Dynamics of Stone Habitats in Coastal Waters of the Southwestern Baltic Sea (Hohwacht Bay)
Abstract
:1. Introduction
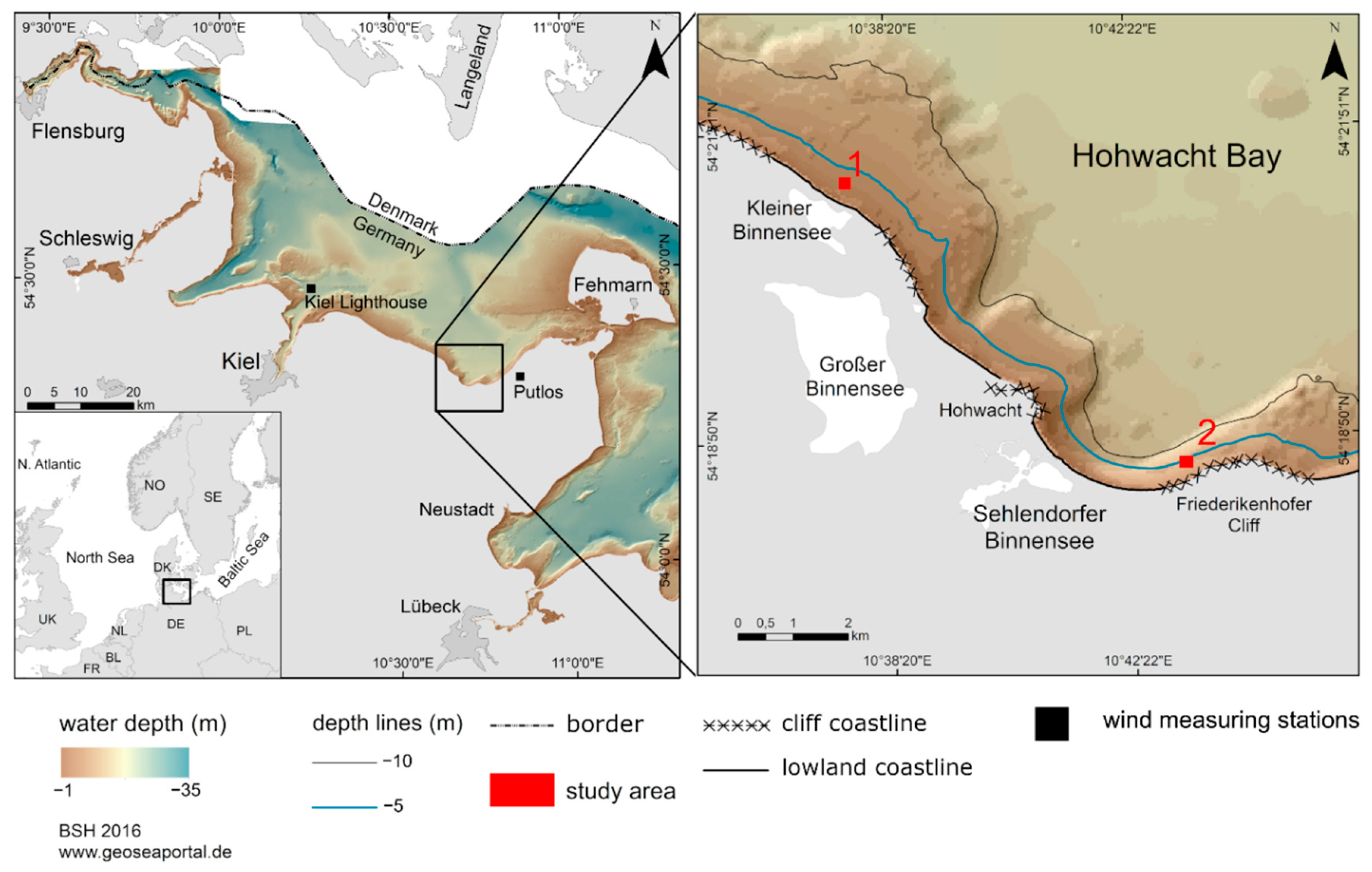
2. Regional Setting
3. Materials and Methods
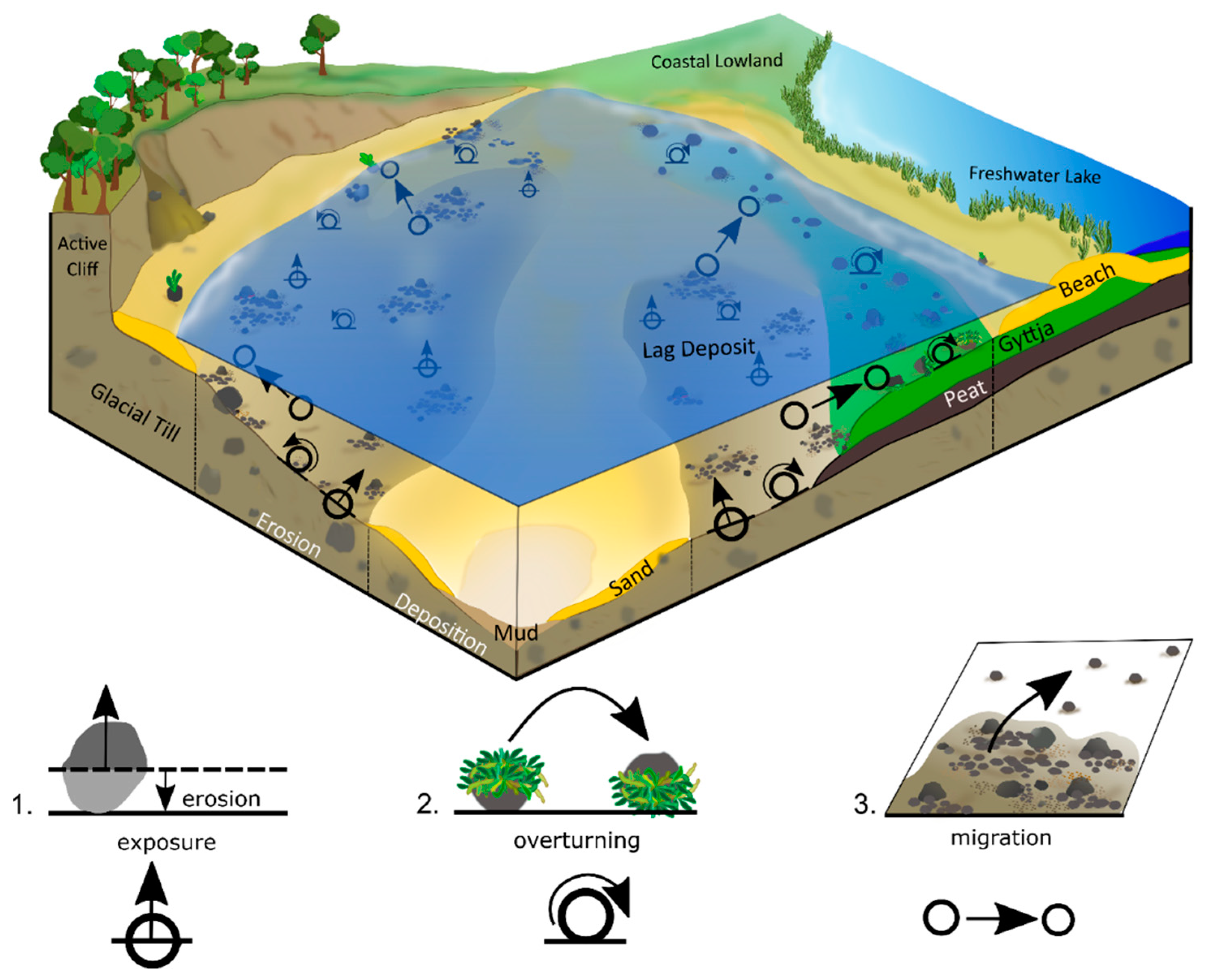
3.1. Area of Investigation
3.2. Hydroacoustic Surveys
3.3. Sediment Samples
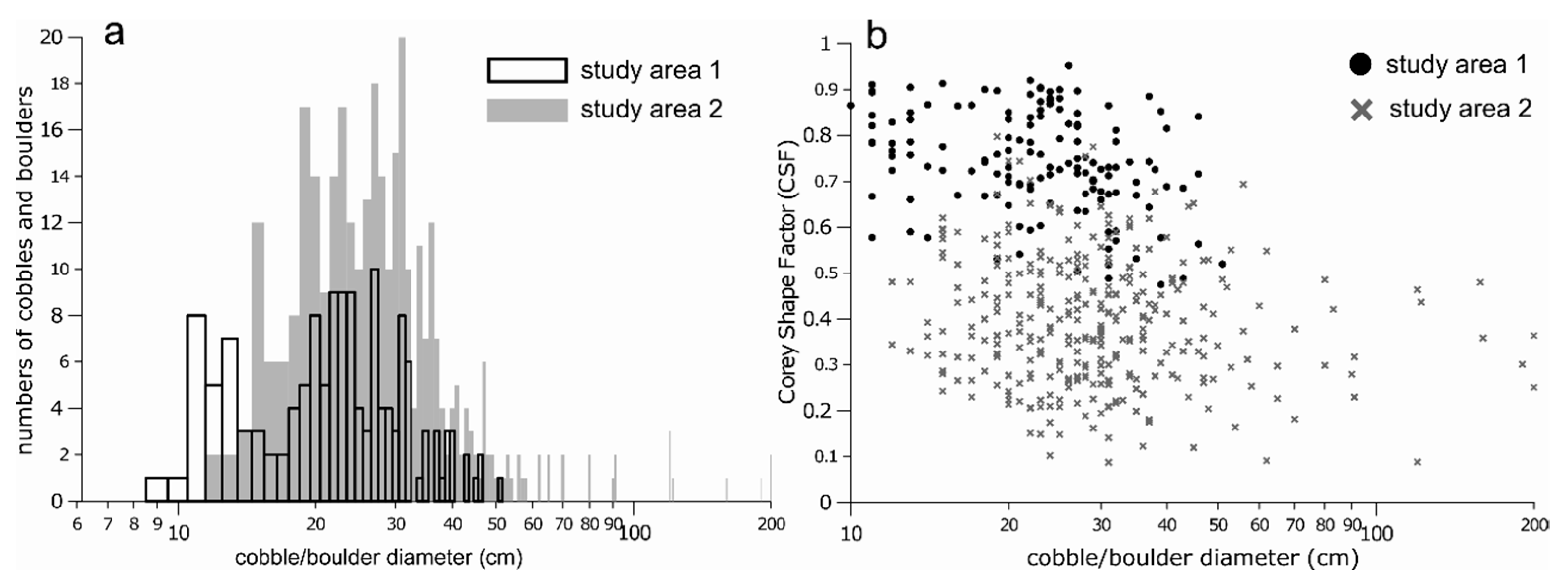
3.4. Size and Roundness of Individual Cobbles and Boulders
3.5. Wave Data
3.6. Biological Sampling and Statistical Analysis
3.7. Underwater Image Enhancement
4. Results
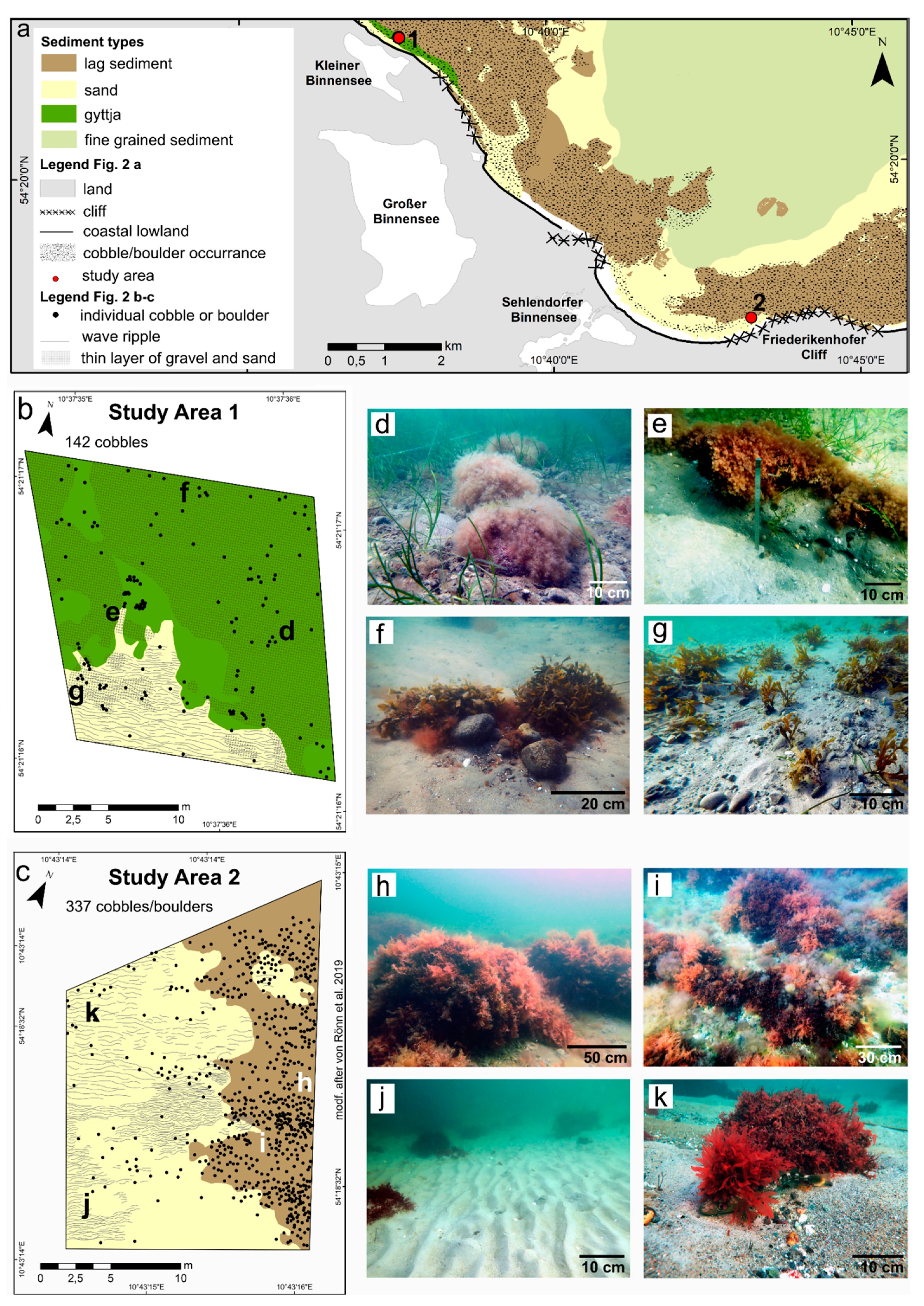
4.1. Sediment Types and Distribution
4.2. Physical Habitat Structure
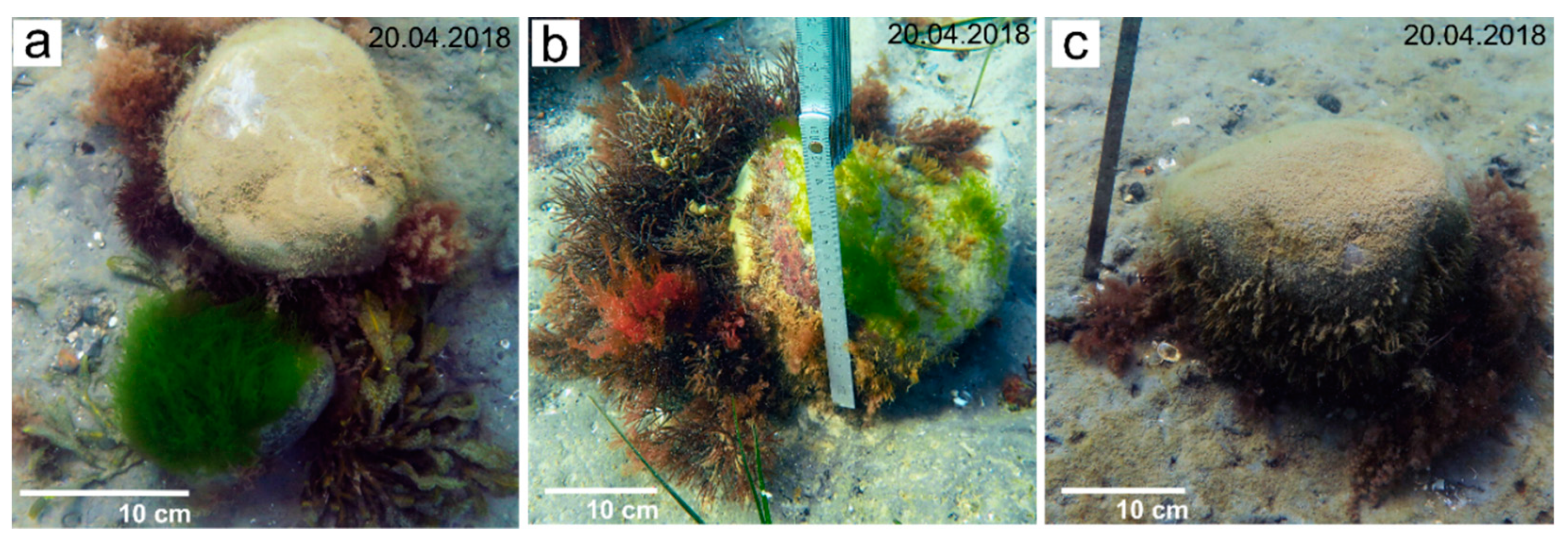
4.3. Benthic Community Structure
4.4. Wind and Wave Conditions during Field Campaigns
Observation Periods
4.5. Overturning of Cobbles
5. Discussion
5.1. Physical Habitat Structure
5.2. Benthic Community Structure
5.3. Exposure of New Cobbles and Boulders
5.4. Overturning of Cobbles
5.5. Migration of Cobbles
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liversage, K. The influence of boulder shape on the spatial distribution of crustose coralline algae (Corallinales, Rhodophyta). Mar. Ecol. 2016, 37, 459–462. [Google Scholar] [CrossRef]
