Use of the Rat Grimace Scale to Evaluate Visceral Pain in a Model of Chemotherapy-Induced Mucositis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
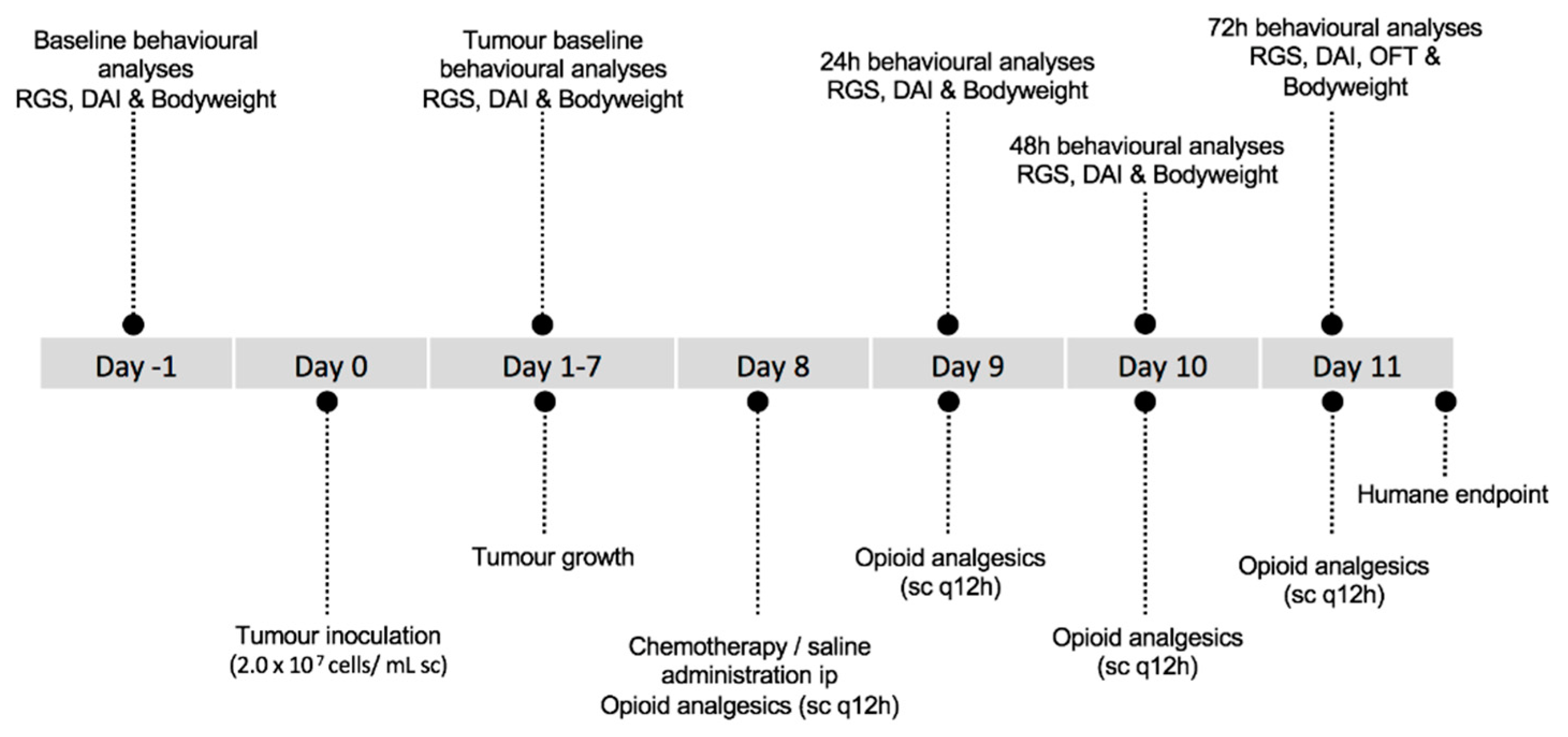
2.1. Animals and Experimental Design
2.2. Facial Image Analysis
2.3. Disease Activity Index
2.4. Open Field Test
2.5. Statistical Analysis
3. Results
3.1. Facial Image Analysis
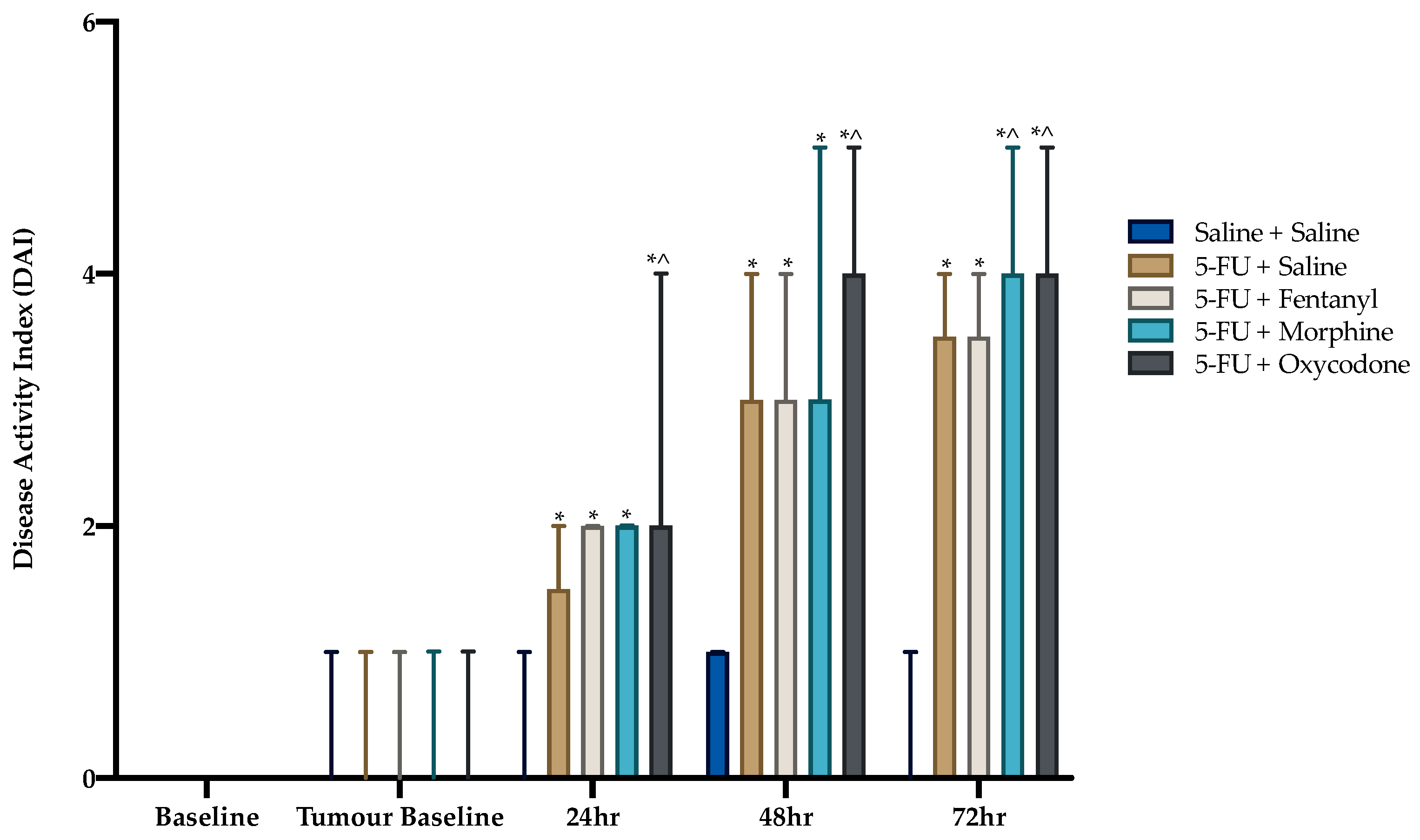
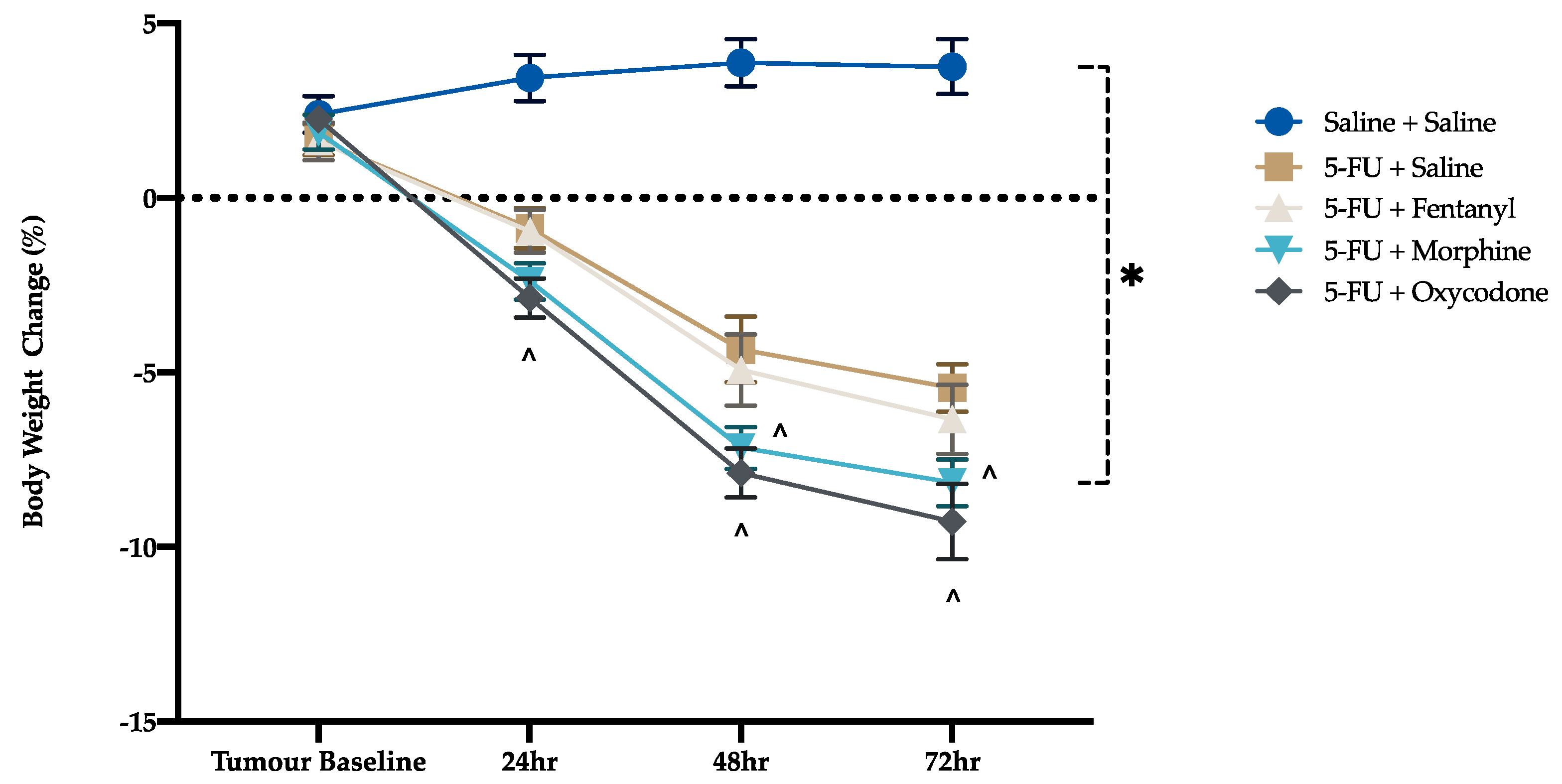
3.2. Disease Activity Index and Body Weight
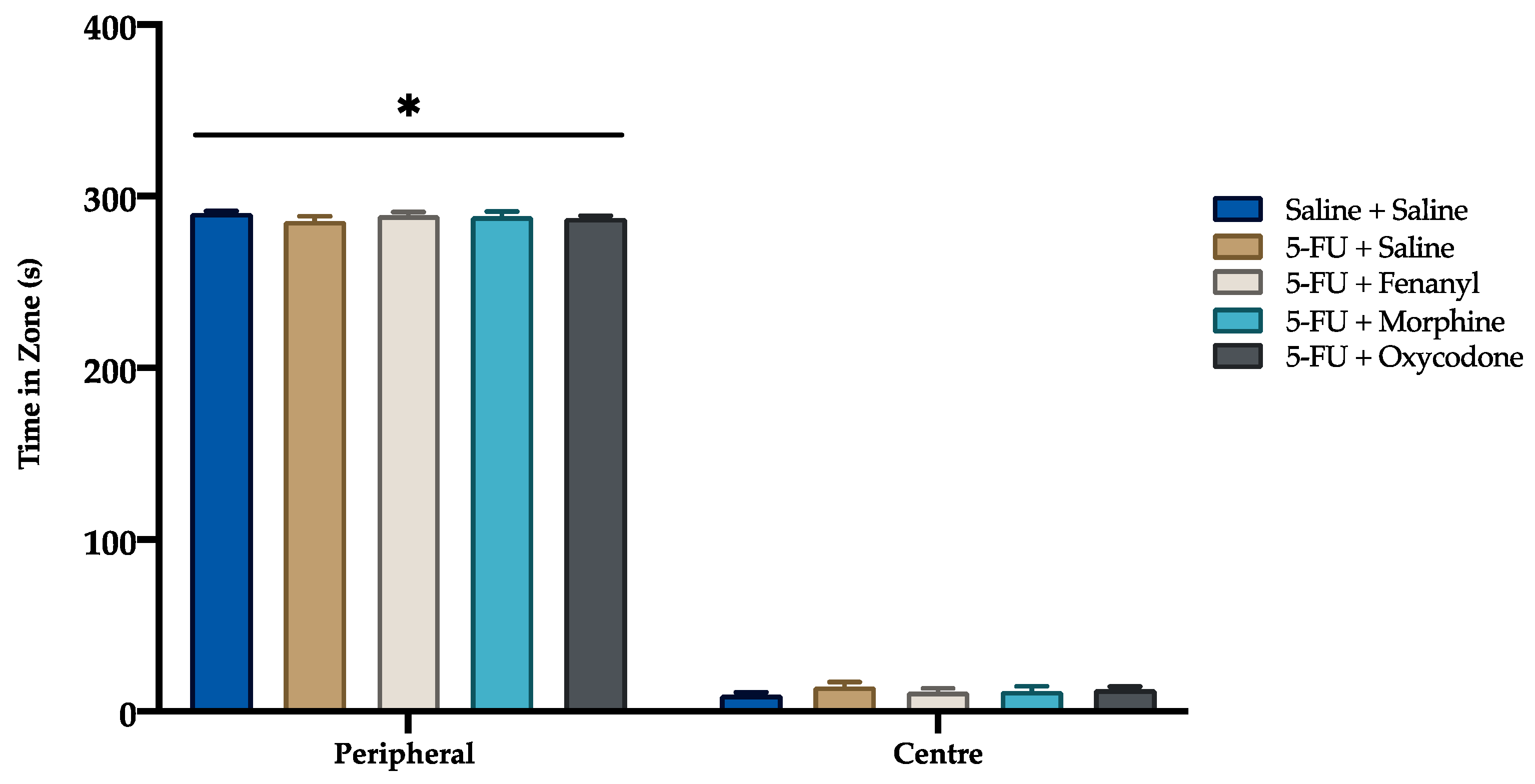
3.3. Open Field Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pico, J.L.; Avila-Garavito, A.; Naccache, P. Mucositis: Its occurrence, consequences, and treatment in the oncologysetting. Oncologist 1998, 3, 446–451. [Google Scholar] [PubMed]
- Sonis, S.T. A biological approach to mucositis. J. Support. Oncol. 2004, 2, 21–32. [Google Scholar] [PubMed]
- Harris, D.J. Cancer treatment-induced mucositis pain: Strategies for assessment and management. Ther. Clin. Risk Manag. 