Sweet Corn Stalk Treated with Saccharomyces Cerevisiae Alone or in Combination with Lactobacillus Plantarum: Nutritional Composition, Fermentation Traits and Aerobic Stability
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Forage Harvest and Silage Preparation
2.2. Sampling and pH Determination
2.3. Determination of Chemical Composition
2.4. Determination of Ammonia Nitrogen, Ethanol and Organic Acid Contents
2.5. Background Microbial Population Analysis
2.6. Aerobic Stability and Quality Assessment
2.7. Data Analysis
3. Results
3.1. Chemical Composition and Microbial Population of Fresh Forage
3.2. Chemical Composition of Silage
3.3. Fermentation Traits, Aerobic Stability and V-Score
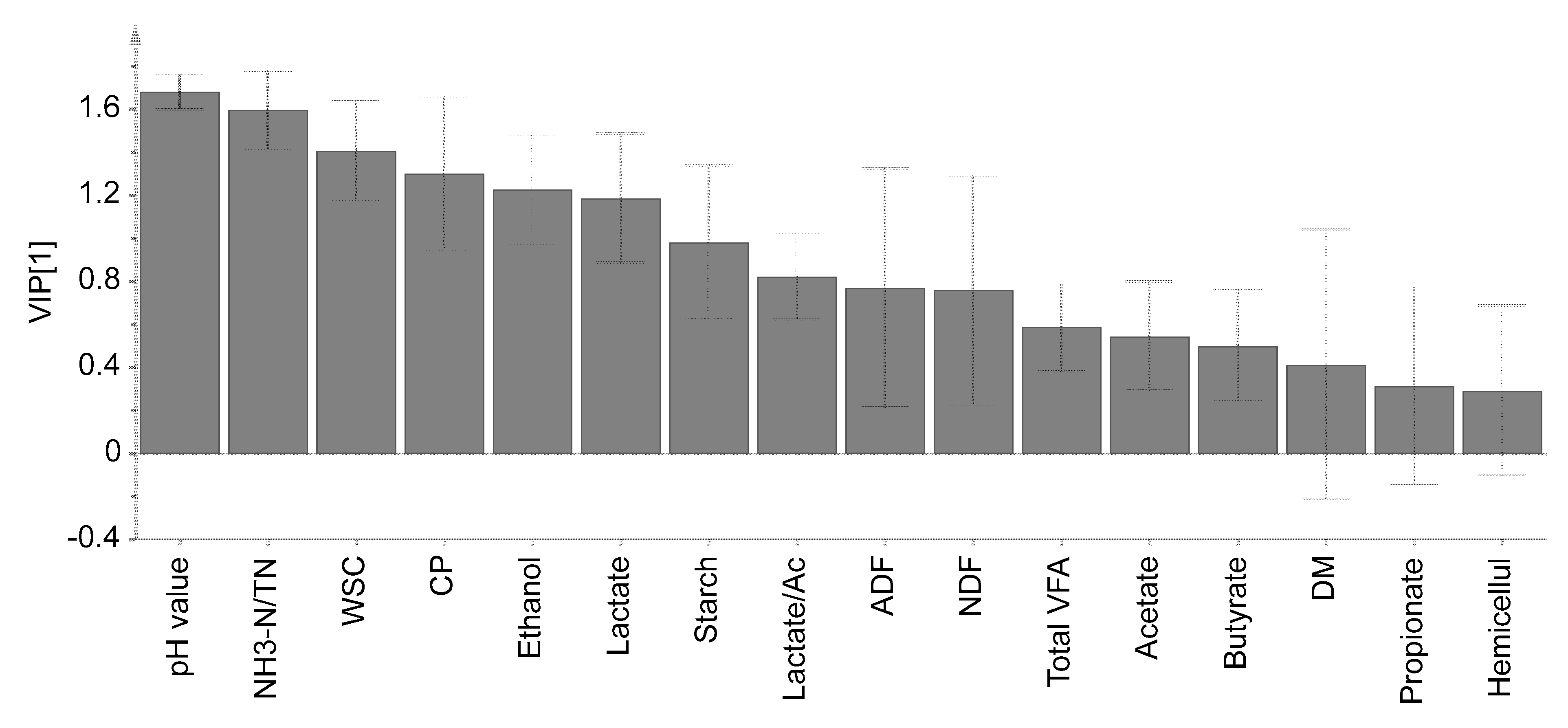
3.4. Principal Component Analysis and VIP Score
4. Discussions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cui, W.; Dong, Z.; Zhang, J.; Wei, J.; Lin, L.; Zhang, M. The nutrient components and ensilage fermentation quality of sweet corn stalks harvested at different times. ACTA Pratacult. Sin. 2011, 20, 208–213. [Google Scholar]
- Idikut, L.; Arikan, B.A.; Kaplan, M.; Guven, I.; Atalay, A.I.; Kamalak, A. Potential nutritive value of sweet corn as a silage crop with or without corn ear. J. Anim. Vet. Adv. 2009, 8, 734–741. [Google Scholar]
- Zhang, M.; Wang, Y.; Tan, Z.; Li, Z.; Li, Y.; Lv, H.; Zhang, B.; Jin, Q. Microorganism profile, fermentation quality and rumen digestibility in vitro of maize-stalk silages produced at different maturity stages. Crop Pasture Sci. 2017, 68, 225–233. [Google Scholar] [CrossRef]
- Queiroz, O.C.M.; Ogunade, I.M.; Weinberg, Z.; Adesogan, A.T. Silage review: Foodborne pathogens in silage and their mitigation by silage additives. J. Dairy Sci. 2018, 101, 4132–4142. [Google Scholar] [CrossRef] [PubMed]
- Muck, R.E.; Nadeau, E.M.G.; McAllister, T.A.; Contreras-Govea, F.E.; Santos, M.C.; Kung, L. Silage review: Recent advances and future uses of silage additives. J. Dairy Sci. 2018, 101, 3980–4000. [Google Scholar] [CrossRef] [PubMed]
- Middelhoven, W.J.; van Baalen, A.H.M. Development of the yeast flora of whole-crop maize during ensiling and during subsequent aerobiosis. J. Sci. Food Agric. 1988, 42, 199–207. [Google Scholar] [CrossRef]
- Shurson, G.C. Yeast and yeast derivatives in feed additives and ingredients: Sources, characteristics, animal responses, and quantification methods. Anim. Feed Sci. Technol. 2018, 235, 60–76. [Google Scholar] [CrossRef]
- Trckova, M.; Faldyna, M.; Alexa, P.; Zajacova, Z.S.; Gopfert, E.; Kumprechtova, D.; Auclair, E.; D’Inca, R. The effects of live yeast Saccharomyces cerevisiae on postweaning diarrhea, immune response, and growth performance in weaned piglets. J. Anim. Sci. 2014, 92, 767–774. [Google Scholar] [CrossRef]
