Assessment of the Photosynthetic Response of Posidonia oceanica (Linneaus) Delile, 1813 along a Depth Gradient in the Northern Tyrrhenian Sea (Latium, Italy)
Abstract
1. Introduction
2. Materials and Methods
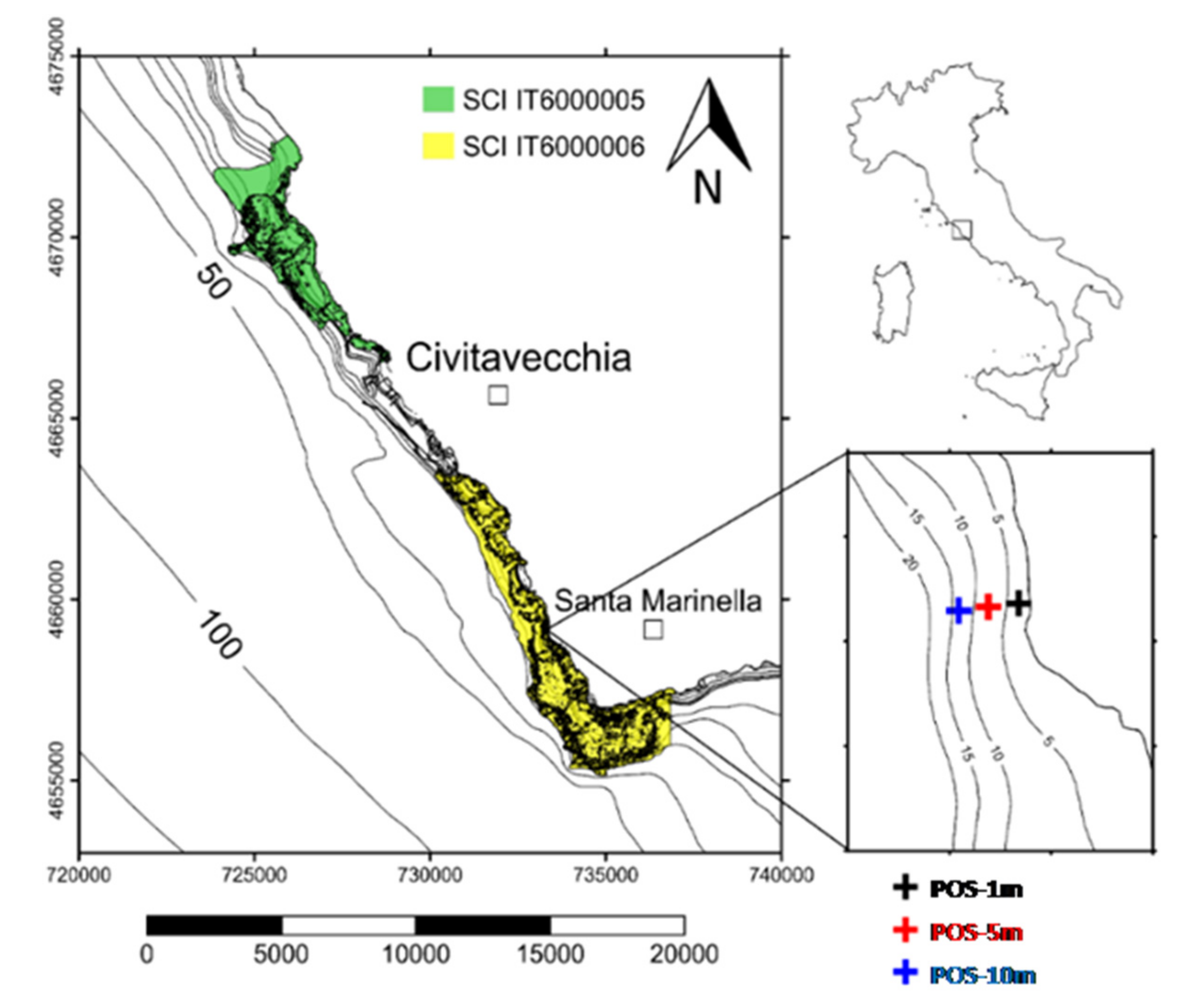
2.1. Analysis of Local Hydrodynamic Conditions
2.2. Sampling Strategy
2.3. PAM Fluorometry
2.4. Morphometric Analysis
2.5. Pigments Content
2.6. Statistical Analysis
3. Results
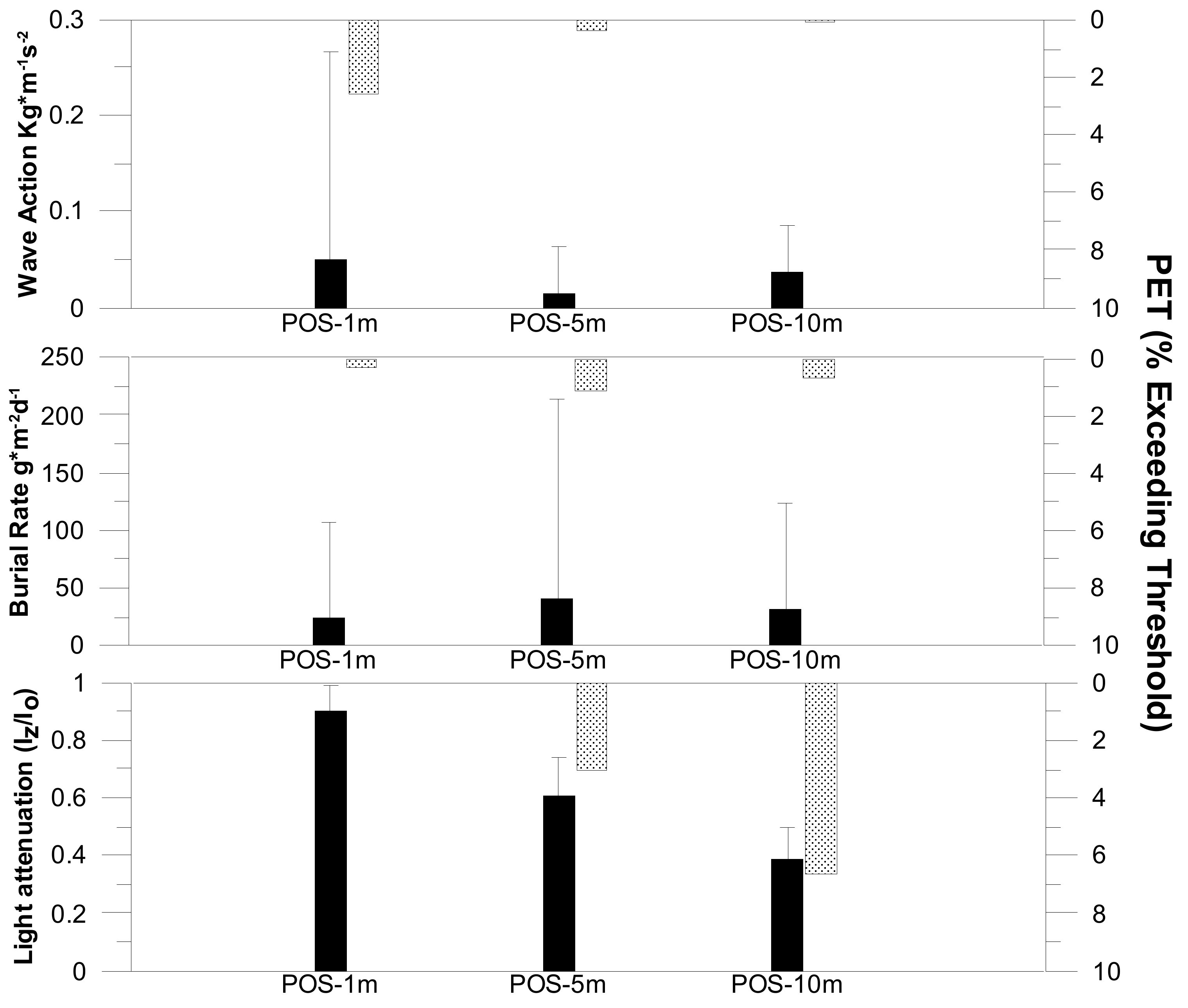
3.1. Assessment of the Environmental Conditions
3.1.1. Abiotic Factors
3.1.2. Biotic Factors
Morphological Traits
Pigments Content
3.2. Photo-Physiological Characterization
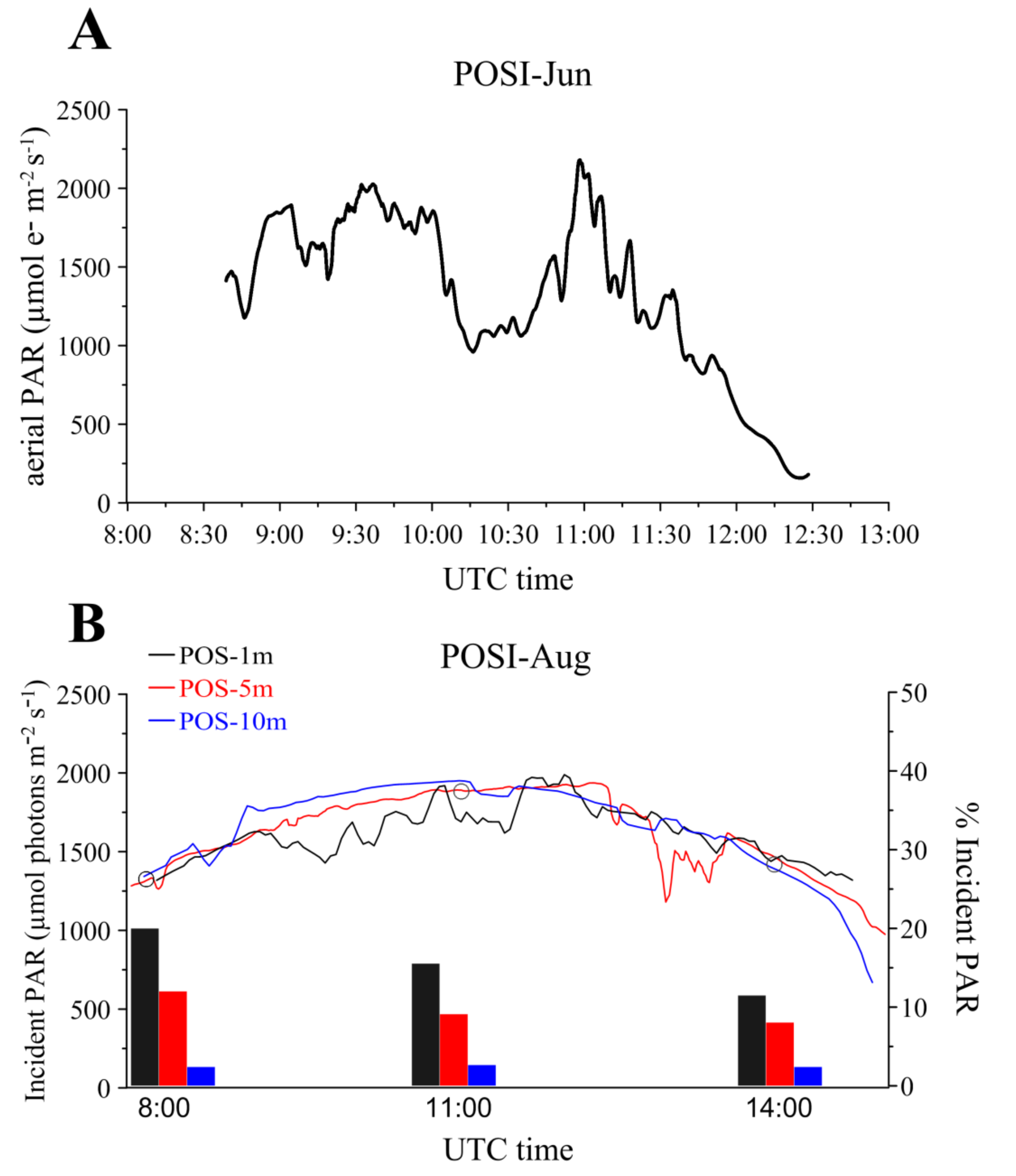
3.2.1. Light Conditions
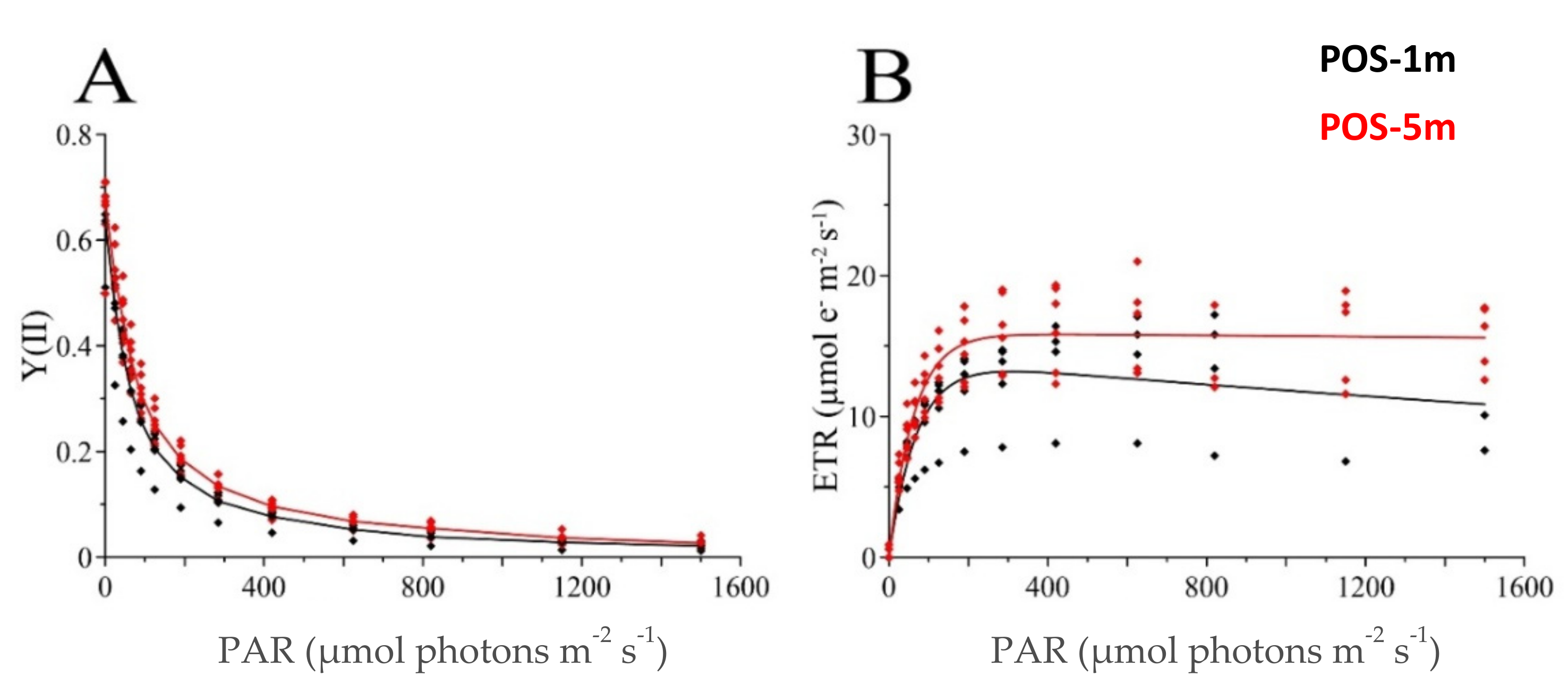
3.2.2. PAM Fluorometry
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Díaz, S.M.; Settele, J.; Brondízio, E.S.; Ngo, H.T.; Guèze, M.; Agard, J.; Arneth, A.; Balvanera, P.; Brauman, K.A.; Butchart, S.H.M.; et al. The Global Assessment Report on Biodiversity and Ecosystem Services: Summary for Policy Makers; IPBES Secretariat: Bonn, Germany, 2019; p. 56. [Google Scholar] [CrossRef]
- Pergent-Martini, C.; Leoni, V.; Pasqualini, V.; Ardizzone, G.D.; Balestri, E.; Bedini, R.; Belluscio, A.; Belsher, T.; Borg, J.; Boudouresque, C.F.; et al. Descriptors of Posidonia oceanica meadows: Use and application. Ecol. Indic. 2005, 5, 213–230. [Google Scholar] [CrossRef]
- Procaccini, G.; Buia, M.C.; Gambi, M.C.; Perez, M.; Pergent, G. The western mediterranean. In World Atlas of Seagrasses; Green, E.P., Short, F.T., Eds.; University of California Press: London, UK, 2003; pp. 48–58. [Google Scholar]
- Pergent-Martini, C.; Pergent, G. Marine phanerogams as a tool in the evaluation of marine trace-metal contamination: An example from the Mediterranean. Int. J. Environ. Pollut. 2000, 13, 126. [Google Scholar] [CrossRef]
- Romero, J.; Martínez-Crego, B.; Alcoverro, T.; Pérez, M.M. A multivariate index based on the seagrass Posidonia oceanica (POMI) to assess ecological status of coastal waters under the water framework directive (WFD). Mar. Pollut. Bull. 2007, 55, 196–204. [Google Scholar] [CrossRef]
- Romero, J. Posidònia: Els Prats Del Fons Del Mar. la Mirada Del Biòleg a Un Ecosistema Mediterrani; Col.lecció Norai 9; Ajuntament de Badalona: Badalona, Spain, 2004; p. 159. [Google Scholar]
- Directive, H. Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Off. J. Eur. Union 1992, 206, 7–50. [Google Scholar]
- Protocol concerning specially protected areas and biological diversity in the Mediterranean. OJEU 1999, L 322, 3–17. Available online: https://www.ecolex.org/details/treaty/protocol-concerning-specially-protected-areas-and-biological-diversity-in-the-mediterranean-tre-001220/ (accessed on 5 May 2021).
- Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy. OJEU 2000, L 327, 1–73.
- Foden, J.; Brazier, D. Angiosperms (seagrass) within the EU water framework directive: A UK perspective. Mar. Pollut. Bull. 2007, 55, 181–195. [Google Scholar] [CrossRef] [PubMed]
- EU. Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008, establishing a framework for community action in the field of marine environmental policy (Marine Strategy Framework Directive). OJEU 2008, L 164/19, 19–40. [Google Scholar]
- Ruiz, J.; Romero, J. Effects of disturbances caused by coastal constructions on spatial structure, growth dynamics and photosynthesis of the seagrass Posidonia oceanica. Mar. Pollut. Bull. 2003, 46, 1523–1533. [Google Scholar] [CrossRef]
- Orth, R.J.; Carruthers, T.J.; Dennison, W.C.; Duarte, C.M.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R.; Kendrick, G.A.; Kenworthy, W.J.; Olyarnik, S. A Global Crisis for Seagrass Ecosystems. Bioscience 2006, 56, 987–996. [Google Scholar] [CrossRef]
- Ardizzone, G.; Belluscio, A.; Maiorano, L. Long-term change in the structure of a Posidonia oceanica landscape and its reference for a monitoring plan. Mar. Ecol. 2006, 27, 299–309. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Mayot, N.; Pergent, G. The outstanding traits of the functioning of the Posidonia oceanica seagrass eco-system. Biol. Mar. Medit. 2006, 13, 109–113. [Google Scholar]