2006, 2, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.G.; Longstreth, G.F.; Drossman, D.A.; Heaton, K.W.; Irvine, E.J.; Müller-Lissner, S.A. Functional bowel disorders and functional abdominal pain. Gut 1999, 45, II43–II47. [Google Scholar] [CrossRef] [PubMed]
- Mashtoub, S.; Tran, C.D.; Howarth, G.S. Emu oil expedites small intestinal repair following 5-fluorouracil-induced mucositis in rats. Exp. Biol. Med. 2013, 238, 1305–1317. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Brook, C.L.; Whittaker, A.L.; Lawrence, A.; Yazbeck, R.; Howarth, G.S. Effects of streptococcus thermophilus th-4 in a rat model of doxorubicin-induced mucositis. Scand. J. Gastroenterol. 2013, 48, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Hager, C.; Keubler, L.M.; Biernot, S.; Dietrich, J.; Buchheister, S.; Buettner, M.; Bleich, A. Time to integrate to nest test evaluation in a mouse dss-colitis model. PLoS ONE 2015, 10, e0143824. [Google Scholar] [CrossRef]
- Lalla, R.V.; Bowen, J.; Barasch, A.; Elting, L.; Epstein, J.; Keefe, D.M.; McGuire, D.B.; Migliorati, C.; Nicolatou-Galitis, O.; Peterson, D.E.; et al. Mascc/isoo clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2014, 120, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Sotocinal, S.G.; Sorge, R.E.; Zaloum, A.; Tuttle, A.H.; Martin, L.J.; Wieskopf, J.S.; Mapplebeck, J.C.S.; Wei, P.; Zhan, S.; Zhang, S.; et al. The rat grimace scale: A partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol. Pain 2011, 7, 55. [Google Scholar] [PubMed]
- Leach, M.C.; Klaus, K.; Miller, A.L.; Scotto di Perrotolo, M.; Sotocinal, S.G.; Flecknell, P.A. The assessment of post-vasectomy pain in mice using behaviour and the mouse grimace scale. PLoS ONE 2012, 7, e35656. [Google Scholar] [CrossRef]
- Langford, D.J.; Bailey, A.L.; Chanda, M.L.; Clarke, S.E.; Drummond, T.E.; Echols, S.; Glick, S.; Ingrao, J.; Klassen-Ross, T.; LaCroix-Fralish, M.L.; et al. Coding of facial expressions of pain in the laboratory mouse. Nat. Meth. 2010, 7, 447. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, A.L.; Leach, M.C.; Preston, F.L.; Lymn, K.A.; Howarth, G.S. Effects of acute chemotherapy-induced mucositis on spontaneous behaviour and the grimace scale in laboratory rats. Lab. Anim. 2016, 50, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Reinke, D.; Kritas, S.; Polychronopoulos, P.; Skaltsounis, A.L.; Aligiannis, N.; Tran, C.D. Herbal substance, acteoside, alleviates intestinal mucositis in mice. Gastroenterol. Res. Pract. 2015, 2015, 327872. [Google Scholar] [CrossRef] [PubMed]
- Leung, V.S.Y.; Benoit-Biancamano, M.-O.; Pang, D.S.J. Performance of behavioral assays: The rat grimace scale, burrowing activity and a composite behavior score to identify visceral pain in an acute and chronic colitis model. Pain Rep. 2019, 4, e718. [Google Scholar] [CrossRef] [PubMed]
- Moloney, R.D.; O’Mahony, S.M.; Dinan, T.G.; Cryan, J.F. Stress-induced visceral pain: Toward animal models of irritable-bowel syndrome and associated comorbidities. Front. Psychiatry 2015, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Vanhoecke, B.; Bateman, E.; Mayo, B.; Vanlancker, E.; Stringer, A.; Thorpe, D.; Keefe, D. Dark agouti rat model of chemotherapy-induced mucositis: Establishment and current state of the art. Exp. Biol. Med. 2015, 240, 725–741. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.J.; Keefe, D.M.K.; Thompson, F.M.; Clarke, J.M.; Goland, G.J.; Cummins, A.G. Effect of interleukin-11 on ameliorating intestinal damage after methotrexate treatment of breast cancer in rats. Dig. Dis. Sci. 2002, 47, 2751–2757. [Google Scholar] [CrossRef] [PubMed]
- Saine, L.; Helie, P.; Vachon, P. Effects of fentanyl on pain and motor behaviors following a collagenase-induced intracerebral hemorrhage in rats. J. Pain Res. 2016, 9, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Thibault, K.; Calvino, B.; Rivals, I.; Marchand, F.; Dubacq, S.; McMahon, S.B.; Pezet, S. Molecular mechanisms underlying the enhanced analgesic effect of oxycodone compared to morphine in chemotherapy-induced neuropathic pain. PLoS ONE 2014, 9, e91297. [Google Scholar] [CrossRef]
- Leung, V.; Zhang, E.; Pang, D.S. Real-time application of the rat grimace scale as a welfare refinement in laboratory rats. Sci. Rep. 2016, 6, 31667. [Google Scholar] [CrossRef] [PubMed]
- National Health and Medical Research Council. Australian Code for the Care and Use of Animals for Scientific Purposes, 8th ed.; Australian Government: Canberra, Australia, 2013.
