A Functional Form for Fine Sediment Interception in Vegetated Environments
Abstract
1. Introduction
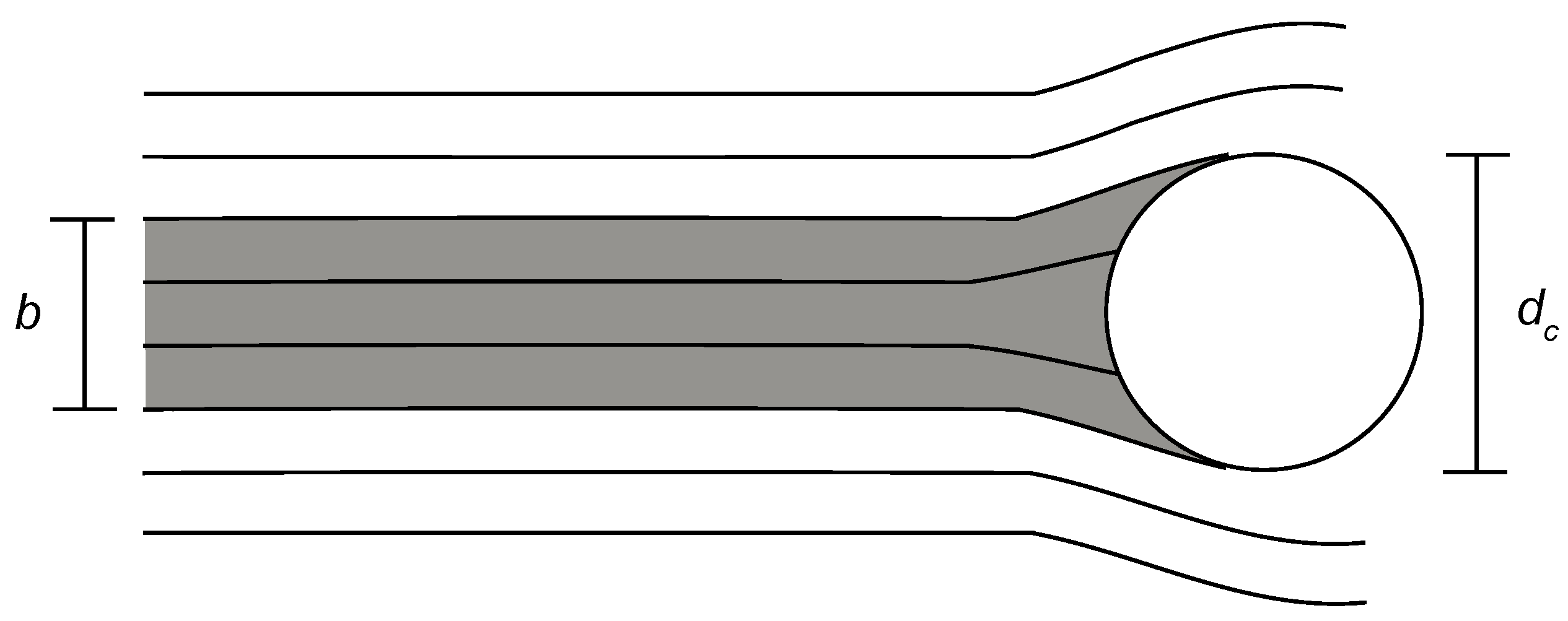
Functional Form for Particle Interception
2. Materials and Methods
2.1. Hypothesized Functional Form for Particle Interception
2.2. Data
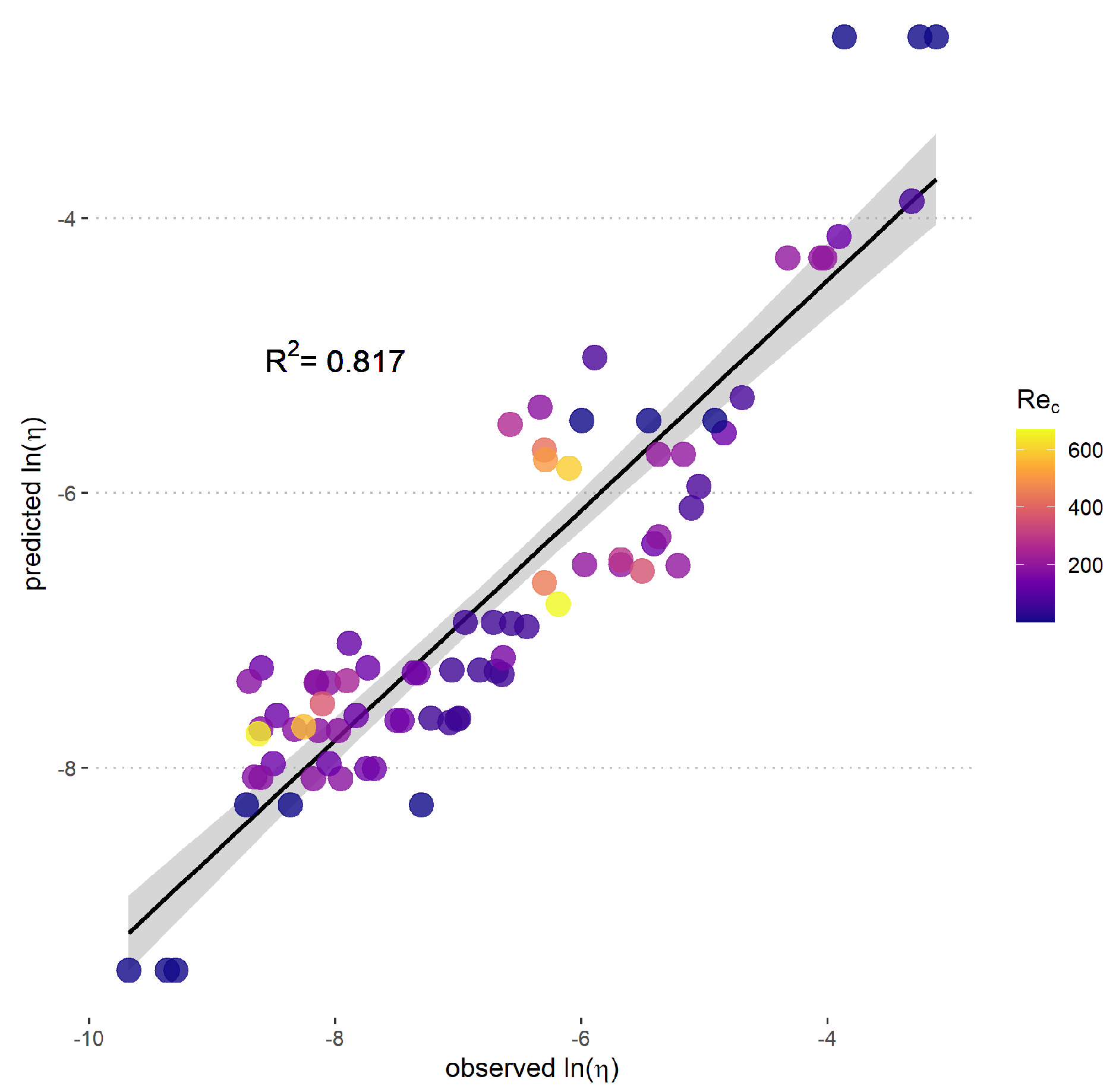
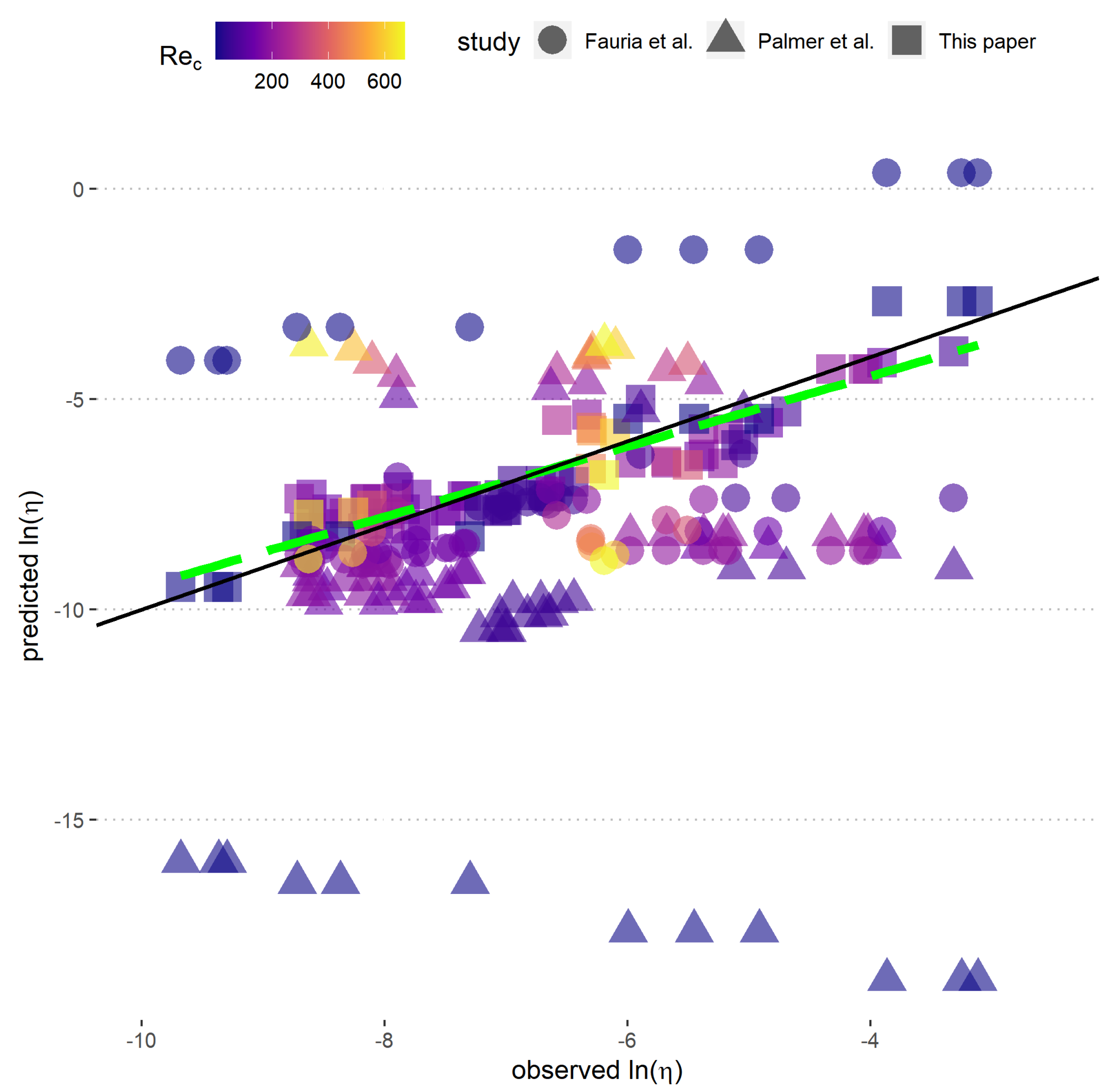
2.3. Model Fitting and Validation
2.4. Integration into Marsh Model
3. Results
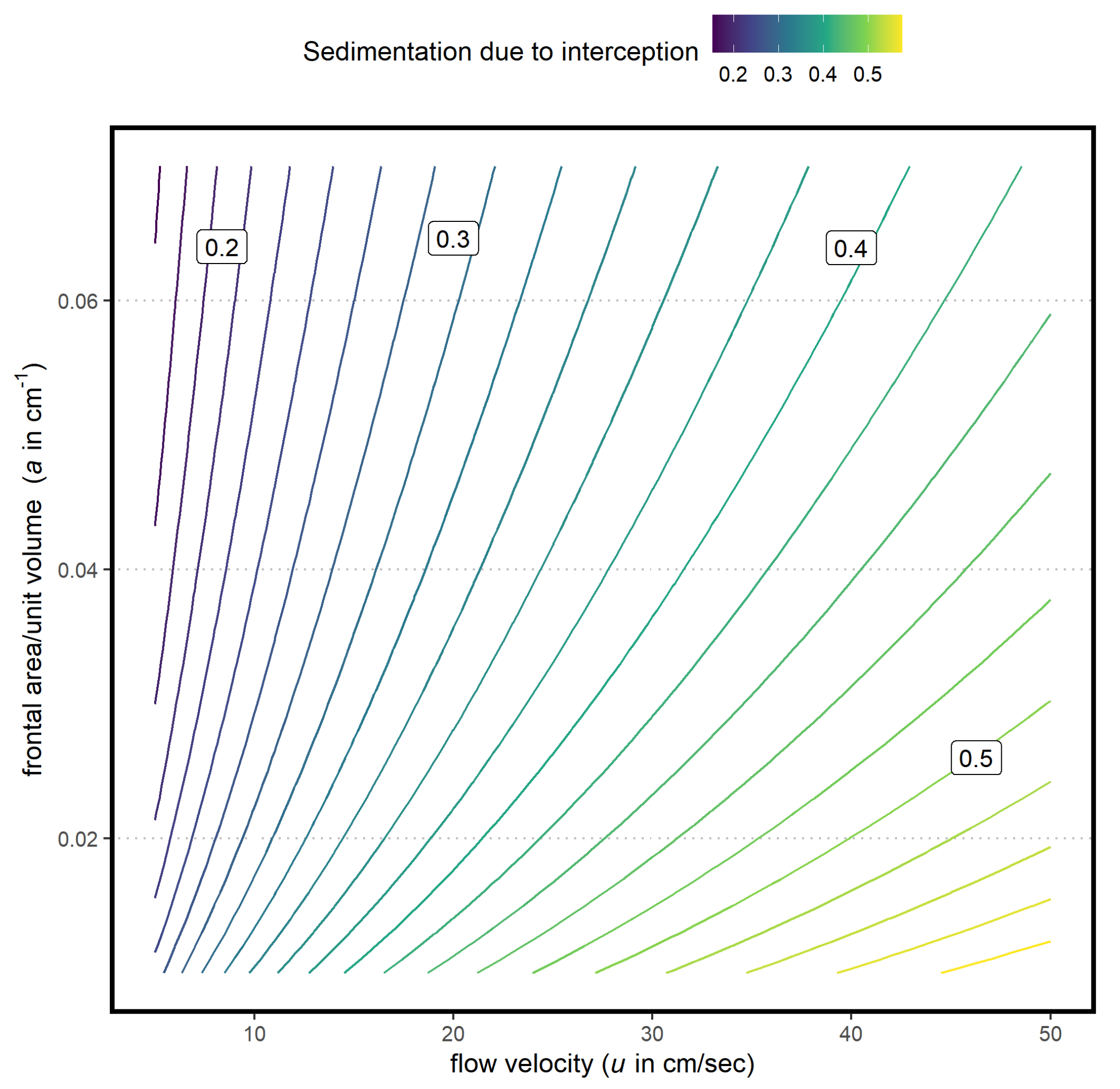
3.1. Predictive Model for
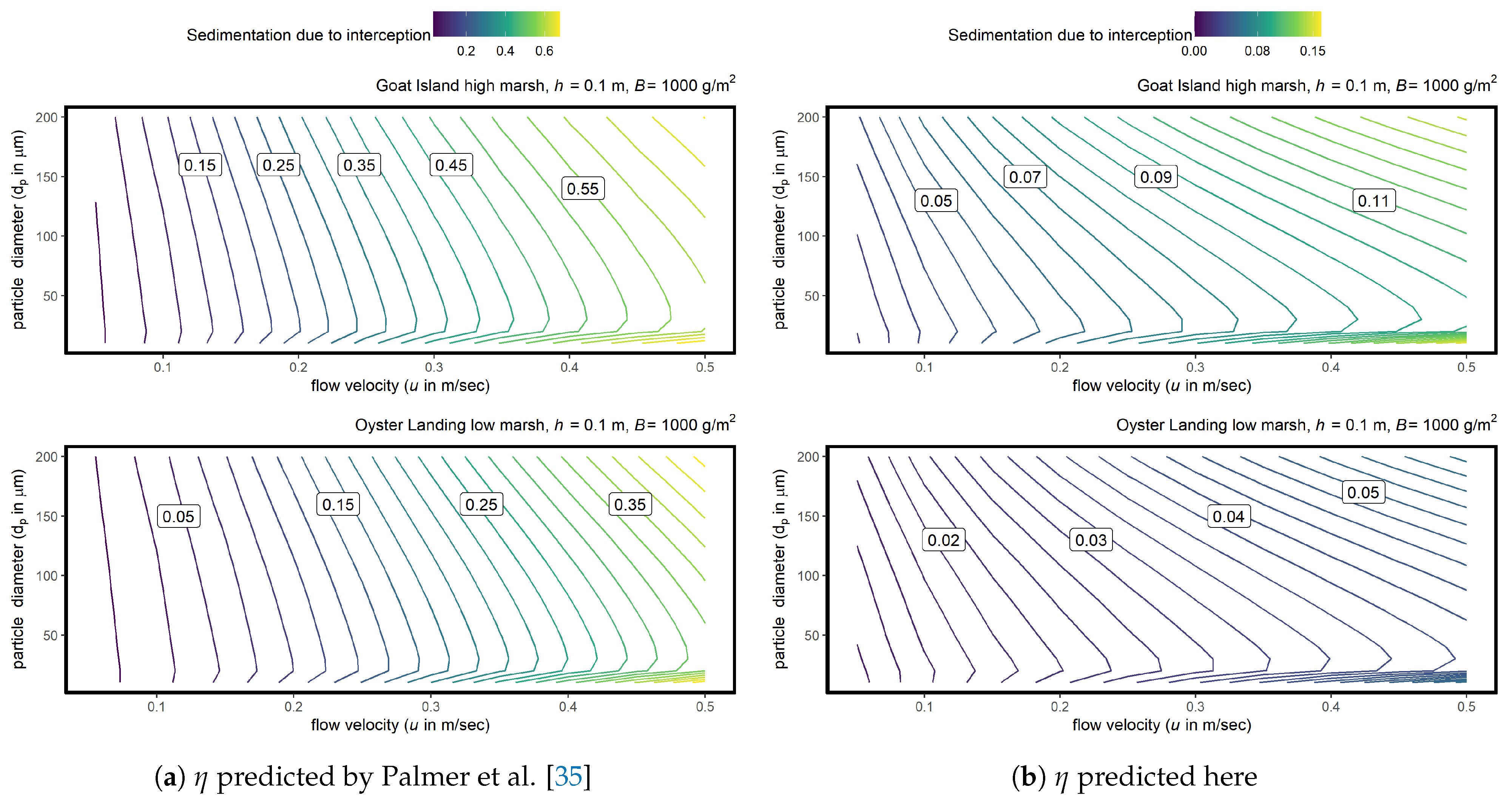
3.2. Marsh System Model
4. Discussion
4.1. Functional Form
4.2. Role of Vegetation Morphology
4.3. Comparison with Previous Models
4.4. Application to Marsh Models
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| a | vegetation frontal area per unit volume (L) |
| attachment efficiency (dimensionless) | |
| b | width of particle streamlines that interact with a collector (L) |
| C | suspended particle concentration (ML) |
| Cmp | compaction rate (LT) |
| collector diameter (L) | |
| particle diameter (L) | |
| E | erosion rate (LT) |
| particle capture efficiency (dimensionless) | |
| particle contact efficiency (dimensionless) | |
| h | length of collectors exposed to flow (L) |
| O | organic material accretion rate (LT) |
| P | ratio of particle to collector density (L) |
| Pe | Péclet number (dimensionless) |
| particle capture flux due to interception (MLT) | |
| particle capture flux due to settling (MLT) | |
| R | ratio of particle to collector diameter (dimensionless) |
| collector Reynold’s number (dimensionless) | |
