Ultrasonic Shaking of Glauconite Pellets with Diverse Reagents for a Comparison of Their K–Ar with Already Published Rb–Sr Results
Abstract
:1. Introduction
2. Description of the Selected Samples and of the Analytical Procedure
3. Results
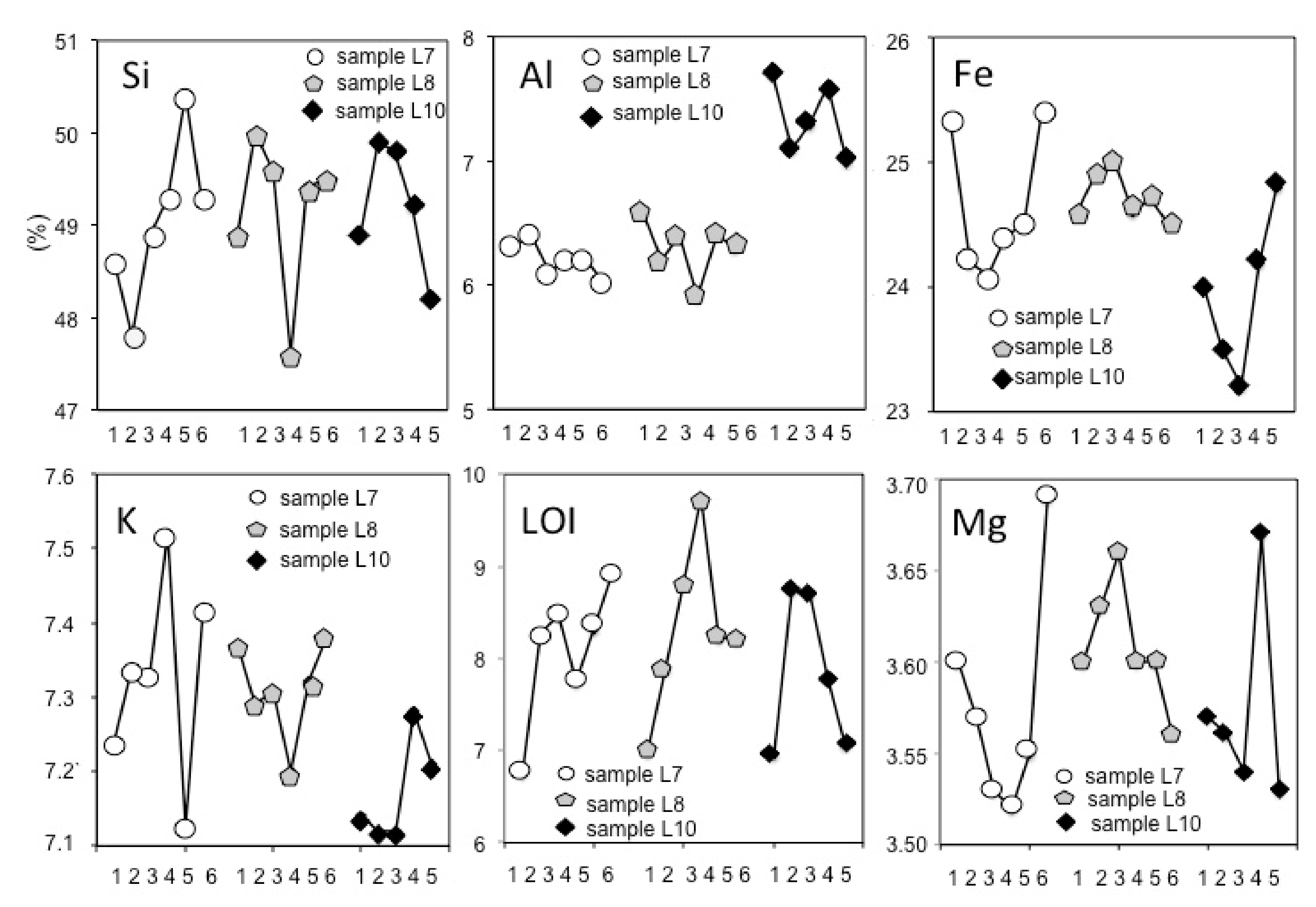
3.1. The Variations of the Major Elements
3.2. The Variations of the Trace Elements
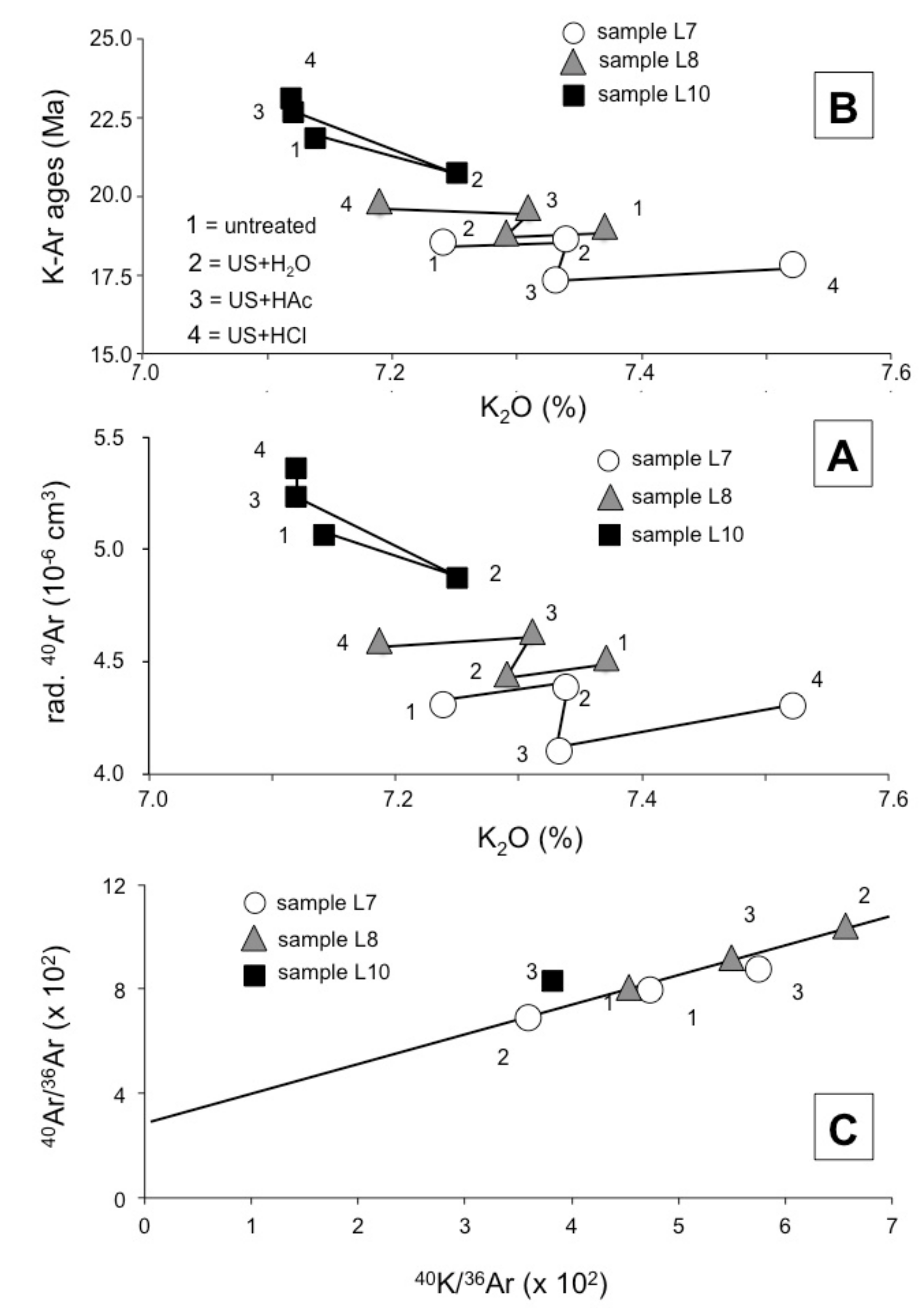
3.3. The K–Ar Data
3.4. The Impact of the Duration of the Ultrasonic Shaking
4. Discussion
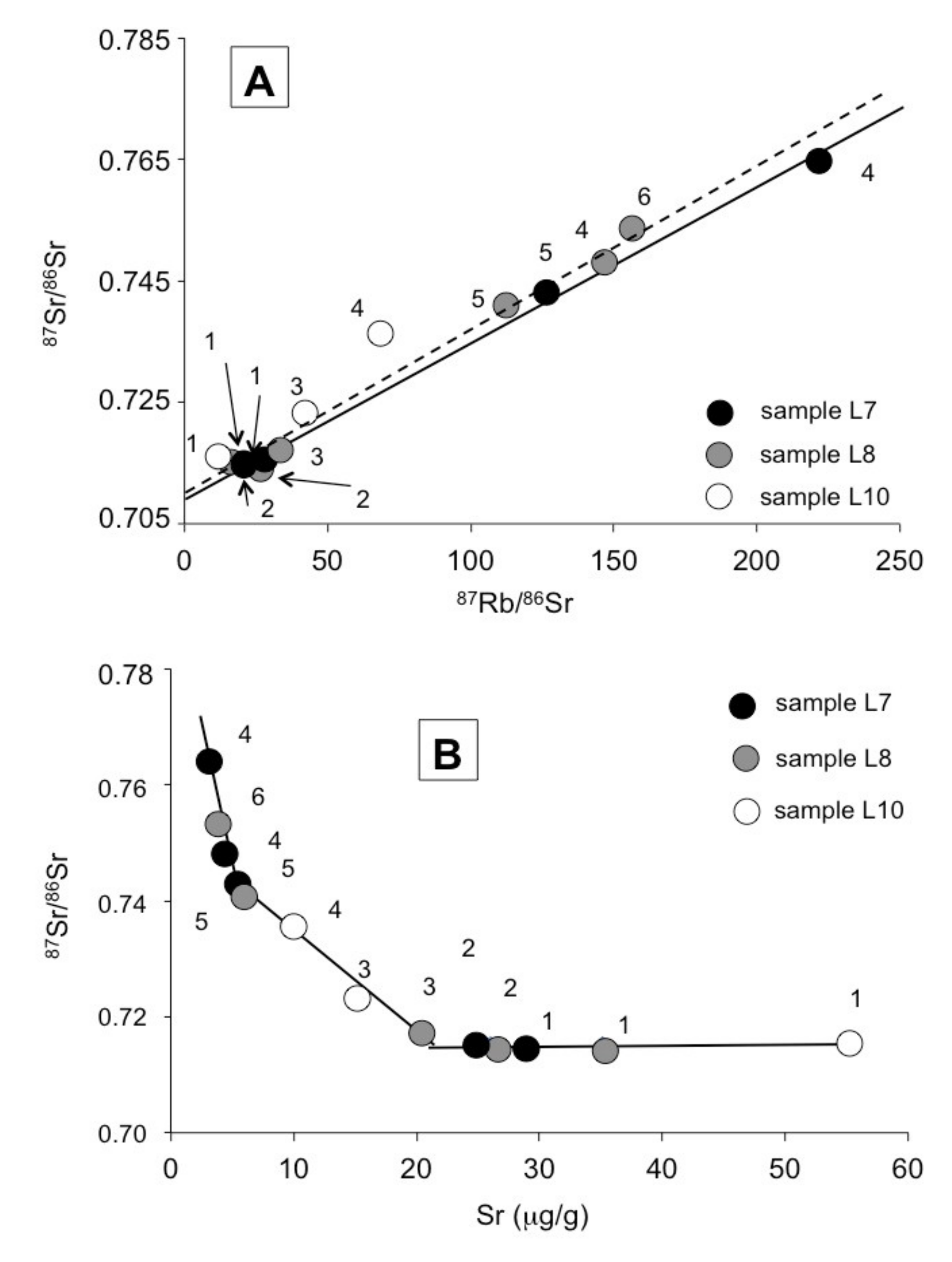
4.1. About the Earlier Published Rb–Sr Data
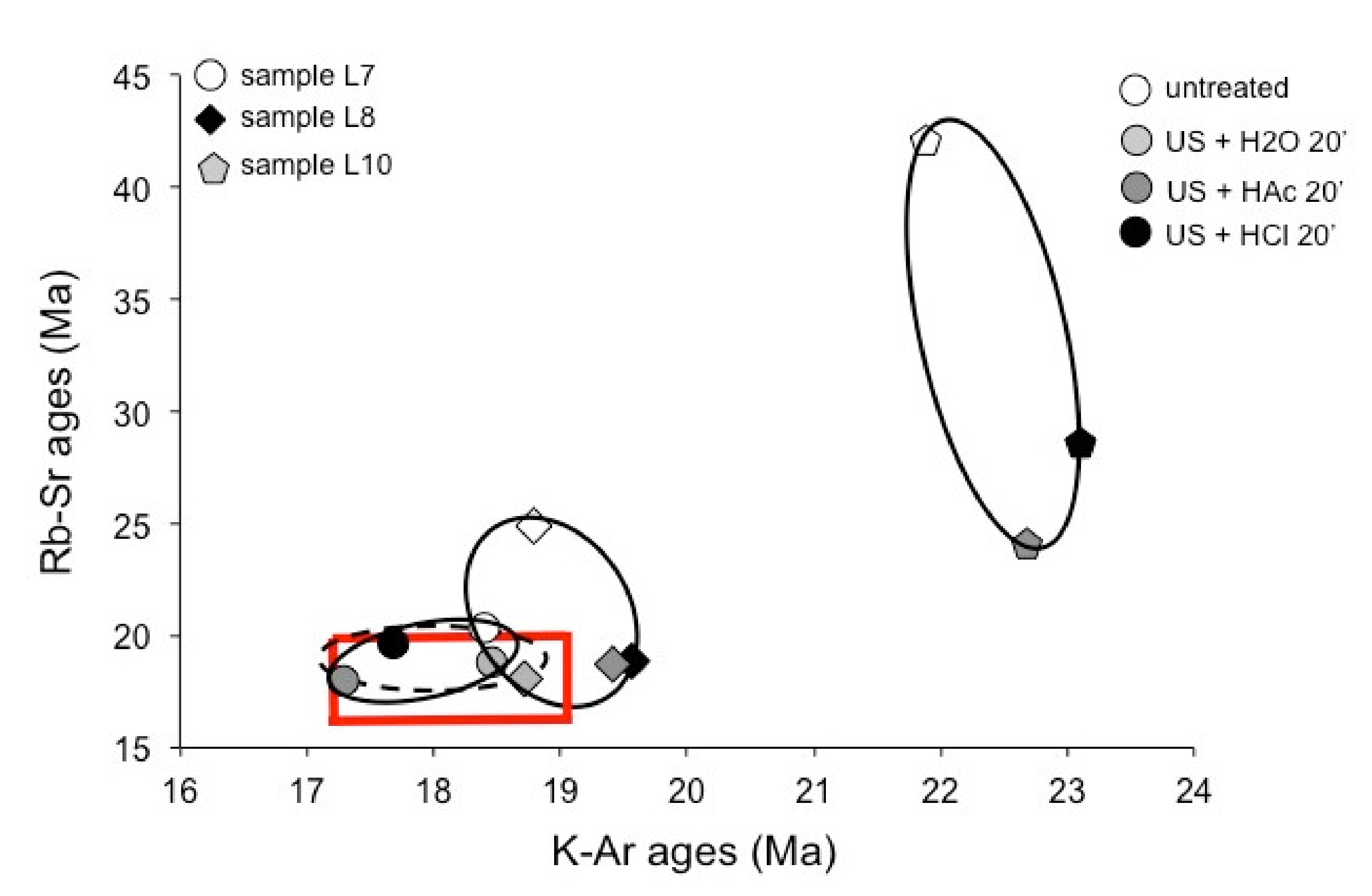
4.2. Comparison of the K–Ar and the Previously Published Rb–Sr Data
4.3. Evaluation of the Sample Preparation Techniques
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ehlmann, A.J.; Hulings, N.C.; Glover, E.D. Stages of glauconite formation in modern foraminiferal sediments. Sedim. Petrol. 1963, 33, 87–96. [Google Scholar]
- Odin, G.S.; Matter, A. De glauconarium origine. Sedimentology 1981, 28, 611–641. [Google Scholar] [CrossRef]
- Velde, B. Green clay minerals. In Treatise on Geochemistry 7. Sediments, Diagnosis and Sedimentary Rocks; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; Volume 7, pp. 309–324. [Google Scholar]
- Cormier, R.F. Rubidium-Strontium ages of glauconite. Bull. Geol. Soc. Am. 1956, 67, 1812. [Google Scholar]
- Thompson, G.R.; Hower, J. An explanation for low radiometric ages from glauconite. Geochim. Cosmochim. Acta 1973, 37, 1473–1491. [Google Scholar] [CrossRef]
- Pasteels, P.; Laga, P.; Keppens, E. Essai d’application de la méthode radiométrique au strontium aux glauconies du Néogène: Le problème du traitement de l’échantillon avant analyse. Comptes Rendus Académie Des. Sci. 1976, 282, 2029–2032. [Google Scholar]
- Morton, J.P.; Long, L.E. Rb-Sr ages of glauconite recrystallization: Dating times of regional emergence above sea level. J. Sedim. Petrol. 1980, 54, 495–506. [Google Scholar]
