Purified β-glucans of Different Molecular Weights Enhance Growth Performance of LPS-challenged Piglets via Improved Gut Barrier Function and Microbiota
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. β-Glucan Samples
2.2. Animals and Diets
2.3. Experimental Design
2.4. Sampling and Analyses
2.5. Jejunal Morphology
2.6. Mucosal D-Lactate, Diamine Oxidase, and Anti-Oxidation Index
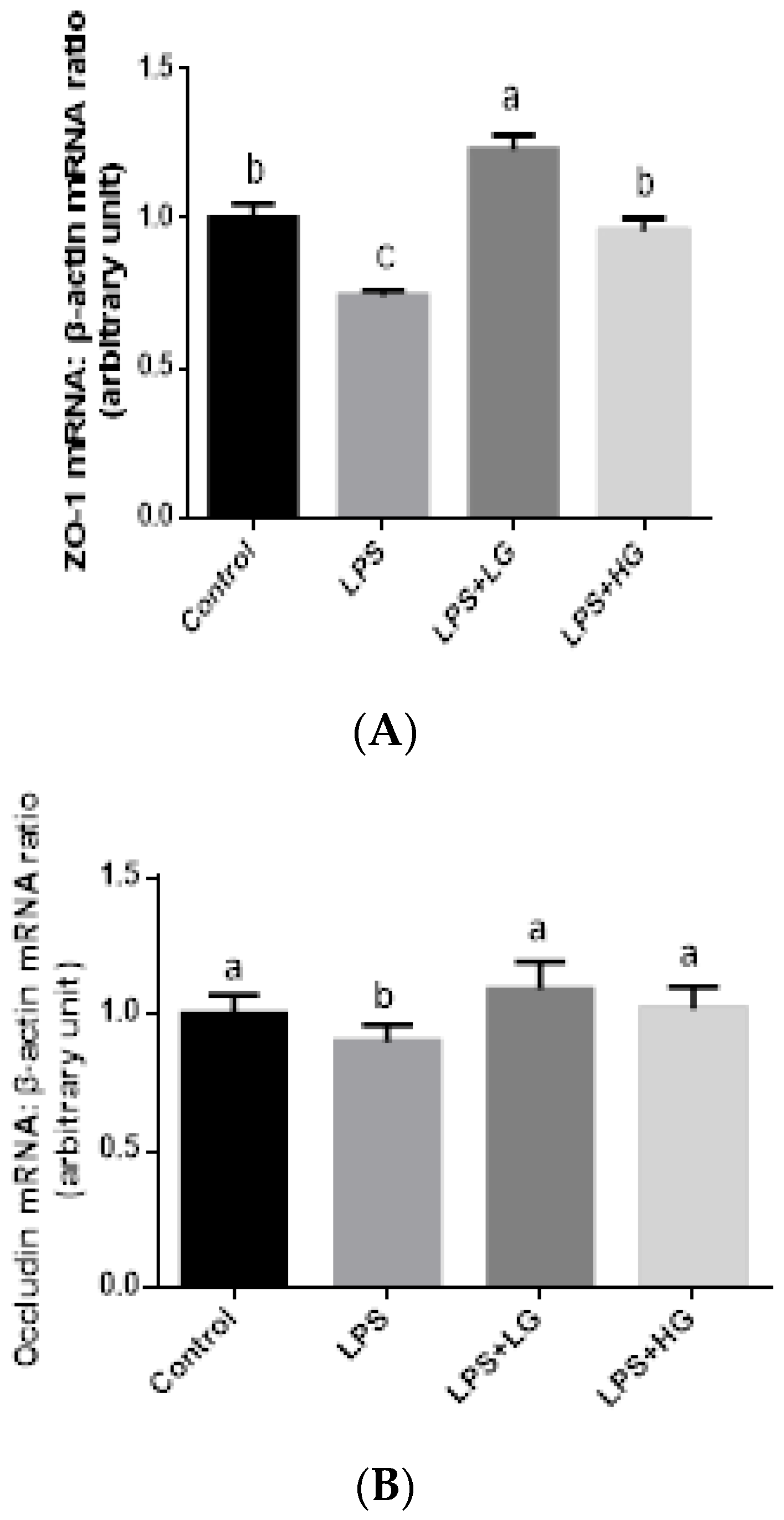
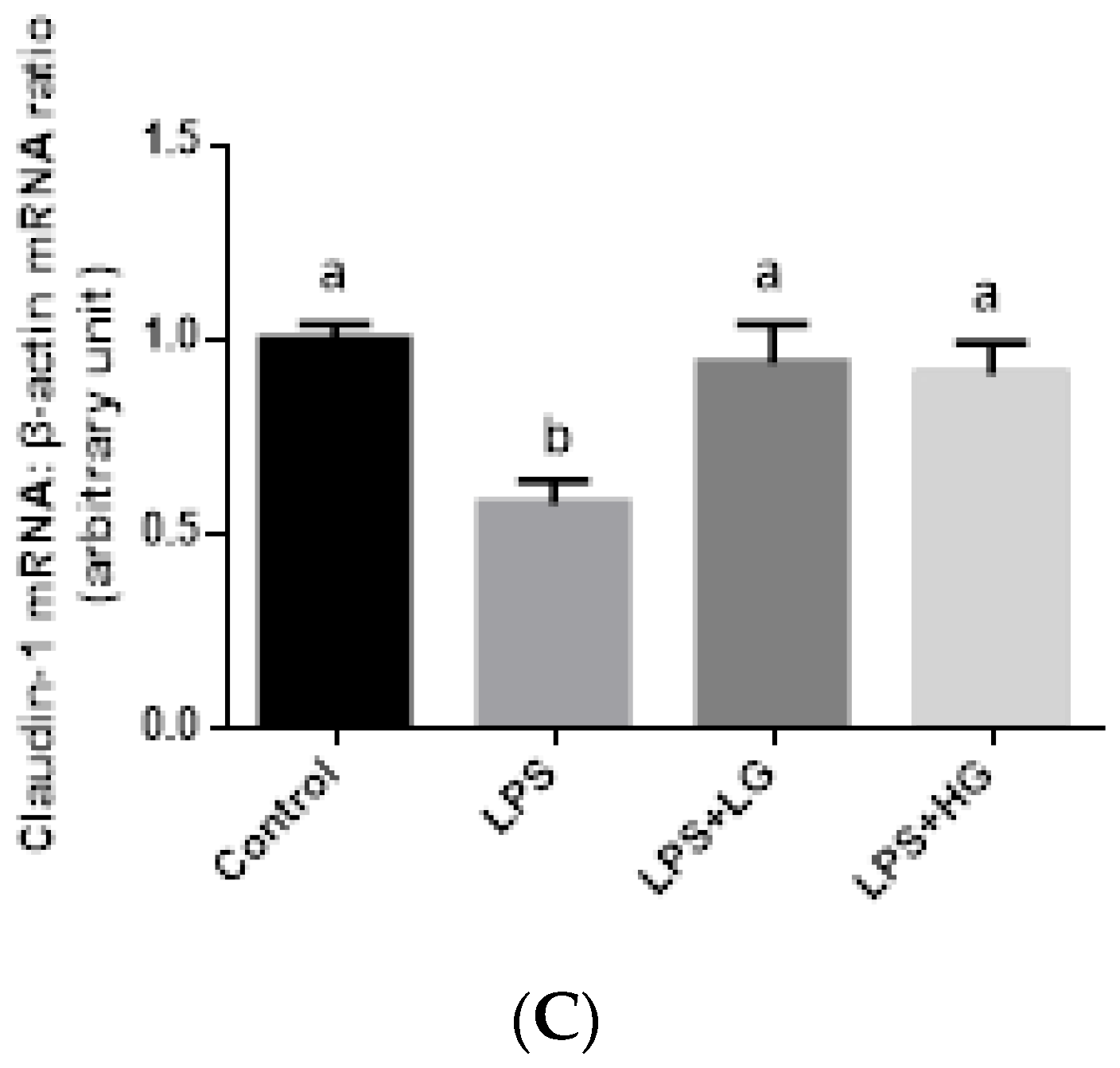
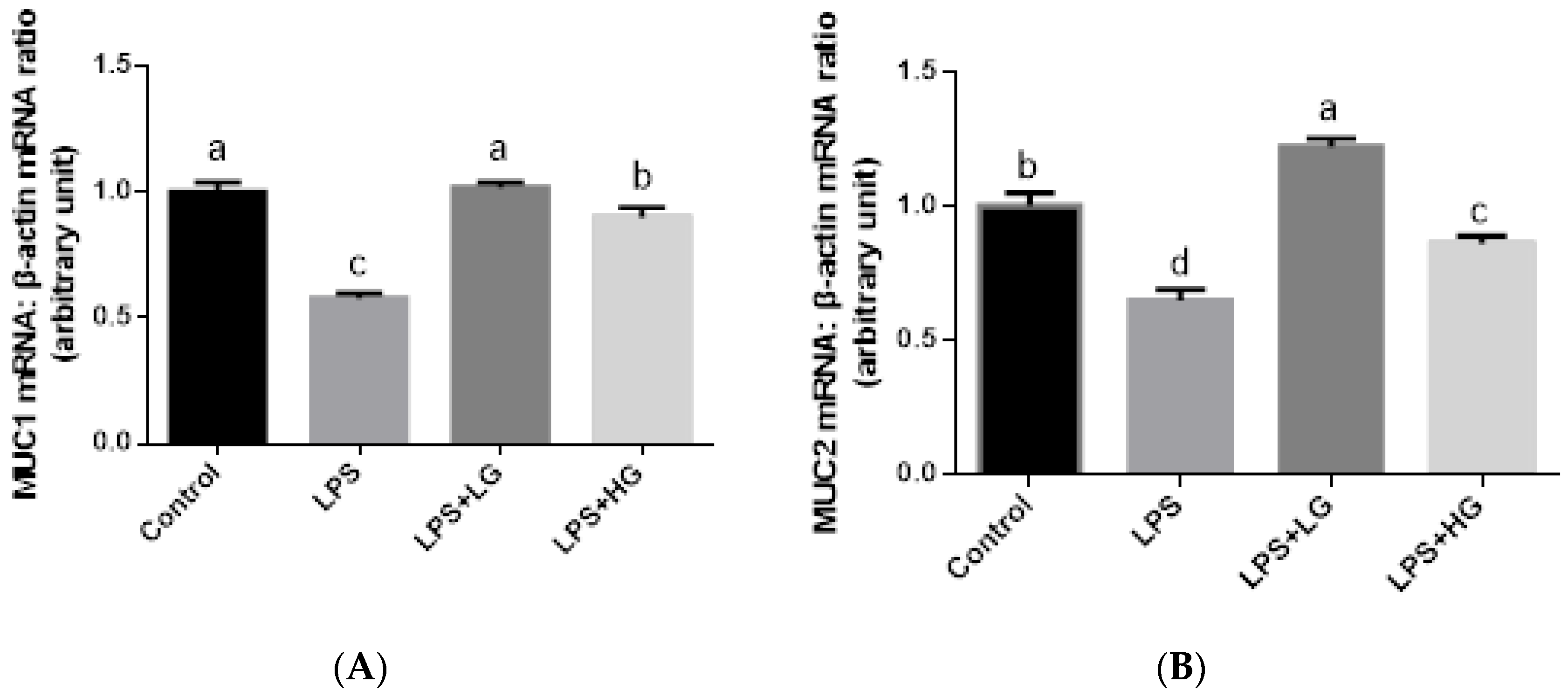
2.7. Mucosal mRNA Expression of ZO-1, Occludin, Claudin-1, and Mucin
2.8. DNA Extraction and Real-Time PCR Analysis of Colonic Bacteria
2.9. Quantification of Volatile Fatty Acids (VFAs)
2.10. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Anti-Oxidation Index
3.3. Morphology of Jejunal Mucosa
3.4. Intestinal Barrier Function
3.5. Quantitative Difference in Bacterial Groups
3.6. Changes in Volatile Fatty Acid Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Yin, J.; Ren, W.K.; Liu, G.; Duan, J.L.; Yang, G.; Wu, L.; Li, T.J.; Yin, Y.L. Birth oxidative stress and the development of an antioxidant system in newborn piglets. Free Radical Res. 2013, 47, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wu, M.M.; Xiao, H.; Ren, W.K.; Duan, J.L.; Yang, G.; Li, T.J.; Yin, Y.L. Development of an antioxidant system after early weaning in piglets. J. Anim. Sci. 2014, 92, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Yang, H.; Li, B.; Liu, G.; Huang, R.; Li, F.; Liao, P.; Zhang, Y.; Nyachoti, C.M.; Deng, D. Dietary supplementation with yeast product improves intestinal function, and serum and ileal amino acid contents in weaned piglets. Livest. Sci. 2015, 171, 20–27. [Google Scholar] [CrossRef]
