Abstract
The paper presents the results of a study of epigenetic changes in technogenic-redeposited coal-bearing rocks of Irkutsk and Kuznetsk coal basin spoil heaps (Russia). Hydrocarbon products formed under high-temperature and low-temperature pyrolysis of coal-bearing rocks were studied by using a chromatography-mass spectrometer GCMS-QP2010NC Plus (made by Shimadzu Company). The average temperature of low-temperature natural pyrolysis does not exceed 120 °C, and its average speed is approximately 2 m/year. In this case, three pyrolysis zones gradually built metamorphic rock mass (from bottom to top) are clearly established: heating (focal) activated and enriched. The average temperature of high-temperature pyrolysis reaches 850 °C, and its average speed is approximately 20 m/year. Unlike low-temperature pyrolysis, high-temperature pyrolysis is accompanied by the presence of two major zones (from bottom to top): pyrogenic (focal) and enriched (coke). The chemical composition of the enriched pyrolysis zone was studied in detail. It has been established that hydrocarbon compounds in samples of the pyrolysis zone are presented by six classes: asphaltic-resinous substances; polycyclic aromatic hydrocarbons, heterocyclic compounds, organic sulphur compounds; pyrolytic hydrocarbon and heavy hydrocarbon residue. Quantitative content of hydrocarbon compounds in the analyzed samples varies from 0.35% to 41.88%.
1. Introduction
For decades, coal underground mining activities on many coal deposits were accompanied by a technogenic deposit of coal-bearing rocks in the form of spoil heaps. Striking examples are Upper Silesian, Appalachian, Donetsk, Kuznetsk and Irkutsk coal basins. Dozens of spoil heaps occurring in these areas have caused and continue to cause significant harm to the environment, polluting it with hazardous products, formed in the process of epigenesis of coal deposits.
Studying the environmental impact of coal dumps has been interesting for a large number of researchers in recent years. First, it is associated with a constant increase in stockpiled mass [1,2,3,4,5,6]. The study of waste is relevanct due to the high environmental hazard to humans. Dumps of coal-bearing rocks occupy and infect vast areas. They are of interest as a source of secondary mineral raw materials. Studying the sequence of mineralogical and geochemical processes and the order in which newly formed technogenic minerals appearing in rock dumps is of great importance for fundamental and applied science. Self-ignition and burning of dump mass in coal deposits is one of the examples of natural disasters that cause distress for both people and all living things in the territory adjacent to it.
Pyrolysis and the following pyrogenesis transform the coal-bearing strata of spoil heaps completely, creating new types of rock and both organic and inorganic compounds. Many researchers dedicated their studies to this topic and opened a new page in the study of mineralogy of techno genesis and pyrometamorphism [7,8,9,10,11,12,13,14]. However, the study of the pyrolysis of natural organic products is still a weak link in the knowledge of epigenetic changes occurring in the sedimentary complex of spoil heaps. The knowledge of these changes is important for the understanding of self-ignition and rock combustion nature, including the environmental and social impact assessment of spoil heaps.
Rock burning in dumps is a powerful source of CO2 [2,3]. It contributes to the development of forest fires, river pollution, the loss of potentially valuable areas, climate change and the entire ecosystem of the region. In addition, emissions of acid gases, particles, organic pollutants and trace elements (As, F, Hg, Se) can cause public health problems [1].
Thus, the main problems of Quaternary geology which this article examines are the problem of the spontaneous combustion of rocks in the dumps of coal mining enterprises, the identification of the zoning of pyrogenesis and the staged nature of its formation. The main tasks faced by the authors are to study the epigenetic changes, as well as the pyrolysis and pyrogenesis of coal deposits occurring in mountain dumps.
The bulk of the field research was carried out in rock dumps of the Cheremkhovsky coal deposit (Irkutsk coal basin). The deposit is composed of Jurassic coal deposits, consisting mainly of sandstones (65%), siltstones (15%), coal (11%), mudstones (5%) and conglomerates (4%). The thickest layers of coal (8–18 m) are uncovered at a depth of 50–450 m. Coals are stone, gas, with an ash content of up to 35.7%, and their sulfur content reaches 1.8%. Sandstones, carbonaceous siltstones, high-ash and low-grade coals, as well as various technogenic products, were dumped. In the process of underground coal mining. In 1965, underground coal mining was discontinued, and the mines closed. The era of open-pit mining of coal seams began. The burning of coal dumps began in the late 1970s.
2. Materials and Methods
The study of epigenetic processes of technogenic-redeposited coal-bearing rocks was conducted both in the field and in laboratory conditions*. Exploring shafts were dug and stripping was made in less accessible areas of pyrolysis of currently active heaps. In total, over the years five expeditions that allowed us to inspect 11 spoil heaps and to pick out extensive collection of 850 samples were organized. Only about 80 samples have been analyzed, and the rest are used as a collection of reference samples. All samples oxidized in air from pyrolysis zones are stored in closed glass jars.
