l-Lactate Dehydrogenase B Chain Associated with Milk Protein Content in Dairy Cows
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Milk Preparation and Milk Epithelial Cells (MECs) Isolation
2.2. Analysis of Protein Expression
2.3. l-Leucine Depletion Experiment in MAC-T Cells
2.4. The mRNA Expression of Candidate Proteins in MAC-T Cells
2.5. Statistical Analysis
3. Results
3.1. Milk Protein Contents for Individual Animals
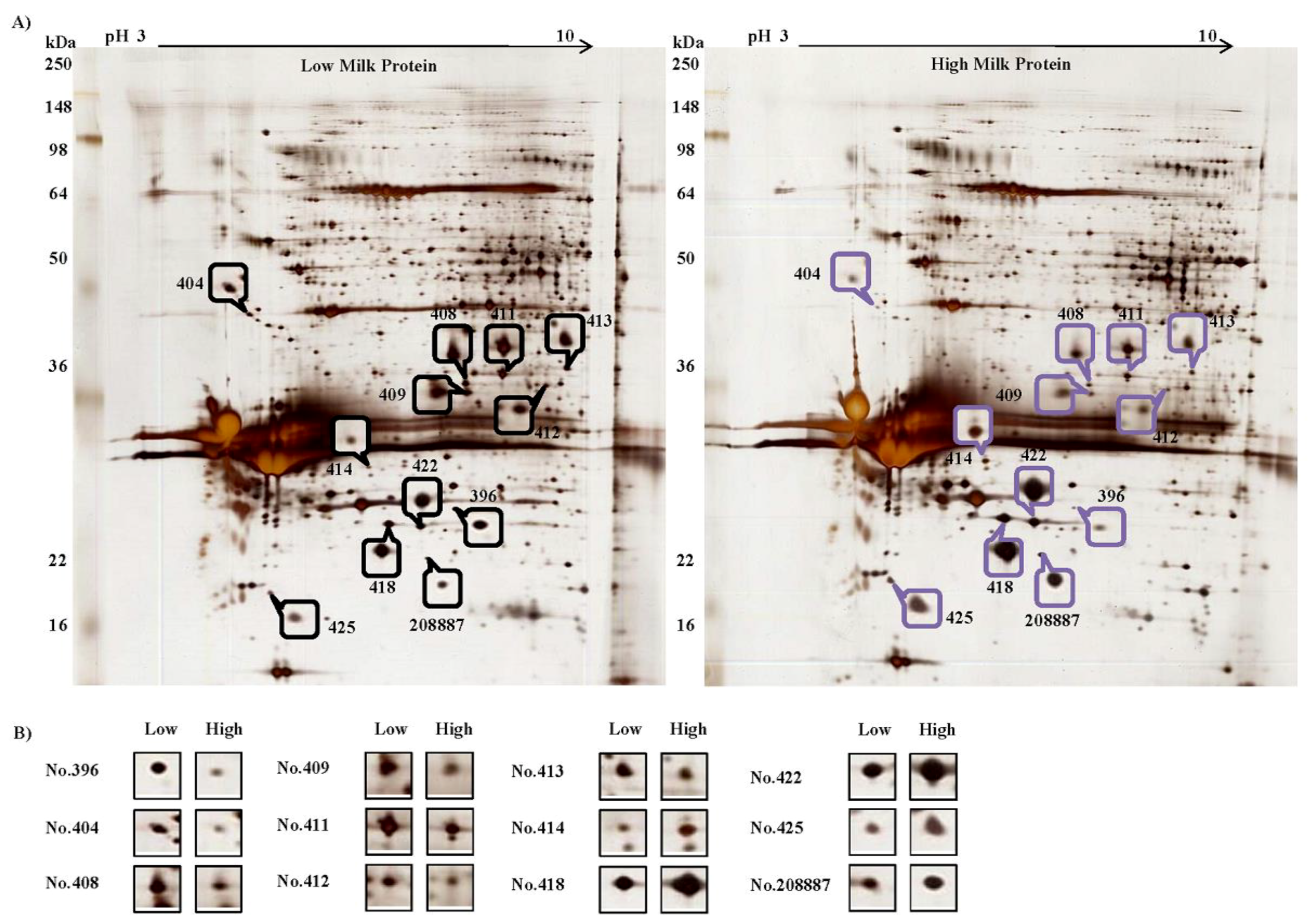
3.2. Differentially Expressed Proteins in Isolated MECs
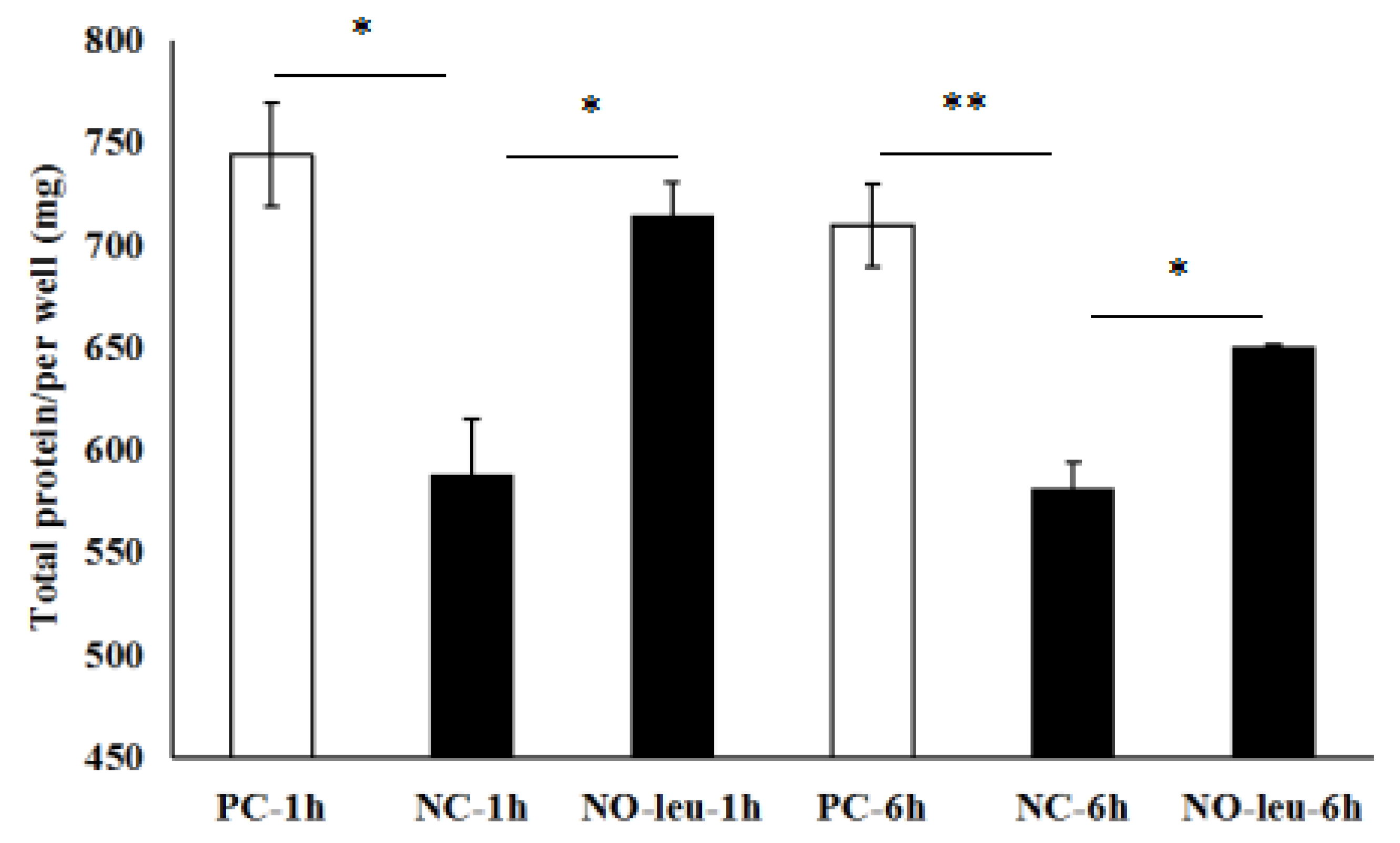
3.3. Effect of l-Leucine Depletion on Total Proteins Content in MAC-T Cells
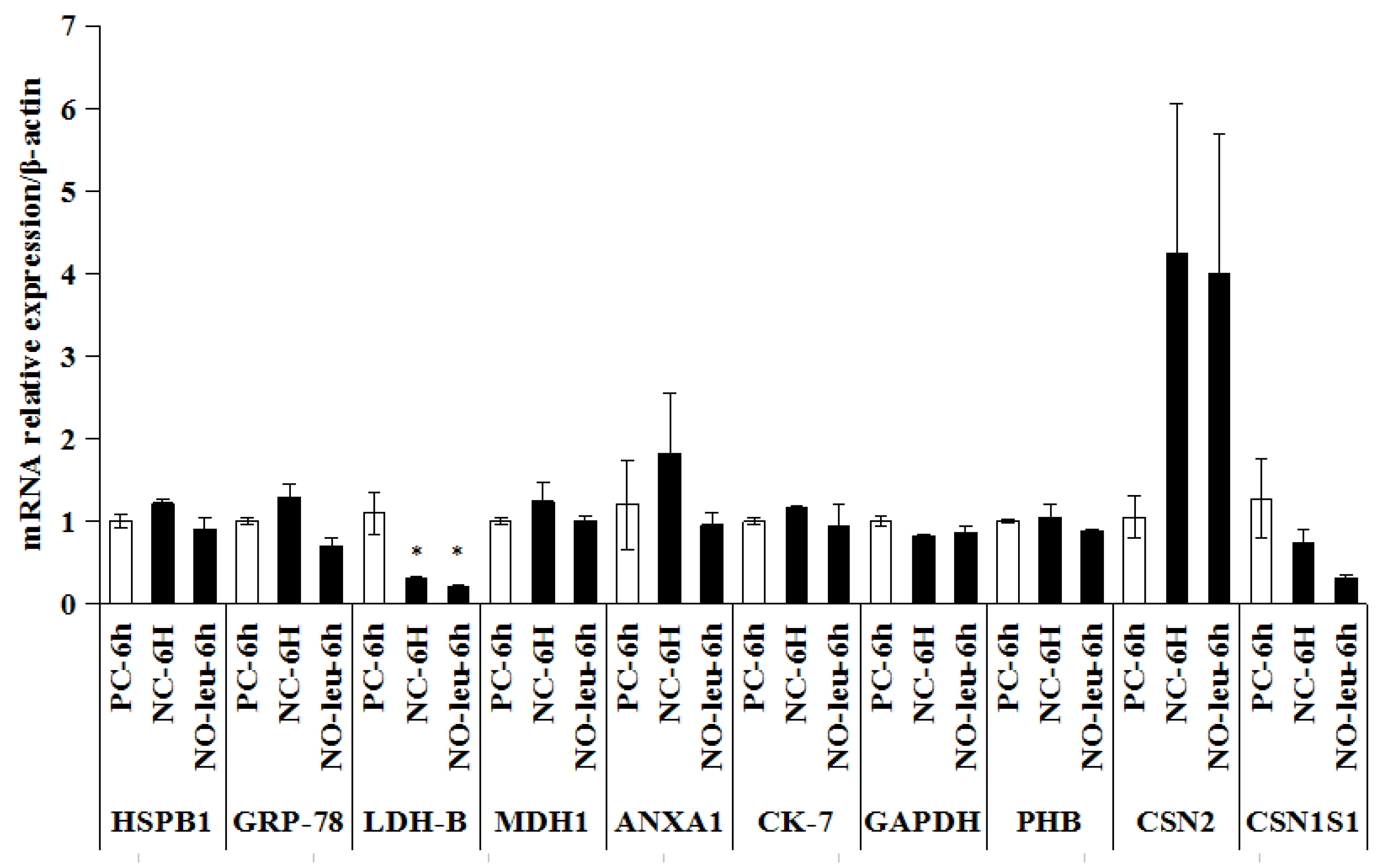
3.4. Differentially Expressed Proteins in Isolated MECs
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Murphy, J.J.; O’Mara, F. Nutritional manipulation of milk protein concentration and its impact on the dairy industry. Livest. Prod. Sci. 1993, 35, 117–134. [Google Scholar] [CrossRef]
- Robinson, P.H. Manipulating milk protein production and level in lactating dairy cows. Part1-Difficult to manipulate factors. Calif. Dairy 1999, 8, 6–7. [Google Scholar]
- Robinson, P.H. Manipulating milk protein percentage and production in lactating dairy cows. Part 2-Factors that can be manipulated. Calif. Dairy 1999, 8, 4–5, 12–13. [Google Scholar]
- Gustavsson, F.; Buitenhuis, A.J.; Johansson, M.; Bertelsen, H.P.; Glantz, M.; Poulsen, N.A.; Lindmark, M.H.; Stålhammar, H.; Larsen, L.B.; Bendixen, C.; et al. Effects of breed and casein genetic variants on protein profile in milk from Swedish Red, Danish Holstein, and Danish Jersey cows. J. Dairy Sci. 2014, 97, 3866–3877. [Google Scholar] [CrossRef] [PubMed]
- Heck, J.M.L. Milk Genomics: Opportunities to Improve the Protein and Fatty Acid Composition in Raw Milk. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2009. [Google Scholar]
