Association of Age with the Expression of Hypoxia-Inducible Factors HIF-1α, HIF-2α, HIF-3α and VEGF in Lung and Heart of Tibetan Sheep
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tibetan Sheep
2.2. Immunohistochemistry Detection of HIF-1α, HIF-2α, HIF-3α and VEGF
2.3. Detection of HIF-1α, HIF-2α, HIF-3α and VEG by ELISA
2.4. RT-PCR
2.5. Measurement and Statistical Analysis
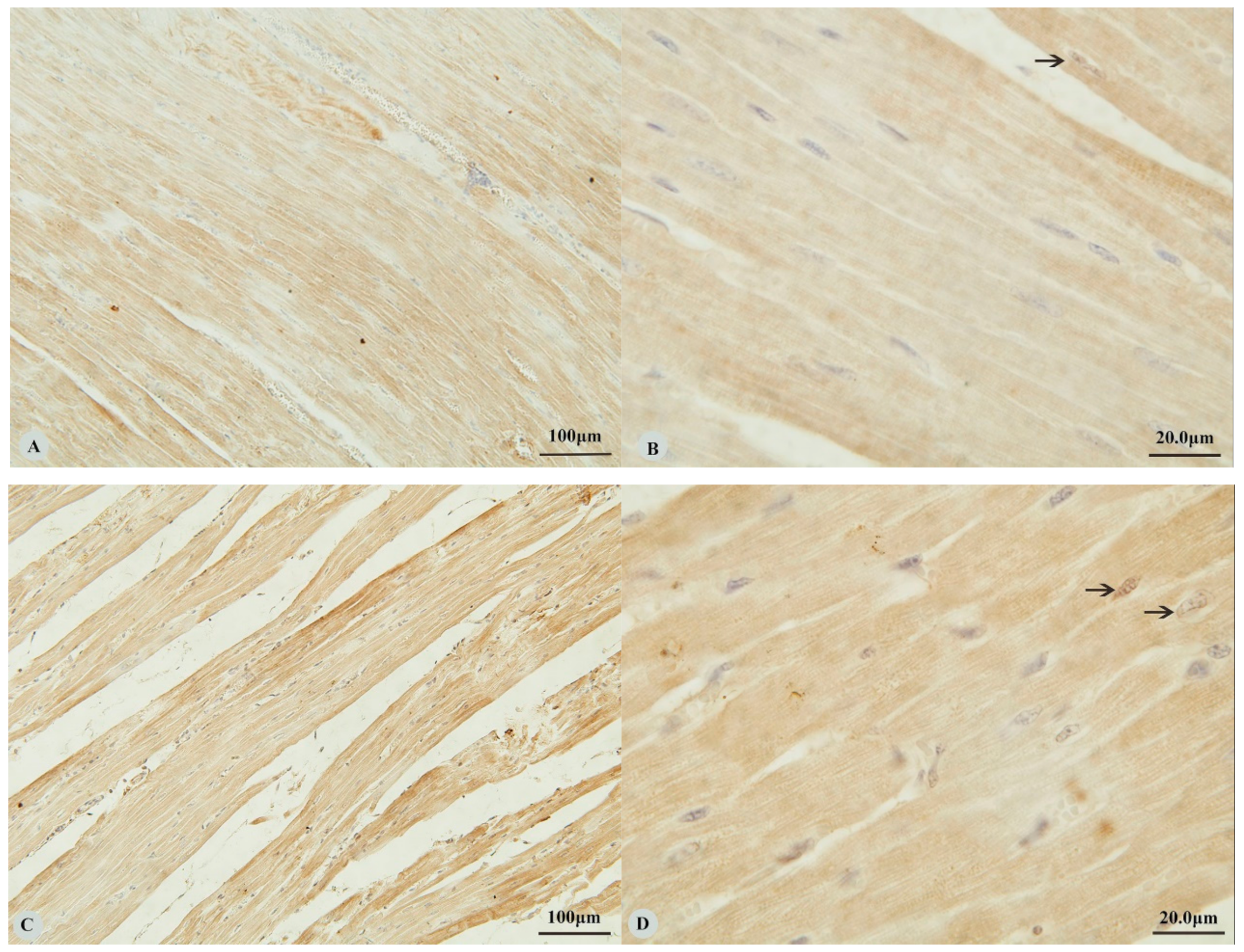
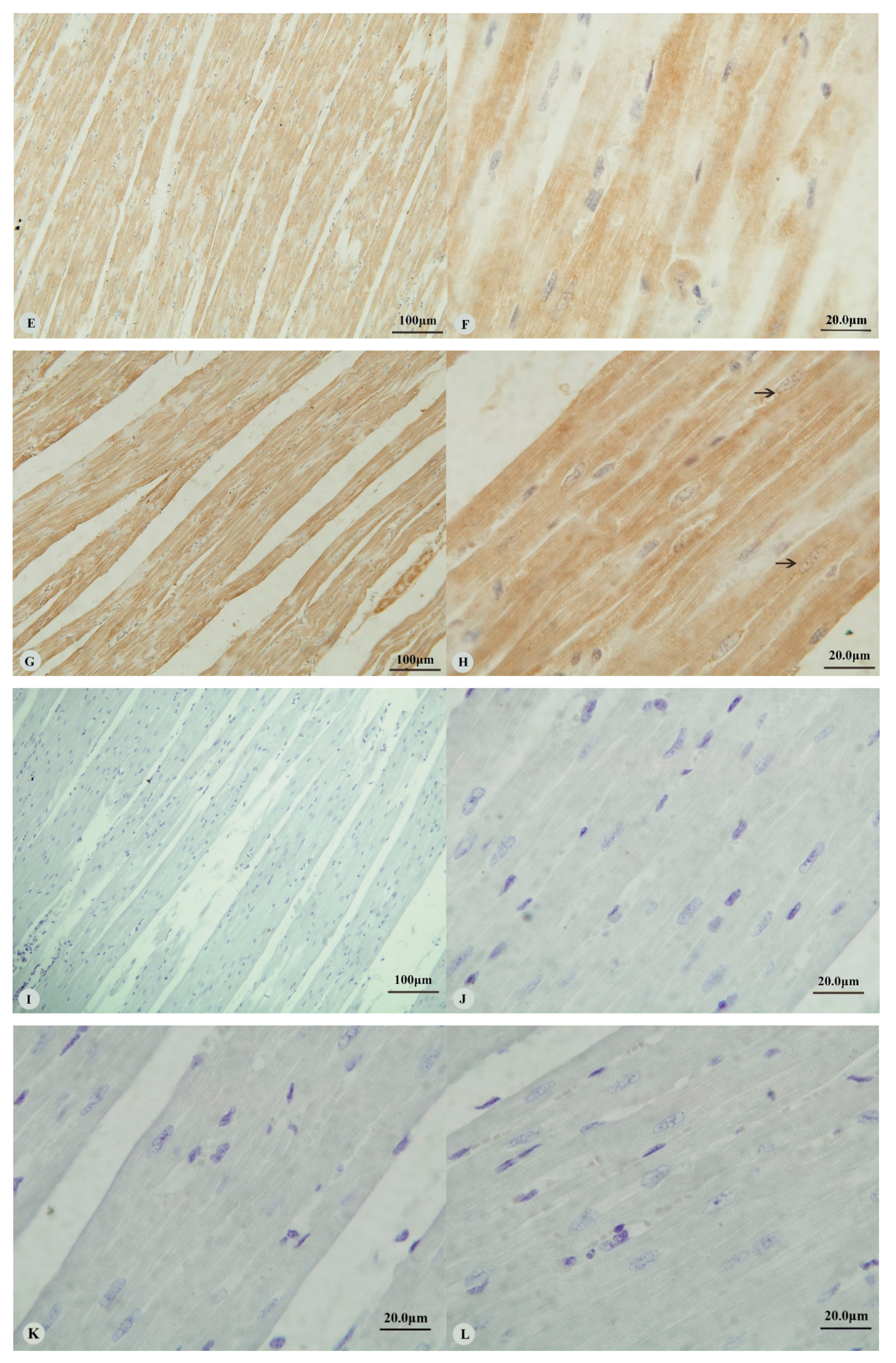
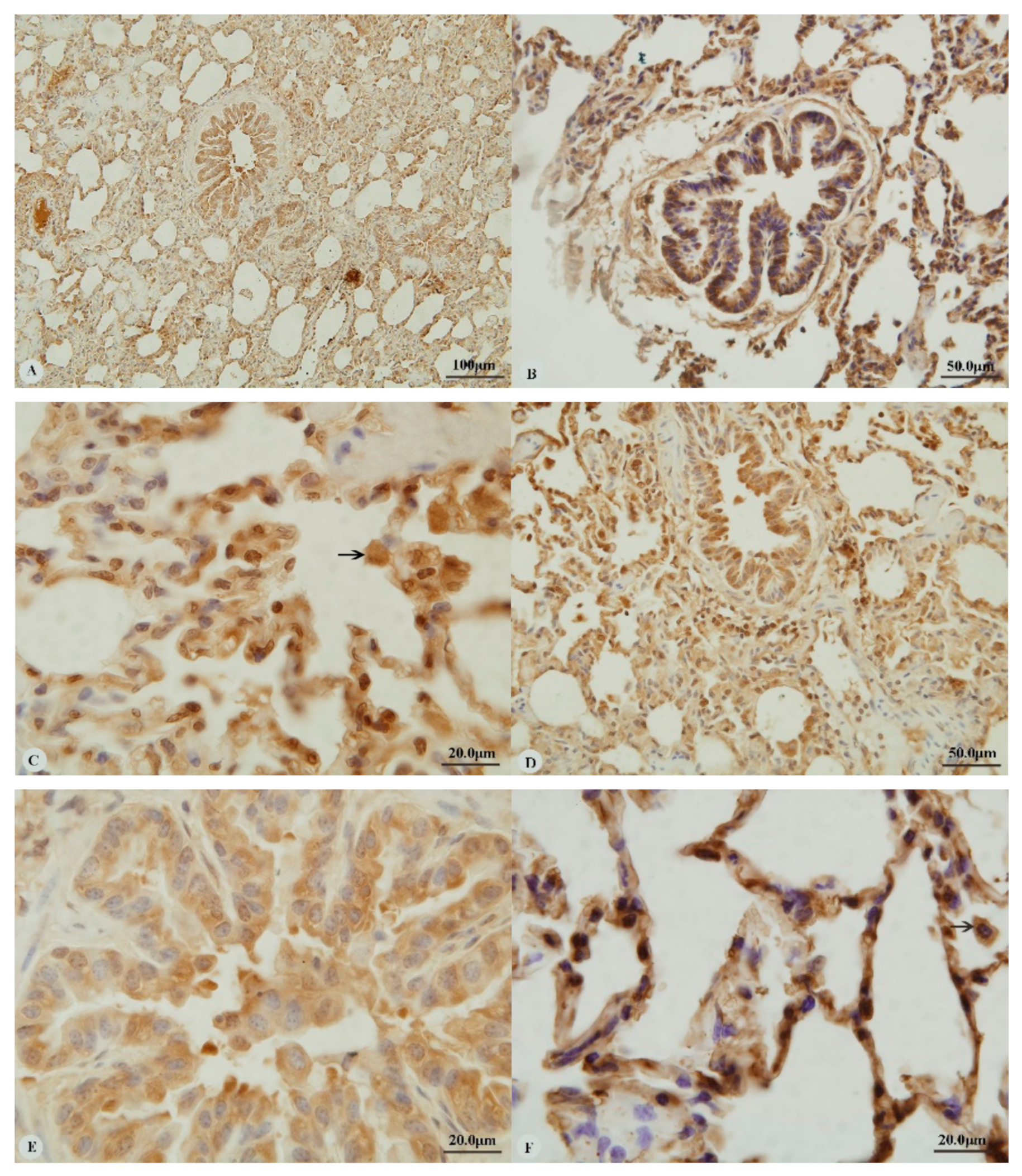
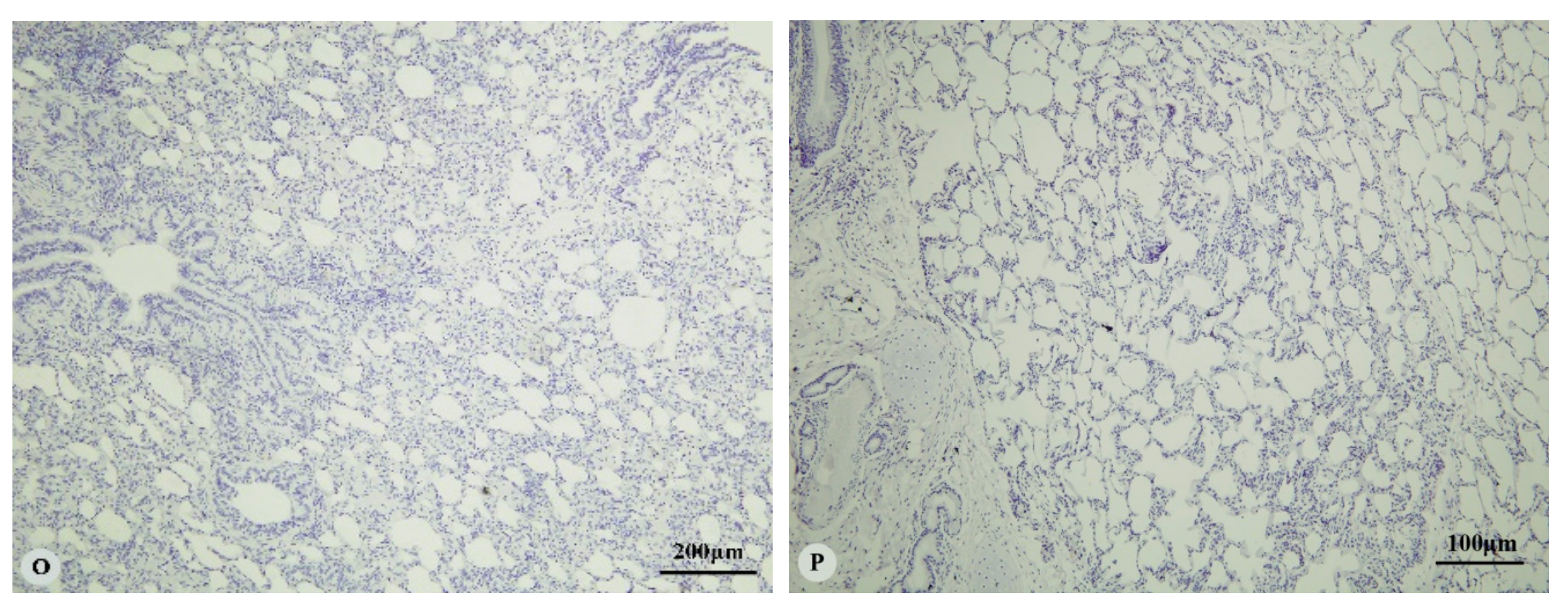
3. Results
3.1. Immunostaining for HIF-1α, HIF-2α, HIF-3α and VEGF in the Heart and Lung of Tibetan Sheep
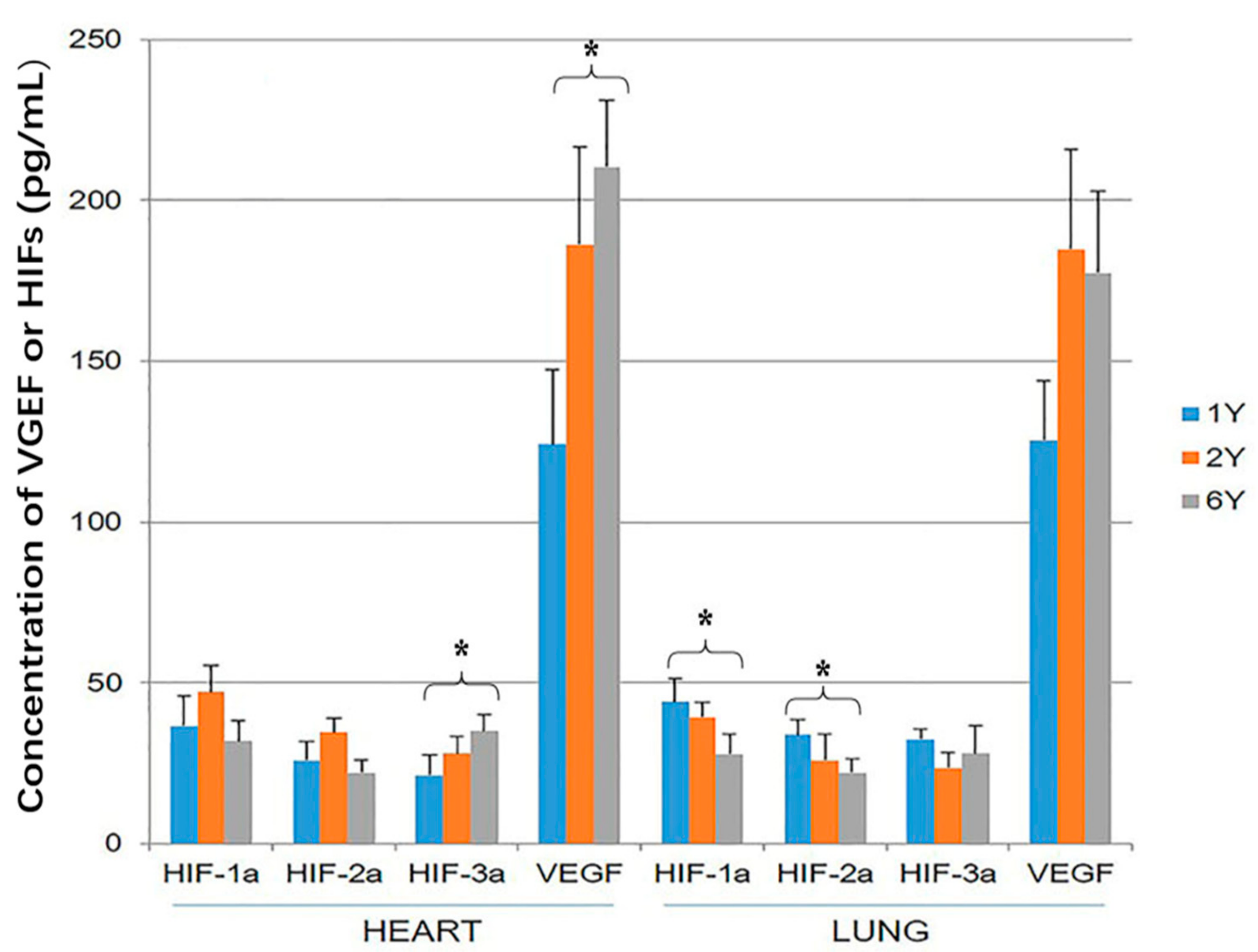
3.2. HIF-1α, HIF-2α, HIF-3α and VEGF ELISA
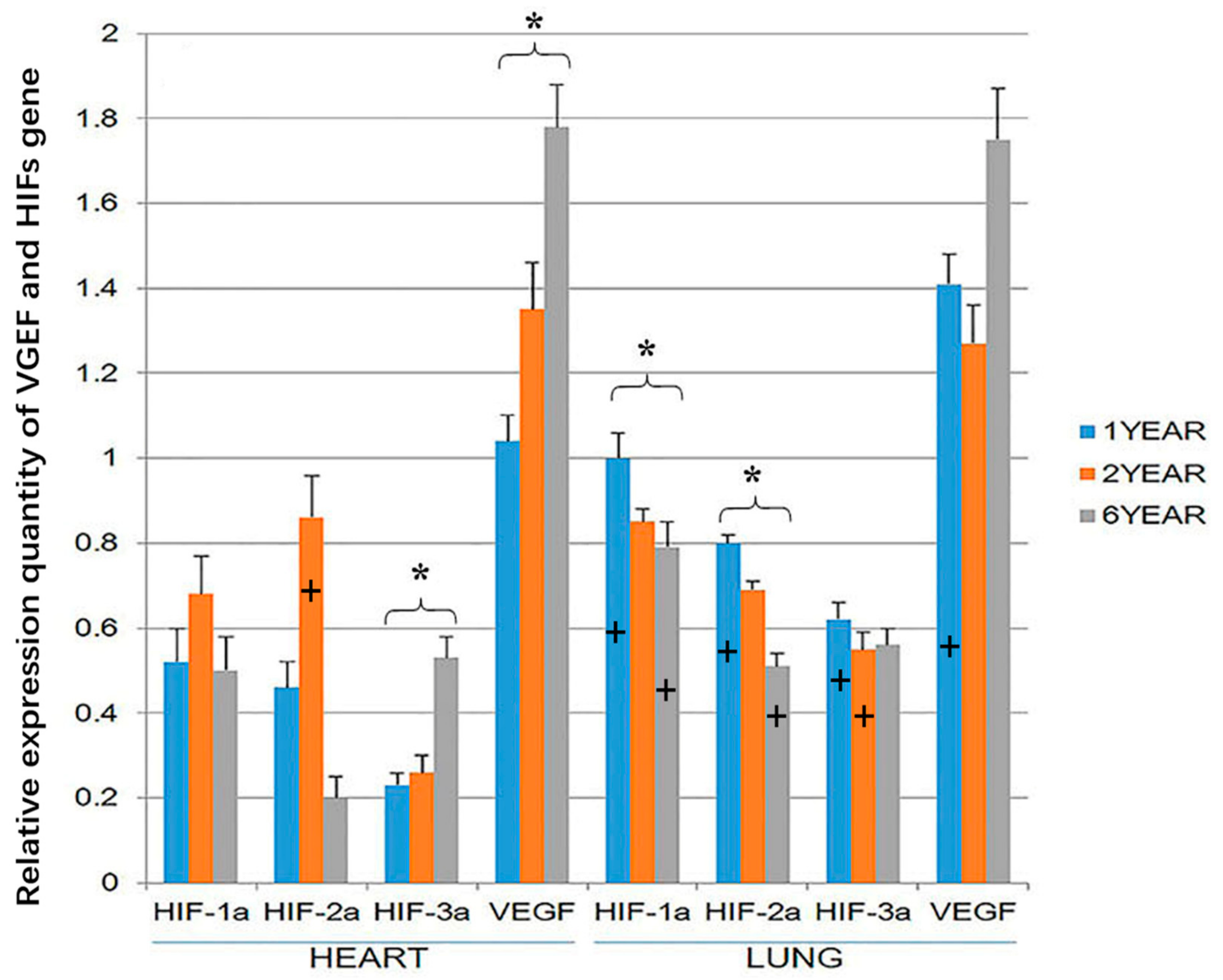
3.3. RT-PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Simonson, T.S.; Yang, Y.Z.; Huff, C.D.; Yun, H.X.; Qin, G.; Witherspoon, D.J.; Bai, Z.Z.; Lorenzo, F.R.; Xing, J.C.; Jorde, L.B.; et al. Genetic evidence for high altitude adaptation in Tibet. Science 2010, 329, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.L.; Cai, Q.; Shen, Y.Y.; San, A.; Ma, L.; Zhang, Y.; Yi, X.; Chen, Y.; Yang, L.F.; Huang, Y.; et al. Draft genome sequence of the Tibetan antelope. Nat. Commun. 2013, 4, 1858. [Google Scholar]