- Lefcheck, J.S.; Hughes, B.B.; Johnson, A.J.; Pfirrmann, B.W.; Rasher, D.B.; Smyth, A.R.; Williams, B.L.; Beck, M.W.; Orth, R.J. Are coastal habitats important nurseries? A meta-analysis. Conserv. Lett. 2019, 12, e12645. [Google Scholar] [CrossRef]
- Gallucci, F.; Christofoletti, A.R.; Fonseca, G.; Dias, M.G. The effects of habitat heterogeneity at distinct spatial scales on hard-bottom-associated communities. Diversity 2020, 12, 39. [Google Scholar] [CrossRef] [Green Version]
- Kolp, O. Die sedimente der westlichen und südlichen ostsee und ihre darstellung. Beiträge Meereskd. 1966, 17–18, 9–60. [Google Scholar]
- Sebens, K.P. Habitat structure and community dynamics in marine benthic systems. In Habitat Structure: The Physical Arrangement of Objects in Space; Bell, S.S., McCoy, E.D., Mushinsky, H.R., Eds.; Population and Community Biology Series; Springer: Dordrecht, The Netherlands, 1991; pp. 211–234. ISBN 978-94-011-3076-9. [Google Scholar]
- Archambault, P.; Bourget, E. Scales of coastal heterogeneity and benthic intertidal species richness, diversity and abundance. Mar. Ecol. Prog. Ser. 1996, 136, 111–121. [Google Scholar] [CrossRef]
