Glucocorticoids and Catecholamines Affect in Vitro Functionality of Porcine Blood Immune Cells
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sampling
2.2. Isolation of Peripheral Blood Mononuclear Cells (PBMC)
2.3. Lymphocyte Proliferation Assay
2.4. Intracellular Cytokine Staining
2.5. Statistical Analysis
3. Results
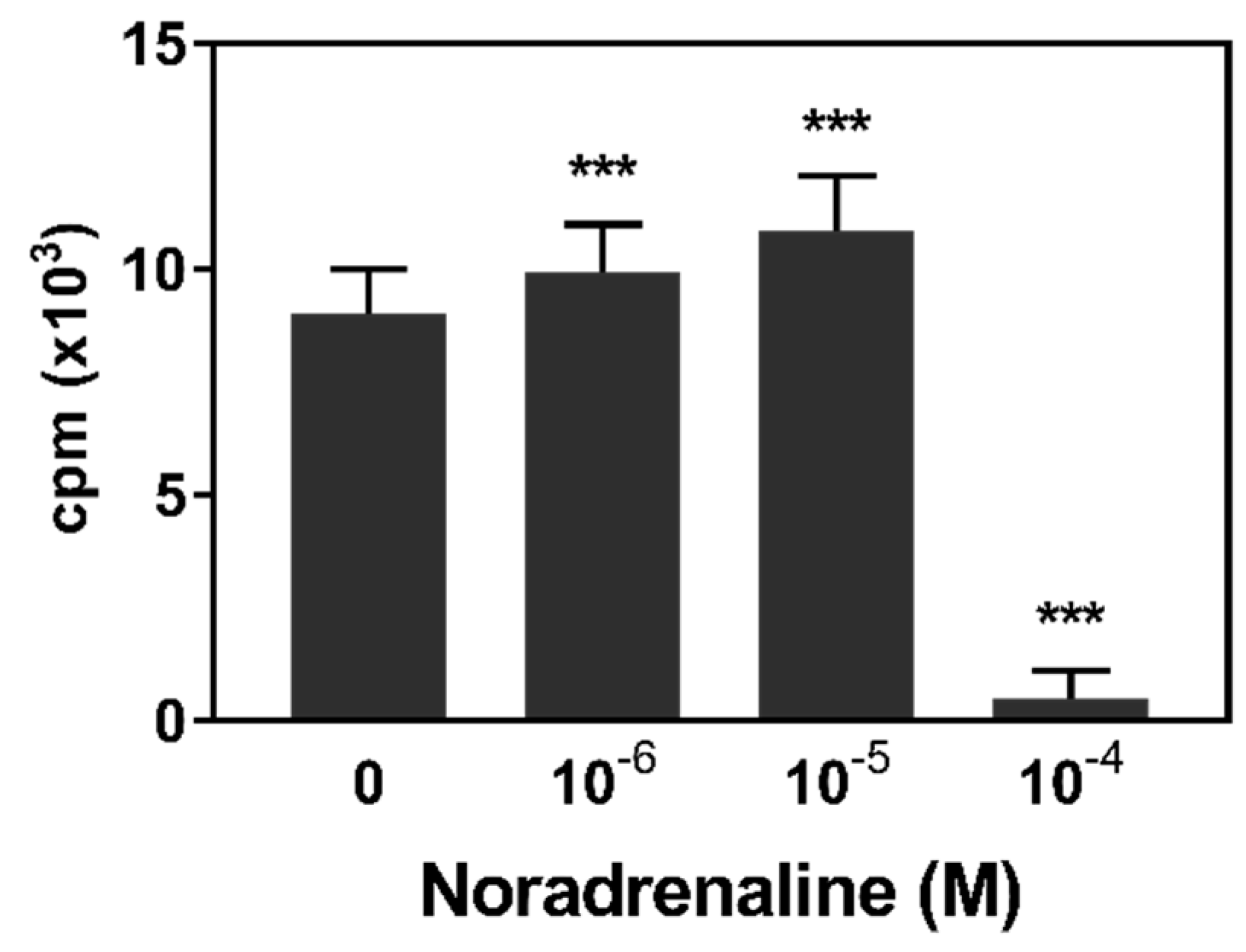
3.1. Lymphocyte Proliferation
3.2. Intracellular Cytokine Staining
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gordan, R.; Gwathmey, J.K.; Xie, L.-H. Autonomic and endocrine control of cardiovascular function. World J. Cardiol. 2015, 7, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Kyrou, I.; Tsigos, C. Stress hormones: Physiological stress and regulation of metabolism. Curr. Opin. Pharmacol. 2009, 9, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Dhabhar, F.S. The short-term stress response—Mother nature’s mechanism for enhancing protection and performance under conditions of threat, challenge, and opportunity. Front. Neuroendocrinol. 2018, 49, 175–192. [Google Scholar] [CrossRef] [PubMed]
- Marsland, A.L.; Bachen, E.A.; Cohen, S.; Rabin, B.; Manuck, S.B. Stress, immune reactivity and susceptibility to infectious disease. Physiol. Behav. 2002, 77, 711–716. [Google Scholar] [CrossRef]
- De Groot, J.; Ruis, M.A.; Scholten, J.W.; Koolhaas, J.M.; Boersma, W.J.A. Long-term effects of social stress on antiviral immunity in pigs. Physiol. Behav. 2001, 73, 145–158. [Google Scholar] [CrossRef]
- Gillis, S.; Crabtree, G.R.; Smith, K.A. Glucocorticoid-induced inhibition of T cell growth factor production. I. The effect on mitogen-induced lymphocyte proliferation. J. Immunol. 1979, 123, 1624–1631. [Google Scholar] [PubMed]
- Strauss, G.; Osen, W.; Debatin, K.-M. Induction of apoptosis and modulation of activation and effector function in T cells by immunosuppressive drugs. Clin. Exp. Immunol. 2002, 128, 255–266. [Google Scholar] [CrossRef]
- Gutsol, A.A.; Sokhonevich, N.A.; Seledtsov, V.I.; Litvinova, L.S. Dexamethasone effects on activation and proliferation of immune memory T cells. Bull. Exp. Biol. Med. 2013, 155, 474–476. [Google Scholar] [CrossRef]
- Ashwell, J.D.; Lu, F.W.; Vacchio, M.S. Glucocorticoids in T cell development and function. Annu. Rev. Immunol. 2000, 18, 309–345. [Google Scholar] [CrossRef]
- Felsner, P.; Hofer, D.; Rinner, I.; Porta, S.; Korsatko, W.; Schauenstein, K. Adrenergic suppression of peripheral blood T cell reactivity in the rat is due to activation of peripheral alpha 2-receptors. J. Neuroimmunol. 1995, 57, 27–34. [Google Scholar] [CrossRef]