- Tang, S.X.; Tayo, G.O.; Tan, Z.L.; Sun, Z.H.; Shen, L.X.; Zhou, C.S.; Xiao, W.J.; Ren, G.P.; Han, X.F.; Shen, S.B. Effects of yeast culture and fibrolytic enzyme supplementation on in vitro fermentation characteristics of low-quality cereal straws. J. Anim. Sci. 2008, 86, 1164–1172. [Google Scholar] [CrossRef]
- Zhu, W.; Wei, Z.; Xu, N.; Yang, F.; Yoon, I.; Chung, Y.; Liu, J.; Wang, J. Effects of Saccharomyces cerevisiae fermentation products on performance and rumen fermentation and microbiota in dairy cows fed a diet containing low quality forage. J. Anim. Sci. Biotechnol. 2017, 8, 36. [Google Scholar] [CrossRef]
- Elghandour, M.M.Y.; Mellado, M.; Kholif, A.E.; Salem, A.Z.M.; Barbabosa, A.; Ballinas, S.; Esquivel, A.; Odongo, N.E. Fecal gas production of ten common horse feeds supplemented with Saccharomyces cerevisiae. J. Equine Vet. Sci. 2016, 47, 1–8. [Google Scholar] [CrossRef]
- Cordonnier, C.; Thévenot, J.; Etienne-Mesmin, L.; Denis, S.; Alric, M.; Livrelli, V.; Blanquet-Diot, S. Dynamic In vitro models of the human gastrointestinal tract as relevant tools to assess the survival of probiotic strains and their interactions with gut microbiota. Microorganisms 2015, 3, 725–745. [Google Scholar] [CrossRef] [PubMed]
- Kwolek-Mirek, M.; Zadrag-Tecza, R. Comparison of methods used for assessing the viability and vitality of yeast cells. FEMS Yeast Res. 2014, 14, 1068–1079. [Google Scholar] [CrossRef] [PubMed]
- Ferraretto, L.F.; Shaver, R.D.; Bertics, S.J. Effect of dietary supplementation with live-cell yeast at two dosages on lactation performance, ruminal fermentation, and total-tract nutrient digestibility in dairy cows. J. Dairy Sci. 2012, 95, 4017–4028. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, C.; Wills, D.A.; Yan, T. Effects of dietary active dried yeast (Saccharomyces cerevisiae) supply at two levels of concentrate on energy and nitrogen utilisation and methane emissions of lactating dairy cows. Anim. Prod. Sci. 2017, 57, 656–664. [Google Scholar] [CrossRef]
- Goncalves, B.L.; Goncalves, J.L.; Rosim, R.E.; Cappato, L.P.; Cruz, A.G.; Oliveira, C.A.F.; Corassin, C.H. Effects of different sources of Saccharomyces cerevisiae biomass on milk production, composition, and aflatoxin M-1 excretion in milk from dairy cows fed aflatoxin B-1. J. Dairy Sci. 2017, 100, 5701–5708. [Google Scholar] [CrossRef]