A weighed portion (30 g) was taken from the clay-sand filler of the initial sediment sample to determine the mineral composition of the light and heavy fractions, and washed first from clay, and then in 5% HCl in a water bath from carbonates. After weighing the weighed portion, and then the carbonate-free sample, it was screened on sieves. Mineral particles 0.25–0.05 mm in size were separated using bromoform into light and heavy fractions. The weighted fractions were subjected to immersion analysis using a polarization microscope according to the traditional method [15]. The mineral content was calculated from 500 randomly selected grains taken as 100%.
The non-contact infrared thermometer (pyrometer) ADA TempPro-1200 was used in the field to measure the temperature in different areas of the pyrolysis process. At rock temperatures from 20 to 500 °C, the measurement accuracy was ±1.0% or ±1.0 °C, and at rock temperatures over 500 °C, it was ±1.5%. The response time of one point was 150 ms. The maximum temperature for measuring rocks using this pyrometer is 1200 °C. This is quite enough to measure the temperature of processes not only of pyrolysis, but also of pyrogenesis, at which the maximum temperature recorded by us in the waste dump did not exceed 854 °C.
Extraction of hydrocarbons (bitumen) from the samples was performed with the use of a non-polar organic solvent. As the content of different types of hydrocarbon and their derivatives are determined by chloroform extraction, it was used as an extractant. Total bitumen and hydrocarbon group analysis was carried out on the chromatography-mass spectrometer GCMS-QP2010NC Plus (Shimadzu Company, Kyoto, Japan), the software system of which is equipped with an extensive library of spectra of chemical compounds. The analysis was performed in the mode of a step change of the polarity gradient with six fronts. The instrument detection limit of hydrocarbons and one-sigma estimate of their determination are 0.001% and 15%, respectively.
The results of silicate analysis of samples were obtained using traditional methods for determining of the substance composition (wet chemistry). Loss on ignition (LOI) was preferably represented by hydrocarbon pyrolysis products.
The methodology for studying pyrometamorphic rocks included the study of their material composition using the JXA-8200 electron probe X-ray microanalyzer (JEOL Ltd, Tokyo, Japan), equipped with five wave spectrometers with crystal analyzers LDE1, LDE2, TAP, LDEBH, TAPH, PETJ, PETH, LiF, LiFH and energy dispersive spectrometer EX-84055MU (JEOL Ltd, Tokyo, Japan). The contents of rock-forming (FeO total, Al2O3, MgO, CaO, SiO2, MnO, TiO2, Na2O, K2O, P2O5, S total) and rare elements were determined by X-ray fluorescence analysis (XRF) on X-ray spectrometer S4 PIONEER (Bruker Company, Karlsruhe, Germany). Standard techniques and methods of the analysis have used. For each element being determined, the optimal angular positions were chosen for measuring the intensities of the analytical lines and the background, the method for taking into account the mutual influences of the elements, and standard rock samples for calculating calibration characteristics [16]. The emitters were made by pressing the analyzed samples into tablets. To assess the accuracy of the chemical analysis results, metrological studies were carried out. The obtained values of the mean square deviations of the analysis results correspond to the III category of accuracy.
Definition of minerals that are difficult to identify was carried out on a diffractometer DRON-3.0 by powder diffraction method. The experimental recording conditions were as follows: Ni—filter, CuKα—radiation, voltage—25 kV and electric current intensity—20 mA.
The hydrocarbon composition of the pyrolysis products was analyzed by the method of quantitative nuclear magnetic resonance spectroscopy [17] at the Analytical Center of the University of Irkutsk using the "Varian VXR-500S" instrument.
3. The Object and Goal of Research
A fieldwork was carried out on terricones (spoil heaps or dumps) of Irkutsk coal basin (Cheremhovo, Ch) and Kuzbass (Osinniki, OS) (Figure 1). Solid products of pyrolysis are presented by sulphur-containing resins, sulphur, asphaltenes and numerous hydrocarbon aromatic compounds concentrated in the "black block" of terricones. "Black blocks" occupy large area of spoil heaps and they can reach a length of hundreds of meters and a height of tens of meters depending on their size. According to Chesnokov B.V. and Shcherbakova E.P. [18], who first used this term during the research of burnt heaps of Chelyabinsk coal basin, basic amounts of undiscovered minerals and organic compounds are concentrated in the "black block". The organic substances are dun oily substances that smell like diesel fuel, lighten from a match and blaze. The goal of this research was to study the natural pyrolysis process and to identify patterns of the distribution of hydrocarbon pyrolysis products.
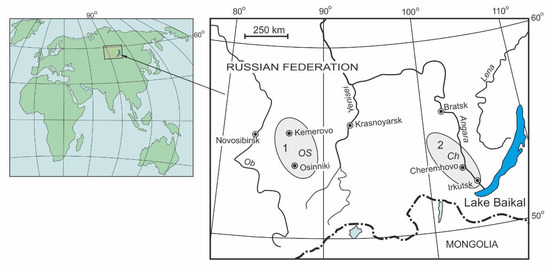
Figure 1.
Sketch map of objects investigated within the Kuznetsk (1) and Irkutsk (2) coal basins.