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G.; NC3Rs Reporting Guidelines Working Group. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1577–1579. [Google Scholar] [PubMed]
- Morton, D.; Griffiths, P. Guidelines on the recognition of pain, distress and discomfort in experimental animals and an hypothesis for assessment. Vet. Rec. 1985, 116, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.M.; Jain, P.; Mayerhofer, R.; Frohlich, E.E.; Farzi, A.; Reichmann, F.; Herzog, H.; Holzer, P. Visceral hyperalgesia caused by peptide yy deletion and y2 receptor antagonism. Sci. Rep. 2017, 7, 40968. [Google Scholar] [CrossRef] [PubMed]
- Privette, T.H.; Terrian, D.M. Kappa opioid agonists produce anxiolytic-like behavior on the elevated plus-maze. Psychopharmacology 1995, 118, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Oliver, V.; De Rantere, D.; Ritchie, R.; Chisholm, J.; Hecker, K.G.; Pang, D.S.J. Psychometric assessment of the rat grimace scale and development of an analgesic intervention score. PLoS ONE 2014, 9, e97882. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, A.L.; Lymn, K.A.; Wallace, G.L.; Howarth, G.S. Differential effectiveness of clinically-relevant analgesics in a rat model of chemotherapy-induced mucositis. PLoS ONE 2016, 11, e0158851. [Google Scholar] [CrossRef] [PubMed]
- De Rantere, D.; Schuster, C.J.; Reimer, J.N.; Pang, D.S. The relationship between the rat grimace scale and mechanical hypersensitivity testing in three experimental pain models. Eur. J. Pain 2016, 20, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Kawano, T.; Eguchi, S.; Iwata, H.; Yamanaka, D.; Tateiwa, H.; Locatelli, F.M.; Yokoyama, M. Effects and underlying mechanisms of endotoxemia on post-incisional pain in rats. Life Sci. 2016, 148, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Kawano, T.; Tamura, T.; Iwata, H.; Takahashi, Y.; Eguchi, S.; Yamazaki, F.; Kumagai, N.; Yokoyama, M. Postoperative pain impairs subsequent performance on a spatial memory task via effects on n-methyl-d-aspartate receptor in aged rats. Life Sci. 2013, 93, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Long, H.; Zhang, L.; Chen, H.; Zhou, Y.; Ye, N.; Lai, W. Evaluation of pain in rats through facial expression following experimental tooth movement. Eur. J. Oral Sci. 2014, 122, 121–124. [Google Scholar] [CrossRef]
- Miller, A.L.; Golledge, H.D.; Leach, M.C. The influence of isoflurane anaesthesia on the rat grimace scale. PLoS ONE 2016, 11, e0166652. [Google Scholar] [CrossRef] [PubMed]
- Dalla Costa, E.; Minero, M.; Lebelt, D.; Stucke, D.; Canali, E.; Leach, M.C. Development of the horse grimace scale (hgs) as a pain assessment tool in horses undergoing routine castration. PLoS ONE 2014, 9, e92281. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.; Michalidis, V.; Lecker, I.; Collymore, C.; Hanwell, D.; Loka, M.; Danesh, M.; Pham, C.; Urban, P.; Bonin, R.P.; et al. Evaluating analgesic efficacy and administration route following craniotomy in mice using the grimace scale. Sci. Rep. 2019, 9, 359. [Google Scholar] [CrossRef] [PubMed]
