Increasing River Temperature Shifts Impact the Yangtze Ecosystem: Evidence from the Endangered Chinese Sturgeon
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
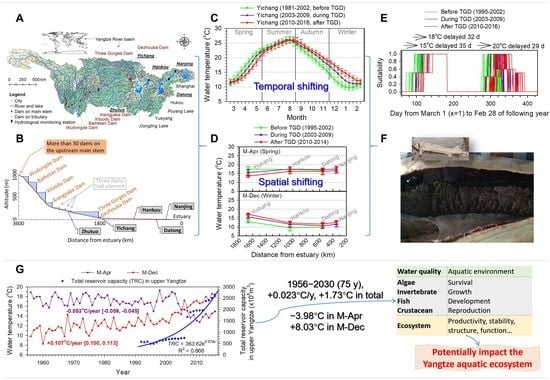
2.1. Study Area
2.2. Water Temperature Measurement
2.3. Assessing the Maturation Process of Spawners
2.4. Description of the Thermal Regime
2.5. Temperature Suitability Evaluation
2.6. Estimating Reservoir Capacity
2.7. Statistical Analysis
3. Results
3.1. River Temperature Shifts
3.2. Maturation of Breeding Population in Relation to the Thermal Regime
3.3. Spawning Delay with the Altered Temperature Regime
3.4. Future Trends
4. Discussion
4.1. Limitations of the Temperature Profile Assessment Method
4.2. Gonad Development and Temperature Regime
4.3. Spawning Timing and Temperature Regime
4.4. Spawning Activity in 2013−2016 and Water Temperature
4.5. Implications for Future River Management
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Caissie, D. The thermal regime of rivers: A review. Freshw. Biol. 2006, 51, 1389–1406. [Google Scholar] [CrossRef]
- Hester, E.T.; Doyle, M.W. Human impacts to river temperature and their effects on biological processes: A quantitative synthesis. JAWRA 2011, 47, 571–587. [Google Scholar] [CrossRef]
- Ellis, L.E.; Jones, N.E. Longitudinal trends in regulated rivers: A review and synthesis within the context of the serial discontinuity concept. Environ. Rev. 2013, 21, 136–148. [Google Scholar] [CrossRef]
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Reidy Liermann, C.; et al. Global threats to human water security and river biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.S.; Wen, L.D.; Li, L.F. Yangtze Conservation and Development Report 2007; Changjiang Press: Wuhan, China, 2007. [Google Scholar]
- Yang, G.S.; Ma, C.D.; Chang, S.Y. Yangtze Conservation and Development Report 2009; Changjiang Press: Wuhan, China, 2009. [Google Scholar]
- Yang, G.S.; Zhu, C.Q.; Jiang, Z.G. Yangtze Conservation and Development Report 2011; Changjiang Press: Wuhan, China, 2011. [Google Scholar]
- Ye, S.W.; Li, Z.J.; Liu, J.S.; Zhang, T.L.; Xie, S.G. Distribution, endemism and conservation status of fishes in the Yangtze River basin, China. In Ecosystems Biodiversity; Grillo, O., Ed.; InTech: Rijeka, Croatia, 2011; pp. 41–66. [Google Scholar]
- Qiu, J. Trouble on the Yangtze. Science 2012, 336, 288–291. [Google Scholar] [CrossRef] [PubMed]
- YARSG (The Yangtze Aquatic Resources Survey Group). The Biology of the Sturgeons and Paddlefish in the Yangtze River and Their Artificial Propagation; Sichuan Scientific and Technical Publishing House: Chengdu, China, 1988. [Google Scholar] [CrossRef]
- Wei, Q.W.; Ke, F.E.; Zhang, J.M.; Zhuang, P.; Luo, J.D.; Zhou, R.Q.; Yang, W.H. Biology, fisheries, and conservation of sturgeons and paddlefish in China. Environ. Biol. Fish. 1997, 48, 241–255. [Google Scholar] [CrossRef]
- Gao, X.; Brosse, S.; Chen, Y.B.; Lek, S.; Chang, J.B. Effects of damming on population sustainability of Chinese sturgeon, Acipenser sinensis: Evaluation of optimal conservation measures. Environ. Biol. Fish. 2009, 86, 325–336. [Google Scholar] [CrossRef]
- Wei, Q.W.; Kynard, B.; Yang, D.G.; Chen, X.H.; Du, H.; Shen, L.; Zhang, H. Using drift nets to capture early life stages and monitor spawning of the Yangtze River Chinese sturgeon (Acipenser sinensis). J. Appl. Ichthyol. 2009, 25 (Suppl. 2), 100–106. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, Q.W.; Du, H. A bedform morphology hypothesis for spawning areas of Chinese sturgeon. Environ. Biol. Fish. 2009, 84, 199–208. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, Q.W.; Kynard, B.E.; Du, H.; Yang, D.G.; Chen, X.H. Spatial structure and bottom characteristics of the only remaining spawning area of Chinese sturgeon in the Yangtze River. J. Appl. Ichthyol. 2011, 27, 251–256. [Google Scholar] [CrossRef]
