Early Feeding Regime of Waste Milk, Milk, and Milk Replacer for Calves Has Different Effects on Rumen Fermentation and the Bacterial Community
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Treatments, and Management
2.2. Sampling and Measurements
2.2.1. Growth Performance and Feed Intake
2.2.2. Blood Metabolites and Hormone Measurements
2.2.3. Assessment of Rumen Organ Development
2.2.4. Rumen Fermentation Parameter Measurements
2.2.5. Identification of the Rumen Bacterial Community
2.2.6. Bioinformatic Analysis
2.3. Statistical Analysis
3. Results
3.1. Growth, Rumen Fermentation, and Blood Indices in Two-Month-Old Female Calves, and Rumen Organ Development in Male Calves
3.2. Rumen Fermentation and Blood Indices in Six-Month-Old Female Calves
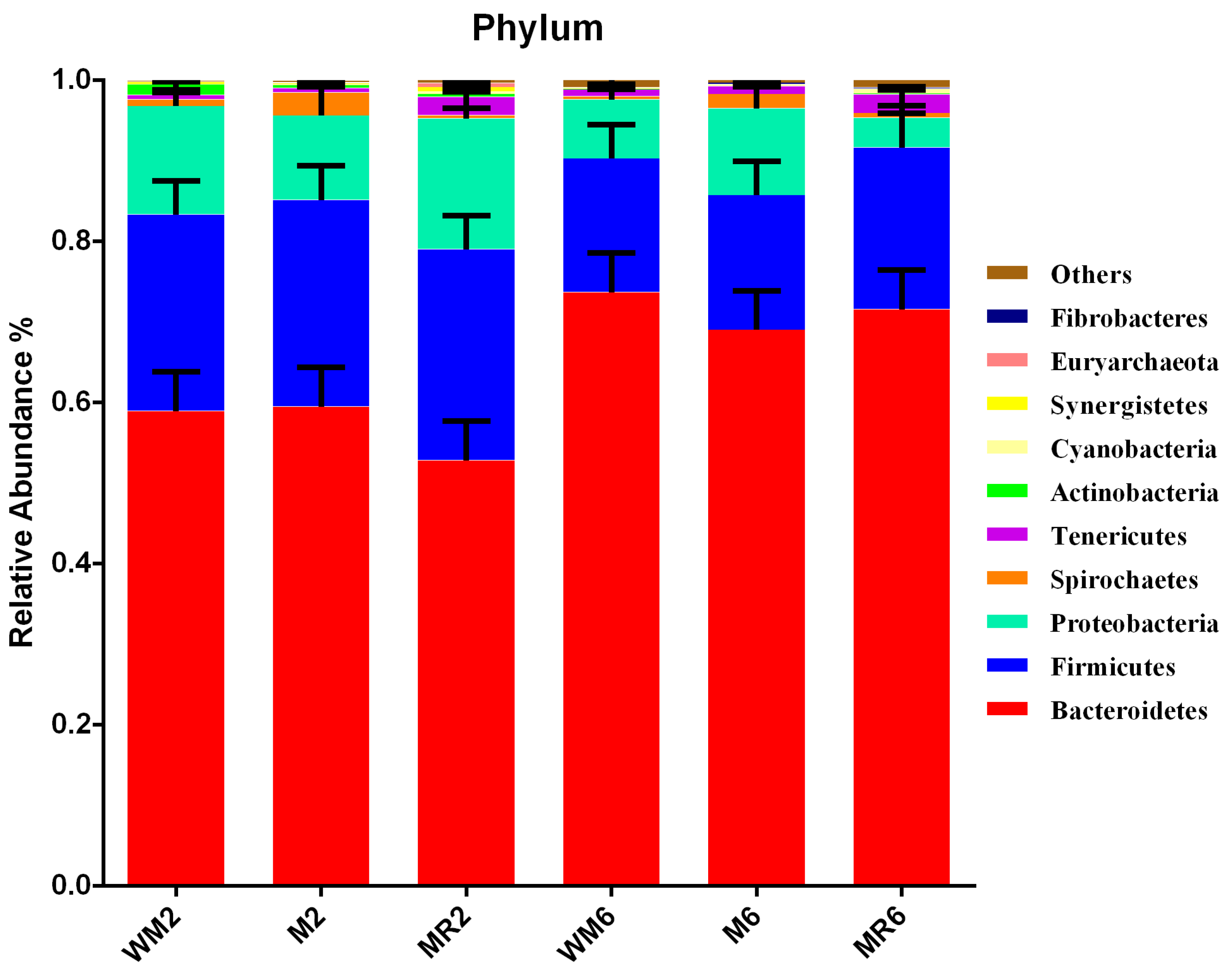
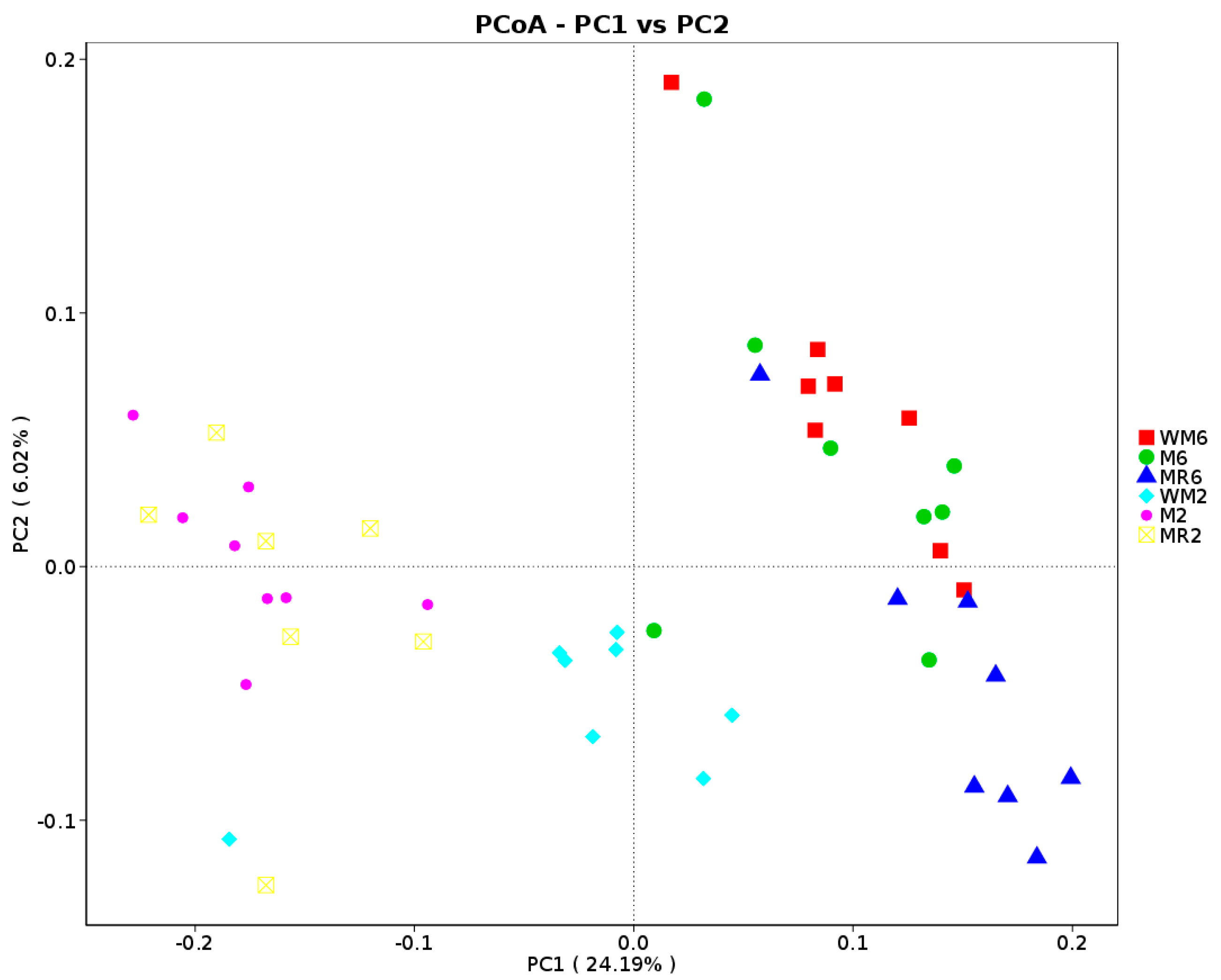
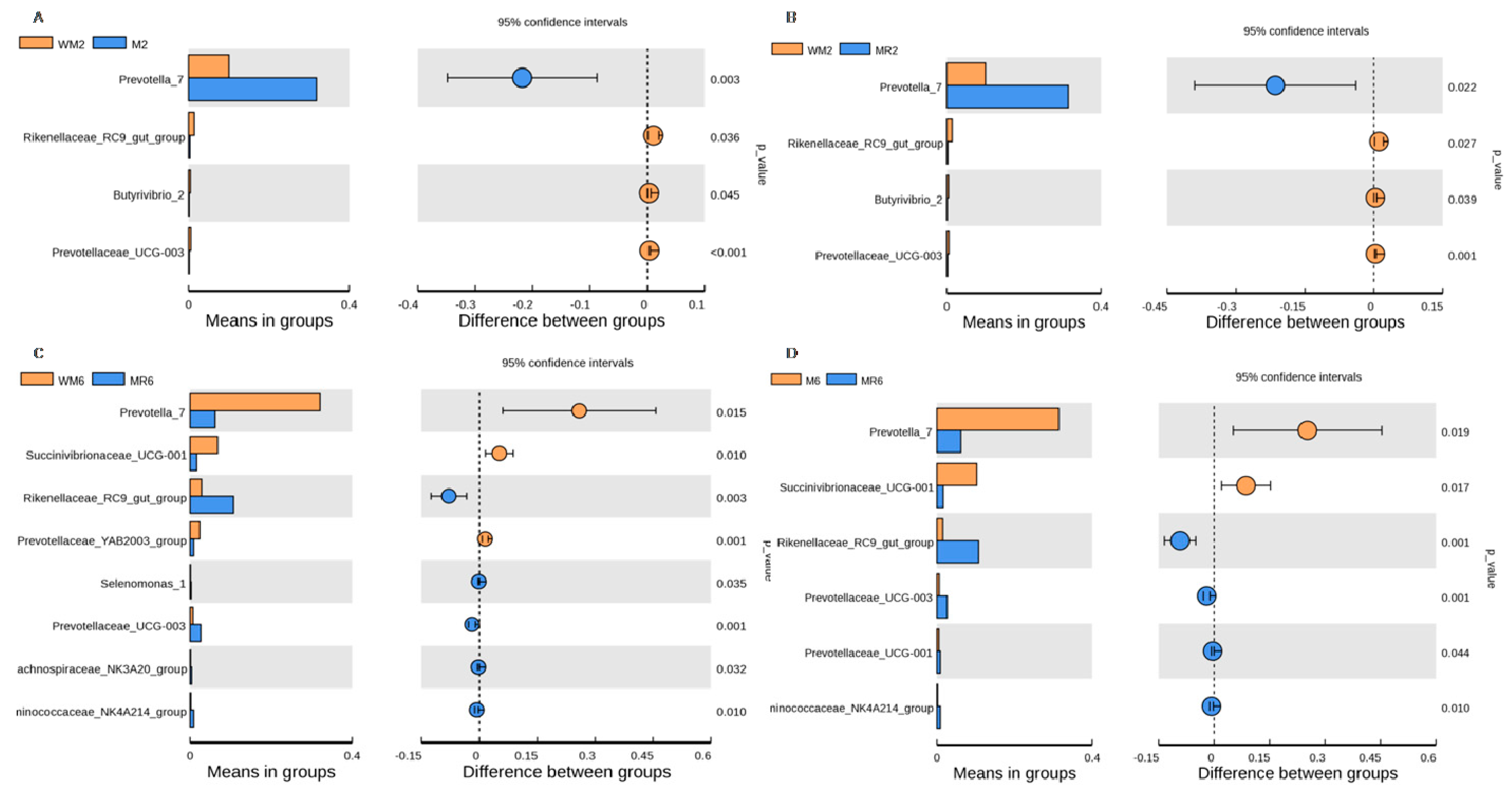
3.3. Ruminal Bacterial Community
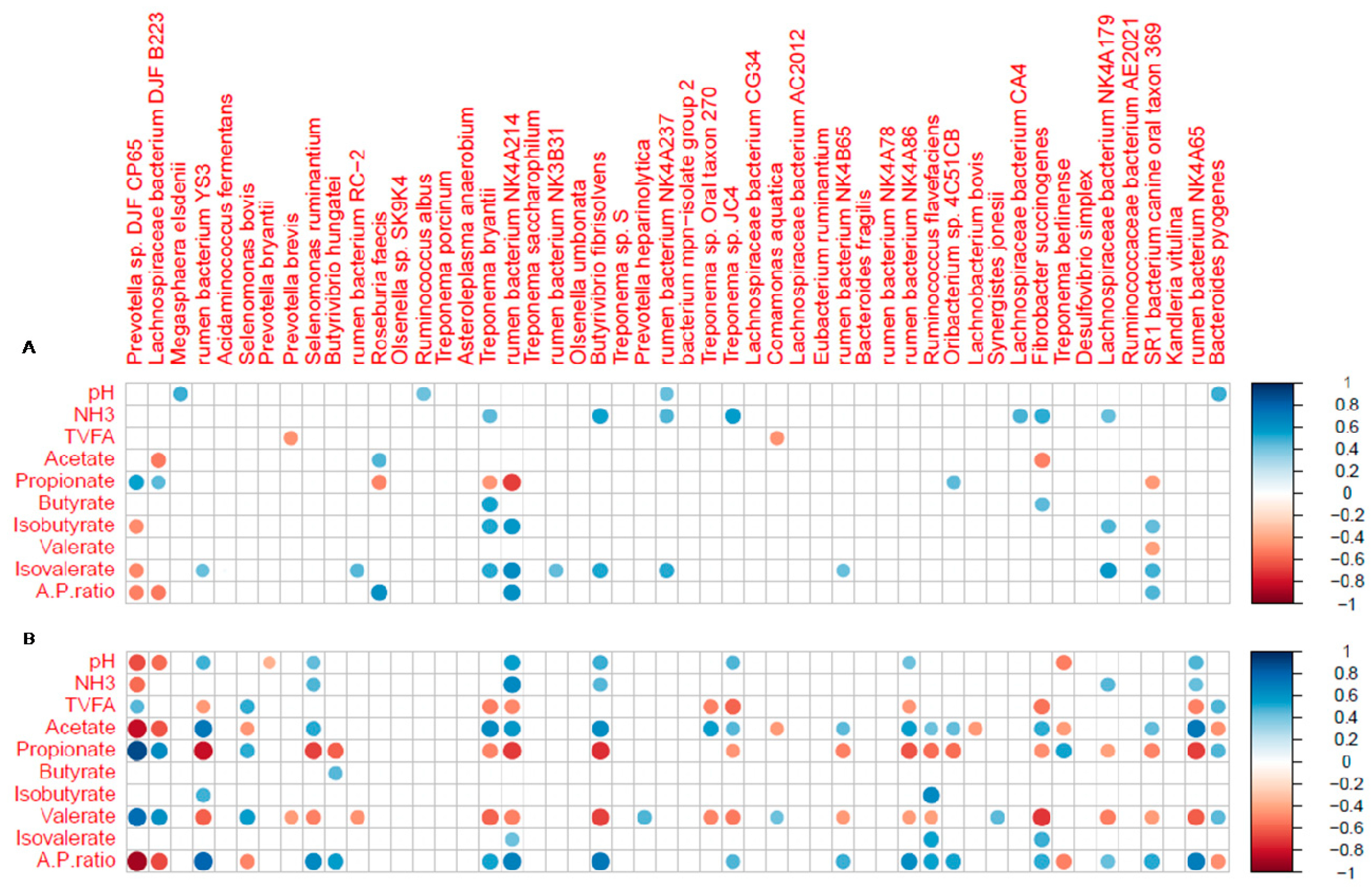
3.4. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chardavoyne, J.R.; Ibeawuchi, J.A.; Kesler, E.M.; Borland, K.M. Waste Milk from Antibiotic Treated Cows as Feed for Young Calves. J. Dairy Sci. 1979, 62, 1285–1289. [Google Scholar] [CrossRef]
- Kesler, E.M. Feeding Mastitic Milk to Calves: Review. J. Dairy Sci. 1981, 64, 719–723. [Google Scholar] [CrossRef]
- Dawson, D.P.; Morrill, J.L.; Reddy, P.G.; Minocha, H.C.; Ramsey, H.A. Soy Protein Concentrate and Heated Soy Flours as Protein Sources in Milk Replacer for Preruminant Calves. J. Dairy Sci. 1988, 71, 1301–1309. [Google Scholar] [CrossRef]