- Montefalcone, M.; Morri, C.; Peirano, A.; Albertelli, G.; Bianchi, C. Substitution and phase shift within the Posidonia oceanica seagrass meadows of NW Mediterranean Sea. Estuar. Coast. Shelf Sci. 2007, 75, 63–71. [Google Scholar] [CrossRef]
- Walker, D.; McComb, A. Seagrass degradation in Australian coastal waters. Mar. Pollut. Bull. 1992, 25, 191–195. [Google Scholar] [CrossRef]
- Infantes, E.; Orfila, A.; Simarro, G.; Terrados, J.; Luhar, M.; Nepf, H. Effect of a seagrass (Posidonia oceanica) meadow on wave propagation. Mar. Ecol. Prog. Ser. 2012, 456, 63–72. [Google Scholar] [CrossRef]
- La Loggia, G.; Calvo, S.; Ciraolo, G.; Mazzola, A.; Pirrotta, M.; Sara, G.; Tomasello, A.; Vizzini, S. Influence of hydrodynamic conditions on the production and fate ofPosidonia oceanicain a semi-enclosed shallow basin (Stagnone di Marsala, Western Sicily). Chem. Ecol. 2004, 20, 183–201. [Google Scholar] [CrossRef]
- Balzano, A.; Calvo, S.; Ciraolo, G.; La Loggia, G. Remote sensing as a tool to calibrate hydrodynamic model-transport numerical models. In Proceedings of the International Conference New Trends in Water and Environmental Engineering for Safety and Life. Eco-compatible Solutions for Aquatic Environments, Capri, Italy, 3–7 July 2000; pp. 1–10. [Google Scholar]
- Vacchi, M.; De Falco, G.; Simeone, S.; Montefalcone, M.; Morri, C.; Ferrari, M.; Bianchi, C.N. Biogeomorphology of the MediterraneanPosidonia oceanicaseagrass meadows. Earth Surf. Process. Landf. 2017, 42, 42–54. [Google Scholar] [CrossRef]
- Cabaço, S.; Santos, R.; Duarte, C.M. The impact of sediment burial and erosion on seagrasses: A review. Estuar. Coast. Shelf Sci. 2008, 79, 354–366. [Google Scholar] [CrossRef]
- Larkum, A.W.D.; West, R.J. Stability, depletion and restoration of seagrass beds. In Proceedings of the Linnean Society of NSW 1983, New South Wales, Sydney, Australia, 27 October 1982; Volume 106, pp. 201–212. [Google Scholar]
- Pulich, W.M.; White, W.A. Decline of submerged vegetation in the Galveston Bay ecosystem: Chronology and relationships to physical processes. J. Coast. Res. 1991, 7, 1125–1138. [Google Scholar]
- Dunton, K.H. Seasonal growth and biomass of the subtropical seagrassHalodule wrightii in relation to continuous measurements of underwater irradiance. Mar. Biol. 1994, 120, 479–489. [Google Scholar] [CrossRef]
- Zimmerman, R.C.; Reguzzoni, J.L.; Alberte, R.S. Eelgrass (Zostera marina L.) transplants in San Francisco Bay: Role of light availability on metabolism, growth and survival. Aquat. Bot. 1995, 51, 67–86. [Google Scholar] [CrossRef]
- Waycott, M.; Duarte, C.M.; Carruthers, T.J.; Orth, R.J.; Dennison, W.C.; Olyarnik, S.; Calladine, A.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R.; et al. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12377–12381. [Google Scholar] [CrossRef]
- Alcoverro, T.; Duarte, C.; Romero, J. Annual growth dynamics of Posidonia oceanica:contribution of large-scale versus local factors to seasonality. Mar. Ecol. Prog. Ser. 1995, 120, 203–210. [Google Scholar] [CrossRef]
- Ralph, P.J. Photosynthetic response of Halophila ovalis (R. Br.) Hook. f. to combined environmental stress. Aquat. Bot. 1999, 65, 83–96. [Google Scholar] [CrossRef]
- Björkman, O. Comparative Studies on Photosynthesis in Higher Plants. Photophysiology 1973, 8, 1–63. [Google Scholar] [CrossRef] [PubMed]
- Sand-Jensen, K. Environmental variables and their effect on photosynthesis of aquatic plant communities. Aquat. Bot. 1989, 34, 5–25. [Google Scholar] [CrossRef]
- Belshe, E.; Durako, M.; Blum, J. Photosynthetic rapid light curves (RLC) of Thalassia testudinum exhibit diurnal variation. J. Exp. Mar. Biol. Ecol. 2007, 342, 253–268. [Google Scholar] [CrossRef]
- Brain, R.A.; Cedergreen, N. Biomarkers in aquatic plants: Selection and utility. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer: New York, NY, USA, 2009; pp. 49–109. [Google Scholar]