- Keppens, E.; Pasteels, P. A comparison of rubidium-strontium and potassium-argon apparent ages on glauconies. In Numerical Dating in Stratigraphy; Odin, G.S., Ed.; Wiley: New York, NY, USA, 1982; pp. 225–239. [Google Scholar]
- Odin, G.S.; Dodson, M.H. Zero isotopic age of glauconies. In Numerical Dating in Stratigraphy; Odin, G.S., Ed.; Wiley: New York, NY, USA, 1982; pp. 277–306. [Google Scholar]
- Foland, K.A.; Hubacher, F.A.; Arehartet, G.B. 40Ar/39Ar dating of very fine-grained samples: An encapsulated-vial procedure to overcome the problem of 39Ar recoil loss. Chem. Geol. Isot. Geosc. Sect. 1992, 102, 269–276. [Google Scholar]
- Smith, P.E.; Evensen, N.M.; York, D. First successful 40Ar/39Ar dating of glauconites: Argon recoil in single grain cryptocrystalline material. Geology 1993, 21, 41–44. [Google Scholar] [CrossRef]
- Clauer, N.; Huggett, J.M.; Hillier, S. How reliable is the K-Ar glauconite chronometer? A case study of Eocene sediments from the Isle of Wight. Clay Min. 2005, 40, 167–176. [Google Scholar] [CrossRef]
- Odin, G.S.; Hunziker, J.C. Potassium-argon dating of washed, leached, weathered and reworked glauconies. In Numerical Dating in Stratigraphy; Odin, G.S., Ed.; J. Wiley: New York, NY, USA, 1982; pp. 363–385. [Google Scholar]
- Keppens, E. Onderzoek van Het Glauconiet als Geochronometer voor de Rb-Sr Dateringsmethode. Ph.D. Thesis, Vrije Universiteit Brussel, Brussel, Belgium, 1981. [Google Scholar]
- Clauer, N.; Chaudhuri, S.; Kralik, M.; Bonnot-Courtois, C. Effects of experimental leaching on Rb-Sr and K-Ar isotopic systems and REE contents of diagenetic illite. Chem. Geol. 1993, 103, 1–16. [Google Scholar] [CrossRef]
- Clauer, N.; Cocker, J.D.; Chaudhuri, S. Isotopic dating of diagenetic illites in reservoir sandstones: Influence of the investigator effect. In Origin, Diagenesis, and Petrophysics of Clay Minerals in Sandstones. Soc. Econ. Pal. Miner. Spec. Pub. 1992, 47, 5–12. [Google Scholar]
- Berggren, W.A. A Cenozoic time-scale—Some implications for regional geology and paleobiogeography. Lethaia 1972, 5, 195–215. [Google Scholar] [CrossRef]