- Kim, E. Mechanisms of amino acid sensing in mTOR signaling pathway. Nutr. Res. Pract. 2009, 3, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Appuhamy, J.A.D.R.N.; Knoebel, N.A.; Nayananjalie, W.A.; Escobar, J.; Hanigan, M.D. Isoleucine and leucine independently regulate mTOR signaling and protein synthesis in MAC-T cells and bovine mammary tissue slices. J. Nutr. 2012, 142, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Appuhamy, J.A.D.R.N. Regulatory Roles of Essential Amino Acids, Energy, and Insulin in Mammary Cell Protein Synthesis. Ph.D. Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 2010. [Google Scholar]
- Wang, T.; Oh, J.J.; Lim, J.N.; Hong, J.E.; Kim, J.H.; Kim, J.H.; Kang, H.S.; Choi, Y.J.; Lee, H.G. Effects of lactation stage and individual performance on milk cis-9, trans-11 conjugated linoleic acids content in dairy cows. Asian Austral J. Anim. 2013, 26, 189–194. [Google Scholar] [CrossRef]
- Wang, T.; Lee, H.G.; Hwang, J.H.; Oh, J.J.; Lim, J.N.; Kang, H.S.; Kang, H.S.; Joo, J.K.; Lee, K.S. Myoglobin: An Exogenous Reference Marker for Proteomics Analysis. Food Sci. Biotechnol. 2013, 22, 393–408. [Google Scholar] [CrossRef]
- Wang, T.; Lim, J.N.; Bok, J.D.; Kim, J.H.; Kang, S.K.; Lee, S.B.; Hwang, J.H.; Lee, K.H.; Kang, H.S.; Choi, Y.J.; et al. Association of protein expression in isolated milk epithelial cells and cis-9, trans-11 CLA concentrations in milk from dairy cows. J. Sci. Food Agric. 2014, 94, 1835–1843. [Google Scholar] [CrossRef]
- Zhang, Q.K.; Lee, H.G.; Han, J.A.; Kang, S.K.; Lee, N.K.; Baik, M.G.; Choi, Y.J. Differentially expressed proteins associated with myogenesis and adipogenesis in skeletal muscle and adipose tissue between bulls and steers. Mol. Biol. Rep. 2012, 39, 953–960. [Google Scholar] [CrossRef]