- Ralph, P.; Burchett, M. Photosynthetic response of Halophila ovalis to heavy metal stress. Environ. Pollut. 1998, 103, 91–101. [Google Scholar] [CrossRef]
- Prange, J.; Dennison, W. Physiological Responses of Five Seagrass Species to Trace Metals. Mar. Pollut. Bull. 2000, 41, 327–336. [Google Scholar] [CrossRef]
- Haynes, D.; Ralph, P.; Prange, J.; Dennison, B. The Impact of the Herbicide Diuron on Photosynthesis in Three Species of Tropical Seagrass. Mar. Pollut. Bull. 2000, 41, 288–293. [Google Scholar] [CrossRef]
- Macinnis-Ng, C.M.; Ralph, P.J. Towards a more ecologically relevant assessment of the impact of heavy metals on the photosynthesis of the seagrass, Zostera capricorni. Mar. Pollut. Bull. 2002, 45, 100–106. [Google Scholar] [CrossRef]
- Macinnis-Ng, C.M.; Ralph, P.J. Short-term response and recovery of Zostera capricorni photosynthesis after herbicide exposure. Aquat. Bot. 2003, 76, 1–15. [Google Scholar] [CrossRef]
- Macinnis-Ng, C.M. In situ impact of multiple pulses of metal and herbicide on the seagrass, Zostera capricorni. Aquat. Toxicol. 2004, 67, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, A.D.; Collier, C.J.; Flores, F.; Negri, A.P. Acute and additive toxicity of ten photosystem-II herbicides to seagrass. Sci. Rep. 2015, 5, 17443. [Google Scholar] [CrossRef] [PubMed]
- Diepens, N.J.; Buffan-Dubau, E.; Budzinski, H.; Kallerhoff, J.; Merlina, G.; Silvestre, J.; Auby, I.; Tapie, N.; Elger, A. Toxicity effects of an environmental realistic herbicide mixture on the seagrass Zostera noltei. Environ. Pollut. 2017, 222, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, N.S.; Buapet, P.; Pernice, M.; Signal, B.; Kahlke, T.; Hardke, L.; Ralph, P.J. Transcriptome profiling analysis of the seagrass, Zostera muelleri under copper stress. Mar. Pollut. Bull. 2019, 149, 110556. [Google Scholar] [CrossRef] [PubMed]
- Beer, S.; Björk, M.; Gademann, R.; Ralph, P. Measurements of photosynthetic rates in seagrasses. In Global Seagrass Research Methods; Elsevier: Amsterdam, The Netherlands, 2001; pp. 183–198. [Google Scholar]
- Campbell, S.; Miller, C.; Steven, A.; Stephens, A. Photosynthetic responses of two temperate seagrasses across a water quality gradient using chlorophyll fluorescence. J. Exp. Mar. Biol. Ecol. 2003, 291, 57–78. [Google Scholar] [CrossRef]
- Bité, J.S.; Campbell, S.J.; McKenzie, L.J.; Coles, R.G. Chlorophyll fluorescence measures of seagrasses Halophila ovalis and Zostera capricorni reveal differences in response to experimental shading. Mar. Biol. 2007, 152, 405–414. [Google Scholar] [CrossRef]
- Mvungi, E.F.; Lyimo, T.J.; Björk, M. When Zostera marina is intermixed with Ulva, its photosynthesis is reduced by increased pH and lower light, but not by changes in light quality. Aquat. Bot. 2012, 102, 44–49. [Google Scholar] [CrossRef]
- Dattolo, E.; Ruocco, M.; Brunet, C.; Lorenti, M.; Lauritano, C.; D’Esposito, D.; De Luca, P.; Sanges, R.; Mazzuca, S.; Procaccini, G. Response of the seagrass Posidonia oceanica to different light environments: Insights from a combined molecular and photo-physiological study. Mar. Environ. Res. 2014, 101, 225–236. [Google Scholar] [CrossRef]
- Davey, P.A.; Pernice, M.; Ashworth, J.; Kuzhiumparambil, U.; Szabó, M.; Dolferus, R.; Ralph, P.J. A new mechanistic understanding of light-limitation in the seagrass Zostera muelleri. Mar. Environ. Res. 2018, 134, 55–67. [Google Scholar] [CrossRef]
- Pagès, J.F.; Pérez, M.M.; Romero, J. Sensitivity of the seagrass Cymodocea nodosa to hypersaline conditions: A microcosm approach. J. Exp. Mar. Biol. Ecol. 2010, 386, 34–38. [Google Scholar] [CrossRef]
- Guerrero-Meseguer, L.; Marín, A.; Sanz-Lázaro, C. Future heat waves due to climate change threaten the survival of Posidonia oceanica seedlings. Environ. Pollut. 2017, 230, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Beer, S.; Vilenkin, B.; Weil, A.; Veste, M.; Susel, L.; Eshel, A. Measuring photosynthetic rates in seagrasses by pulse amplitude modulated (PAM) fluorometry. Mar. Ecol. Prog. Ser. 1998, 174, 293–300. [Google Scholar] [CrossRef]