- Samuel, J.; Rouault, R.; Besnus, Y. Analyse multi-élémentaire standardisée des matériaux géologiques en spectrométrie d’émission par plasma à couplage inductif. Analusis 1985, 13, 312–317. [Google Scholar]
- Odin, G.S. Interlaboratory standards for dating purposes. In Numerical Dating in Stratigraphy; Odin, G.S., Ed.; John Wiley & Sons: Chichester, UK, 1982; pp. 123–148. [Google Scholar]
- Lee, J.Y.; Marti, K.; Severinghaus, J.P.; Kawamura, K.; Yoo, H.S.; Lee, J.B.; Kim, J.S. A redetermination of the isotopic abundances of Atmospheric Ar. Geochim. Cosmochim. Acta 2006, 70, 4507–4512. [Google Scholar] [CrossRef]
- Steiger, R.; Jäger, E. Subcommission on Geochronology: Convention on the use of decay constants in geo- and cosmochronology. Earth Plan. Sci. Lett. 1977, 36, 359–362. [Google Scholar] [CrossRef]
- Bonhomme, M.; Thuizat, R.; Pinault, Y.; Clauer, N.; Wendling, R.; Winkler, R. Méthode de Datation Potassium-Argon. Appareillage et Technique; Rapport technique du Centre de Géochimie de la Surface; Univ. Louis Pasteur: Strasbourg, France, 1975; 53p. [Google Scholar]
- Li, M.; Fang, X.; Galy, A.; Wang, H.; Song, X.; Wang, X. Hydrated sulfate minerals (bloedite and polyhalite): Formation and paleoenvironmental implications. Carbonates Evaporites 2020, 35, 126. [Google Scholar] [CrossRef]
- Harper, C.T. Graphic solution to the problem of 40Ar loss from metamorphic minerals. Eclog. Geol. Helv. 1970, 63, 119–140. [Google Scholar]
- Clauer, N. The rubidium-strontium method applied to sediments: Certitudes and uncertainties. In Numerical Dating in Stratigraphy; Odin, G.S., Ed.; John Wiley and Sons, Ltd.: Hoboken, NJ, USA, 1982; pp. 245–276. [Google Scholar]
- McArthur, J.M.; Howarth, R.J.; Bailey, T.R. Strontium isotope stratigraphy: LOWESS Version 3: Best fit to the marine Sr-isotope curve 0–509 Ma and accompanying look-up table for deriving numerical age. J. Geol. 2001, 109, 155–170. [Google Scholar] [CrossRef] [Green Version]

