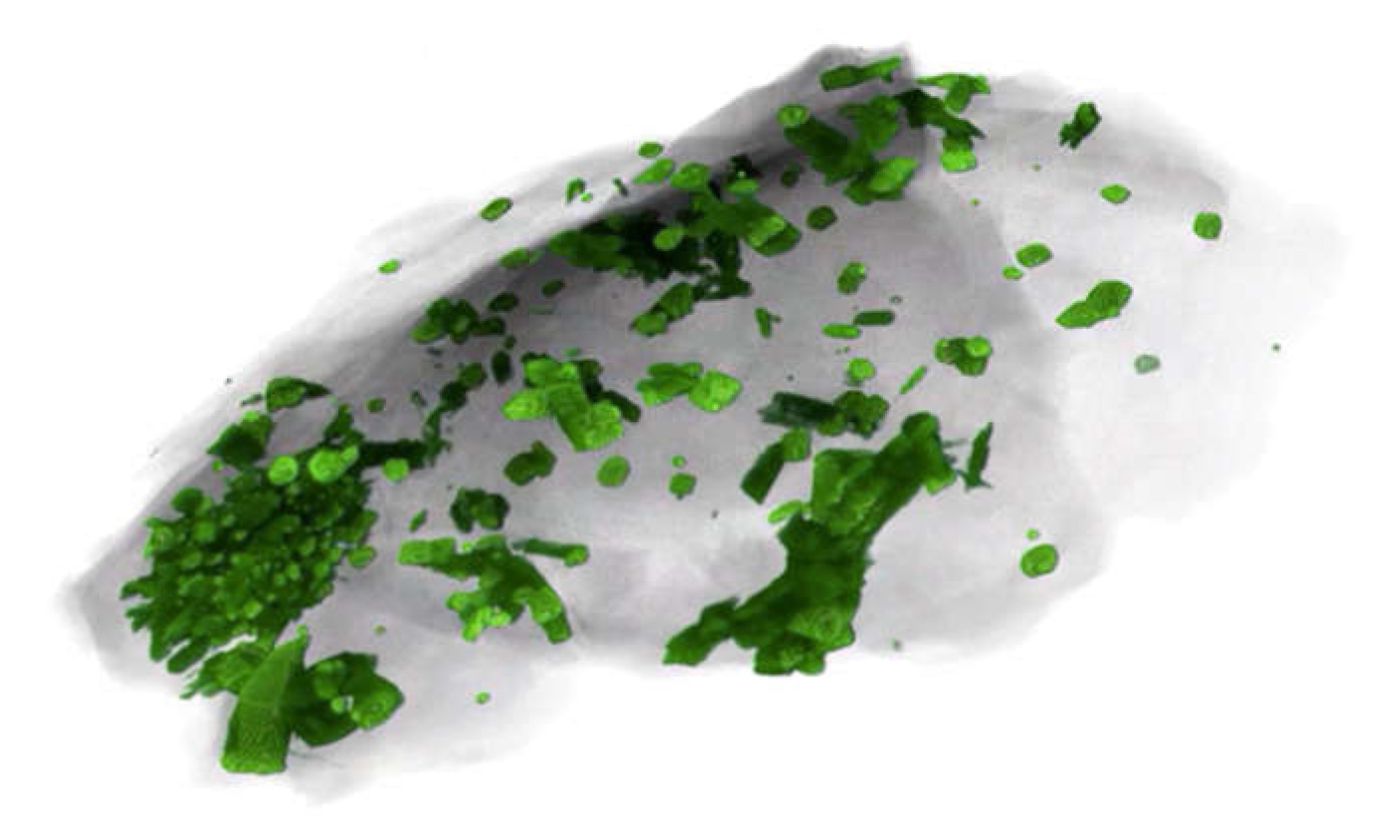
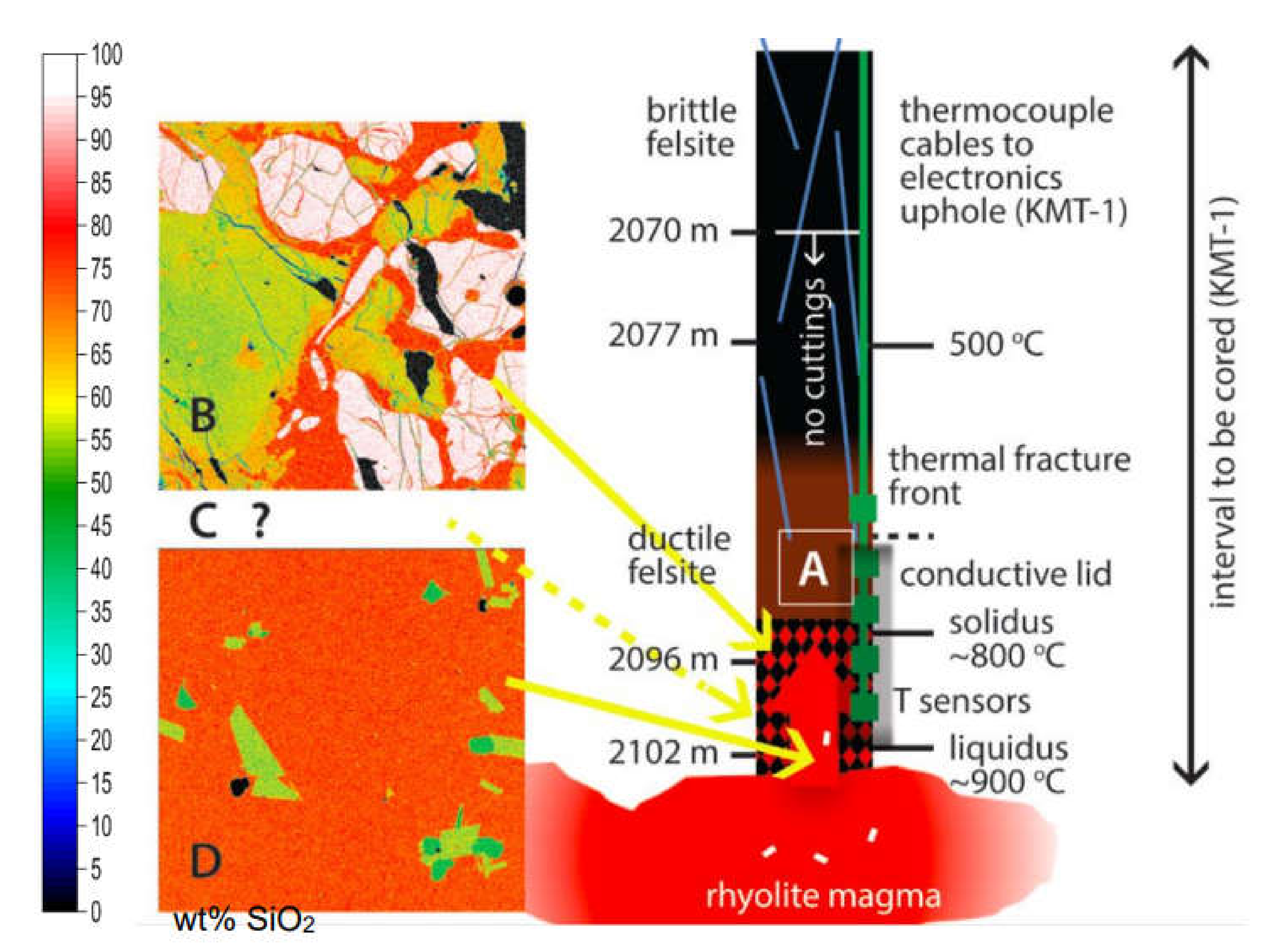
In drilling IDDP-1, circulation was lost at 2070 m, and continued drilling encountered a soft formation at an average depth of 2102 m in the original well and two sidetracks. It is the second sidetrack (third penetration) that is of special interest here because magma flowed up the borehole, the bit became stuck, and for a brief period, restoration of circulation brought glass-bearing chips (
Figure 4) to the surface, confirming that magma was present at the bottom of the well (
Figure 5). Gamma logs indicate that lithology below 2070 m is predominantly felsite (A in
Figure 5, fine-grained crystalline equivalent of rhyolite magma) through this zone [
26]. The deepest temperature measurements are at 2077 m and through multiple measurements are extrapolated to an equilibrium formation temperature of 500 °C (
Figure 5 and
Figure 6) [
26]. Somewhere between that depth and the melt-present zone (B in
Figure 5, checkered) should be a transition from brittle to ductile behavior in the felsite. This should be the lower limit of fracturing. However, high strain rates caused by cooling during drilling would have raised the temperature of the brittle/ductile boundary so that fractures could have propagated deeper. Only two kinds of melt-present (shown by quenched glass) chips were recovered: the partially melted felsite (B) and near-liquidus magma (D), although some chips of D contain micro-xenoliths of B (
Figure 4). Although the actual spatial relationships are unknown, it may be presumed that the high-temperature magma lies below the partially melting felsite. When the magma was penetrated, it rose 8 m from 2104 m to 2096 m within 9 min and then another meter where the bit subsequently became stuck. Lithology C is partially crystallized magma or mush, expected to occur between the melting felsite and the near-liquidus magma (
Figure 5), but it is not represented in the chips recovered. 1 mm × 1 mm elemental electron microprobe maps of B and D are shown to the left of the borehole. SiO
2 contents are displayed as false colors indexed on the vertical bar. In B, interstitial rhyolite melt (red ~75 wt% SiO
2) lies between embayed andesine feldspar (lime green, ~56–58 wt% SiO
2) and quartz (white 100 wt% SiO
2). Black denotes cracks and voids. In D, the main component is rhyolite melt (red ~75 wt% SiO
2), with suspended euhedral crystals of andesine (lime green, ~56 wt% SiO
2) and clinopyroxene (dark green ~50 wt% SiO
2). Small black crystals are titanomagnetite (0 wt% SiO
2).