- Malheiros, J.M.; Enríquez-Valencia, C.E.; da Silva Duran, B.O.; de Paula, T.G.; Curi, R.A.; de Vasconcelos Silva, J.A., II; Dal-Pai-Silva, M.; de Oliveira, H.N.; Chardulo, L.A.L. Association of CAST2, HSP90AA1, DNAJA1 and HSPB1 genes with meat tenderness in Nellore cattle. Meat Sci. 2018, 138, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Norio, K.; Takafumi, S.; Akira, Y.; Toru, M. Distribution of Annexins I, II, and IV in Bovine Mammary Gland. J. Dairy Sci. 1995, 78, 2382–2387. [Google Scholar]
- Wei, Y.C.; Li, X.; Zhang, D.Q.; Liu, Y.F. Comparison of protein differences between high- and low-quality goat and bovine parts based on iTRAQ technology. Food Chem. 2019, 289, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Bionaz, M.; Loor, J.J. Gene Networks Driving Bovine Mammary Protein Synthesis during the Lactation Cycle. Bioinform. Biol. Insights 2011, 5, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Bonfatti, V.; Martino, G.D.; Cecchinato, A.; Vicario, D.; Carnier, P. Effects of β-κ-casein (csn2-csn3) haplotypes and β-lactoglobulin (blg) genotypes on milk production traits and detailed protein composition of individual milk of simmental cows. J. Dairy Sci. 2010, 93, 3797–3808. [Google Scholar] [CrossRef] [PubMed]
- Ti, Z.W.; Li, Z.Y. Glucose regulated protein 78: A critical link between tumor microenvironment and cancer hallmarks. Biochim. Biophys. Acta 2012, 1826, 13–22. [Google Scholar]
- Han, X.; Tong, Y.; Tian, M.; Zhang, Y.; Sun, X.; Wang, S.; Qiu, X.; Ding, C.; Yu, S. Enzymatic Activity Analysis and Catalytic Essential Residues Identification of Brucella abortus Malate Dehydrogenase. Sci. World J. 2014, 8, 973751. [Google Scholar]
- Smith, F.J.; Porter, R.M.; Corden, L.D.; Lunny, D.P.; Lane, E.B.; McLean, W.H. Cloning of human, murine, and marsupial keratin 7 and a survey of K7 expression in the mouse. Biochem. Biophys. Res. Commun. 2002, 297, 818–827. [Google Scholar] [CrossRef]