- Li, H.; Diao, Q.Y.; Zhang, N.F.; Fan, Z.Y. Growth, nutrient utilization and amino acid digestibility of dairy calves fed milk replacers containing different amounts of protein in the preruminant period. Asian Australas. J. Anim. 2008, 21, 1151–1158. [Google Scholar]
- Guilloteau, P.; Huërou-Luron, I.L.; Chayvialle, J.A.; Toullec, R.; Zabielski, R.; Blum, J.W. Gut Regulatory Peptides in Young Cattle and Sheep. J. Vet. Med. A 1997, 44, 1–23. [Google Scholar] [CrossRef]
- Blum, J.; Hammon, H. Colostrum effects on the gastrointestinal tract, and on nutritional, endocrine and metabolic parameters in neonatal calves. Livest. Prod. Sci. 2000, 66, 151–159. [Google Scholar] [CrossRef]
- Edrington, T.S.; Dowd, S.E.; Farrow, R.F.; Hagevoort, G.R.; Callaway, T.R.; Anderson, R.C.; Nisbet, D.J. Development of colonic microflora as assessed by pyrosequencing in dairy calves fed waste milk. J. Dairy Sci. 2012, 95, 4519–4525. [Google Scholar] [CrossRef]
- Smith, R.H.; Sissons, J.W. The effect of different feeds, including those containing soya-bean products, on the passage of digesta from the abomasum of the preruminant calf. Br. J. Nutr. 1975, 33, 329–349. [Google Scholar] [CrossRef]
- Constable, P.D.; Ahmed, A.F.; Misk, N.A. Effect of suckling cow’s milk or milk replacer on abomasal luminal pH in dairy calves. J. Vet. Intern. Med. 2005, 19, 97–102. [Google Scholar] [CrossRef]
- Górka, P.; Kowalski, Z.M.; Pietrzak, P.; Kotunia, A.; Jagusiak, W.; Zabielski, R. Is rumen development in newborn calves affected by different liquid feeds and small intestine development? J. Dairy Sci. 2011, 94, 3002–3013. [Google Scholar] [CrossRef]