4.1. MHB in Krafla Compared to Kilauea Iki
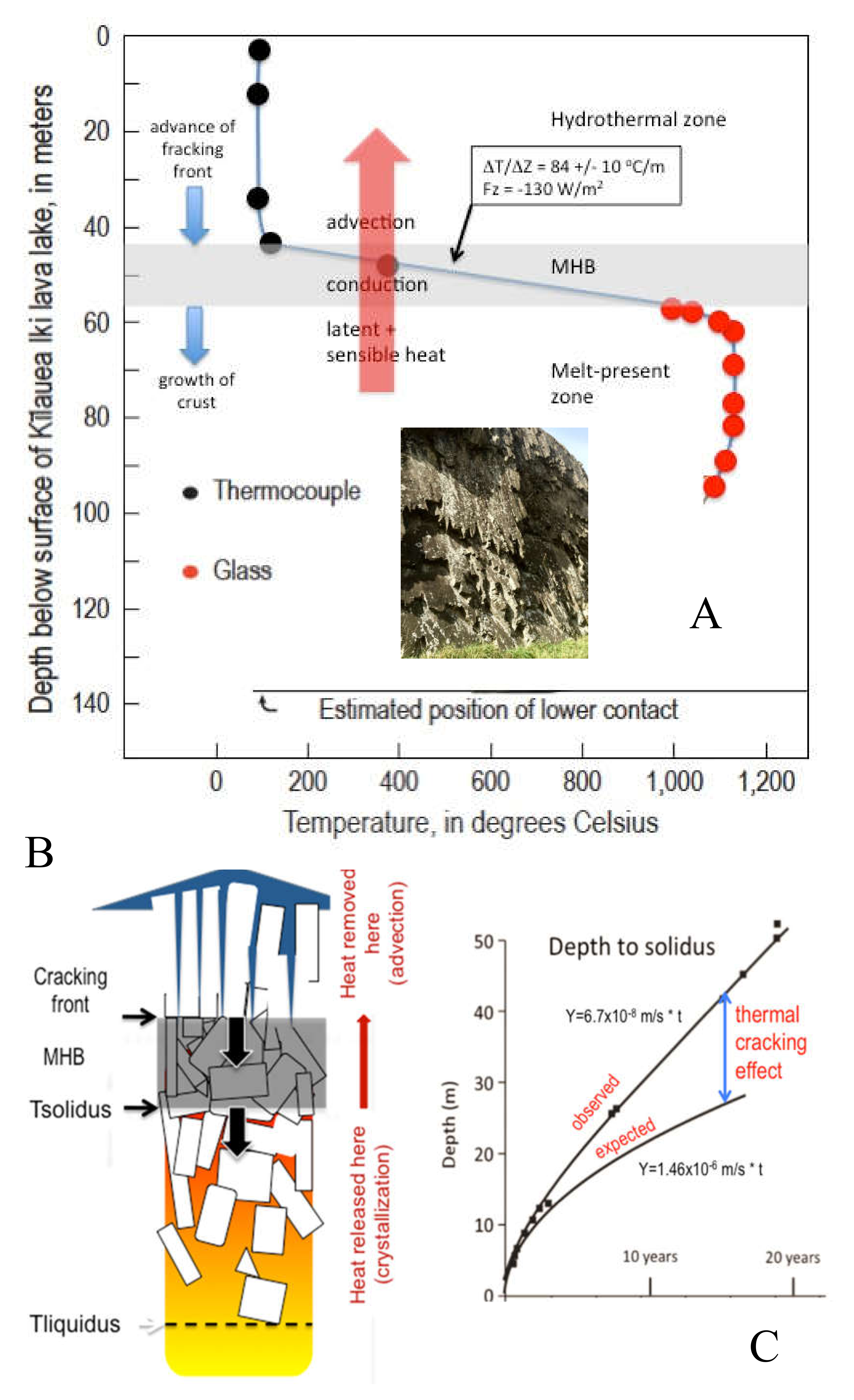
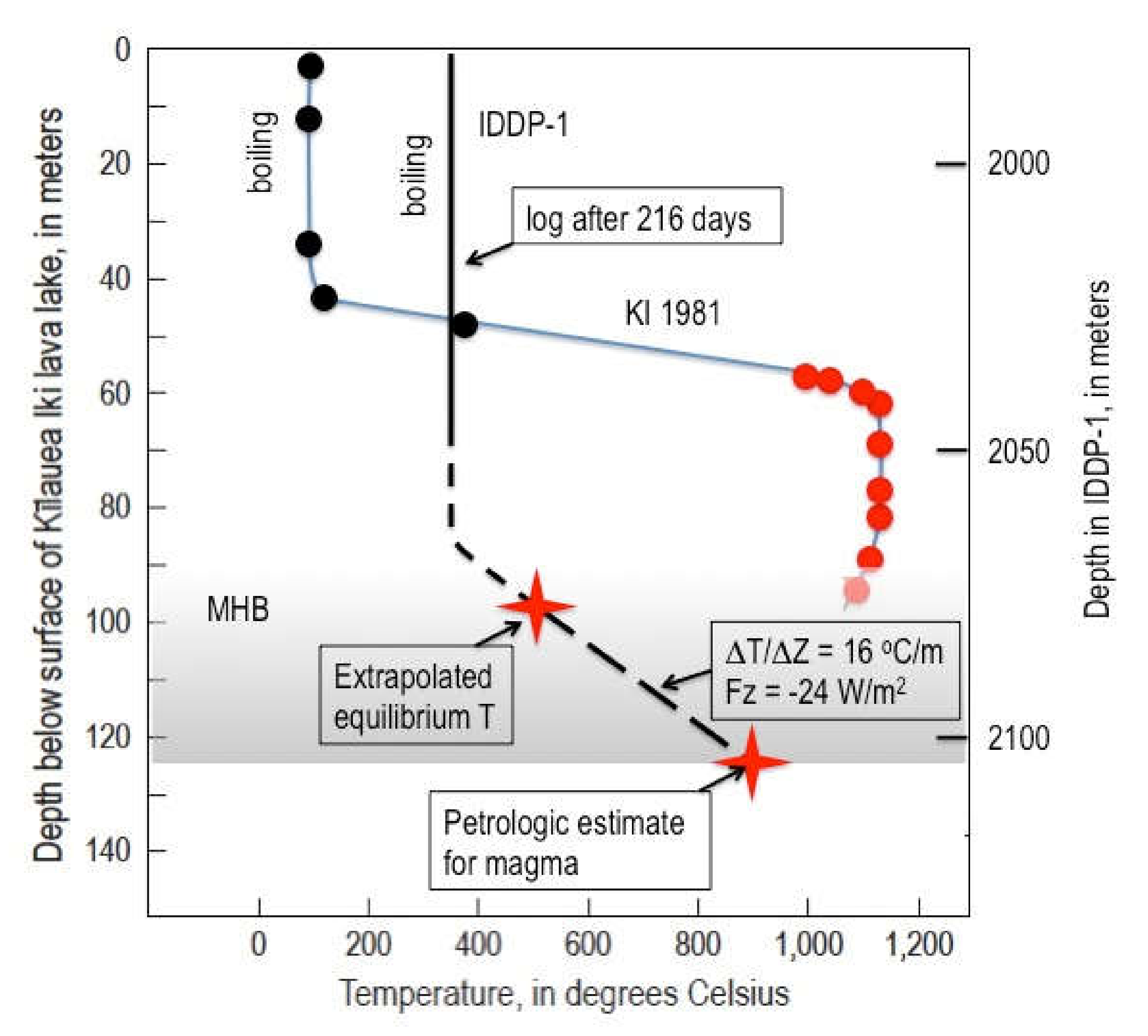
IDDP-1 provides enough data to place some constraints on the Krafla magma–hydrothermal system. Allowing for the fact that the Krafla results are much deeper than for Kilauea Iki and therefore the hydrothermal temperatures much higher, there is a broad similarity in the temperature profiles. Again, we see an upper hydrothermal zone at the boiling point for the prevailing fluid pressure (
Figure 6). Beneath that is a conductive zone with a steep thermal gradient, the MHB. The gradient is constrained by only two temperatures: (1) the time-extrapolated temperature to thermal equilibrium at 2077 m [
26] and (2) petrologic estimates for the near-liquidus rhyolite magma, which can be regarded as about 900 +/−50 °C [
27,
34,
35]. This yields a thermal gradient of 16 °C/m.