- Qiao, Y.; Tang, X.; Brosse, S.; Chang, J. Chinese sturgeon (Acipenser sinensis) in the Yangtze River: A hydroacoustic assessment of fish location and abundance on the last spawning ground. J. Appl. Ichthyol. 2006, 22 (Suppl. 1), 140–144. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, D.G.; Wei, Q.W.; Du, H.; Wang, C.Y. Spatial distribution and spawning stock estimates for adult Chinese sturgeon (Acipenser sinensis Gray, 1835) around the only remaining spawning ground during the trial operation of the newly constructed Three Gorges Project in the Yangtze River, China. J. Appl. Ichthyol. 2013, 29, 1436–1440. [Google Scholar] [CrossRef]
- Zhu, B.; Zhou, F.; Cao, H.; Shao, Z.; Zhao, N.; May, B.; Chang, J. Analysis of genetic variation in the Chinese sturgeon, Acipenser sinensis: Estimating the contribution of artificially produced larvae in a wild population. J. Appl. Ichthyol. 2002, 18, 301–306. [Google Scholar] [CrossRef]
- Yang, D.G.; Wei, Q.W.; Wang, K.; Chen, X.H.; Zhu, Y.J. Downstream migration of tag-released juvenile Chinese sturgeon (Acipenser sinensis) in the Yangtze River. Acta Hydrobiol. Sin. 2005, 29, 26–30. [Google Scholar]
- Wu, J.M.; Wang, C.Y.; Zhang, H.; Du, H.; Liu, Z.G.; Shen, L.; Wei, Q.W.; Rosenthal, H. Drastic decline in spawning activity of Chinese sturgeon Acipenser sinensis Gray 1835 in the remaining spawning ground of the Yangtze River since the construction of hydrodams. J. Appl. Ichthyol. 2015, 31, 839–842. [Google Scholar] [CrossRef]
- Gao, X.; Lin, P.; Li, M.; Duan, Z.; Liu, H. Impact of the Three Gorges Dam on the spawning stock and natural reproduction of Chinese sturgeon in Changjiang River, China. Chin. J. Oceanol. Limnol. 2016, 34, 894–901. [Google Scholar] [CrossRef]
- Wang, N.; Teletchea, F.; Kestemont, P.; Milla, S.; Fontaine, P. Photothermal control of the reproductive cycle in temperate fishes. Rev. Aquacul. 2010, 2, 209–222. [Google Scholar] [CrossRef]
- Chang, T.; Gao, X.; Danley, P.D.; Lin, P.; Li, M.; Liu, H. Longitudinal and temporal water temperature patterns in the Yangtze River and its influence on spawning of the Chinese sturgeon (Acipenser sinensis Gray 1835). River Res. Appl. 2017, 33, 1445–1451. [Google Scholar] [CrossRef]
- Huang, Z.L.; Wang, L.H. Yangtze dams increasingly threaten the survival of the Chinese sturgeon. Curr. Biol. 2018, 28, 3640–3647. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, P.; Wang, C.; Yu, Y.; Kong, N. Potential causes of habitat degradation and spawning time delay of the Chinese sturgeon (Acipenser sinensis). Ecol. Inform. 2018, 43, 96–105. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, D.H.; Tang, D.M.; Guo, B.F. Structural and quality changes in the spawning stock of the Chinese sturgeon Acipenser sinensis below the Gezhouba Dam (Yangtze River). J. Appl. Ichthyol. 2006, 22 (Suppl. 1), 111–115. [Google Scholar]
- Chebanov, M.S.; Galich, E.V. Sturgeon Hatchery Manual; FAO Fisheries and Aquaculture Technical Paper, No. 558; FAO: Rome, Italy, 2013. [Google Scholar]
- TNC (The Nature Conservancy). Indicators of Hydrologic Alteration Version 7.1 User’s Manual. 2009. Available online: https://www.conservationgateway.org/ConservationPractices/Freshwater/EnvironmentalFlows/MethodsandTools/IndicatorsofHydrologicAlteration/Documents/IHAV7.pdf (accessed on 9 July 2019).
- Wei, Q.W. Reproductive Behavioral Ecology of Chinese Sturgeon (Acipenser sinensis Gray) with its Stock Assessment. Ph.D. Thesis, Institute of Hydrobiology, CAS, Wuhan, China, 2003. Available online: http://ir.ihb.ac.cn/handle/342005/19195.
