Abstract
In areas contaminated by potentially toxic elements (PTEs), knowledge of processes of metal mobilisation is the basis for the choice of appropriate remediation methodologies. The mobilisation of metals is a function of several factors, and the response to these factors must be well known during the planning of remediation strategies. The activity of an ore metallurgical plant in South-East Brazil resulted in major contamination by several heavy metals. Reversing the contamination’s negative impact required geochemical assessment of the area, including the physicochemical characterisation, quantification, and delimitation of PTEs, and the rating of the solubilisation/mobilisation capacity of these elements. The definition of spatial patterns for PTEs’ distribution allowed the construction of contamination risk maps which work as a tool for the mitigation and control of the contamination plume. The chemical analysis of interstitial water and selective and sequential extraction methodologies showed that elements that occur in the environment in critical concentrations (Zn, Cd, Pb, As) are mostly associated with easily mobilised forms (soluble, exchangeable cations, associated with Mn oxides). Given the great mobility of the contamination plume, any process of removal of contaminated material becomes unfeasible, thus the strategy of remediation for the stream and associated alluvial deposits must be based on methods of in situ decontamination.
1. Introduction
Mineral extraction and processing activities around the world contribute significantly to solute loads in receiving streams and aquifers [1]. In these streams, sediments are, more often than soils, contaminated with multiple chemicals [2] becoming potential sources of pollution with adverse effects on water resources through the mobilisation of contaminated particles and the release of contaminants into the water phase after natural or artificial resuspension of sediments. However, sediment may also act as an intermediate or permanent repository, using the ability of a sedimentary body to immobilise potentially hazardous substances over the long term [3]. Both distinct functions make risk assessment and sediment management decision-making challenging and complex [3,4]. Water ecosystems also differ in hydrodynamics and geochemistry from terrestrial ecosystems. Although soil and groundwater may often be isolated from receptors during remediation, it is more difficult to implement similar containment or removal methods for contaminated sediment. Contaminated sediments are some of the most challenging site remediation problems today [5].
Among the most common pollutants in our natural environment, heavy metals represent one of the most serious groups because of their toxicity, persistence, and bioaccumulation issues [6]. In uncontaminated environments, heavy metals are bound to silicates and primary minerals, forming relatively immobile mineral species, whereas in contaminated areas, they are usually associated with more mobile mineral phases. Contrary to soils where metals have a great affinity with the mineral and organic constituents on which they are strongly adsorbed or tend to form insoluble precipitates such as oxides [7], in aquatic sediments they can occur in a high diversity of chemical forms, many of them are mobile or bioavailable. Distribution, mobility, and bioavailability of heavy metals in sediments do not simply depend on their nature and total concentrations but, critically, on their chemical and physical associations and on transformation processes they undergo, which depend on several factors, such as, temperature, pH, redox potential (Eh), biological activity, and biological oxygen demand [6]. In sediments there exist mechanisms of element movement via porewater processes such as advection and diffusion, as well particle reworking [1], which makes the associations of metals with other compounds (inorganic or organic), dynamic and reversible, reflecting a change in the physicochemical conditions of the environment. Consequently, even in low-load scenarios, the potential effects of metals released from sediment should be considered a potential risk [6,8]. In aquatic systems and alluvial areas contaminated by heavy metals, knowledge of the complex processes of metal mobilisation and transformation requires detailed studies of release mechanisms [9] in sediments and forms the basis for the choice and planning of appropriate remediation methodologies.
On one of the riverbanks of the longest Brazilian river, São Francisco, in Minas Gerais State, an industrial unit produces zinc alloys, and zinc oxide, through the metallurgical treatment of Zn sulphide and Zn silicate ores. In the first years of operation, in the absence of appropriate regulations and legislation on waste management and disposal systems, this activity triggered numerous environmental problems in the surficial waters, soils, and sediments deposited in alluvial plains and shallow adjacent waterways. Until 1982, waste, rich in heavy metals, was deposited in specific areas near the plant, particularly in the alluvial plains, often mixed with various landfill materials. Several studies carried out on the São Francisco River in the last 15 years [10,11,12,13,14] reported a high concentration of Zn, Cd, Cu, Pb, and Cr in the marginal sediments, directly related to the operation of this major Zn treatment plant. It is worth notice that to avoid negative effects on the local environment, some efforts aimed at reducing pollution were actively pursued by the metallurgical company. However, despite these efforts, high levels of sulphates and potentially toxic metals (PTEs) such as Zn, Pb, Cu, Pb, Cd, and As, still prevail in the soils and alluvial plains in the vicinity of the industrial area [15,16]. Sulphates and the most soluble forms of metals are easily released from residual interstitial water and leached into watercourses.
One of the first mitigation measures to be undertaken by the company is the implementation of adequate methods for the environmental remediation and recovery of the largest and most severely impacted alluvial plain—Consciência creek, one small stream that represents the most important tributary of the São Francisco River in this industrial site. It also included the contiguous alluvial plain of Grota Seca Creek, a small tributary of the Consciência stream. Emphasis is placed on the reduction of negative medium- and long-term impacts on the local and downstream environment. Thus, one of the main goals of this study is the physicochemical characterisation of this alluvial basin, the spatial and vertical quantification and delimitation of pollutant metals, and the evaluating of the solubilisation/mobilisation capacity of these elements in order to assess the most suitable remediation techniques that produce the least disturbance to the system and minimise local environmental disturbances. Therefore, the construction of space–time cartography is pivotal for an accurate visualisation.
A map is always a simplification of reality [17,18]. A two-dimensional map can only gather and display the values of a limited number of variables or attributes up to three. Thus, when considering complex scenarios, such as environmental characterisation, a reduction to a single dimension is mandatory [19]. Risk maps, broadly mentioned in literature often use spatial pattern visualisation of, e.g., pollutant concentration distribution, exposure and its effects, and vulnerability assessment; therefore, they constitute a very powerful tool to support policy-making in a complex environmental risk assessment framework [17,20] Moreover, stochastic approaches have been widely used to provide pivotal information for mitigation strategies [21].
Geostatistical techniques are based on the theory of regionalised variables [22], which states that variables within an area show both random and spatially structured properties [23]. Experimental variograms must be estimated and modelled to quantify the spatial variability of random variables as a function of their separation lag [24]. Geostatistics concerns a broad methodological approach, and it is more than the simple development of mathematical (probabilistic) models and methods and their application. Analysing the practical problems to be solved and formalizing them in terms of concepts is a key issue. When predicting the risk of contamination (e.g., months ahead), it is mandatory to stress the relevance of the chances for the future estimated values exceeding maximum admissible values. The delineation of zones of high and low risk requires the interpolation of risk values to the nodes of a regular grid, making possible proper risk assessments and a prediction model working as guidance to a more sustainable environmental management. Thus, the second main goal of this research is a straightforward geostatistical procedure technique to evaluate the risk of the alluvial plain contamination by PTEs. The geostatistical approach allows the definition of the space–time distribution patterns of the selected PTEs, focusing on the visualisation and delineation of potential zones for future monitoring and remediation.
2. Materials and Methods
2.1. The Study Area
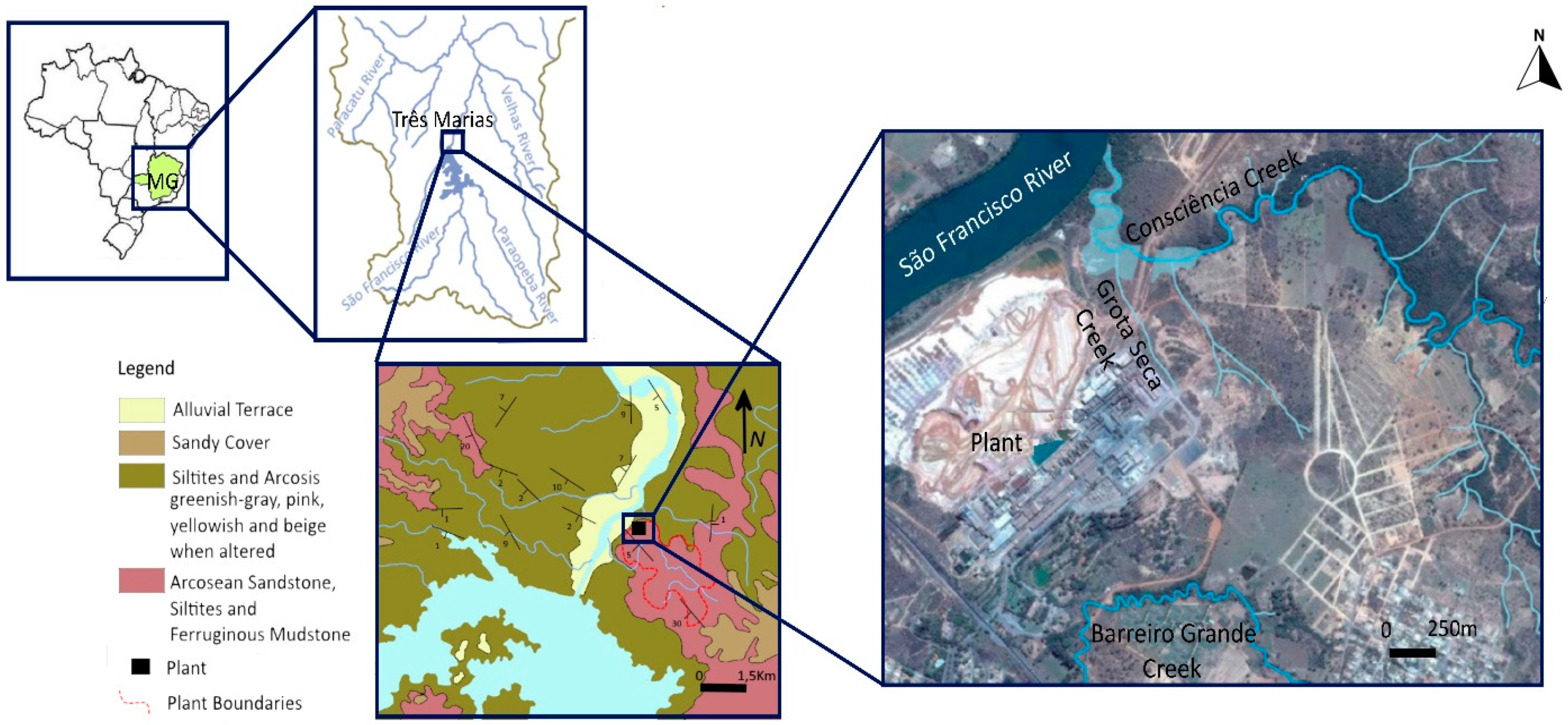
The survey area is in the Minas Gerais State (Brazil), close to the southern bank of the São Francisco River—the largest Brazilian river, about 2800 km long and corresponding to a 640,000 km2 watershed. At an altitude of 563 m and covering approx. 3 km2, the industrial area lies 580 km away from the river source being part of the São Francisco hydrographic system. Confined between the river (west), and Três Marias town (east). The studied contaminated area was selected considering previous studies [25] and includes two contiguous stream floodplains in the northern boundary: one from Consciência creek (2.71 ha) and the other from its tributary, Grota Seca creek (0.51 ha), both near the mouth of the São Francisco River. Consciência creek drains an area of approximately 11.5 km2 that includes the Zn industrial treatment plant. The small gradient and depth of this stream explain its poor hydrodynamics [26].
Geologically, this area is set in the Três Marias Formation (Bambuí Group, approx. 600–650 Ma), which comprises a thick and relatively extensive sedimentary sequence of fine to very fine arkosic sandstones, alternating with arkosic silts rich in quartz, feldspar, and heavy minerals, such as iron oxides, tourmaline, zircon, epidote, and garnet [27,28,29]. This sedimentary sequence overlies low-grade deformation Precambrian rocks of the southern unit of the São Francisco Craton (Figure 1). Water infiltration and circulation in the bedrock occurs mainly through fractures and faults in the horizontal or sub-horizontal weathered sandstone and silt layers [30]. Furthermore, aquifers and surface water bodies are highly vulnerable to contamination due to the absence of any Cretaceous, Tertiary, or Quaternary sedimentary cover.
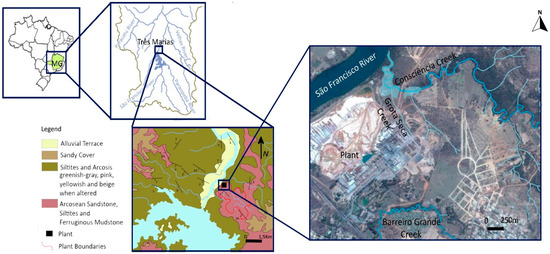
Figure 1.
Geographical location and geological setting of the studied industrial area close to the São Francisco River, Minas Gerais, Brazil.
This situation is aggravated by the tropical climate, characterised by annual average temperatures of ~24 °C, high humidity (50 to 80%) throughout all the year, and an average annual precipitation of ~1000–1500 mm, concentrated in the summer months [30,31,32,33,34,35].