- Kovalenko, K.E.; Thomaz, S.M.; Warfe, D.M. Habitat complexity: Approaches and future directions. Hydrobiologia 2012, 685, 1–17. [Google Scholar] [CrossRef]
- Mikkelsen, L.; Mouritsen, K.; Dahl, J.; Teilmann, J.; Tougaard, J. Re-established stony reef attracts harbour porpoises phocoena phocoena. Mar. Ecol. Prog. Ser. 2013, 238–248. [Google Scholar] [CrossRef] [Green Version]
- Kristensen, L.D.; Støttrup, J.G.; Svendsen, J.C.; Stenberg, C.; Hansen, O.K.H.; Grønkjær, P. Behavioural changes of atlantic cod (gadus morhua) after marine boulder reef restoration: Implications for coastal habitat management and natura 2000 areas. Fish. Manag. Ecol. 2017, 24, 353–360. [Google Scholar] [CrossRef]
- Torn, K.; Herkül, K.; Martin, G.; Oganjan, K. Assessment of quality of three marine benthic habitat types in Northern Baltic Sea. Ecol. Indic. 2017, 73, 772–783. [Google Scholar] [CrossRef]
- Rönnbäck, P.; Kautsky, N.; Pihl, L.; Troell, M.; Söderqvist, T.; Wennhage, H. Ecosystem goods and services from swedish coastal habitats: Identification, valuation, and implications of ecosystem shifts. Ambio 2007, 36, 534–544. [Google Scholar] [CrossRef]
- Snoeijs-Leijonmalm, P.; Schubert, H.; Radziejewska, T. Biological Oceanography of the Baltic Sea; Springer Science & Business Media: Dordrecht, The Netherlands, 2017; ISBN 978-94-007-0668-2. [Google Scholar] [CrossRef]
- Lappalainen, J.; Virtanen, E.A.; Kallio, K.; Junttila, S.; Viitasalo, M. Substrate limitation of a habitat-forming genus fucus under different water clarity scenarios in the Northern Baltic Sea. Estuar. Coast. Shelf Sci. 2019, 218, 31–38. [Google Scholar] [CrossRef]
- Papenmeier, S.; Darr, A.; Feldens, P.; Michaelis, R. Hydroacoustic mapping of geogenic hard substrates: Challenges and review of german approaches. Geosciences 2020, 10, 100. [Google Scholar] [CrossRef] [Green Version]
- The European Parliament; The Council Of The European Union. Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008 Establishing a Framework for Community Action in the Field of Marine Environmental Policy (Marine Strategy Framework Directive). 2008. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32008L0056&from=en (accessed on 7 April 2021).