4. Research Results
In the course of long-standing research of pyrolysis products of technogenic-redeposited coal-bearing series in mine opening of spoil heaps, three successively upward pyrolysis zones were found: the heat zone, activated zone and enriched zone (Figure 2). These conventionally marked zones in the form of halos outline a thermally modified red-colored metamorphic rock mass. Metamorphic mass (MM) is represented by the red-colored clastic mass of thermally altered rocks. Due to the high porosity (about 30%) of the technogenic-redeposited rock mass and the prolonged thermal effect, the fragments of this zone of the terricone are gradually oxidized and turn into a red-colored massif. The average temperature of the metamorphic mass adjoined to the rocks that are under the pyrolysis influence is around 50 °C, although in the areas of contact with the heat zone it reaches 75 °C (Figure 3).
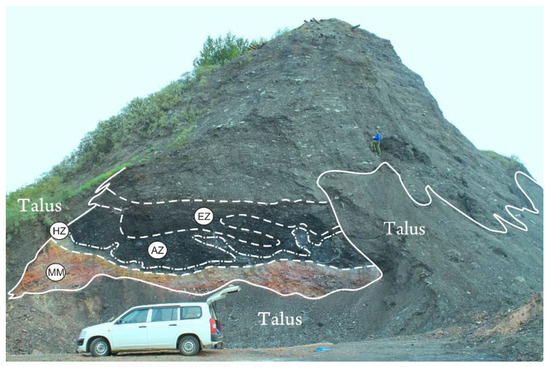
Figure 2.
Type section of quiet spoil heap Ch-09 in Cheremhovo. The red rocks of metamorphic massif (MM) are deposited in the basement. On the metamorphic massif, the “Black block” of pyrolysis rocks is superposed, within which the following zoning is delineated: EZ–enriched zone; AZ–activated zone; HZ–heat zone. The “Black block” is overlapped by sandy and clay deposits with debris of fine and medium sandstones.
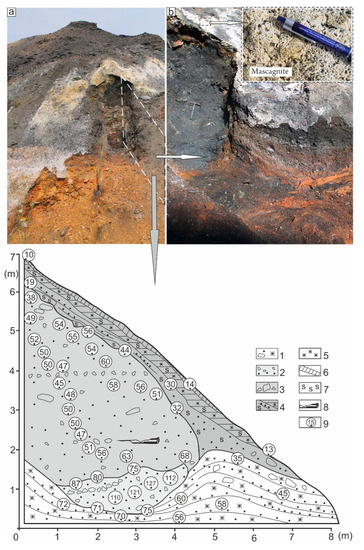
Figure 3.
Sketch sectional drawing of active low-temperature pyrolysis chamber, which shows the pyrolysis zoning and temperature distribution of epigenetically changed coal-bearing rocks (spoil heap Ch-05, the southern hillside). (a)—general view of the excavation site; (b)—an enlarged fragment. Below is a schematic side profile of fragment b with temperature readings over the profile area. 1—metamorphic zone; 2—heat (focal) zone; 3—activated zone; 4—enriched zone; 5—areas impregnated with light brown ferrum hydroxides; 6—areas of concentration of white mascagnite (NH4)2SO4; 7—areas of crystallized sulphur clusters, 8—the location of activated wood, 9—rock temperature measured with pyrometer, the point in the circle indicates the measurement location.
4.1. Heat Zone
The heat zone (HZ) is composed of gray sand and slag remaining products, formed by pyrolysis of coal-bearing deposits in alkali reductive conditions. Its thickness is inconstant and ranges from 0.03 to 0.6 m. An average temperature of rocks in a similar area of the pyrolysis process is about 115 °C, while it was found out that its maximum reaches 127 °C (Figure 3).
4.2. Activated Zone
The activated zone (AZ) is black and consists of solid activated hydrocarbon products of pyrolysis, including fragments of wood modified into charcoal. Most parts of this zone are represented by fragments of coal caked in the form of coal-bearing semi-coke or transformed into heavy fractured coal with bright anthracite lustre (Figure 4). Pyrolysis gases, passing through this zone, colour all the debris of rocks in black. The thickness of the AZ changes over the range of 1.4–2.8 m. The average temperature of rocks is approximately 50 °C, and its fixed maximum does not exceed 63 °C.

Figure 4.
Products from pyrolysis activated zone: (a) activated charcoal; (b) heavy fractured coal with bright anthracite luster.
4.3. Enriched Zone
The enriched zone (EZ) differs enormously from the above-described zones by the presence of variegated solid wet highly porous as well as uliginous oily pyrolysis products. Their variegation is caused by the presence of floury white sulfates (mascagnite and tschermigite), as well as the concentration of greenish-yellow sulphur, black asphaltenes and numerous hydrocarbon compounds, painted in various shades of brown. The unique feature of this zone is the presence of native sulphur, deposited as a well-crystallized near surface feature in the form of nests, the sizes of which reach 0.6 × 0.3 m. The largest (up to 7 mm) sulphur crystals are found near the middle part of the EZ. They are always coincided with large (more than 0.25 m) intensively modified sandstone debris and are arranged in the form of nests in their footprints. It is significant that fine-crystalline (up to 3 mm) sulphur in the form of long acerate crystals penetrates all tender ground mass of pyrolysis products (Figure 5). Terricolytes are very tough metamorphically modified dark gray rocks. According to our RFA data, they have the following composition (%): SiO2 (51.87–71.29); C (20.66–27.87); Al2O3 (0.20–17.15); S(total) (0.41–3.51); Fe2O3 (0.07–1.86); MgO (0.02–1.05); K2O (0.01–0.63); CaO (0.02–0.68); TiO2 (0.02–0.39); Na2O (0.01–0.03); P2O5 (0.03–0.04); F (0.31–0.50). Their inorganic mass is often represented by pure α-quartz with a dash of native sulphur, as well as hematite, smectite, gypsum and anhydrite.