- Sullivan, M.L.; Bradford, B.J. Viable cell yield from active dry yeast products and effects of storage temperature and diluent on yeast cell viability. J. Dairy Sci. 2011, 94, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Duniere, L.; Jin, L.; Smiley, B.; Qi, M.; Rutherford, W.; Wang, Y.; McAllister, T. Impact of adding Saccharomyces strains on fermentation, aerobic stability, nutritive value, and select lactobacilli populations in corn silage. J. Anim. Sci. 2015, 93. [Google Scholar] [CrossRef]
- Xu, S.; Yang, J.; Qi, M.; Smiley, B.; Rutherford, W.; Wang, Y.; McAllister, T.A. Impact of Saccharomyces cerevisiae and Lactobacillus buchneri on microbial communities during ensiling and aerobic spoilage of corn silage1. J. Anim. Sci. 2019, 97, 1273–1285. [Google Scholar] [CrossRef]
- Santos, M.C.; Golt, C.; Joerger, R.D.; Mechor, G.D.; Mourao, G.B.; Kung, L., Jr. Identification of the major yeasts isolated from high moisture corn and corn silages in the United States using genetic and biochemical methods. J. Dairy Sci. 2017, 100, 1151–1160. [Google Scholar] [CrossRef] [Green Version]
- Kumprechtova, D.; Illek, J.; Julien, C.; Homolka, P.; Jancik, F.; Auclair, E. Effect of live yeast (Saccharomyces cerevisiae) supplementation on rumen fermentation and metabolic profile of dairy cows in early lactation. J. Anim. Physiol. Anim. Nutr. (Berl) 2019, 103. [Google Scholar] [CrossRef] [PubMed]
- ter Schure, E.G.; van Riel, N.A.; Verrips, C.T. The role of ammonia metabolism in nitrogen catabolite repression in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2000, 24, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Kamphayae, S.; Kumagai, H.; Bureenok, S.; Narmseelee, R.; Butcha, P. Effects of graded levels of liquid brewer’s yeast on chemical composition and fermentation quality in cassava pulp and rice straw-based total mixed ration silage. Anim. Sci. J. 2017, 88, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, J.; Dong, Z.; Chen, L.; Yuan, X.; Shao, T. The effects of lactic acid bacteria strains isolated from various substrates on the fermentation quality of common vetch (Vicia sativa L.) in Tibet. Grass Forage Sci. 2018, 73, 639–647. [Google Scholar] [CrossRef]
- Porter, M.G.; Murray, R.S. The volatility of components of grass silage on oven drying and the inter-relationship between dry-matter content estimated by different analytical methods. Grass Forage Sci. 2001, 56, 405–411. [Google Scholar] [CrossRef] [Green Version]
- Association of Official Analytical Chemists. Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2002. [Google Scholar]
- Association of Official Analytical Chemists. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Mertens, D.R. Gravimetric determination of amylase-treated neutral detergent fiber in feeds with refluxing in beakers or crucibles: collaborative study. J. AOAC Int. 2002, 85, 1217–1240. [Google Scholar] [PubMed]