- Zhang, H.; Wu, J.M.; Wang, C.Y.; Du, H.; Liu, Z.G.; Shen, L.; Chen, D.; Wei, Q.W. River temperature variations and potential effects on fish in a typical Yangtze River reach: Implications for management. Appl. Ecol. Environ. Res. 2016, 14, 553–567. [Google Scholar] [CrossRef]
- Wang, J.; Li, C.; Duan, X.; Chen, D.; Feng, S.; Luo, H.; Peng, Q.; Liao, W. Variation in the significant environmental factors affecting larval abundance of four major Chinese carp species: Fish spawning response to the Three Gorges Dam. Freshw. Biol. 2014, 59, 1343–1360. [Google Scholar] [CrossRef]
- Yi, B.; Yu, Z.; Liang, Z. Gezhouba Hydraulic Project and Four Major Chinese Carps in the Yangtze River; Hubei Science and Technology Press: Wuhan, China, 1988. [Google Scholar]
- Chen, J. Evolution and Water Resources Utilization in the Yangtze River; Changjiang Press: Wuhan, China, 2012. [Google Scholar]
- Wang, C.Y.; Wei, Q.W.; Kynard, B.; Du, H.; Zhang, H. Migrations and movements of adult Chinese sturgeon Acipenser sinensis in the Yangtze River, China. J. Fish Biol. 2012, 81, 696–713. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.F.; Chang, J.B.; Xiao, H.; Zhu, B.; Wan, J.Y.; Tian, J.Y.; Shu, D.B. The reproductive biology of first filial generation of Acipenser sinensis growing up in the freshwater environment. Acta Hydrobiol. Sin. 2011, 35, 940–945. [Google Scholar]
- Wei, Q.W.; Li, L.X.; Du, H.; Zhang, X.Y.; Xiong, W.; Zhang, H.; Shen, L.; Wu, J.M.; Zhang, S.H.; Wang, C.Y.; et al. Research on technology for controlled propagation of cultured Chinese sturgeon (Acipenser sinensis). J. Fish. Sci. China 2013, 20, 1–11. [Google Scholar] [CrossRef]
- SGFRYR (Survey group on fishery resources in the Yangtze River). Fishery Resources of Yangtze River System; China Ocean Press: Beijing, China, 1990. [Google Scholar]
- Kynard, B.; Bronzi, P.; Rosenthal, H. Life History and Behaviour of Connecticut River Shortnose and Other Sturgeons; No. 4; World Sturgeon Conservation Society Special Publication: Norderstedt, Germany, 2012. [Google Scholar]
