Geochronology and Stable Isotope Analysis of Fracture-Fill and Karst Mineralization Reveal Sub-Surface Paleo-Fluid Flow and Microbial Activity of the COSC-1 Borehole, Scandinavian Caledonides
Abstract
1. Introduction
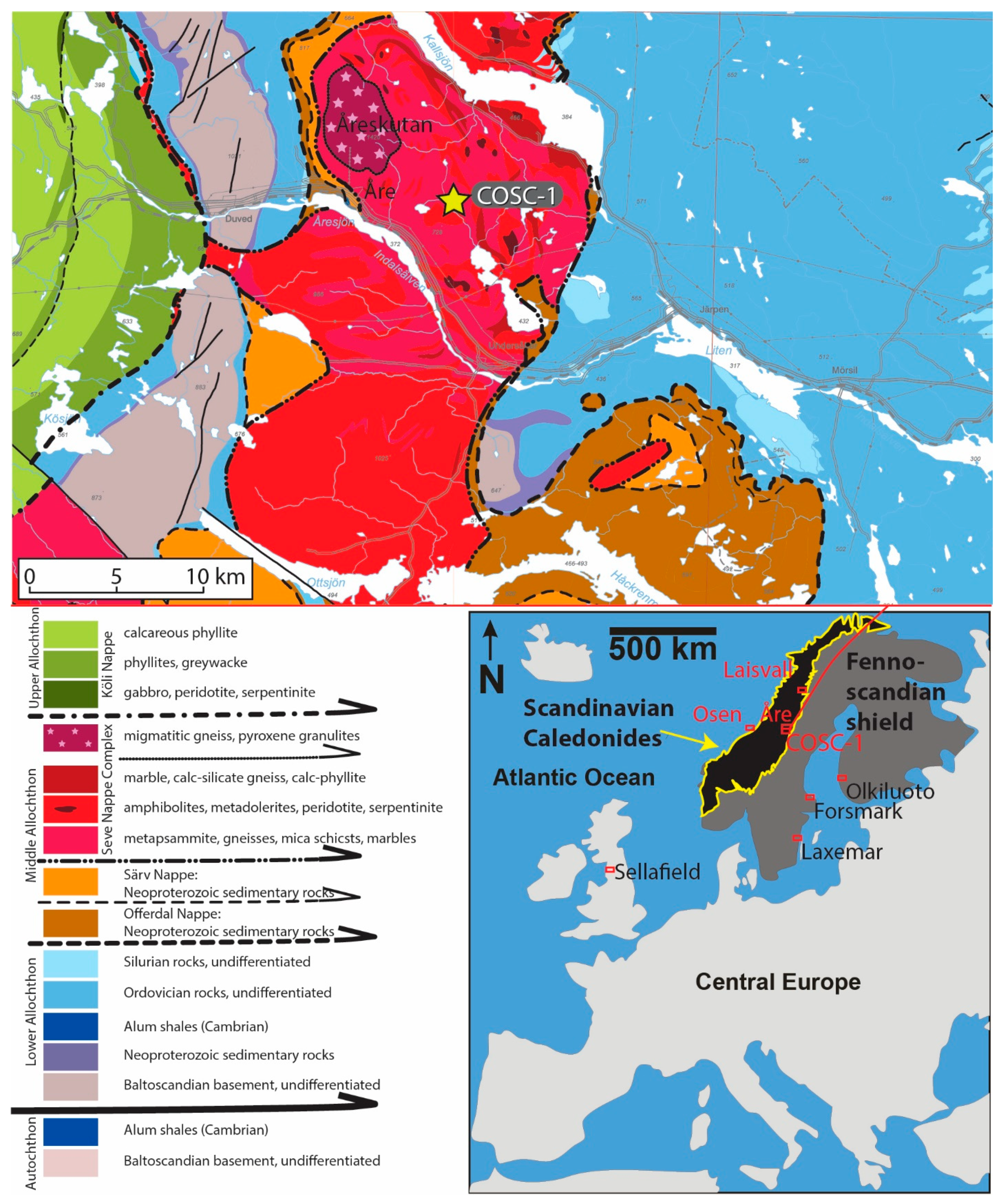
2. Geological Setting and the COSC-1 (Collisional Orogeny in the Scandinavian Caledonides) Borehole
3. Materials and Methods
3.1. Scanning Electron Microscopy (SEM)
3.2. Secondary Ion Mass Spectrometry for δ13C, δ18O, δ34S
3.3. Laser Ablataion Inductively Coupled Plasma Mass Spectrometry (LA-ICP-MS) for U-Pb Geochronology
4. Results
4.1. Mineralogy
4.1.1. Micro-Karst
4.1.2. Veins
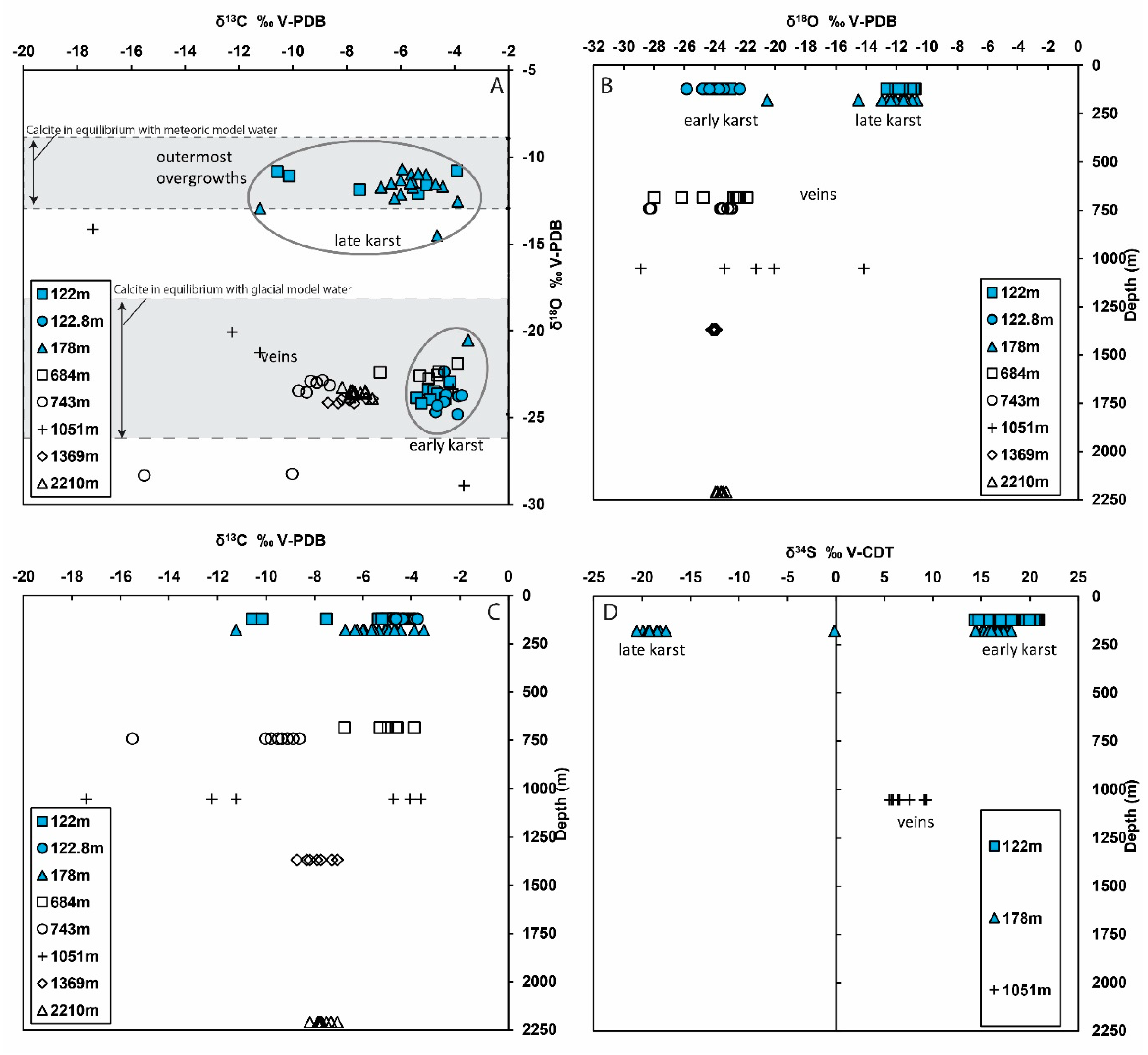
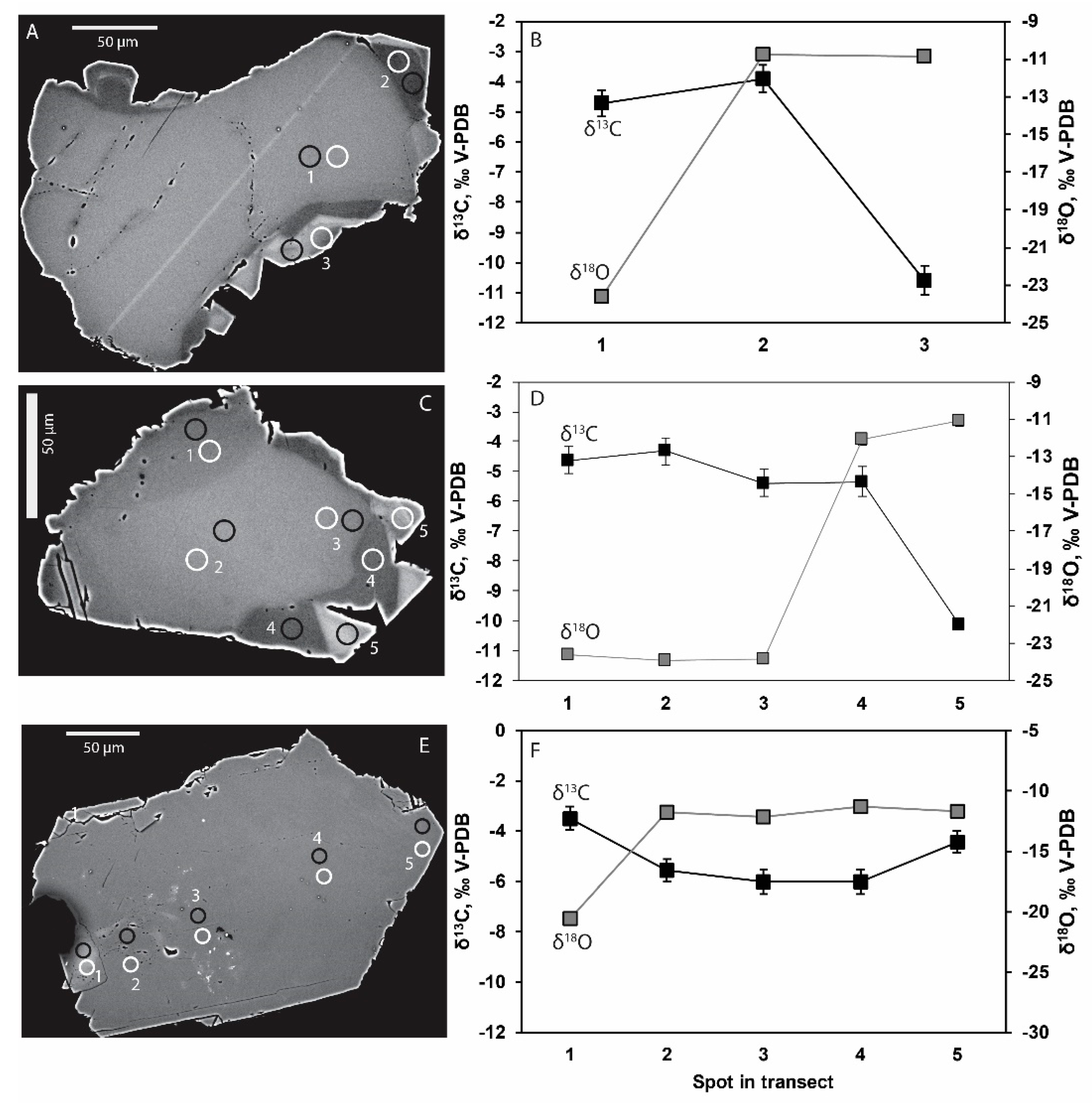
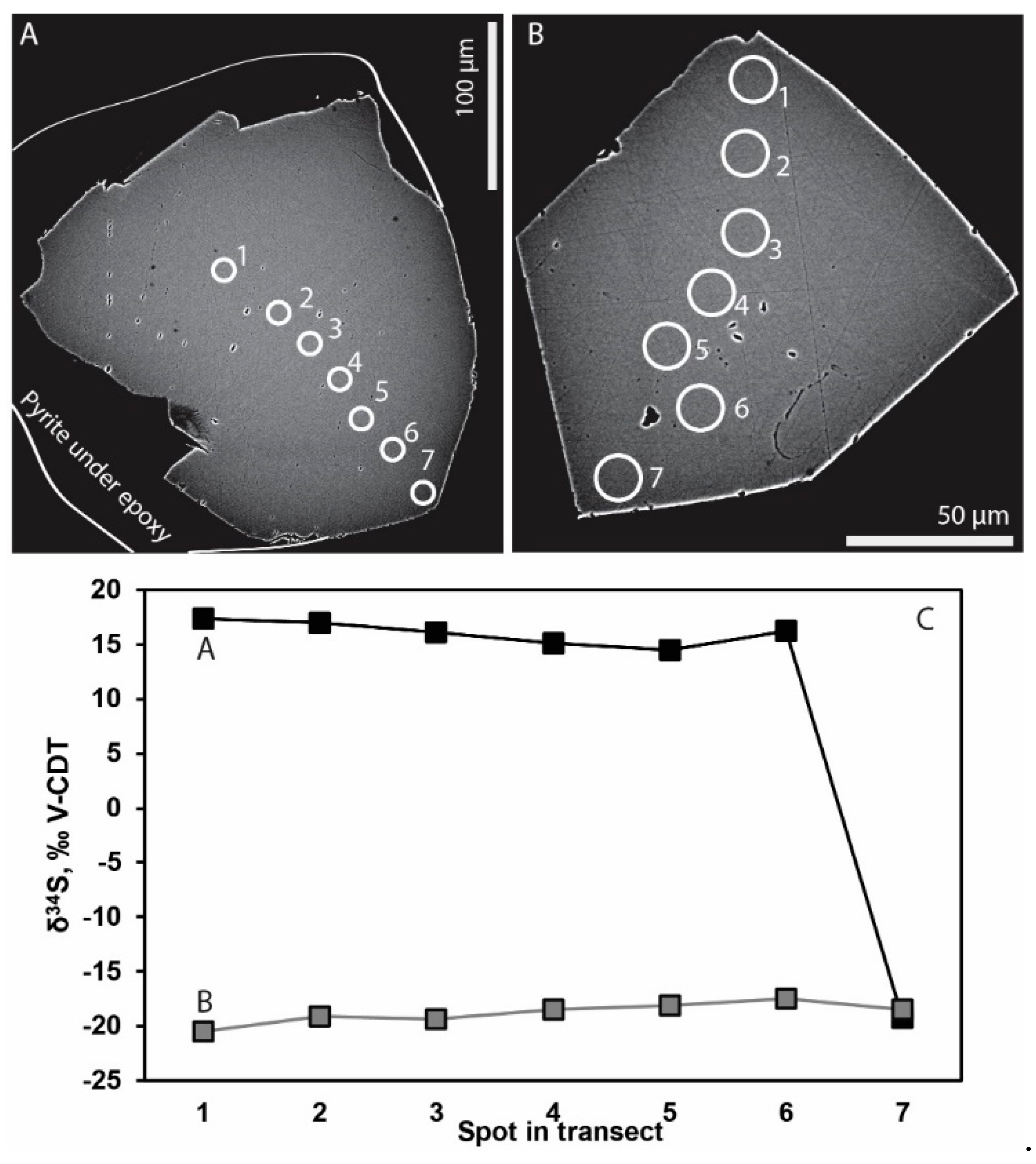
4.2. Stable Isotopes
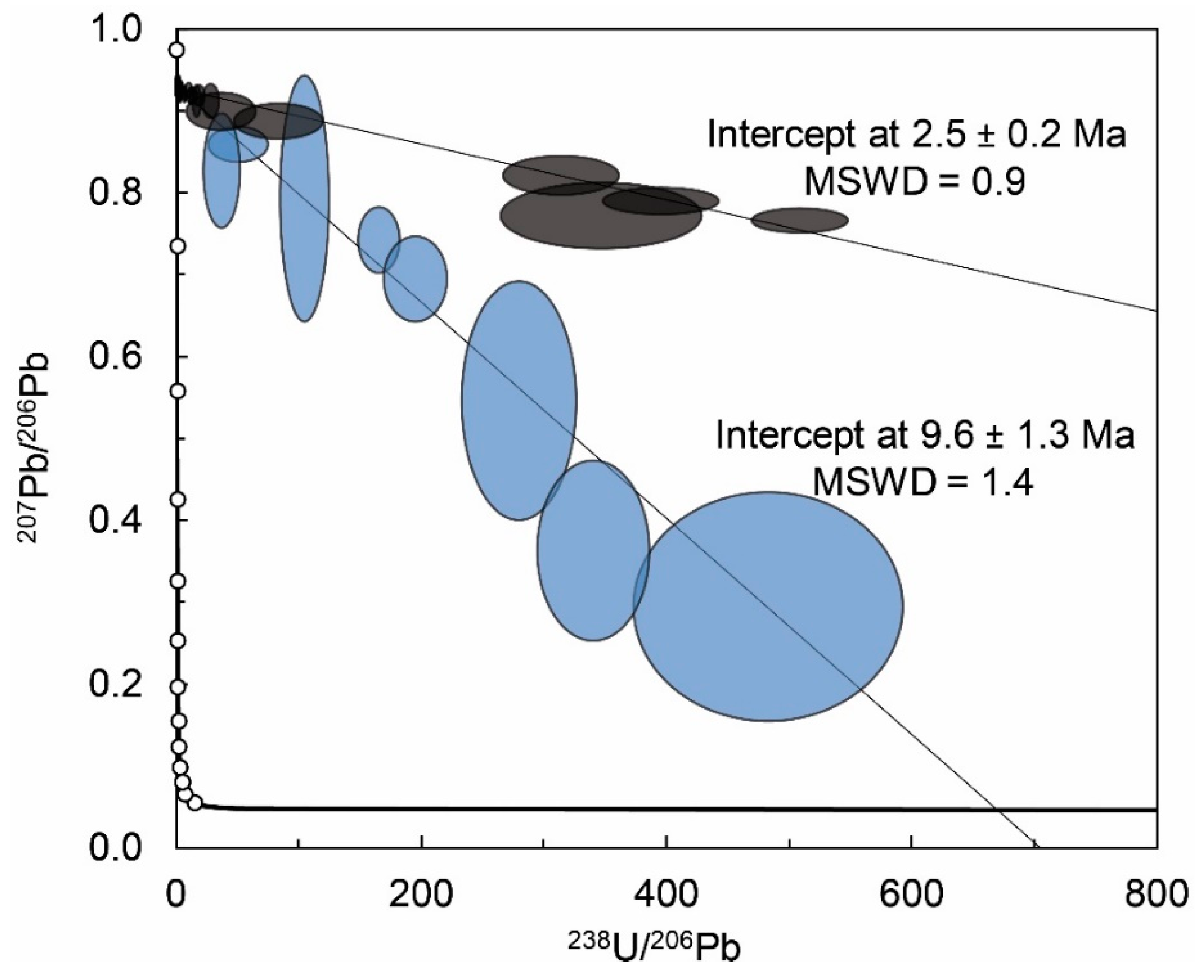
4.3. U-Pb Geochronology
5. Discussion
5.1. Microbial Activity
5.2. Paleo-Fluid Flow and Water Types
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Graaf, S.; Lüders, V.; Banks, D.A.; Sośnicka, M.; Reijmer, J.J.; Kaden, H.; Vonhof, H.B. Fluid evolution and ore deposition in the Harz Mountains revisited: Isotope and crush-leach analyses of fluid inclusions. Miner. Depos. 2019, 1–16. [Google Scholar] [CrossRef]
- Ivarsson, M.; Kilias, S.P.; Broman, C.; Neubeck, A.; Drake, H.; Fru, E.C.; Bengtson, S.; Naden, J.; Detsi, K.; Whitehouse, M.J. Exceptional Preservation of Fungi as H2-Bearing Fluid Inclusions in an Early Quaternary Paleo-Hydrothermal System at Cape Vani, Milos, Greece. Minerals 2019, 9, 749. [Google Scholar] [CrossRef]
- Dexter-Dyer, B.; Kretzschmar, M.; Krumbein, W.E. Possible microbial pathways in the formation of Precambrian ore deposits. J. Geol. Soc. 1984, 141, 251–262. [Google Scholar] [CrossRef]
- Martini, A.M.; Budai, J.M.; Walter, L.M.; Schoell, M. Microbial generation of economic accumulations of methane within a shallow organic-rich shale. Nature 1996, 383, 155–158. [Google Scholar] [CrossRef]
- Drake, H.; Roberts, N.M.W.; Heim, C.; Whitehouse, M.J.; Siljeström, S.; Kooijman, E.; Broman, C.; Ivarsson, M.; Åström, M.E. Timing and origin of natural gas accumulation in the Siljan impact structure, Sweden. Nat. Commun. 2019, 10, 4736. [Google Scholar] [CrossRef] [PubMed]
- Tullborg, E.-L.; Drake, H.; Sandström, B. Palaeohydrogeology: A methodology based on fracture mineral studies. Appl. Geochem. 2008, 23, 1881–1897. [Google Scholar] [CrossRef]