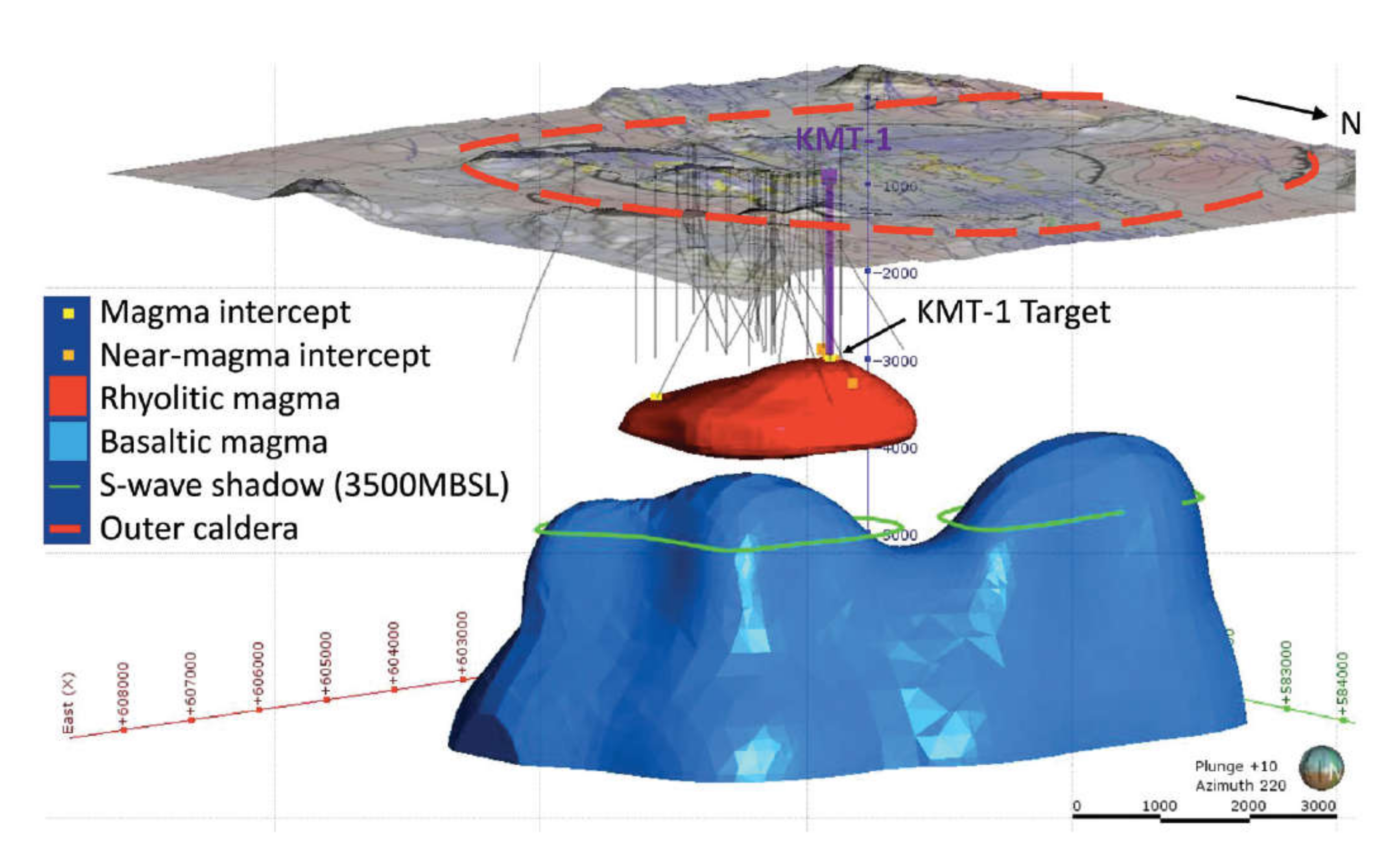
One of the great mysteries about the rhyolite magma is that it there: shallow yet undetected prior to drilling. Another is that there is no mush zone represented in the cuttings. There is, however, partially melted roof rock, formation of which would seem to require heat from partial crystallization of magma. Krafla is a dominantly basaltic central shield volcano with a summit caldera and essentially no rhyolite eruptions in the last 104 a. The very low crystal content would suggest the magma is “new”, yet since the time of the Krafla Fires of 1975 to 1984, the volcano has been among the best monitored in the world [. It is unlikely that a substantial body of magma could have arrived at only 2.1 km beneath the center of the volcano during that time period without detection. However, if it were small enough to avoid detection, it should be entirely crystallized.
The existence of a conductive MHB with temperature constraints allows an approach at Krafla analogous to Kilauea Iki to predict how fast crystallization should be occurring in the magma below.
Using Equation (3) and values adopted by Axelsson [
28]:
where 800 J/kg·°C, sensible heat capacity of rhyolite magma
= 400,000 J/kg, latent heat of crystallization of rhyolite magma
TL = 900 °C, liquidus temperature and
T
S = 800 °C, solidus temperature [
36]
= 2300 kg/m3, mass density of rhyolite magma
= 2700 kg/m3, mass density of crystallized rhyolite (felsite)
Equation (4) yields (adjusted to the volume of crystallized rhyolite):
We are neglecting here a contribution from the transfer of energy in any released magmatic vapor phase (MVP [
7]), but the amount of vapor exsolved and the pressure and temperature drop it undergoes should be negligible at this local scale.