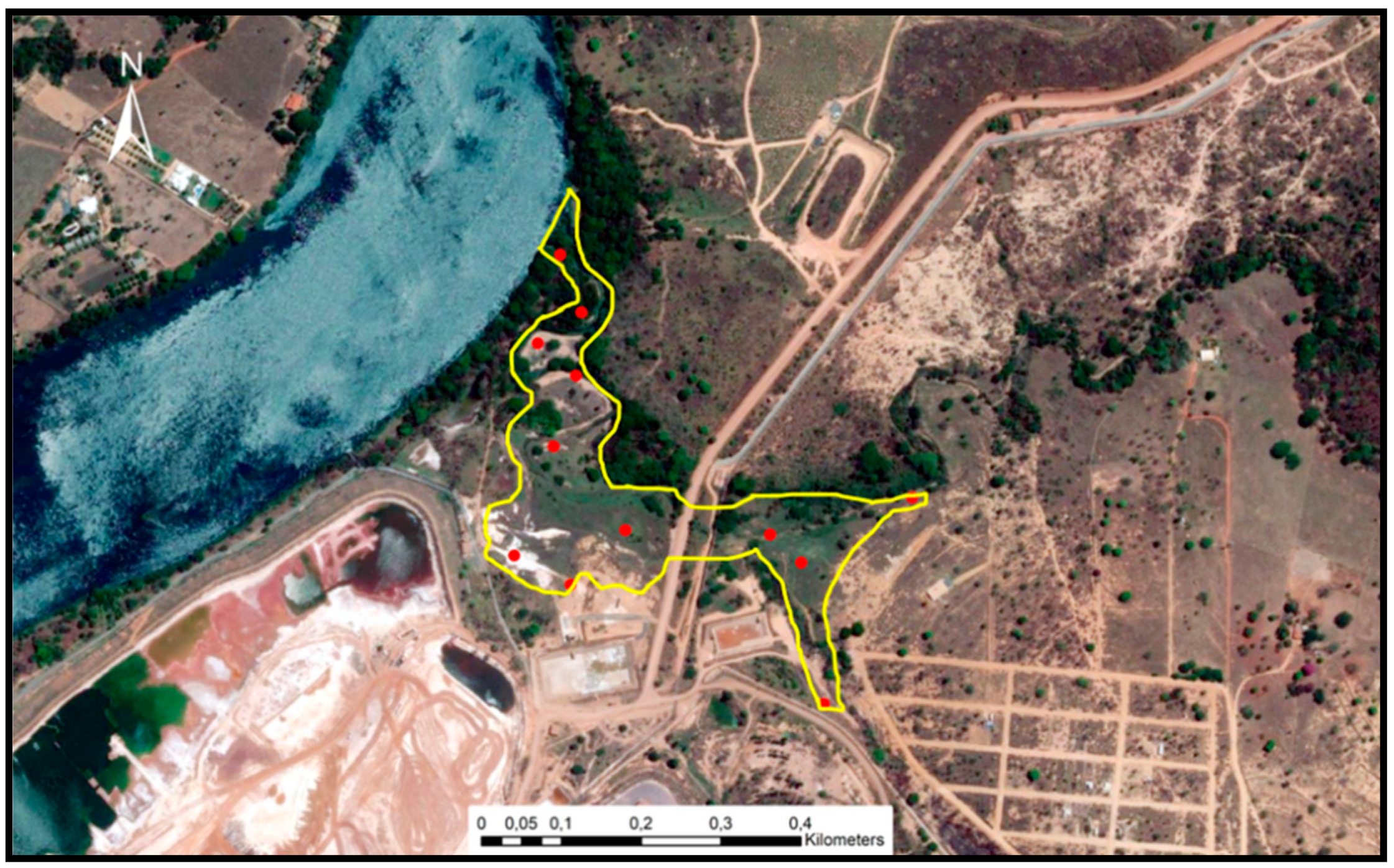
Sediment sampling (Figure 2) was simultaneous with in situ readings of the physical and chemical parameters, most influencing metal mobilisation and retention (pH and redox potential). A portable multi-parameter Consort, C5020 model, probes (SP10T model for pH, SP50X model for redox potential) was used together with the extraction of porewater through rhizome samplers.
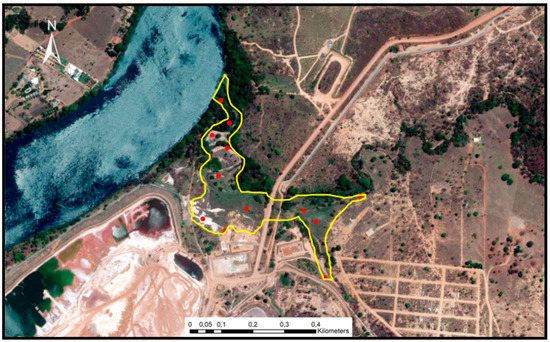
Figure 2.
Study area and sampling points.
Sample analysis followed the overview of traceability for chemical analysis defined by [3] for the assessment and management of contaminated sediments in metals. Another group of contaminants in the area are the sulphates whose origin is the chemical oxidation processes of sphalerite, used to extract zinc alloys, and therefore were also analysed.
Metal contents were determined in samples preserved at about 4 °C (from the time of collection), including both potentially toxic elements (As, Cd, Co, Cr, Cu, Ni, Pb, Zn), and endogenous metallic elements (Fe, Mn). Indeed, due to their high concentrations and geochemical behaviour, they may control pathways and cycles of contaminants. The evaluation of the contamination degree was based on the quantification of the metallic forms with high to moderate mobility, which correspond to the forms that can be extracted in nature by weathering and through chemical modifications of the environment (potential redox, pH). These forms are obtained from the digestion of sediments with hydrogen peroxide and aqua regia (HNO3 + HCl) in a high-pressure microwave digestion unit (Anton Paar Multiwave PRO) following the US EPA Method 3051A [36]. This is a type of digestion in which only the crystalline nets of silicate minerals or resistant oxides are not destroyed.
The evaluation of metals’ mobility was based on (1) the quantification of the soluble contents in interstitial water extracted simultaneously with sampling and (2) the percentage determination of the mineral and organic components with which the different metals are bounded. A sequential extraction protocol was adopted from [37] where the Tessier Method is optimised [38] for heavily contaminated soils. The procedure of sequential chemical extraction gives a series of 5 fractions dissolved by the selected mixture of reagents [37,39]: F1—ammonium acetate (1 M NH4Ac, pH 4.5): water-soluble metals and dissolved exchangeable ions, specifically adsorbed and bounded to carbonates; F2—hydroxylamine hydrochloride (0.1 M NH4OHHCl, pH 2): metals bounded to Mn oxyhydroxides; F3—ammonium oxalate (0.175 M (NH4)2C2O4—0.1 M H2C2O4, pH 3.3 in darkness): metals bounded to amorphous Fe oxides; F4—H2O2 35%: metals bounded to organic matter; sulphide (primary sulphide minerals could not be leached out in this step); F5—ammonium oxalate (0.175 M (NH4)2C2O4—0.1 M H2C2O4, pH 3.3 under U.V. radiation): metals bounded to crystalline Fe oxides.
The metals contents obtained through the 3 types of methodologies (interstitial water, aqua regia digestion, and sequential extraction) were analysed by optical emission spectroscopy with inductive plasma source (ICP-OES, Perkin-Elmer OPTIMA 8300), operated under the following conditions: plasma gas flow—10 L/min; auxiliary gas flow—0.2 L/min; nebulizer gas flow—0.70 L/min; sample flow—1.50 mL/min; RF Power—1450 watts; viewing modes—radial and axial; reading time—2–5 min; read delay—60 s; normal resolution; internal standard—yttrium. The accuracy and analytical precision for all the performed analysis were checked by reference materials’ analytics through duplicate samples for each analytical set. The accuracy of the sequential treatment may be estimated by comparing the total amounts sum for each step, with the well-defined amount obtained after a hot mixed-acid sample attack (aqua regia digestion). The overall recovery rates (ratio between the sum of the 5 fractions and the independent total concentration) ranged from 85 to 110%.
Determination of the sulphate ion in the sedimentary materials followed the method described by [40]: sulphate was extracted by an acidified ammonium acetate solution and converted to a BaSO4 suspension under controlled conditions; the resulting turbidity was measured at a wavelength of 420 nm on a Thermo Scientific (Evolution 201) spectrophotometer using a K2SO4 solution as standard.
2.2. Spatial Modelling—Geostatistical Approach
The geostatistical approach for assessing the contamination risk of the alluvial plain was based on a group of 5 potentially toxic elements (Zn, Cd, As, Cu, Pb), tucked in the sediments, in two depth levels: the first up to 40 cm and the second above 40 cm during the dry time of the year. Data were used to compute a hundred simulated scenarios, through Sequential Gaussian Simulation (SGA), to study how high concentrations of PTEs linked up and to study how the presence of these factors can be mutually decided. Moreover, among the samples taken in during the rainy season, 2 were selected and were overlapped to the final Mean Image map (MI) to evaluate the associated spatial risk variability, along with the hydrologic cycle.
The spatial distribution patterns of the PTEs were established using a two-step geostatistical modelling methodology:
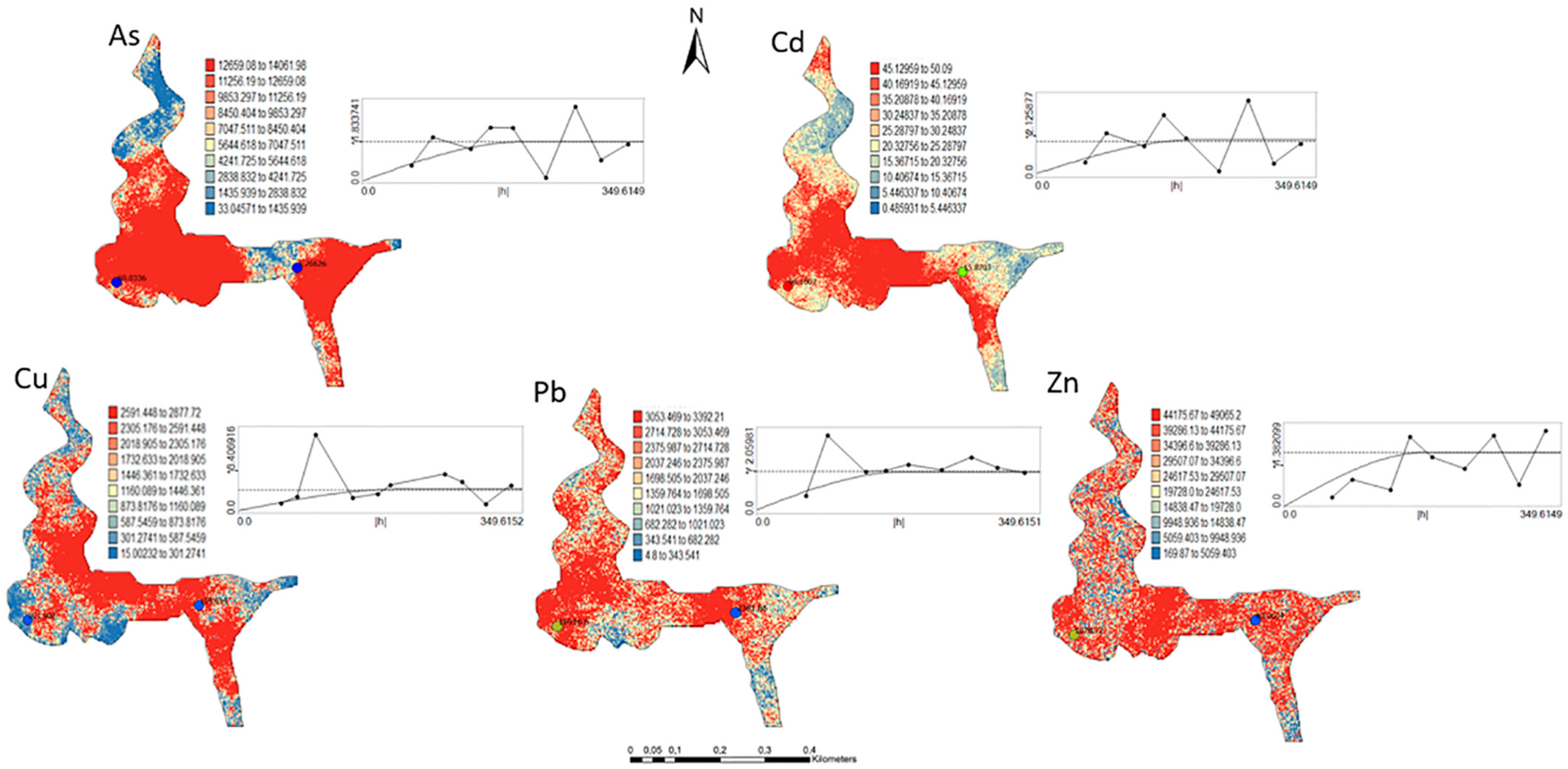
- Structural analysis and experimental variograms were carried out on the selected attribute. Variograms are vectorial functions used to calculate the spatial variation structure of regional variables [22,23]. Its argument is h (distance), where Z(xi) and Z(xi+h) are the numeric values of the variable observed in points xi and xi + h. The number of forming pairs for an h distance is N (h). Thus, it is the median value of the square of the differences between all pairs of points existing in the geometric field spaced at an h distance [23]. The graphical study of the behaviour of the variogram gives an overview of the spatial structure of the variable. One of the parameters that supply this information is the nugget effect (C0), which shows the behaviour at the origin. The other two parameters are the sill (C1) and the amplitude (a) which define correspondently the inertia used in the interpolation process and the influence radius of the variable (Table 1).
 Table 1. Variogram parameters for the fitted isotropic models.
Table 1. Variogram parameters for the fitted isotropic models. - Sequential Gaussian Simulation (SGS) was employed as a stochastic simulation algorithm. SGS begins by setting the univariate value distribution by performing a normal score transformation of the original values into standard normal distribution. Normal scores at grid node sites were sequentially simulated with simple kriging (SK) using normal score data and a zero mean [41] After all normal scores were simulated, they were retro converted to original rank values. For the calculus, the Space-Stat V software. 4.0.18, Biomedwere, was used [42]. The output of a simulation is a misrepresented variant of an estimation process, which reproduces the statistics of the known data, producing a realistic feel of the exemplar, but supplying a low prediction behaviour. If multiple sequences in the sequential simulation process are developed, more reliable probabilistic maps can be obtained.
3. Results
3.1. Geochemical Assessment of Metal Contamination
3.1.1. Discrete Data Evaluation
The physicochemical parameters of sediment most affecting solubility, mobility, and the precipitation of potentially toxic metals [6,43] are pH and redox potential. Table 2 synthetises the corresponding descriptive statistics for the two alluvial plains in both seasonal periods.

Table 2.
pH and redox potential values of the sediments of the two alluvial plains in the dry and rainy periods.