- Sahlstedt, E.; Karhu, J.A.; Pitkänen, P. Indications for the past redox environments in deep groundwaters from the isotopic composition of carbon and oxygen in fracture calcite, Olkiluoto, SW Finland. Isot. Environ. Heal. Stud. 2010, 46, 370–391. [Google Scholar] [CrossRef] [PubMed]
- Budai, J.M.; Walter, L.M.; Ku, T.C.W.; Martini, A.M. Fracture?fill calcite as a record of microbial methanogenesis and fluid migration: A case study from the Devonian Antrim Shale, Michigan Basin. Geofluids 2002, 2, 163–183. [Google Scholar] [CrossRef]
- Feng, D.; Chen, D.; Peckmann, J.; Bohrmann, G. Authigenic carbonates from methane seeps of the northern Congo fan: Microbial formation mechanism. Mar. Pet. Geol. 2010, 27, 748–756. [Google Scholar] [CrossRef]
- Natalicchio, M.; Birgel, D.; Pierre, F.D.; Martire, L.; Clari, P.; Spötl, C.; Peckmann, J. Polyphasic carbonate precipitation in the shallow subsurface: Insights from microbially-formed authigenic carbonate beds in upper Miocene sediments of the Tertiary Piedmont Basin (NW Italy). Palaeogeogr. Palaeoclim. Palaeoecol. 2012, 329, 158–172. [Google Scholar] [CrossRef]
- Caesar, K.H.; Kyle, J.R.; Lyons, T.W.; Tripati, A.; Loyd, S.J. Carbonate formation in salt dome cap rocks by microbial anaerobic oxidation of methane. Nat. Commun. 2019, 10, 808. [Google Scholar] [CrossRef] [PubMed]
- Kohn, M.J.; Riciputi, L.R.; Stakes, D.; Orange, D.L. Sulfur isotope variability in biogenic pyrite; reflections of heterogeneous bacterial colonization? Am. Mineral. 1998, 83, 1454–1468. [Google Scholar] [CrossRef]
- Parnell, J.; Boyce, A.; Thackrey, S.; Muirhead, D.; Lindgren, P.; Mason, C.; Taylor, C.; Still, J.; Bowden, S.; Osinski, G.R.; et al. Sulfur isotope signatures for rapid colonization of an impact crater by thermophilic microbes. Geology 2010, 38, 271–274. [Google Scholar] [CrossRef]
- Drake, H.; Whitehouse, M.J.; Heim, C.; Reiners, P.W.; Tillberg, M.; Hogmalm, K.J.; Dopson, M.; Broman, C.; Åström, M.E. Unprecedented 34 S-enrichment of pyrite formed following microbial sulfate reduction in fractured crystalline rocks. Geobiology 2018, 16, 556–574. [Google Scholar] [CrossRef]
- Knittel, K.; Boetius, A. Anaerobic Oxidation of Methane: Progress with an Unknown Process. Annu. Rev. Microbiol. 2009, 63, 311–334. [Google Scholar] [CrossRef]
- Machel, H.G.; Krouse, H.R.; Sassen, R. Products and distinguishing criteria of bacterial and thermochemical sulfate reduction. Appl. Geochem. 1995, 10, 373–389. [Google Scholar] [CrossRef]
- Drake, H.; Tullborg, E.-L. Paleohydrogeological events recorded by stable isotopes, fluid inclusions and trace elements in fracture minerals in crystalline rock, Simpevarp area, SE Sweden. Appl. Geochem. 2009, 24, 715–732. [Google Scholar] [CrossRef]
