Benthic Foraminiferal Assemblages and Rhodolith Facies Evolution in Post-LGM Sediments from the Pontine Archipelago Shelf (Central Tyrrhenian Sea, Italy)
Abstract
1. Introduction
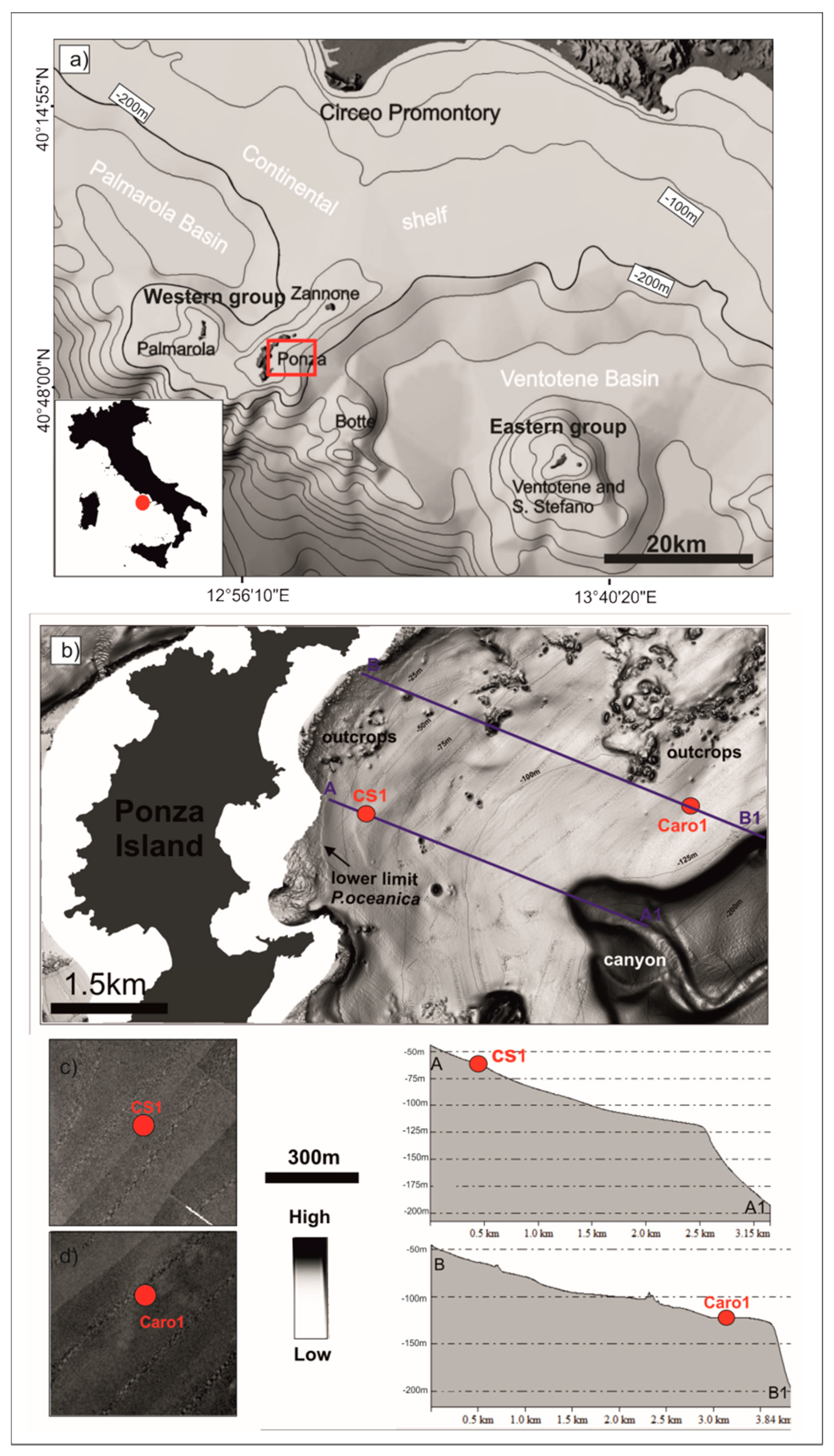
2. Study Area
3. Materials and Methods
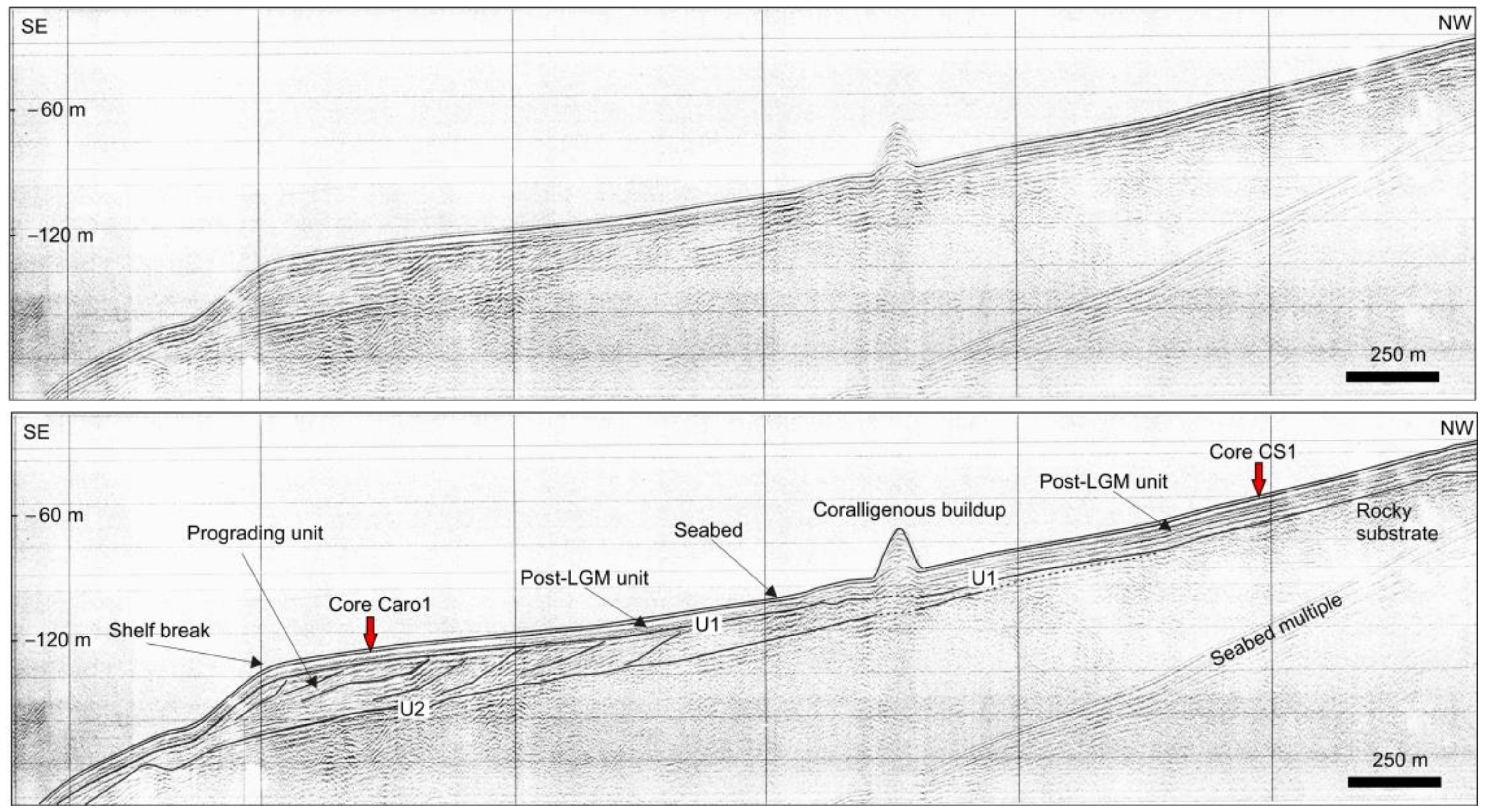
3.1. Geophysical Data
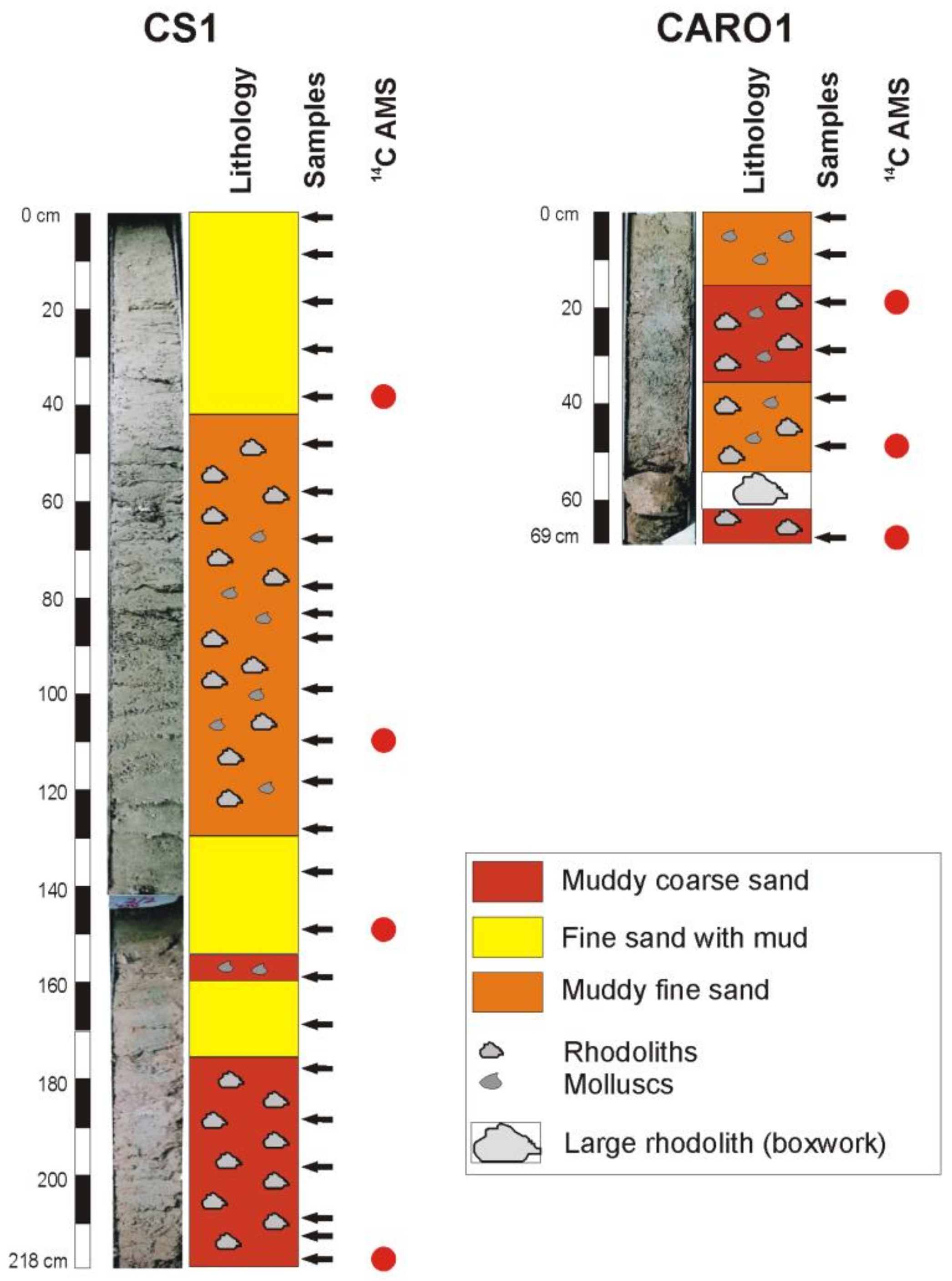
3.2. Sediment Samples
3.3. Radiocarbon Dates
4. Results
4.1. Seabed Morphology and Seismo-Stratigraphic Evidences
4.2. Lithology and Sediment Composition
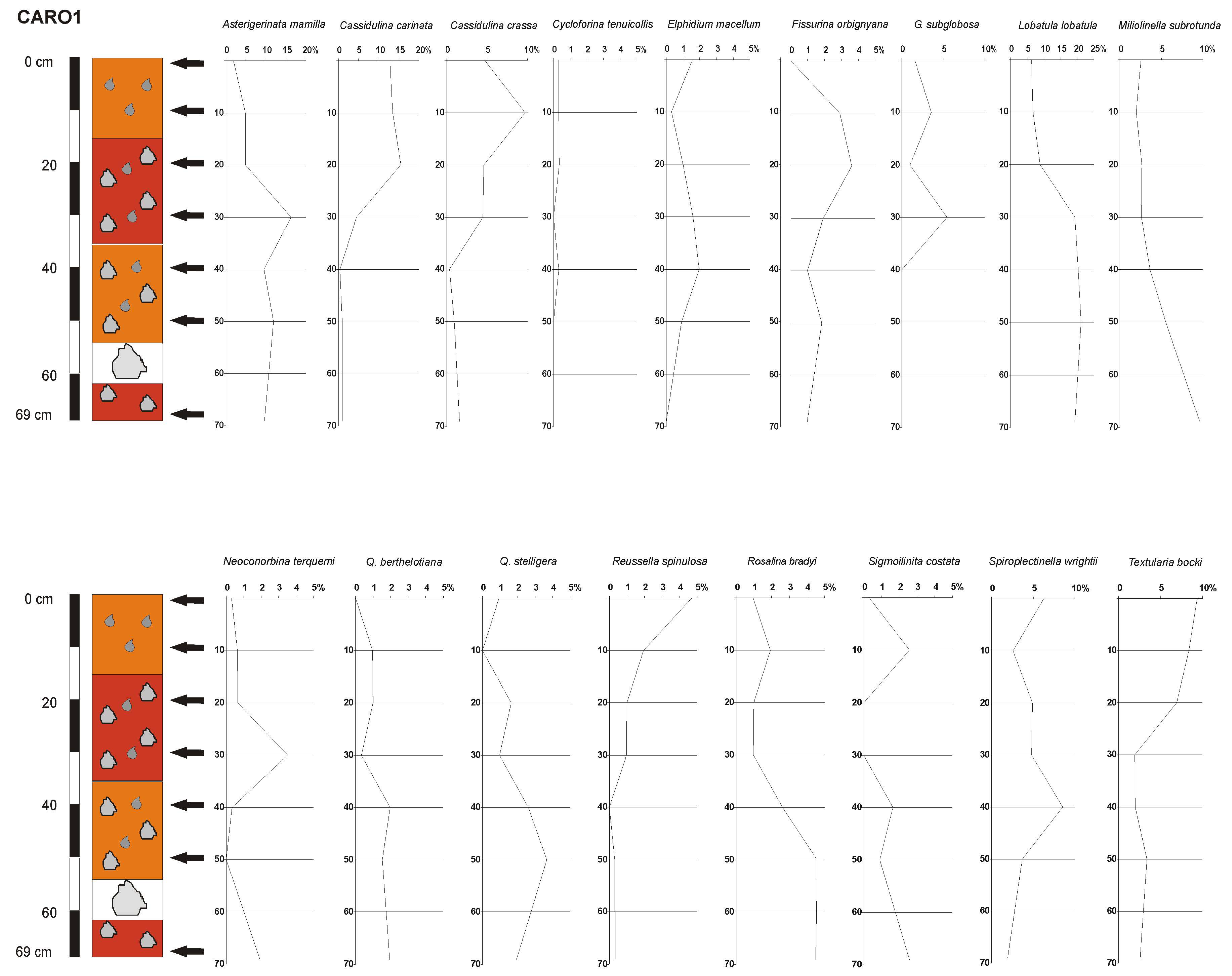
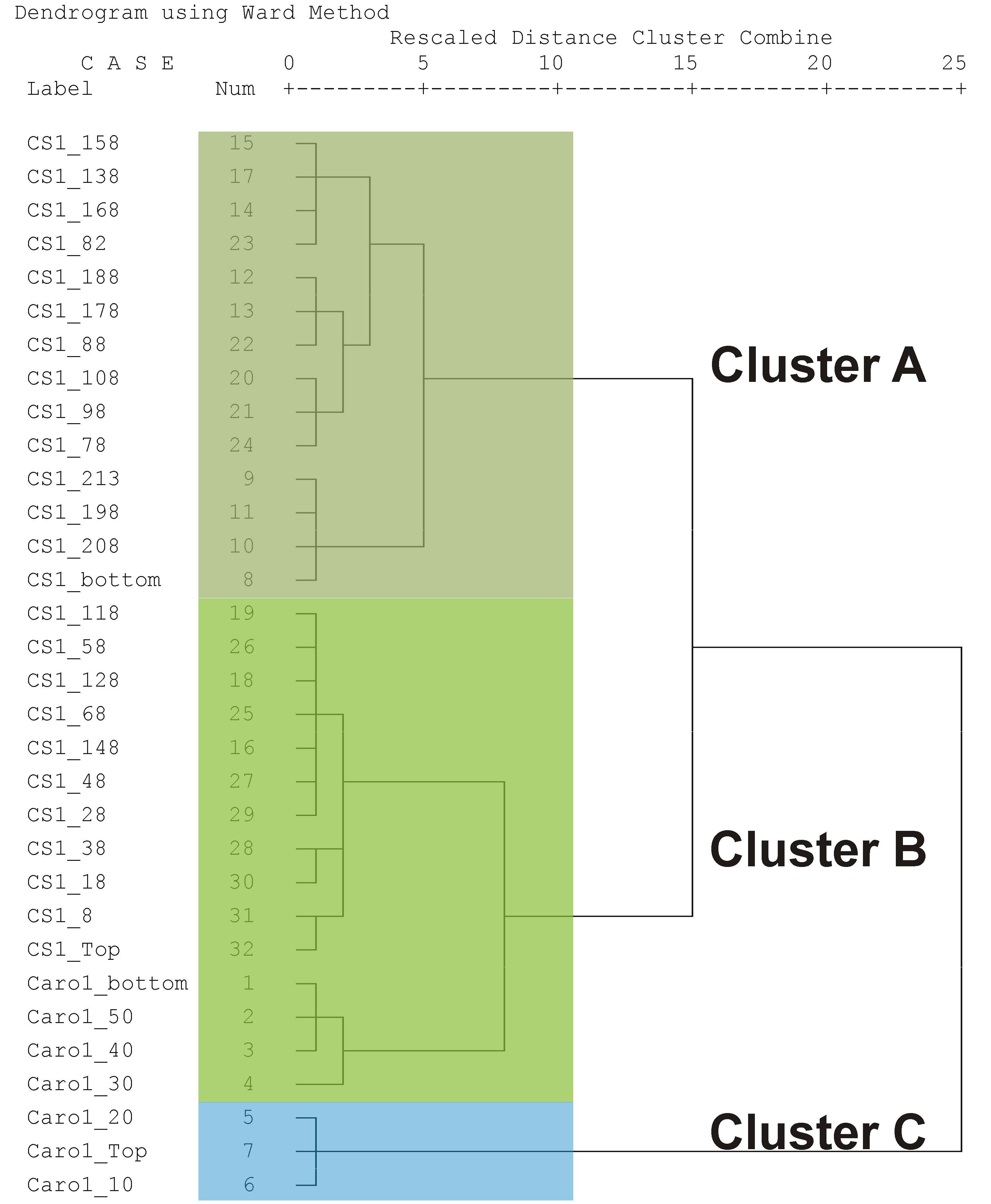
4.3. Benthic Foraminifera
4.4. Coralline Red Algae
4.5. Age Model and Sedimentation Rates
5. Discussion
5.1. Ecological Considerations Based on Benthic Foraminiferal Assemblages
5.2. Environmental Conditions Required for the Free-Living Red Algae Growth
5.3. Paleoenvironmental Reconstruction
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nelson, C.S. An introductory perspective on non-tropical shelf carbonates. Sed. Geol. 1988, 60, 3–12. [Google Scholar] [CrossRef]
- Tropeano, M.; Spalluto, L. Present-day temperate-type carbonate sedimentation on Apulia shelves (southern Italy). Geoacta 2006, 5, 129–142. [Google Scholar]
- James, N.P. The cool-water carbonate depositional realm. In Cool-Water Carbonates; James, N.P., Clarke, J.A.D., Eds.; SEPM Special Publications: Broken Arrow, OK, USA, 1997; Volume 56, pp. 1–20. [Google Scholar]
- Betzler, C.; Brachert, T.C.; Nebelsick, J. The warm temperate carbonate province. A review of the facies, zonations, and delimitations. Cour. Forsch. Senckenberg 1997, 201, 83–99. [Google Scholar]
- Pérès, J.M.; Picard, J. Nouveau manuel de bionomie marine benthique de la Mer Méditerranée. Rec. Trav. Stat. Mar. Endoume 1964, 31, 5–138. [Google Scholar]
- Carannante, G.; Esteban, M.; Milliman, J.D.; Simone, L. Carbonate lithofacies as paleolatitude indicators: Problems and limitations. Sediment. Geol. 1988, 60, 333–346. [Google Scholar] [CrossRef]
- Milker, Y.; Schmiedl, G.; Betzler, C.; Römer, M.; Jaramillo-Vogel, D.; Siccha, M. Distribution of recent benthic foraminifera in shelf carbonate environments of the Western Mediterranean Sea. Mar. Micropaleontol. 2009, 73, 207–225. [Google Scholar] [CrossRef]
- Martorelli, E.; Falese, F.; Chiocci, F.L. Overview of the variability of Late Quaternary continental shelf deposits of the Italian peninsula. In Continental Shelves of the World: Their Evolution During the Last Glacio-Eustatic Cycle; Chiocci, F.L., Chivas, A.R., Eds.; Geological Society Memoirs: London, UK, 2014; Volume 41, pp. 171–186. [Google Scholar]
- Colantoni, P.; Cremona, G.; Ligi, M.; Borsetti, A.M.; Cati, F. The Adventure Bank (off Southwestern Sicily): A present day example of carbonate shelf sedimentation. Giorn. Geol. 1985, 47, 165–180. [Google Scholar]
- Corselli, C.; Basso, D.; Garzanti, E. Paleobiological and sedimentological evidence of Pleistocene/Holocene hiatuses and ironstone formation at the Pontian islands shelfbreak (Italy). Mar. Geol. 1994, 117, 317–328. [Google Scholar] [CrossRef]
- Basso, D. Deep rhodolith distribution in the Pontian Islands, Italy: A model for the paleoecology of a temperate sea. Palaeogeogr. Palaeoclim. Palaeoecol. 1998, 137, 173–187. [Google Scholar] [CrossRef]