Figure 5.
The main products of active fumaroles of spoil heap Ch-02: (a) a general view of the pyrolysis products and conical structures of active fumaroles; (b) a “crateral” part of fumaroles contoured by greenish-yellow authigenic sulphur; (c) oily resinous pyrolysis products; (d) a section of active pyrolysis fumarole, consisting of sulphur and hydrocarbon compounds, with an indication of sampling locations.
The thickness of the EZ does not exceed more than 5.5 m. The average temperature in a similar area of low-temperature pyrolysis is 30 °C, and the maximum temperature near to the AZ reaches 40 °C.
It is important to note that the metamorphic zone consists of weakly cemented iron hydroxides of gravelly-sandy-clayey yellowish-brown deposits, with an average temperature of about 50 °C; the heat (focal) zone is represented by grey-colored dry loose rubble-sandy intensively modified coal-bearing mass with an average temperature of about 115 °C; the activated zone is composed of grey-colored dry loose rubble-sandy coal-bearing material with sticks of activated wood (an average temperature is about 50 °C); the enriched zone is represented by dark grey wet and porous pyrolysis products, commonly impregnated with sulphur, and occasionally with ferrum hydroxides and ammonium sulphate (average temperature is about 30 °C).
4.4. Fumarole Channels
The point to be emphasized is that a large part of gasified organic and inorganic compounds in conjunction with pyrolysis water vapor penetrated through a porous structure of pyrolysis products of this zone, escapes into the atmosphere. In the event that EZ is superposed by a significant mass of rocks, gassed pyrolysis products penetrate them through numerous fumaroles, and then escape into the atmosphere. Concentration of fumaroles on the surface of spoil heaps is easily found by vapor jets coming from them, oily resinous pyrolysis aureole spread around them and specific smudge of pyrolysis gases. A lot of pyrolysis products gather around the fumaroles that conduce pyrolysis hills building, the accumulation of which often forms range of pyrolysis spoil heaps (Figure 5). The surface of such ranges is covered with black highly porous and poorly cemented asphaltenes, containing numerous sulphur crystals. Fumaroles temperature measurements showed that pyrolysis products temperature in their “craters” is 62 °C, and it reaches 85 °C in their “necks” at a depth of one meter from the surface.
According to the data obtained as a result of mass spectrometry analysis, samples Ch-02/1 and Ch-02/2, selected nearby and on the surface of the enriched zone (Figure 5d), contain asphaltic-resinous substances with relative intensities of peaks: for 57 from 81% to 100%, for 155 at 3% (Table 1 and Table 2). Polycyclic aromatic compounds included in their composition are presented by phenanthrene, anthracene, acenaphthene, pyrene, fluoranthene, chrysene, benzaanthracene and naphthalene. Where mass content of phenanthrene is twice as much as mass contents of other, polycyclic aromatic compounds. The total pyrolysis products content reaches 220,000 mg/kg.

Table 1.
The relative intensities of main components of pyrolysis products of coal-bearing deposits of spoil heaps according to mass spectrograms.

Table 2.
Polycyclic aromatic hydrocarbon content (mg/kg).
Unconsolidated light gray hot (up to 83 °C) and wet authigenic minerals with numerous crystals and hollow druses of sulphur are revealed in relatively deep parts of the enriched zone (0.25 m—Ch-02/3 and 0.5 m—Ch-02/4). Group composition of polycyclic aromatic compounds is similar to samples collected nearby and on the surface of the enriched zone, but their contents are one order less. Furthermore, in these samples, negligible contents of benzo (b) fluoran, benzo (k) fluoran, benzo (a) fluoran are revealed, but naphthalene is missing. The total pyrolysis products content decreases to 27,375 mg/kg.
Light-brown oily pyrolysis units of the enriched zone are coincided with the fumaroles, where they cover the surface of spoil heaps in the form of small spots with a thin (1.5 cm) crust. They are presented by the congeries of encrustations of various sizes and forms (Figure 5b). The sample Ch-02/5 has a high content of the pyrolysis hydrocarbon compounds that reaches 328,125 mg/kg. The group composition of polycyclic hydrocarbons is similar to the sample Ch-02/2, selected near the surface of the enriched zone, except for benzo (a) pyrene. It is worth noting that these light-brown oily pyrolysis units have the highest content of phenanthrene—1074 mg/kg—and anthracene—109.7 mg/kg.