In the dry period, the pH values of sediments are slightly acid, with average and median values ranging between 5.91 and 5.94 in Consciência floodplain and between 6.35 and 6.45 in Grota Seca. The minimum values are similar, approximately 4.8–4.9, and the maximum ones are about 7.5, corresponding to environments already mildly alkaline. After the rainy season, a slightly more alkaline environment was identified in Consciência alluvium, with median values of 6.4. At each point, although there are no significant differences in pH values from the upper to the deepest layers, the variation in depth does not follow a homogeneous pattern, and the highest acidity levels can be in more superficial or deeper layers, independent of the period of the year. Identical heterogeneity was observed concerning redox distribution in depth. Eh values that correspond to conditions of major reduction occur naturally in the layers where the highest moisture content was observed, located randomly over the entire length of the sedimentary profile regardless of the period of the year. Most values indicate a prevailing oxidizing environment in both sampling periods, with a slightly more reducing condition after the rainy season due to the higher water saturation of sediments.
From the bulk geochemical data obtained in the dry and rainy seasons (Table 3), samples were classified accordingly to the degree of contamination based on the guideline values established by the Minas Gerais State laws for the quality of dredged sediments [44].

Table 3.
Minimum, maximum, average, and median levels of metals and arsenic in sediments from the background area, Consciência and Grota Seca alluvial plains, obtained in aqua regia digested samples in both seasonal periods. Guideline values for dredged sediments for Minas Gerais State [44].
In both alluvial plains, sediments show concentrations of Zn, Cd, Pb, Cu, and As far exceeding the background levels and critical limits. Ni is also significant, although this element rarely reaches excessive levels. Zn is by far the most important contaminant, exhibiting much higher concentrations than the legislated limits, and Cd, although a vestigial element in most geological materials and substantially lower than Zn, is another very harmful and significant contaminant, sometimes reaching values 50 times higher than the permitted by law. Arsenic shows a heterogeneous behaviour, with very low contents in a few samples, while critical in others, corresponding the highest contents in the Grota Seca alluvial plain, where it preferably concentrates in surface layers up to 60 cm deep.
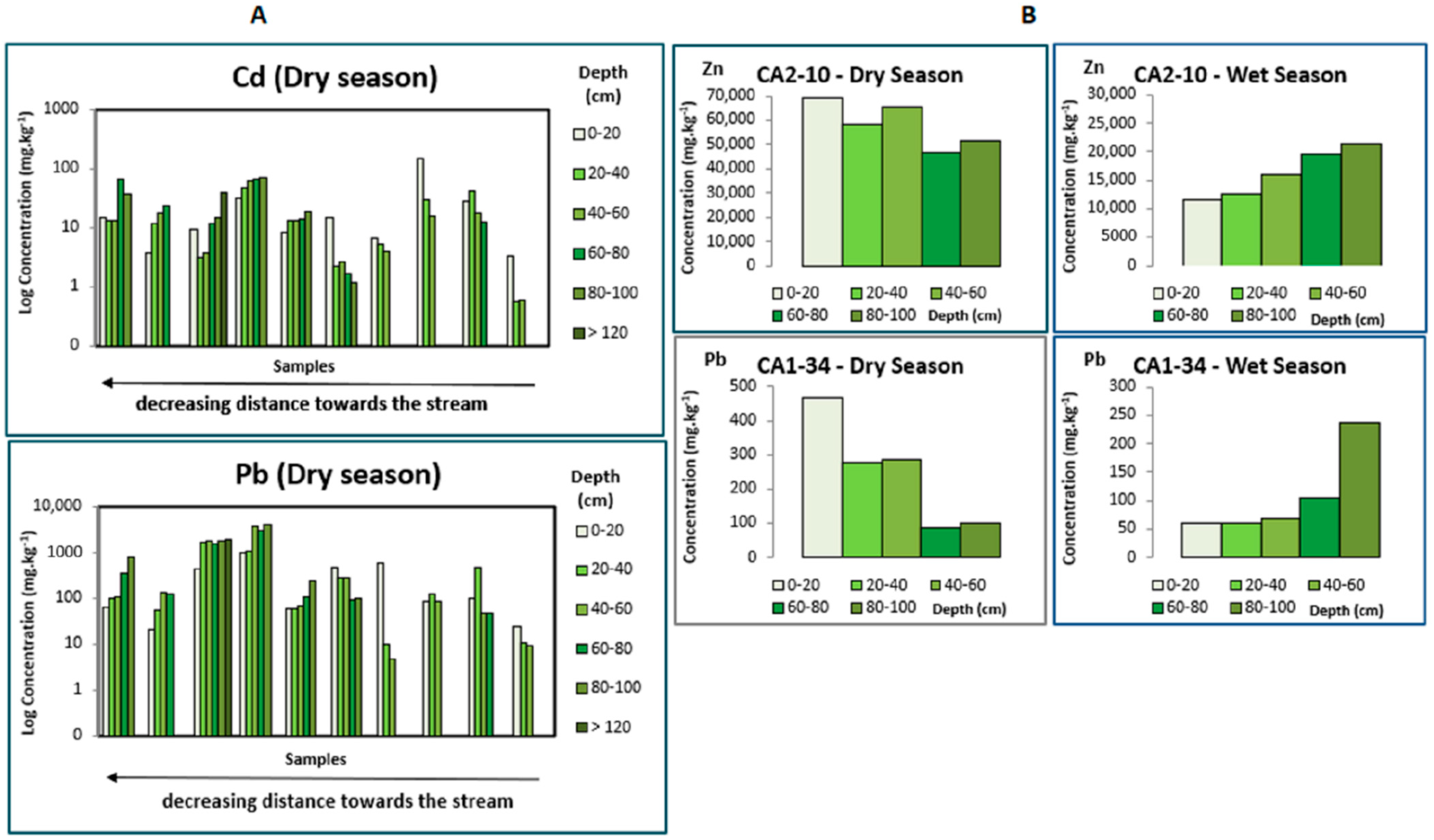
To understand the influence of climate on concentrations of metallic elements and their chemical associations, the levels in both seasonal periods were compared. It is notorious for a general increase in the post-dry period, although this variation is not uniform for all elements nor all sampling points. The seasonal effect on metal distribution is also observed through the content fluctuation at depth. A preferential accumulation of a few elements, especially Zn and Pb, and, in a few cases, Cd, at the less deep layers during the dry season increases in deeper areas after the rainy period (Figure 3A). This seasonal fluctuation reveals the mobility of these elements dependent on the amount of water available.
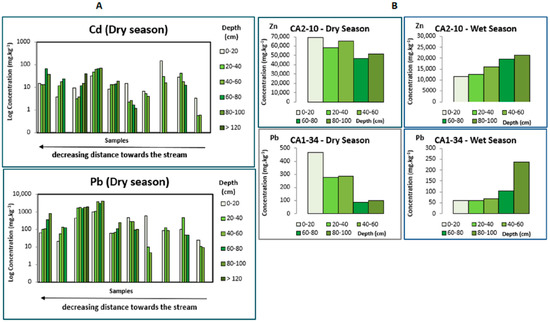
Figure 3.
Evolution of metal concentration at depth according to the seasonal periods. In (A), both graphs represent the evolution at depth for Cd and Pb in several samples in the dry season. Depending on the proximity of the stream, higher levels occur in the deeper layers or at the surface. In (B), graphs on the left represent the dry season, and on the right the rainy season, where, along with a clear decrease in metal contents in the rainy season, an increase in surface levels in the dry period and higher concentrations in deeper levels after the rain are evident. Zn and Pb were depicted as an example, respectively, for the first (CA2-10) and second samples (CA1-34).
Although there is an important influence of the climate on metal distribution at the surface and at depth, a few differences can be observed according to the deposits’ location and topography.
Depending on the proximity to the stream, higher PTEs levels occur (1) in the sediment deposits of the stream banks, the metal distribution at depth shows a slightly different pattern concerning pH and Eh values. Moreover, high concentrations on the bottom are often stable regardless of the season. (Figure 3A). This behaviour may be due to an increase in the degree of wetting and infiltration capacity in the sedimentary columns, which are emerged for most of the year, with a consequent drag of the metals in more soluble phases towards the deeper layers. (2) The most homogeneous distribution in depth is found in flat or almost flat areas flooding during the rainy season, leading to the rainwater accumulation, and consequently possible stagnation at the deposits’ surface. In these cases, there is a clear increase in the concentrations at the surface level during the dry period as a higher concentration in the deeper levels is observed after the rain (Figure 3B). The slow water’s infiltration, due to embedding sediments’ low permeability, drags at depth the elements in more labile forms, being the seasonality responsible for its uniform accumulation along the hydrological year.
Sulphates represent another contaminant group, produced during chemical oxidation of sphalerite (zinc sulphide ore) used in the industrial plant for zinc alloy’s metallurgical concentration (Table 4). Over the entire length of the alluvial basins, these compounds have high contents and are heterogeneously distributed, though the higher concentration is observed in the vicinity of the industrial plant. The distribution in depth is also very irregular, depending on the topography and sediment composition, namely concerning the content of the metal. Throughout the alluvial plains, sulphates occur either in soluble form or as a set of different precipitates; a few of them contain metallic elements [16]. Extensive deposits of sulphated precipitates are observed at the surface, especially during the dry period.

Table 4.
Minimum, maximum, average, and median values of sulphate concentration in alluvial sediments in the dry season.
Although with lower concentrations, but still high, the sediments on the right bank of Consciência stream are worth mentioning, corroborating the high mobility of these compounds.
3.1.2. Continuous Data Evaluation—Spatial Patterns and Spatial Uncertainty
In the first step, experimental variograms for each selected PTE for the two computed levels were computed for variable structural characterisation. No clear evidence of anisotropies was found, and isotropic variograms were computed and corresponding models were fitted. The quality of the model of uncertainty provided by simple kriging (SK) (zero mean) was assessed using the same source and destination geography approach, whereby SK results at sampled locations were compared to observations. Correlation indices ranged between 0.70 and 0.85. Therefore, cross-validation results were considered satisfactory for the selected models, thereby indicating consistency between the estimated and observed values. The graphic behaviour of the variogram function provides an overview of the spatial variation structure of the variable [45]. One of the parameters that provides such information is the nugget effect (C0), which shows the behaviour at the origin [46]. The other two parameters are the sill (C1) and the Range (a), which define correspondingly the inertia used in the interpolation process and the variable structure influence zone (Table 1).