- The Council Of The European Communities. Directive 92/43/EEC on the Conservation of Natural Habitats and of Wild Fauna and Flora (Habitat Directive). 1992. Available online: http://extwprlegs1.fao.org/docs/pdf/eur34772.pdf (accessed on 7 April 2021).
- Winter, C. Monitoring concepts for an evaluation of marine environmental states in the german bight. Geo. Mar. Lett 2017, 37, 75–78. [Google Scholar] [CrossRef] [Green Version]
- Brown, E.J.; Vasconcelos, R.P.; Wennhage, H.; Bergström, U.; Støttrup, J.G.; van de Wolfshaar, K.; Millisenda, G.; Colloca, F.; Le Pape, O. Conflicts in the coastal zone: Human impacts on commercially important fish species utilizing coastal habitat. ICES J. Mar. Sci. 2018, 75, 1203–1213. [Google Scholar] [CrossRef] [Green Version]
- Bertocci, I.; Dell’Anno, A.; Musco, L.; Gambi, C.; Saggiomo, V.; Cannavacciuolo, M.; Martire, M.L.; Passarelli, A.; Zazo, G.; Danovaro, R. Multiple human pressures in coastal habitats: Variation of meiofaunal assemblages associated with sewage discharge in a post-industrial area. Sci. Total Environ. 2019, 655, 1218–1231. [Google Scholar] [CrossRef]
- Bock, G.; Thiermann, F.; Rumohr, H.; Karez, R. Ausmaß der Steinfischerei an der schleswig-holsteinischen Ostseeküste. Jahresber. Landesamt Natur Umwelt Landes Schleswig-Holst. 2003, 8, 111–116. [Google Scholar]
- Karez, R.; Schories, D. Die Steinfischerei und ihre Bedeutung für die Wiederansiedlung von Fucus vesiculosus in der Tiefe. Rostocker Meeresbiol. Beiträge 2005, 14, 95–107. [Google Scholar]
- Schwarzer, K.; Bohling, B.; Heinrich, C. Submarine hard-bottom substrates in the Western Baltic Sea—Human impact versus natural development. J. Coast. Res. 2014, 70, 145–150. [Google Scholar] [CrossRef]
- Niedermeyer, R.-O.; Lampe, R.; Jahnke, W.; Schwarzer, K.; Duphorn, K.; Kliewe, H.; Werner, F. Die Deutsche Ostseeküste; Gebr. Borntraeger: Stuttgart, Germany, 2011; Volume 105, p. 377. ISBN 978-3443-15091-4. [Google Scholar]
- Diesing, M.; Schwarzer, K. Identification of submarine hard-bottom substrates in the German North Sea and Baltic Sea EEZ with high-resolution acoustic seafloor imaging. In Progress in Marine Conservation in Europe; Springer: Berlin/Heidelberg, Germany, 2006; pp. 111–125. [Google Scholar]
- Bohling, B.; May, H.; Mosch, T.; Schwarzer, K. Regeneration of submarine hard-bottom substrate by natural abrasion in the Western Baltic Sea. Marbg. Geogr. Schr. 2009, 145, 66–79. [Google Scholar]
- Papenmeier, S.; Hass, H.; Papenmeier, S.; Hass, H.C. Detection of stones in marine habitats combining simultaneous hydroacoustic surveys. Geosciences 2018, 8, 279. [Google Scholar] [CrossRef] [Green Version]
- Werner, F. Sedimentation und abrasion am mittelgrund (eckernförder bucht, westliche ostsee). Meyniana 1967, 17, 101–110. [Google Scholar]
- Flemming, B.; Wefer, G. Tauchbeobachtungen an wellenrippeln und abrasionserscheinungen in der westlichen ostsee südöstlich bokniseck. Meyniana 1973, 23, 9–18. [Google Scholar]
- Healy, T.R.; Sneyd, A.D.; Werner, F. First approximation sea-level dependent mathematical model for volume eroded and submarine profile development in a semienclosed sea: Kiel Bay, Western Baltic. Math. Geol. 1987, 19, 41–56. [Google Scholar] [CrossRef]
- Schrottke, K.; Schwarzer, K.; Fröhle, P. Mobility and transport directions of residual sediments on abrasion platforms in front of active cliffs (Southwestern Baltic Sea). J. Coast. Res. 2006, 7, 459–464. [Google Scholar]
- Canessa, M.; Bavestrello, G.; Trainito, E.; Navone, A.; Cattaneo-Vietti, R. Lithology could affect benthic communities living below boulders. J. Mar. Biol. Assoc. UK 2020, 100, 879–888. [Google Scholar] [CrossRef]
- Leclerc, J.-C. Patterns of spatial variability between contrasting substrata: A boulder-field study. Mar. Ecol. Prog. Ser. 2018, 597, 23–38. [Google Scholar] [CrossRef]
- Bessey, C.; Rule, M.J.; Dasey, M.; Brearley, A.; Huisman, J.M.; Wilson, S.K.; Kendrick, A.J. Geology is a significant indicator of algal cover and invertebrate species composition on intertidal reefs of Ngari Capes Marine Park, South-Western Australia. Mar. Freshw. Res. 2019, 70, 270. [Google Scholar] [CrossRef]