Lacunas of fuming fumaroles are also correlated with dark brown oily pyrolysis aureole (Figure 5c). Generally fumaroles begin in activated zone of pyrolysis, but occasionally their “roots” can be traced in the area of ignition. Dark brown oily viscous gatherings (sample Ch-02/6) differ from other pyrolysis products by the presence of heterocyclic compounds, the relative peak intensity of which is 337 is 60%. Maximum contents of polycyclic hydrocarbons as well as the maximum total amount of pyrolysis products (418,750 mg/kg) are detected in their composition.
It is significant that qualitative composition of the hydrocarbons in shiny black coal debris of activated zone (sample Ch-01/19) and coal coke (sample Ch-01/20), selected in the enriched zone of high-temperature pyrolysis, is close to the abovementioned samples, but their concentrations are negligible. The total content of the pyrolysis products is significantly lower than in the zone of low-temperature (soft) pyrolysis and ranges from 3469 to 3594 mg/kg.
Research of high-temperature pyrolysis products of the enriched zone, conducted on spoil heaps OS-03, showed that their composition is similar to the abovementioned samples. Thermally modified debris of shiny coal with well-preserved flora molds (sample OS-03/1) contains higher concentration of heavy carbon residue than fragile intensely fractured debris caked as coal coke (sample OS-03/2). The relative peak intensity of heavy carbon residue for 365–512 is 46%. For both samples, polycyclic aromatic hydrocarbons are only presented by acenaphthene, phenanthrene, anthracene, fluoranthene and pyrene, moreover phenanthrene content is two to four times more than the content of other ones. The total quantity of pyrolysis products in the zone does not exceed 7187 mg/kg.
4.5. High-Temperature Pyrolysis
We managed to make a clearing of the current fire source in the zone of high-temperature pyrolysis and identify the pyrolysis zonal sequence in its section (Figure 6 and Figure 7). It has been established that its heating (focal) area is much larger than the same zones under low-temperature pyrolysis, and reaches more than 3.5 m. Statistical analysis of temperature measurement results in this zone showed that its average value is 790 °C, and recorded maximum temperature reaches 854 °C. The size of the activated zone is substantially reduced, moreover in some places this zone is missing, and there is a slow high-temperature combustion of the rock mass at the contact with the pyrolysis products of enriched zone. In the places of oxygen access, pyrolysis products inflammation and open combustion of rocks are observed. The average temperature of the rocks in the activated zone is 230 °C, and its fixed maximum temperature is 264 °C. Under natural high-temperature pyrolysis, the basement of the enriched zone is extremely wavy, and the thickness of the zone reaches four meters. The average temperature of hydrocarbon products in this zone is 60 °C, and near to the basement its maximum value reaches 177 °C. It is observed that the closer to the surface of the enriched zone, the lower temperature of the rock is. Every ten centimeters, the temperature drops by 12 °C sharply, and the average temperature of the ground surface is 20 °C.

Figure 6.
Section of pyrogenesis mass of coal-bearing deposits on the eastern hillside of spoil heap Ch-05. The bottom fragment shows the area of high-temperature pyrolysis, occurring under pyrogenesis that promotes coking, and then high-temperature burning of rock fragments and their subsequent caking.

Figure 7.
Sketch sectional drawing of active high-temperature pyrolysis focus, in the western part of which pyrogenesis of hard pyrolysis products of coal-bearing rocks (spoil heap Ch-05, the western hillside) occurs: 1—hydrocarbon products of enriched pyrolysis zone (average temperature is about 60 °C), 2—ctivated zone (average temperature is about 230 °C), 3—heat zone (average temperature is about 790 °C); 4—temperature measured with pyrometer in the point indicated in the circle; 5—talus; 6—pyrogenesis of rocks in the zone enriched by hydrocarbon compounds.
In conclusion, it is worth emphasizing that the numerous conducted chemical analyses of metamorphically modified rocks under pyrolysis showed that the metamorphic rocks are close to unaltered protolith sandstones and protolith coals are similar to the rocks of the pyrolysis enriched zone in their composition. In the performance of the silicate analysis, the entire mass of organic pyrolysis products burnt down, changing over to loss on ignition. Therefore, it was found that under natural pyrolysis, the distribution of the silica in different rocks of pyrolysis zones is inversely proportional to the amount of the pyrolysis products that is clearly confirmed by their polynominal curves (Figure 8).
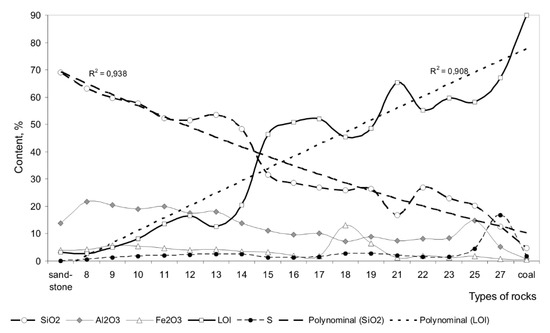
Figure 8.