- Murphy, P.; Bello, F.D.; O’Doherty, J.; Arendt, E.K.; Sweeney, T.; Coffey, A. Analysis of bacterial community shifts in the gastrointestinal tract of pigs fed diets supplemented with β-glucan from Laminaria digitata, Laminaria hyperborea and Saccharomyces cerevisiae. Animal 2013, 7, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wang, S.; Liu, Y.; Li, B.; Wang, B.; Peng, Y. Long-term effect of pH on short-chain fatty acids accumulation and microbial community in sludge fermentation systems. Bioresour. Technol. 2015, 197, 56–63. [Google Scholar] [CrossRef]
- Belobrajdic, D.P.; Jobling, S.A.; Morell, M.K.; Taketa, S.; Bird, A.R. Wholegrain barley β-glucan fermentation dose not improve glucose tolerance in rats fed a high-fat diet. Nutr. Res. 2015, 35, 162–168. [Google Scholar] [CrossRef]
- Tian, X.; Shao, Y.; Wang, Z.; Guo, Y. Effects of dietary yeast β-glucans supplementation on growth performance, gut morphology, intestinal Clostridium perfringens population and immune response of brioler chickens challenged with necrotic enteritis. Anim. Feed Sci. Tech. 2016, 215, 144–155. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, P.; Li, M.; Liu, S.; Zhang, S. Dietary β-glucan enhances the contents of complement component 3 and factor B in eggs of zebrafish. Dev. Comp. Immunol. 2016, 65, 107–113. [Google Scholar] [CrossRef]
- Lee, S.I.; Kim, J.K.; Hancock, J.D.; Kim, I.H. β-glucan from mulberry leaves and curcuma can improve growth performance and nutrient digestibility in early weaned pigs. J. Appl. Anim. Res. 2017, 45, 209–214. [Google Scholar] [CrossRef]
- Ma, T.; Tu, Y.; Zhang, N.; Guo, J.; Deng, K.; Zhou, Y.; Yun, Q.; Diao, Q. Effects of dietary yeasr β-glucan on nutrient digestibility and serum profiles in pre-reminant Holstein calves. J. Integr. Agric. 2015, 14, 749–757. [Google Scholar] [CrossRef]
- Sweeney, T.; Collins, C.B.; Reilly, P.; Pierce, K.M.; Ryan, M.; O’Doherty, J.V. Effect of purified β-glucans derived from Laminaria digitata, Laminaria hyperborean and Saccharomyces cerevisiae on piglet performance, selected bacterial populations, volatile fatty acids and pro-inflammatory cytokines in the gastrointestinal tract of pigs. Brit. J. Nutr. 2012, 108, 1226–1234. [Google Scholar] [PubMed]