- Kitajima, M.B.W.L.; Butler, W.L. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by di-bromothymoquinone. Bioenergetics 1975, 376, 105–115. [Google Scholar] [CrossRef]
- Larkum, A.W.; Drew, E.A.; Ralph, P.J. Photosynthesis and metabolism in Seagrasses at the cellular level. In Seagrasses: Biology, Ecologyand Conservation; Metzler, J.B., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 323–345. [Google Scholar]
- Meravi, N.; Prajapati, S.K. Biomonitoring the genotoxicity of heavy metals/metalloids present n soil contaminated by fly ash from coal-fired thermal power plant using Tradescantia pallida. In Phytoremediation; Springer: Berlin/Heidelberg, Germany, 2015; pp. 169–176. [Google Scholar]
- Gao, L.N. Comparison of chlorophyll fluorescence characteristics between two submerged macrophytes in Jiuzhaigou National Nature Reserve. Acta Agric. Zhejiangensis 2017, 29, 951–958. [Google Scholar]
- Gao, L.-N. Photosynthetic characteristics of Marestail using PAM fluorometry. In Proceedings of the 2nd International Conference on Biomedical and Biological Engineering 2017 (BBE 2017), Guilin, China, 26–28 May 2017; pp. 134–139. [Google Scholar]
- Ranawakage, V.P.; Asaeda, T. Evaluation of the physiological alterations in Ceratophyllum demersum L. along a diurnally changing solar irradiance gradient. J. Plant Interact. 2020, 15, 8–16. [Google Scholar] [CrossRef]
- Figueroa, F.L.; Jerez, C.G.; Korbee, N. Use of in vivo chlorophyll fluorescence to estimate photosynthetic activity and biomass productivity in microalgae grown in different culture systems. Lat. Am. J. Aquat. Res. 2013, 41, 801–819. [Google Scholar] [CrossRef]
- Machado, M.D.; Lopes, A.R.; Soares, E.V. Responses of the alga Pseudokirchneriella subcapitata to long-term exposure to metal stress. J. Hazard. Mater. 2015, 296, 82–92. [Google Scholar] [CrossRef]
- Jerez, C.G.; Malapascua, J.R.; Sergejevová, M.; Masojídek, J.; Figueroa, F.L. Chlorella fusca (Chlorophyta) grown in thin-layer cascades: Estimation of biomass productivity by in-vivo chlorophyll a fluorescence monitoring. Algal Res. 2016, 17, 21–30. [Google Scholar] [CrossRef]
- Elso, M.Z.; Garcia-Jimenez, P.; Robaina, R.; Robaina, R. Endogenous polyamine content and photosynthetic performance under hypo-osmotic conditions reveal Cymodocea nodosa as an obligate halophyte. Aquat. Biol. 2012, 17, 7–17. [Google Scholar] [CrossRef][Green Version]
- Govers, L. The Effects of Biogeochemical Stressors on Seagrass Ecosystems. Ph.D. Thesis, Radboud University, Nijmegen, The Netherlands, 2014. [Google Scholar]
- Repolho, T.; Duarte, B.; Dionísio, G.; Paula, J.R.; Lopes, A.R.; Rosa, I.C.; Grilo, T.F.; Caçador, I.; Calado, R.; Rosa, R. Seagrass ecophysiological performance under ocean warming and acidification. Sci. Rep. 2017, 7, 41443. [Google Scholar] [CrossRef] [PubMed]
- Mota, C.F.; Engelen, A.H.; Serrao, E.A.; Coelho, M.A.G.; Marbà, N.; Krause-Jensen, D.; Pearson, G.A. Differentiation in fitness-related traits in response to elevated temperatures between leading and trailing edge populations of marine macrophytes. PLoS ONE 2018, 13, e0203666. [Google Scholar] [CrossRef] [PubMed]
- Bonamano, S.; Piermattei, V.; Madonia, A.; De Mendoza, F.P.; Pierattini, A.; Martellucci, R.; Stefanì, C.; Zappalà, G.; Caruso, G.; Marcelli, M. The Civitavecchia Coastal Environment Monitoring System (C-CEMS): A new tool to analyze the conflicts between coastal pressures and sensitivity areas. Ocean Sci. 2016, 12, 87–100. [Google Scholar] [CrossRef]
- Bonamano, S.; Madonia, A.; Borsellino, C.; Stefanì, C.; Caruso, G.; De Pasquale, F.; Piermattei, V.; Zappalà, G.; Marcelli, M. Modeling the dispersion of viable and total Escherichia coli cells in the artificial semi-enclosed bathing area of Santa Marinella (Latium, Italy). Mar. Pollut. Bull. 2015, 95, 141–154. [Google Scholar] [CrossRef] [PubMed]
- de Mendoza, F.P.; Fontolan, G.; Mancini, E.; Scanu, E.; Scanu, S.; Bonamano, S.; Marcelli, M. Sediment dynamics and resuspension processes in a shallow-water Posidonia oceanica meadow. Mar. Geol. 2018, 404, 174–186. [Google Scholar] [CrossRef]