- Blyth, A.; Frape, S.; Ruskeeniemi, T.; Blomqvist, R. Origins, closed system formation and preservation of calcites in glaciated crystalline bedrock: Evidence from the Palmottu natural analogue site, Finland. Appl. Geochem. 2004, 19, 675–686. [Google Scholar] [CrossRef]
- Mathurin, F.A.; Åström, M.E.; Laaksoharju, M.; Kalinowski, B.E.; Tullborg, E.-L. Effect of Tunnel Excavation on Source and Mixing of Groundwater in a Coastal Granitoidic Fracture Network. Environ. Sci. Technol. 2012, 46, 12779–12786. [Google Scholar] [CrossRef]
- O’Neil, J.R. Oxygen Isotope Fractionation in Divalent Metal Carbonates. J. Chem. Phys. 1969, 51, 5547. [Google Scholar] [CrossRef]
- Watkins, J.M.; Hunt, J.D.; Ryerson, F.J.; DePaolo, D.J. The influence of temperature, pH, and growth rate on the δ18O composition of inorganically precipitated calcite. Earth Planet. Sci. Lett. 2014, 404, 332–343. [Google Scholar] [CrossRef]
- Kim, S.-T.; O’Neil, J.R. Equilibrium and nonequilibrium oxygen isotope effects in synthetic carbonates. Geochim. Cosmochim. Acta 1997, 61, 3461–3475. [Google Scholar] [CrossRef]
- Drake, H.; Ivarsson, M.; Tillberg, M.; Whitehouse, M.J.; Kooijman, E. Ancient Microbial Activity in Deep Hydraulically Conductive Fracture Zones within the Forsmark Target Area for Geological Nuclear Waste Disposal, Sweden. Geosciences 2018, 8, 211. [Google Scholar] [CrossRef]
- Drake, H.; Ivarsson, M.; Bengtson, S.; Heim, C.; Siljeström, S.; Whitehouse, M.J.; Broman, C.; Belivanova, V.; Åström, M.E. Anaerobic consortia of fungi and sulfate reducing bacteria in deep granite fractures. Nat. Commun. 2017, 8, 55. [Google Scholar] [CrossRef]
- Drake, H.; Åström, M.E.; Tullborg, E.-L.; Whitehouse, M.; Fallick, A.E. Variability of sulphur isotope ratios in pyrite and dissolved sulphate in granitoid fractures down to 1km depth—Evidence for widespread activity of sulphur reducing bacteria. Geochim. Cosmochim. Acta 2013, 102, 143–161. [Google Scholar] [CrossRef]
- Sahlstedt, E.; Karhu, J.A.; Pitkänen, P.; Whitehouse, M. Biogenic processes in crystalline bedrock fractures indicated by carbon isotope signatures of secondary calcite. Appl. Geochem. 2016, 67, 30–41. [Google Scholar] [CrossRef]
- Sahlstedt, E.; Karhu, J.; Pitkanen, P.; Whitehouse, M. Implications of sulfur isotope fractionation in fracture-filling sulfides in crystalline bedrock, Olkiluoto, Finland. Appl. Geochem. 2013, 32, 52–69. [Google Scholar] [CrossRef]