| u | flow velocity (LT) |
| change in marsh elevation surface over time (LT) |
Appendix A. Marsh Sedimentation Equations
| Value | Goat Island High Marsh | Oyster Landing Low Marsh | All Applicable Sites |
|---|---|---|---|
| - | - | 11 | |
| - | - | 0.46 | |
| - | - | 3.8 | |
| 0.00066 | 0.0019 | - | |
| 0.55 | 0.12 | - | |
| 0.29 | 0.18 | - | |
| 0.40 | 0.53 | - |
References
- Fagherazzi, S.; Marani, M.; Blum, L.K. Introduction: The coupled evolution of geomorphological and ecosystem structures in salt marshes. In The Ecogeomorphology of Tidal Marshes, Coastal and Estuarine Studies; American Geophysical Union: Washington, DC, USA, 2004; pp. 1–4. [Google Scholar]
- Larsen, L.G.; Harvey, J.W. How vegetation and sediment transport feedbacks drive landscape change in the Everglades and wetlands worldwide. Am. Nat. 2010, 176, E66–E79. [Google Scholar] [CrossRef]
- Mudd, S.M.; D’Alpaos, A.; Morris, J.T. How does vegetation affect sedimentation on tidal marshes? Investigating particle capture and hydrodynamic controls on biologically mediated sedimentation. J. Geophys. Res. Earth Surf. 2010, 115. [Google Scholar] [CrossRef]
- Olliver, E.A.; Edmonds, D.A.; Shaw, J.B. Influence of Floods, Tides, and Vegetation on Sediment Retention in Wax Lake Delta, Louisiana, USA. J. Geophys. Res. Earth Surf. 2020, 125. [Google Scholar] [CrossRef]
- Fagherazzi, S.; Kirwan, M.L.; Mudd, S.M.; Guntenspergen, G.R.; Temmerman, S.; D’Alpaos, A.; Koppel, J.V.D.; Rybczyk, J.M.; Reyes, E.; Craft, C.; et al. Numerical models of Salt Marsh Evolution: Ecological, Geomorphic, and Climatic Factors. Rev. Geophys. 2012, 50. [Google Scholar] [CrossRef]