- Marcelli, M.; Scanu, S.; Frattarelli, F.M.; Mancini, E.; Carli, F.M. A Benthic Zonation System as a Fundamental Tool for Natural Capital Assessment in a Marine Environment: A Case Study in the Northern Tyrrhenian Sea, Italy. Sustainability 2018, 10, 3786. [Google Scholar] [CrossRef]
- Gnisci, V.; De Martiis, S.C.; Belmonte, A.; Micheli, C.; Piermattei, V.; Bonamano, S.; Marcelli, M. Assessment of the ecological structure of Posidonia oceanica (L.) Delile on the northern coast of Lazio, Italy (central Tyrrhenian, Mediterranean). Ital. Bot. 2020, 9, 1–19. [Google Scholar] [CrossRef]
- Booij, N.R.R.C.; Ris, R.C.; Holthuijsen, L.H. A third-generation wave model for coastal regions: 1. Model description and val-idation. J. Geophys. Res. Ocean. 1999, 104, 7649–7666. [Google Scholar] [CrossRef]
- Infantes, E.; Cañellas, B.; Álvarez-Ellacuria, A.; Terrados, J.; Orfila, A. Wave energy and the upper depth limit distribution of Posidonia oceanica. Bot. Mar. 2009, 52, 419–427. [Google Scholar] [CrossRef]
- Manzanera, M.; Alcoverro, T.; Tomás, F.; Romero, J. Response of Posidonia oceanica to burial dynamics. Mar. Ecol. Prog. Ser. 2011, 423, 47–56. [Google Scholar] [CrossRef]
- Gera, A.; Alcoverro, T.; Mascaró, O.; Pérez, M.; Romero, J. Exploring the utility of Posidonia oceanica chlorophyll fluorescence as an indicator of water quality within the European Water Framework Directive. Environ. Monit. Assess. 2011, 184, 3675–3686. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.-M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta (BBA) Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Platt, T.; Gallegos, C.L.; Harrison, W.G. Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J. Mar. Res. 1980, 38, 687–701. [Google Scholar]
- Buia, M.C.; Gambi, M.C.; Dappiano, M. Seagrass systems. Biol. Mar. Mediterr. 2004, 11 (Suppl. 1), 133–183. [Google Scholar]
- Giraud, G.; Boudouresque, C.-F.; Cinelli, F.; Fresi, E.; Mazzella, L. Observations sur l’herbier de Posidonia oceanica (L.) Delile autour de l’Ile d’Ischia (Italie). G. Bot. Ital. 1979, 113, 261–274. [Google Scholar] [CrossRef]
- Dennison, W.C. Chlorophyll content. In Seagrass Research Methods; Phillips, R.C., McRoy, C.P., Eds.; UNESCO: Paris, France, 1990; pp. 83–85. [Google Scholar]
- Porra, R.; Thompson, W.; Kriedemann, P. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta (BBA) Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Bonamano, S.; Piazzolla, D.; Scanu, S.; Mancini, E.; Madonia, A.; Piermattei, V.; Marcelli, M. Modelling approach for the evaluation of burial and erosion processes on Posidonia oceanica meadows. Estuar. Coast. Shelf Sci. 2021, 254, 107321. [Google Scholar] [CrossRef]
- Short, F.T.; Neckles, H.A. The effects of global climate change on seagrasses. Aquat. Bot. 1999, 63, 169–196. [Google Scholar] [CrossRef]
- Fonseca, M.; Bell, S. Influence of physical setting on seagrass landscapes near Beaufort, North Carolina, USA. Mar. Ecol. Prog. Ser. 1998, 171, 109–121. [Google Scholar] [CrossRef]
- Frederiksen, M.; Krause-Jensen, D.; Holmer, M.; Laursen, J.S. Spatial and temporal variation in eelgrass (Zostera marina) landscapes: Influence of physical setting. Aquat. Bot. 2004, 78, 147–165. [Google Scholar] [CrossRef]
- Alcoverro, T.; Manzanera, M.; Romero, J. Annual metabolic carbon balance of the seagrass Posidonia oceanica: The importance of carbohydrate reserves. Mar. Ecol. Prog. Ser. 2001, 211, 105–116. [Google Scholar] [CrossRef]
- Ruban, A.V.; Johnson, M.P. Dynamics of higher plant photosystem cross-section associated with state transitions. Photosynth. Res. 2008, 99, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Marbà, N.; Santiago, R.; Díaz-Almela, E.; Álvarez, E.; Duarte, C.M. Seagrass (Posidonia oceanica) vertical growth as an early indicator of fish farm-derived stress. Estuar. Coast. Shelf Sci. 2006, 67, 475–483. [Google Scholar] [CrossRef]
- Major, K.M.; Dunton, K.H. Variations in light-harvesting characteristics of the seagrass, Thalassia testudinum: Evidence for photoacclimation. J. Exp. Mar. Biol. Ecol. 2002, 275, 173–189. [Google Scholar] [CrossRef]