- Wang, M.; Wang, R.; Xie, T.Y.; Janssen, P.H.; Sun, X.Z.; Beauchemin, K.A.; Tan, Z.L.; Gao, M. Shifts in rumen fermentation and microbiota are associated with dissolved ruminal hydrogen concentrations in lactating dairy cows fed different types of carbohydrates. J. Nutr. 2016, 146, 1714–1721. [Google Scholar] [CrossRef] [PubMed]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Chen, L.; Ren, A.; Zhou, C.; Tan, Z. Effects of Lactobacillus acidophilus supplementation for improving in vitro rumen fermentation characteristics of cereal straws. Ital. J. Anim. Sci. 2016, 16, 52–60. [Google Scholar] [CrossRef]
- Playne, M.J. Determination of ethanol, volatile fatty acids, lactic and succinic acids in fermentation liquids by gas chromatography. J. Sci. Food Agric. 1985, 36, 638–644. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yilmaz, P.; Parfrey, L.W.; Yarza, P.; Gerken, J.; Pruesse, E.; Quast, C.; Schweer, T.; Peplies, J.; Ludwig, W.; Glockner, F.O. The SILVA and “All-species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Res. 2014, 42, D643–D648. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, O.C.M.; Arriola, K.G.; Daniel, J.L.P.; Adesogan, A.T. Effects of 8 chemical and bacterial additives on the quality of corn silage. J. Dairy Sci. 2013, 96, 5836–5843. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y. Analysis method for silage. In Guidebook for Forage Evaluation, 3rd ed.; Association of Self-Supply Feed Evaluation, Ed.; Japan Grassland Agriculture and Forage Seed Association: Tokyo, Japan, 2009; pp. 64–78. [Google Scholar]
- Lee, S.; Lee, D.K. What is the proper way to apply the multiple comparison test? Korean J. Anesthesiol. 2018, 71, 353–360. [Google Scholar] [CrossRef] [Green Version]
- Afanador, N.L.; Tran, T.N.; Buydens, L.M.C. Use of the bootstrap and permutation methods for a more robust variable importance in the projection metric for partial least squares regression. Anal. Chim. Acta 2013, 768, 49–56. [Google Scholar] [CrossRef]
- Liu, Q.H.; Shao, T.; Zhang, J.G. Determination of aerobic deterioration of corn stalk silage caused by aerobic bacteria. Anim. Feed Sci. Technol. 2013, 183, 124–131. [Google Scholar] [CrossRef]
- Li, F.; Ding, Z.; Ke, W.; Xu, D.; Zhang, P.; Bai, J.; Mudassar, S.; Muhammad, I.; Guo, X. Ferulic acid esterase-producing lactic acid bacteria and cellulase pretreatments of corn stalk silage at two different temperatures: Ensiling characteristics, carbohydrates composition and enzymatic saccharification. Bioresour. Technol. 2019, 282, 211–221. [Google Scholar] [CrossRef]