- Toscano, F.; Sorgente, B. Rhodalgal-Bryomol temperate carbonates from the Apulian Shelf (Southeastern Italy), relict and modern deposits on a current dominated shelf. Facies 2002, 46, 103–118. [Google Scholar] [CrossRef]
- Lecca, L.; De Muro, S.; Cossellu, M.; Pau, M. I sedimenti terrigeno-carbonatici attuali della piattaforma continentale del Golfo di Cagliari. Il Quat. 2005, 18, 201–221. [Google Scholar]
- Toscano, F.; Vigliotti, M.; Simone, L. Variety of coralline algal deposits (rhodalgal facies) from the Bays of Naples and Pozzuoli (northern Tyrrhenian Sea, Italy). In Cool-Water Carbonates: Depositional Systems and Palaeoenvironmental Controls; Pedley, H.M., Carannante, G., Eds.; Geological Society Special Publication: London, UK, 2006; Volume 255, pp. 85–94. [Google Scholar]
- Brandano, M.; Civitelli, G. Non-seagrass meadow sedimentary facies of the Pontinian Islands, Tyrrhenian Sea: A modern example of mixed carbonate-siliciclastic sedimentation. Sediment. Geol. 2007, 201, 286–301. [Google Scholar] [CrossRef]
- Bracchi, V.A.; Basso, D. The contribution of calcareous algae to the biogenic of the continental shelf: Pontian Islands, Tyrrhenian sea, Italy. Geodiversitas 2012, 34, 61–76. [Google Scholar] [CrossRef]
- Gaglianone, G.; Brandano, M.; Mateu-Vicens, G. The sedimentary facies of Posidonia oceanica seagrass meadows from the central Mediterranean Sea. Facies 2017, 63, 1–21. [Google Scholar] [CrossRef]
- Frezza, V.; Carboni, M.G.; Matteucci, R. Recent foraminiferal assemblages near Ponza Island (Central Tyrrhenian Sea, Italy). Boll. Soc. Paleontol. Ital. 2005, 44, 155–173. [Google Scholar]
- Frezza, V.; Pignatti, J.S.; Matteucci, R. Benthic foraminiferal biofacies in temperate carbonate sediment in the Western Pontine Archipelago (Tyrrhenian Sea, Italy). J. Foraminifer. Res. 2010, 40, 313–326. [Google Scholar] [CrossRef]
- Frezza, V.; Baldassarre, A.; Gaglianone, G.; Mateu-Vicens, G.; Brandano, M. Mixed carbonate-siliclastic sediments and benthic foraminiferal assemblages from Posidonia oceanica seagrass meadows of the central Tyrrhenian continental shelf (Latium, Italy). Ital. J. Geosci. 2011, 130, 352–369. [Google Scholar]
- Martorelli, E.; D’Angelo, S.; Fiorentino, A.; Chiocci, F.L. Nontropical Carbonate Shelf Sedimentation. The Archipelago Pontino (Central Italy) Case History. In Seafloor Geomorphology as Benthic Habitat; Harris, P.T., Baker, E.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 449–456. [Google Scholar]
- Sañé, E.; Chiocci, F.L.; Basso, D.; Martorelli, E. Environmental factors controlling the distribution of rhodoliths: An integrated study based on seafloor sampling, ROV and side scan sonar data, offshore the W-Pontine Archipelago. Cont. Shelf Res. 2016, 129, 10–22. [Google Scholar] [CrossRef]
- Ingrassia, M.; Martorelli, E.; Sañé, E.; Falese, F.G.; Bosman, A.; Bonifazi, A.; Argenti, L.; Chiocci, F.L. Coralline algae on hard and soft substrata of a temperate mixed siliciclastic-carbonatic platform: Sensitive assemblages in the Zannone area (western Pontine Archipelago; Tyrrhenian Sea). Mar. Environ. Res. 2019, 147, 1–12. [Google Scholar] [CrossRef]
- Council Directive. Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Off. J. Eur. Communities Legis. 1992, 206, 7–50. [Google Scholar]