- Yáñez-Ruiz, D.R.; Abecia, L.; Newbold, C.J. Manipulating rumen microbiome and fermentation through interventions during early life: A review. Front. Microbiol. 2015, 6, 1133. [Google Scholar] [CrossRef] [PubMed]
- Abecia, L.; Martín-García, A.I.; Martínez, G.; Newbold, C.J.; Yáñez-Ruiz, D.R. Nutritional intervention in early life to manipulate rumen microbial colonization and methane output by kid goats postweaning. J. Anim. Sci. 2013, 91, 4832–4840. [Google Scholar] [CrossRef] [PubMed]
- Abecia, L.; Waddams, K.E.; Martínez-Fernandez, G.; Martín-García, A.I.; Ramos-Morales, E.; Newbold, C.J.; Yáñez-Ruiz, D.R. An Antimethanogenic Nutritional Intervention in Early Life of Ruminants Modifies Ruminal Colonization by Archaea. Archaea 2014, 2014, 841463. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Ruiz, D.R.; Macías, B.; Pinloche, E.; Newbold, C.J. The persistence of bacterial and methanogenic archaeal communities residing in the rumen of young lambs. FEMS Microbiol. Ecol. 2010, 72, 272–278. [Google Scholar] [CrossRef] [PubMed]
- De Barbieri, I.; Hegarty, R.S.; Silveira, C.; Gulino, L.M.; Oddy, V.H.; Gilbert, R.A.; Klieve, A.V.; Ouwerkerk, D. Programming rumen bacterial communities in newborn Merino lambs. Small Rumin. Res. 2015, 129, 48–59. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, W.B.; Bi, Y.L.; Tu, Y.; Ma, T.; Dong, L.F.; Du, H.C.; Diao, Q.Y. Sanguinarine and resveratrol affected rumen fermentation parameters and bacterial community in calves. Anim. Feed Sci. Technol. 2019, 251, 64–75. [Google Scholar] [CrossRef]
- Kwak, H.S.; Jeon, I.J. Comparison of high-performance liquid chromatography and enzymatic method for the measurement of lactose in milk. J. Food Sci. 1988, 53, 975–976. [Google Scholar] [CrossRef]