Applying this to Equation (5) gives:
This amount of crystallization expected at the roof of the rhyolite magma body over a period of three decades, before which emplacement of the magma might have gone unnoticed, would have been seen in chips from IDDP-1. It is hard to imagine randomly bringing up chips of magma and partially melted felsite without a substantial portion of mush, hypothetical lithology C in
Figure 5, if C were present. The simplest interpretation, and one that can be tested by coring, is a stratigraphy comprised downward of felsite (A), partially melted felsite (B), and rhyolite magma (D) with no intervening mush (C). The hotter and higher enthalpy (more melt) rhyolite magma is actively melting its lid (less melt), which in turn means it is releasing heat to the MHB faster than the overlying hydrothermal system can take it away. Presumably, this would be moving MHB upward by effectively dissolving the lower face of MHB and closing fractures by thermal expansion at the cracking front at the upper face. In Kilauea Iki, we clearly see the progress of crystallization yielding mush and then solid crust at the roof of the molten lava lens, and its rate of downward growth is consistent with simple conduction through the MHB. Why not at Krafla? Likely because the magma of Krafla is convecting, and the molten lava of Kilauea Iki is stagnant.
One alternative to the convection of magma under Krafla is that the partially melted felsite is itself the source of the rhyolite magma. Rhyolitic melt could be percolating out of the walls of the bore hole or coming from preexisting segregation veins like those that flowed into the borehole at Kilauea Iki [
14]. In this scenario, which aligns with quite a bit of thought about the source of near-liquidus rhyolite [
37], the volume below MHB is mostly crystals with interstitial rhyolite melt. However, percolation is a slow process, all the more so with the walls of the borehole quenched. Segregation of melt into veins prior to sampling by drilling seems to be required [
38]. However, the magma contains sparse euhedral phencrysts, totally unlike what is present in the melting felsite. Percolating these through the crystal network of felsite is a physical impossibility. The phenocrysts are larger than the pathways. However, growing them after segregation into veins is equally implausible. The magma and felsite are not in thermal equilibrium. Crystals in the felsite are melting and those in the magma are growing. Heat is being transferred. This could not be so in the segregation vein scenario where veins and their partially melting host should be at the same temperature. There must be flow within the magma relative to the felsite to maintain thermal disequilibrium.
It is worth noting that the calculated minimum heat flux of 24 W/m
2 in IDDP-1, if applied to the entire area of 3.5 km
2 that A. Mortensen (2012, unpublished data) suggested, which might be underlain by shallow rhyolitic magma, amounts to only 100 MW thermal power output, or less than the output of IDDP-1 during flow tests and less that the Krafla power plant uses to generate 60 MW electrical power. There are many uncertainties; for example, there may be other magmatic sources of heat in the caldera, the thermal gradient in MHB could be much higher, or there may be advective transport of heat by magmatic vapor phase through episodic rupturing of MHB [
7,
9] in addition to continuous conductive transport of heat across MHB. However, if the inferred values approximate reality, then realizing an order of continuous magnitude power output from this magma-sourced geothermal field likely requires penetrating and thermally fracturing MHB, as apparently occurred with IDDP-1.
4.2. MHB Represents a Discontinuity in the Stress Field as Well as in Temperature
Below the MHB is magma with properties of a liquid. As the interval between magma and thermal cracking, MHB is expected to be ductile and should not support a difference between the lithostatic load on it and pressure within the magma. Pressure within the magma can therefore be expected to be lithostatic and isotropic. Above MHB, stress in the rock is lithostatic and anisotropic, whereas fluid in cracks and connected pores is hydrostatic. Hydrostatic pressure in a borehole is a straightforward measurement or calculation. Neglecting additives to water in the drilling fluid (which for some boreholes are substantial) and density variations with temperature, the hydrostatic pressure (P
H) at the bottom of IDDP-1, 2100 m, with fluid standing 400 m below the surface and the density of water
, acceleration of gravity
, and depth of water column
is:
Taking 2500 kg/m
3 as a common approximation for shallow, porous, and fractured volcanic rock, the lithostatic pressure, P
L, at
is:
Zierenberg et al. [
27] concluded that the magma is at about 40 MPa, less than the lithostatic pressure of 53 MPa, though significantly higher than hydrostatic pressure at 17 MPa, because the volatile content of the quenched melt falls below (i.e., at lower volatile content than) the vapor saturation surface in CO
2 + H
2O vs. P space. Taking this at face value, there are three ways to explain the discrepancy in inferred pressures (1) The rock envelope containing the magma is strong enough to protect it from the full weight of the overburden. This has already been suggested to be unlikely. (2) The magma is vapor undersaturated: i.e., P
CO2 +P
H2O < P
L. This seems unlikely as well, because the magma appears to have been generated at greater depth by partial melting of hydrothermally altered basalt protolith [
27,
34,
35]. The source rock contained chemically bound water that would be preferentially partitioned into the melt phase. Both partial melting and fractional crystallization concentrate water in melt just as they concentrate other incompatible elements. (3) The magma ascended to a higher level (lower pressure), degassed, and then descended to where it was sampled. This unlikely scenario would be necessary because degassing requires vapor saturation so that volatiles can escape as vapor.
A more likely conclusion is that the magma is vapor saturated at local lithospheric pressure, but the calculated vapor and/or lithostatic pressures are in error. There are multiple possible sources of errors: (1) The actual overburden pressure is unknown. Certainly, the density profile is not constant, and 2500 kg/m
3 is an arbitrary figure. (2) There are uncertainties in the experimentally determined solubility values. (3) Likewise, there are uncertainties in the analytical determination of the CO
2 and H
2O contents, as shown by error bars that overlap the vapor saturation surface at +1 σ [
27]. (4) Finally, the solubilities of volatiles are dependent on melt temperature, and this is not known with confidence to within 50 °C. If the magma is hotter than 900 °C, the solubilities of H
2O and CO
2 will be lower and hence the pressure inferred from the volatile contents higher
There is another intriguing possibility and it arises because of active rifting, the regional stress field is anisotropic with the least principal stress,
oriented approximately east–west, causing dikes from the main basaltic magma system to propagate north–south. Or, if the caldera fill is decoupled from this field,
may be vertical within the caldera, resulting in the formation of sills. The stress field changes with time during rifting and magma intrusion events. This leads to further uncertainties, not only as to the current confining pressure on the magma but also the history of that pressure. It is possible that the magma degasses if it experiences a lower
during a rifting event and so becomes vapor undersaturated when pressure returns to equilibrium. The upper limit for pressure in the magma may be
plus the critical overpressure to initiate a magmatic hydraulic fracture or dike, thought to be
[
39]. Therefore, we do not know the pressure on the magma accurately. With the progress presently underway in developing extreme sensors, direct measurement of magma pressure may become possible. This will provide a vast advance in understanding both the coupling of magma dynamics to tectonic events and changes in magma pressure that drive seismicity, surface deformation, and presage eruptions.