- Zhao, F.; Zhuang, P.; Zhang, T.; Xu, J.M.; Liu, J.Y.; Zhang, L.Z.; Wang, M.; Shi, Q. New timing record of juvenile Acipenser sinensis appearing in the Yangtze Estuary. Mar. Fish. 2015, 37, 288–292. [Google Scholar]
- Zhang, S.H.; Yang, H.C.; Xin, M.M.; Wu, J.M.; Dai, Z.G.; Du, H.; Liu, Z.G.; Wei, Q.W. External morphology and molecular identification of wild juvenile Acipenser sinensis newly found in the Jiangsu Xupu section of the Yangtze River. J. Fish. Sci. China 2016, 23, 1–9. [Google Scholar]
- Wu, J.M.; Wang, C.Y.; Zhang, S.H.; Zhang, H.; Du, H.; Liu, Z.G.; Wei, Q.W. From continuous to occasional: Small-scale natural reproduction of Chinese sturgeon occurred in the Gezhouba spawning ground, Yichang, China. J. Fish. Sci. China 2017, 24, 425–431. [Google Scholar] [CrossRef]
- Dettlaff, T.A.; Ginsburg, A.S.; Schmalhausen, O.I. Sturgeon Fishes: Developmental Biology and Aquaculture; Springer: Berlin, Germany, 1993. [Google Scholar]
- CWRPI (Changjiang Water Resources Protection Institute). Environmental Impact Report of Wudongde Hydropower Station in the Jinsha River; CWRPI: Wuhan, China, 2014. [Google Scholar]
- Guo, W.X.; Xia, Z.Q.; Wang, H.X.; Zhang, Y.X. Multiple-scale analysis on water temperature variation of Yichang Hydrological Station in recent 50 years. J. Hydraul. Eng. 2008, 39, 1197–1203. [Google Scholar]
- Olden, J.D.; Naiman, R.J. Incorporating thermal regimes into environmental flows assessments: Modifying dam operations to restore freshwater ecosystem integrity. Freshw. Biol. 2010, 55, 86–107. [Google Scholar] [CrossRef]
- Winemiller, K.O.; McIntyre, P.B.; Castello, L.; Fluet-Chouinard, E.; Giarrizzo, T.; Nam, S.; Baird, I.G.; Darwall, W.; Lujan, N.K.; Harrison, I.; et al. Balancing hydropower and biodiversity in the Amazon, Congo, and Mekong. Science 2016, 351, 128–129. [Google Scholar] [CrossRef] [PubMed]
- Sabo, J.L.; Ruhi, A.; Holtgrieve, G.W.; Elliott, V.; Arias, M.E.; Ngor, P.B.; Räsänen, T.A.; Nam, S. Designing river flows to improve food security futures in the Lower Mekong Basin. Science 2017, 358, eaao1053. [Google Scholar] [CrossRef] [PubMed]