- Wilson, S.; Blake, C.; Berges, J.A.; Maggs, C.A. Environmental tolerances of free-living coralline algae (maerl): Implications for European marine conservation. Biol. Conserv. 2004, 120, 279–289. [Google Scholar] [CrossRef]
- Curiel, D.; Rismondo, A.; Falace, A.; Kaleb, S. Affioramenti rocciosi sommersi (tegnùe) e la Rete Natura 2000: Possibili S.I.C. marini per il Nord Adriatico. Biol. Mar. Mediterr. 2009, 16, 103–106. [Google Scholar]
- Van der Heijden, L.H.; Kamenos, N.A. Reviews and syntheses: Calculating the global contribution of coralline algae to total carbon burial. Biogeosciences 2015, 12, 6429–6441. [Google Scholar] [CrossRef]
- Hill, R.; Bellgrove, A.; Macreadie, P.I.; Petrou, K.; Beardall, J.; Steven, A.; Ralph, P.J. Can macroalgae contribute to blue carbon? An Australian perspective. Limnol. Oceanogr. 2015, 60, 1689–1706. [Google Scholar] [CrossRef]
- Macreadie, P.I.; Jarvis, J.; Trevathan-Tackett, S.M.; Bellgrove, A. Seagrasses and macroalgae: Importance, vulnerability and impacts. In Climate Change Impacts on Fisheries and Aquaculture: A Global Analysis; Phillips, B.F., Pérez-Ramírez, M., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2017; pp. 729–770. [Google Scholar]
- Chiocci, F.L.; Martorelli, E. Carta Geologica D’Italia Alla Scala 1:50.000. F° 412. Borgo Grappa Isole Ponziane; Geologico d’Italia: Catania, Italy, 2018; p. 413. [Google Scholar]
- Basso, D.; Morbioli, C.; Corselli, C. Rhodolith facies evolution and burial as a response to Holocene transgression at the Pontian Islands shelf break. In Cool-Water Carbonates: Depositional Systems and Palaeoenvironmental Controls; Pedley, H.M., Carannante, G., Eds.; Geological Society Special Publication: London, UK, 2006; Volume 255, pp. 23–34. [Google Scholar]
- Ragazzola, F.; Caragnano, A.; Basso, D.; Schmidt, D.N.; Fietzke, J. Establishing temperate crustose early Holocene coralline algae as archives for palaeoenvironmental reconstructions of the shallow water habitats of the Mediterranean Sea. Palaeontology 2020, 63, 155–170. [Google Scholar] [CrossRef]
- Conti, M.A.; Girasoli, D.E.; Frezza, V.; Conte, A.M.; Martorelli, E.; Matteucci, R.; Chiocci, F.L. Repeated events of hardground formation and colonisation by endo-epilithozoans on the sediment-starved Pontine continental slope (Tyrrhenian Sea, Italy). Mar. Geol. 2013, 336, 184–197. [Google Scholar] [CrossRef]
- De Rita, D.; Funiciello, R.; Pantosti, D.; Salvini, F.; Sposato, A.; Velonà, M. Geological and structural characteristics of the Pontine Islands (Italy) and implications with the evolution of the tyrrhenian margin. Mem. Soc. Geol. Ital. 1986, 36, 55–65. [Google Scholar]
- Marani, M.; Zitellini, N. Rift structures and wrench tectonics along the continental slope between Civitavecchia and C. Circeo. Mem. Soc. Geol. Ital. 1986, 35, 453–457. [Google Scholar]
- Bellucci, F.; Lirer, L.; Munno, R. Geology of Ponza, Ventotene and Santo Stefano islands (with a 1:15,000 scale geological map). Acta Vulcanol. 1999, 11, 197–222. [Google Scholar]
- Conte, A.M.; Dolfi, D. Petrological and geochemical characteristics of Plio-Pleistocene Volcanics from Ponza Island (Tyrrhenian Sea, Italy). Mineral. Pet. 2002, 74, 75–94. [Google Scholar]
- Cadoux, A.; Pinti, D.L.; Aznar, C.; Chiesa, S.; Gillot, P.Y. New chronological and geochemical contraints on the genesis and geological evolution of Ponza and Palmarola Volcanic Islands (Tyrrhenian Sea, Italy). Lithos 2005, 81, 121–151. [Google Scholar] [CrossRef]