| (A) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample IDs | SiO2 (%) | Al2O3 (%) | MgO (%) | CaO (%) | Fe2O3 (%) | Mn3O4 (%) | TiO2 (%) | Na2O (%) | K2O (%) | LOI (%) | Total (%) |
| L7 untreated (=1) | 48.6 | 6.30 | 3.60 | 0.40 | 25.3 | 0.010 | 0.07 | 0.12 | 7.24 | 6.76 | 98.40 |
| US H2O 20′ (=2) | 47.8 | 6.40 | 3.57 | 0.50 | 24.2 | 0.010 | 0.06 | 0.12 | 7.34 | 8.27 | 98.27 |
| US HAc 20′ (=3) | 48.9 | 6.10 | 3.53 | 0.30 | 24.1 | 0.013 | 0.07 | 0.11 | 7.33 | 8.47 | 98.92 |
| US HCl 20′ (=4) | 49.3 | 6.20 | 3.52 | bdl | 24.4 | 0.014 | 0.08 | 0.14 | 7.52 | 7.75 | 98.92 |
| US HCl 60′ (=5) | 50.4 | 6.20 | 3.55 | bdl | 24.5 | 0.01 | 0.06 | 0.13 | 7.13 | 8.33 | 100.3 |
| US H2O + US HAc (=6) | 49.3 | 6.00 | 3.69 | 0.30 | 25.4 | 0.015 | 0.06 | 0.10 | 7.42 | 8.92 | 101.2 |
| L8 untreated (=1) | 48.9 | 6.60 | 3.60 | 0.40 | 24.6 | 0.016 | 0.07 | 0.09 | 7.37 | 6.99 | 98.64 |
| US H2O 20′ (=2) | 50.0 | 6.20 | 3.63 | 0.40 | 24.9 | 0.012 | 0.07 | 0.09 | 7.29 | 7.89 | 100.5 |
| US HAc 20′ (=3) | 49.6 | 6.40 | 3.66 | 0.30 | 25.0 | 0.013 | 0.07 | 0.05 | 7.31 | 8.80 | 101.2 |
| US HCl 20′ (=4) | 47.6 | 5.90 | 3.60 | bdl | 24.6 | 0.016 | 0.06 | 0.07 | 7.20 | 9.73 | 98.78 |
| US H2O 60′ (=5) | 49.4 | 6.40 | 3.60 | bdl | 24.7 | 0.010 | 0.06 | 0.07 | 7.32 | 8.23 | 99.79 |
| US H2O 24h (=6) | 49.5 | 6.30 | 3.56 | bdl | 24.5 | 0.010 | 0.07 | 0.09 | 7.38 | 8.20 | 99.61 |
| L10 untreated (=1) | 48.9 | 7.70 | 3.57 | 0.50 | 24.0 | 0.015 | 0.14 | 0.08 | 7.14 | 6.96 | 99.01 |
| US HAc 20′ (=3) | 49.9 | 7.10 | 3.56 | 0.30 | 23.5 | 0.011 | 0.08 | 0.30 | 7.12 | 8.74 | 100.6 |
| US HCl 20′ (=4) | 49.8 | 7.30 | 3.54 | bdl | 23.2 | 0.014 | 0.12 | 0.16 | 7.12 | 8.71 | 99.96 |
| US H2O + US HAc (=5) | 49.2 | 7.60 | 3.67 | 0.30 | 24.2 | 0.012 | 0.10 | 0.07 | 7.28 | 7.78 | 100.2 |
| USH2O + USHAc (2×) (=6) | 48.2 | 7.00 | 3.53 | 0.20 | 24.8 | 0.016 | 0.09 | 0.11 | 7.21 | 7.07 | 98.23 |
| (B) | |||||||||||
| Sample IDs | SiO2 (%) | Al2O3 (%) | MgO (%) | CaO (%) | Fe2O3 (%) | Mn3O4 (%) | TiO2 (%) | Na2O (%) | K2O (%) | LOI (%) | Total (%) |