- Huang, Y.H.; Saiers, J.E.; Harvey, J.W.; Noe, G.B.; Mylon, S. Advection, dispersion, and filtration of fine particles within emergent vegetation of the Florida Everglades. Water Resour. Res. 2008, 44. [Google Scholar] [CrossRef]
- Zhang, J.; Lei, J.; Huai, W.; Nepf, H. Turbulence and Particle Deposition Under Steady Flow Along a Submerged Seagrass Meadow. J. Geophys. Res. Ocean. 2020, 125. [Google Scholar] [CrossRef]
- Harvey, J.W.; Noe, G.B.; Larsen, L.G.; Nowacki, D.J.; McPhillips, L.E. Field flume reveals aquatic vegetation’s role in sediment and particulate phosphorus transport in a shallow aquatic ecosystem. Geomorphology 2011, 126, 297–313. [Google Scholar] [CrossRef]
- Simon, M.; Grossart, H.P.; Schweitzer, B.; Ploug, H. Microbial ecology of organic aggregates in aquatic ecosystems. Aquat. Microb. Ecol. 2002, 28, 175–211. [Google Scholar] [CrossRef]
- Yallop, M.L.; Paterson, D.M.; Wellsbury, P. Interrelationships between rates of microbial production, exopolymer production, microbial biomass, and sediment stability in biofilms of intertidal sediments. Microb. Ecol. 2000, 39, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, R.C.; Droppo, I.G.; Wharton, G. Erodibility of cohesive sediment: The importance of sediment properties. Earth-Sci. Rev. 2011, 105, 101–120. [Google Scholar] [CrossRef]
- Lundkvist, M.; Grue, M.; Friend, P.L.; Flindt, M.R. The relative contributions of physical and microbiological factors to cohesive sediment stability. Cont. Shelf Res. 2007, 27, 1143–1152. [Google Scholar] [CrossRef]