- Conte, A.M.; Perinelli, C.; Bianchini, G.; Natali, C.; Martorelli, E.; Chiocci, F.L. New insights on the petrology of submarine volcanics from the Western Pontine Archipelago (Tyrrhenian Sea, Italy). J. Volcanol. Geotherm. Res. 2016, 327, 223–239. [Google Scholar] [CrossRef]
- Chiocci, F.L.; Orlando, L. Lowstand terraces on Tyrrhenian Sea steep continental slopes. Mar. Geol. 1996, 134, 127–143. [Google Scholar] [CrossRef]
- Ingrassia, M.; Martorelli, E.; Bosman, A.; Macelloni, L.; Sposato, A.; Chiocci, F.L. The Zannone Giant Pockmark: First evidence of a giant complex seeping structure in shallow-water, central Mediterranean Sea, Italy. Mar. Geol. 2015, 363, 28–51. [Google Scholar] [CrossRef]
- Martorelli, E.; Italiano, F.; Ingrassia, M.; Macelloni, L.; Bosman, A.; Conte, A.M.; Beaubien, S.E.; Graziani, S.; Sposato, A.; Chiocci, F.L. Evidence of a shallow water submarine hydrothermal field off Zannone Island from morphological and geochemical characterization: Implications for Tyrrhenian Sea Quaternary volcanism. J. Geophys. Res. Solid Earth 2016, 121, 8396–8414. [Google Scholar] [CrossRef]
- Rastelli, E.; Corinaldesi, C.; Dell’Anno, A.; Tangherlini, M.; Martorelli, E.; Ingrassia, M.; Chiocci, F.L.; Lo Martire, M.; Danovaro, R. High potential for temperate viruses to drive carbon cycling in chemoautotrophy-dominated shallow-water hydrothermal vents. Environ. Microbiol. 2017, 19, 4432–4446. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, L.; Ingrassia, M.; Frezza, V.; Chiocci, F.L.; Martorelli, E. The response of benthic meiofauna to hydrothermal emissions in the Pontine Archipelago, Tyrrhenian Sea (central Mediterranean Basin). J. Mar. Syst. 2016, 164, 53–66. [Google Scholar] [CrossRef]
- Di Bella, L.; Ingrassia, M.; Frezza, V.; Chiocci, F.L.; Pecci, R.; Bedini, R.; Martorelli, E. Spiculosiphon oceana (Foraminifera) a new bio-indicator of acidic environments related to fluid emissions of the Zannone Hydrothermal Field (central Tyrrhenian Sea). Mar. Environ. Res. 2018, 136, 89–98. [Google Scholar] [CrossRef]
- Martorelli, E.; Chiocci, F.L.; Civitelli, G.; Chimenz, C.; Ventura, G.; Altobelli, C.; Balocco, A.; Bosman, A.; Cassata, L.; Raspagliosi, M. Mid-latitude carbonate sedimentation on a volcanic island shelf (Pontine Islands, Tyrrhenian Sea). In Proceedings of the Abstracts Volume of 2nd IGCP464 Annual Conference, San Paulo/Cananeia, Brasil, 30 August–3 September 2002; p. 1. [Google Scholar]
- Chiocci, F.L.; Martorelli, E.; Bosman, A. Cannibalization of a continental margin by regional scale mass wasting: An example from the central Tyrrhenian Sea. In Submarine Mass Movements and Their Consequences; Locat, J., Mienert, J., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; pp. 409–416. [Google Scholar]
- Ardizzone, G.D.; Belluscio, A. Le praterie di Posidonia oceanica lungo le coste laziali. In Il Mare del Lazio. Oceanografia Fisica e Chimica, Biologia e Geologia Marina, Clima Meteomarino, Dinamica dei Sedimenti e apporti Continentali; Università degli Studi di Roma “La Sapienza”, Regione Lazio, Assessorato Opere e Reti di Servizi e Mobilità: Roma, Italy, 1996; pp. 194–217. [Google Scholar]