- Olesen, B.; Enríquez, S.; Duarte, C.; Sand-Jensen, K. Depth-acclimation of photosynthesis, morphology and demography of Posidonia oceanica and Cymodocea nodosa in the Spanish Mediterranean Sea. Mar. Ecol. Prog. Ser. 2002, 236, 89–97. [Google Scholar] [CrossRef]
- Collier, C.; Lavery, P.; Masini, R.; Ralph, P. Morphological, growth and meadow characteristics of the seagrass Posidonia sinuosa along a depth-related gradient of light availability. Mar. Ecol. Prog. Ser. 2007, 337, 103–115. [Google Scholar] [CrossRef]
- Collier, C.J.; Lavery, P.S.; Ralph, P.J.; Masini, R.J. Shade-induced response and recovery of the seagrass Posidonia sinuosa. J. Exp. Mar. Biol. Ecol. 2009, 370, 89–103. [Google Scholar] [CrossRef]
- Olivé, I.; Vergara, J.J.; Pérez-Lloréns, J.L. Photosynthetic and morphological photoacclimation of the seagrass Cymodocea nodosa to season, depth and leaf position. Mar. Biol. 2013, 160, 285–297. [Google Scholar] [CrossRef]
- Abal, E.; Loneragan, N.; Bowen, P.; Perry, C.; Udy, J.; Dennison, W. Physiological and morphological responses of the seagrass Zostera capricorni Aschers, to light intensity. J. Exp. Mar. Biol. Ecol. 1994, 178, 113–129. [Google Scholar] [CrossRef]
- Peralta, G.; Pérez-Lloréns, J.L.; Hernández, I.; Vergara, J.J. Effects of light availability on growth, architecture and nutrient content of the seagrass Zostera noltii Hornem. J. Exp. Mar. Biol. Ecol. 2002, 269, 926. [Google Scholar] [CrossRef]
- Ralph, P.J.; Gademann, R. Rapid light curves: A powerful tool to assess photosynthetic activity. Aquat. Bot. 2005, 82, 222–237. [Google Scholar] [CrossRef]
- Sandoval-Gil, J.M.; Ruiz, J.M.; Marín-Guirao, L.; Bernardeau-Esteller, J.; Sánchez-Lizaso, J.L. Ecophysiological plasticity of shallow and deep populations of the Mediterranean seagrasses Posidonia oceanica and Cymodocea nodosa in response to hypersaline stress. Mar. Environ. Res. 2014, 95, 39–61. [Google Scholar] [CrossRef] [PubMed]
- Schubert, N.; Freitas, C.; Silva, A.; Costa, M.M.; Barrote, I.; Horta, P.A.; Silva, J. Photoacclimation strategies in northeastern Atlantic seagrasses: Integrating responses across plant organizational levels. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Lorenti, M.; Lassauque, J.; Mattera, F.; Buia, M.C. Photosynthetic activity of Posidonia oceanica during a diurnal cycle of irra-diance. Biol. Mar. Mediterr. 2006, 13, 597–600. [Google Scholar]
- Procaccini, G.; Ruocco, M.; Marín-Guirao, L.; Dattolo, E.; Brunet, C.; D’Esposito, D.; Lauritano, C.; Mazzuca, S.; Serra, I.A.; Bernardo, L.; et al. Depth-specific fluctuations of gene expression and protein abundance modulate the photophysiology in the seagrass Posidonia oceanica. Sci. Rep. 2017, 7, srep42890. [Google Scholar] [CrossRef]
- Ruocco, M.; De Luca, P.; Marín-Guirao, L.; Procaccini, G. Differential Leaf Age-Dependent Thermal Plasticity in the Keystone Seagrass Posidonia oceanica. Front. Plant Sci. 2019, 10, 1556. [Google Scholar] [CrossRef]
- Ralph, P.J.; Wilhelm, C.; Lavaud, J.; Jakob, T.; Petrou, K.; Krans, S.A. Fluorescence as a tool to understand changes in photo-synthetic electron flow regulation. In Chlorophyll a Fluorescence in Aquatic Sciences: Methods and Applications; Suggett, D.J., Borowitzka, M.A., Prášil, O., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 75–89. [Google Scholar]
- Pergent, G.; Romero, J.; Pergent-Martini, C.; Mateo, M.-A.; Boudouresque, C.-F. Primary production, stocks and fluxes in the Mediterranean seagrass Posidonia oceanica. Mar. Ecol. Prog. Ser. 1994, 106, 139–146. [Google Scholar] [CrossRef]
- Lobban, C.S.; Harrison, P.J. Seaweed Ecology and Physiology; Cambridge University Press: Cambridge, UK, 1994; p. 384. [Google Scholar]
- Falkowski, P.G.; Raven, J.A. Aquatic Photosynthesis, 2nd ed.; Princeton University Press: Princeton, NJ, USA, 2007. [Google Scholar]
- Kirk, J.T.O. Light and Photosynthesis in Aquatic Ecosystems, 3rd ed.; Cambridge University: Cambridge, UK, 2011; p. 662. [Google Scholar]