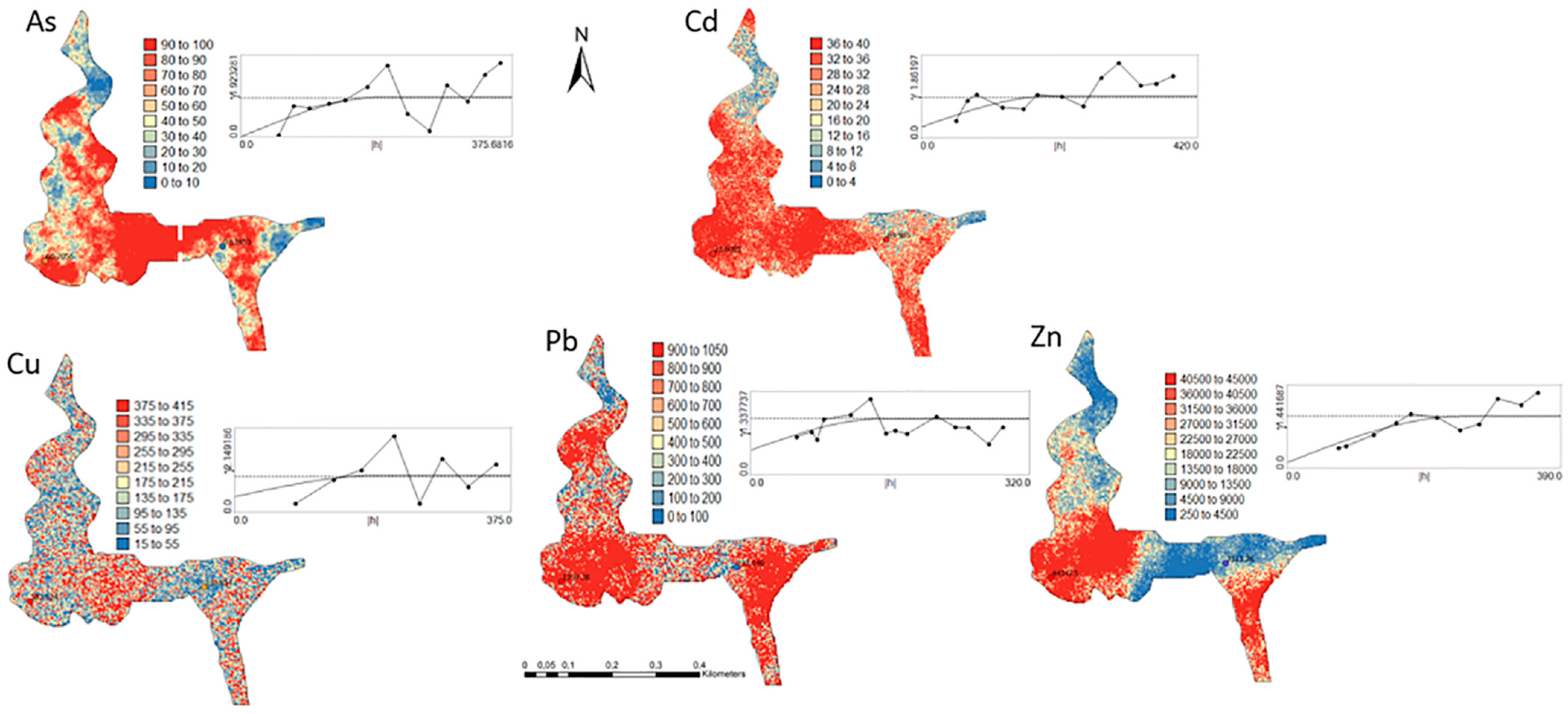
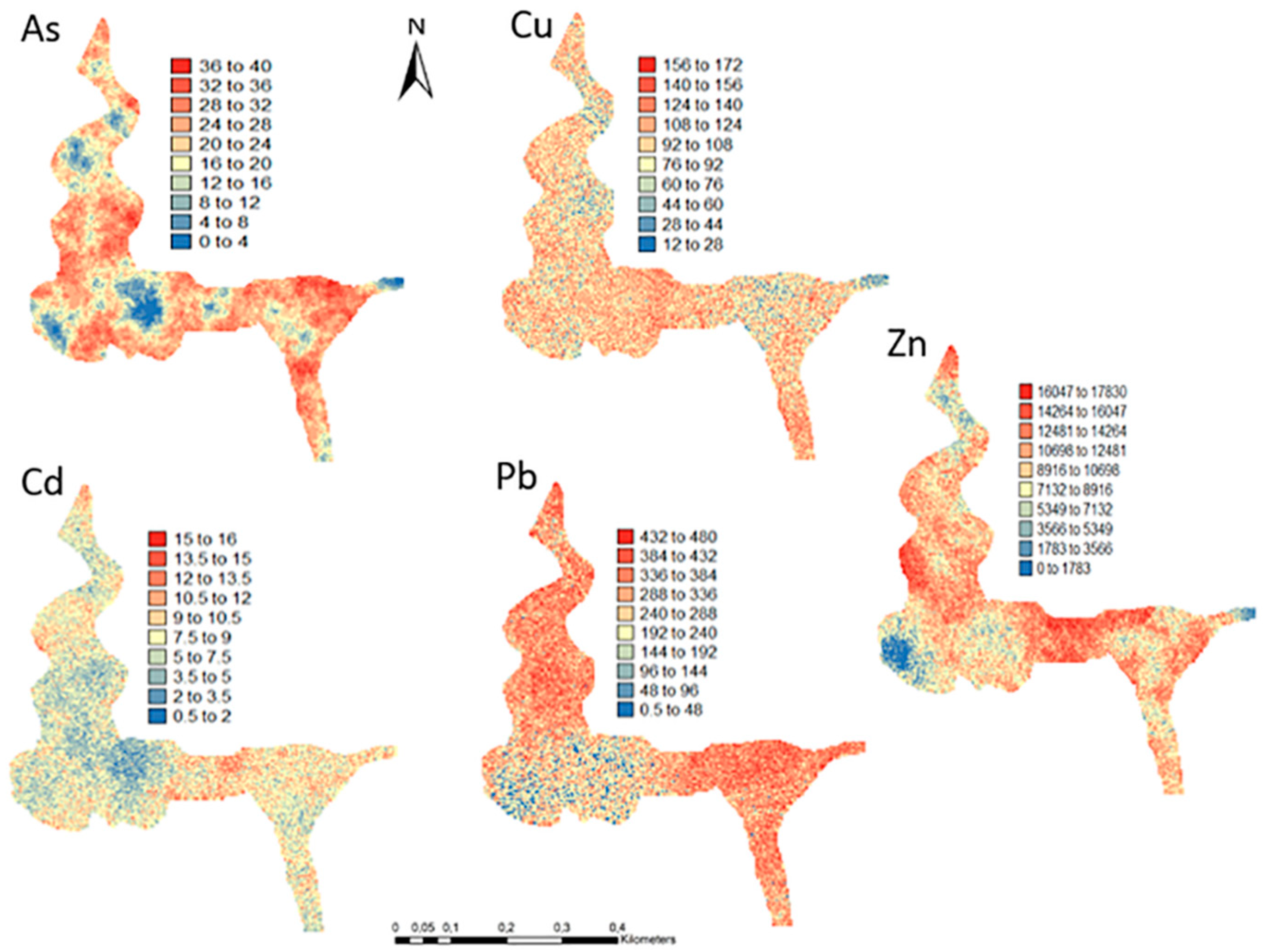
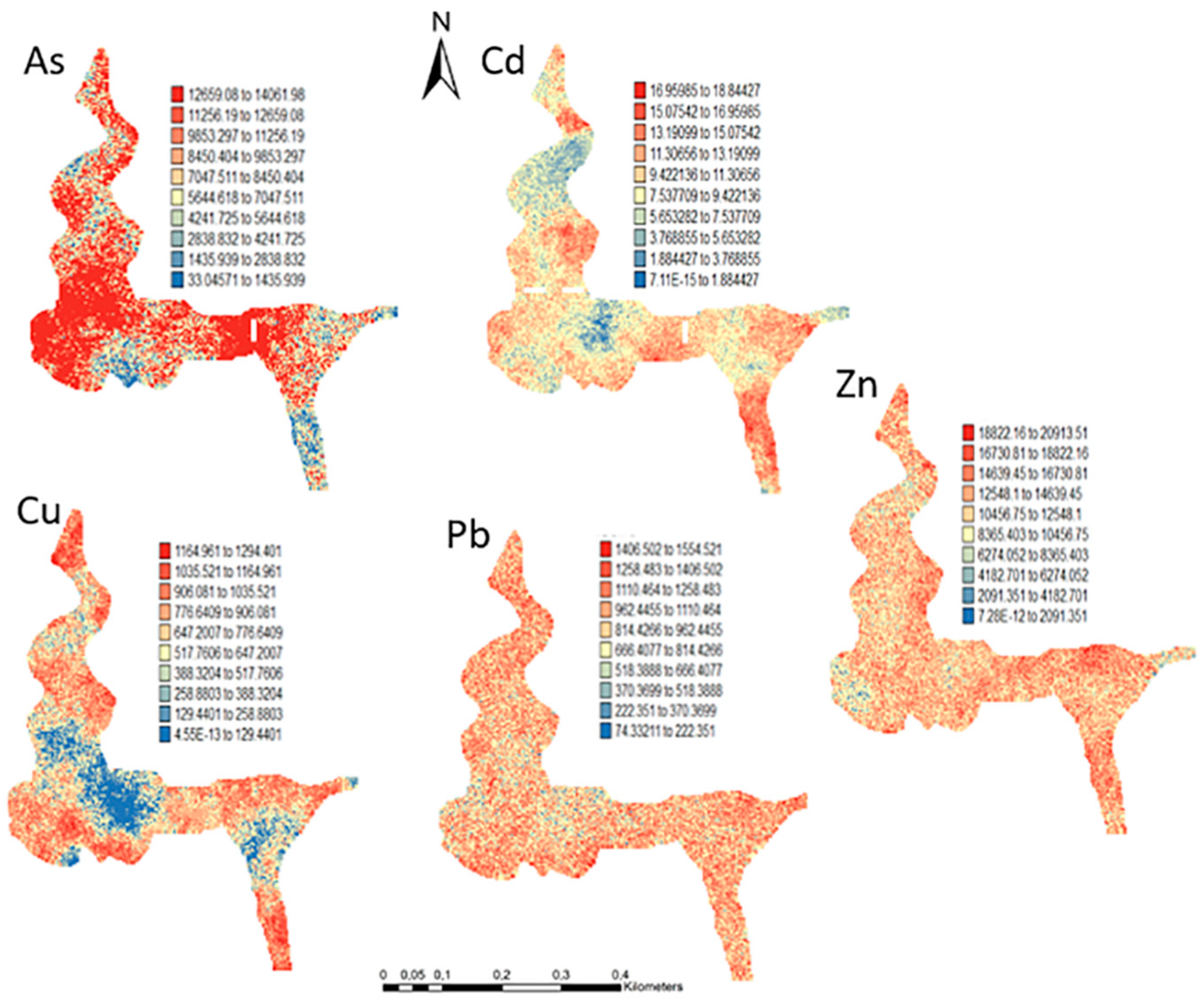
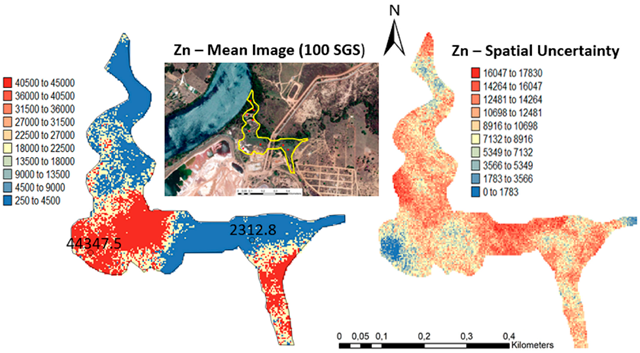
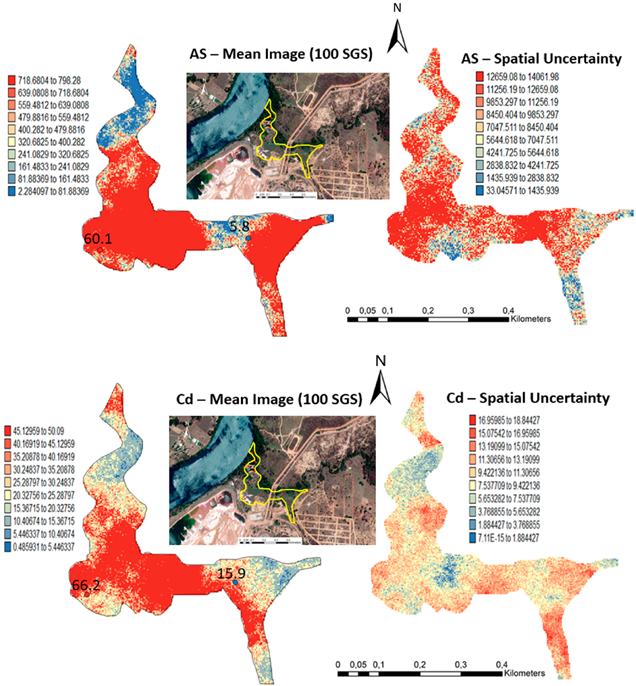
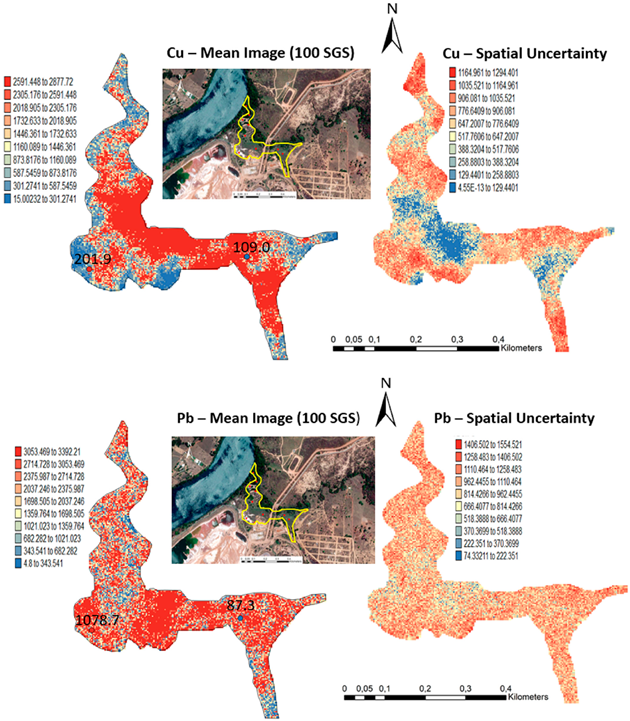
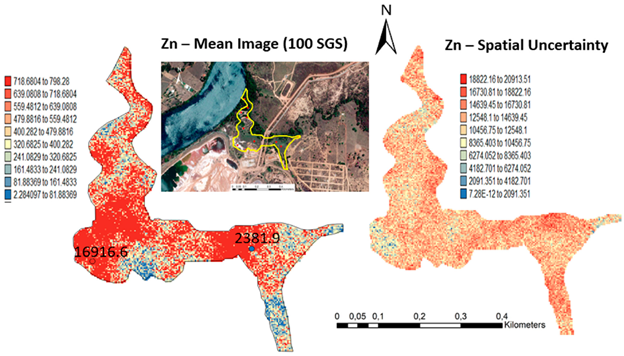
One hundred simulations were performed using Sequential Gaussian Simulation (SGS) as a conditional stochastic simulation of the As, Cd, Cu, Pb, and Zn content. The calculation of the spatial uncertainty—the standard deviation of each pixel—set aside the discussion of local accuracy. Geostatistics allows finding answers to problems with space–time indexation [47]. A stochastic SGS model on a 100 × 100 m grid was used to generate 100 equiprobable scenarios. However, the issue is that no single realisation can be taken as a better representation of reality than any other, and the mean spatial images (MI)—average maps—are afterward used to assess the spatial pattern of each variable (Figure 4 and Figure 5), while the spatial variability images (standard deviation maps) allow the quantification of spatial uncertainty for each attribute (Figure 6 and Figure 7). The spatial patterns are shown, and the computed clusters allowed classification in growing zones of risk. The overlapped points, corresponding to sediments deposited in the margins of the streams and collected during the rainy season show a decreasing risk of contamination for all the PTEs mainly on the deeper level, thereby indicating that in areas having a high wetting degree throughout the year, contamination spreads at various levels of depth to the most superficial layers during the dry season, thus affecting a larger area than what was previously assumed. Low to moderate spatial uncertainty is generally associated with the prediction scenarios, indicating accurate representations for risk contamination with PTEs.
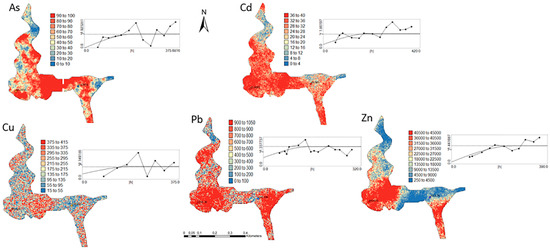
Figure 4.
Level 1 (Appendix A) (0–40 cm)—Sequential Gaussian Simulation Mean Image for the concentration spatial distribution of As, Cd, Cu, Pb, and Zn.