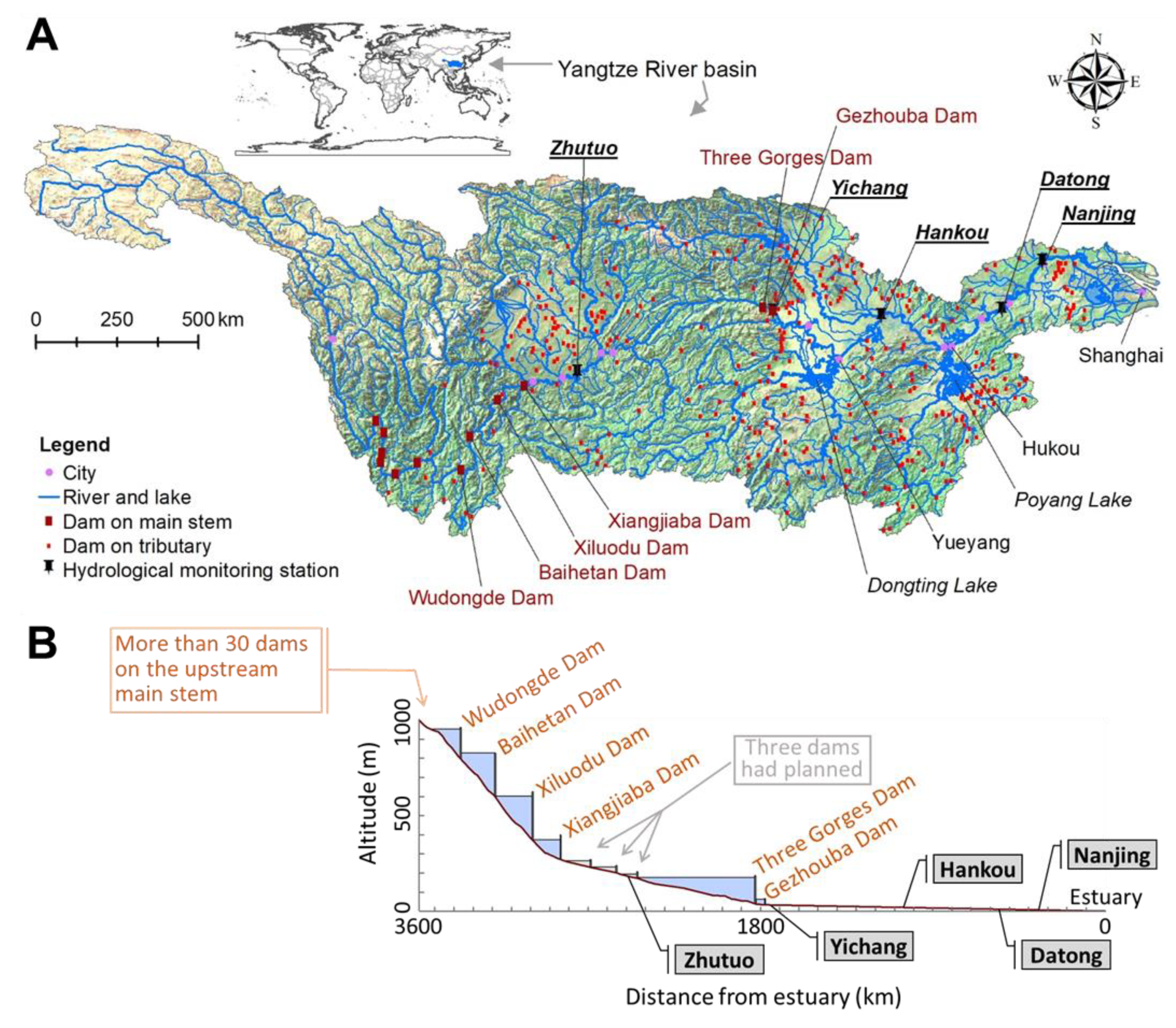
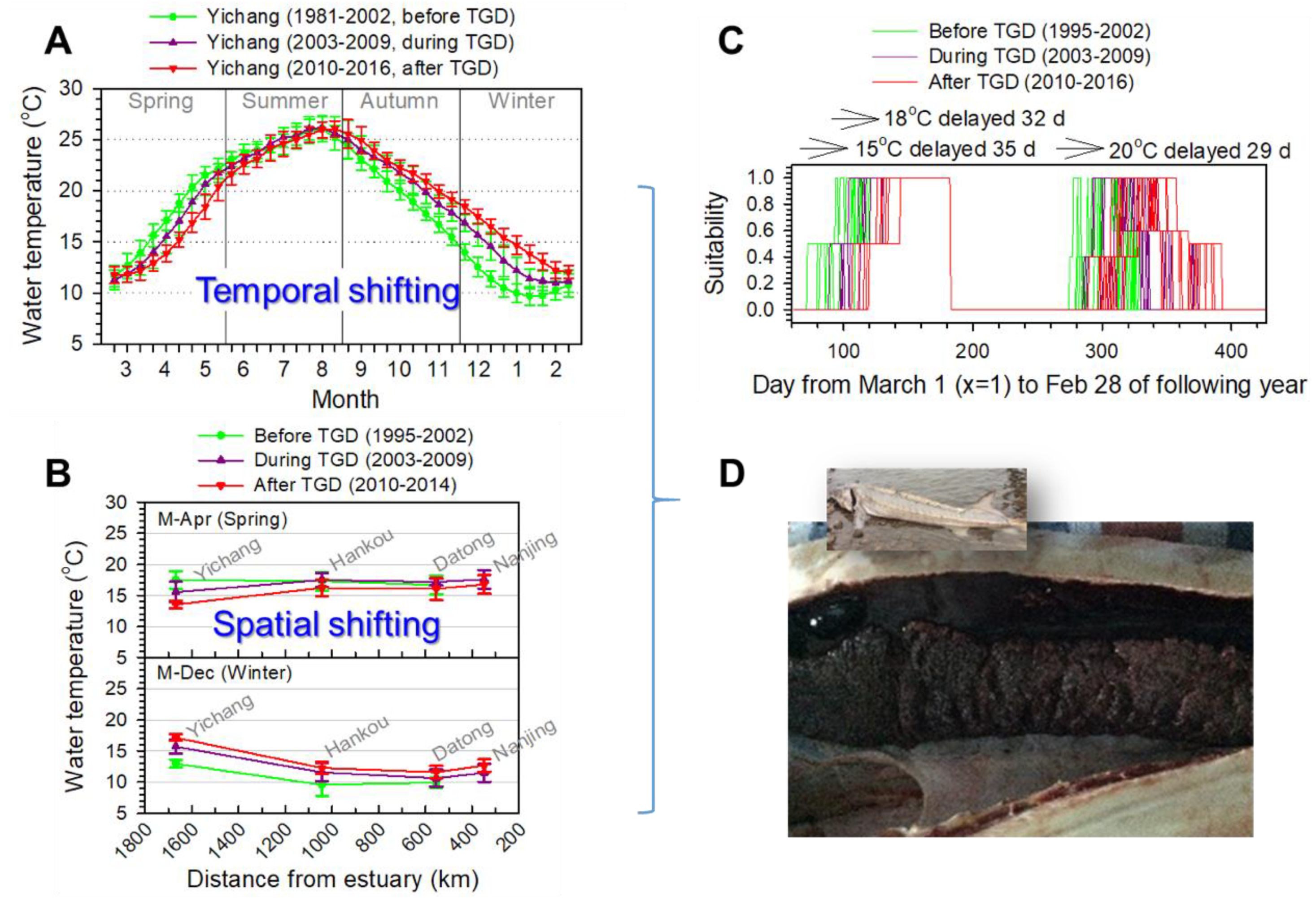
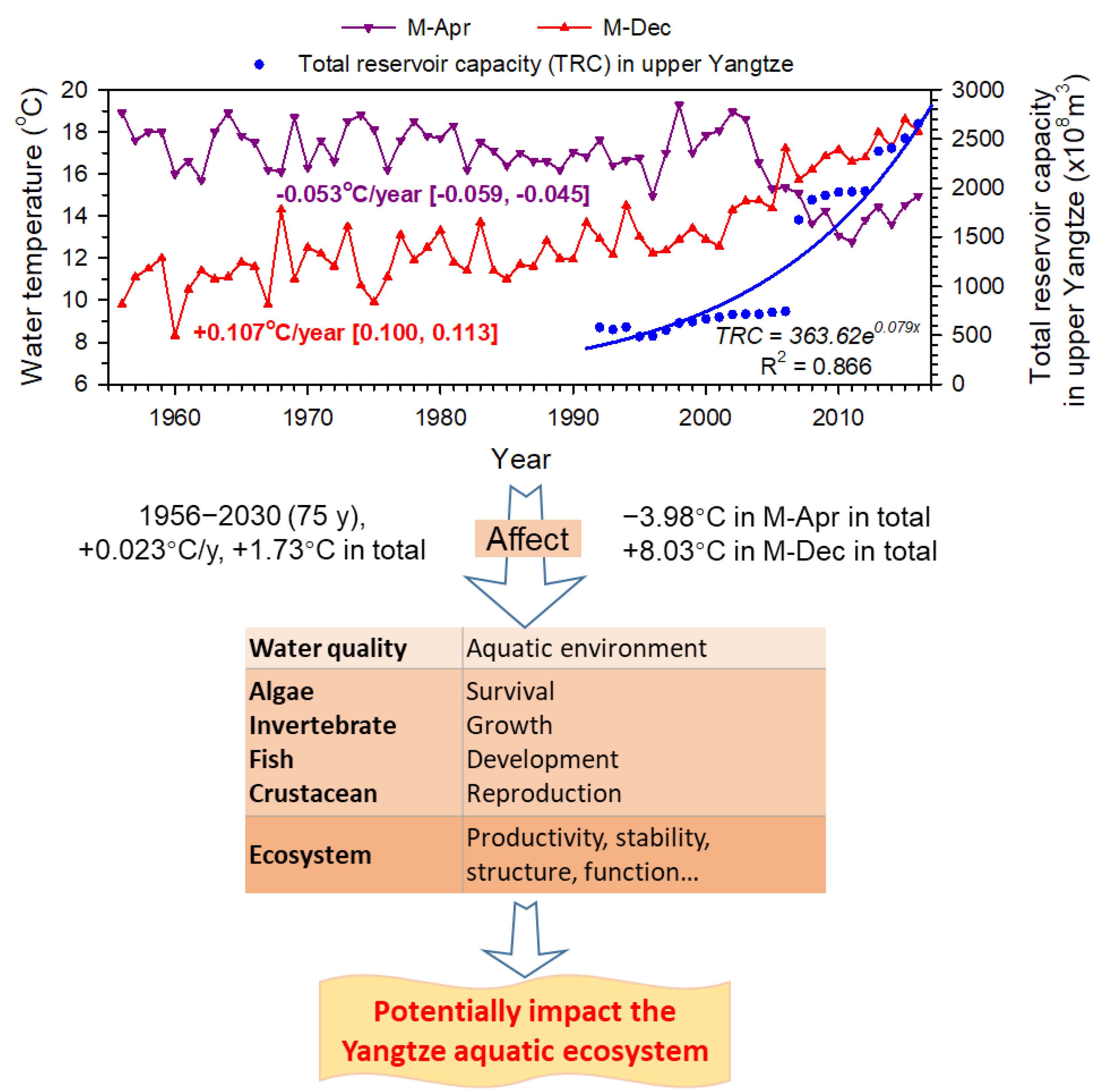
| Indicator Group | Temperature Regime Indicator | Reason for Indicator Definition |
|---|---|---|
| (1) Magnitude of monthly water temperature amplitude | Mean temperature for each calendar month, from November to October, subtotal 12 parameters | The adults need about one year to reach maturity, which month is important as trigger is unknown. |
| (2) High temperature | Annual maximum temperature: 1-day mean, 3-day means, 7-day means, 30-day means, 90-day means | To see whether the high temperature and duration of that are related with gonad development. |
| (3) Low temperature | Annual minimum temperature: 1-day mean, 3-day means, 7-day means, 30-day means, 90-day means | To test whether the low temperature and duration of that are related to gonad development. |
| (4) Cumulative temperature | The sum of temperature from November 1 to October 31 of the following year | To see the cumulative effects of temperature on gonad development. |
| (5) Timing of extreme temperature | Days of 1-day maximum and 1-day minimum from November 1 to October 31 | To see whether the occurrence time of extreme temperature is related to gonad development. |
| (6) Relative temperature difference | Base temperature index: 7-day minimum temperature/mean temperature of the year | To see the effects of relative temperature difference on gonad development. This definition refers to the concept of Base flow in IHA (Indicators of Hydrologic Alteration). |
| Indicator Group | Indicator | 1982–2001, 2003 a (n = 21) | 1984–1993 b (n = 10) | 2010–2016 (n = 7) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean ± S.D. | Correlation M (p, 2-Tailed) | Min | Max | Mean ± S.D. | Correlation D (p, 2-Tailed) | Min | Max | Mean ± S.D. | ||
| (1) Magnitude of monthly water temperature | Nov Mean | 15.10 | 17.52 | 16.60 ± 0.65 | 0.429 (0.053) | 15.60 | 16.96 | 16.33 ± 0.49 | 0.298 (0.403) | 18.91 | 20.79 | 19.85 ± 0.61 |