- McGarvey, J.A.; Franco, R.B.; Palumbo, J.D.; Hnasko, R.; Stanker, L.; Mitloehner, F.M. Bacterial population dynamics during the ensiling of Medicago sativa (alfalfa) and subsequent exposure to air. J. Appl. Microbiol. 2013, 114, 1661–1670. [Google Scholar] [CrossRef]
- Xu, Z.; He, H.; Zhang, S.; Kong, J. Effects of inoculants Lactobacillus brevis and Lactobacillus parafarraginis on the fermentation characteristics and microbial communities of corn stover silage. Sci. Rep. 2017, 7, 13614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero, J.J.; Zhao, Y.; Balseca-Paredes, M.A.; Tiezzi, F.; Gutierrez-Rodriguez, E.; Castillo, M.S. Laboratory silo type and inoculation effects on nutritional composition, fermentation, and bacterial and fungal communities of oat silage. J. Dairy Sci. 2017, 100, 1812–1828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallo, A.; Bertuzzi, T.; Giuberti, G.; Moschini, M.; Bruschi, S.; Cerioli, C.; Masoero, F. New assessment based on the use of principal factor analysis to investigate corn silage quality from nutritional traits, fermentation end products and mycotoxins. J. Sci. Food Agric. 2016, 96, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Muck, R.E. Factors influencing silage quality and their implications for management. J. Dairy Sci. 1988, 71, 2992–3002. [Google Scholar] [CrossRef]
- Kung, L.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef]
- Gervasi, T.; Pellizzeri, V.; Calabrese, G.; Di Bella, G.; Cicero, N.; Dugo, G. Production of single cell protein (SCP) from food and agricultural waste by using Saccharomyces cerevisiae. Nat. Prod. Res. 2018, 32, 648–653. [Google Scholar] [CrossRef]
- Yamakawa, S.-i.; Yamada, R.; Tanaka, T.; Ogino, C.; Kondo, A. Repeated fermentation from raw starch using Saccharomyces cerevisiae displaying both glucoamylase and α-amylase. Enzyme Microb. Technol. 2012, 50, 343–347. [Google Scholar] [CrossRef]
- Chaucheyras, F.; Fonty, G.; Gouet, P.; Bertin, G.; Salmon, J.-M. Effects of a strain of Saccharomyces cerevisiae (Levucell® SC), a microbial additive for ruminants, on lactate metabolism in vitro. Can. J. Microbiol. 1996, 42, 927–933. [Google Scholar] [CrossRef]
- Lau, M.W.; Dale, B.E. Cellulosic ethanol production from AFEX-treated corn stover using Saccharomyces cerevisiae 424A(LNH-ST). Proc. Natl. Acad. Sci. USA 2009, 106, 1368–1373. [Google Scholar] [CrossRef]
- Kung, L.; Robinson, J.R.; Ranjit, N.K.; Chen, J.H.; Golt, C.M.; Pesek, J.D. Microbial populations, fermentation end-products, and aerobic stability of corn silage treated with ammonia or a propionic acid-based preservative. J. Dairy Sci. 2000, 83, 1479–1486. [Google Scholar] [CrossRef]