- Salant, N.L. ‘Sticky business’: The influence of streambed periphyton on particle deposition and infiltration. Geomorphology 2011, 126, 350–363. [Google Scholar] [CrossRef]
- Winterwerp, J.C.; van Kesteren, W.G. Introduction to the Physics of Cohesive Sediment in the Marine Environment; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Hu, Z.; Lei, J.; Liu, C.; Nepf, H. Wake structure and sediment deposition behind models of submerged vegetation with and without flexible leaves. Adv. Water Resour. 2018, 118, 28–38. [Google Scholar] [CrossRef]
- Liu, X.; Zeng, Y.; Huai, W. Modeling of Interactions Between Floating Particles and Emergent Stems in Slow Open Channel Flow. Water Resour. Res. 2018, 54, 7061–7075. [Google Scholar] [CrossRef]
- McCombe, D.; Ackerman, J.D. Collector motion affects particle capture in physical models and in wind pollination. Am. Nat. 2018, 192, 81–93. [Google Scholar] [CrossRef]
- Larsen, L.G. Multiscale flow-vegetation-sediment feedbacks in low-gradient landscapes. Geomorphology 2019, 334, 165–193. [Google Scholar] [CrossRef]
- Tinoco, R.O.; Coco, G. A laboratory study on sediment resuspension within arrays of rigid cylinders. Adv. Water Resour. 2016, 92, 1–9. [Google Scholar] [CrossRef]
- Coco, G.; Zhou, Z.; Maanen, B.V.; Olabarrieta, M.; Tinoco, R.; Townend, I. Morphodynamics of tidal networks: Advances and challenges. Mar. Geol. 2013, 346, 1–16. [Google Scholar] [CrossRef]
- Tanino, Y.; Nepf, H. Laboratory Investigation of Mean Drag in a Random Array of Rigid, Emergent Cylinders. J. Hydraul. Eng. 2008, 134, 34–41. [Google Scholar] [CrossRef]
