic matter content follow the classical trend, which fixes increase of primary water saturation with increase of content of clay minerals (hydromica in particular).
However, samples with increased organic matter content are classified into a separate group.
Figure 7 is presented to illustrate the non-typical behavior of organic-rich carbonates. Analyzing the behavior of samples in constructing the Sw = f(SiO
2) dependence showed that the rock samples enriched in organic matter (in which presence of the organo-mineral association is fixed by the electronic microscopy method) and that demonstrate the phenomenon of “non-separated” S4–S5 peak in the oxidation stage in the course of conducting the pyrolysis are classified into a separate group.
4. Discussion
Lithological-geochemical laboratory examinations of Domanik deposits demonstrated non-uniformity of the organo-mineral matrix across the section. In addition, pyrolysis data showed that atypical behavior of the measured parameters is observed for carbonate rocks of the lithotypes D4 and D5 (see
Figure 3): in pyrograms of samples before extraction merging of the peaks S4 (amount of liberated CO
2 during destruction of OM) and S5 (amount of CO
2 during destruction of carbonates) is observed.
In other words, on pyrograms of non-extracted rocks of the lithotypes D4 and D5, a confluent non-separated “hump” of peaks S4 and S5 is observed (see
Figure 5 and
Figure 6). This takes place due to a very high-melting coke or polymerization of the organic matter and the mineral matrix [
22]. Supposedly, at the stage of fossilization or even sedimentation, organic compounds are able to create agglomerates with a carbonate or silicate gel. In a mature organic matter, long-chain heteroatomic compounds are capable of oxidation and splitting into hydrocarbon compounds soluble in organic solvents. After chloroform extraction, a doubled peak of CO
2 yield at the oxidation stage segregates into S4 and S5, which is evidential of the presence of soluble compounds amalgamating the carbonate or siliceous matter and the hydrocarbons.
Thus, unseparability of peaks S4 and S5 on pyrograms may be evidential of the fact that the carbonate/siliceous and the organic components are in a kind of agglomeration that was described in [
23] and were fixed by the authors of this work by methods of optical and electronic microscopy in the course of conducting lithological studies.
Electronic microscopic and micro-X-ray spectral analyses (with the use of scanning electronic microscope SEM) showed that of all the appearances the organo-mineral association in the studied samples is the only matter whose main elements are Ca, C, O, and Si (
Figure 8) and that this organo-mineral association is rather porous [
24]. Despite the high resolution of SEM it is necessary for further research to use subatomic microscopy methods such as transmission electron microscopy.
In addition, experimental geochemical studies showed that in case of absence or a small amount of kerogen, the S4 peak is unavailable on pyrograms. This implies absence (or a small amount) of heteroatomic elements in the organic matter. In this case, the S2 peak will correspond to “heavy oils” containing resin-asphaltene compounds. In practically all samples of the Domanik Formation with low TOC values (less than 1 wt.%), a significant amount of resin-asphaltene components is detected in deposits though kerogen is absent. In thin sections, OM fills thin fissures, present in the form of irregular-shaped inclusions and veinlets.
Figure 9 shows that mineral matrix and organic matter are in a close association, particularly within brown organic matter light mineral inclusions were observed. Optical microscopy does not allow detailing their relationship. SEM in turn shows that this is a single material.
Distribution of OM across the section of the Domanik deposits is very non-uniform (see
Figure 3); in close vicinity there may be located interlayers enriched and impoverished in organic carbon (which, in its turn, contained both in kerogen and autochthonous bitumoids on the one hand and in migrational hydrocarbons on the other hand), which is confirmed by lithological studies as well.
However, comparison of specific lithological features of the described lithotypes in the Domanik deposits makes it impossible to identify any single parameter that will cardinally discriminate the lithotypes D4–D5 and explain their “distinctive from others” geochemical behavior. Such identifiers do not manifest themselves in either biota or organic detritus, or secondary processes, though some differences in fabric are fixed: for example, rocks of the lithotype D1 have micro-clotted, pelitomorphic, bioclastic fabric, while in rocks of the lithotypes D2–D5, fabric is bioclastic and fine-grained. The only essential distinction of the lithotypes D4 and D5 is filling of the intercrystalline space with organic matter, as well as presence of veinlets of OM in which individual crystals of calcite less than 0.01 mm in size were detected. In other words, for the lithotypes D4-D5 there are fixed increase of amount of organic matter and changes in the form of its interaction with the carbonate-siliceous matrix (OM is present in an association with minerals or the OM plays the role of cement?).