- Jin, Y.; Jang, J.W.; Han, C.H.; Lee, M.H. Development of ELISA and immunochromatographic assay for the detection of gentamicin. Agric. Food Chem. 2005, 53, 7639–7643. [Google Scholar] [CrossRef]
- Liu, Y.F.; Sun, F.F.; Wan, F.C.; Zhao, H.B.; Liu, X.M.; You, W.; Cheng, H.J.; Liu, G.F.; Tan, X.W.; Song, E.L. Effects of three feeding systems on production performance, rumen fermentation and rumen digesta particle structure of beef cattle. Asian Australas. J. Anim. Sci. 2016, 29, 659–665. [Google Scholar] [CrossRef]
- Berson, S.A.; Yalow, R.S. Assay of plasma insulin in human subjects by immunological methods. Nature 1959, 184, 1648–1649. [Google Scholar]
- Lequin, R.M. Enzyme Immunoassay (EIA)/Enzyme-Linked Immunosorbent Assay (ELISA). Clin. Chem. 2005, 51, 2415–2418. [Google Scholar] [CrossRef] [PubMed]
- Broderick, G.; Kang, J. Automated stimultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef]
- Zhang, R.; Dong, X.; Zhou, M.; Tu, Y.; Zhang, N.F.; Deng, K.D.; Diao, Q.Y. Oral administration of Lactobacillus plantarum and Bacillus subtilis on rumen fermentation and the bacterial community in calves. Anim. Sci. J. 2016, 88, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Native Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009. [Google Scholar]
- Clarke, K.R. Non-parametric multivariate analysis of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Wei, T. Corrplot: Visualization of a Correlation Matrix. R Package Version 0.73. 2013. Available online: http://CRAN.R-project.org/package=corrplot (accessed on 4 July 2019).