- Nepf, H.M. Drag, turbulence, and diffusion in flow through emergent vegetation. Water Resour. Res. 1999, 35, 479–489. [Google Scholar] [CrossRef]
- Yager, E.M.; Schmeeckle, M.W. The influence of vegetation on turbulence and bed load transport. J. Geophys. Res. Earth Surf. 2013, 118, 1585–1601. [Google Scholar] [CrossRef]
- Elliot, A. Settling of Fine Sediment in a Channel with Emergent Vegetation. J. Hydraul. Eng. 2000, 126, 570–577. [Google Scholar] [CrossRef]
- Temmerman, S.; Govers, G.; Wartel, S.; Meire, P. Modelling estuarine variations in tidal marsh sedimentation: Response to changing sea level and suspended sediment concentrations. Mar. Geol. 2004, 212, 1–19. [Google Scholar] [CrossRef]
- D’Alpaos, A.; Lanzoni, S.; Marani, M.; Rinaldo, A. Landscape evolution in tidal embayments: Modeling the interplay of erosion, sedimentation, and vegetation dynamics. J. Geophys. Res. Earth Surf. 2007, 112. [Google Scholar] [CrossRef]
- Nepf, H.M. Flow and Transport in Regions with Aquatic Vegetation. Annu. Rev. Fluid Mech. 2012, 44, 123–142. [Google Scholar] [CrossRef]
- Tinoco, R.O.; Coco, G. Observations of the effect of emergent vegetation on sediment resuspension under unidirectional currents and waves. Earth Surf. Dyn. 2014, 2, 83–96. [Google Scholar] [CrossRef]
- Follett, E.; Nepf, H. Particle Retention in a Submerged Meadow and Its Variation Near the Leading Edge. Estuaries Coasts 2018, 41, 724–733. [Google Scholar] [CrossRef]