- Alcoverro, T.; Manzanera, M.; Romero, J. Seasonal and age-dependent variability of Posidonia oceanica (L.) Delile photosynthetic parameters. J. Exp. Mar. Biol. Ecol. 1998, 230, 1–13. [Google Scholar] [CrossRef]
- Via, J.D.; Sturmbauer, C.; Schönweger, G.; Sötz, E.; Mathekowitsch, S.; Stifter, M.; Rieger, R. Light gradients and meadow structure in Posidonia oceanica: Ecomorphological and functional correlates. Mar. Ecol. Prog. Ser. 1998, 163, 267–278. [Google Scholar] [CrossRef]
- Marín-Guirao, L.; Bernardeau-Esteller, J.; Ruiz, J.M.; Sandoval-Gil, J.M. Resistance of Posidonia oceanica seagrass meadows to the spread of the introduced green alga Caulerpa cylindracea: Assessment of the role of light. Biol. Invasions 2015, 17, 1989–2009. [Google Scholar] [CrossRef]

| POSI-Jun | |||||||
|---|---|---|---|---|---|---|---|
| Site | ANOVA | ||||||
| Type of Leaf | Parameters | Units | POS-1m | POS-5m | p | ||
| Mean | (±) SD | Mean | (±) SD | ||||
| Intermediate | width | cm | 0.726 | 0.025 | 0.906 | 0.056 | <0.001 |
| leaf blade length | cm | 23.5 | 6.9 | 35.8 | 15.4 | ns | |
| green tissue length | cm | 23.3 | 6.7 | 35.3 | 15.1 | ns | |
| brown tissue length | cm | 0.200 | 0.447 | 0.500 | 0.769 | ns | |
| n. of leaves | shoot−1 | 1.67 | 0.58 | 2.67 | 0.58 | ns | |
| total leaf area | cm2 shoot−1 | 28.4 | - | 88.3 | - | ||
| green leaf area | cm2 shoot−1 | 28.1 | - | 87.1 | - | ||
| photosynthetic tissue | % | 99.1 | - | 98.6 | - | ||
| Adult | width | cm | 0.721 | 0.039 | 0.963 | 0.048 | <0.001 |
| leaf blade length | cm | 19.5 | 10.2 | 20.1 | 11.6 | ns | |
| leaf base length | cm | 2.24 | 0.32 | 2.74 | 0.76 | ns | |
| total leaf length | cm | 21.7 | 10.1 | 22.9 | 11.2 | ns | |
| green tissue length | cm | 16.8 | 8.7 | 20.1 | 11.6 | ns | |
| brown tissue length | cm | 2.73 | 4.6 | 0.058 | 0.151 | ns | |
| n. of leaves | shoot−1 | 2.33 | 0.58 | 4.00 | 0.00 | ||
| total leaf area | cm2 shoot−1 | 32.5 | - | 77.6 | - | ||
| green leaf area | cm2 shoot−1 | 28.0 | - | 77.3 | - | ||
| photosynthetic tissue | % | 86.2 | - | 99.7 | - | ||
| Site | n. of leaves | shoot−1 | 4.67 | 0.58 | 8.00 | 0.00 | ns |
| total leaf area | cm2 shoot−1 | 60.9 | - | 166 | - | ns | |
| green leaf area | cm2 shoot−1 | 56.2 | - | 164 | - | ||
| photosynthetic tissue | % | 92.2 | - | 99.0 | - | ||
| POSI-Aug | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Site | ANOVA | ||||||||
| Type of Leaf | Parameters | Units | POS-1m | POS-5m | POS-10m | p | |||
| Mean | (±) SD | Mean | (±) SD | Mean | (±) SD | ||||
| Intermediate | width | cm | 0.771 | 0.071 | 0.917 | 0.090 | 0.939 | 0.058 | <0.001 |
| leaf blade length | cm | 11.5 | 8.1 | 30.8 | 22.3 | 20.4 | 12.3 | <0.01 | |
| green tissue length | cm | 11.0 | 7.3 | 30.8 | 22.3 | 20.4 | 12.3 | <0.01 | |
| brown tissue length | cm | 0.494 | 2.04 | 0.000 | 0.000 | 0.00 | 0.00 | ns | |
| n. of leaves | shoot−1 | 1.89 | 0.60 | 2.56 | 1.01 | 2.56 | 1.24 | ||
| total leaf area | cm2 shoot−1 | 16.9 | - | 73.7 | - | 49.1 | - | ||
| green leaf area | cm2 shoot−1 | 16.2 | - | 73.7 | - | 49.1 | - | ||
| photosynthetic tissue | % | 95.9 | - | 100 | - | 100 | - | ||
| Adult | width | cm | 0.763 | 0.087 | 0.931 | 0.079 | 0.910 | 0.048 | <0.001 |
| leaf blade length | cm | 19.3 | 6.2 | 57.2 | 22.2 | 48.6 | 20.0 | <0.001 | |
| leaf base length | cm | 2.08 | 0.55 | 3.72 | 0.66 | 3.84 | 0.81 | <0.001 | |
| total leaf length | cm | 21.3 | 6.5 | 60.9 | 22.2 | 52.4 | 20.3 | <0.001 | |
| green tissue length | cm | 16.4 | 5.2 | 52.7 | 20.2 | 46.0 | 21.4 | <0.001 | |
| brown tissue length | cm | 2.86 | 2.88 | 4.49 | 8.43 | 2.54 | 8.24 | ns | |
| n. of leaves | shoot−1 | 1.89 | 0.60 | 3.56 | 2.07 | 4.89 | 1.45 | ||
| total leaf area | cm2 shoot−1 | 16.9 | - | 551 | - | 216 | - | ||
| green leaf area | cm2 shoot−1 | 16.2 | - | 535 | - | 205 | - | ||
| photosynthetic tissue | % | 95.9 | - | 97.1 | - | 94.8 | - | ||
| Site | n. of leaves | shoot−1 | 6.89 | 1.17 | 6.78 | 2.73 | 8.22 | 3.60 | <0.001 |
| total leaf area | cm2 shoot−1 | 89.5 | - | 625 | - | 268 | - | ns | |