In any case, the magma is at or close to vapor saturation and at a much higher pressure than hydrostatic, as dramatically demonstrated by the magma flowing 8 m up IDDP-1 in 9 minutes or less after it was penetrated by drilling [
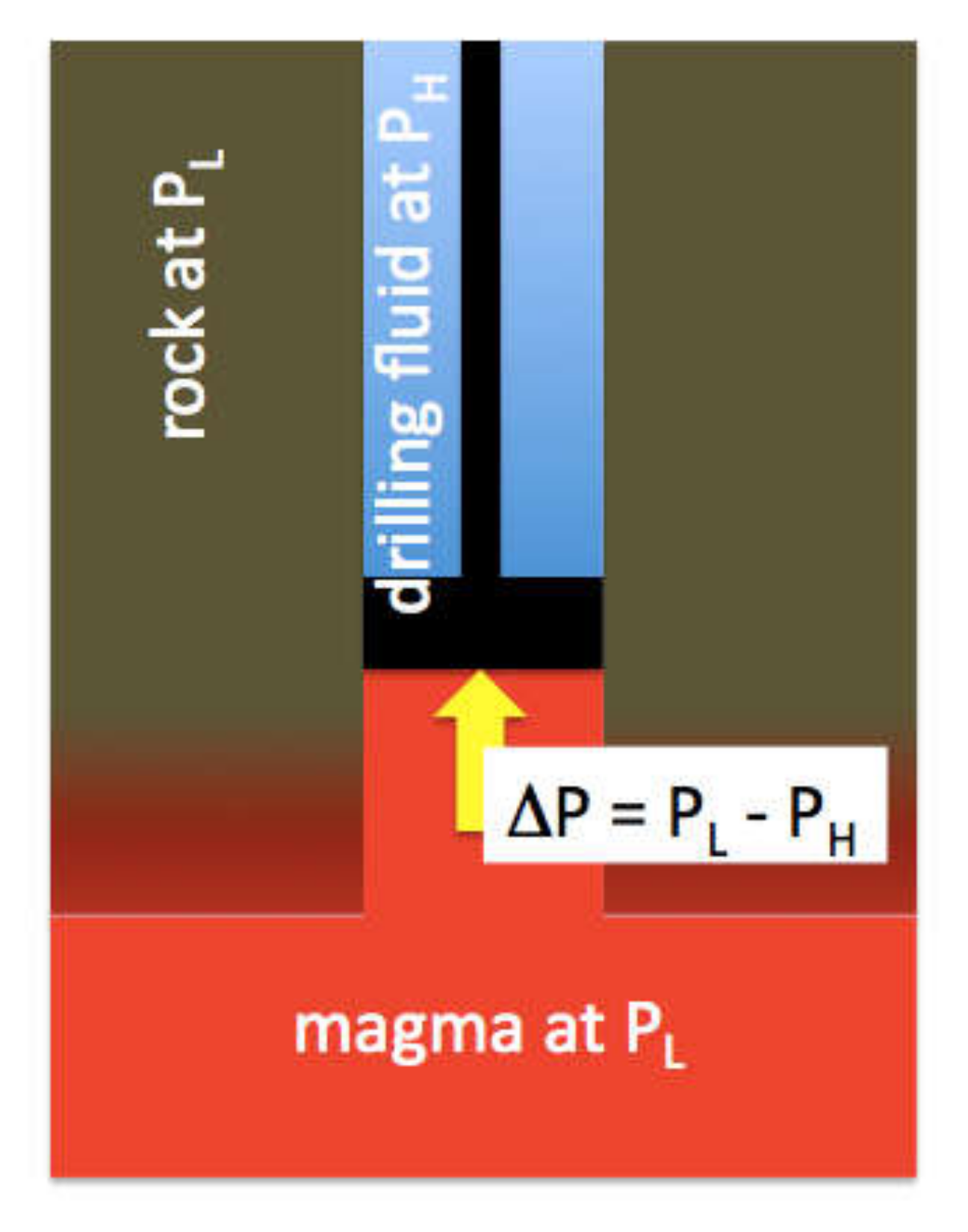
26]. This provides a first direct measurement of magma pressure, although only a crude minimum. When the drill bit reached the magma, there was a weight loss from the drill string (hook load) of 50,000 kg. The magma pushed back with a force in N of (50,000 kg) × g. We can treat the drill bit as a piston, albeit an ill-fitting one (
Figure 7). Net upward force (F) on the 12 ¼” diameter drill bit is produced by Δ
P acting on the area (
A) of the bit face:
With a bit area of 0.076 m
2 (r = 0.15 m):
We know that PH = 17 MPa, so this gives a minimum for PM of 24 MPa. In fact, magma apparently flowed around and ended up on top of the drill bit at the same time it was pushing against the bit, so clearly the cylinder/piston leaked and PM > 24 MPa. This leakage of magma and the fragments of partially melted borehole wall material it carried are the likely sources of the chips that were recovered. The pressure at the top of the rising magma column was also likely reduced by viscous drag as the magma cooled against the borehole walls and drilling fluid.
Note that the large change in the stress field across MHB in Krafla, or between the fluid-filled borehole and its wall, is in contrast to the lava lake case. In the latter, a shallow depth means that the difference between hydrostatic and lithostatic pressure is <2 MPa. The much larger pressure contrast at 2.1 km depth has implications for the stability of the borehole within the MHB at Krafla. Without extensive chilling or emplacement of casing to support
of >30 MPa, the hottest portion of KMT-1 will close much faster than coreholes at Kilauea Iki. This can be used to advantage in understanding the MHB if a thermocouple string can be emplaced quickly (
Figure 5) before the borehole closes on it.
4.3. Suppression of Upward Flow of Magma in the Borehole
There is always concern if the fluid in a well is overpressured. However, in general, “overpressured” is relative to hydrostatic. Here, we are dealing with a situation where the fluid, magma, could become overpressured with respect to lithostatic. If the Krafla magma were a simple liquid of ρ = 2300 kg/m
3, then P
L = 53 MPa at 2100 m would support a column of magma 2280 m high, 180 m higher than the surface. However, decompression during ascent would cause exsolution of the CO
2 and H
2O, forming a foam and then a dusty gas (because expansion of magmatic foam is limited by the strength of bubble walls to about 4 X), expanding about 200 X if the pre-eruption magma contains 2 wt% dissolved H
2O, e.g., [
40]. This very low-density material within the borehole would reduce the pressure below it, establishing a condition similar to an airlift in a water well, that is, it becomes self-pumping, erupting at high velocity. An eruption, or in drilling terms a blowout, clearly did not happen, and from a safety standpoint in future exploration and use of magma the successful suppression of upward flow needs to be understood. Just as boiling in the drilling fluid column must be prevented, boiling, that is vesiculation, in the magma column, must be prevented as well.
Merely dropping the pressure on the magma to that prevailing at the bottom of the borehole, 17 MPa, results in significant foaming. Neglecting CO
2, which will not add much to the vapor volume, results from Zierenberg et al. [
27] indicate that dropping the pressure of the magma to the hydrostatic pressure of 17 MPa will produce exsolution of about 0.5 wt% H
2O, or 0.005 kg of vapor in 1 kg of magma originally comprising melt (neglecting sparse crystals) with 1.8 wt% dissolved water. For magma density of 2300 kg/m
3 [
29], the specific volume is V
M =
. The specific volume of vapor for ideal gas behavior is V
v = (R
T)/P where R = 456 J kg
−1 oK
−1, T = 1173
oK, and P = 17 MPa, giving V
v = 3.1
10
−2 m
3/kg (and consistent with extrapolation from tables of Burnham et al. [
41]). The specific volume of the bubble-in-melt suspension is:
and porosity,
, is:
This gives a porosity of 0.27, i.e., 27 vol% bubbles. The magma chips have less porosity than this, and many have no bubble content at all (
Figure 4). This is clearly because the chips were effectively quenched by the drilling fluid before vapor exsolution and bubble growth due to decompression could occur. It was the cooling effect of the drilling fluid, rather than the pressure it exerted on the rising magma, that stopped the ascent.
The event described is essentially a small intrusion through an artificial perforation in the MHB. In the same way, a natural breach in MHB will cause quenching of magma unless it has sufficient volume and force to get through the formidable heat sink provided by the hydrothermal system and erupt.
