Animal Research, Accountability, Openness and Public Engagement: Report from an International Expert Forum
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Recruitment of Expert Participants
2.2. Discussion Groups and Facilitation
2.3. Analysis
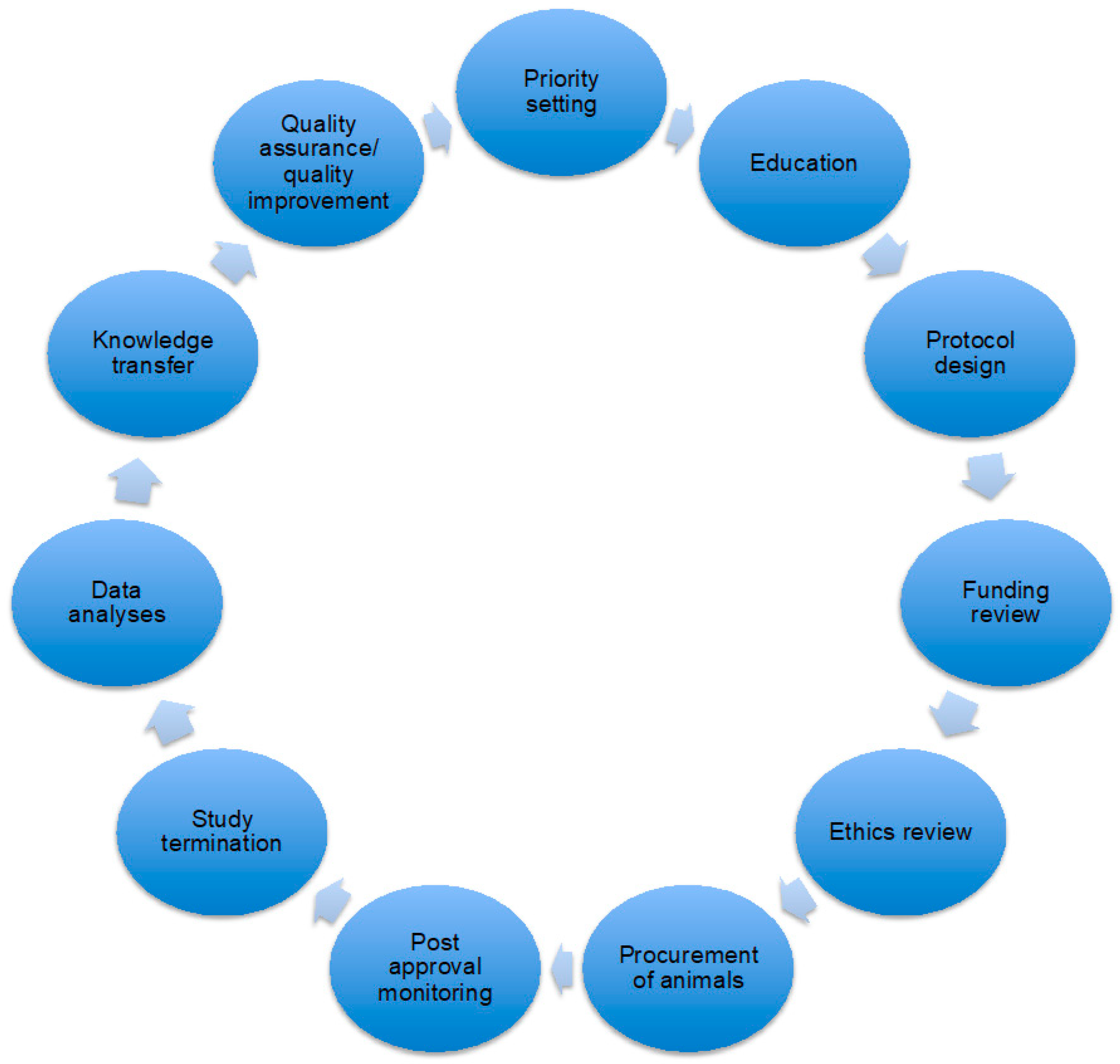
3. Results and Discussion
3.1. Ethics Review Committee
3.1.1. Protocol Review Committee Structure
3.1.2. Protocol Review Committee Function
3.2. Institutional Culture
3.2.1. Openness within Institutions
3.2.2. Openness between Institutional Research Community and the Public
3.3. Policy and Oversight
3.3.1. Openness at All Levels
3.3.2. Findings for Canada
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Auger, G.A. Trust Me, Trust Me Not: An Experimental Analysis of the Effect of Transparency on Organizations. J. Public Relat. Res. 2014, 26, 325–343. [Google Scholar] [CrossRef]
- Vastag, B. Openness in biomedical research collides with heightened security concerns. J. Am. Med. Assoc. 2003, 289, 686–690. [Google Scholar]
- Holmberg, T.; Ideland, M. Secret and lies: “Selective openness” in the apparatus of animal experimentation. Public Underst. Sci. 2012, 21, 354–368. [Google Scholar] [CrossRef] [PubMed]
- Understanding Animal Research. Available online: http://www.animalrightsextremism.info (accessed on 13 December 2018).