- Takaoka, Y.; Goto, S.; Nakano, T.; Tseng, H.P.; Yang, S.M.; Kawamoto, S.; Ono, K.; Chen, C.L. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) prevents lipopolysaccharide (LPS)-induced, sepsis-related severe acute lung injury in mice. Sci. Rep. 2014, 4, 5204. [Google Scholar] [CrossRef]
- Wang, Y.J.; Guo, X.L.; Li, S.A.; Zhao, Y.Q.; Liu, Z.C.; Lee, W.H.; Xiang, Y.; Zhang, Y. Prohibitin is involved in the activated internalization and degradation of protease-activated receptor 1. Biochim. Biophys. Acta 2014, 1843, 1393–1401. [Google Scholar] [CrossRef]
- Moshel, Y.; Rhoads, R.E.; Barash, I. Role of amino acids in translational mechanisms governing milk protein synthesis in murine and ruminant mammary epithelial cells. J. Cell Biochem. 2006, 98, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.N.; Hu, H.; Zheng, N.; Wang, J.Q. Leucine and histidine independently regulate milk protein synthesis in bovine mammary epithelial cells via mTOR signaling pathway. J. Zhejiang Univ. Sci. B 2015, 16, 560–572. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.W.; Goldberg, A.L. Leucine inhibits oxidation of glucose and pyruvate in skeletal muscles during fasting. J. Biol. Chem. 1978, 253, 3696–3701. [Google Scholar] [PubMed]

| Gene 1 | Accession Number | Primers | |
|---|---|---|---|
| ACTB | NM_173979.3 | forward | GCGTGGCTACAGCTTCACC |
| reverse | TTGATGTCACGGACGATTTC | ||
| HSPB1 | NM_001025569.1 | forward | GCCGGAAACAAGTAAAGACC |
| reverse | GGTGAGGATGTCCAGTGATG | ||
| GRP-78 | NM_001075148.1 | forward | CTTCTCGGAGACCCTGACTC |
| reverse | CACTTTCTGGACAGGCTTCA | ||
| LDH-B | NM_174100.1 | forward | GTGGAGTGGAGTGAATGTGG |
| reverse | TGTCTGTTCCCATTTCTGGA | ||
| MDH1 | NM_001034628.2 | forward | CAACCATGCCAAAGTGAAAC |
| reverse | GCCAGCTGTCATCTTTCAGA | ||
| ANXA1 | NM_175784.3 | forward | TTCTTTGCTGAGAAGCTCCA |
| reverse | CAGAGCGGGAAACCATAATC | ||