- Khan, A.A.; Gani, A.; Masoodi, F.A.; Amin, F.; Wani, I.A.; Khanday, F.A.; Gani, A. Structural thermal, functional, antioxidant & antimicrobial properties of β-d-glucan extracted from baker’s yeast (Saccharomyces cereviseae)—Effect of γ-irradiation. Carbohyd. Polym. 2016, 140, 442–450. [Google Scholar]
- Rieder, A.; Grimmer, S.; Aachmann, F.L.; Westereng, B.; Kolset, S.O.; Knutsen, S.H. Generic tools to assess genuine carbohydrate specific effects on in vitro immune modulation exemplified by β-glucans. Carbohyd. Polym. 2013, 92, 2075–2083. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xia, L.; Pang, W.; Wang, T.; Chen, P. A novel soluble β-1,3-d-glucan salecan reduces adiposity and improves glucose tolerance in high-fat diet-fed mice. Brit. J. Nutr. 2013, 109, 254–262. [Google Scholar] [CrossRef] [PubMed]
- DuBios, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Qiao, J.; Li, H.; Wang, Z.; Wang, W. Effects of Lactobacillus acidophilus dietary supplementation on the performance, intestinal barrier function, rectal microflora and serum immune function in weaned piglets challenged with Escherichia coli lipopolysaccharide. Anton. Van. Leeuw. 2015, 107, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Y.; Li, Y.; Yan, H.; Zhang, H. L-Tryptophan Enhances Intestinal Integrity in Diquat-Challenged Piglets Associated with Improvement of Redox Status and Mitochondrial Function. Animal 2019, 9, 266. [Google Scholar] [CrossRef]
- Metzler-Zebeli, B.U.; Ganzle, M.G.; Mosenthin, R.; Zijlstra, R.T. Oat β-glucan and dietary Calcium and phosphorus differentially modify intestinal expression of proinflammatory cytokines and monocarboxylate transporter 1 and cecal morphology in weaned pigs. J. Nutr. 2012, 142, 668–674. [Google Scholar] [CrossRef]