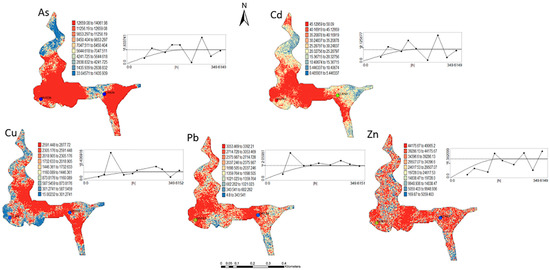
Figure 5.
Level 2 (Appendix B) (+40 cm)—Sequential Gaussian Simulation Mean Image for the concentration spatial distribution of As, Cd, Cu, Pb, and Zn.
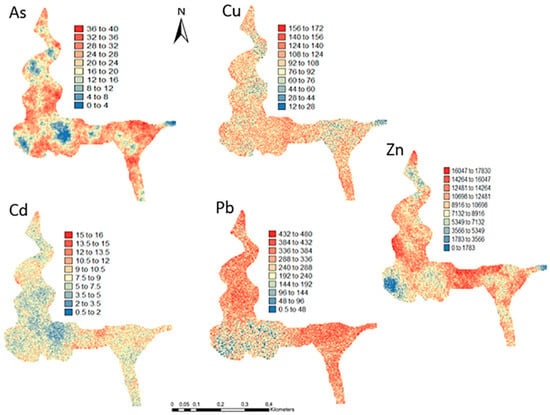
Figure 6.
Level 1 (Appendix A) (0–40 cm)—Sequential Gaussian Simulation Mean Image for the spatial uncertainty of As, Cd, Cu, Pb, and Zn.
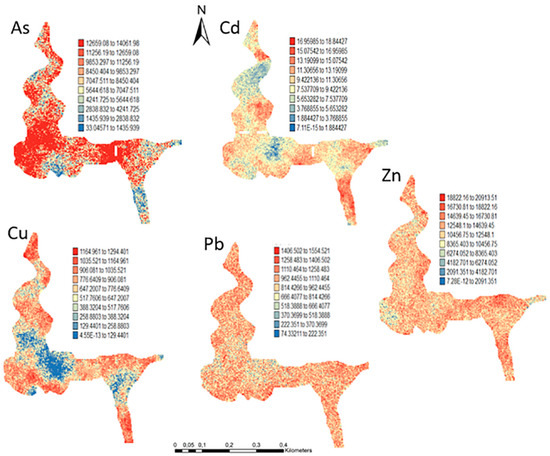
Figure 7.
Level 2 (Appendix B) (+40 cm)—Sequential Gaussian Simulation Mean Image for the spatial uncertainty of As, Cd, Cu, Pb, and Zn.
3.2. Assessment of the Geochemical Mobility of Metals
The assessment of metals’ mobility in the alluvial sediments has been made based on (1) quantification of elements’ soluble content through chemical analysis of the interstitial water and (2) determination of the proportion between the main mineral and organic components (where the different metals are associated) through selective and sequential extraction procedures.
3.2.1. Geochemistry of the Interstitial Water
Interstitial waters correspond to the aqueous fraction found in sediments’ pores, where the interaction “sediment–water” takes place. Metals dissolved in porewaters are in their higher soluble form, and therefore show the highest mobility and quick leaching into the hydrographic system. Rainfall, or only advection and diffusion processes, justifies this at-depth movement [43]. Its chemistry enables the identification, in the first step, of the PTEs in the most harmful forms to the environment [6].
The pH of interstitial waters ranges between 5.02 and 7.09, with a median value of 5.90 in the dry season and a slight increase in the post-rain period (pH: 5.88–7.48, median: 6.94). The electrical conductivity is relatively low (0.58–2.75 mS·cm−1), with higher values in a few areas of the alluvium near the plant.
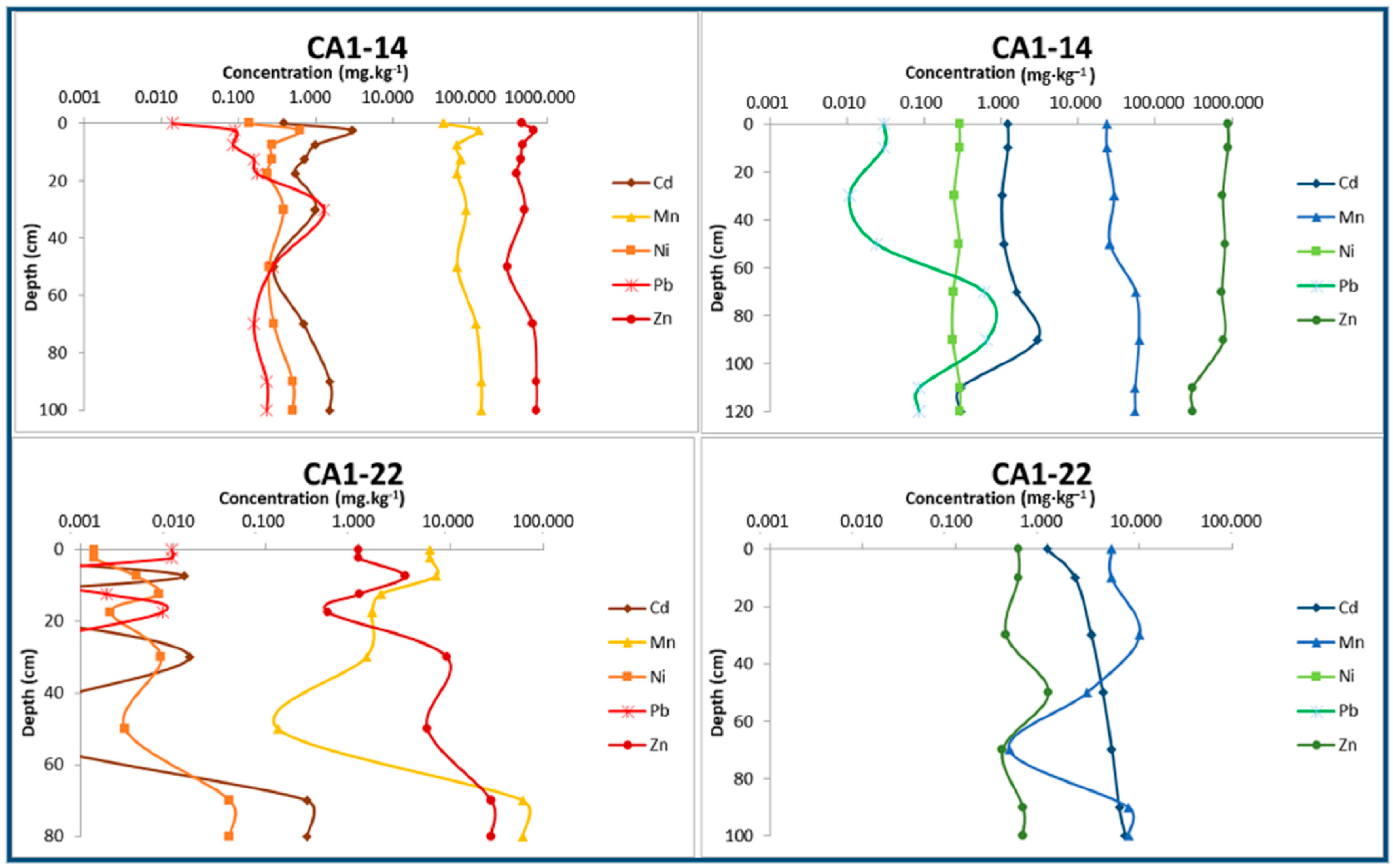
Considering the hydrogeological definition of groundwater as the water stored in the pores and cracks of the rock substrates and sedimentary materials [48], the chemical composition of the extracted alluvium’s interstitial water, in the absence of specific legislation, was regulated by the limits imposed by the legal provisions for groundwater in Brazil [49]. The soluble contents of metals are very often higher than those permitted by law. The values that are most in non-compliance correspond to Cd, Mn, Zn, and, to a lesser extent, Ni and Pb. As an example, Zn, whose normative limit is 1.05 mg·L−1, shows soluble contents of 800–900 mg·L−1 measured in samples along the banks of Consciência stream and represents a value of almost 900 times higher than the legally admitted threshold. In these samples, also the concentrations of Cd in the order of 2–3 mg·L−1 correspond to values about 600 times higher than the legal threshold (0.005 mg·L−1). The distribution of metal contents in soluble forms is consistent with their concentration in the extractable fraction of sediments (fraction digested with aqua regia). Although with very high contents in the extractable fraction of some samples, As does not show significant concentrations in the soluble phase of the materials, seldom exceeding the permitted limits for groundwater. Compared to the values measured in the background area [15], in these alluvial plains there is a clear enrichment in all metallic elements, including those not considered as contaminants in this study (Ni, Cr).
The environmental chemical conditions and the sediments’ chemical composition, especially related to the levels of Fe and Mn oxides, differ greatly within the alluvial plains and from the surface to the deepest layers. Thus, the solubility balance of the metals may vary significantly by a few cm or mm, either in depth or at surface. This local variability may explain the high heterogeneity in the dissolved metal content. Regarding the high spatial variability, the great variability between minimum, maximum, average, and median values for all the elements in the same alluvial plain is noteworthy.
Comparing the metal content in their dissolved forms corresponding to the post-dry and post-rain periods (Figure 8), it is possible to denote that most metals (Cd, Cr, Cu, Mn, Ni, Pb, and Zn) have a higher content in the dry period, while elements such as Fe tend to increase their concentration after the rain.
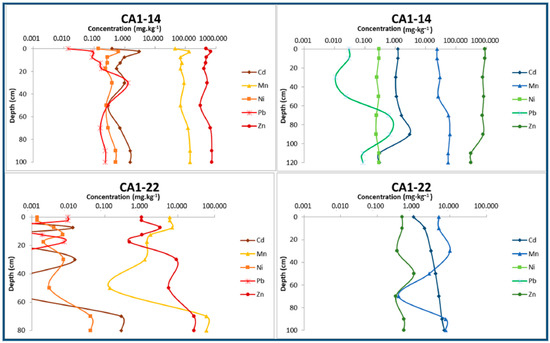
Figure 8.
Concentrations of the main potentially toxic metals dissolved in interstitial waters of two samples representing the alluvial sediments set. On the left, metal profiles correspond to the dry season, and on the right to the rainy season.
3.2.2. Geochemistry of Metals Speciation
Metals can be found in sediments in various chemical forms or bounded in different ways. In unpolluted materials, they are preferably bounded to silicates and other primary minerals, forming relatively immobile mineral species, while in contaminated areas they are usually associated with more mobile phases [50,51].
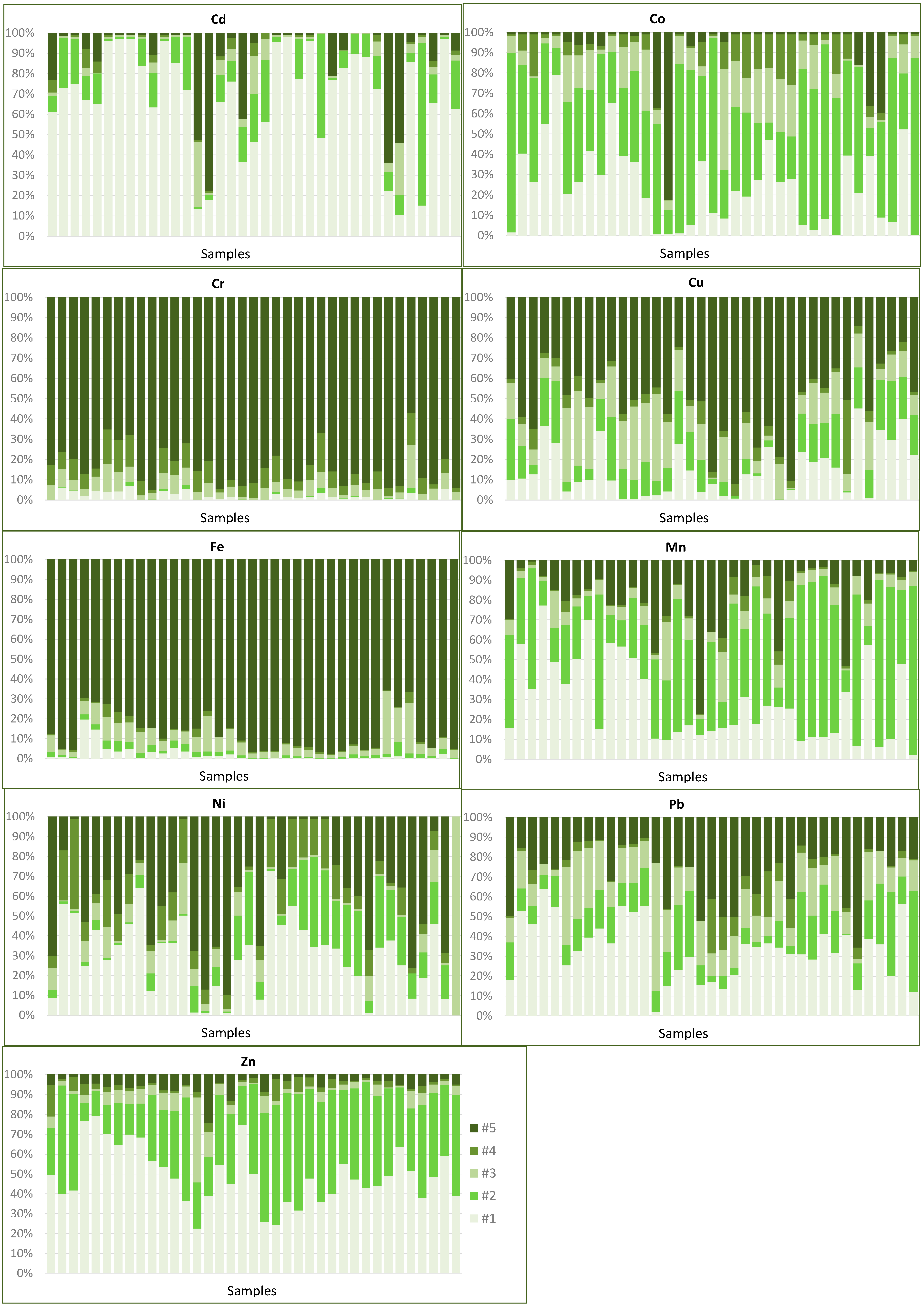
In environmental programs for the remediation of contaminated areas, the mobility and bioavailability assessment of potentially toxic metals in soils and sediment are of particular importance, which makes their analysis through selective extraction techniques [6,52,53] fundamental. The methodology used in this study [37] enabled the quantification of each metal into distinct chemical forms: (1) labile forms (#1)—water-soluble, adsorbed in exchangeable sites and bounded to carbonates; (2) easily reducible (#2)—bounded to Mn oxides; (3) moderately reducible (#3)—bounded by adsorption, precipitation, or occluded in amorphous Fe oxides; (4) oxidizable (#4)—bounded to sulphides and organic compounds; and (5) weakly reducible (#5)—bounded by adsorption, precipitation, or occluded in crystalline Fe oxides.