- Michaelis, R.; Hass, H.C.; Mielck, F.; Papenmeier, S.; Sander, L.; Ebbe, B.; Gutow, L.; Wiltshire, K.H. Hard-substrate habitats in the german bight (South-Eastern North Sea) observed using drift videos. J. Sea Res. 2019, 144, 78–84. [Google Scholar] [CrossRef]
- Beisiegel, K.; Darr, A.; Zettler, M.; Friedland, R.; Gräwe, U.; Gogina, M. Spatial variability in subtidal hard substrate assemblages across horizontal and vertical gradients: A multi-scale approach using seafloor imaging. Mar. Ecol. Prog. Ser. 2020, 633, 23–36. [Google Scholar] [CrossRef]
- Sousa, W.P. Disturbance in marine intertidal boulder fields: The nonequilibrium maintenance of species diversity. Ecology 1979, 60, 1225. [Google Scholar] [CrossRef] [Green Version]
- Davis, A.N.; Wilce, R.T. Algal diversity in relation to physical disturbance: A mosaic of successional stages in a subtidal cobble habitat. Mar. Ecol. Prog. Ser. 1987, 37, 229–237. [Google Scholar] [CrossRef]
- Scheibling, R.E.; Kelly, N.E.; Raymond, B.G. Physical disturbance and community organization on a subtidal cobble bed. J. Exp. Mar. Biol. Ecol. 2009, 368, 94–100. [Google Scholar] [CrossRef]
- Liversage, K.; Kotta, J. Disturbance-related patterns in unstable rocky benthic habitats of the north-eastern baltic coast. Proc. Est. Acad. Sci. 2015, 64, 53. [Google Scholar] [CrossRef] [Green Version]
- Hupfer, P.; Harff, J.; Sterr, H.; Stigge, H.-J. Die Wasserstände an der Ostseeküste; Sonderheft Die Küste; Boysen & Co.: Bremen, Germany, 2003; Volume 66, p. 332. ISBN 3-8042-1057-0. [Google Scholar]
- Harris, P.T. Shelf and deep-sea sedimentary environments and physical benthic disturbance regimes: A review and synthesis. Mar. Geol. 2014, 353, 169–184. [Google Scholar] [CrossRef]
- Björck, S. The late quaternary development of the baltic sea basin. In Assessment of Climate Change for the Baltic Sea Basin; Springer: Berlin/Heidelberg, Germany, 2008; pp. 398–407. [Google Scholar]
- Harff, J.; Meyer, M. Coastlines of the Baltic Sea—Zones of competition between geological processes and a changing climate: Examples from the Southern Baltic. In The Baltic Sea Basin; Central and Eastern European Development Studies (CEEDES); Springer: Berlin/Heidelberg, Germany, 2011; pp. 149–164. ISBN 978-3-642-17219-9. [Google Scholar]
- Bayerl, K.; Schwarzer, K.; Lübker-Bammann, U. Das Küstenholozän an der inneren Lübecker Bucht. Meyniana 1992, 44, 97–110. [Google Scholar]
- Schwarzer, K.; Reimers, H.-C.; Störtenbecker, M.; Waldow, K.-R. Das Küstenholozän in der westlichen Hohwachter Bucht. Meyniana 1993, 45, 131–144. [Google Scholar]
- Larsson, R. Behaviour of Organic Clay and Gyttja; Report No. 38; Swedish Geotechnical Institute: Linköping, Sweden, 1990. [Google Scholar]
- Blume, H.-P.; Brümmer, G.W.; Horn, R.; Kandeler, E.; Kögel-Knabner, I.; Kretzschmar, R.; Stahr, K.; Wilke, B.-M. Scheffer/Schachtschabel: Lehrbuch der Bodenkunde, 16th ed.; Springer: Berlin/Heidelberg, Germany, 2010; ISBN 978-3-662-49959-7. [Google Scholar]
- Kreuzburg, M.; Ibenthal, M.; Janssen, M.; Rehder, G.; Voss, M.; Naumann, M.; Feldens, P. Sub-marine continuation of peat deposits from a coastal peatland in the Southern Baltic Sea and its holocene development. Front. Earth Sci. 2018, 6. [Google Scholar] [CrossRef] [Green Version]
- Reisch, F.; Schmoll, D. Morphologische und sedimentologische Untersuchungen von Strand und Seegrund im Bereich der Geltinger Birk (Flensburger Außenförde). Schr. Naturwiss. Ver. Schlesw. Holst. 1997, 67, 16. [Google Scholar]
- von Rönn, G.A.; Schwarzer, K.; Reimers, H.-C.; Winter, C. Limitations of boulder detection in shallow water habitats using high-resolution sidescan sonar images. Geosciences 2019, 9, 390. [Google Scholar] [CrossRef] [Green Version]
- Blondel, P.; Murton, B.J. Handbook of Seafloor Sonar Imagery; John Wiley & Sons, Inc.: Chichester, UK, 1997; ISBN 978-3-540-42967-8. [Google Scholar]
- Lurton, X. An Introduction to Underwater Acoustics: Principles and Applications; Springer: Berlin/Heidelberg, Germany, 2002; ISBN 978-3-540-42967-8. [Google Scholar]
- Katsnelson, B.; Petnikov, V.; Lynch, J. Fundamentals of Shallow Water Acoustics; Springer: Boston, MA, USA, 2012; ISBN 978-1-4419-9776-0. [Google Scholar]
- Chesapeake Technology SonarWiz 7.3 User Guide. Available online: www.cheasapeaketech.com/index.htm (accessed on 20 August 2019).