| L7 untreated (=1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| US H2O 20′ (=2) | −1.6 | +1.6 | +0.8 | −25 | −4.3 | 0 | −14.3 | 0 | +1.4 | −22.3 | +0.1 |
| US HAc 20′ (=3) | +0.6 | −3.2 | +1.9 | −25 | −4.7 | −30.0 | 0 | −8.3 | +1.2 | −25.3 | +0.1 |
| US HCl 20′ (=4) | +1.4 | +1.6 | +2.2 | −3.6 | −40.0 | −14.3 | −16.7 | −3.9 | −11.6 | +0.5 | |
| US HCl 60′ (=5) | +3.7 | +1.6 | +1.4 | −3.2 | −90.0 | −14.3 | +8.3 | +1.5 | −23.2 | +1.9 | |
| US H2O + US HAc (=6) | +1.4 | −4.8 | +2.5 | −25 | +0.4 | −50.0 | −14.3 | −16.7 | +2.5 | −32.8 | +2.8 |
| L8 untreated (=1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| US H2O 20′ (=2) | +2.2 | −6.1 | +0.8 | 0 | +1.2 | −25.0 | 0 | 0 | +1.1 | −12.9 | +2.0 |
| US HAc 20′ (=3) | +1.4 | −3.1 | +1.7 | −25 | +1.6 | −18.8 | 0 | −44.4 | +0.8 | −25.9 | −2.8 |
| US HCl 20′ (=4) | −2.7 | −10.6 | 0 | 0 | 0 | −14.3 | −22.2 | +2.3 | −39.2 | +0.1 | |
| US H2O 1h (=5) | +1.0 | −3.1 | 0 | +0.4 | −37.5 | −14.3 | −22.2 | +0.7 | −17.7 | +1.2 | |
| US H2O 24h (=6) | >1.2 | −4.5 | +1.1 | +0.4 | −37.5 | 0 | 0 | +0.1 | +1.0 | ||
| L10 untreated (=1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| US HAc 20′ (=3) | +2.0 | −7.8 | +0.3 | −40 | +2.1 | −26.7 | −42.9 | −2750 | +0.3 | −25.6 | +1.6 |
| US HCl 20′ (=4) | +1.9 | −5.2 | +0.8 | +3.3 | −6.7 | −14.3 | −2000 | +0.3 | −25.1 | +1.0 | |
| US H2O + US HAc (=5) | +0.6 | +1.3 | −2.8 | −40 | +0.8 | −20.0 | −28.6 | −125 | +2.2 | −11.8 | +1.0 |
| USH2O + USHAc (2×) (=6)) | −1.4 | −9.1 | +1.1 | −60 | −3.3 | −6.7 | −35.7 | −375 | +1.0 | +1.6 | +0.8 |
| (A) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample IDs | Rb (μg/g) | Sr (μg/g) | Ba (μg/g) | V (μg/g) | Ni (μg/) | Co (μg/g) | Cr (μg/) | Zn (μg/g) | Cu (μg/) | Sc (μg/g) | Y (μg/g) | Zr (μg/g) |
| L7 untreated (=1) | 222 | 28.7 | ||||||||||
| US + H2O 20′ (=2) | 231 | 24.6 | 11.0 | 129 | 113 | 15.0 | 174 | 108 | 7.00 | 10.5 | 9.00 | 22.0 |
| US + HAc 20′ (=3) | 14.0 | 8.00 | 131 | 107 | 14.0 | 190 | 86.0 | 4.00 | 11.0 | 8.00 | 26.0 | |