- Alexander, A.; Jensen, R. Gross cardiac changes in cattle with high mountain (brisket) disease and in experimental cattle maintained at high altitudes. Am. J. Vet. Res. 1959, 20, 680–689. [Google Scholar]
- Recavarren, S.; Arias-Stella, J. Right ventricular hypertrophy in people born and living at high altitudes. Br. Heart. J. 1964, 26, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.L.; Mo, V.Y.; Januzzi, J.L.; Jin, G.; Yang, Y.; Han, S.; Wood, M.J.; Levine, B.D. B-type natriuretic peptide, vascular endothelial growth factor, endothelin-1, and nitric oxide synthase in chronic mountain sickness. Am. J. Physiol. Heart. Circ. Physiol. 2011, 300, H1427–H1433. [Google Scholar] [CrossRef] [PubMed]
- Du, X.L.; Li, J.Q.; Ma, N.; Ma, Y.H.; Wang, J.M.; Yin, C.G.; Luo, J.; Liu, N.; Jia, Z.H.; Fu, C.X. Animal Genetic Resources in China: Sheep and Goats; China Agriculture Press: Beijing, China, 2011. [Google Scholar]
- Yu, A.; Shimoda, L.A.; Iyer, N.V.; Huso, D.L.; Sun, X.; McWilliams, R.; Beaty, T.; Sham, J.S.K.; Wiener, C.M.; Sylvester, J.T.; et al. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha. J. Clin. Investig. 1999, 103, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Tipoe, G.L.; Fung, M.L. Expression of HIF-1αlpha, VEGF and VEGF receptors in the carotid body of chronically hypoxic rat. Respir. Physiol. Neurobiol. 2003, 138, 143–154. [Google Scholar] [CrossRef]
- Maynard, M.A.; Evans, A.J.; Shi, W.; Kim, W.Y.; Liu, F.F.; Ohh, M. Dominant-negative HIF-3 alpha 4 suppresses VHL-null renal cell carcinoma progression. Cell Cycle 2007, 6, 2810–2816. [Google Scholar] [CrossRef] [PubMed]
- Markel, T.A.; Wang, Y.; Herrmann, J.L.; Crisostomo, P.R.; Wang, M.; Novotny, N.M.; Herring, C.M.; Tan, J.N.; Laham, T.; Meldrum, D.R. VEGF is critical for stem cell-mediated cardioprotection and a crucial paracrine factor for defining the age threshold in adult and neonatal stem cell function. Am. J. Physiol. Heart. Circ. Physiol. 2008, 295, H2308–H2314. [Google Scholar] [CrossRef]
- Duan, D.; Yu, S.; Cui, Y. Morphological study of the sinus node and its artery in yak. Anat. Rec. 2012, 295, 2045–2056. [Google Scholar] [CrossRef]