| CK-7 | NM_001046411.1 | forward | CATGAACAAGGTGGAGTTGG |
| reverse | AGCTCTTTCAGCTCCTGCTC | ||
| GAPDH | NM_001034034.2 | forward | CGTTCGACAGATAGCCGTAA |
| reverse | TCACCATCTTGTCTCAGGGA | ||
| PHB | NM_001034572.2 | forward | GAGATCCTCAAGTCCGTGGT |
| reverse | ACCAGCTCTCTCTGGGTGAT | ||
| CSN2 | NM_181008.2 | forward | GTGAGGAACAGCAGCAAACA |
| reverse | TTTTGTGGGAGGCTGTTAGG | ||
| CSN1S1 | NM_181029.2 | forward | GCTGAGGAACGACTTCACAG |
| reverse | AGGCCAGTTCCTGATTCACT | ||
| Spot No. | UniProtKB/Swiss-Prot Entry | Protein Name 1 | Theory; Calculation Mol. Mass (kDa)/PI | MASCOT Score | Peptides Matched | Sequence Coverage (%) | Molecular Functions | Protein Expression (Area) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| H | L | H/L | ||||||||
| 396 | Q3T149 | HSPB1 | 22.4/5.98; | 750.45 | 233 | 60.70 | Stress resistance and actin organization | 0.0423 | 0.2046 | 0.207 |
| 22.4/6.40 | ||||||||||
| 404 | Q0VCX2 | GRP-78 | 72.4/5.07; | 131.03 | 38 | 10.53 | Facilitating the assembly of multimeric protein complexes inside the ER | 0.0242 | 0.2217 | 0.109 |
| 72.4/5.16 | ||||||||||
| 408 | Q5E9B1 | LDH-B | 36.7/6.02; | 150.54 | 56 | 17.37 | Oxidoreductase in cellular carbohydrate metabolic process and glycolysis | 0.0934 | 0.3852 | 0.242 |
| 36.7/6.44 | ||||||||||
| 409 | Q3T145 | MDH1 | 36.4/6.16; | 20.44 | 9 | 3.59 | Oxidoreductase in cellular carbohydrate metabolic process and malate metabolic process | 0.1291 | 0.5164 | 0.250 |
| 36.4/6.58 | ||||||||||
| 411 | P46193 | ANXA1 | 39.0/6.37; | 2103.24 | 522 | 46.24 | Plays important roles in the innate immune response and has anti-inflammatory activity | 0.2705 | 0.6448 | 0.420 |
| 39.0/6.81 | ||||||||||
| 412 | Q29S21 | CK-7 | 51.5/5.79; | 59.14 | 17 | 2.58 | Blocks interferon-dependent interphase and stimulates DNA synthesis in cells | 0.0742 | 0.1984 | 0.374 |
| 51.5/5.97 | ||||||||||