| Dec Mean | 11.41 | 14.16 | 12.60 ± 0.79 | 0.602 ** (0.004) | 11.49 | 13.27 | 12.38 ± 0.65 | −0.288 (0.419) | 16.62 | 18.47 | 17.27 ± 0.71 | |
| Jan Mean | 8.51 | 11.90 | 10.07 ± 0.82 | 0.528 * (0.014) | 8.51 | 10.76 | 9.79 ± 0.73 | −0.441 (0.202) | 13.67 | 16.08 | 14.64 ± 0.87 | |
| Feb Mean | 8.02 | 11.75 | 10.21 ± 0.93 | 0.580 ** (0.006) | 8.02 | 11.23 | 9.85 ± 0.96 | −0.605 (0.064) | 11.44 | 13.49 | 12.47 ± 0.74 | |
| Mar Mean | 10.77 | 14.50 | 12.66 ± 0.97 | 0.514 * (0.017) | 10.77 | 13.82 | 12.29 ± 1.04 | −0.595 (0.069) | 10.59 | 12.79 | 11.93 ± 0.80 | |
| Apr Mean | 15.69 | 18.80 | 17.09 ± 0.68 | 0.362 (0.107) | 16.33 | 17.54 | 17.00 ± 0.43 | −0.468 (0.172) | 12.93 | 14.85 | 14.02 ± 0.72 | |
| May Mean | 19.96 | 22.73 | 21.43 ± 0.70 | 0.205 (0.373) | 19.96 | 22.16 | 21.20 ± 0.67 | 0.186 (0.607) | 17.21 | 20.03 | 18.64 ± 1.08 | |
| Jun Mean | 22.65 | 24.47 | 23.55 ± 0.56 | 0.326 (0.149) | 22.65 | 24.47 | 23.73 ± 0.65 | −0.591 (0.072) | 21.51 | 23.53 | 22.45 ± 0.79 | |
| Jul Mean | 23.45 | 26.43 | 24.57 ± 0.88 | 0.158 (0.494) | 23.53 | 26.43 | 24.66 ± 0.87 | −0.001 (0.998) | 23.31 | 26.25 | 24.61 ± 1.06 | |
| Aug Mean | 24.14 | 28.70 | 25.73 ± 1.05 | 0.348 (0.123) | 24.14 | 26.43 | 25.43 ± 0.84 | 0.285 (0.425) | 24.69 | 26.54 | 25.84 ± 0.67 | |
| Sep Mean | 22.06 | 24.64 | 23.16 ± 0.78 | 0.381 (0.088) | 22.06 | 24.23 | 22.80 ± 0.65 | −0.411 (0.239) | 23.15 | 26.16 | 24.82 ± 1.11 | |
| Oct Mean | 18.85 | 21.52 | 19.95 ± 0.77 | 0.470 * (0.031) | 18.85 | 20.00 | 19.33 ± 0.40 | −0.628 (0.052) | 21.39 | 23.24 | 22.31 ± 0.65 | |
| (2) High temperature | 1-day Max | 25.80 | 29.70 | 27.28 ± 1.03 | 0.302 (0.196) | 26.20 | 28.20 | 27.10 ± 0.76 | −0.217 (0.547) | 26.00 | 27.40 | 26.84 ± 0.53 |
| 3-day Max | 25.57 | 29.50 | 27.07 ± 1.00 | 0.275 (0.241) | 25.87 | 28.07 | 26.88 ± 0.79 | −0.155 (0.669) | 25.93 | 27.33 | 26.80 ± 0.52 | |
| 7-day Max | 25.29 | 29.34 | 26.86 ± 1.03 | 0.294 (0.208) | 25.46 | 27.87 | 26.61 ± 0.83 | −0.090 (0.805) | 25.87 | 27.17 | 26.70 ± 0.51 | |
| 30-day Max | 24.56 | 28.82 | 26.09 ± 1.00 | 0.307 (0.188) | 24.56 | 26.79 | 25.77 ± 0.73 | 0.106 (0.770) | 25.30 | 26.76 | 26.13 ± 0.60 | |