- Zhou, J.X.; Yu, S.J.; He, J.F.; Cui, Y. Segmentation features and structural organization of the intrapulmonary artery of the yak. Anat. Rec. 2013, 296, 1755–1788. [Google Scholar] [CrossRef]
- He, Y.; Yu, S.; Hu, J.; Cui, Y.; Liu, P. Changes in the anatomic microscopic structure and the expression of HIF-1α and VEGF of the yak heart with aging and hypoxia. PLoS ONE 2016, 11, e0149947. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, N.R.; Semenza, G.L. Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol. Rev. 2012, 92, 967–1003. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Hypoxia-inducible factors in physiology and medicine. Cell 2012, 148, 399–408. [Google Scholar] [CrossRef]
- Brahimi-Horn, M.C.; Pouysségur, J. HIF at a glance. J. Cell Sci. 2009, 122, 1055–1057. [Google Scholar] [CrossRef]
- Zhang, P.; Yao, Q.; Lu, L.; Li, Y.; Chen, P.J.; Duan, C. Hypoxia-inducible factor 3 is an oxygen-dependent transcription activator and regulates a distinct transcriptional response to hypoxia. Cell Rep. 2014, 6, 1110–1121. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.F.; Wang, X.R.; Yang, Y.W.; Lin, H. Hypoxia upregulates hypoxia inducible factor (HIF)-1a expresion in lung eptthelial cells: Characterization and comparision with HIF-1a. Cell Res. 2006, 16, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, M.R.; Lin, Y.Q.; Zhu, J.J. Comparative analysis of tissue expression and methylation reveal the crucial hypoxia genes in hypoxia resistant animals. Can. J. Anim. Sci. 2018, 98, 204–212. [Google Scholar] [CrossRef]
- Drevytska, T.; Gavenauskas, B.; Drozdovska, S.; Nosar, V.; Dosenko, V.; Mankovska, I. HIF-3αlpha mRNA expression changes in different tissues and their role in adaptation to intermittent hypoxia and physical exercise. Pathophysiology 2012, 19, 205–214. [Google Scholar] [CrossRef]
- Kiang, J.G.; Bowman, P.D.; Wu, B.W. Geldanamycin treatment inhibits hemorrhage-induced increase in KLF6 and Inos expression in unresuscitated mouse organs: Role of inducible HSPJ. Appl. Physiol. 2004, 97, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Bunn, H.F.; Poyton, R.O. Oxygen sensing and molecular adaption to hypoxia. Physiol. Rev. 1996, 76, 839–885. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L.; Wang, G.L. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell. Biol. 1992, 12, 5447–5454. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Xu, M.; Wang, Y.; Pasha, Z.; Li, T.; Ashraf, M. HIF-1αlpha induced-VEGF overexpression in bone mar- row stem cells protects cardiomyocytes against ischemia. J. Mol. Cell. Cardiol. 2007, 42, 1036–1044. [Google Scholar] [CrossRef]
- He, J.F.; Cui, Y. Distribution of vascular endothelial growth factor in plateau yak’s lung. Chin. Vet. Sci. 2008, 12, 1086–1088. [Google Scholar]
- Chao, J.; Wood, J.G.; Gonzalea, N.C. Alveolar hypoxia, alveolar macrophages, and systemic inflammation. Respir. Res. 2009, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Zee, E.D.; Schomberg, S.; Carpenter, T.C. Hypoxia upregulates lung microvascular neurokinin-1 receptor expression. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2006, 29, L102–L110. [Google Scholar] [CrossRef] [PubMed]
- Suresh, M.V.; Ramakrishnan, S.K.; Tomas, B.; Machado-Aranda, D.; Bi, Y.; Talarico, N.; Anderson, E.; Yatrik, S.M.; Raghavendran, K. Activation of hypoxia-inducible factor-1a in type 2 alveolar epithelial cells is a major driver of acute inflammation following lung contusion. Crit. Care. Med. 2014, 42, e642–e653. [Google Scholar] [CrossRef] [PubMed]