| US + HCl 20′ (=4) | 230 | 3.03 | 8.00 | 129 | 112 | 14.0 | 199 | 80.0 | 5.00 | 9.50 | 2.00 | 22.0 |
| US + HCl 60′ (=5) | 231 | 5.34 | 7.00 | 129 | 111 | 13.0 | 194 | 80.0 | 5.00 | 10.5 | 2.00 | 25.0 |
| US+H2O + US+HAc (=6) | 15.0 | 6.00 | 126 | 109 | 15.0 | 174 | 95.0 | 12.0 | 10.0 | 8.00 | 20.0 | |
| L8 untreated (=1) | 226 | 35.1 | 17.0 | 128 | 115 | 15.0 | 208 | 93.0 | 5.00 | 10.5 | 7.00 | 33.0 |
| US + H2O 20′ (=2) | 230 | 26.0 | ||||||||||
| US + HAc 20′ (=3) | 227 | 20.2 | 5.00 | 130 | 117 | 11.0 | 197 | 84.0 | 4.00 | 10.5 | 7.00 | 28.0 |
| US + HCl 20′ (=4) | 225 | 4.43 | 7.00 | 135 | 113 | 16.0 | 175 | 79.0 | 3.00 | 9.50 | 2.00 | 22.0 |
| US + H2O 60′ (=5) | 3.00 | 6.00 | 131 | 30.0 | 17.0 | 169 | 74.0 | bdl | 10.5 | bdl | 24.0 | |
| US + H2O 24h (=6) | 3.00 | 7.00 | 135 | 37.0 | 14.0 | 173 | 74.0 | bdl | 10.0 | bdl | 24.0 | |
| L10 untreated (=1) | 224 | 22.0 | 13.0 | 160 | 124 | 17.0 | 348 | 84.0 | 25.0 | 13.5 | 11.0 | 43.0 |
| US + HAc 20′ (=3) | 225 | 15.4 | ||||||||||
| US + HCl 20′ (=4) | 224 | 9.60 | 12.0 | 152 | 39 | 15.0 | 228 | 84.0 | 10.0 | 11.5 | 9.00 | 75.0 |
| US+H2O + US+HAc (=5) | 16.0 | 11.0 | 139 | 110 | 16.0 | 235 | 91.0 | 15.0 | 11.5 | 9.00 | 75.0 | |
| US+H2O + US+HAc (2×) (=6) | 14.0 | 9.00 | 134 | nd | 16.0 | nd | 67.0 | 13.0 | 12.0 | 10.0 | 35.0 | |
| (B) | ||||||||||||
| Sample IDs | Rb (%) | Sr (%) | Ba (%) | V (%) | Ni (%) | Co (%) | Cr (%) | Zn (%) | Cu (%) | Sc (%) | Y (%) | Zr (%) |
| L7 untreated (=1) | 0 | 0 | ||||||||||
| US + H2O 20′ (=2) | +4.1 | −14.6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| US + HAc 20′ (=3) | −51.2 | +1.6 | −5.3 | −6.7 | +9.2 | −20.4 | −42.9 | +4.8 | −11.1 | +18.2 | ||
| US + HCl 20′ (=4) | +3.6 | −96.1 | 0 | −0.9 | −6.7 | +14.4 | −25.9 | −28.6 | −9.5 | −77.8 | 0 | |
| US + HCl 60′ (=5) | +4.1 | −81.4 | 0 | −1.8 | −13.3 | +11.5 | −25.9 | −28.6 | 0 | −77.8 | +13.6 | |
| US H2O + US HAc (=6) | −47.7 | −1.6 | −3.5 | 0 | 0 | −12.0 | +71.4 | −4.8 | −11.1 | −9.1 | ||