| 90-day Max | 24.07 | 26.76 | 24.89 ± 0.62 | 0.320 (0.169) | 24.07 | 25.50 | 24.76 ± 0.44 | −0.027 (0.940) | 23.94 | 26.15 | 25.16 ± 0.81 | |
| (3) Low temperature | 1-day Min | 7.40 | 10.90 | 9.05 ± 0.96 | 0.584 ** (0.007) | 7.40 | 10.30 | 8.67 ± 0.90 | −0.499 (0.142) | 10.20 | 12.50 | 11.37 ± 0.89 |
| 3-day Min | 7.53 | 10.97 | 9.15 ± 0.94 | 0.568 ** (0.009) | 7.53 | 10.40 | 8.79 ± 0.88 | −0.478 (0.162) | 10.20 | 12.50 | 11.44 ± 0.88 | |
| 7-day Min | 7.64 | 11.04 | 9.29 ± 0.92 | 0.559 * (0.010) | 7.64 | 10.56 | 8.95 ± 0.87 | −0.475 (0.166) | 10.29 | 12.53 | 11.55 ± 0.85 | |
| 30-day Min | 7.88 | 11.55 | 9.67 ± 0.93 | 0.575 ** (0.008) | 7.88 | 10.76 | 9.32 ± 0.87 | −0.531 (0.114) | 10.58 | 12.78 | 11.78 ± 0.79 | |
| 90-day Min | 9.26 | 12.09 | 10.69 ± 0.75 | 0.670 ** (0.001) | 9.26 | 11.21 | 10.31 ± 0.63 | −0.740 * (0.014) | 11.41 | 13.58 | 12.64 ± 0.76 | |
| (4) Cumulative temperature | Sum from Nov 1 to Oct 31 | 6392.7 | 6844.6 | 6637.7 ± 145.8 | 0.830 ** (0.000) | 6446.7 | 6672.9 | 6551.7 ± 77.7 | −0.891 ** (0.001) | 6782.7 | 7250.6 | 6980.0 ± 158.9 |
| (5) Timing of extreme temperature | Days of 1-day max to Oct 31 | 62 | 128 | 85.55 ± 16.69 | 0.348 (0.132) | 62 | 122 | 83.20 ± 17.43 | −0.364 (0.301) | 48 | 86 | 66.14 ± 12.67 |
| Days of 1-day min to Oct 31 | 249 | 289 | 273.55 ± 13.08 | 0.312 (0.181) | 249 | 289 | 271.30 ± 16.75 | −0.364 (0.301) | 228 | 253 | 239.00 ± 8.94 | |
| (6) Relative temperature difference | 7-day min/year mean | 0.43 | 0.59 | 0.51 ± 0.04 | 0.459 * (0.042) | 0.43 | 0.59 | 0.50 ± 0.05 | −0.398 (0.254) | 0.55 | 0.64 | 0.60 ± 0.03 |
| Season | Month | Kendall’s Tau | Trend | p-Value (Two-Tailed) | Sen’s Slope (95% Confidence Interval) |
|---|---|---|---|---|---|
| Spring | March | −0.201 | Yes | 0.022 | −0.017 (−0.021, −0.013) |
| April | −0.414 | Yes | <0.0001 | −0.050 (−0.055, −0.045) | |
| May | −0.203 | Yes | 0.021 | −0.019 (−0.022, −0.014) | |
| Summer | June | −0.146 | No | 0.099 | −0.009 (−0.011, −0.006) |
| July | −0.095 | No | 0.282 | −0.007 (−0.010, −0.004) | |