- Connor, T.J.; Brewer, C.; Kelly, J.P.; Harkin, A. Acute stress suppresses pro-inflammatory cytokines TNF-alpha and IL-1 beta independent of a catecholamine-driven increase in IL-10 production. J. Neuroimmunol. 2005, 159, 119–128. [Google Scholar] [CrossRef]
- Strahler, J.; Rohleder, N.; Wolf, J.M. Acute psychosocial stress induces differential short-term changes in catecholamine sensitivity of stimulated inflammatory cytokine production. Brain Behav. Immun. 2015, 43, 139–148. [Google Scholar] [CrossRef]
- Kouassi, E.; Li, Y.S.; Boukhris, W.; Millet, I.; Revillard, J.P. Opposite effects of the catecholamines dopamine and norepinephrine on murine polyclonal B-cell activation. Immunopharmacology 1988, 16, 125–137. [Google Scholar] [CrossRef]
- Torres, K.C.L.; Antonelli, L.R.V.; Souza, A.L.S.; Teixeira, M.M.; Dutra, W.O.; Gollob, K.J. Norepinephrine, dopamine and dexamethasone modulate discrete leukocyte subpopulations and cytokine profiles from human PBMC. J. Neuroimmunol. 2005, 166, 144–157. [Google Scholar] [CrossRef]
- Elenkov, I.J.; Wilder, R.L.; Chrousos, G.P.; Vizi, E.S. The Sympathetic Nerve—An Integrative Interface between Two Supersystems: The Brain and the Immune System. Pharmacol. Rev. 2000, 52, 595–638. [Google Scholar]
- Kick, A.R.; Tompkins, M.B.; Almond, G.W. Stress and immunity in the pig. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2011, 6, 1–17. [Google Scholar] [CrossRef]
- Deguchi, E.; Akuzawa, M. Effects of fighting after grouping on plasma cortisol concentration and lymphocyte blastogenesis of peripheral blood mononuclear cells induced by mitogens in piglets. J. Vet. Med. Sci. 1998, 60, 149–153. [Google Scholar] [CrossRef]
- Li, L.-A.; Xia, D.; Bao, E.-D.; Wei, S.; Xiao, J.-S.; Bao, J.-W.; Chen, W.-H.; Chen, J.; Hartung, J.; Zhao, R.-Q. Erhualian and Pietrain pigs exhibit distinct behavioral, endocrine and biochemical responses during transport. Livest. Sci. 2008, 113, 169–177. [Google Scholar] [CrossRef]
- Rosochacki, S.J.; Piekarzewska, A.B.; Poloszynowicz, J.; Sakowski, T. The Influence of Restraint Immobilization Stress on the Concentration of Bioamines and Cortisol in Plasma of Pietrain and Duroc Pigs. J. Vet. Med. Physiol. Pathol. Clin. Med. 2000, 47, 231–242. [Google Scholar] [CrossRef]
- Althen, T.G.; Ono, K.; Topel, D.G. Effect of Stress Susceptibility or Stunning Method on Catecholamine Levels in Swine. J. Anim. Sci. 1977, 44, 985–989. [Google Scholar] [CrossRef]
- Bacou, E.; Haurogné, K.; Mignot, G.; Allard, M.; de Beaurepaire, L.; Marchand, J.; Terenina, E.; Billon, Y.; Jacques, J.; Bach, J.-M.; et al. Acute social stress-induced immunomodulation in pigs high and low responders to ACTH. Physiol. Behav. 2017, 169, 1–8. [Google Scholar] [CrossRef]