Behavior of the main chemical components in the process of natural low-temperature pyrolysis of coal-bearing deposits. Types of rocks: sandstone and coal—the most important protolites of technogenic-redeposited deposits; 8–12—low-temperature formed red rocks of metamorphic mass; 13–14—grey-colored coal bearing deposits of heat zone; 15–16—lean coal-bearing black deposits of activated zone; 17–19, 21–23, 25, 27—variegated products of enriched pyrolysis zone.
5. Discussion of the Obtained Results
5.1. Burnt Rocks
According to Callegari E. and Pertsev N.N. [19], the term of burnt rocks is correlated with such old names as thermantide, thermantide porcellanite, fused shale, porcellanite, clinker, porcelain jasper. Vitreous or vitrified types of combustion metamorphic rocks were formerly called buchite (coal-fire buchite) or fritted rock, respectively. However, grayish red low-temperature formations appeared in the process of low-temperature (soft) pyrolysis decomposition of coal-bearing rocks in spoil heaps also belong to combustion metamorphic rocks. Notably, coal thermal decomposition is accompanied by the irreversible processes of its organic mass decomposition forming water pyrogenetic, resinous substance, gas and solid residue. According to studies of Shevkoplyas V.N. and his colleagues [20] brown coal decomposition begins at temperature of 160–200 °C and is accompanied by opening the weakest chemical bonds. According to the previous undertaken studies [21], when the temperature of the coal-bearing mass in coals rises, molecule energy increases and this conduces the forming of thermal decomposition products of different aggregative states. Successive heating of coal sample to 800 °C in pyrolysis furnace allowed us to establish that at a temperature of 370 °C, the resins coming out are 9.6% or 50% of its total amount, the gas coming out is 18.2 % (or 42.5%), and total change ratio reaches 27.8% (or 44.8%) [20]. This experiment showed that 370 °C is an optimum temperature for getting the primary pyrolysis products. Experiment or flash pyrolysis lasts 58 min.
5.2. Source of Pyrolysis Heat Energy
The temperature of active natural low-temperature pyrolysis on our investigated spoil heaps did not exceed 127 °C, and the time of the reaction is measured by years, not by hours or months. Therefore, we had to deal with a slow low-temperature spontaneous pyrolysis of coal-bearing rocks. The surveillance study of slow low-temperature pyrolysis of spoil heap Ch-02 conducted for three years enabled us to find out a vast net of constantly developing and expanding fumaroles in the pyrolysis zones. Spontaneous but stable exhalation of heated vapors and pyrolysis gases that have passed all the pyrolysis zones is shed from holes of fumaroles that are, as a rule, situated on the tops of spoil heaps. Due to this amount of hydrocarbon products, native sulphur and sulfate compounds in near-surface zone of the spoil heap increases from the bottom up continuously. Over the years, all zones of the natural pyrolysis process move up (to the top of spoil heap) gradually and progressively, and in consequence there is a constant build-up of metamorphic rock mass. Thermal energy sources encouraging low-temperature pyrolysis followed by the forming of low-temperature metamorphic rocks have not been studied yet, although it is believed, and we adhere to, that heat energy is a product of thionic bacteria activity [7], but no one has managed to carry out such pyrolysis in a laboratory yet. On the other hand, some researchers are convinced that coal appearing on the earth’s surface oxidizes rapidly, heats up and inflames spontaneously [22]. Coal, when it is exposed to atmospheric conditions, oxidizes and its properties change. In some cases, that can lead to self-heating that is present in coal heaps causing various problems. The same is relevant to coal waste heaps. Some of the heaps undergo self-heating, others not [23,24,25].
5.3. Hydrocarbon Pyrolysis Products
All samples analyzed by mass-spectrometer contain asphaltic-resinous substances and polycyclic aromatic hydrocarbons. Almost all of the analyzed samples contain organic sulphur compounds such as thiophene (C4H4S) and methylthiophene (C7H7S). Numerous experiments conducted by Telyashev I.R. and his colleagues [26] showed that the sulphur interreacts with aromatic hydrocarbons and resins. The determinative factor influencing on this reaction is low-temperature conditions (no more than 100–130 °C). At a temperature of 150 °C, sulphur rings (clusters) decompose, radicals consisting of sulphur atoms form, then they join to hydrocarbons of alkene type, or there is a dehydration interreacting with a residual oil hydrocarbons, a sign of which is a segregating of sulphurated hydrogen.
Resin density of pyrolysis tar formations is close to one (0.845–1.076), and its color varies from dun to dark-brown. Condensated under pyrolysis aromatic hydrocarbon compounds are represented by naphthalene, acenaphthylene, acenaphthene, fluorene, phenanthrene, fluoranthene, anthracene, pyrene, benzo(a)anthracene, chrysene, benzo(b)fluoran, benzo(k)fluoranthene and benzo(a)pyrene. It should be pointed out that the experiment conducted by Butuzova L.F. and her colleagues [27] to study the composition of the pyrolysis products of coal allowed them to discover and identify almost ten times more (120) individual compounds than are found in environmental conditions. Thus, a large number of natural pyrolysis products are not kept in EZ of spoil heaps, but escape into the atmosphere.