- Filya, I. The effect of Lactobacillus buchneri and Lactobacillus plantarum on the fermentation, aerobic stability, and ruminal degradability of low dry matter corn and sorghum silages. J. Dairy Sci. 2003, 86, 3575–3581. [Google Scholar] [CrossRef]
- Arriola, K.G.; Kim, S.C.; Adesogan, A.T. Effect of applying inoculants with heterolactic or homolactic and heterolactic bacteria on the fermentation and quality of corn silage. J. Dairy Sci. 2011, 94, 1511–1516. [Google Scholar] [CrossRef] [PubMed]

| Genus | Relative Abundance (%) |
|---|---|
| Pseudomonas | 46.70 |
| Pantoea | 14.37 |
| Unclassified | 8.02 |
| Klebsiella | 4.73 |
| Raoultella | 4.22 |
| Enterobacter | 3.15 |
| Stenotrophomonas | 2.59 |
| Sphingomonas | 2.19 |
| Acinetobacter | 1.79 |
| Sphingobacterium | 1.59 |
| Burkholderia | 1.50 |
| Delftia | 0.87 |
| Pectobacterium | 0.79 |
| Asaia | 0.73 |
| Serratia | 0.56 |
| Rhizobium | 0.55 |
| Ochrobactrum | 0.46 |
| Methylobacterium | 0.44 |
| Gluconobacter | 0.43 |
| Tatumella | 0.38 |
| Lactobacillus | 0.35 |
| Curtobacterium | 0.29 |
| Herbaspirillum | 0.25 |
| Lactococcus | 0.24 |
| Brevundimonas | 0.24 |
| Total | 97.45 |
| Item 2 (g/kg) | Days | Treatment 1 | SEM 3 | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| U | S | SL | Treatment | Period | Interaction | |||
| DM | 30 | 269 Aa | 260 Aa | 244 Bb | 2.7 | <0.001 | <0.001 | 0.048 |
| 60 | 263 a | 256 a | 256 a | |||||
| 90 | 223 b | 219 b | 213 c | |||||
| CP | 30 | 65 B | 85 Ab | 80 Aa | 1.2 | <0.001 | <0.001 | <0.001 |
| 60 | 65 C | 80 Ac | 72 Bb | |||||
| 90 | 67 C | 93 Aa | 84 Ba | |||||
| NH3-N/TN | 30 | 55.8 C | 138.6 Bb | 177.2 Ab | 5.08 | <0.001 | <0.001 | 0.001 |
| 60 | 63.8 B | 171.0 Aa | 177.4 Ab | |||||
| 90 | 73.3 C | 177.7 Ba | 209.9 Aa | |||||
| Starch | 30 | 74.1 Aab | 72.6 Ba | 73.6 Aa | 0.18 | <0.001 | <0.001 | 0.021 |
| 60 | 74.8 Aa | 72.8 Ba | 73.1 Ba | |||||
| 90 | 72.9 Ab | 71.6 Bb | 72.1 ABb | |||||
| WSCs | 30 | 14 Ac | 4 B | 2 Bb | 0.9 | <0.001 | <0.001 | <0.001 |
| 60 | 21 Ab | 7 B | 8 Ba | |||||
| 90 | 32 Aa | 6 B | 9 Ba | |||||
| NDF | 30 | 559 Ca | 592 Ba | 626 Aa | 7.8 | <0.001 | <0.001 | 0.072 |
| 60 | 571 Ba | 610 Aa | 629 Aa | |||||
| 90 | 451 Bb | 481 Bb | 548 Ab | |||||
| ADF | 30 | 373 a | 430 a | 412 a | 10.2 | <0.001 | <0.001 | 0.101 |
| 60 | 389 Ba | 424 Aa | 429 Aa | |||||
| 90 | 288 Cb | 328 Bb | 363 Ab | |||||
| Hemicelluloses | 30 | 187 | 161 ab | 214 | 9.2 | 0.001 | 0.003 | 0.450 |
| 60 | 182 | 186 a | 200 | |||||
| 90 | 163 AB | 153 Bb | 185 A | |||||
| Item | Days | Treatment 1 | SEM 2 | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| U | S | SL | Treatment | Period | Interaction | |||