- Kanitz, E.; Tuchscherer, M.; Puppe, B.; Tuchscherer, A.; Stabenow, B. Consequences of repeated early isolation in domestic piglets (Sus scrofa) on their behavioural, neuroendocrine, and immunological responses. Brain Behav. Immun. 2004, 18, 35–45. [Google Scholar] [CrossRef]
- Blecha, F.; Pollmann, D.S.; Nichols, D.A. Weaning pigs at an early age decreases cellular immunity. J. Anim. Sci. 1983, 56, 396–400. [Google Scholar] [CrossRef]
- Westly, H.J.; Kelley, K.W. Physiologic concentrations of cortisol suppress cell-mediated immune events in the domestic pig. Proc. Soc. Exp. Biol. Med. 1984, 177, 156–164. [Google Scholar] [CrossRef]
- Ciepielewski, Z.M.; Stojek, W.; Glac, W.; Wrona, D. Restraint effects on stress-related hormones and blood natural killer cell cytotoxicity in pigs with a mutated ryanodine receptor. Domest. Anim. Endocrinol. 2013, 44, 195–203. [Google Scholar] [CrossRef]
- Tuchscherer, M.; Kanitz, E.; Puppe, B.; Tuchscherer, A.; Viergutz, T. Changes in endocrine and immune responses of neonatal pigs exposed to a psychosocial stressor. Res. Vet. Sci. 2009, 87, 380–388. [Google Scholar] [CrossRef]
- Jozsa, R.; Olah, A.; Cornélissen, G.; Csernus, V.; Otsuka, K.; Zeman, M.; Nagy, G.; Kaszaki, J.; Stebelova, K.; Csokas, N.; et al. Circadian and extracircadian exploration during daytime hours of circulating corticosterone and other endocrine chronomes. Biomed. Pharmacother. 2005, 59, S109–S116. [Google Scholar] [CrossRef]
- Ruis, M.A.; Te Brake, J.H.; Engel, B.; Ekkel, E.D.; Buist, W.G.; Blokhuis, H.J.; Koolhaas, J.M. The circadian rhythm of salivary cortisol in growing pigs: Effects of age, gender, and stress. Physiol. Behav. 1997, 62, 623–630. [Google Scholar] [CrossRef]
- Engert, L.C.; Weiler, U.; Pfaffinger, B.; Stefanski, V.; Schmucker, S.S. Diurnal rhythms in peripheral blood immune cell numbers of domestic pigs. Dev. Comp. Immunol. 2018, 79, 11–20. [Google Scholar] [CrossRef]
- Griffin, J.F. Stress and immunity: A unifying concept. Vet. Immunol. Immunopathol. 1989, 20, 263–312. [Google Scholar] [CrossRef]
- Roth, J.A.; Flaming, K.P. Model systems to study immunomodulation in domestic food animals. Adv. Vet. Sci. Comp. Med. 1990, 35, 21–41. [Google Scholar]
- Kanitz, E.; Otten, W.; Nürnberg, G.; Brüssow, K.P. Effects of age and maternal reactivity on the stress response of the pituitary-adrenocortical axis and the sympathetic nervous system in neonatal pigs. Anim. Sci. 1999, 68, 519–526. [Google Scholar] [CrossRef]