- Han, G.Q.; Xiang, Z.T.; Yu, B.; Chen, D.W.; Qi, H.W.; Mao, X.B.; Chen, H.; Mao, Q.; Huang, Z.Q. Effects of different starch sources on Bacillus spp. in intestinal tract and expression of intestinal development related genes of weanling piglets. Mol. Biol. Rep. 2012, 39, 1869–1876. [Google Scholar] [CrossRef]
- Qi, H.W.; Xiang, Z.T.; Han, G.Q.; Yu, B.; Huang, Z.Q.; Chen, D.W. Effects of different dietary protein sources on cecal microflora in rats. Afr. J. Biotechnol. 2011, 10, 3704–3708. [Google Scholar]
- Franklin, M.; Mathew, A.; Vickers, J.; Clift, R. Characterization of microbial populations and volatile fatty acid concentrations in the jejunum, ileum, and cecum of pigs weaned at 17 vs 24 days of age. J. Anim. Sci. 2002, 80, 2904–2910. [Google Scholar] [CrossRef]
- Suchecka, D.; Blaszczyk, K.; Harasym, J.; Gudej, S.; Wilczak, J.; Gromadzka-Ostrowska, J. Impact of purified oat 1-3,1-4-β-d-glucan of different molecular weight on alleviation of inflammation parameters during gastritis. J. Funct. Foods. 2017, 28, 11–18. [Google Scholar] [CrossRef]
- Zhou, T.X.; Jung, J.H.; Zhang, Z.F.; Kim, I.H. Effect of dietary β-glucan on growth performance, fecal microbial shedding and immunological responses after lipopolysaccharide challenge in weaned pigs. Anim. Feed Sci. Tech. 2013, 179, 85–92. [Google Scholar] [CrossRef]
- Hester, S.N.; Comstock, S.S.; Thorum, S.C.; Monaco, M.H.; Pence, B.D.; Woods, J.A.; Donovan, S.M. Intestinal and systemic immune development and response to vaccination are unaffected by dietary (1,3/1,6)-β-D-glucan supplementation in neonatal piglets. Clin. Vaccine Immunol. 2012, 19, 1499–1508. [Google Scholar] [CrossRef]
- Suchecka, D.; Harasym, J.; Wilczak, J.; Gromadzka-Ostrowska, J. Hepato- and gastro- protective activity of purified oat 1-3,1-4-β-D-glucans of different molecular weight. Int. J. Biol. Macromol. 2016, 91, 1177–1185. [Google Scholar] [CrossRef]
- Yang, H.; Wu, F.; Long, L.; Li, T.; Xiong, X. Effects of yeast products on the intestinal morphology, barrier function, cytokine expression, and antioxidant system of weaned piglets. Zhejiang Univ-Sci. B (Biomed. Biotechnol.) 2016, 17, 752–762. [Google Scholar]