- Drake, H.; Heim, C.; Roberts, N.M.; Zack, T.; Tillberg, M.; Broman, C.; Ivarsson, M.; Whitehouse, M.J.; Åström, M.E. Isotopic evidence for microbial production and consumption of methane in the upper continental crust throughout the Phanerozoic eon. Earth Planet. Sci. Lett. 2017, 470, 108–118. [Google Scholar] [CrossRef]
- Drake, H.; Åström, M.E.; Heim, C.; Broman, C.; Åström, J.; Whitehouse, M.; Ivarsson, M.; Siljestrom, S.; Sjövall, P. Extreme 13C depletion of carbonates formed during oxidation of biogenic methane in fractured granite. Nat. Commun. 2015, 6, 7020. [Google Scholar] [CrossRef]
- Lorenz, H.; Rosberg, J.-E.; Juhlin, C.; Bjelm, L.; Almqvist, B.S.G.; Berthet, T.; Conze, R.; Gee, D.G.; Klonowska, I.; Pascal, C.; et al. COSC-1 – drilling of a subduction-related allochthon in the Palaeozoic Caledonide orogen of Scandinavia. Sci. Drill. 2015, 19, 1–11. [Google Scholar] [CrossRef]
- Gee, D.G.; Juhlin, C.; Pascal, C.; Robinson, P. Collisional Orogeny in the Scandinavian Caledonides (COSC). GFF 2010, 132, 29–44. [Google Scholar] [CrossRef]
- Corfu, F.; Andersen, T.B.; Gasser, D. The Scandinavian Caledonides: Main features, conceptual advances and critical questions. Geol. Soc. Lond. Spéc. Publ. 2014, 390, 9–43. [Google Scholar] [CrossRef]
- Roberts, D. The Scandinavian Caledonides: Event chronology, palaeogeographic settings and likely modern analogues. Tectonophysics 2003, 365, 283–299. [Google Scholar] [CrossRef]
- Lidmar-Bergström, K.; Näslund, J.O.; Ebert, K.; Neubeck, T.; Bonow, J.M. Cenozoic landscape development on the passsive margin of northern Scandinavia. Nor. J. Geol. 2007, 87, 181–196. [Google Scholar]
- Lidmar-Bergström, K. Long term morphotectonic evolution in Sweden. Geomorphology 1996, 16, 33–59. [Google Scholar] [CrossRef]
- Kendrick, M.; Burgess, R.; Harrison, D.; Bjørlykke, A. Noble gas and halogen evidence for the origin of Scandinavian sandstone-hosted Pb-Zn deposits. Geochim. Cosmochim. Acta 2005, 69, 109–129. [Google Scholar] [CrossRef]
- Hedin, P.; Almqvist, B.; Berthet, T.; Juhlin, C.; Buske, S.; Simon, H.; Giese, R.; Krauß, F.; Rosberg, J.-E.; Alm, P.-G. 3D reflection seismic imaging at the 2.5km deep COSC-1 scientific borehole, central Scandinavian Caledonides. Tectonophysics 2016, 689, 40–55. [Google Scholar] [CrossRef]
- Lorenz, H.; Rosberg, J.E.; Juhlin, C.; Bjelm, L.; Almqvist, B.G.S.; Berthet, T.; Conze, R.; Gee, D.; Klonowska, I.; Pascal, C.; et al. Operational report about phase 1 of the Collisional Orogeny in the Scandinavian Caledonides scientific drilling project (COSC-1). ICDP Oper. Rep. Potsdam GFZ Ger. Res. Cent. Geosci. 2015, 52. [Google Scholar]