| green leaf area | cm2 shoot−1 | 59.0 | - | 609 | - | 254 | - | ||
| photosynthetic tissue | % | 65.9 | - | 97.4 | - | 94.9 | - | ||
| * Density | shoot m−2 | 82.6 | 37.3− | 350.7 | 92.6− | 206.3 | 68.8 | ||
| Campaign | Site | Sampling Time(UTC) | Parameters | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Chl a | Chl b | Chl a + b | Cx+c | |||||||
| Mean | (±) SD | Mean | (±) SD | Mean | (±) SD | Mean | (±) SD | |||
| POSI-Jun | POS-1m | 1.54 | 0.47 | 0.57 | 0.16 | 2.16 | 0.64 | 0.44 | 0.10 | |
| POS-5m | 1.80 | 0.51 | 0.68 | 0.21 | 2.53 | 0.74 | 0.57 | 0.11 | ||
| POSI-Aug | POS-1m | Entire dataset | 1.07 | 0.48 | 0.40 | 0.21 | 1.68 | 0.80 | 0.40 | 0.19 |
| 8:00 | 1.16 | 0.37 | 0.48 | 0.18 | 1.70 | 0.54 | 0.37 | 0.15 | ||
| 11:00 | 0.83 | 0.19 | 0.29 | 0.18 | 1.37 | 0.35 | 0.38 | 0.14 | ||
| 14:00 | 1.42 | 0.98 | 0.51 | 0.30 | 2.29 | 1.75 | 0.50 | 0.40 | ||
| POS-5m | Entire dataset | 1.17 | 0.52 | 0.42 | 0.23 | 1.78 | 0.72 | 0.36 | 0.13 | |
| 8:00 | 0.86 | 0.35 | 0.31 | 0.16 | 1.38 | 0.67 | 0.27 | 0.10 | ||
| 11:00 | 0.96 | 0.21 | 0.27 | 0.25 | 1.74 | 0.24 | 0.37 | 0.01 | ||
| 14:00 | 1.44 | 0.60 | 0.54 | 0.23 | 2.02 | 0.86 | 0.41 | 0.16 | ||
| POS-10m | Entire dataset | 1.02 | 0.17 | 0.42 | 0.08 | 1.53 | 0.24 | 0.27 | 0.09 | |
| 8:00 | 1.14 | 0.22 | 0.45 | 0.14 | 1.72 | 0.30 | 0.35 | 0.08 | ||
| 11:00 | 0.87 | 0.12 | 0.41 | 0.4 | 1.34 | 0.19 | 0.17 | 0.05 | ||
| 14:00 | 1.03 | 0.01 | 0.41 | 0.05 | 1.53 | 0.04 | 0.29 | 0.03 | ||
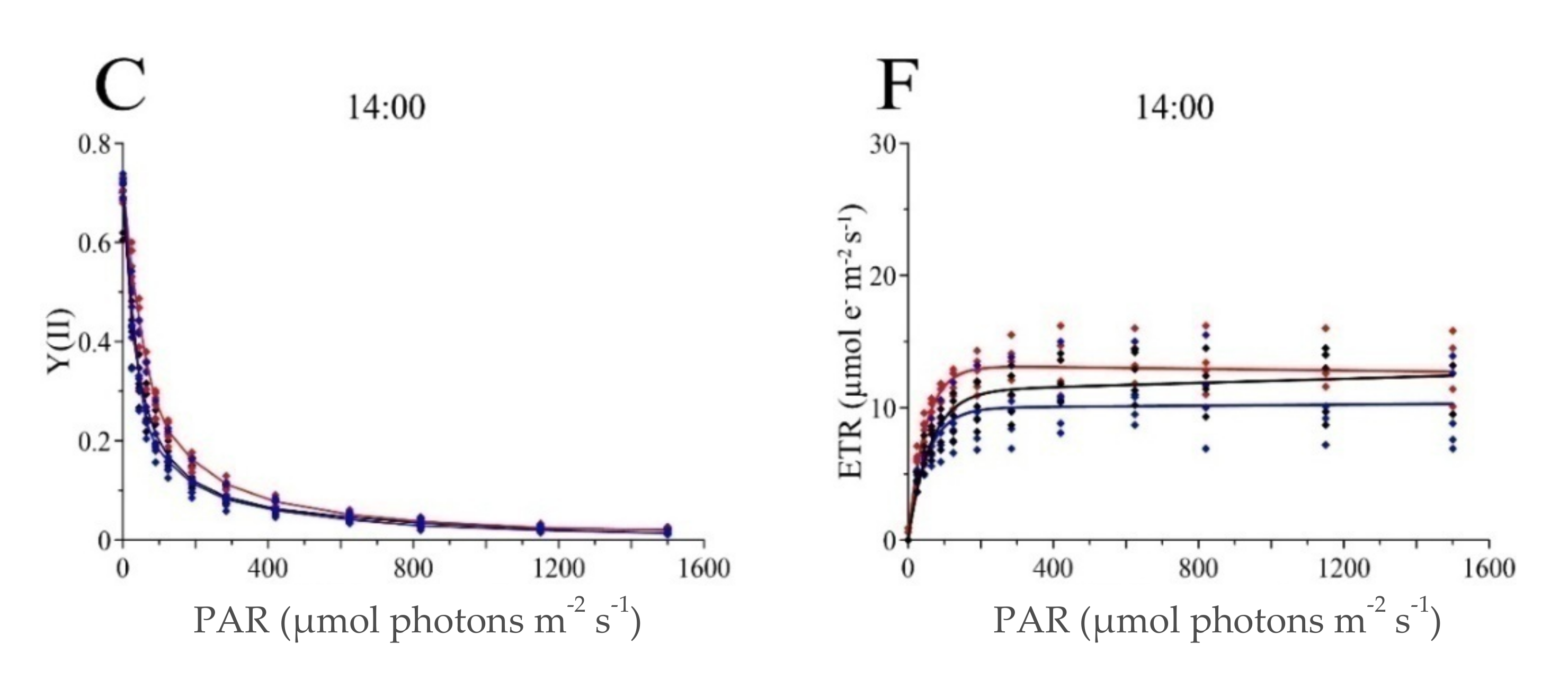
| Campaign | Site | Sampling Time | Fv/Fm | ETR | ETRmax | α | Ek | N | ||
|---|---|---|---|---|---|---|---|---|---|---|
| UTC | Mean | (±) SD | Mean | (±) SD | ||||||
| POSI-Jun | POS-1m | 9:00 | 0.633 | 0.067 | 10.8 *** | 2.2 | 12.8 | 0.217 | 58.8 | 65 |
| POS-5m | 11:00 | 0.673 | 0.034 | 14.1 *** | 2.0 | 15.6 | 0.259 | 60.2 | 59 | |
| POSI-Aug | POS-1m | Entire dataset | 0.715 * | 0.025 | 7.49 *** | 1.4 | - | - | - | - |
| 8:00 | 0.706 | 0.026 | 7.39 **b | 1.63 | 10.3 ** | 0.162 | 63.5 | 64 | ||
| 11:00 | 0.726 ***a | 0.024 | 7.31 ***c | 1.05 | 9.29 | 0.234 | 39.7 | 59 | ||
| 14:00 | 0.713 | 0.021 | 7.84 ***d | 1.53 | 11.3 | 0.187 | 60.4 | 62 | ||
| POS-5m | Entire dataset | 0.722 * | 0.027 | 11.2 *** | 1.9 | - | - | - | - | |
| 8:00 | 0.720 | 0.031 | 11.0 **b | 2.5 | 12.8 | 0.234 | 54.6 | 65 | ||
| 11:00 | 0.728 ***a | 0.026 | 12.0 ***c | 1.5 | 16.3 | 0.260 | 62.7 | 62 | ||
| 14:00 | 0.719 | 0.025 | 10.6 ***d | 1.4 | 13.2 | 0.282 | 46.9 | 65 | ||
| POS-10m | Entire dataset | 0.698 * | 0.076 | 6.97 *** | 2.93 | - | - | - | - | |
| 8:00 | 0.725 | 0.020 | 9.42 ** | 0.9 | 11.2 | 0.237 | 47.0 | 65 | ||
| 11:00 | 0.640 ***a | 0.108 | 3.84 ***c | 2.24 | 5.31 | 0.126 | 42.1 | 65 | ||
| 14:00 | 0.729 | 0.019 | 7.66 ***d | 1.96 | 10.0 | 0.193 | 51.9 | 65 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madonia, A.; Caporale, G.; Penna, M.; Bonamano, S.; Marcelli, M. Assessment of the Photosynthetic Response of Posidonia oceanica (Linneaus) Delile, 1813 along a Depth Gradient in the Northern Tyrrhenian Sea (Latium, Italy). Geosciences 2021, 11, 202. https://doi.org/10.3390/geosciences11050202
Madonia A, Caporale G, Penna M, Bonamano S, Marcelli M. Assessment of the Photosynthetic Response of Posidonia oceanica (Linneaus) Delile, 1813 along a Depth Gradient in the Northern Tyrrhenian Sea (Latium, Italy). Geosciences. 2021; 11(5):202. https://doi.org/10.3390/geosciences11050202
Chicago/Turabian StyleMadonia, Alice, Giulia Caporale, Marina Penna, Simone Bonamano, and Marco Marcelli. 2021. "Assessment of the Photosynthetic Response of Posidonia oceanica (Linneaus) Delile, 1813 along a Depth Gradient in the Northern Tyrrhenian Sea (Latium, Italy)" Geosciences 11, no. 5: 202. https://doi.org/10.3390/geosciences11050202
APA StyleMadonia, A., Caporale, G., Penna, M., Bonamano, S., & Marcelli, M. (2021). Assessment of the Photosynthetic Response of Posidonia oceanica (Linneaus) Delile, 1813 along a Depth Gradient in the Northern Tyrrhenian Sea (Latium, Italy). Geosciences, 11(5), 202. https://doi.org/10.3390/geosciences11050202