- Mateu-Vicens, G.; Brandano, M.; Gaglianone, G.; Baldassarre, A. Seagrass-meadow sedimentary facies in a mixed siliciclastic-carbonate temperate system in the Tyrrhenian Sea (Pontinian Islands, western Mediterranean). J. Sediment. Res. 2012, 82, 451–463. [Google Scholar] [CrossRef]
- Schönfeld, J.; Alve, E.; Geslin, E.; Jorissen, F.; Korsun, S.; Spezzaferri, S. Members of the FOBIMO group, 2012. The FOBIMO (FOraminiferal BIo-MOnitoring) initiative–towards a standardized protocol for soft-bottom benthic foraminiferal monitoring studies. Mar. Micropaleontol. 2012, 94, 1–13. [Google Scholar] [CrossRef]
- Van der Zwaan, G.J.; Jorissen, F.J.; De Stigter, H.C. The depth dependency of planktonic/benthic foraminiferal ratios: Constraints and applications. Mar. Geol. 1990, 95, 1–16. [Google Scholar] [CrossRef]
- Bressan, G.; Babbini, L. Corallinales del mar Mediterraneo: Guida alla determinazione. Biol. Mar. Mediterr. 2003, 10, 237. [Google Scholar]
- Loeblich, A.R., Jr.; Tappan, H. Foraminiferal Genera and their Classification; Van Nostrand Reinhold Company: New York, NY, USA, 1987; p. 970. [Google Scholar]
- Cimerman, F.; Langer, M.R. Mediterranean Foraminifera. Acad. Sci. Artium Slov. 1991, 30, 1–118. [Google Scholar]
- Sgarrella, F.; Moncharmont Zei, M. Benthic Foraminifera of the Gulf of Naples (Italy): Systematics and autoecology. Boll. Soc. Paleontol. Ital. 1993, 32, 145–264. [Google Scholar]
- Fiorini, F.; Vaiani, S.C. Benthic foraminifers and transgressive-regressive cycles in the Late Quaternary subsurface sediments of the Po Plain near Ravenna (Northern Italy). Boll. Soc. Paleontol. Ital. 2001, 40, 357–403. [Google Scholar]
- Fisher, R.A.; Corbet, A.S.; Williams, C.B. The relationship between the number of species and the number of individuals in a random sample of an animal population. J. Anim. Ecol. 1943, 12, 42–58. [Google Scholar] [CrossRef]
- Murray, J.W. Ecology and Paleoecology of Benthic Foraminifera; Longman Scientific and Technical: New York, NY, USA, 1991; p. 312. [Google Scholar]
- Murray, J.W. Ecology and Applications of Benthic Foraminifera; Cambridge University Press: Cambridge, UK, 2006; p. 426. [Google Scholar]
- Walton, W.R. Recent foraminiferal ecology and paleoecology. In Approaches to Paleoecology; Imbrie, J., Newell, N.D., Eds.; John Wiley: New York, NY, USA, 1964; pp. 151–237. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Kovach, W.L. Multivariate methods of analyzing paleoecological data. Paleontol. Soc. Spec. Publ. 1987, 3, 72–104. [Google Scholar] [CrossRef]
- Kovach, W.L. Comparisons of multivariate analytical techniques for use in Pre-Quaternary plant paleoecology. Rev. Palaeobot. Palynol. 1989, 60, 255–282. [Google Scholar] [CrossRef]
- Parker, W.C.; Arnold, A.J. Quantitative methods of analysis in foraminiferal ecology. In Modern Foraminifera; Sen Gupta, B.K., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1999; pp. 71–89. [Google Scholar]
- Stuiver, M.; Reimer, P.J. Extended 14C database and revised CALIB radiocarbon calibration program. Radiocarbon 1993, 35, 215–230. [Google Scholar] [CrossRef]
- Lambeck, K.; Antonioli, F.; Anzidei, M.; Ferranti, L.; Leoni, G.; Scicchitano, G.; Silenzi, S. Sea level change along the Italian coast during the Holocene and projections for the future. Quat. Int. 2011, 232, 250–257. [Google Scholar] [CrossRef]