To summarize, it should be emphasized that the study of pyrolysis of coal-bearing deposits of spoil heaps represents one of the most interesting and important multidisciplinary problems. A large number of hydrocarbon compounds and native sulphur generated during long-term low-temperature pyrolysis of technogenic-redeposited coal-bearing mass contribute to the spontaneous combustion of sulphur pyrolysis products and the beginning of the pyrogenic process. Hydrocarbon compounds combustion is accompanied by high-temperature transformations, sometimes liquefaction of sedimentary rock debris with the formation of paralava and high-temperature flash pyrolysis of superincumbent coal-bearing deposits with the formation of semi-coke and coke.
Importantly, due to relatively rapid combustion in the process of pyrogenesis, in the enriched pyrolysis zone, the formation of such a large number of hydrocarbon products as under low-temperature pyrolysis goes behind. The above process of epigenetic changes of technogenic-redeposited coal-bearing deposits of spoil heaps allowed the following chain of sequential irreversible transformation to be established:
6. Conclusions
The study of spontaneous pyrolysis of coal-bearing rocks of different spoil heaps allowed the following regularities to be identified.
1. The average temperature of low-temperature natural pyrolysis does not exceed 120 °C, and an average speed of its course is about 2 m/year. Furthermore, the presence of three pyrolysis zones which build metamorphic rock mass (from bottom to top) gradually is clearly established: heating (focal), activated and enriched.
2. In the products of low-temperature pyrolysis, natural sulphur and asphaltic-resinous substances dominate. The polycyclic hydrocarbons class is represented by 13 types of hydrocarbons compounds. Pyrolytic carbon and heterocyclic compounds are important products for natural low-temperature pyrolysis.
3. High-temperature pyrolysis temperature reaches 854 °C, and an average speed of its course is about 20 m/year. High-temperature pyrolysis is accompanied by the presence of two major zones (from bottom to top): pyrogenic (focal) and enriched (coke).
4. The distribution of silica content in the rocks of various pyrolysis zones is inversely proportional to the number of pyrolysis products.
Author Contributions
N.I.A. and V.V.A. designed the study, all authors performed the study, N.I.A. and V.V.A. prepared the draft manuscript, all authors edited and commented on the manuscript. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
The authors express their respect to Elena V. Khudonogova (chemical composition), Maria N. Rubtsova (X-ray structure), Irina G. Neretina (polycyclic aromatic hydrocarbons) and Alexander V. Rohin (nuclear magnetic resonance spectrometer “Varian VXR-500S”) for the analyzes. The studies were carried out using the equipment of the “Isotope-Geochemical Research” Collective Use Centers of the Institute of Geochemistry of the SB RAS and the “Geodynamics and Geochronology” of the Institute of the Earth’s Crust of the SB RAS under the basic program “The Latest Geodynamics, Geosphere and Biosphere Evolutionary and Catastrophic Natural Changes”.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing the manuscript, and in the decision to publish the results.
References
- Misz-Kennan, M.; Fabiańska, M.J. Thermal transformation of organic matter in coal waste from Rymer Cones (Upper Silesian Coal Basin, Poland). Int. J. Coal Geol. 2010, 81, 343–358. [Google Scholar] [CrossRef]
- Finkelman, R.B. Potential health impacts of burning coal beds and waste banks. Int. J. Coal Geol. 2004, 59, 19–24. [Google Scholar] [CrossRef]
- Carras, J.N.; Day, S.J.; Saghafi, A.; Williams, D.J. Greenhouse gas emissions from low-temperature oxidation and spontaneous combustion at open-cut coal mines in Australia. Int. J. Coal Geol. 2009, 78, 161–168. [Google Scholar] [CrossRef]
- Misz-Kennan, M. Thermal alterations of organic matter in coal wastes from Upper Silesia, Poland. Mineralogia 2010, 41, 105–236. [Google Scholar] [CrossRef]
- Fabiańska, M.J.; Misz-Kennan, M.; Ciesielczuk, J.; Pierwoła, J.; Nitecka, N.; Brzoznowski, J. Thermal history of coal wastes reflected in their organic geochemistry and petrography; the case study: The Katowice-Wełnowiec dump, Poland. Int. J. Coal Geol. 2017, 184, 11–26. [Google Scholar] [CrossRef]