- Kraetzl, W.D.; Weiler, U. Erfahrungen mit einem implantierbaren Kathetersystem zur frequenten und chronischen Blutentnahme bei Schafen in Gruppenhaltung und bei säugenden Sauen. Tierarztl. Umsch. 1998, 53, 567–574. [Google Scholar]
- Schalk, C.; Pfaffinger, B.; Schmucker, S.; Weiler, U.; Stefanski, V. Effects of repeated social mixing on behavior and blood immune cells of group-housed pregnant sows (Sus scrofa domestica). Livest. Sci. 2018, 217, 148–156. [Google Scholar] [CrossRef]
- Felten, D.L.; Felten, S.Y.; Bellinger, D.L.; Carlson, S.L.; Ackerman, K.D.; Madden, K.S.; Olschowki, J.A.; Livnat, S. Noradrenergic sympathetic neural interactions with the immune system: Structure and function. Immunol. Rev. 1987, 100, 225–260. [Google Scholar] [CrossRef]
- Bergquist, J.; Tarkowski, A.; Ewing, A.; Ekman, R. Catecholaminergic suppression of immunocompetent cells. Immunol. Today 1998, 19, 562–567. [Google Scholar] [CrossRef]
- Kenward, M.G.; Roger, J.H. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 1997, 53, 983–997. [Google Scholar] [CrossRef]
- Faraway, J.J. Extending the Linear Model with R: Generalized Linear, Mixed Effects and Nonparametric Regression Models; Chapman & Hall/CRC: Boca Raton, FL, USA, 2006. [Google Scholar]
- Roess, D.A.; Bellone, C.J.; Ruh, M.F.; Nadel, E.M.; Ruh, T.S. The effect of glucocorticoids on mitogen-stimulated B-lymphocytes: Thymidine incorporation and antibody secretion. Endocrinology 1982, 110, 169–175. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [CrossRef]
- Hadden, J.W.; Hadden, E.M.; Middleton, E. Lymphocyte blast transformation. I. Demonstration of adrenergic receptors in human peripheral lymphocytes. Cell. Immunol. 1970, 1, 583–595. [Google Scholar] [CrossRef]
- Kohm, A.; Sanders, V.M. Norepinephrine: A messenger from the brain to the immune system. Trends Immunol. 2000, 21, 539–542. [Google Scholar] [CrossRef]
- Gerner, W.; Talker, S.C.; Koinig, H.C.; Sedlak, C.; Mair, K.H.; Saalmüller, A. Phenotypic and functional differentiation of porcine αβ T cells: Current knowledge and available tools. Mol. Immunol. 2015, 66, 3–13. [Google Scholar] [CrossRef]
- Murtaugh, M.P.; Johnson, C.R.; Xiao, Z.; Scamurra, R.W.; Zhou, Y. Species specialization in cytokine biology: Is interleukin-4 central to the TH1–TH2 paradigm in swine? Dev. Comp. Immunol. 2009, 33, 344–352. [Google Scholar] [CrossRef]
- Skjolaas, K.A.; Grieger, D.M.; Hill, C.M.; Minton, J.E. Glucocorticoid regulation of type 1 and type 2 cytokines in cultured porcine splenocytes. Vet. Immunol. Immunopathol. 2002, 87, 79–87. [Google Scholar] [CrossRef]