- Roberts, D.; Gee, D.G. An introduction to the structure of the Scandinavian Caledonides. Caledonide Orogen–Scand. Relat. Areas 1985, 1, 55–68. [Google Scholar]
- Andréasson, P.-G.; Gorbatschev, R. Metamorphism in extensive nappe terrains: A study of the Central Scandinavian Caledonides. Geologiska Föreningen i Stockholm Förhandlingar 1980, 102, 335–357. [Google Scholar] [CrossRef]
- Be’Eri-Shlevin, Y.; Gee, D.; Claesson, S.; Ladenberger, A.; Majka, J.; Kirkland, C.; Robinson, P.; Frei, D. Provenance of Neoproterozoic sediments in the Särv nappes (Middle Allochthon) of the Scandinavian Caledonides: LA-ICP-MS and SIMS U–Pb dating of detrital zircons. Precambrian Res. 2011, 187, 181–200. [Google Scholar] [CrossRef]
- Gee, D.G.; Fossen, H.; Henriksen, N.; Higgins, A.K. From the Early Paleozoic Platforms of Baltica and Laurentia to the Caledonide Orogen of Scandinavia and Greenland. Episodes 2008, 31, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Ladenberger, A.; Be’eri-Shlevin, Y.; Claesson, S.; Gee, D.G.; Majka, J.; Romanova, I.V. Tectonometamorphic evolution of the Åreskutan Nappe–Caledonian history revealed by SIMS U–Pb zircon geochronology. Geol. Soc. Lond. Spec. Publ. 2014, 390, 337–368. [Google Scholar] [CrossRef]
- Gromet, L.; Sjöström, H.; Bergman, S.; Claesson, S.; Essex, R.; Andréasson, P.; Albrecht, L. Contrasting ages of metamorphism in the Seve nappes: U-Pb results from the central and northern Swedish Caledonides. GFF 1996, 118, 36–37. [Google Scholar] [CrossRef]
- Williams, I.S.; Claesson, S. Isotopic evidence for the Precambrian provenance and Caledonian metamorphism of high grade paragneisses from the Seve Nappes, Scandinavian Caledonides. Contrib. Mineral. Petrol. 1987, 97, 205–217. [Google Scholar] [CrossRef]
- Bender, H.; Glodny, J.; Ring, U. Absolute timing of Caledonian orogenic wedge assembly, Central Sweden, constrained by Rb–Sr multi-mineral isochron data. Lithos 2019, 344–345, 339–359. [Google Scholar] [CrossRef]
- Klonowska, I.; Janák, M.; Majka, J.; Petrík, I.; Froitzheim, N.; Gee, D.G.; Sasinková, V. Microdiamond on Åreskutan confirms regional UHP metamorphism in the Seve Nappe Complex of the Scandinavian Caledonides. J. Metamorph. Geol. 2017, 35, 541–564. [Google Scholar] [CrossRef]
- Tsang, C.-F.; Rosberg, J.-E.; Sharma, P.; Berthet, T.; Juhlin, C.; Niemi, A. Hydrologic testing during drilling: Application of the flowing fluid electrical conductivity (FFEC) logging method to drilling of a deep borehole. Hydrogeol. J. 2016, 24, 1333–1341. [Google Scholar] [CrossRef]