- PEW Research Centre. Americans are Divided over the Use of Animals in Scientific Research. Available online: https://www.pewresearch.org/fact-tank/2018/08/16/americans-are-divided-over-the-use-of-animals-in-scientific-research/ (accessed on 17 July 2019).
- Speaking of Research. 52% of American Public Opposes the Use of Animals in Scientific Research. Available online: https://speakingofresearch.com/2018/08/30/52-of-american-public-opposes-the-use-of-animals-in-scientific-research/ (accessed on 17 July 2019).
- Hadley, J. Telling it like it is: A proposal to improve the transparency in biomedical research. Between Species 2012, 15, 103–126. [Google Scholar] [CrossRef]
- Ormandy, E.H. Openness and accountability of animal research: A focus group study with local stakeholders at a Canadian University. In Proceedings of the 9th World Congress on Alternatives and Animal Use in the Life Sciences, Prague, Czech Republic, 24–28 August 2014. [Google Scholar]
- Dietrich, H.; Schibeci, R. Beyond public perceptions of gene technology: Community participation I public policy in Australia. Public Underst. Sci. 2003, 12, 381–401. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, F.; Furger, F. Beyond Bioethics: A Proposal for Modernizing the Regulation of Human Biotechnologies; Paul H. Nitze School of Advanced International Studies: Washington, DC, USA, 2007; pp. 1–355. Available online: http://ieet.org/archive/Fukuyama-BiotechReg2006.pdf (accessed on 26 November 2018).
- House of Lords. Science and Technology—Third Report. In Proceedings of the Science and Technology Select Committee, London, UK, 23 February 2000; Available online: http://www.publications.parliament.uk/pa/ld199900/ldselect/ldsctech/38/3801.htm (accessed on 24 August 2019).
- Walmsley, H. Biobanking, public consultation, and the discursive logics of deliberation: Five lessons from British Columbia. Public Underst. Sci. 2009, 19, 452–468. [Google Scholar] [CrossRef] [PubMed]
- Gaskell, G.; Allum, N.; Bauer, M.; Jackson, J.; Howard, S.; Lindsey, N. Ambivalent GM Nation? Public Attitudes to Biotechnology in the UK, 1991–2002. Lond. Sch. Econ. Politi. Sci. Life Sci. Europ. Societ. Rep. 2003, 1–19. Available online: https://collection.sciencemuseumgroup.org.uk/documents/aa110107203/ambivalent-nation-public-attitudes-to-biotechnology-in-the-uk-1991-2002 (accessed on 24 August 2019).