Based on the results achieved through the sequential extraction analysis, a summary can be made concerning the main associations of the metallic elements in the sediments of these contiguous floodplains (Figure 9):
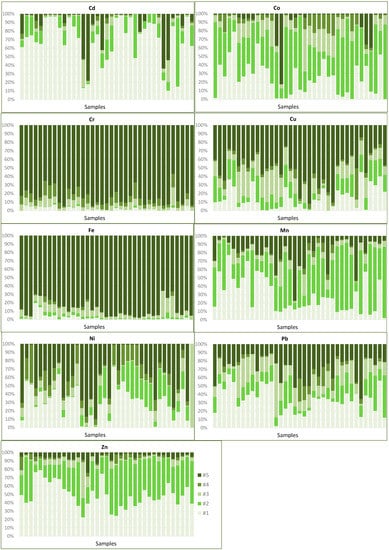
Figure 9.
Fractions of the alluvial sediments extracted in each of the sequential phases for Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb, and Zn. Data for the dry season. #1: soluble fraction, as exchange cation and bounded to carbonates; #2: fraction bounded to manganese oxides; #3: fraction bounded to amorphous iron oxides; #4: fraction bounded to organic material and partially to sulphides; #5: fraction bounded to crystalline iron oxides.
Cd and Zn can be distinguished as the most dangerous elements in the environment because, besides the high contamination levels, approximately 90% of both elements are associated with the most soluble phases (#1) and (#2). Based on the average values, 69% of Cd and 45% of Zn are in soluble and exchangeable forms, while 14% for Cd and 46% for Zn are linked to Mn oxides. The distribution of Zn among the various fractions is much more homogeneous than that of Cd, both spatially and vertically throughout the entire thickness of the alluvial material, with no significant variations between the two seasonal periods. The heterogeneity of Cd levels is clearer in both labile fractions, with a significant increase after the rainy season.
Two other elements with critical concentrations, Pb, and Cu, present less danger in the environment as they are preferably associated with crystalline Fe oxides. These elements show irregularity in the distribution between the different mineral phases, particularly in the two most mobile fractions: (#1) and (#2):
i-Cu is more stable given the higher proportion in the more immobile phase (approximately 60%), with about 20% in forms of greater solubility. It is mostly precipitated or co-precipitated in Fe oxides, either in crystalline or amorphous forms. However, in a few samples, very high percentages are extracted in the easily solubilised fractions, which should be associated with local contamination.
ii-Pb is mostly associated with less stable phases (about 30% in the soluble phase or as exchangeable cation), although in some samples it occurs in more stable mineral forms, associated with crystalline Fe oxides (about 35%) and, to a lesser extent, is associated with sulphides (about 9%). Considering the high levels of Pb in these sedimentary materials, the proportion existing in more mobile forms needs some attention.
Arsenic has a very irregular distribution, although most of it is in more stable forms, with about 45% linked to crystalline and 27–30% to amorphous Fe oxides, remaining only 20% in more mobile fractions. In these alluvial sediments, only if chemical modifications occur (such as a pH decrease and an increase in reducing conditions) can Fe oxides be solubilised with the subsequent release of As. Only under these conditions can this element be leached towards the streams, representing a danger to the environment.
Ni occurs in association with more labile mineral phases (extractable in the first two steps of sequential extraction #1 and #2), especially in soluble or exchangeable forms. However, in a few samples, between 40% and 60% of this element can be found in more stable forms, linked to crystalline Fe oxides. The heterogeneous behaviour verified between samples may be due to local contamination or due to small variations in the physical–chemical parameters which can lead to the dissolution of the more stable phases.
Mn, with very high concentrations and very heterogeneous distributions in these alluvial sediments due to its solubility much influenced by the chemical conditions of the environment, occurs preferentially in the form of oxide, but there is a relevant proportion (21%) linked to crystalline Fe oxides, and approximately 25% linked to the soluble phase or as an exchangeable cation. These forms of greater mobility are the most influenced by climatic conditions, showing a significant increase after the rainy season.
Co, although occurring preferentially in association with the most labile fractions (Mn oxides in greater proportion, between 50% and 60%) and therefore having high mobility, does not present a danger to the environment, given the low concentrations found in most sediments.
Cr and Fe are highly immobile, so there is no danger in the case of Cr, which is a potentially toxic metal. Cr mainly occurs precipitated in crystalline Fe oxides (85%), and only 7–8% as inclusions or precipitated in sulphides. For Fe, about 90% occurs as crystalline oxides, and only 7.5% as amorphous forms.
4. Discussion
4.1. Geochemical Behaviour and Risk Assessment of Potentially Toxic Metals in the Alluvial Sediments
The sediment pH observed in areas under the influence of the plant’s activities are generally higher than the common values of sedimentary materials in a laterite soils region under a tropical climate [54]. This is probably due to the accumulation of Ca sulphates, which have been observed in all areas, especially in the Consciência stream, and identified through the mineralogical analyses carried out by [16]. The slightly more alkaline environment especially identified in the deeper layers of the alluvium after the rainy season might be a result of the increased leaching rate of sulphates accumulated in areas closer to the plant, with the subsequent accumulation in the lower less porous and impermeable deposits. Due to the high mobility, sulphates may circulate throughout the area via processes of run-off and/or infiltration.
The high metal contents found along the floodplain reflect the chemical nature of the old tailings’ deposits, which were disposed of in inappropriate places for such practice, without adequate treatment or packaging. In Grota Seca’s alluvium, the high concentration of As in the sediments reflects the existence of a Zn purification plant a few years ago that used As and Sb trioxide, and at this time the tailings were placed in basins without any waterproofing. The surface and at-depth distribution of metals must be also related to the approach to the Zn treatment plant, as well as the run-off and groundwater’s principal flow directions from industrial activities and tailing dumps.
The fine-grained alluvial sediments characterised by median values of 41.9% silt, 27.5% clay, and 30.6% sand, contain a range of different minerals [15,16] with different properties and surface groups which could account for the high sorption of the metal cations. During the transport of these pollutants throughout the surface, subsurface, and sediments’ porewater, the colloidal phase can play a major role due to the high specific surface area and high reactivity [55]. Additionally, both pH and redox potential in sediment/water interaction are significant parameters in the metals’ mobilisation and transformation [56]. These contiguous alluvial plains are characterised by very high evaporation rates and limited hydrodynamics, favourable to reducing conditions [6]; however, this is only observed in the deeper layers of the riverine sediments with a regime of constant water saturation, which may lead to sulphide precipitation and metal concentration in the host sediments. Due to metallurgical processing in the plant or to natural mechanisms in areas further from the streams, oxidizing conditions prevail, and then fine particles of primary Zn sulphides, the main Zn ore material processed in this plant, might be quickly decomposed, releasing metals and hydrogen ions. The released H+ will further acidify some deeper layers of the sediments, which show the lowest pH values (pH 5–5.5) in the dryer season. Moreover, metals released during oxidation may be adsorbed by fine-grained particles, such as Fe and Mn oxides and hydroxides, clay minerals, and even organic matter [6,57], so that metal mobility and/or retention are strongly controlled by the presence of such materials.
The slight increase in most metallic elements after the dry season followed a period of heavy rainfall. The latter season led to the leaching of elements associated with high mobility phases from the alluvial plains towards the streams and to the deeper levels of sediments. Thereafter, the sudden drought period led to the deposition of these elements under particulate or dissolved phases, mainly in the surface layers of the sediments near the stream, leading to the increase in metal content. The seasonal variations observed in the levels of the various elements and data obtained from the sequential extraction analyses suggest different geochemical behaviour. Elements with high variation occur in sediments in a more labile form, and those with higher uniformity are associated with more stable mineral phases. The evolution of contents at depth suggests the phenomena of transport and underground circulation of metals. Since these sediments are saturated or under-saturated in water, and given the pH and Eh values favourable to the mobility of most metallic elements, they certainly circulate in more soluble forms at different depths of the alluvium, becoming immobilised in the most superficial layers when evaporation occurs and in the deepest layers when there is an increase in the water retained.
Although sulphates are not potentially toxic compounds, their high levels in this alluvial plain may lead to serious environmental problems in the Consciência stream, whenever they are leached towards it, causing an increase in the conductivity and salinity of the water column. Another negative impact of their presence is related to their association with metallic elements such as Cd, Zn, and Cu, forming soluble compounds. This association may increase the mobility of these metals which, through diffusion mechanisms, may reach areas far from the source, increasing their dispersion.
4.2. Metal Mobilisation in the Alluvial Sediments
Physical and chemical parameters such as pH and Eh are determining factors, affecting the solubility, mobility, and precipitation of potentially toxic metals in sedimentary materials [6,43]. High pH values enhance the adsorption and precipitation of most metals as oxides, hydroxides, and carbonates [58], whereas metal solubility, occurring as hydrated free cations (Fe, Cu, Zn, Pb, Cd, Ni, Mn), usually increases with decreasing pH. However, the pH values at which metal transport starts for a specific element are determined together with the Eh values [6].
4.2.1. Geochemistry of the Soluble Phase
Determination of chemical concentrations of potentially toxic elements in porewater is recommended, in addition to the regular contaminant measurements conducted in the sediments as a means of providing information on the pathways and levels of exposure, aiding the interpretation of results and the choice of the most suitable technology for the remediation of a contaminated area [3,7].
Analyses of the interstitial water from these alluvial deposits have shown that concentrations of Zn, Cd, Mn, Ni, Pb, and sometimes, As, are often above the legal values for groundwater [49] and reinforce the assumption that the contamination in this area is primarily due to the circulation of metal-rich subsurface waters. The high concentrations of these elements in the aqueous phase of most sediments reflect their high contents in the extractable form. The absence of clay minerals with a high retention capacity for metallic cations (mineralogy dominated by Fe oxides and kaolinite) [15,16]), the low content of organic compounds (average Corg values of 0.7%, [15]), the chemical conditions of the floodplain, and the average pH values between 5 and 6 must also be stressed. Although showing generally high levels, As concentration in the soluble phase is not significant. This may be due, along with the oxidizing conditions not favourable to its mobility, to the high levels of Fe oxides [15,16], which ensure a strong immobilisation of As by anionic adsorption and block its availability in the aqueous phase.
The irregular vertical distribution of metals corroborates the heterogeneity also verified in sediment profiles concerning (1) metal contents in all extractable forms (obtained through digestion by aqua regia), (2) pH and redox potential values, (3) proportion of clay fraction [15], and (4) concentrations of Fe and Mn oxides [15], which are influencing factors for metal solubility. It should also be noted that the dispersion of the mobile phase of metals results from the combination of two processes [52]: longitudinal and transverse dispersion, with the solubility equilibria of each element varying along narrow breaks at depth. The mechanical dispersion and the diffusion in the interstitial water flow leads to significant differences in the elements’ concentration along the vertical profiles. Thus, for each sample, the vertical distribution of each metal in the soluble phase does not strictly coincide with the profile obtained for the same element, in forms associated with Fe and Mn oxides, sulphides, organic compounds, and exchange complexes (extractable forms).
The transport of the metallic elements in mobile forms through the alluvial sediments, according to [43], should be performed by mechanisms of advection, molecular diffusion, and the movement of the solution by mass or leaching flows. The advection will transport the elements at different speeds towards deeper layers, depending on their concentration in the interstitial water and the amount of water that moves. By diffusion, the elements move whenever there are concentration gradients, migrating from the areas of highest concentration to the areas where the concentration is lower. Via these transport mechanisms, metals may migrate through the length and thickness of the deposited materials, from areas of the source where the concentration is very high, to areas of lower concentration, thus leading to high mobilisation of these elements to areas further away from their source. These alluvial plains have a large accumulation of sedimentary materials, with a high humidity level, thus facilitating the transport of metallic elements along with the sedimentary columns by interstitial water. These circulating mechanisms must have occurred across the bed of Consciência stream, which justifies the high concentration of heavy metals in the deeper layers of the sediments deposited on the right bank of the stream, opposite to the unit. Metal enrichment in these sediments could also be the result of the deposition of elements in soluble or particulate form from the water of the stream during flood periods.
In the rainy season, the general decrease in the soluble metal contents in the various areas of the floodplains may be related to a dilution effect by rainwater and by the increase in sediment-water saturation due to the river’s rising flow. The only exception to this tendency is Fe, which shows an increase in the wet period, especially in the sediments located in areas closer to the stream, where the lowest redox potential values were found. Under these more reductive conditions, iron oxides will tend to dissolve, increasing their concentration in interstitial water. During the dry season, by evaporation, the water retained in the pores becomes more concentrated, significantly increasing the concentrations of most metallic elements, while Fe tends to precipitate as Fe oxyhydroxides.
The slight increase in pH and electrical conductivity values in the post-rain period may be related to an increase in the streamflow and consequent solubilisation of the leached sulphates from the industrial area.