- Abe, M.; Iriki, T.; Kondoh, K.; Shibui, H. Effects of nipple or bucket feeding of milk-substitute on rumen by-pass and on rate of passage in calves. Br. J. Nutr. 1979, 41, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Wise, G.H.; Anderson, G.W.; Linnerud, A.C. Relationship of milk intake by sucking and by drinking to reticular groove reactions and ingestion behavior in calves. J. Dairy Sci. 1984, 67, 1983–1992. [Google Scholar] [CrossRef]
- Herrli-Gygi, M.; Hammon, H.M.; Zbinden, Y.; Steiner, A.; Blum, J.W. Ruminal Drinkers: Endocrine and Metabolic Status and Effects of Suckling from a Nipple Instead of Drinking from a Bucket. J. Vet. Med. Ser. A 2006, 53, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Suárez, B.J.; Van Reenen, C.G.; Stockhofe, N.; Dijkstra, J.; Gerrits, W.J.J. Effect of Roughage Source and Roughage to Concentrate Ratio on Animal Performance and Rumen Development in Veal Calves. J. Dairy Sci. 2007, 90, 2390–2403. [Google Scholar] [CrossRef] [PubMed]
- Labussière, E.; Berends, H.; Gilbert, M.S.; van den Borne, J.J.G.C.; Gerrits, W.J.J. Estimation of milk leakage into the rumen of milk-fed calves through an indirect and repeatable method. Animal 2014, 8, 1643–1652. [Google Scholar] [CrossRef] [PubMed]
- Berends, H.; van den Borne, J.J.; Stockhofe-Zurwieden, N.; Gilbert, M.S.; Zandstra, T.; Pellikaan, W.F.; van Reenen, C.G.; Bokkers, E.A.; Gerrits, W.J. Effects of solid feed level and roughage-to-concentrate ratio on ruminal drinking and passage kinetics of milk replacer, concentrates, and roughage in veal calves. J. Dairy Sci. 2015, 98, 5621–5629. [Google Scholar] [CrossRef]
- Ellingsen, K.; Mejdell, C.M.; Ottesen, N.; Larsen, S.; Grondahl, A.M. The effect of large milk meals on digestive physiology and behaviour in dairy calves. Physiol. Behav. 2016, 154, 169–174. [Google Scholar] [CrossRef]
- Berends, H.; van Reenen, C.G.; Stockhofe-Zurwieden, N.; Gerrits, W.J.J. Effects of early rumen development and solid feed composition on growth performance and abomasal health in veal calves. J. Dairy Sci. 2012, 95, 3190–3199. [Google Scholar] [CrossRef]
- Gentile, A.; Sconza, S.; Lorenz, I.; Otranto, G.; Rademacher, G.; Famigli-Bergamini, P.; Klee, W. d-Lactic acidosis in calves as a consequence of experimentally induced ruminal acidosis. J. Vet. Med. Ser. A Physiol. Pathol. Clin. Med. 2004, 51, 64–70. [Google Scholar] [CrossRef]
- Plaizier, J.C.; Krause, D.O.; Gozho, G.N.; McBride, B.W. Subacute ruminal acidosis in dairy cows: The physiological causes, incidence and consequences. Vet. J. 2008, 176, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Zebeli, Q.; Dijkstra, J.; Tafaj, M.; Steingass, H.; Ametaj, B.N.; Drochner, W. Modeling the adequacy of dietary fiber in dairy cows based on the responses of ruminal pH and milk fat production to composition of the diet. J. Dairy Sci. 2008, 91, 2046–2066. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Lee, H.J.; Lee, W.S.; Kim, H.S.; Kim, S.B.; Park, S.B.; Baek, K.S.; Ha, J.K.; Choi, Y.J. Starch source evaluation in calf starter: II. Ruminal parameters, rumen development, nutrient digestibilities and nitrogen utilization in Holstein calves. J. Dairy Sci. 2008, 91, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Rosener, D.L.; Uhlenhopp, E.K. Isoacids—An overview. Iowa State Univ. Vet. 1987, 49, 15–20. [Google Scholar]