- Hopkinson, C.S.; Morris, J.T.; Fagherazzi, S.; Wollheim, W.M.; Raymond, P.A. Lateral Marsh Edge Erosion as a Source of Sediments for Vertical Marsh Accretion. J. Geophys. Res. Biogeosci. 2018, 123, 2444–2465. [Google Scholar] [CrossRef]
- Kondziolka, J.M.; Nepf, H.M. Vegetation wakes and wake interaction shaping aquatic landscape evolution. Limnol. Oceanogr. Fluids Environ. 2014, 4, 106–119. [Google Scholar] [CrossRef]
- Meire, D.W.; Kondziolka, J.M.; Nepf, H.M. Interaction between neighboring vegetation patches: Impact on flow and deposition. Water Resour. Res. 2014, 50, 3809–3825. [Google Scholar] [CrossRef]
- Searcy, K.E.; Packman, A.I.; Atwill, E.R.; Harter, T. Capture and retention of Cryptosporidium parvum oocysts by Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 2006, 72, 6242–6247. [Google Scholar] [CrossRef] [PubMed]
- Fauria, K.E.; Kerwin, R.E.; Nover, D.; Schladow, S.G. Suspended particle capture by synthetic vegetation in a laboratory flume. Water Resour. Res. 2015, 51, 9112–9126. [Google Scholar] [CrossRef]
- Palmer, M.R.; Nepf, H.M.; Pettersson, T.J.R. Observations of particle capture on a cylindrical collector: Implications for particle accumulation and removal in aquatic systems. Limnol. Oceanogr. 2004, 49, 76–85. [Google Scholar] [CrossRef]
- Purich, A. The Capture of Suspended Particles by Aquatic Vegetation. Ph.D. Thesis, University of Western Australia, Crawley, Australia, 2006. [Google Scholar]
- Wingenroth, J.; Yee, C.; Nghiem, J.A.; Larsen, L. Effects of Stem Density and Reynolds Number on Fine Sediment Interception by Emergent Vegetation. Geosciences 2021, 11, 136. [Google Scholar] [CrossRef]