4.2.2. Geochemistry of Metal Speciation
A combined analysis of the data obtained by partial digestion (aqua regia) and by sequential extraction shows that elements having concentrations far above the legal values are those that are preferably concentrated in more soluble forms, which considerably increases their environmental hazard. By contrast, elements such as Cr or Ni, which have moderate or low levels, occur in higher proportion in stable chemical forms, associated with crystalline Fe oxides or in residual forms (in the structure of silicate minerals or as resistant oxides). In the first case are Zn and Cd which, besides the high contamination levels, have approximately 90% of their content associated with soluble and exchangeable forms and Mn oxides. These elements, as well as the fraction of all other elements associated with these phases, correspond to the most soluble and easily mobilised elements/fractions in the sediments, representing a major environmental hazard. For these labile forms, any change in the chemistry of the environment may allow its solubilisation.
(Hydr)oxides of iron (III) and manganese (III, IV) are recognized as very important sinks of heavy metals in soils and sedimentary materials [43,56], existing with high abundance in all the studied sediments. Mineralogical studies carried out on these samples [15,16] revealed a large abundance of mineral oxides, especially Fe oxides of different natures (goethite, hematite, magnetite, ilmenite). Partial chemical analyses (aqua regia digestion) also showed, in addition to Fe, high levels of Mn, and sequential extraction procedures quantified a ratio of between 40% and 85% of this element occurring as oxides.
These oxides, in crystalline or amorphous forms, can occur in these materials as fine discrete particles or as films covering the surface of other minerals. Mineralogical analyses carried out with electronic microprobe have revealed the presence of these cases in these sediments [15,16]. The binding of metals to these oxides is performed through a combination of several mechanisms: co-precipitation, adsorption, surface complexes, ion exchange, and fixation inside the crystalline structure [6], and thus the presence of these compounds contributes to the existence of a dynamic heavy metal fraction [52]. In a reducing environment, such as in the alluvial marginal sediments during periods of higher wetting, metals associated with these oxides may be released; on the other hand, during periods of higher oxidation, there may occur re-precipitation of amorphous oxides, with co-precipitation and immobilisation of metals. Fe and Mn oxides have an affinity for retaining cations that have approximately the same dimensions as their ions—Mn3+, Fe2+, and Fe3+. Among the metals with higher affinity, the following are highlighted: Co2+, Co3+, Ni2+, Cu2+, Zn2+, Cd2+, Pb2+, [43,52]. All these elements were identified as contaminants in the sedimentary materials of these floodplains, which corroborates the high proportions that occur coupled to these oxides.
Associated with Mn oxides, in addition to the two elements that have the most critical levels in these materials (Zn and Cd), there is also a significant proportion of Pb and Co (35–50%). This represents the most easily soluble fraction of these elements, and those more dependent on oxidation–reduction and pH conditions.
With the most reductive fractions, amorphous and crystalline Fe oxides, some of the elements existing in very high concentrations in these alluvial materials, such as Cu and Pb, and others existing in less critical levels, such as Ni and Cr, are preferably associated. Significant proportions of an important set of elements linked to these oxides follow the average pH values between 5 and 6.5 which, as already mentioned, enhance the adsorption and precipitation of most metals as oxides and hydroxides. Regardless of the proportion in which they occur, any of these elements are preferentially associated with crystalline Fe oxide forms, which are the most stable, most difficult to solubilise, and most resistant to changes in pH or redox conditions and are therefore the ones that hold metals the most strongly.
The existence of variable loads on the surface of Fe oxides allows the adsorption of anions, such as arsenates (AsO4)3−, which explains why about 50% of this element occurs associated with these oxides. This adsorption is highly dependent on the pH, and only a small oscillation is enough to pass to the soluble phase, denoting the great potential for contamination of this element by slight changes in the chemical conditions of the environment.
The fractions that release metals more easily under conditions of increased oxidation are sulphides and organic compounds, represented by step #4 of the sequential extraction methodology. In these sediments, the metals that most easily precipitate with these compounds are Ni and Co, in which a slight decrease in their content in the most oxidised levels of the sediments and in the most oxidised period (dry season) is most noticeable, due to their release by the transformation of sulphides into sulphates.
5. Conclusions
The activity of an ore metallurgical plant in South-East Brazil (Minas Gerais State) has resulted in contamination by several heavy metals, which extends through the alluvial plain of a small stream (Consciência creek) and its affluent (Grota Seca creek).
As a result of the chemical processes used in the plant to extract and purify the ore, large quantities of metallic elements are mobilised, many of which are transported in soluble form through the sedimentary materials in both alluvial plains, in the banks and bed of Consciência creek, which may even be mobilised through the bed of the main river of this region, São Francisco, and therefore reach the opposite bank. Considering the elements which represent most of the processed ores in the unit (Zn, Cd, Cu, and Pb), the levels found in most sediments, from the surface to the deepest layers, are above the legal limits. In addition to the excessive concentrations, they occur preferably in soluble and/or adsorbed forms in different mineral phases. Preferential adsorption of Pb in Mn oxides, and Zn, Cd, and Cu in clay minerals [16] are common. These forms are easily mobilised and spread rapidly to areas further from the source of contamination, thus representing the elements with the greatest environmental hazard. Co and Mn are also in easily soluble phases; however, the contamination of these elements is more occasional. Cr, Fe, and Ni are in more stable phases and therefore there are no serious environmental problems in the sediments of the area concerning those elements. Arsenic has an especially high concentration in Grota Seca’s alluvium because of the existence of a no longer active Zn purification plant. However, the occurrence under the soluble form is low, being mainly adsorbed on the surface of Fe oxides. As this adsorption mechanism is highly dependent on pH, any small change of this parameter is enough to solubilise the host mineral, with the subsequent release of arsenic. Thus, this element may have a high potential for contamination since any change in the chemical conditions of the environment could lead to its mobilisation.
The seasonal variation stresses a slight concentration decrease for almost all the PTEs in the floodplain after the rainy season, indicating a fluctuation of their contents throughout the sedimentary columns. The elements’ accumulation is preferentially in the surface layers during the summer season, due to the ascending movement of interstitial water, followed by evaporation and subsequent accumulation at depth after the rainy season as a consequence of the increase of the infiltration and presence of water filling the sediments’ pores.
The geostatistical approach was used with the five key elements (As, Cd, Cu, Pb, and Zn) through Sequential Gaussian Simulation, which allowed the computation of space–time distribution patterns, considering two vertical layers (up to 40 cm, level 1; and above 40 cm, level 2), for the dry season. Furthermore, two points gathered during the rainy season were overlapped to the five Mean Images (MI) allowing the observation of a diminishing risk of contamination during the rainy season, mainly at depth. Future monitoring actions must be carried out and a set of climatic soft covariates considered for modelling purposes.
Changes in pH or redox potential for the most labile forms of metals (soluble, exchangeable cations, and bounded to Mn oxides) may increase the elements’ mobility, and therefore their bioavailability. Thus, sediments act as traps or sources of PTEs, depending on the physical, chemical, or biological processes that affect them and any changes in the chemical conditions of the water or sediments can have serious environmental consequences.
Given the company’s need to implement mitigation measures to reduce the medium- and long-term negative impacts on the environment, this study showed that due to the high mobility of the contamination plume, any process of removal of the contaminated material becomes unfeasible. The remediation strategy for the Consciência stream (and its tributary) and associated alluvial deposits must be based on in situ decontamination methods, which are inevitably time-consuming, involving several years of treatment. Moreover, to ensure that contamination of the São Francisco River is stopped, measures must be taken to ensure that the water of the stream is of good quality and that the São Francisco River is kept free of contamination from run-off and groundwater coming from the alluvial sediments.
Author Contributions
Conceptualization, R.F. and T.A.; methodology, R.F., C.P., T.A. and J.A.; software, T.A. and J.A.; validation, R.F. and T.A.; formal analysis, R.F., C.P. and J.A.; investigation, R.F., C.P., T.A. and J.A.; resources, R.F.; data curation, R.F.; writing original draft preparation, R.F. and T.A.; writing—R.F. and T.A.; visualization, R.F., C.P., T.A. and J.A.; supervision, R.F.; project administration, R.F.; funding acquisition, R.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Votorantim Metais S.A. Company through the consultant Project “Proposal of remediation strategy of Consciência and Barreiro Grande streams-phase 2”. Some of the equipment used in this study was purchased under the project INALENTEJO–Quadro de Referência Estratégia Nacional 2007-2013 (QREN) through the projects ALENT-07-0262-FEDER-001867 and ALENT-07-0262-FEDER-001876.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable due to industrial confidentiality.
Acknowledgments
The authors acknowledge the funding provided by ICT, under contract with FCT (the Portuguese Science and Technology Foundation) and by the European Regional Development Fund through COMPETE 2020–Programa Operacional Competitividade e Internacionalização (POCI). The authors are also grateful for the helpful comments and suggestions of two anonymous reviewers.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A. Level 1
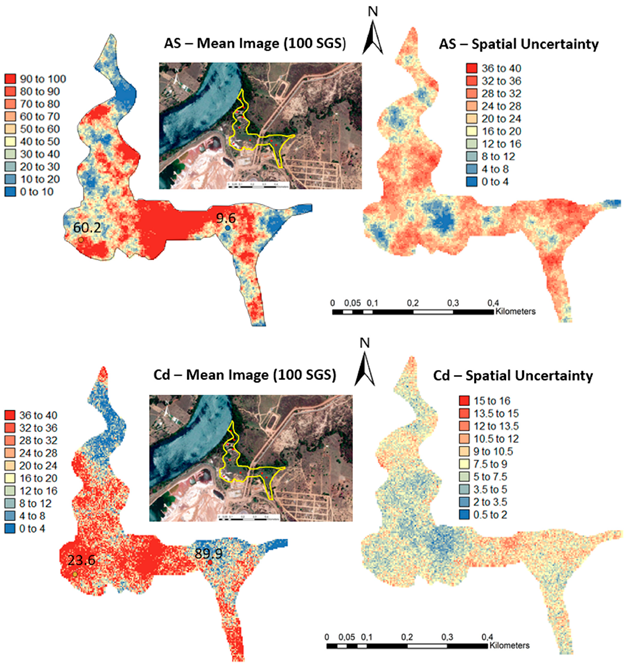
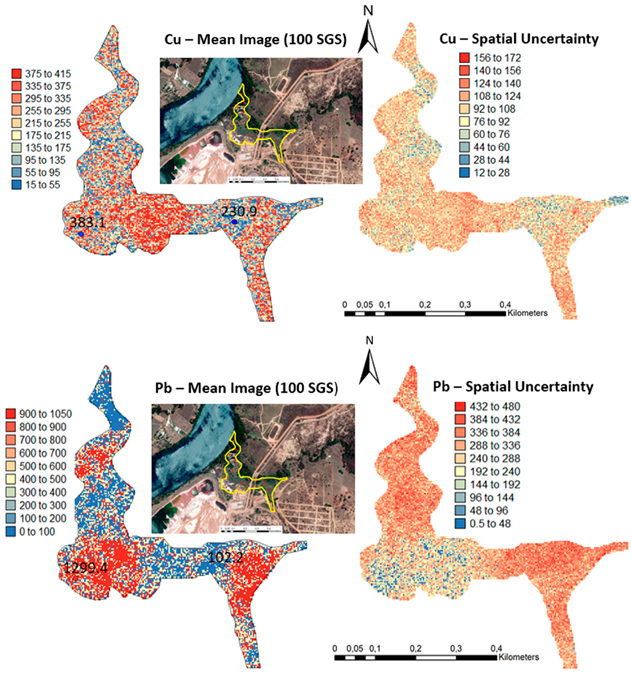
Appendix B. Level 2
References
- Bentley, S.; Thibodeaux, L.; Adriaens, P.; Li, M.-Y.; Romero-González, M.; Banwart, S.A.; Filip, Z.; Demnerova, K.; Reible, D. Physicochemical and Biological Assessment and Characterization of Contaminated Sediments. In Assessment and Remediation of Contaminated Sediments; Nato Series; IV Earth and Environment; Reible, D., Lanczos, T., Eds.; Springer Science & Business Media: Berlin, Germany, 2006; Volume 3, pp. 83–136. [Google Scholar]
- Long, E.R.; MacDonald, D.D.; Smith, S.L.; Calder, E.D. Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ. Manag. 1995, 19, 81–97. [Google Scholar] [CrossRef]
- Förstner, U.; Heise, S. Assessing and Managing Contaminated Sediments: Requirements on Data Quality–from Molecular to River Basin Scale. Croat. Chem. Acta 2006, 79, 5–14. [Google Scholar]
- Apitz, S.E.; Brils, J.; Marcomi, A.; Critto, A.; Agostini, P.; Micheletti, C.; Pippa, R.; Zuin, P.; Lánczos, T.; Dercová, K.; et al. Approaches and Frameworks for managing contaminated sediments–a European Perspective. In Assessment and Remediation of Contaminated Sediments; Nato Series; IV Earth and Environment; Reible, D., Lanczos, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 3, pp. 5–82. [Google Scholar]
- Reible, D.; Lanczos, T. Assessment and Remediation of Contaminated Sediments; Nato Series; IV Earth and Environment; Springer: Berlin/Heidelberg, Germany, 2006; Volume 3, p. 268. [Google Scholar]
- Siegel, F. Environmental Geochemistry of Potentially Toxic Metals; Springer: Berlin/Heidelberg, Germany, 2002; p. 218. [Google Scholar]
- Siepmann, R.; Von der Kammer, F.; Calmano, F. Determination of Heavy Metal Mobility from Resuspended Sediments Using Simulated Natural Experimental Conditions. In Sediment Dynamics and Pollutant Mobility in Rivers. An Interdisciplinary Approach, 1st ed.; Westrich, B., Förstner, U., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 258–268. [Google Scholar]
- Sahuquillo, A.; Rauret, G.; Rehnert, A.; Muntau, H. Solid sample graphite furnace atomic absorption spectroscopy for supporting arsenic determination in sediments following a sequential extraction procedure. Anal. Chim. Acta 2003, 476, 15–24. [Google Scholar] [CrossRef]
- Calmano, W.; Von der Kammer, F.; Schwartz, R. Characterization of redox conditions in soils and sediments: Heavy metals. In Soil and Sediment Remediation; Lens, P., Grotenhuis, T., Malina, G., Tabak, H., Eds.; IWA Publ.: London, UK, 2005; pp. 102–120. [Google Scholar]
- Tundisi, J.G. Avaliação das Condições Físicas, Químicas, Biológicas e Toxicológicas da Represa de Três Marias e do Rio São Francisco (Trecho Represa Três Marias–Rio Abaeté); Instituto Internacional de Ecologia e Gerenciamento Ambiental: Belo Horizonte, Minas Gerais, Brazil, 2005. [Google Scholar]
- Mozeto, A.A.; Nascimento, M.D.; Silva, E.F.A.; Fioravanti, M.I.A. Avaliação da Contaminação por Metais e Metalóides (Água, Sedimento e Peixe) no Rio São Francisco em Três Marias (MG-Brasil): Projeto de Pesquisa Participativa com a Comunidade Local; Relatório Final Técnico-Científico; Departamento de Química, Universidade Federal de São Carlos: São Carlos, State of São Paulo, Brazil, 2007; Available online: http://worldfish.org/PPA/PDFs/Semi-Annual%20VII/E-6b%20UFSCar%20Metals%20Project%20Technical%20Report-%20port.pdf (accessed on 14 January 2021).