- Liu, Q.; Wang, C.; Pei, C.X.; Li, H.Y.; Wang, Y.X.; Zhang, S.L.; Zhang, Y.L.; He, J.P.; Wang, H.; Yang, W.Z.; et al. Effects of isovalerate supplementation on microbial status and rumen enzyme profile in steers fed on corn stover based diet. Livest. Sci. 2014, 161, 60–68. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, C.; Zhang, Y.L.; Pei, C.X.; Zhang, S.L.; Wang, Y.X.; Zhang, Z.W.; Yang, W.Z.; Wang, H.; Huo, W.J. Effects of isovalerate supplementation on growth performance and ruminal fermentation in pre- and post-weaning dairy calves. J. Agric. Sci. 2016, 154, 1499–1508. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Q.; Zhang, Y.L.; Pei, C.X.; Zhang, S.L.; Wang, Y.X.; Yang, W.Z.; Bai, Y.S.; Shi, Z.G.; Liu, X.N. Effects of isobutyrate supplementation on ruminal microflora, rumen enzyme activities and methane emissions in Simmental steers. J. Anim. Physiol. Anim. Nutr. 2015, 99, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Miron, J.; Ben-Ghedalia, D.; Morrison, M. Invited review: Adhesion mechanisms of rumen cellulolytic bacteria. J. Dairy Sci. 2001, 84, 1294–1309. [Google Scholar] [CrossRef]
- Deng, Y.F.; Wang, Y.J.; Zou, Y.; Azarfar, A.; Wei, X.L.; Ji, S.K.; Zhang, J.; Wu, Z.H.; Wang, S.X.; Dong, S.Z.; et al. Influence of dairy by-product waste milk on the microbiomes of different gastrointestinal tract components in pre-weaned dairy calves. Sci. Rep. 2017, 7, 42689. [Google Scholar] [CrossRef] [PubMed]
- Fieo, A.G.; Sweeney, T.F.; Kensinger, R.S.; Muller, L.D. Metabolic and digestion effects of the addition of ammonium salts of volatile fatty acids to the diets of cows in early lactation. J. Dairy Sci. 1984, 67 (Suppl. 1), 117. [Google Scholar]
- Towns, R.; Cook, R.M. Isoacids, a new growth hormone releasing factor. Proc. Annu. Mig. Am. Assoc. Adv. Sci. 1984. (Abstr. 347). [Google Scholar]
- Liu, Q.; Wang, C.; Yang, W.Z.; Zhang, B.; Yang, X.M.; He, D.C.; Zhang, P.; Dong, K.H.; Huang, Y.X. Effects of isobutyrate on rumen fermentation, lactation performance and plasma characteristics in dairy cows. Anim. Feed Sci. Technol. 2009, 154, 58–67. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, C.; Guo, G.; Huo, W.J.; Zhang, Y.L.; Pei, C.X.; Zhang, S.L.; Wang, H. Effects of isovalerate supplements on morphology and functional gene expression of rumen mucosa in pre- and post-weaning dairy calves. Animal 2017, 12, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Andries, J.I.; Buysse, F.X.; De Brabander, D.L.; Cottyn, B.G. Isoacids in ruminant nutrition: Their role in ruminal and intermediary metabolism and possible influences on performances—A review. Anim. Feed Sci. Technol. 1987, 18, 169–180. [Google Scholar] [CrossRef]
- Hamamdzic, M. Recent knowledge on the role of isoacids in rumen metabolism and milk production. Veterinaria (Sarajevo) 1989, 38, 3–17. [Google Scholar]
- Jansson, L.; Karlson, F.A.; Westermark, B. Mitogenic activity and epidermal growth factor content in human milk. Acta Paediatr. Scand. 1985, 74, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Rauprich, A.B.E.; Hammon, H.M.; Blum, J.M. Effects of feeding colostrums and a formula with nutrient contents as colostrums on metabolic and endocrine traits in neonatal calves. Biol. Neonatol. 2000, 78, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, R.L. The proliferative actions of insulin, insulin-like growth factor-I, epidermal growth factor, butyrate and propionate on ruminal epithelial cells in vitro. Small Rumin. Res. 1999, 32, 261–268. [Google Scholar] [CrossRef]
- Zitnan, R.; Kuhla, S.; Sanftleben, P.; Bilska, A.; Schneider, F.; Zupcanova, M.; Voigt, J. Diet induced ruminal papillae development in neonatal calves not correlating with rumen butyrate. Vet. Med.-Czech 2005, 50, 472–479. [Google Scholar] [CrossRef]
- Nagaraja, T.G.; Taylor, M.B. Susceptibility and resistance of ruminal bacteria to antimicrobial feed additives. Appl. Environ. Microbiol. 1987, 53, 1620–1625. [Google Scholar]
- Baldwin, K.A.; Bitman, J.; Thompson, M.J. Comparison of N,N-dimethyldodecanamine with antibiotics on in virto cellulose digestion and volatile fatty acid production by ruminal microorganisms. J. Anim. Sci. 1982, 55, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Weimer, P.J.; Stevenson, D.M.; Mertens, D.R.; Thomas, E.E. Effect of monensin feeding and withdrawal on populations of individual bacterial species in the rumen of lactating dairy cows fed high-starch rations. Appl. Microbiol. Biotechnol. 2008, 80, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Coe, M.L.; Nagaraja, T.G.; Sun, Y.D.; Wallace, N.; Towne, E.G.; Kemp, K.E.; Hutcheson, J.P. Effect of virginiamycin on ruminal fermentation in cattle during adaptation to a high concentrate diet and during an induced acidosis. J. Anim. Sci. 1999, 77, 2259–2268. [Google Scholar] [CrossRef] [PubMed]