- Giaccone, G.; Giaccone, T.; Catra, M. Maërl or free calcareous Algae (Melobesiae) facies (association with Lithothamnion corallioides and Phymatolithon calcareum: Phymatolitho-Lithothamnietum corallioidis Giaccone 1965). In Priority Habitats According to the SPA/BIO Protocol (Barcelona Convention) Present in Italy. Identification Sheets; Relini, G., Giaccone, G., Eds.; Italian National Research Council: Rome, Italy, 2009; Volume 16, pp. 122–126. [Google Scholar]
- Jorissen, F.J. The distribution of benthic foraminifera in the Adriatic Sea. Mar. Micropaleontol. 1987, 12, 21–48. [Google Scholar] [CrossRef]
- Langer, M.R. Recent epiphytic foraminifera from Vulcano (Mediterranean Sea). Rev. Paléobiol. Vol. Spéc. 1988, 2, 827–832. [Google Scholar]
- Langer, M.R. Epiphytic foraminifera. Mar. Micropaleontol. 1993, 20, 235–265. [Google Scholar] [CrossRef]
- Jorissen, F.J. Benthic Foraminifera from the Adriatic Sea; principles of phenotypic variation. Utrecht Micropaleontol. Bull. 1988, 37, 1–174. [Google Scholar]
- Moulfi-El-Houari, L.; Ambroise, D.; Mathieu, R. Distribution of recent Benthic Foraminifera of the continental margin of Algeria (Bou-Ismaïl Bay). Rev. De Micropaléontol. 1999, 42, 315–327. [Google Scholar] [CrossRef]
- Ferraro, L.; Alberico, I.; Lirer, F.; Vallefuoco, M. Distribution of benthic foraminifera from the southern Tyrrhenian continental shelf (South Italy). Rend. Lincei 2012, 23, 103–119. [Google Scholar] [CrossRef]
- Vénec-Peyré, M.T.; Le Calvez, Y. Les Foraminifères épiphytes de l’herbier de Posidonies de Banyuls-sur-Mer (Méditerranée occidentale) étude des variations spatiotemporelles du peuplement. Cah. Micropaléont. 1988, 3, 21–40. [Google Scholar]
- Frezza, V.; Carboni, M.G. Distribution of Recent foraminiferal assemblages near the Ombrone River mouth (northern Tyrrhenian Sea, Italy). Rev. De Micropaléontol. 2009, 52, 43–66. [Google Scholar] [CrossRef]
- Buosi, C.; Armynot Du Châtelet, E.; Cherchi, A. Benthic foraminiferal assemblages in the current-dominated Strait of Bonifacio (Mediterranean Sea). J. Foraminifer. Res. 2012, 42, 39–55. [Google Scholar] [CrossRef]
- Benedetti, A.; Frezza, V. Benthic foraminiferal assemblages from shallow-water environments of northeastern Sardinia (Italy, Mediterranean Sea). Facies 2016, 62, 1–17. [Google Scholar] [CrossRef]
- Ribes, T.; Gracia, M.P. Foraminifères des herbiers de posidonies de la Méditerranée occidentale. Vie Et Milieu 1991, 41, 117–126. [Google Scholar]
- Langer, M.R.; Frick, H.; Silk, M.T. Photophile and sciaphile foraminiferal assemblages from marine plant communities of Lavezzi Islands (Corsica, Mediterranean Sea). Rev. Paléobiol. 1998, 17, 525–530. [Google Scholar]
- Mateu-Vicens, G.; Box, A.; Deudero, S.; Rodríguez, B. Comparative analysis of epiphytic foraminifera in sediments colonized by seagrass Posidonia oceanica and invasive macroalgae Caulerpa spp. J. Foraminifer. Res. 2010, 40, 134–147. [Google Scholar] [CrossRef]
- Mateu-Vicens, G.; Khokhlova, A.; Sebastián-Pastor, T. Epiphytic foraminiferal indices as bioindicators in Mediterranean seagrass meadows. J. Foraminifer. Res. 2014, 44, 325–339. [Google Scholar] [CrossRef]