4.4. Convection in Krafla Magma
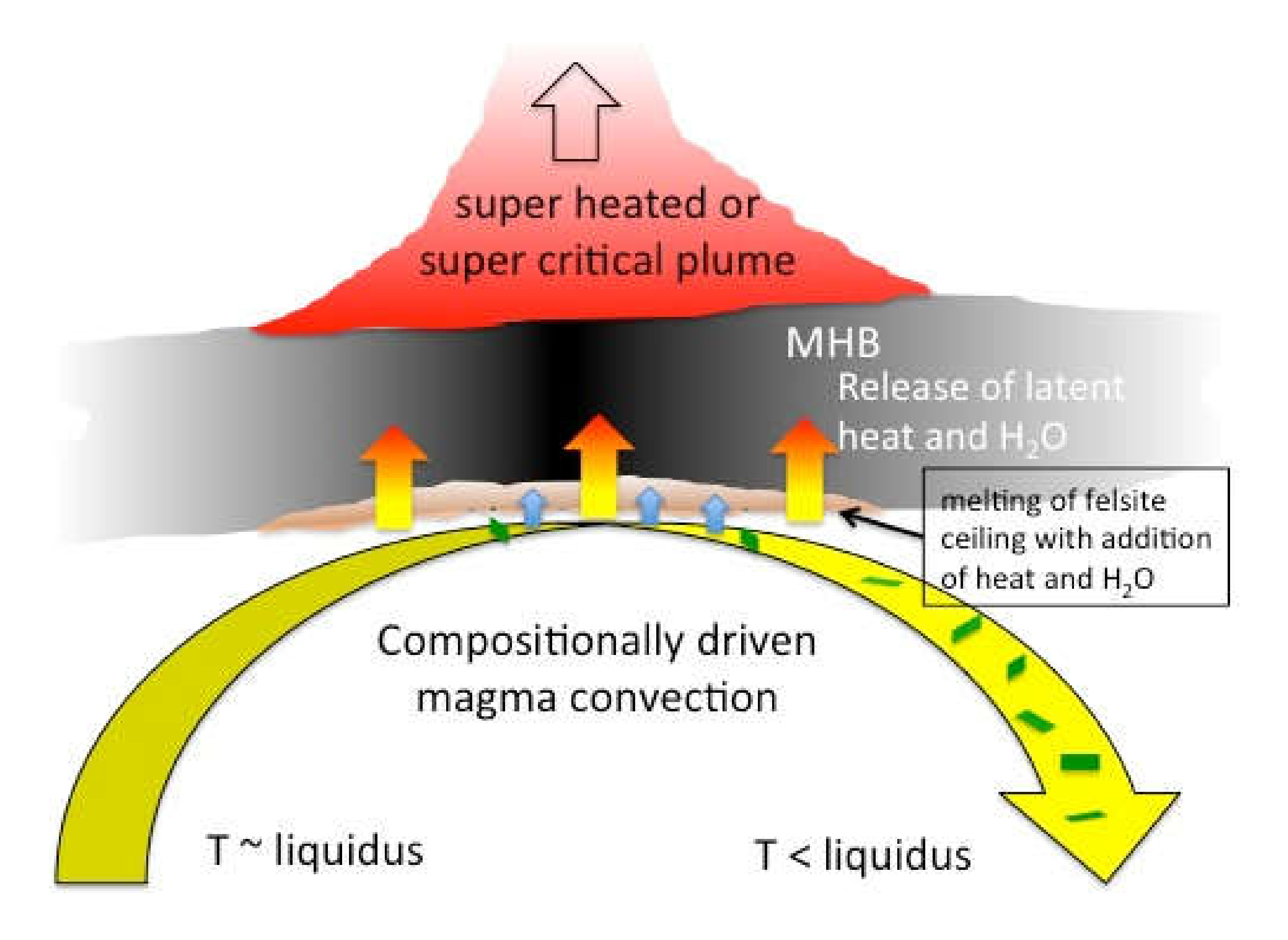
To explain the absence of crystallization at the top of Krafla magma, Axelsson et al. [
29] suggested, and I agree, that there must be convection within the magma body, continually sweeping away denser crystallizing magma and replacing it with uncooled magma at the roof zone (
Figure 8).
An independent line of evidence supporting convection is the crystallization required to produce the observed melting in the roof at Krafla. In a static system, crystallization of magma should approximately balance melting in the roof because the latent heat of crystallization dominates the energy budget. Yet the meager crystallization observed at the top of the magma (~1 vol%) cannot account for the much more significant melting observed in some of the felsite (
Figure 5). Convection of magma below the static roof would allow the crystallization that is driving the melting to be spread out over a much larger volume of magma, and so crystals would be less abundant in any volume element of magma than melt in a volume element of the roof.
But, melting of the roof requires delivery not only of heat but of water as well. The felsite contains no hydrous phases; it is anhydrous. Without water, the felsite solidus should be about 950 °C [
36], well above that of the rhyolite magma with 1.8 wt% H
2O. Two mechanisms might accomplish this transfer of water. One is if the magmatic vapor phase exsolves in the upwelling magma because of decreasing pressure or increasing crystallization (second boiling) or both and enters the overlying felsite along grain boundaries. There it becomes a key though minor (in wt% but not mole%) ingredient in the melt phase as melting develops between quartz and feldspar crystals, which provide the other ingredients necessary to form the eutectic melt. Alternatively, this might be accomplished by diffusion of water within the melt phase. Given the presumed impermeability of MHB and the fact that the diffusivity of water in melt is five orders of magnitude smaller than for heat (10
−11 m
2/s vs. 10
−6 m
2/s), it is probably the transfer of water rather than heat to the felsite that is the rate-controlling step for melting. The extent of melting may therefore be quite limited, perhaps even to only occurring in fragments of roof rock that were engulfed by the magma. As a layer, the partially melted felsite would be a thin transient heat sink, blocking some of the upward heat flow as long as melting is occurring.
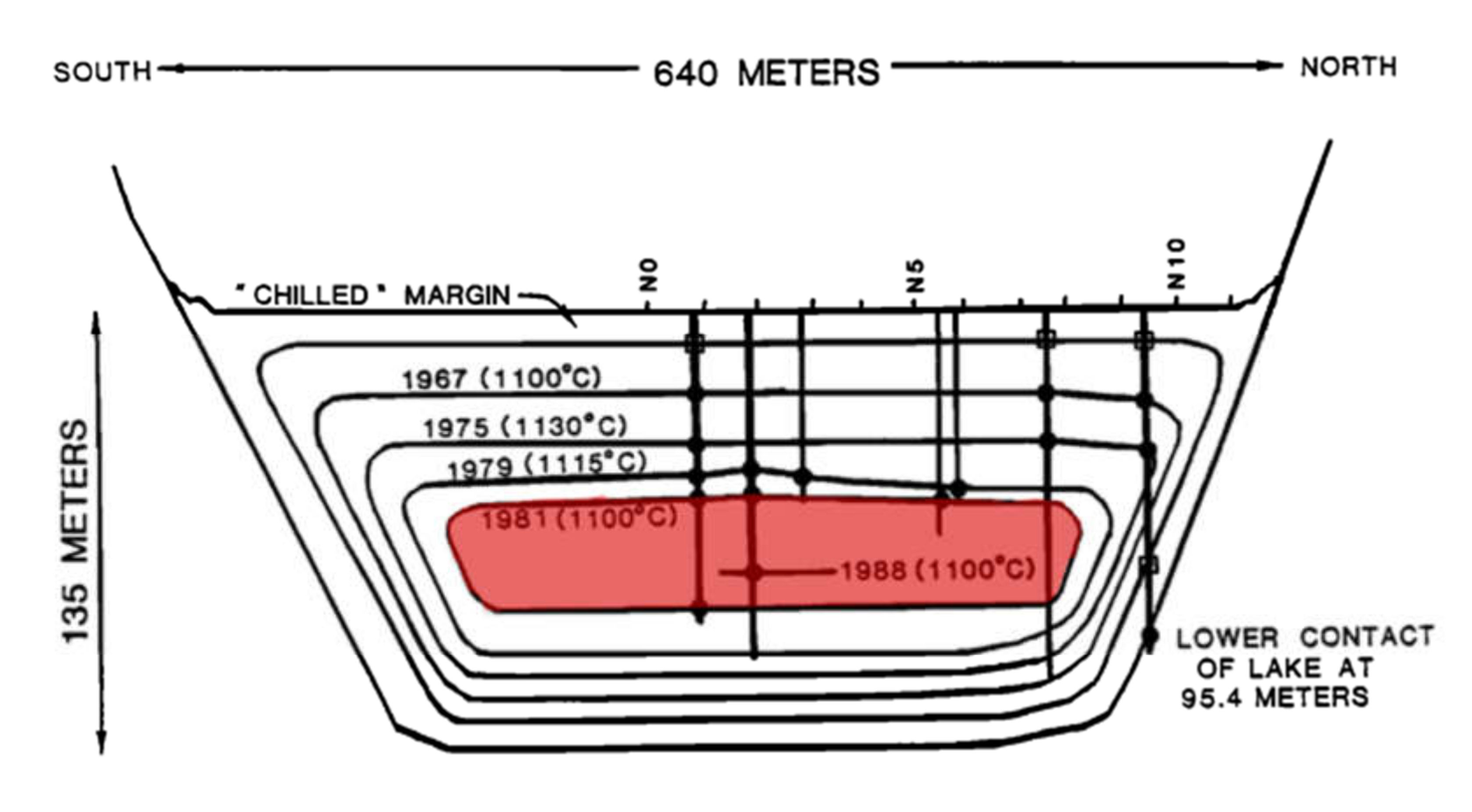
The convection hypothesis is consistent with the presence of an upper mush zone in Kilauea Iki and absence in magma bodies. Convection in the former may be limited in time and vigor due to the smaller size and to cooling from the bottom as well as the top in a lava lake, whereas in the magma chamber case, convection may be more vigorous and longer long-term [
5].