- Kanjan, P.; Sahasrabudhe, N.M.; de Haan, B.J.; de Vos, P. Immune effects of β-glucan are determined by combied effects on Dectin-1, TLR2, 4 and 5. J. Funct. Foods. 2017, 37, 433–440. [Google Scholar] [CrossRef]
- Holtekjolen, A.K.; Vhile, S.G.; Sahlstrom, S.; Knutsen, S.H.; Uhlen, A.K.; Assveen, M.; Kjos, N.P. Changes inrelative molecular weight distribution of soluble barley beta-glucan during passage through the small intestine of pigs. Livest. Sci. 2014, 168, 102–108. [Google Scholar] [CrossRef][Green Version]
- Luo, Y.; Chen, H.; Yu, B.; He, J.; Zheng, P.; Mao, X.; Yu, J.; Luo, J.; Huang, Z.; Chen, D. Dietary pea fibre alters the microbial community and fermentation with increase in fibre degradation-associated bacterial groups in the colon of pigs. J. Anim. Physiol. Anim. Nutr. 2018, 102, e254–e261. [Google Scholar] [CrossRef]
- Chen, H.; Chen, D.; Michiels, J.; De Smet, S. Dietary fiber affects intestinal mucosal barrier function and regulated intestinal bacteria in weaning piglets. Brit. J. Nutr. 2013, 110, 1837–1848. [Google Scholar] [CrossRef]
- Che, L.; Chen, H.; Yu, B.; He, J.; Zheng, P.; Mao, X.; Yu, J.; Huang, Z.; Chen, D. Long-term intake of pea fiber affects colonic barrier function, bacterial and transcriptional profile in pig model. Nutr. Cancer 2014, 66, 388–399. [Google Scholar] [CrossRef]
- Smith, A.G.; O’Doherty, J.V.; Reilly, P.; Ryan, M.T.; Bahar, B.; Sweeney, T. The effects of laminarin derived from Laminaria digitat on measurements of gut health: Selected bacterial populations, intestinal fermentation, mucin gene expression and cytokine gene expression in the pig. Brit. J. Nutr. 2011, 105, 669–677. [Google Scholar] [CrossRef]
| Beta-Glucan Source | MW (kDa) | Content (%) | Structure |
|---|---|---|---|
| Yeast | 5–80 | ≤30% | β-1,3/1,6 β-1,3/1,4 |
| Oat | 5–250 | ≤85% | β-1,3/1,4 |