- Mazel, C. Side Scan Sonar Record Interpretation; Klein and Associates. Inc.: Salem, NH, USA, 1985. [Google Scholar]
- ASTM E11-09. Standard Specification for Wire Cloth and Sieves for Testing Purposes; ASTM International: West Conshohocken, PA, USA, 2009. [Google Scholar]
- Blott, S.J.; Pye, K. GRADISTAT: A grain size distribution and statistics package for the analysis of unconsolidated sediments. Earth Surf. Process. Landf. 2001, 26, 1237–1248. [Google Scholar] [CrossRef]
- Wentworth, C.K. A laboratory and field study of cobble abrasion. J. Geol. 1919, 27, 507–521. [Google Scholar] [CrossRef] [Green Version]
- Udden, J.A. Mechanical composition of clastic sediments. Geol. Soc. Am. Bull. 1914, 25, 655–744. [Google Scholar] [CrossRef]
- ISO. 14688-1: 2017: Geotechnical Investigation and Testing–Identification and Classification of Soil–Part 1: Identification and Description; International Organization for Standardization: Geneva, Switzerland, 2017. [Google Scholar]
- Beisiegel, K.; Darr, A.; Zettler, M.L.; Friedland, R.; Gräwe, U.; Gogina, M. Understanding the spatial distribution of subtidal reef assemblages in the Southern Baltic Sea using towed camera platform imagery. Estuar. Coast. Shelf Sci. 2018, 207, 82–92. [Google Scholar] [CrossRef]
- Corey, A.T. Influence of Shape on the Fall Velocity of Sand Grains. Ph.D. Thesis, Colorado State University, Fort Collins, CO, USA, 1949. [Google Scholar]
- Tucker, L.; Griffiths, C.L.; Schroeter, F.; Vetter, H.D. Boulder shores in South Africa—A distinct but poorly documented coastal habitat type. Afr. J. Mar. Sci. 2017, 39, 193–202. [Google Scholar] [CrossRef]
- USACE; U.S.Corps of Engeneers. Shore Protection Manual; USACE Publication: Washington, DC, USA, 1984; Volume 1. [Google Scholar]
- Bretschneider, C.L. Generation of Wind Waves Over a Shallow Bottom. U.S. Army Corps of Engineers, Beach Erosion Board, T.M. No. 51. 1954. Available online: https://apps.dtic.mil/dtic/tr/fulltext/u2/046517.pdf (accessed on 7 April 2021).
- Deutscher Wetterdienst 10 Minutes Wind Data. Available online: https://Opendata.Dwd.de/Climate_environment/CDC/Observations_germany/Climate/10_minutes/Wind/Historical/ (accessed on 10 August 2020).
- EMODnet Bathymetry Consortium EMODnet Digital Bathymetry (DTM 2020). 2020. Available online: https://portal.emodnet-bathymetry.eu/ (accessed on 20 August 2020).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P. Package Vegan, R Package Version 2.5-6. 2019. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 2 March 2021).
- R Core Team. A Language and Environment for Statistical Computing, Version 3.6.3; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.R-project.org (accessed on 7 April 2021).