| L8 untreated (=1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| US + H2O 20′ (=2) | +1.8 | −25.9 | ||||||||||
| US + HAc 20′ (=3) | +0.4 | −42.5 | −70.6 | +1.6 | +1.7 | −26.7 | −5.3 | −9.7 | −20.0 | 0 | 0 | −15.2 |
| US + HCl 20′ (=4) | +0.1 | −87.4 | −58.8 | +5.5 | −1.7 | +6.6 | −15.9 | −15.1 | −40.0 | 9.5 | −71.4 | −33.3 |
| US + H2O 60′ (=5) | −91.5 | −64.7 | +2.3 | −73.9 | +13.3 | −18.8 | −20.4 | bdl | 0 | bdl | −27.3 | |
| US + H2O 24h (=6) | −91.5 | −58.8 | +5.5 | −67.8 | −6.7 | −16.8 | −20.4 | bdl | −4.8 | bdl | −27.3 | |
| L10 untreated (=1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| US + HAc 20′ (=3) | +0.4 | −30.0 | ||||||||||
| US + HCl 20′ (=4) | 0 | −56.4 | −7.7 | −5.0 | −68.5 | −11.8 | −34.5 | 0 | −60.0 | −17.4 | −18.2 | +74.4 |
| US H2O + US HAc (=5) | −27.3 | −15.4 | −13.1 | −11.3 | −5.9 | −32.5 | +8.3 | −40.0 | −14.8 | −18.2 | +74.4 | |
| US H2O + US HAc (2×) (=6) | −36.4 | −30.8 | −16.3 | nd | −5.9 | nd | −20.2 | −48.0 | −8.9 | −9.1 | −18.6 | |
| Sample IDs | K2O | 40Ar * | rad 40Ar | 40Ar/36Ar | 40K/36Ar | Age |
|---|---|---|---|---|---|---|
| (%) | (%) | (10−6 cm3/g) | (×10−6) | (Ma +/−2σ) | ||
| L7 untreated (=1) | 7.24 | 63.03 | 4.33 | 799.3 | 466.6 | 18.4 (0.6) |
| US + H2O 20′ (=2) | 7.34 | 57.66 | 4.41 | 696.4 | 356.2 | 18.5 (0.7) |
| US + HAc 20′ (=3) | 7.33 | 66.33 | 4.12 | 877.6 | 572.3 | 17.3 (0.6) |
| US + HCl 20′ (=4) | 7.52 | 66.39 | 4.31 | 17.7 (0.6) | ||
| L8 untreated (=1) | 7.37 | 62.77 | 4.49 | 793.7 | 453.7 | 18.8 (0.6) |
| US + H2O 20′ (=2) | 7.29 | 70.99 | 4.43 | 1018.6 | 660.0 | 18.7 (0.6) |
| US + HAc 20′ (=3) | 7.31 | 67.94 | 4.61 | 921.7 | 549.6 | 19.4 (0.6) |
| US + HCl 20′ (=4) | 7.19 | 57.74 | 4.57 | 19.6 (0.7) | ||
| L10 untreated (=1) | 7.14 | 67.59 | 5.08 | 21.9 (0.7) | ||
| US + H2O 20′ (=2) | 7.25 | 73.84 | 4.88 | 20.7 (0.7) | ||
| US + HAc 20′ (=3) | 7.12 | 64.31 | 5.23 | 828.0 | 385.4 | 22.7 (0.8) |
| US + HCl 20′ (=4) | 7.12 | 72.28 | 5.37 | 23.1 (0.7) |
| Sample IDs | Rb | Sr | 87Sr * | 87Rb/86Sr | 87Sr/86Sr | Age (+/−2σ) |