The simplest form of convection occurs due to thermal contraction of magma on cooling and therefore negative buoyancy at the top of the cooling body. The coefficient of thermal expansion,
, is defined as:
where
V is specific volume, about 4 × 10
−5 °C
−1 for magma [
5]. The Rayleigh number for thermal convection,
, which indicates whether a fluid body across which a vertical temperature drop exists will convect, is the dimensionless ratio of the time scale for heat transport by conduction (
cd) divided by the time scale for heat transport by convective flow (
cv).
The time scale for conduction (t
cd) can be approximated as d
2/K, where d is the thickness of the magma layer and K is thermal diffusivity of magma. The time scale for convection (t
cv) is d/u, where u is the terminal velocity of the sinking cooled fluid, dependent in turn on the density change with cooling (
) and magma viscosity (
. This gives:
Application of the viscosity model of [
42] for the Krafla melt composition and T = 900 °C and P = 50 MPa gives viscosity
. Taking
,
, K = 10
−6 m
2/s, and
as above gives:
That is well within the convective regime that begins above Ra ~ 2000.
The value of is critically dependent upon the thickness of the magma layer, d. A 10-m thickness can be ruled out because the entire body would have crystallized since the Krafla Fires. A thickness of 1000 m is plausible for a long-lived rhyolite magma body. The 100-m thickness is chosen as being intermediate and conservative.
Another route to obtaining magma viscosity is the observation that magma quickly rose 8 m up the borehole after drilling penetrated magma on the second sidetrack [
26]. After encountering magma, the drill stem was pulled back and then went back in, encountering magma 8 m higher in the well 9 minutes later. The magma continued to rise as the drill string was pulled back again, and 50,000 kg was lost from the hook load (see
Section 4.2). The bit became stuck in magma 9.4 m above the previous level.
The Hagen–Poiseuille equation for fluid flow through a pipe gives a first approximation for this situation, although it applies to steady-state flow rather than abrupt entry of magma into the borehole, wherein the pressure gradient will start high but decrease with time and cooling of magma against the borehole wall and drilling fluid will cause the viscosity to rise.
Using the same values as in
Section 4.2 and velocity V = 0.015 m/s and L = 4 m, the midpoint in flow up the borehole to give an intermediate pressure gradient between the beginning and end of flow, gives:
This value is about an order of magnitude greater than that calculated from the laboratory measurement-based model of Giodano et al. [
42] noted above. However, flow was being impeded by cooling of magma against the borehole walls and the drilling fluid, both raising its viscosity and constricting the radius (r) of flow. Zierenberg et al. [
27] obtained quench temperatures averaging 850 °C based on speciation of water in melt. Dingwell et al. [
43] found that a temperature decrease from 900 °C to 800 °C increases viscosity by more than an order of magnitude. So the estimate for magma viscosity from borehole flow is reasonable, but likely higher than that of the undisturbed magma.
There is an additional buoyancy force affecting convection due to crystallization of magma flowing below the roof. As is the case with heat capacity where the effect of heat from crystallization can be added to define an effective heat capacity, the effect of crystallization of crystals denser than melt can be added to obtain an effective thermal expansivity. Just as crystallization dominates heat transport through the release of latent heat, crystallization also dominates mass flow by increasing the bulk density of the melt + crystal mixture, magma.
We should first establish the validity of considering the bulk density of the melt+crystals suspension that is magma, wherein relative motion between melt and crystals can be neglected. The Stokes settling relationship, where buoyancy and drag forces are balanced for a sphere of r radius, is:
with
r = 1 × 10
−4 m (
Figure 5),
= 400 kg/m
3 [
29], and η = 3 × 10
5 Pa·s [
43].
This works out to 1 mm/a, trivial even for convection in a lava lake [
5].
The crystallization equivalent for the coefficient of normal thermal expansion, Equation (10), where we will assume linear crystallization with decreasing temperature (not accurate [
36] but a useful approximation) from a weight fraction of
Xwtc = 0 at the liquidus of 900 °C to
Xwtc = 1 (so that
XwtL +
XwtC = 1) at the solidus of 800 °C, is:
where
is the specific bulk density of the melt + crystal mixture, i.e., magma:
Because
XwtL +
XwtC = 1, we can deal only with
XwtC as crystallinity, eliminating
XwtL as (1 -
XwtC):
Because of the relatively small temperature interval, we can treat
VC and
VL as constant with
T. Differentiating with respect to
T:
Using
VL = 4.35 × 10
−4 m
3/kg,
VC = 3.70 × 10
−4 m
3/kg, and
we get:
Converting to the form of Equation (15) and using a 90:10 liquid to crystal mixture for Vmix:
This is almost two orders of magnitude larger than the thermal expansivity of melt, so it will obviously dominate convection if crystallization is occurring, as it must. The compositional Rayleigh number, as it is called, analogous to Equation (12), is then:
This means that convection will occur in a thinner magma body or at a lower thermal gradient than in the melt-only case.
During convection, there is another process that helps to ensure that the hottest magma is delivered to the base of MHB. That is, the preferential migration of lower viscosity fluid within a fluid of variable viscosity to regions of high shear during flow. This has been observed in the chemical zonation of a volcanic conduit [
44] and in pipes in an industrial setting [
45]. In convecting magma, the highest shear will be at the base of MHB [
45], driving—in addition to the buoyancy forces at work—the hottest magma with least crystallinity there.
However, some caveats should be recognized. One is that crystal growth must be sufficiently rapid to release the latent heat of fusion as the magma is flowing under the roof. If there is a time lag so that crystallization occurs when the magma has already been returned by thermally driven convection to deep in the magma chamber, then it will not contribute to convection as a whole. In fact, it may retard it. Also, in a vapor-saturated system, crystallization of anhydrous phases, as all the crystalline phases in Krafla magma are, will induce vapor exsolution, that is, bubble growth with the vapor fraction dependent upon depth (pressure). This would tend to compensate for the negative buoyancy effect of growing crystals. These complications have been treated in a number of sophisticated models, e.g., [
46] and are beyond the scope of this simple analysis. The conclusion here is that the release of latent heat and forcing convection by crystallization are linked and can contribute greatly to heat transport from magma into the suprajacent hydrothermal system. Arguments against convection of magma, both experimental and theoretical, rely on growth crystals at the roof of the magma body in response to heat lost through MHB. Thus far, drilling encounters with magma indicate that this is not the case. A difference in views is whether convection is limited to the earliest stage of a magma body and therefore can be ignored in its overall cooling history [
47] or whether it can continue until flow is locked as the crystal content approaches 50 vol% [
4].
To get an idea of how effective convection is in delivering heat to MHB, we can ask how large the upwelling limb of magma convection cell needs to be to continuously deliver 1 GW of thermal energy. Assumptions are a vertical velocity in cross sectional area A, rising at vertical velocity u, say 0.01 m/s, and releasing heat to the roof by cooling 10 °C.