- Amadori, M.; Cristiano, A.; Ferrari, M. Constitutive expression of interferons in swine leukocytes. Res. Vet. Sci. 2010, 88, 64–71. [Google Scholar] [CrossRef]
- Sutherland, M.A.; Niekamp, S.R.; Johnson, R.W.; van Alstine, W.G.; Salak-Johnson, J.L. Heat and social rank impact behavior and physiology of PRRS-virus-infected pigs. Physiol. Behav. 2007, 90, 73–81. [Google Scholar] [CrossRef]
- Skjolaas, K.A.; Minton, J.E. Does cortisol bias cytokine production in cultured porcine splenocytes to a Th2 phenotype? Vet. Immunol. Immunopathol. 2002, 87, 451–458. [Google Scholar] [CrossRef]
- Fairbairn, L.; Kapetanovic, R.; Sester, D.P.; Hume, D.A. The mononuclear phagocyte system of the pig as a model for understanding human innate immunity and disease. J. Leukoc. Biol. 2011, 89, 855–871. [Google Scholar] [CrossRef]
- Mair, K.H.; Essler, S.E.; Patzl, M.; Storset, A.K.; Saalmüller, A.; Gerner, W. NKp46 expression discriminates porcine NK cells with different functional properties. Eur. J. Immunol. 2012, 42, 1261–1271. [Google Scholar] [CrossRef]
- Sedlak, C.; Patzl, M.; Saalmüller, A.; Gerner, W. CD2 and CD8α define porcine γδ T cells with distinct cytokine production profiles. Dev. Comp. Immunol. 2014, 45, 97–106. [Google Scholar] [CrossRef]
- Kunicka, J.E.; Talle, M.A.; Denhardt, G.H.; Brown, M.; Prince, L.A.; Goldstein, G. Immunosuppression by glucocorticoids: Inhibition of production of multiple lymphokines by in vivo administration of dexamethasone. Cell. Immunol. 1993, 149, 39–49. [Google Scholar] [CrossRef]
- Maes, M.; Lin, A.; Kenis, G.; Egyed, B.; Bosmans, E. The effects of noradrenaline and alpha-2 adrenoceptor agents on the production of monocytic products. Psychiatry Res. 2000, 96, 245–253. [Google Scholar] [CrossRef]
- Van der Poll, T.; Jansen, J.; Endert, E.; Sauerwein, H.P.; van Deventer, S.J. Noradrenaline inhibits lipopolysaccharide-induced tumor necrosis factor and interleukin 6 production in human whole blood. Infect. Immun. 1994, 62, 2046–2050. [Google Scholar]
- Maisel, A.S.; Harris, T.; Rearden, C.A.; Michel, M.C. Beta-adrenergic receptors in lymphocyte subsets after exercise. Alterations in normal individuals and patients with congestive heart failure. Circulation 1990, 82, 2003–2010. [Google Scholar] [CrossRef]
- Ben-Eliyahu, S.; Shakhar, G.; Page, G.G.; Stefanski, V.; Shakhar, K. Suppression of NK Cell Activity and of Resistance to Metastasis by Stress: A Role for Adrenal Catecholamines and β-Adrenoceptors. Neuroimmunomodulation 2000, 8, 154–164. [Google Scholar] [CrossRef]
- Irwin, M. Stress-induced immune suppression: Role of brain corticotropin releasing hormone and autonomic nervous system mechanisms. Adv. Neuroimmunol. 1994, 4, 29–47. [Google Scholar] [CrossRef]