| 413 | P10096 | GAPDH | 35.8/8.51; | 17.58 | 7 | 7.93 | Oxidoreductase and transferase in apoptosis glycolysis, translation regulation | 0.1343 | 0.3234 | 0.415 |
| 24.2/8.53 | ||||||||||
| 414 | Q3T165 | PHB | 29.8/5.57; | 703.84 | 225 | 52.57 | Has a role in regulating proliferation | 0.1909 | 0.0777 | 2.457 |
| 29.8/5.76 | ||||||||||
| 418 | P02666 | CSN2 | 25.1/5.26; | 224.15 | 61 | 19.14 | Antioxidant activity; negative regulation of catalytic activity; transporter activity | 0.8430 | 0.3449 | 2.444 |
| 23.6/5.34 | ||||||||||
| 422 | P02666 | CSN2 | 25.1/5.26; | 106.40 | 30 | 19.14 | 0.6392 | 0.2882 | 2.218 | |
| 23.6/5.34 | ||||||||||
| 425 | P02662 | CSN1S1 | 24.5/4.98; | 94.57 | 41 | 19.83 | Antioxidant activity; transporter activity; important role in the capacity of milk to transport calcium phosphate | 0.2055 | 0.1024 | 2.008 |
| 13.8/5.55 | ||||||||||
| 208887 | P02662 | CSN1S1 | 24.5/4.98; | 79.25 | 32 | 19.83 | 0.2066 | 0.0820 | 2.518 | |
| 13.8/5.55 | ||||||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Jeon, S.W.; Jung, U.S.; Kim, M.J.; Lee, H.G. l-Lactate Dehydrogenase B Chain Associated with Milk Protein Content in Dairy Cows. Animals 2019, 9, 442. https://doi.org/10.3390/ani9070442
Wang T, Jeon SW, Jung US, Kim MJ, Lee HG. l-Lactate Dehydrogenase B Chain Associated with Milk Protein Content in Dairy Cows. Animals. 2019; 9(7):442. https://doi.org/10.3390/ani9070442
Chicago/Turabian StyleWang, Tao, Seung Woo Jeon, U Suk Jung, Min Jeong Kim, and Hong Gu Lee. 2019. "l-Lactate Dehydrogenase B Chain Associated with Milk Protein Content in Dairy Cows" Animals 9, no. 7: 442. https://doi.org/10.3390/ani9070442
APA StyleWang, T., Jeon, S. W., Jung, U. S., Kim, M. J., & Lee, H. G. (2019). l-Lactate Dehydrogenase B Chain Associated with Milk Protein Content in Dairy Cows. Animals, 9(7), 442. https://doi.org/10.3390/ani9070442