| Algal | <5 | ≤80% | β-1,3/1,6 |
| Agrobacterium sp. ZX09 | 200–3000 | ≥90% | β-1,3 |
| Ingredients | Composition, g/kg |
|---|---|
| Corn | 301.8 |
| Extruded corn | 290.0 |
| Fish meal | 40.0 |
| Whey powder | 40.0 |
| Soybean meal | 107.6 |
| Extruded full-fat soybean | 100.0 |
| Soy protein concentrate | 50.0 |
| Wheat bran | 20.0 |
| Corn starch | 5.0 |
| L-Lysine·HCL (78%) | 3.3 |
| L-Threonine (98.5%) | 1.5 |
| DL-Methionine (99%) | 0.9 |
| L-Tryptophan | 0.3 |
| Choline chloride | 1.0 |
| Sodium chloride | 3.0 |
| Calcium carbonate | 7.0 |
| Dicalcium phosphate | 5.5 |
| Soybean oil | 17.8 |
| Vitamin premix 1 | 0.3 |
| Mineral premix 2 | 5.0 |
| Nutrient composition, g/kg | |
| Digestible energy 3, MJ/kg | 14.83 |
| Crude protein 4 | 205.6 ± 3.51 |
| Total lysine 4 | 13.5 ± 0.20 |
| Total methionine and cysteine 3 | 7.4 |
| Total tryptophan 3 | 2.2 |
| Total threonine 3 | 7.9 |
| Calcium 3 | 8.0 |
| Phosphorus available 3 | 4.0 |
| Gene | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) | Accession Number. | Product Length |
|---|---|---|---|---|
| ZO-1 | CAGCCCCCGTACATGGAGA | GCGCAGACGGTGTTCATAGTT | XM_005659811 | 114bp |
| Occludin | CTACTCGCTCAACGGGAAAG | ACGCCTCCAAGTTACCZCTG | NM_001163647.2 | 158bp |
| Claudin-1 | TCTTAGTTGCCACAGCATGG | CCAGTGAAGAGAGCCTGACC | NM001244539 | 106bp |
| Mucin 1 | GTGCCGCTGCCCACAACCTG | AGCCGGGTACCCCAGACCCA | XM_001926883.4 | 141bp |
| Mucin 2 | GGTCATGCTGGAGCTGGACAGT | TGCCTCCTCGGGGTCGTCAC | XM_003122394.1 | 181bp |
| β-actin | TCTGGCACCACACCTTCT | TGATCTGGGTCATCTTCTCAC | DQ178122 | 114bp |
| Item | Primers/Probes and Sequence (5′–3′) | Product Length |
|---|---|---|
| Total bacteria | Eub338F: ACTCCTACGGGAGGCAGCAG | 200bp |
| Eub518R: ATTACCGCGGCTGCTGG | ||
| Lactobacillus | F: GAGGCAGCAGTAGGGAATCTTC | 126bp |
| R: CAACAGTTACTCTGACACCCGTTCTTC | ||
| P: (FMA)AAGAAGGGTTTCGGCTCGTAAAACTCTGTT(BHQ-1) | ||
| Bifidobacterium | F: CGCGTCCGGTGTGAAAG | 121bp |
| R: CTTCCCGATATCTACACATTCCA | ||