- Almeida, D.F. Gestão Ambiental dos Sedimentos de Corrente do Rio São Francisco na Região de Três Marias/Minas Gerais. Ph.D. Thesis, Engenharia Metalúrgica e de Minas, Universidade de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil, 2010; p. 94. [Google Scholar]
- Ribeiro, E.V. Avaliação da Qualidade da Água do rio São Francisco No Segmento Entre Três Marias e Pirapora-MG: Metais Pesados e Actividades Antropogênicas. Master’s Thesis, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil, 2010; p. 196. [Google Scholar]
- Trindade, W.M. Concentração e Distribuição de Metais Pesados em Sedimentos do rio São Francisco Entre Três Marias e Pirapora/MG: Factores Naturais e Antrópicos. Master’s Thesis, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil, 2010; p. 111. [Google Scholar]
- Fonseca, R.; Araújo, A.; Martins, L.; Dias, N.; Pinho, C.; Carneiro, J.; Cavacundo, O.; Borges, J.; Caldeira, B.; Matos, J.; et al. Remediation Strategy of the Consciência and Barreiro Grande Creeks-2nd Phase. Final Report to Votorantim Metais Zinco S/A; University of Évora: Évora, Portugal, 2015; p. 588. [Google Scholar]
- Ribeiro da Costa, I.; Fonseca, R.; Pinho, C.; Araújo, A.; Martins, L.; Dias, N.; Janeiro, A.I.; Freitas, G. Contaminated soils and sediments associated with Zn ore metallurgy near the São Francisco River, Minas Gerais (Brazil). Environ. Earth Sci. 2018, 77, 202. [Google Scholar] [CrossRef]
- Lahr, J.; Kooistra, L. Environmental risk mapping of pollutants: State of the art and communication aspects. Sci. Total Environ. 2010, 408, 3899–3907. [Google Scholar] [CrossRef] [PubMed]
- Woodbury, P.B. DOs and DON’Ts of spatially explicit ecological risk assessment. Environ. Toxicol. Chem. 2003, 22, 77–82. [Google Scholar] [CrossRef]
- Moen, J.E.T.; Ale, B.J.M. Risk maps and communication. J. Hazard. Mater. 1998, 61, 271–278. [Google Scholar] [CrossRef]
- Li, Y.; Cai, Y. Mobility of toxic metals in sediments: Assessing methods and controlling factors. J. Environ. Sci. 2015, 31, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Zambon, I.; Colantoni, A.; Carlucci, M.; Morrow, N.; Sateriano, A.; Salvati, L. Land quality, sustainable development and environm;ntal degradation in agricultural districts: A computational approach based on entropy indexes. EIA Rev. 2017, 64, 37–46. [Google Scholar] [CrossRef]
- Matheron, G. The Theory of Regionalized Variables and Its Applications; Les Cahiers du Centre de Morphologie Mathématique, no. 5, Ecole des Mines de Paris: Paris, France, 1971; p. 211. [Google Scholar]
- Journel, A.G.; Huijbregts, C.J. Mining Geostatistics; Academic Press: San Diego, CA, USA, 1978. [Google Scholar]
- Antunes, I.M.H.R.; Albuquerque, M.T.D. Using indicator kriging for the evaluation of arsenic potential contamination in an abandoned mining area (Portugal). Sci. Total Environ. 2013, 442, 545–552. [Google Scholar] [CrossRef]
- Golder Associates. Zoneamento da Distribuição da Contaminação de Sedimentos do Leito Submerso do Rio São Francisco; Final Report to Votorantim Metais Zinco S/A, Três Marias–MG, RT-079-515-6012-0015-00-J; Golder Associates Brasil Consultoria e Projetos LTDA: Belo Horizonte, Minas Gerais, Brazil, 2007; p. 124. [Google Scholar]
- Mozeto, A.A.; Silva, E.F.A. Diagnóstico Preliminar De Contaminação Ambiental por Metais na Área de Influência da VM na Bacia do Rio São Francisco, Região de Três Marias (MG). Final Scientific Report; Laboratory of Environmental Biogeochemistry, DQ/UFS Carlos: São Carlos, State of São Paulo, Brazil, 2005. [Google Scholar]
- Chiavegatto, J.R. Análise Estratigráfica das Seqüências Tempestíticas da Formação Três Marias (Proterozóico Superior), na Porção Meridional da Bacia do São Francisco. Master’s Thesis, Depto. de Geol., Esc. de Minas, Univ. Fed. Ouro Preto, Ouro Preto, Minas Gerais, Brazil, 1992; p. 216. [Google Scholar]
- Signorelli, N.; Tuller, M.P.; Silva, P.C.; Justo, L.J. Carta Geológica Escala 1:250000 Folha SE.23-Y-B-Três Marias; CPRM-Serviço Geológico do Brasil, Ministério de Minas e Energia: Belo Horizonte, Minas Gerais, Brazil, 2003. [Google Scholar]
- Costa, R.D.; Knauer, L.G.; Prezotti, F.P.S.; Paula, F.L.; Duarte, F.T.; Teixeira, L.F. Mapa Geológico Folha Três Marias-SE.23-Y-B-III Escala 1:100.000. Ministério de Minas e Energia, CPRM-Serviço Geológico do Brasil; CODEMIG-Companhia de Desenvolvimento Econômico de Minas Gerais: Belo Horizonte, Minas Gerais, Brazil, 2011. [Google Scholar]
- Oliveira, M.R. Investigação da Contaminação por Metais Pesados da Água e do Sedimento de Corrente Nas Margens do Rio São Francisco e Tributários, a Jusante da Represa da Cemig, no Município de Três-Marias, Minas Gerais. Ph.D. Thesis, Federal University of Minas Gerais, Belo Horizonte, Minas Gerais, Brazil, 2007; p. 150. [Google Scholar]
- Carvalho, D.A.C.; Filho, A.T.O.; Vilela, E.A.; Curi, N.; Van den Berg, E.; Fontes, A.L.; Botezelli, L. Distribuição de espécies arbóreo-arbustivas ao longo de um gradiente de solos e topografia em um trecho de floresta ripária do Rio São Francisco em Três Marias, MG. Brasil. Rev. Bras. Botânica 2005, 28, 329–345. [Google Scholar] [CrossRef]
- Marinho, A.O.T.; Abreu, A.V.; Pol, A.; Costa, A.L.; Costa, D.A.A.; Ramos, D.B.S.A.; Nascimento, F.S.; Eccard, G.H.A.; Meyer, G.; Christofidis, H.V.; et al. Caderno da região hidrográfica do São Francisco. Ministério do Meio Ambiente, Secretaria de Recursos Hídricos; MMA: Brasília, Brazil, 2006; p. 148. [Google Scholar]
- Oliveira, M.A.; Horn, A.H. Comparação da Concentração de Metais Pesados nas Águas do Rio São Francisco em Três Marias, desde 1991 até hoje, relacionando a atuação da CMM-Três Marias. Geonomos 2006, 14, 55–63. [Google Scholar] [CrossRef][Green Version]
- Silva, D.F.; Galvíncio, J.D.; Silva, D.F.; Almeida, H.C. Análise espaço-temporal de parâmetros de qualidade de água no Alto São Francisco e sua relação com intervenções antrópicas. Eng. Ambient. Espírito Santo do Pinhal 2009, 6, 492–518. [Google Scholar] [CrossRef]
- Amaral, D.C. Estudos Ultraestruturais e da Capacidade Bioacumuladora de Zn, Cd e PB POR PLANTAS em Área de Mineração de Zinco. Master’s Thesis, Universidade Federal de Lavras, Lavras, Minas Gerais, Brazil, 2013. [Google Scholar]
- US EPA (US Environmental Protection Agency). Method 3051A, Microwave Assisted Acid Digestion of Sediments, Sludges, Soils, and Oils; US EPA (US Environmental Protection Agency): Washington, DC, USA, 2007.
- Cardoso Fonseca, E.; Ferreira da Silva, E. Application of selective extraction in metal-bearing phases identification: A South European case study. J. Geochem. Explor. 1998, 6, 203–212. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential Extraction Procedure for the Speciation of Particulate Trace Metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Fonseca, R.; Patinha, C.; Barriga, F.J.A.S.; Morais, M. Role of the sediments of two tropical dam reservoirs in the flux of metallic elements to the water column. Water Sci. Technol. 2012, 66, 254–266. [Google Scholar] [CrossRef]
- Singh, R.; Bhumbla, D.K.; Keefer, R.F. Recommended Soil Sulfate-S Tests, Chapter 7; The Northeast Coordinating Committee for Soil Testing: Madison, WI, USA, 2011. [Google Scholar]
- Goovaerts, P. Geostatistics for Natural Resources Evaluation; Oxford University Press: New York, NY, USA; Oxford, UK, 1997. [Google Scholar]
- Albuquerque, M.T.D.; Gerassis, S.; Sierra, C.; Taboada, J.; Martín, J.E.; Antunes, I.M.H.R.; Gallego, J.R. Developing a new Bayesian Risk Index for risk evaluation of soil contamination. Sci. Total Environ. 2017, 603–604, 167–177. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010; p. 548. ISBN 9781420093681. [Google Scholar]
- CONAMA. Resoluções do CONAMA: Resoluções Vigentes Publicadas Entre Setembro de 1984 e Janeiro de 2012; Ministério do Meio Ambiente: Brasília, Brazil, 2012; p. 1125.
- Chica-Olmo, M. La Geoestadística Como Herramienta de Análisis Espacial de Datos de Inventario Forestal. Cuadernos De La Sociedad Espanola De Ciencias Forestales 2005, 19, 47–55. [Google Scholar] [CrossRef]
- Pereira, H.G.; Brito, M.G.; Albuquerque, T.; Ribeiro, J. Geostatistical Estimation of a Summary Recovery Index for Marble Quarries; Geostatistics Troia’92 5; Kluwer Academic Publishers: Lisbon, Portugal, 1993; pp. 1029–1040. [Google Scholar]
- Kyriakidis, P.C.; Journel, A.G. Geostatistical space–time models: A review. Math. Geol. 1999, 31, 651–684. [Google Scholar] [CrossRef]
- Schmoll, O.; Howard, G.; Chilton, G.; Chorus, I. Protecting Groundwater for Health: Managing the Quality of Drinking-Water Sources; World Health Organization: Geneva, Switzerland; IWA Publishing: London, UK, 2006; p. 697. [Google Scholar]
- COPAM. Deliberação Normativa COPAM n 166, de 29 de Junho de 2011; Conselho Estadual de Política Ambiental, 2011; p. 5. [Google Scholar]
- Evanko, C.R.; Dzombak, D.A. Remediation of Metals-Contaminated Soils and Groundwater; Ground-Water Remediation Technologies Analysis Center: Pittsburgh, PA, USA, 1997; p. 53. [Google Scholar]
- Rauret, G. Extraction procedures for the determination of heavy metals in contaminated soil and sediment. Talanta 1998, 46, 449–455. [Google Scholar] [CrossRef]
- Hooda, P.S. Trace Elements in Soils, 1st ed.; Wiley Online Library: Chichester, UK, 2010; p. 596. [Google Scholar]
- Kabata-Pendias, A.; Mukherjee, A.B. Trace Elements from Soil to Human, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2007; p. 550. [Google Scholar]
- Buringh, P. Introduction to the Study of Soils in Tropical and Subtropical Regions, 3rd ed.; Centre for Agricultural Publishing and Documentation: Wageningen, The Netherlands, 1979; p. 148. [Google Scholar]
- Buffle, J.; Leppard, G.G. Characterization of Aquatic Colloids and Macromolecules. 1. Structure and Behavior of Colloidal Material. Environ. Sci. Technol. 1995, 29, 2169–2175. [Google Scholar] [CrossRef] [PubMed]
- Westrich, B. Managing River Sediments. In Sediment Dynamics and Pollutant Mobility in Rivers. An Interdisciplinary Approach, 1st ed.; Westrich, B., Förstner, U., Eds.; Springer: Berlin/Heidelberg, 2007; pp. 35–66. [Google Scholar]
- Blowes, D.W.; Ptacek, C.J.; Benner, S.G.; McRae, C.W.; Bennett, T.A.; Puls, R.W. Treatment of inorganic contaminants using permeable reactive barriers. J. Contam. Hydrol. 2000, 45, 123–137. [Google Scholar] [CrossRef]
- McBride, B. Environmental Chemistry of Soils, 1st ed.; Oxford University Press: Oxford, UK, 1994; ISBN 0195070119. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).