- Jirkof, P. Side effects of pain and analgesia in animal experimentation. Lab. Anim. 2017, 46, 123–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNicol, E.; Horowicz-Mehler, N.; Fisk, R.A.; Bennett, K.; Gialeli-Goudas, M.; Chew, P.W.; Lau, J.; Carr, D. Management of opioid side effects in cancer-related and chronic noncancer pain: A systematic review. J. Pain 2003, 4, 231–256. [Google Scholar] [CrossRef]
- George, R.P.; Barker, T.H.; Lymn, K.A.; Bigatton, D.A.; Howarth, G.S.; Whittaker, A.L. A judgement bias test to assess affective state and potential therapeutics in a rat model of chemotherapy-induced mucositis. Sci. Rep. 2018, 8, 8193. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, A.L.; Howarth, G.S. Use of spontaneous behaviour measures to assess pain in laboratory rats and mice: How are we progressing? Appl. Anim. Behav. Sci. 2014, 151, 1–12. [Google Scholar] [CrossRef]

| Overall Appearance * | Weight Loss (%) | Stool Consistency | Rectal Bleeding | Score |
|---|---|---|---|---|
| Normal | No weight loss | Normal Stools | No observable blood | 0 |
| Mild | 0–5% | Loose | Blood spots in faeces | 1 |
| Moderate | 5–10% | Mild diarrhoea | Clear evidence of blood in faeces | 2 |
| Severe | >10% | Overt diarrhoea | Gross bleeding | 3 |
| RGS | DAI | |
|---|---|---|
| Saline + Saline | P Value | P Value |
| Tumour baseline vs. 24hr | 0.28 | 0.54 |
| Tumour baseline vs. 48hr | 0.07 | 0.07 |
| Tumour baseline vs. 72hr | 0.03 * | 0.06 |
| 5-FU + Saline | ||
| Tumour baseline vs. 24hr | 0.06 | <0.001 * |
| Tumour baseline vs. 48hr | 0.53 | <0.001 * |
| Tumour baseline vs. 72hr | 0.03 * | <0.001 * |
| 5-FU + Fentanyl | ||
| Tumour baseline vs. 24hr | 0.91 | <0.001 * |
| Tumour baseline vs. 48hr | 0.12 | <0.001 * |
| Tumour baseline vs. 72hr | 0.001 * | <0.001 * |
| 5-FU +Morphine | ||
| Tumour baseline vs. 24hr | 0.03 * | <0.001 * |
| Tumour baseline vs. 48hr | 0.47 | <0.001 * |
| Tumour baseline vs. 72hr | 0.01 * | <0.001 * |
| 5-FU + Oxycodone | ||
| Tumour baseline vs. 24hr | 0.11 | <0.001 * |
| Tumour baseline vs. 48hr | 0.12 | <0.001 * |
| Tumour baseline vs. 72hr | 0.001 * | <0.001 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
George, R.P.; Howarth, G.S.; Whittaker, A.L. Use of the Rat Grimace Scale to Evaluate Visceral Pain in a Model of Chemotherapy-Induced Mucositis. Animals 2019, 9, 678. https://doi.org/10.3390/ani9090678
George RP, Howarth GS, Whittaker AL. Use of the Rat Grimace Scale to Evaluate Visceral Pain in a Model of Chemotherapy-Induced Mucositis. Animals. 2019; 9(9):678. https://doi.org/10.3390/ani9090678
Chicago/Turabian StyleGeorge, Rebecca P., Gordon S. Howarth, and Alexandra L. Whittaker. 2019. "Use of the Rat Grimace Scale to Evaluate Visceral Pain in a Model of Chemotherapy-Induced Mucositis" Animals 9, no. 9: 678. https://doi.org/10.3390/ani9090678