- Van Vleck, P.R.; Lima, S.; Siler, J.D.; Foditsch, C.; Wamick, L.D.; Bicalho, R.C. Ingestion of milk containing very low concentration of antimicrobials: Longitudinal effects on fecal microbiota composi-tion in preweaned calves. PLoS ONE 2016, 11, e0147525. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, M.A.; Hoffman, P.C.; Nytes, A.J. A Field Survey of On-Farm Milk Pasteurization Efficacy. Prof. Anim. Sci. 2006, 22, 472–476. [Google Scholar] [CrossRef]
- Anumala, D.; Pasupuleti, M.K.; Nagireddy, R.R. Detection and Characterization of Prevotella Intermedia and Its In Vitro Susceptibility to Selected Antimicrobial Agents in Chronic Periodontitis and Acute Myocardial Infarction. Perio J. 2019, 3, 1–6. [Google Scholar] [CrossRef]
- Li, J.H.; Yousif, M.H.; Li, Z.Q.; Wu, Z.H.; Li, S.L.; Yang, H.J.; Wang, Y.J.; Cao, Z.J. Effects of antibiotic residues in milk on growth, ruminal fermentation, and microbial community of preweaning dairy calves. J. Dairy Sci. 2019, 102, 2298–2307. [Google Scholar] [CrossRef]
- Holmes, C.W.; Davey, A.W.F. The energy metabolism of young Jersey and Friesian calves fed fresh milk. Anim. Prod. 1976, 23, 43–53. [Google Scholar] [CrossRef]
- Morrill, J.L. The calf: Birth to 12 weeks. In Large Dairy Herd Management; van Horn, H.H., Wilcox, C.J., Eds.; American Dairy Science Association: Savoy, IL, USA, 1992; pp. 401–410. [Google Scholar]
- Seegraber, F.J.; Morrill, J.L. Effect of protein source in calf milk replacers on morphology and absorptive ability of small intestine. J. Dairy Sci. 1986, 69, 460–469. [Google Scholar] [CrossRef]
- Reddy, K.E.; Jeong, J.Y.; Baek, Y.-C.; Oh, Y.K.; Kim, M.; So, K.M.; Kim, M.J.; Kim, D.W.; Park, S.K.; Lee, H.J. Early weaning of calves after different dietary regimens affects later rumen development, growth, and carcass traits in Hanwoo cattle. Asian Australas. J. Anim. Sci. 2017, 30, 1425–1434. [Google Scholar] [CrossRef]



| Age | Feeding Amount (L) |
|---|---|
| 7–14 d | 5.0 |
| 14–28 d | 5.4 |
| 28–42 d | 6.2 |
| 42–60 d | 7.3 |
| 61 d | 5.5 |
| 62 d | 3.7 |
| 63 d | 1.8 |
| Chemical Analysis | Milk 1 | Waste Milk 1 | Milk Replacer Emulsion 2 |
|---|---|---|---|
| Crude Protein (%) | 3.3 ± 0.27 | 4.29 ± 0.61 | 2.71 ± 0.01 |
| Crude Fat (%) | 4.25 ± 0.25 | 3.91 ± 0.56 | 1.90 ± 0.04 |
| Crude Lactose (%) | 4.47 ± 0.27 | 3.85 ± 0.52 | - |
| Ingredients | Concentrate Feed | Forage 2 | ||
|---|---|---|---|---|
| 0–3 Months | 4–6 Months | Alfalfa Hay | Oat Hay | |
| Corn (%) | 55.65 | 56.5 | ||
| Soybean Meal (%) | 26.2 | 23.15 | ||
| Extruded Soy (%) | 7 | 0 | ||
| Wheat bran (%) | 3.9 | 10 | ||
| Distillers Dried Grains with Solubles (%) | 3 | 6 | ||
| Calcium Carbonate (%) | 2.25 | 2.302 | ||
| Phospate Dicalcium (%) | 0.6 | 0.55 | ||
| Salt (%) | 0.4 | 0.5 | ||
| Premix (%) 1 | 1 | 1 | ||
| Items | 0–3 Months | 4–6 Months | Alfalfa hay | Oat hay |
| Dry Matter (%) | 87.94 | 87.77 | 90.22 | 89.50 |
| Crude Protein (%) | 20.00 | 18.00 | 18.77 | 7.18 |
| Crude Fat (%) | 3.86 | 3.21 | 2.32 | 2.27 |
| Neutral Detergent Fiber (%) | 9.79 | 11.41 | 35.31 | 50.33 |
| Acid Detergent Fiber (%) | 3.77 | 3.95 | 26.22 | 32.36 |
| Crude Ash (%) | 6.94 | 6.99 | 7.35 | 5.57 |
| Calcium (%) | 1.00 | 1.00 | 1.28 | 0.32 |
| Total Phosphate (%) | 0.45 | 0.45 | 0.26 | 0.20 |
| Salt (%) | 0.47 | 0.58 | - | - |
| Items | Age | Treatment 3 | SEM | p value | ||
|---|---|---|---|---|---|---|
| WM | M | MR | ||||
| pH | 2-month-old | 6.28 | 6.15 | 5.67 | 0.174 | 0.0651 |
| 6-month-old | 6.38 b | 6.36 b | 6.71 a | 0.077 | 0.0038 | |