- Proper, S.P.; Saini, Y.; Greenwood, K.K.; Bramble, L.A.; Downing, N.J.; Harkema, J.R.; LaPres, J.J. Loss of hypoxia-inducible factor 2 alpha in the lung alveolar epithelium of mice leads to enhanced eosinophilic inflammation in cobalt-induced lung injury. Toxicol. Sci. 2014, 137, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Cummins, E.P.; Keogh, C.E.; Crean, D.; Taylor, C.T. The role of HIF in immunity and inflammation. Mol. Aspects Med. 2016, 47–48, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Curi, R.; Mendees, R.D.S.; Crispin, L.A.D.C.; Norata, G.D.; Sampaio, S.C.; Newsholme, P. A past and present overview of macrophage, metabolism and functional outcomes. Clin. Sci. 2017, 131, 1329–1342. [Google Scholar] [CrossRef]
- Huamg, S.C.; Smith, A.M.; Everts, B.; Colonna, M.; Pearce, E.L.; Schilling, J.D. Metabolic reprogramming mediated by the Mtorc2-irf4 signaling axis is essential for macrophage alternatiove activiation. Immunity 2016, 45, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.K.; Huang, S.C.; Sergushichev, A.; Lampropoulou, V.; Ivanova, Y.; Loginicheva, E. Network intergration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity 2015, 42, 419–430. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Munday, J.S.; Perrott, M.; Wang, G.; Liu, X. Association of Age with the Expression of Hypoxia-Inducible Factors HIF-1α, HIF-2α, HIF-3α and VEGF in Lung and Heart of Tibetan Sheep. Animals 2019, 9, 673. https://doi.org/10.3390/ani9090673
He Y, Munday JS, Perrott M, Wang G, Liu X. Association of Age with the Expression of Hypoxia-Inducible Factors HIF-1α, HIF-2α, HIF-3α and VEGF in Lung and Heart of Tibetan Sheep. Animals. 2019; 9(9):673. https://doi.org/10.3390/ani9090673
Chicago/Turabian StyleHe, Yanyu, John S Munday, Matthew Perrott, Guan Wang, and Xiu Liu. 2019. "Association of Age with the Expression of Hypoxia-Inducible Factors HIF-1α, HIF-2α, HIF-3α and VEGF in Lung and Heart of Tibetan Sheep" Animals 9, no. 9: 673. https://doi.org/10.3390/ani9090673
APA StyleHe, Y., Munday, J. S., Perrott, M., Wang, G., & Liu, X. (2019). Association of Age with the Expression of Hypoxia-Inducible Factors HIF-1α, HIF-2α, HIF-3α and VEGF in Lung and Heart of Tibetan Sheep. Animals, 9(9), 673. https://doi.org/10.3390/ani9090673