- Kamber, B.S.; Whitehouse, M.J. Micro-scale sulphur isotope evidence for sulphur cycling in the late Archean shallow ocean. Geobiology 2007, 5, 5–17. [Google Scholar] [CrossRef]
- Ding, T.; Valkiers, S.; Kipphardt, H.; De Bièvre, P.; Taylor, P.; Gonfiantini, R.; Krouse, R. Calibrated sulfur isotope abundance ratios of three IAEA sulfur isotope reference materials and V-CDT with a reassessment of the atomic weight of sulfur. Geochim. Cosmochim. Acta 2001, 65, 2433–2437. [Google Scholar] [CrossRef]
- Roberts, N.M.W.; Rasbury, E.T.; Parrish, R.R.; Smith, C.J.; Horstwood, M.S.A.; Condon, D.J. A calcite reference material for LA-ICP-MS U-Pb geochronology. Geochem. Geophys. Geosystems 2017, 18, 2807–2814. [Google Scholar] [CrossRef]
- Ludwig, K. Isoplot version 4.15: A geochronological toolkit for microsoft Excel. Berkeley Geochronol. Cent. Spec. Publ. 2008, 4, 247–270. [Google Scholar]
- Hill, C.A.; Polyak, V.J.; Asmerom, Y.; Provencio, P.P. Constraints on a Late Cretaceous uplift, denudation, and incision of the Grand Canyon region, southwestern Colorado Plateau, USA, from U-Pb dating of lacustrine limestone. Tectonics 2016, 35, 896–906. [Google Scholar] [CrossRef]
- Horstwood, M.S.A.; Kosler, J.; Gehrels, G.; Jackson, S.E.; McLean, N.M.; Paton, C.; Pearson, N.J.; Sircombe, K.; Sylvester, P.; Vermeesch, P.; et al. Community-Derived Standards for LA-ICP-MS U-(Th-)Pb Geochronology—Uncertainty Propagation, Age Interpretation and Data Reporting. Geostand. Geoanalytical Res. 2016, 40, 311–332. [Google Scholar] [CrossRef]
- Peckmann, J.; Thiel, V. Carbon cycling at ancient methane–seeps. Chem. Geol. 2004, 205, 443–467. [Google Scholar] [CrossRef]
- Sandström, B.; Tullborg, E.-L. Episodic fluid migration in the Fennoscandian Shield recorded by stable isotopes, rare earth elements and fluid inclusions in fracture minerals at Forsmark, Sweden. Chem. Geol. 2009, 266, 126–142. [Google Scholar] [CrossRef]
- Campbell, K.; Farmer, J.D.; Marais, D.D. Ancient hydrocarbon seeps from the Mesozoic convergent margin of California: Carbonate geochemistry, fluids and palaeoenvironments. Geofluids 2002, 2, 63–94. [Google Scholar] [CrossRef]
- Tullborg, E.-L.; Landström, O.; Wallin, B. Low-temperature trace element mobility influenced by microbial activity—Indications from fracture calcite and pyrite in crystalline basement. Chem. Geol. 1999, 157, 199–218. [Google Scholar] [CrossRef]