- Sherwin, S. Toward setting an adequate ethical framework for evaluating biotechnology policy. Presented at the Canadian Biotechnology Advisory Committee, Ottawa, ON, Canada, 4 January 2001. [Google Scholar]
- Burgess, M.M.; Tansey, J. Democratic deficit and the politics of “informed and inclusive consultation”. In Hindsight and Foresight on Emerging Technologies; Einseidel, E., Parker, R., Eds.; UBC Press: Vancouver, BC, Canada, 2008; pp. 275–288. [Google Scholar]
- Rowe, G.; Frewer, L.J. A typology of public engagement mechanisms. Sci. Technol. Hum. Values 2005, 30, 251–290. [Google Scholar] [CrossRef]
- Mayer, S. Science out of step with the public: The need for public accountability of science in the UK. Sci. Public Policy 2003, 30, 177–181. [Google Scholar] [CrossRef]
- McLeod, C.; Hobson-West, P. Opening up animal research and science-society relations? A thematic analysis of transparency discourses in the United Kingdom. Public Underst. Sci. 2016, 25, 791–806. [Google Scholar] [CrossRef] [PubMed]
- Basel Declaration. Available online: http://www.basel-declaration.org/doc/basle%20declation_en.pdf (accessed on 20 December 2018).
- Leenaars, M.; Ritske-Hoitinga, M.; Ormandy, E.H.; Griffin, G. Background to the Montréal Declaration on the synthesis of evidence to advance the 3Rs principles in science, as adopted by the 8th World Congress on Alternatives and Animal Use in the Life Sciences, Montréal, Canada on August 2011. ALTEX Proc. WC8 2012, 3–6. [Google Scholar] [CrossRef]
- UK Concordat Public Consultation Report. Available online: http://www.understandinganimalresearch.org.uk/files/9314/1207/5101/concordat-consultation-report-download-only.pdf (accessed on 18 December 2014).
- European Animal Research Association. Belgian Research Organisations Unite in Support of Animal Research. Available online: http://eara.eu/en/21-belgian-research-organisations-unite-in-support-of-animal-research/ (accessed on 24 August 2019).
- European Animal Research Association. Transparency Agreement on Animal Research Launched in Spain. Available online: http://eara.eu/en/campaigns/transparency-agreement-spain/ (accessed on 6 November 2018).
- European Animal Research Association. Transparency Agreement on Animal Research in Portugal. Available online: http://eara.eu/en/transparency-agreement-on-animal-research-in-portugal/ (accessed on 26 November 2018).
- Ipsos MORI. Openness in Animal Research: The Public’s Views on Openness and Transparency in Animal Research. Available online: http://www.understandinganimalresearch.org.uk/files/3014/1041/0713/openness-in-animal-r.pdf (accessed on 18 December 2014).
- Coffey, A.; Atkinson, P. Concepts and coding. In Making Sense of Qualitative Data: Complementary Research Strategies; Coffey, A., Atkinson, P., Eds.; Sage: Thousand Oaks, CA, USA, 2006; pp. 26–53. [Google Scholar]
- Burnard, P.; Gill, P.; Stewart, K.; Treasure, E.; Chadwick, B. Analysing and presenting qualitative data. Br. Dent. J. 2008, 204, 429–432. [Google Scholar] [CrossRef]
- Miles, M.B.; Huberman, A.M. Qualitative Data Analysis: An. Expanded Sourcebook, 2nd ed.; Sage: Thousand Oaks, CA, USA, 1994. [Google Scholar]
- Groling, J. University animal ethics committees and the Animals (Scientific Procedures) Act: Using the Freedom of Information Act as a research tool. In Proceedings of the 2012 Conference Critical Perspectives on Animals in Society; held at the University of Exeter, UK; Calvert, C., Groling, J., Eds.; Critical Perspectives on Animals in Society (CPAS): Exeter, UK, 2013; pp. 49–62. [Google Scholar]
- Hansen, L.A.; Goodman, J.R.; Chandna, A. Analysis of animal ethics committee membership at American institutions. Animals 2012, 2, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Schuppli, C.A.; Fraser, D. Factors influencing the effectiveness of research ethics committees. J. Med. Ethic. 2007, 33, 294–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawkins, P.; Hobson-West, P. Delivering Effective Ethical Review: The AWERB as a Forum for Discussion. Available online: https://view.pagetiger.com/AWERB/AWERB (accessed on 24 August 2019).