- Wu, L.; Gao, B.; Muñoz-Carpena, R. Experimental analysis of colloid capture by a cylindrical collector in laminar overland flow. Environ. Sci. Technol. 2011, 45, 7777–7784. [Google Scholar] [CrossRef]
- Wu, L.; Gao, B.; Muñoz-Carpena, R.; Pachepsky, Y.A. Single collector attachment efficiency of colloid capture by a cylindrical collector in laminar overland flow. Environ. Sci. Technol. 2012, 46, 8878–8886. [Google Scholar] [CrossRef]
- Wu, L.; Muñoz-Carpena, R.; Gao, B.; Yang, W.; Pachepsky, Y.A. Colloid filtration in surface dense vegetation: Experimental results and theoretical predictions. Environ. Sci. Technol. 2014, 48, 3883–3890. [Google Scholar] [CrossRef] [PubMed]
- Nepf, H.M. Vegetated Flow Dynamics Introduction: Scales of Morphology and Flow in a Tidal Marsh. Coast. Estuar. Stud. 2004, 59, 137–163. [Google Scholar]
- Durham, W.M.; Climent, E.; Barry, M.; De Lillo, F.; Boffetta, G.; Cencini, M.; Stocker, R. Turbulence drives microscale patches of motile phytoplankton. Nat. Commun. 2013, 4, 2148. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, C.; Nepf, H. Turbulent Kinetic Energy in Submerged Model Canopies Under Oscillatory Flow. Water Resour. Res. 2018, 54, 1734–1750. [Google Scholar] [CrossRef]
- Espinosa-Gayosso, A.; Ghisalberti, M.; Ivey, G.N.; Jones, N.L. Density-ratio effects on the capture of suspended particles in aquatic systems. J. Fluid Mech. 2015, 783, 191–210. [Google Scholar] [CrossRef]
- Kuhn, M. Caret: Classification and Regression Training. 2020. Available online: https://cran.r-project.org/package=caret (accessed on 11 January 2021).