Using the same parameters as before and solving for the radius of the heat pipe:
There may be time-discontinuous mechanisms of heat transport as well, for example, magma breaching the MHB and cooling within the hydrothermal system, transferring its energy directly. Based upon observations at Yellowstone, including inflation and deflation cycles and migrating seismic swarms, Fournier [
9] postulated that build up of vapor pressure in a magma body due to crystallization would periodically rupture the MHB, injecting the magmatic vapor phase into the hydrothermal system (and occasionally causing phreatomagmatic eruptions).
4.5. Conceptual Model of Krafla as a Closely Coupled Magma–Hydrothermal System
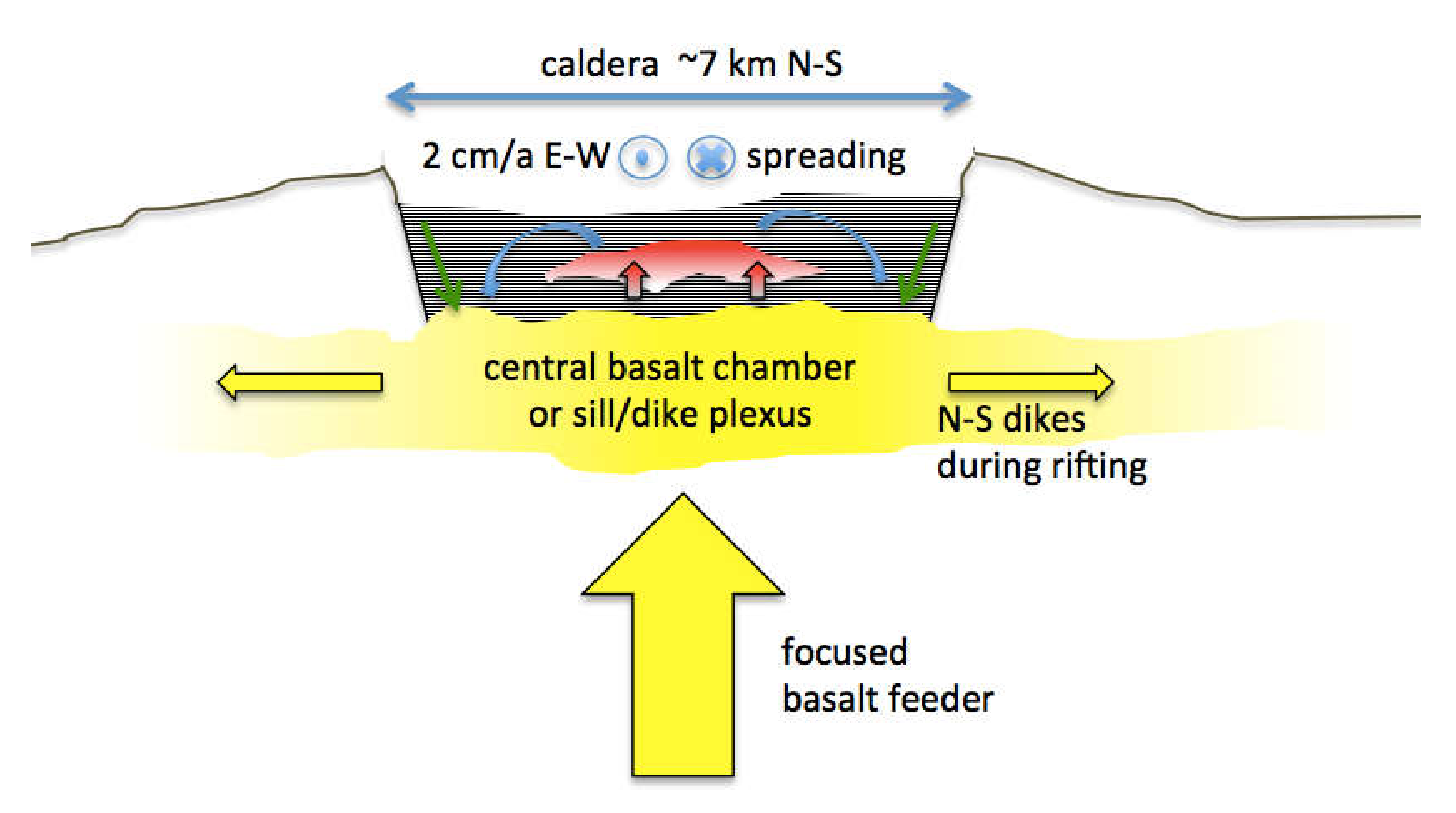
A view of the Krafla system that is consistent with, but not unique to, most of the observations cited is presented in
Figure 9. Like its nearby companion, Askja Caldera [
33], Krafla is an essentially basaltic system lying astride Iceland’s northern rift zone. Although one might be tempted to think of rift magmatism as uniformly distributed along the spreading plate boundary, these central volcanoes are clearly foci of basaltic magma upwelling that then feed dikes laterally at shallow depth along the rift structure. If there is a deeper zone of uniform along-strike magma generation, the magma must gather into plumes or heat pipes, which in this case then feeds into chambers or dike/sill complexes that in turn redisperse magma laterally at shallower depth. In a way, this is like subduction zone volcanism where existing models suggest uniform, along-strike magma generation above the subducting slab that coalesces upward to focus into discrete, long-lived centers rooted in the crust.
Sustained, focused magmatism produces impressive edifices, but less so in rifts because the structures are being torn apart as they are built. Focused magmatism also produces calderas that are the surface expression of shallow magma chambers. As such, calderas reflect subsidence above shallow magma bodies because over time the magma entering the chamber is more than balanced by magma dispersed beyond the immediate volcanic center. The two processes that do this are lateral diking that drains the central chamber in the subsurface and large volume explosive eruptions that disperse its contents widely on the surface. The former is more frequent and the latter more catastrophic. Both processes appear to have contributed to the topographically rather subtle Krafla Caldera.
The association of rhyolitic magmas with an isotopic signature suggestive of a hydrothermally altered basalt protolith [
27] fits with this view. Intense hydrothermal circulation is concentrated within these caldera structures, and the gradual or episodic subsidence feeds hydrothermally altered basaltic caldera fill towards the hot plate of focused basaltic magmatism. Partial melting ensues, generating rhyolitic melt that accumulates at shallow depth, perhaps at its level of neutral buoyancy, as a coherent body. This may be a continuous process and requires only local vertical rearrangement of materials. Thus it produces little by way of surface deformation or gravity signals. Being viscous and at neutral buoyancy, the magma accumulation remains stable over millennia, requiring a massive “kick” by a basaltic intrusion as occurred at Askja [
33] in 1875 or on a smaller scale at Krafla’s Viti Crater in 1724 [
20]. To remain near liquidus, these rhyolitic accumulations must themselves be continuously heated by the basaltic furnace, either through their own intervening conductive boundary zone, or directly, because the strong contrast in density and viscosity and relatively low water content inhibit mixing [
48]. Indeed, Iceland is one of the classic sites exhibiting bimodal volcanism [
49]. This is in contrast to the subduction zone case where high water contents in the basalt cause foaming and mixing due to the temperature contrast at the boundary [
50].
This is an admittedly simplistic picture, and three variants in the magma–hydrothermal relationship seem obvious. One is where the shallow magma body maintains an open pathway to the surface. This prevents the hydrothermal system from forming as a cap above the magma, but allows it to develop as a lateral fluid outflow zone. Mutnovsky Volcano, Kamchatka, Russia, is an example [
51]. The direct connection between Mutnovsky and its adjacent geothermal system is supported both by oxygen isotope composition of fluids and evidence that changes in geothermal energy extraction appear to modulate the behavior of the volcano itself (A. Kiryukhin, unpublished data, 2019). Another is the rift setting, such as Reykjanes or in submarine midocean ridges, where frequent unfocused diking events maintain high temperature fluid circulation in the crust. The development of a magma chamber requires frequent, focused input [
11]. Finally, as the end stage of a silicic system, the melt may disappear and with hydrothermal circulation driven by residual heat. However, in this case, shallow heat is not replenished by the combination of the latent heat of crystallization and magma convection.