- Clauer, N.; Frape, S.K.; Fritz, B. Calcite veins of the Stripa granite (Sweden) as records of the origin of the groundwaters and their interactions with the granitic body. Geochim. Cosmochim. Acta 1989, 53, 1777–1781. [Google Scholar] [CrossRef]
- Canfield, D. Isotope fractionation by natural populations of sulfate-reducing bacteria. Geochim. Cosmochim. Acta 2001, 65, 1117–1124. [Google Scholar] [CrossRef]
- Ries, J.B.; Fike, D.A.; Pratt, L.M.; Lyons, T.W.; Grotzinger, J.P. Superheavy pyrite (δ34Spyr> δ34SCAS) in the terminal Proterozoic Nama Group, southern Namibia: A consequence of low seawater sulfate at the dawn of animal life. Geology 2009, 37, 743–746. [Google Scholar] [CrossRef]
- Jones, D.S.; Fike, D.A. Dynamic sulfur and carbon cycling through the end-Ordovician extinction revealed by paired sulfate–pyrite δ34S. Earth Planet. Sci. Lett. 2013, 363, 144–155. [Google Scholar] [CrossRef]
- Sim, M.S.; Bosak, T.; Ono, S. Large Sulfur Isotope Fractionation Does Not Require Disproportionation. Sci. 2011, 333, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Wortmann, U.G.; Bernasconi, S.M.; Böttcher, M.E. Hypersulfidic deep biosphere indicates extreme sulfur isotope fractionation during single-step microbial sulfate reduction. Geology 2001, 29, 647. [Google Scholar] [CrossRef]
- Drake, H.; Tullborg, E.-L.; Whitehouse, M.; Sandberg, B.; Blomfeldt, T.; Åström, M.E. Extreme fractionation and micro-scale variation of sulphur isotopes during bacterial sulphate reduction in deep groundwater systems. Geochim. Cosmochim. Acta 2015, 161, 1–18. [Google Scholar] [CrossRef]
- Canfield, D.E.; Farquhar, J.; Zerkle, A.L. High isotope fractionations during sulfate reduction in a low-sulfate euxinic ocean analog. Geology 2010, 38, 415–418. [Google Scholar] [CrossRef]
- Laaksoharju, M.; Smellie, J.; Tullborg, E.-L.; Gimeno, M.; Molinero, J.; Gurban, I.; Hallbeck, L. Hydrogeochemical evaluation and modelling performed within the Swedish site investigation programme. Appl. Geochem. 2008, 23, 1761–1795. [Google Scholar] [CrossRef]
- Machel, H. Bacterial and thermochemical sulfate reduction in diagenetic settings—Old and new insights. Sediment. Geol. 2001, 140, 143–175. [Google Scholar] [CrossRef]
- Goldstein, R.H. Fluid inclusions in sedimentary and diagenetic systems. Lithos 2001, 55, 159–193. [Google Scholar] [CrossRef]
- Johansson, E.; Gustafsson, L.-G.; Berglund, S.; Lindborg, T.; Selroos, J.-O.; Liljedahl, L.C.; Destouni, G. Data evaluation and numerical modeling of hydrological interactions between active layer, lake and talik in a permafrost catchment, Western Greenland. J. Hydrol. 2015, 527, 688–703. [Google Scholar] [CrossRef]