|---|---|---|---|---|---|---|
| (μg/g) | (μg/g) | (+/−1%) | (+/−10−5) | (i = 0.7086) | ||
| L7 untreated (=1) | 222 | 28.7 | 18.5 (1.0) | 22.42 | 0.7151 (4) | 20.3 (1.5) |
| US H2O 20′ (=2) | 231 | 24.6 | 17.6 (1.5) | 27.22 | 0.7159 (7) | 18.8 (2.2) |
| US HCl 20′ (=4) | 230 | 3.03 | 16.7 (0.5) | 220.1 | 0.7649 (17) | 18.1 (0.6) |
| US HCl 60′ (=5) | 231 | 5.34 | 18.3 (0.3) | 125.4 | 0.7436 (5) | 19.7 (0.6) |
| L8 untreated (=1) | 226 | 35.1 | 23.0 (1.2) | 18.67 | 0.7152 (3) | 25.0 (1.9) |
| US H2O 20′ (=2) | 230 | 26.0 | 16.9 (0.6) | 25.64 | 0.7151 (2) | 18.3 (1.2) |
| duplicate | 0.7151 (2) | |||||
| US HAc 20′ (=3) | 227 | 20.2 | 17.5 (0.4) | 32.53 | 0.7174 (2) | 18.8 (0.7) |
| US HCl 20′ (=4) | 225 | 4.43 | 17.4 (0.2) | 147.2 | 0.7482 (4) | 18.9 (0.5) |
| US HCl 60′ (=5) | 228 | 5.86 | 18.7 (1.0) | 112.8 | 0.7411 (14) | 20.7 (1.6) |
| US HCl 24h (=6) | 227 | 4.20 | 18.5 (0.3) | 156.7 | 0.7534 (6) | 20.1 (0.6) |
| L10 untreated (=1) | 224 | 54.9 | 38.6 (0.4) | 11.83 | 0.7157 (8) | 42.1 (1.6) |
| US HAc 20′ (=3) | 225 | 15.4 | 22.3 (1.0) | 42.36 | 0.7232 (6) | 24.2 (1.3) |
| US HCl 20′ (=4) | 224 | 9.60 | 26.1 (0.6) | 67.64 | 0.7363 (3) | 28.7 (1.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clauer, N.; Keppens, E.; Uysal, I.T.; Aubert, A. Ultrasonic Shaking of Glauconite Pellets with Diverse Reagents for a Comparison of Their K–Ar with Already Published Rb–Sr Results. Geosciences 2021, 11, 439. https://doi.org/10.3390/geosciences11110439
Clauer N, Keppens E, Uysal IT, Aubert A. Ultrasonic Shaking of Glauconite Pellets with Diverse Reagents for a Comparison of Their K–Ar with Already Published Rb–Sr Results. Geosciences. 2021; 11(11):439. https://doi.org/10.3390/geosciences11110439
Chicago/Turabian StyleClauer, Norbert, Edward Keppens, I. Tonguç Uysal, and Amélie Aubert. 2021. "Ultrasonic Shaking of Glauconite Pellets with Diverse Reagents for a Comparison of Their K–Ar with Already Published Rb–Sr Results" Geosciences 11, no. 11: 439. https://doi.org/10.3390/geosciences11110439