- Getreuer, P. Automatic color enhancement (ACE) and its fast implementation. Image Process. On Line 2012, 2, 266–277. [Google Scholar] [CrossRef] [Green Version]
- Bartholomä, A. Acoustic bottom detection and seabed classification in the german bight, Southern North Sea. Geo Mar. Lett. 2006, 26, 177. [Google Scholar] [CrossRef]
- Healy, T.; Wefer, G. The efficacy of submarine abrasion vs. cliff retreat as a supplier of marine sediment in the kieler bucht, Western Baltic. Meyniana 1980, 32, 89–96. [Google Scholar]
- Wallin, A.; Qvarfordt, S.; Norling, P.; Kautsky, H. Benthic communities in relation to wave exposure and spatial positions on sublittoral boulders in the Baltic Sea. Aquat. Biol. 2011, 12, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Maughan, B.C. The Effects of sedimentation and light on recruitment and development of a temperate, subtidal, epifaunal community. J. Exp. Mar. Biol. Ecol. 2001, 256, 59–71. [Google Scholar] [CrossRef]
- Oganjan, K.; Lauringson, V.; Kotta, J.; Rostin, L.; Martin, G. Factors Affecting the recruitment of amphibalanus improvisus and dreissena polymorpha in a highly eutrophic Brackish Bay. Estuar. Coast. Shelf Sci. 2017, 184, 37–45. [Google Scholar] [CrossRef]
- Franz, M.; von Rönn, G.A.; Barboza, F.R.; Karez, R.; Reimers, H.-C.; Schwarzer, K.; Wahl, M. How do geological structure and biological diversity relate? Benthic communities in boulder fields of the Southwestern Baltic Sea. Estuaries Coasts 2021. [Google Scholar] [CrossRef]
- Wefer, G.; Flemming, B.; Tauchgruppe, K. Submarine abrasion des geschiebemergels vor bokniseck (Westl. Ostsee). Meyniana 1976, 28, 87–94. [Google Scholar]
- McGuinness, K.A. Disturbance and organisms on boulders. I. Patterns in the environment and the community. Oecologia 1987, 71, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Addessi, L. Human disturbance and long-term changes on a rocky intertidal community. Ecol. Appl. 1994, 4, 786–797. [Google Scholar] [CrossRef]
- Krumbein, W.C. The effects of abrasion on the size, shape and roundness of rock fragments. J. Geol. 1941, 49, 482–520. [Google Scholar] [CrossRef]
- Nott, J. Waves, coastal boulder deposits and the importance of the pre-transport setting. Earth Planet. Sci. Lett. 2003, 210, 269–276. [Google Scholar] [CrossRef]
- Oetjen, J.; Engel, M.; Pudasaini, S.; Schüttrumpf, H. Significance of boulder shape, shoreline configuration and pre-transport setting for the transport of boulders by tsunamis. Earth Surf. Process. Landf. 2020, 45, 2118–2133. [Google Scholar] [CrossRef] [Green Version]
- Kudrass, H.-R. Experimental study of nearshore transportation of pebbles with attached algae. Mar. Geol. 1974, 16, M9–M12. [Google Scholar] [CrossRef]
- Frey, S.E.; Dashtgard, S.E. Seaweed-assisted, benthic gravel transport by tidal currents. Sediment. Geol. 2012, 265–266, 121–125. [Google Scholar] [CrossRef]
- Masteller, C.C.; Finnegan, N.J.; Warrick, J.A.; Miller, I.M. Kelp, cobbles, and currents: Biologic reduction of coarse grain entrainment stress. Geology 2015, 43, 543–546. [Google Scholar] [CrossRef]

| Research Vessel. | Water Depth (m) | SSS | Frequency (kHz) | Survey Speed (ms−1) | Range (m) | Spatial Resolution (cm) |
|---|---|---|---|---|---|---|
| Littorina | 5–18 | Benthos 1624 | 400 | 2.3 | 100 | 20 |
| Rubber Boat | 0.6–5 | StarFish | 452 | 2.3 | 80 | 20 |
| Size Class | Diameter (cm) | Size Class | Diameter (cm) |
|---|---|---|---|
| small cobble | 1–6.3 | small boulder | 63–100 |
| medium cobble | 6.3–20 | medium boulder | 100–200 |
| large cobble | 20–63 | large boulder | 200–630 |
| Camera | Focal Length (mm) | Polynomial Order | a | b | c | d |
|---|---|---|---|---|---|---|
| Canon Power Shot G9 | 10.2 | 4 | 0.0263809 | −0.1398290 | 0.0743743 | 1.00 |
| Olympus TG-5 | 4.5 | 3 | 0 | −0.0466100 | 0 | 1.00 |
| GoPro HERO 5 Black | 3.0 | 3 | 0 | −0.0301200 | 0 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
von Rönn, G.A.; Krämer, K.; Franz, M.; Schwarzer, K.; Reimers, H.-C.; Winter, C. Dynamics of Stone Habitats in Coastal Waters of the Southwestern Baltic Sea (Hohwacht Bay). Geosciences 2021, 11, 171. https://doi.org/10.3390/geosciences11040171
von Rönn GA, Krämer K, Franz M, Schwarzer K, Reimers H-C, Winter C. Dynamics of Stone Habitats in Coastal Waters of the Southwestern Baltic Sea (Hohwacht Bay). Geosciences. 2021; 11(4):171. https://doi.org/10.3390/geosciences11040171
Chicago/Turabian Stylevon Rönn, Gitta Ann, Knut Krämer, Markus Franz, Klaus Schwarzer, Hans-Christian Reimers, and Christian Winter. 2021. "Dynamics of Stone Habitats in Coastal Waters of the Southwestern Baltic Sea (Hohwacht Bay)" Geosciences 11, no. 4: 171. https://doi.org/10.3390/geosciences11040171
APA Stylevon Rönn, G. A., Krämer, K., Franz, M., Schwarzer, K., Reimers, H.-C., & Winter, C. (2021). Dynamics of Stone Habitats in Coastal Waters of the Southwestern Baltic Sea (Hohwacht Bay). Geosciences, 11(4), 171. https://doi.org/10.3390/geosciences11040171