- Stuevold, L.M.; Eldholm, O. Cenozoic uplift of Fennoscandia inferred from a study of the mid-Norwegian margin. Glob. Planet. Chang. 1996, 12, 359–386. [Google Scholar] [CrossRef]
- Pitkänen, P.; Partamies, S.; Luukkonen, A. Hydrogeochemical Interpretation of Baseline Groundwater Conditions at the Olkiluoto Site; Posiva Olkiluto: Helsinki, Finland, 2004. [Google Scholar]
- Milodowski, A.E.; Bath, A.; Norris, S. Palaeohydrogeology using geochemical, isotopic and mineralogical analyses: Salinity and redox evolution in a deep groundwater system through Quaternary glacial cycles. Appl. Geochem. 2018, 97, 40–60. [Google Scholar] [CrossRef]
- Bird, D.K.; Spieler, A.R. Epidote in geothermal systems. Rev. Mineral. Geochem. 2014, 56, 235–300. [Google Scholar] [CrossRef]
- Liou, J.G.; Maruyama, S.; Cho, M. Phase equilibria and mixed parageneses of metabasites in lowgrade metamorphism. Mineral. Mag. 1985, 49, 321–333. [Google Scholar] [CrossRef]
- Dennis, P.F.; Myhill, D.J.; Marca, A.; Kirk, R. Clumped isotope evidence for episodic, rapid flow of fluids in a mineralized fault system in the Peak District, UK. J. Geol. Soc. 2019, 176, 447–461. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drake, H.; Roberts, N.M.W.; Whitehouse, M.J. Geochronology and Stable Isotope Analysis of Fracture-Fill and Karst Mineralization Reveal Sub-Surface Paleo-Fluid Flow and Microbial Activity of the COSC-1 Borehole, Scandinavian Caledonides. Geosciences 2020, 10, 56. https://doi.org/10.3390/geosciences10020056
Drake H, Roberts NMW, Whitehouse MJ. Geochronology and Stable Isotope Analysis of Fracture-Fill and Karst Mineralization Reveal Sub-Surface Paleo-Fluid Flow and Microbial Activity of the COSC-1 Borehole, Scandinavian Caledonides. Geosciences. 2020; 10(2):56. https://doi.org/10.3390/geosciences10020056
Chicago/Turabian StyleDrake, Henrik, Nick M. W. Roberts, and Martin J. Whitehouse. 2020. "Geochronology and Stable Isotope Analysis of Fracture-Fill and Karst Mineralization Reveal Sub-Surface Paleo-Fluid Flow and Microbial Activity of the COSC-1 Borehole, Scandinavian Caledonides" Geosciences 10, no. 2: 56. https://doi.org/10.3390/geosciences10020056
APA StyleDrake, H., Roberts, N. M. W., & Whitehouse, M. J. (2020). Geochronology and Stable Isotope Analysis of Fracture-Fill and Karst Mineralization Reveal Sub-Surface Paleo-Fluid Flow and Microbial Activity of the COSC-1 Borehole, Scandinavian Caledonides. Geosciences, 10(2), 56. https://doi.org/10.3390/geosciences10020056