| pH | 30 | 3.57 C | 4.42 B | 4.56 A | 0.021 | <0.001 | 0.042 | 0.003 |
| 60 | 3.66 C | 4.45 B | 4.57 A | |||||
| 90 | 3.70 C | 4.37 B | 4.58 A | |||||
| Acetate [g/kg] | 30 | 10.5 Bb | 18.3 Ab | 21.1 Ab | 1.36 | <0.001 | <0.001 | <0.001 |
| 60 | 19.6 Aa | 8.7 Bc | 7.2 Bc | |||||
| 90 | 8.6 Bb | 29.6 Aa | 28.8 Aa | |||||
| Propionate [g/kg] | 30 | 1.1 Ba | 1.7 Aa | 1.8 Aa | 0.12 | 0.028 | <0.001 | <0.001 |
| 60 | 1.0 Aa | 0.3 Bc | 0.3 Bb | |||||
| 90 | 0.4 Cb | 0.9 Bb | 1.5 Aa | |||||
| Butyrate [g/kg] | 30 | 0.5 | 0.7 a | 0.7 b | 0.26 | <0.001 | <0.001 | <0.001 |
| 60 | 0.5 A | 0.2 Bb | 0.2 Bc | |||||
| 90 | 0.2 B | 0.4 Bab | 10.6 Aa | |||||
| VFAs [g/kg]3 | 30 | 12.1 Bb | 20.6 Ab | 23.6 Ab | 1.59 | <0.001 | <0.001 | <0.001 |
| 60 | 21.2 Aa | 9.2 Bc | 7.7 Bc | |||||
| 90 | 9.2 Bb | 30.9 Aa | 40.9 Aa | |||||
| Lactate [g/kg] | 30 | 64.2 A | 43.0 Bb | 44.5 Bab | 2.57 | <0.001 | 0.001 | 0.222 |
| 60 | 56.8 A | 44.4 Bb | 41.0 Bb | |||||
| 90 | 63.9 A | 55.9 ABa | 50.7 Ba | |||||
| Lactate:acetate ratio | 30 | 5.6 Aa | 2.1 Bb | 1.9 Bb | 0.08 | <0.001 | 0.014 | <0.001 |
| 60 | 2.7 Bb | 4.7 Aa | 5.3 Aa | |||||
| 90 | 6.9 Aa | 1.8 Ba | 1.7 Bb | |||||
| Ethanol [g/kg] | 30 | 0.9 B | 16.5 Ab | 12.0 A | 3.00 | <0.001 | 0.019 | 0.269 |
| 60 | 1.8 C | 24.7 Aa | 16.6 B | |||||
| 90 | 1.2 B | 34.8 Aa | 24.7 A | |||||
| V-score | 30 | 91.5 A | 61.5 Ba | 45.7 Ca | 2.22 | <0.001 | <0.001 | <0.001 |
| 60 | 90.3 A | 52.7 Bb | 49.8 Ca | |||||
| 90 | 93.0 A | 46.5 Bb | 30.7 Bb | |||||
| Aerobic stability [h] | 90 | 106.8 A | 53.4 B | 124.8 A | 11.48 | 0.011 | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Ouyang, Z.; Zhang, X.; Wei, Y.; Tang, S.; Ma, Z.; Tan, Z.; Zhu, N.; Teklebrhan, T.; Han, X. Sweet Corn Stalk Treated with Saccharomyces Cerevisiae Alone or in Combination with Lactobacillus Plantarum: Nutritional Composition, Fermentation Traits and Aerobic Stability. Animals 2019, 9, 598. https://doi.org/10.3390/ani9090598
Zhou X, Ouyang Z, Zhang X, Wei Y, Tang S, Ma Z, Tan Z, Zhu N, Teklebrhan T, Han X. Sweet Corn Stalk Treated with Saccharomyces Cerevisiae Alone or in Combination with Lactobacillus Plantarum: Nutritional Composition, Fermentation Traits and Aerobic Stability. Animals. 2019; 9(9):598. https://doi.org/10.3390/ani9090598
Chicago/Turabian StyleZhou, Xiaoling, Zhu Ouyang, Xiaoli Zhang, Yuqing Wei, Shaoxun Tang, Zhiyuan Ma, Zhiliang Tan, Nong Zhu, Tsegay Teklebrhan, and Xuefeng Han. 2019. "Sweet Corn Stalk Treated with Saccharomyces Cerevisiae Alone or in Combination with Lactobacillus Plantarum: Nutritional Composition, Fermentation Traits and Aerobic Stability" Animals 9, no. 9: 598. https://doi.org/10.3390/ani9090598