- Carboni, M.G.; Frezza, V.; Bergamin, L. Distribution of Recent benthic foraminifers in the Ombrone River Basin (Tuscany Continental Shelf, Tyrrhenian Sea, Italy): Relations with fluvial run-off. In Proceedings in the 1st Italian Meeting on Environmental Micropaleontology; Coccioni, R., Galeotti, S., Lirer, F., Eds.; Grzybozsky Foundation Special Publication: Kraków, Poland, 2004; Volume 9, pp. 7–16. [Google Scholar]
- Basso, D.; Babbini, L.; Ramos-Esplá, A.A.; Salomidi, M. Mediterranean rhodolith beds. In Rhodolith/Maerl Beds: A Global Perspective; Riosmena-Rodríguez, R., Nelson, W., Aguirre, J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 281–298. [Google Scholar]
- Brandano, M.; Vannucci, G.; Mateu-Vicens, G. Le alghe rosse calcaree come indicatori paleoambientali: L’esempio della rampa carbonatica Laziale-Abruzzese (Burdigaliano, Appennino centrale). Boll. Soc. Geol. Ital. 2007, 126, 55–69. [Google Scholar]
- Bosence, D.W.; Pedley, H.M. Sedimentology and palaeoecology of a Miocene coralline algal biostrome from the Maltese islands. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1982, 38, 9–43. [Google Scholar] [CrossRef]
- Basso, D.; Favega, P.; Piazza, M.; Vannucci, G. Biostratigraphic, paleobiogeographic and paleoecological implications in the taxonomic review of Corallinaceae. Rend. Lincei 1998, 9, 201–211. [Google Scholar] [CrossRef]
- Melbourne, L.A.; Denny, M.W.; Harniman, R.L.; Rayfield, E.J.; Schmidt, D.N. The importance of wave exposure on the structural integrity of rhodoliths. J. Exp. Mar. Biol. Ecol. 2018, 503, 109–119. [Google Scholar] [CrossRef]
- Foster, M.S. Rhodoliths: Between rocks and soft places. J. Phycol. 2001, 37, 659–667. [Google Scholar] [CrossRef]
- Dutertre, M.; Grall, J.; Ehrhold, A.; Hamon, D. Environmental factors affecting maerl bed structure in Brittany (France). Eur. J. Phycol. 2015, 50, 371–383. [Google Scholar] [CrossRef]
- Steller, D.L.; Foster, M.S. Environmental factors influencing distribution and morphology of rhodoliths in Bahía Concepción, BCS, México. J. Exp. Mar. Biol. Ecol. 1995, 194, 201–212. [Google Scholar] [CrossRef]
- Cabioch, J. Le maërl des côtes de Bretagne et le problème de sa survie. Penn Ar Bed 1970, 7, 421–429. [Google Scholar]
- Basso, D.; Babbini, L.; Kaleb, S.; Bracchi, V.; Falace, A. Monitoring deep Mediterranean rhodolith beds. Aquat. Conserv. Mar. Freshwat. Ecosyst. 2016, 26, 549–561. [Google Scholar] [CrossRef]
- Hall-Spencer, J.M.; Moore, P.G. Scallop dredging has profound, long-term impacts on maërl habitats. Ices J. Mar. Sci. 2000, 57, 1407–1415. [Google Scholar] [CrossRef]
- Bellan-Santini, D. The Mediterranean benthos: Reflections and problems raised by a classification of the benthic assemblages. In Mediterranean Marine Ecosystems; Moraitou-Apostolopoulou, M., Kiortis, V., Eds.; Nato Conference Series; Springer: Boston, MA, USA, 1985; Volume 8, pp. 19–48. [Google Scholar]
- Bracchi, V.A.; Basso, D.; Savini, A.; Corselli, C. Algal reefs (Coralligenous) from glacial stages: Origin and nature of a submerged tabular relief (Hyblean Plateau, Italy). Mar. Geol. 2019, 411, 119–132. [Google Scholar] [CrossRef]

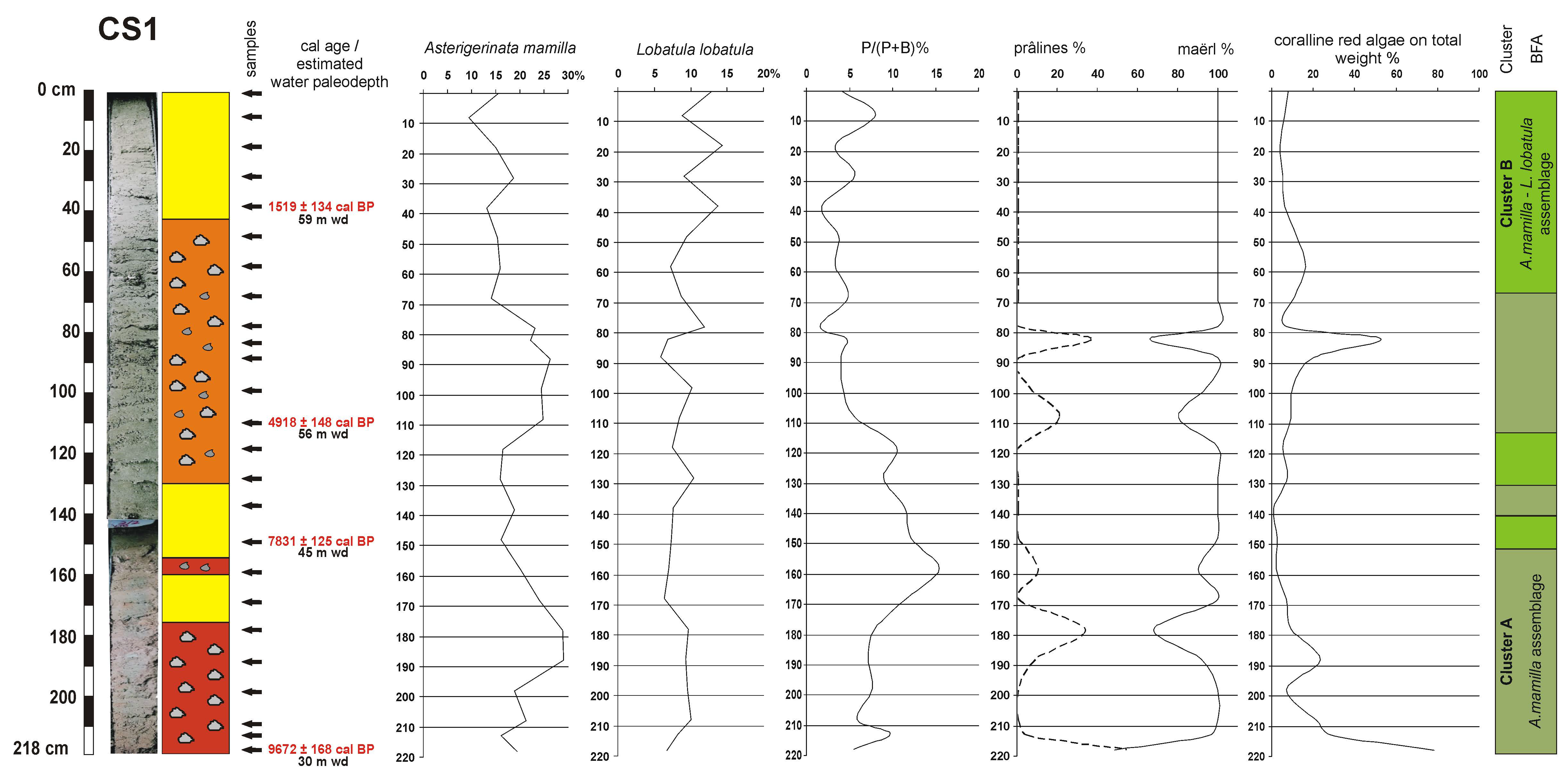
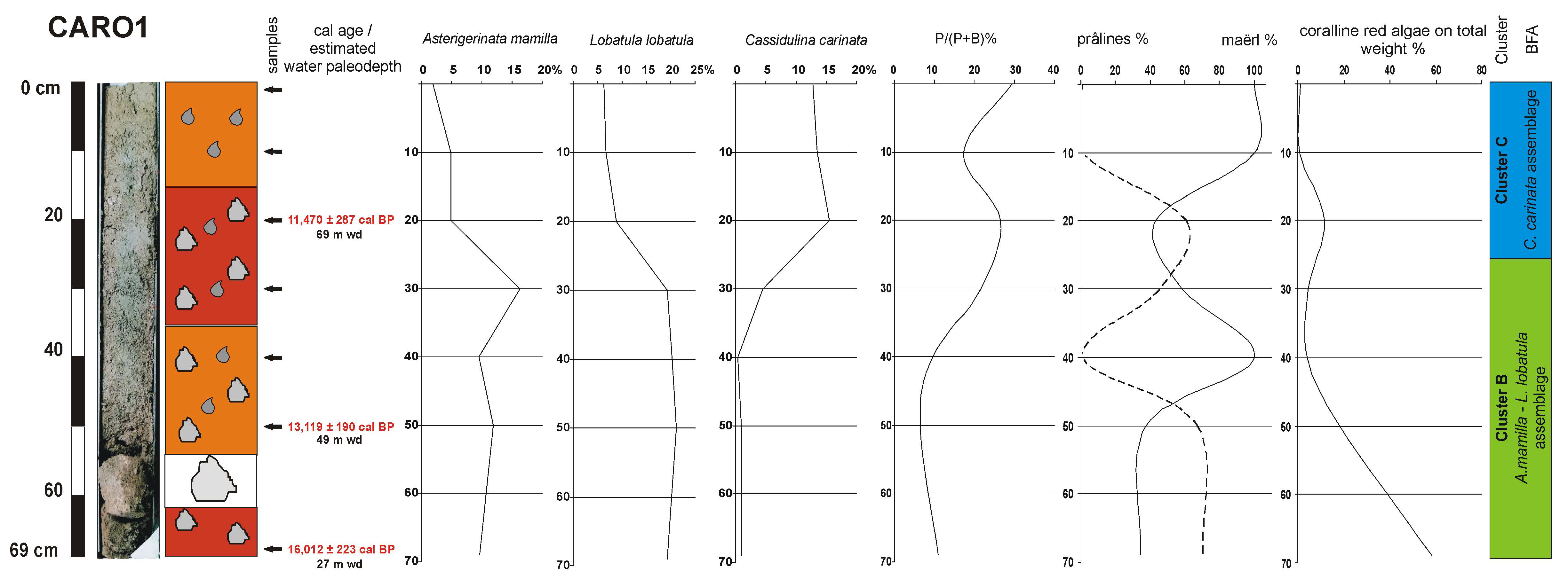
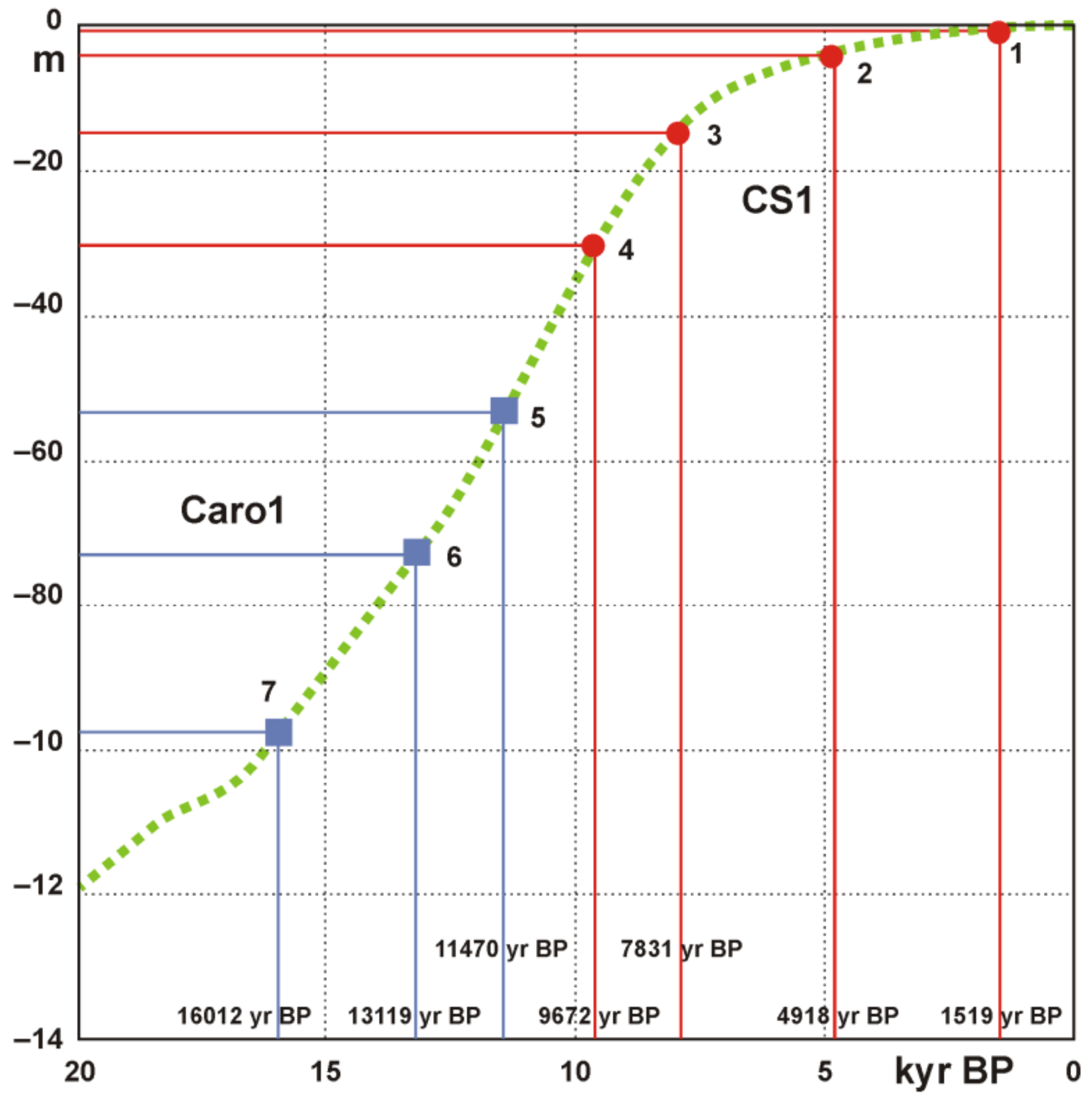
| Cores/Samples | Radiocarbon 14C Age (yr BP) | Calibrated 14C Age (yr BP, 2σ) |
|---|---|---|
| CS1 | ||
| 38 cm | 2014 ± 45 | 1519 ± 134 |
| 108 cm | 4712 ± 55 | 4918 ± 148 |
| 148 cm | 7431 ± 55 | 7831 ± 125 |
| 218 cm | 9024 ± 55 | 9672 ± 168 |