| August | 0.071 | No | 0.426 | 0.005 (0.001, 0.008) | |
| Autumn | September | 0.284 | Yes | 0.001 | 0.027 (0.022, 0.031) |
| October | 0.497 | Yes | <0.0001 | 0.048 (0.044, 0.053) | |
| November | 0.638 | Yes | <0.0001 | 0.068 (0.063, 0.072) | |
| Winter | December | 0.670 | Yes | <0.0001 | 0.103 (0.096, 0.110) |
| January | 0.701 | Yes | <0.0001 | 0.086 (0.080, 0.093) | |
| February | 0.506 | Yes | <0.0001 | 0.051 (0.047, 0.055) | |
| Year | 0.548 | Yes | <0.0001 | 0.023 (0.022, 0.024) | |
| Season | Month | Rkm 2741.7 (Below Xiangjiaba Dam) | Rkm 2474.2 (Zhutuo, about 100 km to the Tail of Three Gorges Reservoir) |
|---|---|---|---|
| Spring | March | −0.2 | 0.8 |
| April | −3.3 | −1.2 | |
| May | −3.7 | −1.8 | |
| Summer | June | −2.9 | −1.7 |
| July | −1.4 | −1.1 | |
| August | −1.0 | −1.2 | |
| Autumn | September | 0.6 | −0.1 |
| October | 1.3 | 0.2 | |
| November | 3.5 | 1.1 | |
| Winter | December | 5.3 | 2.6 |
| January | 5.5 | 3.8 | |
| February | 2.9 | 3.6 | |
| Year | 0.55 | 0.42 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Kang, M.; Wu, J.; Wang, C.; Li, J.; Du, H.; Yang, H.; Wei, Q. Increasing River Temperature Shifts Impact the Yangtze Ecosystem: Evidence from the Endangered Chinese Sturgeon. Animals 2019, 9, 583. https://doi.org/10.3390/ani9080583
Zhang H, Kang M, Wu J, Wang C, Li J, Du H, Yang H, Wei Q. Increasing River Temperature Shifts Impact the Yangtze Ecosystem: Evidence from the Endangered Chinese Sturgeon. Animals. 2019; 9(8):583. https://doi.org/10.3390/ani9080583
Chicago/Turabian StyleZhang, Hui, Myounghee Kang, Jinming Wu, Chengyou Wang, Junyi Li, Hao Du, Haile Yang, and Qiwei Wei. 2019. "Increasing River Temperature Shifts Impact the Yangtze Ecosystem: Evidence from the Endangered Chinese Sturgeon" Animals 9, no. 8: 583. https://doi.org/10.3390/ani9080583
APA StyleZhang, H., Kang, M., Wu, J., Wang, C., Li, J., Du, H., Yang, H., & Wei, Q. (2019). Increasing River Temperature Shifts Impact the Yangtze Ecosystem: Evidence from the Endangered Chinese Sturgeon. Animals, 9(8), 583. https://doi.org/10.3390/ani9080583