Thus, comparison of results of pyrolysis and of lithological studies makes it possible to suppose that the mineral limy matrix and the organic matter in some cases form a single high-molecular compound. It is possible that at the stage of sedimentation and diagenesis, carbonate sediments/rocks are capable of interaction with acids of the organic matter and formation of natural organo-mineral polymers.
Analysis of literature sources showed that the question of the possibility of immobilization of organic matter into carbonate matrix at the stage of fossilization or deposition and creation of organo-silicate-carbonate agglomerates is of interest for many researchers.
For example, in the works [
24,
25], in the course of studying limestones of P1art age (Artinskian Stage of Early Permian) of the Orenburg petroliferous complex, the following non-standard properties of carbonate rocks were described:
Presence of ordered supramolecular (globular) microstructures of carbonate grains of nanometer size detected with increase of magnification ratio on a SEM microgram from 1 μm to 200 nm. Such globular nanometer microstructures are characteristic of organic block-copolymers and bitumens.
Aberration of the stoichiometric formula of calcium carbonate: when zoning the surface of the crystals of limestone, which visually have the form of calcite, the energy-dispersive analyzer of element composition showed that the content of C increased (23.34% atomic instead of 20% atomic) and the content of Ca decreased (16.88% atomic instead of 20% atomic).
Swelling of grains of limestone in hydrocarbons that is accompanied by a significant increase of volume (1.5–4 times) and of mass of grains of a sample i.e., the ability was experimentally fixed of carbonate matter to swell in hydrocarbons in analogy with organic polymers cross-linked to various extent.
By results of studies, the authors of [
24,
25] supposed that a carbonate rock is a polymer of complex structure and that it has not only inorganic but also organic nature.
In another work, [
26] described the effect of increase of open porosity on several samples of dolomite from the Preobrazhensky Horizon (Upper Proterozoic, Vendian, East Siberia, Russia) associated both with widening of existing pores and with the formation of new voids inside the mineral matrix after extraction with pure benzene. In other words, microtomographic scanning of a dolomite sample detected “washing-out” of the mineral part of the rock by a solvent from the middle part of the sample. In the initial shot, a uniform mineral structure is observed because in reconstructed images these organic inclusions are indiscernible in the mineral matric, which has much higher X-ray absorption coefficient. The procedure of extraction washes out organic inclusions and as a result small particles of the mineral matrix may be evacuated from the sample by flow of the solvent. By supposition of the authors [
26], in the studied samples, organic inclusions are immobilized into carbonate grains, i.e., are bonded with the mineral matrix by chemical and/or physical bonds. In other words, the observed phenomenon is completely explained if one keeps to the non-standard idea that a carbonate is a natural organo-mineral polymer, which is formed in the process of sedimentogenesis and as early as at early diagenesis stage has two components bonded chemically: inorganic (calcium carbonate or dolomite) and organic.
Carbonates or salts of the carbonic acid, as components of mineral associations, are widespread in the earth’s crust. Carbonate deposits compose from 18% to 29% of the total amount of sedimentary rocks and a huge number of appropriate publications are devoted to them. Nonetheless, the issue of genesis, for example, of dolomites as multi-facies, polygenic and multi-stage rocks, still attracts interest of researchers [
27].
So, how widespread in the nature are substances having in their composition organo-mineral associations; what are the conditions for origination of such “comradeship”; and what are the properties of such substances?
The most easily identifiable and commonly encountered organo-mineral aggregate is mother-of-pearl—the essential component of shells of mollusks and or pearls proper. Mother-of-pearl consists of calcium carbonate (aragonite 85–90%), organic matter of protein type (conchiolin 4–14%), and water (2–4%). The organic matter forms the carcass of the mother-of-pearl structure—a fine net in which small grids microscopic crystals of aragonite are formed [
28].
Another organo-carbonate abundantly used in medicine (surgery, stomatology) is hydroxyapatite (HA) entering in the composition of bone tissue. Laboratory investigations aimed at improvement of mechanical and biological properties of artificial bone tissue [
29] showed that organic molecules have an effect on setting of hydroxyapatite in water solutions. For synthesizing the biopolymer HA, the following are necessary: time t = 24 h, temperature T = 55 °C, and a basic water solution with pH = 9.5. At that, by data of studying IR and XRF spectra, adsorption interactions exist between the organic and inorganic components of the obtained samples of the composite.