| Caro1 | ||
| 20 cm | 10399 ± 75 | 11470 ± 287 |
| 50 cm | 11702 ± 75 | 13119 ± 190 |
| 69 cm | 13771 ± 65 | 16012 ± 223 |
| Summary of Sample Characteristics | CS1 | Caro1 | Total (CS1 + Caro1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Median | Min | Max | Median | Min | Max | Median | |
| species number | 41 | 65 | 52 | 48 | 61 | 53 | 41 | 65 | 52 |
| α-Fisher index | 12.66 | 25.16 | 17.79 | 15.51 | 22.39 | 18.39 | 12.66 | 25.16 | 17.79 |
| Shannon index | 2.99 | 3.59 | 3.26 | 3.17 | 3.54 | 3.32 | 2.99 | 3.59 | 3.30 |
| Dominance% | 9.4 | 29.1 | 18.8 | 12.8 | 21.1 | 19.2 | 9.4 | 29.1 | 18.9 |
| P/(P + B)% | 1.5 | 15.4 | 5.6 | 6.3 | 29.5 | 17.3 | 1.5 | 29.5 | 7.4 |
| A. mamilla | 9.4 | 29.1 | 18.8 | 1.9 | 16.3 | 9.5 | 1.9 | 29.1 | 16.3 |
| C. carinata | 0.0 | 2.0 | 0.6 | 0.3 | 15.5 | 4.5 | 0.0 | 15.5 | 0.9 |
| L. lobatula | 5.9 | 14.4 | 8.8 | 6.3 | 21.1 | 19.2 | 5.9 | 21.1 | 9.0 |
| Q. stelligera | 0.0 | 10.3 | 1.6 | 0.0 | 3.7 | 1.6 | 0.0 | 10.3 | 1.6 |
| R. bradyi | 1.6 | 10.0 | 4.0 | 0.9 | 4.6 | 1.9 | 0.9 | 10.0 | 3.8 |
| Cluster | Values | A | B | C |
|---|---|---|---|---|
| Dominant species | A. mamilla (16.2–29.1%) | A. mamilla (9.4–18.7%) and L. lobatula (7.3–21.1%) | C. carinata (12.8–15.5%) | |
| Accompanying species | L. lobatula (5.9–11.9%), Q. stelligera (0–10.3%) | R. bradyi (1.0–10.0%), S. wrighti (1.9–8.5%) | C. crassa (4.6–9.7%), T. bocki (6.9–9.4%), L. lobatula (6.3–8.9%), S. wrighti (2.6–6.3%) | |
| Species number | 41–56 | 48–65 | 49–61 | |
| α-Fisher Index | range | 12.66–20.13 | 15.51–25.16 | 16.38–22.39 |
| median | 16.98 | 19.53 | 22.35 | |
| Shannon Index | range | 2.99–3.37 | 3.17–3.59 | 3.32–3.54 |
| median | 3.23 | 3.37 | 3.46 | |
| Dominance% | range | 16.2–29.1 | 9.4–21.1 | 12.8–15.5 |
| median | 22.7 | 15.9 | 13.5 | |
| P/(P + B)% | range | 1.5–16.4 | 1.7–21.6 | 17.3–29.5 |
| median | 6.5 | 6.3 | 26.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frezza, V.; Argenti, L.; Bonifazi, A.; Chiocci, F.L.; Di Bella, L.; Ingrassia, M.; Martorelli, E. Benthic Foraminiferal Assemblages and Rhodolith Facies Evolution in Post-LGM Sediments from the Pontine Archipelago Shelf (Central Tyrrhenian Sea, Italy). Geosciences 2021, 11, 179. https://doi.org/10.3390/geosciences11040179
Frezza V, Argenti L, Bonifazi A, Chiocci FL, Di Bella L, Ingrassia M, Martorelli E. Benthic Foraminiferal Assemblages and Rhodolith Facies Evolution in Post-LGM Sediments from the Pontine Archipelago Shelf (Central Tyrrhenian Sea, Italy). Geosciences. 2021; 11(4):179. https://doi.org/10.3390/geosciences11040179
Chicago/Turabian StyleFrezza, Virgilio, Letizia Argenti, Andrea Bonifazi, Francesco L. Chiocci, Letizia Di Bella, Michela Ingrassia, and Eleonora Martorelli. 2021. "Benthic Foraminiferal Assemblages and Rhodolith Facies Evolution in Post-LGM Sediments from the Pontine Archipelago Shelf (Central Tyrrhenian Sea, Italy)" Geosciences 11, no. 4: 179. https://doi.org/10.3390/geosciences11040179
APA StyleFrezza, V., Argenti, L., Bonifazi, A., Chiocci, F. L., Di Bella, L., Ingrassia, M., & Martorelli, E. (2021). Benthic Foraminiferal Assemblages and Rhodolith Facies Evolution in Post-LGM Sediments from the Pontine Archipelago Shelf (Central Tyrrhenian Sea, Italy). Geosciences, 11(4), 179. https://doi.org/10.3390/geosciences11040179