- Querol, X.; Izquierdo, M.; Monfort, E.; Alvarez, E.; Font, O.; Moreno, T.; Alastuey, A.; Zhuang, X.; Lud, W.; Wang, Y. Environmental characterization of burnt coal gangue banks at Yangquan, Shanxi Province, China. Int. J. Coal Geol. 2008, 75, 93–104. [Google Scholar] [CrossRef]
- Zborschik, M.P.; Osokin, V.V. Combustion of Coal Deposit Rocks and Fire Extinguishing; DonNTU: Donetsk, Ukraine, 2000; p. 180. [Google Scholar]
- Sokol, E.V.; Kalugin, V.M.; Nigmatulina, E.N.; Volkova, N.I.; Frenkel, A.E.; Maksimova, N.V. Ferrospheres from fly ashes of Chelyabinsk coals: Chemical composition, morphology and formation conditions. Fuel 2002, 81, 867–876. [Google Scholar] [CrossRef]
- Heffern, E.L.; Coates, D.A. Geologic history of natural coal-bed fires, Powder River basin, USA. Coal Fires Burning around the World: A Global Catastrophe. Int. J. Coal Geol. 2004, 59, 25–47. [Google Scholar] [CrossRef]
- Stracher, G.B.; Taylor, T.P. Coal fires burning out of control around the world: Thermodynamic recipe for environmental catastrophe. Coal Fires Burning around the World: A Global Catastrophe. Int. J. Coal Geol. 2004, 59, 7–17. [Google Scholar] [CrossRef]
- Grapes, R. Pyrometamorphism; Springer: Berlin/Heidelberg, Germany, 2006; p. 276. [Google Scholar]
- Stracher, G.B. Coal fires burning around the World: Opportunity for innovative and interdisciplinary research. GSA Today 2007, 17, 36–37. [Google Scholar] [CrossRef]
- Akulov, N.I.; Akulova, V.V.; Khudonogova, E.V. Pyrogenic metamorphism of the carbonaceous rocks in the south of the Siberian platform. In Coal Combustion Research; Nova Science Publishers: New York, NY, USA, 2010; pp. 219–234. ISBN 978-1-61668-646-8. [Google Scholar]
- Hrselova, P.; Cempirek, J.; Houzar, S.; Sejkora, J. S,F,Cl-rich mineral assemblages from Burned Spoil Heaps in the Rosice-Oslavany Coalfield, Crech Republic. Can. Mineral. 2013, 351, 171–188. [Google Scholar] [CrossRef]
- Akulov, N.I.; Agafonov, B.P.; Rubtsova, M.N. Glacial deposits and “watershed pebbles” in the Western Baikal area. Russ. Geol. Geophys. 2008, 49, 28–39. [Google Scholar] [CrossRef]
- Revenko, A.G.; Khudonogova, E.V. X-ray fluorescence determination of minor and tracer element contents in various types of rocks, soils, and sediments using the S4 PIONEER spectrometer. Ukr. Chem. Mag. 2005, 71, 39–45. [Google Scholar]
- Kalabin, G.A.; Kanitskaya, L.V.; Kushnarev, D.F. Quantitative NMR Spectroscopy of Natural Organic Raw Materials and Products of Its Processing; Chemistry: Moscow, Russia, 2000; p. 408. ISBN 5-7245-1169-X. [Google Scholar]
- Chesnokov, B.V.; Scherbakova, E.P. Burnt Heaps Mineralogy of Chelyabinsk Coal Basin; (Experiment of Mineralogy of Technogenesis); Nauka: Moscow, Russia, 1991; p. 152. [Google Scholar]
- Callegari, E.; Pertsev, N.N. A systematic nomenclature for metamorphic rocks: 10. Contact metamorphic rocks. Recommendations by the IUGS Subcommission on the Systematics of Metamorphic Rocks. 2007. SCMR. Available online: https://www.bgs.ac.uk/scmr/products.html (accessed on 4 March 2019).
- Shevkoplias, V.N.; Butuzova, L.F.; Liaschuk, S.N.; Pismenova, N.E. Structural and chemical transformations of brown coal under heating. Probl. Chem. Chem. Technol. 2008, 5, 87–92. [Google Scholar]
- Kamneva, A.I.; Platonov, V.V. Theoretical Fundamentals of Anthracides Chemical Technology; Himiia: Moscow, Russia, 1990; p. 287. [Google Scholar]
- Martysevich, U.V.; Sokol, E.V.; Maksimova, N.V.; Zolnikov, I.D. Dimentional analysis of residual thermal phenomena in coal heaps based on GIS technologies. Geoinformatika 2004, 3, 31–37. [Google Scholar]
- Grossman, S.L.; Davidi, S.; Cohen, H. Emission of toxic and fire hazardous gases from open air coal stockpiles. Fuel 1994, 73, 1184–1188. [Google Scholar] [CrossRef]
- Krishnaswamy, S.; Bhat, S.; Gunn, R.D.; Agarwal, P.K. Low—Temperature oxidation of coal. 1. Single—Particle reaction—Diffusion model. Fuel 1996, 75, 333–343. [Google Scholar] [CrossRef]
- Krishnaswamy, S.; Agarwal, P.K.; Gunn, R.D. Low—Temperature oxidation of coal. 3. Modelling spontaneous combustion in coal stockpiles. Fuel 1996, 75, 353–362. [Google Scholar] [CrossRef]
- Teliashev, I.R.; Davletshin, A.R.; Obukhova, S.A. Study of the interaction of heavy oil residue and elemental sulphur. Oil Refin. Petrochem. 2000, 1, 31–34. [Google Scholar]
- Butuzova, L.F.; Safin, V.A.; Kipria, A.V.; Butuzov, G.N.; Shakir, S.H.M.; Marinov, S.; Stefanova, M. Environmental aspects of sulphur coals processing. Chem. Technol. Anthracides Process. 2003, 6, 90–97. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).