- Zhou, L.; Li, Y.; Li, X.; Chen, G.; Liang, H.; Wu, Y.; Tong, J.; Ouyang, W. Propranolol Attenuates Surgical Stress-Induced Elevation of the Regulatory T Cell Response in Patients Undergoing Radical Mastectomy. J. Immunol. 2016, 196, 3460–3469. [Google Scholar] [CrossRef]
- Sojka, D.K.; Huang, Y.-H.; Fowell, D.J. Mechanisms of regulatory T-cell suppression—A diverse arsenal for a moving target. Immunology 2008, 124, 13–22. [Google Scholar] [CrossRef]
- Takamatsu, H.-H.; Denyer, M.S.; Stirling, C.; Cox, S.; Aggarwal, N.; Dash, P.; Wileman, T.E.; Barnett, P.V. Porcine gammadelta T cells: Possible roles on the innate and adaptive immune responses following virus infection. Vet. Immunol. Immunopathol. 2006, 112, 49–61. [Google Scholar] [CrossRef]
- Girardi, M. Immunosurveillance and immunoregulation by gammadelta T cells. J. Investig. Dermatol. 2006, 126, 25–31. [Google Scholar] [CrossRef]


| Frequency (%) | Control | Hormone | Pooled SEM | Treatment p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Cortisol | Noradrenaline | Adrenaline | |||||||
| 10−8 M | 10−6 M | 10−8 M | 10−6 M | 10−8 M | 10−6 M | ||||
| Naive TH cells † | 1.37 | 1.31 | 1.07 *** | 1.37 | 1.32 | 1.31 | 1.31 | 0.48 | <0.001 |
| Ag-exp. TH cells | 12.36 | 12.20 | 10.09 *** | 12.48 | 11.92 | 11.89 | 11.60 | 1.99 | <0.001 |
| Cytotoxic T cells | 2.42 | 2.46 | 1.93 *** | 2.54 | 2.41 | 2.39 | 2.25 | 0.67 | <0.001 |
| γδ T cells ‡ | 1.34 | 1.31 | 1.08 *** | 1.34 | 1.27 | 1.25 | 1.00 *** | 0.08 | <0.001 |
| NK cells ‡ | 4.32 | 4.47 | 3.82 | 4.18 | 3.95 | 4.46 | 3.94 | 0.85 | 0.307 |
| Monocytes † | 26.35 | 22.15 *** | 27.00 | 23.96 * | 2.62 | <0.001 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reiske, L.; Schmucker, S.; Steuber, J.; Stefanski, V. Glucocorticoids and Catecholamines Affect in Vitro Functionality of Porcine Blood Immune Cells. Animals 2019, 9, 545. https://doi.org/10.3390/ani9080545
Reiske L, Schmucker S, Steuber J, Stefanski V. Glucocorticoids and Catecholamines Affect in Vitro Functionality of Porcine Blood Immune Cells. Animals. 2019; 9(8):545. https://doi.org/10.3390/ani9080545
Chicago/Turabian StyleReiske, Lena, Sonja Schmucker, Julia Steuber, and Volker Stefanski. 2019. "Glucocorticoids and Catecholamines Affect in Vitro Functionality of Porcine Blood Immune Cells" Animals 9, no. 8: 545. https://doi.org/10.3390/ani9080545
APA StyleReiske, L., Schmucker, S., Steuber, J., & Stefanski, V. (2019). Glucocorticoids and Catecholamines Affect in Vitro Functionality of Porcine Blood Immune Cells. Animals, 9(8), 545. https://doi.org/10.3390/ani9080545