| NH3-N(mg/dL) | 2-month-old | 30.08 | 20.15 | 19.17 | 4.337 | 0.1787 |
| 6-month-old | 9.67 | 10.22 | 11.92 | 1.063 | 0.3484 | |
| TVFA (mmol/L) 1 | 2-month-old | 39.21 b | 40.45 b | 62.13 a | 5.269 | 0.0215 |
| 6-month-old | 71.82 | 70.29 | 61.5 | 4.888 | 0.3263 | |
| Acetate (%) 2 | 2-month-old | 49.48 | 47.15 | 44.39 | 2.693 | 0.4483 |
| 6-month-old | 60.92 | 61.86 | 66.72 | 1.698 | 0.0683 | |
| Propionate (%) 2 | 2-month-old | 31.03 | 35.29 | 38.87 | 2.39 | 0.1051 |
| 6-month-old | 25.52 a | 25.49 a | 19.14 b | 1.63 | 0.0221 | |
| Butyrate (%) 2 | 2-month-old | 11.95 | 10.59 | 11.49 | 1.863 | 0.8731 |
| 6-month-old | 9.46 | 8.76 | 10.18 | 0.759 | 0.4580 | |
| Isobutyrate (%) 2 | 2-month-old | 1.57 | 0.91 | 0.79 | 0.314 | 0.2055 |
| 6-month-old | 0.90 | 0.83 | 1.08 | 0.129 | 0.3980 | |
| Valerate (%) 2 | 2-month-old | 2.94 | 4.37 | 3.02 | 0.608 | 0.2041 |
| 6-month-old | 1.57 | 1.66 | 1.21 | 0.125 | 0.0565 | |
| Isovalerate (%) 2 | 2-month-old | 3.03 a | 1.69 b | 1.45 b | 0.399 | 0.0256 |
| 6-month-old | 1.63 | 1.41 | 1.67 | 0.148 | 0.4450 | |
| Acetate/propionate | 2-month-old | 1.77 | 1.36 | 1.2 | 0.204 | 0.1624 |
| 6-month-old | 2.44 b | 2.55 b | 3.70a | 0.255 | 0.0055 | |
| Items | Age | Treatment 7 | SEM | p-value | ||
|---|---|---|---|---|---|---|
| WM | M | MR | ||||
| SUN (mmol/L) 1 | 2-month-old | 5.64 | 5.65 | 5.03 | 0.5232 | 0.6866 |
| 6-month-old | 8.09 a | 8.17 a | 6.78 b | 0.3896 | 0.0303 | |
| NEFA (mmol/L) 2 | 2-month-old | 0.37 b | 0.49 a | 0.44 a | 0.0232 | 0.0037 |
| 6-month-old | 0.41 | 0.42 | 0.41 | 0.0162 | 0.8815 | |
| GH (ng/mL) 3 | 2-month-old | 4.70 a | 3.93 b | 3.94 b | 0.1385 | 0.0005 |
| 6-month-old | 4.58 | 4.10 | 3.98 | 0.2647 | 0.2449 | |
| Insulin (IU/mL) | 2-month-old | 9.17 b | 18.53 a | 15.29 ab | 2.1231 | 0.0141 |
| 6-month-old | 14.53 | 14.95 | 13.82 | 1.8822 | 0.9128 | |
| GH/insulin 4 | 2-month-old | 0.63 a | 0.26 b | 0.27 b | 0.0482 | <0.0001 |
| 6-month-old | 0.36 | 0.30 | 0.33 | 0.0447 | 0.6181 | |
| h-EGF (ng/mL) 5 | 2-month-old | 0.95 a | 0.82 b | 0.81 b | 0.0181 | <0.0001 |
| 6-month-old | 0.93 | 0.76 | 0.87 | 0.083 | 0.3506 | |
| IGF-1 (ng/mL) 6 | 2-month-old | 219.31 a | 168.46 b | 167.44 b | 7.1208 | <0.0001 |
| 6-month-old | 186.54 | 177.06 | 195.43 | 19.245 | 0.7977 | |
| Items | Treatments 3 | SEM | p value | ||
|---|---|---|---|---|---|
| WM | M | MR | |||
| Stomach mass 1 | 1.26 b | 2.12 a | 2.19 a | 0.21 | 0.0395 |
| Rumen % 2 | 42.05 b | 51.89 a | 55.05 a | 1.88 | 0.0065 |
| Reticulum % 2 | 7.75 | 8.53 | 8.83 | 0.50 | 0.3603 |
| Omasum % 2 | 13.28 | 11.13 | 10.55 | 0.93 | 0.1746 |
| Abomasum % 2 | 36.93 a | 28.45 b | 25.58 b | 1.58 | 0.0056 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Zhang, W.-b.; Bi, Y.-l.; Tu, Y.; Beckers, Y.; Du, H.-c.; Diao, Q.-y. Early Feeding Regime of Waste Milk, Milk, and Milk Replacer for Calves Has Different Effects on Rumen Fermentation and the Bacterial Community. Animals 2019, 9, 443. https://doi.org/10.3390/ani9070443
Zhang R, Zhang W-b, Bi Y-l, Tu Y, Beckers Y, Du H-c, Diao Q-y. Early Feeding Regime of Waste Milk, Milk, and Milk Replacer for Calves Has Different Effects on Rumen Fermentation and the Bacterial Community. Animals. 2019; 9(7):443. https://doi.org/10.3390/ani9070443
Chicago/Turabian StyleZhang, Rong, Wei-bing Zhang, Yan-liang Bi, Yan Tu, Yves Beckers, Han-chang Du, and Qi-yu Diao. 2019. "Early Feeding Regime of Waste Milk, Milk, and Milk Replacer for Calves Has Different Effects on Rumen Fermentation and the Bacterial Community" Animals 9, no. 7: 443. https://doi.org/10.3390/ani9070443
APA StyleZhang, R., Zhang, W.-b., Bi, Y.-l., Tu, Y., Beckers, Y., Du, H.-c., & Diao, Q.-y. (2019). Early Feeding Regime of Waste Milk, Milk, and Milk Replacer for Calves Has Different Effects on Rumen Fermentation and the Bacterial Community. Animals, 9(7), 443. https://doi.org/10.3390/ani9070443