In the work of Academician N.P. Yushkin [
30], studies are analyzed whose result was identification of amino acids in all genetic and structural types of high-carbonic substances; a supposition is made concerning self-organization of polymerized monomers into multi-functional biopolymer structures. In other words, within the presented concept of mineral organobiosis, chemical, energetic, and structural-functional correspondence of some minerals and complex organic compounds is discussed.
Thus, analysis of literary sources makes it possible to conclude that no special thermobaric conditions and long time are needed for immobilization of organic matter into the volume of carbonate minerals, i.e., for the formation of an organo-mineral carbonate association. We suppose that the processes and abnormal behavior of the carbonates described in the results are only seen for the described cases. However, the researchers have no clear vision of the sedimentation conditions for the formation of such an organo-mineral polymer, which is the direction of future research.
5. Conclusions
Organic-rich carbonate rocks of the Domanik Formation are considered both as source rocks and unconventional hydrocarbon reservoirs. The Upper Devonian Domanik Formation within the Upper Kama depression is represented by carbonate rocks and mixed siliceous-carbonate varieties. The mineral matrix is characterized by crystalline and bioclastic texture. Fossilized organic matter is an important part of the rock fabric. OM fills the intercrystalline space and forms lenticular shapes or laminaes. The number of samples are characterized by the presence of a close association of calcite or siliceous mineral matrix with organic matter. The heterogeneous organo-mineral composition of the matrix affects the distribution over the section of oil-producing kerogen and of newly generated hydrocarbons, both heavy and light.
The carbonate material facilitates retaining in the deposits resin-asphaltene high-viscosity components whose content may reach 1/3 of the total amount of pyrolyzable hydrocarbons. Heavy hydrocarbons, in turn, block the light petroleum fraction and hamper migration.
In layers with increased organic matter content, the phenomenon of “non-separated” S4–S5 peaks in the oxidation stage is encountered when conducting pyrolytic analysis. In the geological section under investigation, such samples are confined to lithotypes represented by siliceous limestones.
The conducted geochemical investigations show that conventional pyrolytic examinations are insufficient for characterizing organic-rich siliceous-carbonate deposits. Heterogeneity (even at the microlevel), big amount of non-uniformly distributed high-viscosity hydrocarbon components and specific behavior of the organo-mineral matrix, complicates the conducting of conventional analyses and classical interpretation of pyrolysis Rock-Eval.
Analysis of literary sources makes it possible to conclude that organo-mineral carbonate associations are rather widespread in the nature, while no special pressure-and-temperature conditions and extended periods of time are required for the formation thereof, i.e., for immobilization of organic matter in the volume of carbonate minerals.
Nonetheless, researchers have no clear picture of depositional conditions required for the formation of such an organo-mineral carbonate polymer and this is the line of future research efforts.
The lithological, geochemical, and physicochemical studies of heterogeneity of the organo-mineral matrix of carbonate rocks of the Domanik Formation made it possible to identify, in the section under investigation, intervals where organic matter forms a complex association with the siliceous-carbonate matrix.
We suppose that the revealed organo-mineral associations are complex polymers resembling the substances known in the chemistry, which are composed of multiple recurring macromolecules. The chemists are familiar with variants of spatial structure of polymer molecules where atoms combine into a structure resembling atoms in crystals, as well as with network polymers, which contain crosslinking nodes that are able to swell in particular solvents.
Thus, confirmation, in the course of subsequent micro-examinations, of the fact of immobilization of organic matter into the structure of carbonates modifies the insight into many physical properties of such rocks. For example, such an organo-mineral surface cannot be purged by extraction without destroying the structure of the carbonate mineral, and hence, presence of non-extractable hydrophobic localities does not make it possible to evaluate, by the present-time techniques, wettability, water saturation, electric, stress–strain, and poroperm properties of polymer-comprising rocks.
Hence, presence of such organo-mineral associations or polymer-comprising zones in the section of carbonate rocks should be taken into account in the course of well logging data interpretation, drilling-in, in forming the reservoir development system, and assessment of reserves.