| P: (FMA) ATTCCACCGTTACACCGGGAA(BHQ-1) | ||
| Bacillus | F: GCAACGAGCGCAACCCTTGA | 92bp |
| R: TCATCCCCACCTTCCTCCGGT | ||
| P: (FMA)CGGTTTGTCACCGGCAGTCACCT(BHQ-1) | ||
| Escherichia coli | F: CATGCCGCGTGTATGAAGAA | 96bp |
| R: CGGGTAACGTCAATGAGCAAA | ||
| P: (FMA)AGGTATTAACTTTACTCCCTTCCTC(BHQ-1) |
| Item | Control | LPS 1 | LG 2 | HG 3 | SEM | p-Value |
|---|---|---|---|---|---|---|
| 1–21d 6 | ||||||
| ADG(g) | 305 a | 371 b | 348 ab | 29.12 | 0.048 | |
| ADFI(g) | 538 a | 597 b | 582 b | 24.73 | 0.039 | |
| F/G | 1.78 | 1.63 | 1.67 | 0.12 | 0.338 | |
| LPS + LG 4 | LPS + HG 5 | SEM | p-value | |||
| 22–28d 7 | ||||||
| ADG(g) | 415 a | 325 b | 390 a | 411 a | 27.47 | 0.035 |
| ADFI(g) | 656 | 602 | 621 | 637 | 29.35 | 0.55 |
| F/G | 1.60 a | 1.88 b | 1.58 a | 1.56 a | 0.09 | 0.027 |
| Score of diarrheas | 3.50 a | 7.50 b | 4.75 a | 5.00 a | 0.55 | 0.041 |
| Item | Control | LPS 1 | LPS + LG 2 | LPS + HG 3 | SEM | p-Value |
|---|---|---|---|---|---|---|
| T-AOC (U/mg prot) | 3.26 a | 1.84 b | 3.86 a | 3.72 a | 0.23 | <0.01 |
| CAT (U/mg prot) | 3.58 a | 2.69 b | 3.88 a | 3.43 a | 0.32 | 0.039 |
| GSH-Px (U/mg prot) | 50.86 a | 40.69 b | 53.21 a | 51.64 a | 2.05 | <0.01 |
| SOD (U/mg prot) | 51.18 b | 29.35 c | 67.14 a | 54.22 b | 3.95 | 0.025 |
| MDA (nmol/mg prot) | 2.33 b | 3.57 a | 1.59 c | 2.41 b | 0.21 | 0.047 |
| Item | Control | LPS 1 | LPS + LG 2 | LPS + HG 3 | SEM | p-Value |
|---|---|---|---|---|---|---|
| Villus height (μm) | 319 b | 269 c | 368 a | 325 b | 6.11 | 0.031 |
| Crypt depth (μm) | 178 b | 213 a | 177 b | 178 b | 3.32 | 0.042 |
| VH:CD 4 | 1.80 a | 1.26 b | 2.09 a | 1.84 a | 0.05 | 0.040 |
| Item. | Control | LPS 1 | LPS + LG 2 | LPS + HG 3 | SEM | p-Value |
|---|---|---|---|---|---|---|
| D-lactate (μg/mL) | 8.87 b | 10.99 a | 8.46 b | 8.75 b | 0.45 | <0.01 |
| DAO (U/L) | 14.68 b | 17.14 a | 14.15 b | 13.07 b | 0.40 | 0.026 |
| Item | Control | LPS 1 | LPS + LG 2 | LPS + HG 3 | SEM | p-Value |