- Ideland, M. Different views on ethics: How animal ethics is situated in committee culture. J. Med. Ethic. 2009, 35, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Schuppli, C.A. Decisions about the use of animals in research: Ethical reflection by animal ethics committee members. Anthrozoos 2011, 24, 409–425. [Google Scholar] [CrossRef]
- Rose, M. Ethical decision making: Do we need to reset the GPS? In Proceedings of the 2013 ANZCAART Conference, Sydney, Australia, 23–25 July 2013. [Google Scholar]
- Ormandy, E.H.; Kwok, Y.K.E.; Weary, D.M. Public openness in laboratory research: A model for soliciting public input into protocol review. In Proceedings of the 9th World Congress on Alternatives and Animal Use in the Life Sciences, Prague, Czech Republic, 24–28 August 2014. [Google Scholar]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959; p. 238. [Google Scholar]
- Anderson, J.A.; Swatzky-Girling, B.; McDonald, M.A.; Pullman, D.; Saginur, R.; Sampson, H.A.; Willison, D.J. Research Ethics, Broadly Writ. Health Law Rev. 2011, 19, 12–24. [Google Scholar]
- Johnson, J. Some challenges with Animal Ethics Committees: Can greater transparency help? In Proceedings of the 2013 ANZCAART Conference, Sydney, Australia, 23–25 July 2013. [Google Scholar]
- Kimmelmann, J. The XV Collection: Ethical Oversights in Ethical Oversight of Animal Research. PLoS. Blogs 2019. Available online: https://blogs.plos.org/biologue/2019/01/11/the-xv-collection-ethical-oversights-in-ethical-oversight-of-animal-research/ (accessed on 24 August 2019).
- Pound, P.; Nicol, C.J. Retrospective harm benefit analysis of pre-clinical animal research for six treatment interventions. PLoS ONE 2018. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.F.; Greenhough, B.J.; Hobson-West, P.; Kirk, R.G.W.; Applebee, K.; Bellingan, L.C.; Wolfensohn, S. Developing a Collaborative Agenda for Humanities and Social Scientific Research on Laboratory Animal Science and Welfare. PLoS ONE 2016, 11, e0158791. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ormandy, E.H.; Weary, D.M.; Cvek, K.; Fisher, M.; Herrmann, K.; Hobson-West, P.; McDonald, M.; Milsom, W.; Rose, M.; Rowan, A.; et al. Animal Research, Accountability, Openness and Public Engagement: Report from an International Expert Forum. Animals 2019, 9, 622. https://doi.org/10.3390/ani9090622
Ormandy EH, Weary DM, Cvek K, Fisher M, Herrmann K, Hobson-West P, McDonald M, Milsom W, Rose M, Rowan A, et al. Animal Research, Accountability, Openness and Public Engagement: Report from an International Expert Forum. Animals. 2019; 9(9):622. https://doi.org/10.3390/ani9090622
Chicago/Turabian StyleOrmandy, Elisabeth H., Daniel M. Weary, Katarina Cvek, Mark Fisher, Kathrin Herrmann, Pru Hobson-West, Michael McDonald, William Milsom, Margaret Rose, Andrew Rowan, and et al. 2019. "Animal Research, Accountability, Openness and Public Engagement: Report from an International Expert Forum" Animals 9, no. 9: 622. https://doi.org/10.3390/ani9090622
APA StyleOrmandy, E. H., Weary, D. M., Cvek, K., Fisher, M., Herrmann, K., Hobson-West, P., McDonald, M., Milsom, W., Rose, M., Rowan, A., Zurlo, J., & von Keyserlingk, M. A. G. (2019). Animal Research, Accountability, Openness and Public Engagement: Report from an International Expert Forum. Animals, 9(9), 622. https://doi.org/10.3390/ani9090622