- Venables, W.; Ripley, B. Modern Applied Statistics with S, 4th ed.; Springer: Berlin, Germany, 2002. [Google Scholar]
- Muggeo, V.M. Interval estimation for the breakpoint in segmented regression: A smoothed score-based approach. Aust. N. Z. J. Stat. 2017, 59, 311–322. [Google Scholar] [CrossRef]
- Hurvich, C.; Tsai, C.L. Regression and Time Series Model Selection in Small Samples. Biometrika 1989, 76, 297–307. [Google Scholar] [CrossRef]
- Oosterlee, L.; Cox, T.J.S.; Vandenbruwaene, W.; Maris, T.; Temmerman, S.; Meire, P. Tidal Marsh Restoration Design Affects Feedbacks Between Inundation and Elevation Change. Estuaries Coasts 2018, 41, 613–625. [Google Scholar] [CrossRef]
- Amos, C.L.; Bergamasco, A.; Umgiesser, G.; Cappucci, S.; Cloutier, D.; Denat, L.; Flindt, M.; Bonardi, M.; Cristante, S. The stability of tidal flats in Venice Lagoon - The results of in-situ measurements using two benthic, annular flumes. J. Mar. Syst. 2004, 51, 211–241. [Google Scholar] [CrossRef]
- Bradley, P.; Morris, J. Physical characteristics of salt marsh sediments: Ecological implications. Mar. Ecol. Prog. Ser. 1990, 61, 245–252. [Google Scholar] [CrossRef]
- Carr, J.A.; D’Odorico, P.; McGlathery, K.J.; Wiberg, P.L. Spatially explicit feedbacks between seagrass meadow structure, sediment and light: Habitat suitability for seagrass growth. Adv. Water Resour. 2016, 93, 315–325. [Google Scholar] [CrossRef]
- Harvey, M.; Bourget, E.; Ingram, R.G. Experimental evidence of passive accumulation of marine bivalve larvae on filamentous epibenthic structures. Limnol. Oceanogr. 1995, 40, 94–104. [Google Scholar] [CrossRef]
- Tinoco, R.O.; San Juan, J.E.; Mullarney, J.C. Simplification bias: Lessons from laboratory and field experiments on flow through aquatic vegetation. Earth Surf. Process. Landf. 2020, 45, 121–143. [Google Scholar] [CrossRef]
- Camenen, B. Simple and general formula for the settling velocity of particles. J. Hydraul. Eng. 2007, 133, 229–233. [Google Scholar] [CrossRef]
| Paper | Data Points | Range | Particle Size (m) | Frontal Area/Unit Volume (cm) |
|---|---|---|---|---|
| Purich [36] | 18 | 71–657 | 231 | 0.06–0.25 |
| Wu et al. [40] | 12 | 0.02–1.2 | 1.05 | 0.002–0.1 |
| Fauria et al. [34] | 36 | 55–184 | 9.9–13.8 | 0.06 |
| Wingenroth et al. [37] | 14 | 67–200 | 32 | 0.008–0.039 |
| Min | −3.88 | −0.55 | −1.71 | 0.33 | 1.79 |
| Fitted | −1.13 | −0.38 | −1.54 | 1.00 | 2.10 |
| Max | 1.21 | −0.17 | −1.36 | 1.49 | 2.50 |
| Standard | 1.29 | −0.47 | −1.35 | 0.24 | 1.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stein, S.; Wingenroth, J.; Larsen, L. A Functional Form for Fine Sediment Interception in Vegetated Environments. Geosciences 2021, 11, 157. https://doi.org/10.3390/geosciences11040157
Stein S, Wingenroth J, Larsen L. A Functional Form for Fine Sediment Interception in Vegetated Environments. Geosciences. 2021; 11(4):157. https://doi.org/10.3390/geosciences11040157
Chicago/Turabian StyleStein, Samuel, Jordan Wingenroth, and Laurel Larsen. 2021. "A Functional Form for Fine Sediment Interception in Vegetated Environments" Geosciences 11, no. 4: 157. https://doi.org/10.3390/geosciences11040157
APA StyleStein, S., Wingenroth, J., & Larsen, L. (2021). A Functional Form for Fine Sediment Interception in Vegetated Environments. Geosciences, 11(4), 157. https://doi.org/10.3390/geosciences11040157