|---|---|---|---|---|---|---|
| Lactobacillus | 8.27 a | 7.73 c | 8.05 b | 8.25 a | 0.12 | 0.012 |
| Bifidobacterium | 5.66 b | 5.02 d | 5.33 c | 6.03 a | 0.28 | <0.01 |
| Bacillus | 8.79 b | 8.45 c | 8.70 b | 9.47 a | 0.08 | <0.01 |
| Escherichia coli | 7.47 b | 8.08 a | 7.99 a | 7.42 b | 0.15 | 0.011 |
| Total bacteria | 11.16 a | 9.61 c | 10.75 b | 11.18 a | 0.20 | <0.01 |
| Item | Control | LPS 1 | LPS + LG 2 | LPS + HG 3 | SEM | p-Value |
|---|---|---|---|---|---|---|
| Acetate | 58.02 a | 52.49 c | 55.69 b | 59.08 a | 2.90 | 0.021 |
| Propionate | 25.95 a | 22.55 b | 23.61 ab | 25.92 a | 1.37 | 0.048 |
| Butyrate | 13.21 b | 11.10 c | 13.29 b | 14.72 a | 1.13 | 0.033 |
| Total VFAs | 97.18 a | 86.14 c | 92.59 b | 99.72 a | 4.48 | 0.042 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, J.; Chen, D.; Mao, X.; He, J.; Yu, B.; Cheng, L.; Zeng, D. Purified β-glucans of Different Molecular Weights Enhance Growth Performance of LPS-challenged Piglets via Improved Gut Barrier Function and Microbiota. Animals 2019, 9, 602. https://doi.org/10.3390/ani9090602
Luo J, Chen D, Mao X, He J, Yu B, Cheng L, Zeng D. Purified β-glucans of Different Molecular Weights Enhance Growth Performance of LPS-challenged Piglets via Improved Gut Barrier Function and Microbiota. Animals. 2019; 9(9):602. https://doi.org/10.3390/ani9090602
Chicago/Turabian StyleLuo, Junqiu, Daiwen Chen, Xiangbing Mao, Jun He, Bing Yu, Long Cheng, and Dafu Zeng. 2019. "Purified β-glucans of Different Molecular Weights Enhance Growth Performance of LPS-challenged Piglets via Improved Gut Barrier Function and Microbiota" Animals 9, no. 9: 602. https://doi.org/10.3390/ani9090602
APA StyleLuo, J., Chen, D., Mao, X., He, J., Yu, B., Cheng, L., & Zeng, D. (2019). Purified β-glucans of Different Molecular Weights Enhance Growth Performance of LPS-challenged Piglets via Improved Gut Barrier Function and Microbiota. Animals, 9(9), 602. https://doi.org/10.3390/ani9090602





